PREAMBLE
Sarcoidosis is a systemic disorder of unknown etiology whose clinical presentation is characterized by the heterogeneous contributions of nonnecrotizing granulomatous inflammation and concomitant fibrosis. Cardiac involvement portends an adverse prognosis and may account for approximately 25% of deaths from sarcoidosis. The diagnosis of cardiac sarcoidosis (CS) remains a challenge because of the combination of a nonspecific clinical presentation (e.g., conduction abnormalities, tachy- and bradyarrhythmias, and heart failure) and focal infiltration of granulomas and scarring in the heart, which limit the diagnostic utility of endomyocardial biopsy (EMB). Potentiating these challenges is the fact that data on the benefit of immunosuppressive therapy are limited.
It is well recognized that glucose metabolism is increased in inflammatory cells; thus, cellular inflammation can be detected by PET/CT using 18F-FDG. Consequently, there is growing interest in the use of PET/CT for diagnosis and management in patients with known or suspected CS. The current imaging paradigm includes a cardiac PET/CT study of metabolism and perfusion to provide information on the inflammatory and fibrotic components of CS. Numerous reports have highlighted the potential of this approach for improving the ability to identify and treat patients with this disease. However, these studies are constrained by a host of factors including, but not limited to, variability in design, variability in imaging methodology, small sample sizes, and lack of prospective data.
Given the absence of randomized prospective trials, and the fact that such studies are unlikely to be performed for this rare disease, a comprehensive evidence-based clinical guideline on this topic is not feasible. Accordingly, we were assembled by the Cardiovascular Council of the Society of Nuclear Medicine and Molecular Imaging (SNMMI) and the American Society of Nuclear Cardiology (ASNC) as a panel of experts in cardiovascular imaging, clinical cardiology, cardiac electrophysiology, and systemic sarcoidosis to develop a joint consensus document on the role of 18F-FDG PET/CT in the management of patients with CS. The aims of this document are to discuss the indications for cardiac PET/CT within the context of disease detection and management; provide recommendations on image acquisition, processing, and interpretation; and discuss clinical scenarios in which PET/CT may help guide patient management.
BACKGROUND
Sarcoidosis is a systemic disease characterized by significant clinical heterogeneity. Although most frequently involving the lungs, sarcoidosis may affect any organ. The multicenter ACCESS study (A Case Control Study of Sarcoidosis) concluded that approximately 1 in 4 sarcoidosis patients will present with one or more newly involved organs during the first 2 y after diagnosis (1).
The true prevalence of sarcoidosis remains unknown and is potentially underestimated, given the existence of an unmeasured pool of individuals with incidentally discovered subclinical disease. A recent prospective cohort study estimated a baseline prevalence of 100 in 100,000 women aged 25–44 y (2). Other data suggest a higher prevalence, at 330 cases per 100,000, in certain regions of the United States (3). A variegated disease prevalence defined by ethnicity, sex, and geographic region identifies an important interaction between genetic and environmental factors influencing the risk for developing sarcoidosis (4). Worldwide, the lifetime risk of developing sarcoidosis has been estimated at 1%–2% in Western developed nations (5–7). Once considered a rare disease (7–9), recent estimates suggest a prevalence rate of sarcoidosis that is increasing (e.g., 141.4 per 100,000 in U.S. African Americans and 49.8 per 100,000 in U.S. Caucasians). The highest incidence of sarcoidosis occurs between the ages of 20 and 40 y (6,7,10). Overall, sarcoidosis is associated with a low mortality rate; however, its clinical course and prognosis are highly variable and dependent on age, on disease severity at presentation (in nonacute sarcoidosis), and on the distribution of major organ involvement.
Cardiac involvement is considered an infrequent manifestation and may be clinically evident in less than 10% of patients with sarcoidosis without cardiac symptoms, although autopsy and imaging series report a substantially higher occurrence ranging from more than 20% in the United States to more than 50% in Japan (11,12). Consistent with prior autopsy findings, recent studies using cardiac MRI reported that late gadolinium enhancement identified cardiac involvement in 25%–30% of individuals referred for testing, with lower rates being found for nonselected cohorts (13–16).
Postmortem studies have confirmed that sarcoidosis may involve any part of the heart but most commonly involves the myocardium, particularly the basal ventricular septum, the left ventricular free wall, the papillary muscles, and the right ventricle, in descending order of frequency (17,18). Depending on the type and extent of involvement, CS can present as conduction abnormalities, ventricular arrhythmias, sudden cardiac death, systolic and diastolic heart failure, or valvular disorders, which may be related to papillary muscle involvement (19–24). Sarcoidosis of the pericardium can present as a pericardial effusion with or without hemodynamic instability (25,26). Myocardial ischemia and infarction due to coronary artery involvement have also been described but are rare (27), and myocardial fibrosis due to CS itself typically occurs in a nonvascular distribution and in some cases is the only evidence of prior active CS.
The overall survival of patients with symptomatic CS is variable. Studies from Japan identified the extent of left ventricular systolic dysfunction as the most significant independent predictor of mortality (23,28). Patients with a normal left ventricular ejection fraction (LVEF) (≥50%) at the time of CS presentation have a 10-y survival rate of more than 80% (23,28). Conversely, those with severe left ventricular dysfunction (LVEF < 30%) have a significantly lower 10-y survival, at 19% (28). Other determinants of prognosis in those studies included left ventricular dilatation, a high New York Heart Association class, and sustained ventricular tachycardia. The anticipated benefits of adjunct therapies for heart failure, including implantable cardioverter defibrillator (ICD) placement and resynchronization therapy, have not been rigorously studied.
Several criteria have been proposed for the diagnosis of CS, but all have limited supporting data and lack prospective validation. The most commonly used are those revised in 2006 by the Japanese Ministry of Health and Welfare (JMHW) and those published more recently (in 2014) by the Heart Rhythm Society (HRS) (Table 1) (29,30). Both sets of criteria have a histologic pathway whereby a diagnosis of CS is confirmed by an EMB showing noncaseating granulomas. Traditionally, EMB has low sensitivity (<25%) for CS when compared retrospectively with autopsy, probably because of the patchy involvement of CS, the typical midwall distribution of inflammation, and the limited number of biopsy sites using standard techniques (31). Newer techniques guided by PET, cardiac MRI, electrocardiography, or electroanatomic voltage mapping may increase the sensitivity of EMB for CS (32–35).
TABLE 1.
| JMHW | HRS |
| Histologic diagnosis group | Histologic diagnosis from myocardial tissue |
| CS confirmed by EMB, and histologic or clinical diagnosis of extraCS | Noncaseating granuloma on EMB with no alternative cause identified |
| Clinical diagnosis group | Clinical diagnosis |
| Histologic or clinical diagnosis of extraCS and | Probable diagnosis of CS exists if |
| Two or more major criteria or | There is histologic diagnosis of extraCS* and |
| One major criterion and two or more minor criteria | One or more of the following is present: |
| Major criteria | Cardiomyopathy or atrioventricular block responsive to immunosuppressive treatment* |
| Advanced atrioventricular block | Unexplained reduced LVEF (<40%) |
| Basal thinning of intraventricular septum | Unexplained ventricular tachycardia |
| 67Ga uptake in heart | Mobitz II second- or third-degree heart block |
| Depressed LVEF (<50%) | Patchy 18F-FDG uptake on cardiac PET consistent with CS* |
| Minor criteria | Late gadolinium enhancement on cardiac MRI consistent with CS |
| Electrocardiography: ventricular tachycardia, PVCs, RBBB, abnormal axis, abnormal Q wave | Cardiac 67Ga uptake and |
| Echocardiography: structural or wall motion abnormality | Exclusion of other causes of cardiac manifestations |
| Nuclear medicine: perfusion defect, 201Tl, 99mTc* | |
| Cardiac MRI: late gadolinium enhancement | |
| EMB: moderate fibrosis or monocyte infiltration |
Significant difference between JMHW and HRS criteria.
PVC = premature ventricular contractions; RBBB = right bundle branch block.
Acknowledging the possibility of false-negative EMBs, both sets of criteria allow cases of suspected or “probable” (defined as >50% likelihood) CS to be diagnosed via a clinical pathway. Importantly, both sets of criteria still require a histologic or clinical diagnosis of extracardiac sarcoidosis (extraCS) and therefore remain of limited utility for identifying isolated CS, which may be more common than previously reported, with estimates of 20%–50% in various studies (36). In addition to requiring histologic confirmation of extraCS, the JMHW criteria require a combination of major and minor electrocardiographic, imaging, and nonspecific EMB findings, and the HRS criteria require at least 1 of 7 clinical, electrocardiographic, or imaging diagnostic criteria. In contrast to the JMHW criteria, the diagnostic criteria of HRS include advanced cardiac imaging with dedicated cardiac PET and responsiveness to immunosuppressive treatment and thus may have a higher sensitivity for CS (30). Future studies are needed to determine whether these HRS provisions improve accuracy for diagnosing CS.
Although the JMHW criteria include 201Tl and 99mTc-based imaging findings, they are not part of the HRS criteria, probably because of the low sensitivity and specificity of these findings for CS, but they may be combined with 18F-FDG imaging in the evaluation of CS when perfusion PET is not available (37). 201Tl and 99mTc-based findings include focal perfusion defects at rest, with either a fixed or a reverse-redistribution pattern with vasodilator stress. The latter is believed to involve microvascular disturbances (reversible with vasodilators) by granulomatous inflammation (38–40).
67Ga scintigraphy is also included in several diagnostic algorithms and has high specificity but low sensitivity (<50%) for CS, in part because of the challenges in distinguishing cardiac from pulmonary and mediastinal 67Ga uptake (41,42). Although not extensively evaluated, combined 99mTc-based or 201Tl-plus-67Ga scintigraphy may have a higher diagnostic sensitivity than that of the individual tracers alone (43,44). Furthermore, a pattern of absence of 67Ga uptake associated with at least a moderately severe 99mTc or 201Tl perfusion defect may represent fibrosis in CS and is less likely to respond to immunosuppressive treatment (44). The higher radiation exposure from 67Ga, combined with its low contrast resolution, has limited its use in North America for the evaluation of CS. Nevertheless, 67Ga scintigraphy remains in the JMHW and HRS criteria for the diagnosis of CS and may have a potential role for monitoring disease activity and response to therapy.
Over the past few decades, the nuclear imaging modality of choice for CS has evolved from planar scintigraphy and SPECT to 18F-FDG PET combined with perfusion imaging to detect inflammation and fibrogranulomatous replacement of the myocardium. In the remainder of this document, the term PET refers both to combined PET/CT systems and to PET systems with line sources, unless otherwise specified. The ability of 18F-FDG to image inflammation in sarcoidosis is due to the increased uptake of 18F-FDG in macrophage-dense regions; macrophages have high metabolic activity and are more reliant than normal cells on external glucose as a source of fuel (45). Combining resting PET myocardial perfusion imaging or SPECT (when PET myocardial perfusion imaging is not available) with 18F-FDG PET permits differentiation of the spectrum of CS and provides valuable diagnostic and prognostic information (46).
In the subsequent sections of this document, we detail the indications for performing 18F-FDG PET with myocardial perfusion imaging for suspected or known CS, the technical aspects of PET for CS, and the role of 18F-FDG PET in clinical diagnosis, assessment of disease activity, monitoring of therapeutic response, and assessment of prognosis in patients with known or suspected CS.
INDICATIONS FOR CARDIAC PET FOR CS
The current literature provides good evidence that PET is useful for investigating suspected CS (30,35,37,46–50). In the absence of tissue confirmation of CS, there is agreement that the diagnosis of cardiac involvement requires integrating multiple sources of data, including 18F-FDG PET in some cases. Consequently, the diagnosis of CS should not be based on 18F-FDG PET alone. Similarly, 18F-FDG PET may also be useful as an adjunct in monitoring response to therapy. In one series of CS patients with left ventricular dysfunction who underwent serial 18F-FDG PET examinations, a reduction in 18F-FDG uptake was associated with improvements in LVEF (50). In patients who undergo immunosuppressive treatment, quantification of inflammation with serial 18F-FDG PET may also be useful in assessing treatment response, thereby informing decisions on duration, intensity, or choice of immunosuppressive therapy. However, more data are needed on the efficacy of serial imaging, as well as on how various immunosuppressive regimens compare. Recognizing the limited observational literature in this area, we have identified the following clinical scenarios in which cardiac PET may be useful for suspected or known CS (1,30,51). These scenarios are summarized in Table 2.
TABLE 2.
Clinical Scenarios in Which Cardiac PET May Be Useful in Suspected or Known CS
| Scenario | Specifics |
| Patients with histologic evidence of extraCS, and abnormal screening for CS, defined as one or more of following: | Abnormal electrocardiographic findings of complete left or right bundle branch block or presence of unexplained pathologic Q waves in two or more leads |
| Echocardiographic findings of regional wall motion abnormality, wall aneurysm, basal septum thinning, or LVEF ≤ 50% | |
| Holter findings of sustained or nonsustained ventricular tachycardia | |
| Cardiac MRI findings suggestive of CS | |
| Unexplained palpitations or syncope | |
| Young patients (<60 y) with unexplained, new onset, significant conduction system disease (such as sustained second- or third-degree atrioventricular block) | |
| Patients with idiopathic sustained ventricular tachycardia, defined as not fulfilling any of the following criteria: | Typical outflow tract ventricular tachycardia |
| Fascicular ventricular tachycardia | |
| Ventricular tachycardia secondary to other structural heart disease (coronary artery disease or any cardiomyopathy other than idiopathic) | |
| Patients with proven CS as adjunct to follow response to treatment |
Patients with Histologic Evidence of ExtraCS and Abnormal Screening for CS
In this scenario, abnormal screening for CS is defined as one or more of the following: electrocardiographic findings of complete left or right bundle branch block or the presence of unexplained pathologic Q waves in two or more leads; echocardiographic findings of regional wall motion abnormality, wall aneurysm, basal septum thinning, or an LVEF of no more than 50%; Holter findings of sustained or nonsustained ventricular tachycardia; cardiac MRI findings suggestive of CS; and unexplained palpitations or syncope.
Patients with an Unexplained, New Onset of Significant Conduction System Disease
This scenario applies to patients younger than 60 y. An example of significant conduction system disease is a sustained second- or third-degree atrioventricular block.
Patients with Idiopathic Sustained Ventricular Tachycardia
This scenario is defined as not fulfilling any of the following criteria: typical outflow tract ventricular tachycardia, fascicular ventricular tachycardia, or ventricular tachycardia secondary to other structural heart disease (coronary artery disease, any cardiomyopathy other than idiopathic).
Patients with Proven CS
In this scenario, cardiac PET is used as an adjunct to follow the response to treatment. Although there are promising data demonstrating a relationship between cardiac PET findings and outcomes, the data are insufficient to recommend the use of cardiac PET alone for the purpose of stratifying the risk of sudden cardiac death.
PATIENT PREPARATION FOR 18F-FDG PET FOR CS
An important consideration before performing cardiac PET for CS is to exclude the presence of significant coronary artery disease, which can result in myocardial ischemia and consequently lead to abnormalities in both perfusion and 18F-FDG uptake. In addition, resting perfusion defects may be due to either prior myocardial infarction or the presence of CS, thereby reducing the usefulness of stress myocardial perfusion imaging in distinguishing underlying coronary artery disease from CS. Therefore, depending on the patient’s age and risk factors, invasive angiography or CT coronary angiography may be best suited for excluding anatomic stenosis. In some cases, cardiac MRI may be helpful in distinguishing prior infarction from infiltrative disease, although in rare instances CS can mimic an infarct pattern (i.e., subendocardial late gadolinium enhancement).
Because the heart uses a mixture of free fatty acids and glucose for energy production under normal resting conditions, assessment of myocardial inflammation on a background of physiologic myocardial 18F-FDG uptake is challenging. To improve specificity in identifying pathologic glucose uptake, several methods to reduce physiologic myocardial glucose uptake have been proposed, though none has been systematically studied or adequately standardized for CS. On the basis of the current literature and our expert consensus, the most common components in preparing patients to undergo 18F-FDG PET for inflammation include prolonged fasting, dietary manipulation, and intravenous heparin, often in combination. Patient preparation for the 18F-FDG PET study is critical and should ideally commence 24 h before the study, to provide sufficient time for application of at least one of these approaches (52).
Prolonged Fasting
The myocardium is a metabolic omnivore whose substrate utilization (carbohydrates, fats, ketones, or amino acids) varies with the nutritional state. More fat is used during periods of prolonged fasting (53). Hence, several investigators have used prolonged fasting (12–18 h) to reduce physiologic myocardial 18F-FDG uptake (52). Although the reduction in physiologic uptake is significantly greater after more than 18 h of fasting than after a shorter fast, patient compliance with the longer fast can be challenging. Furthermore, one study showed that despite undergoing an 18-h fast, 38% of individuals still demonstrated physiologic 18F-FDG uptake, necessitating additional measures (54).
Dietary Manipulation
A high-fat, low-carbohydrate diet may facilitate the switching of myocardial substrate metabolism from glucose to fatty acids. Williams et al. compared fasting (overnight or minimum of 4 h) to a high-fat, low-carbohydrate, protein-permitted diet in individuals undergoing 18F-FDG PET scans primarily for lymphoma and showed that myocardial SUVmax was significantly lower in the diet group than in the fasting-alone group (55). These findings were confirmed in another study, which used a similar diet (starting 12 h before the 18F-FDG scan and including a meal 4 h before) and found a higher frequency of adequately suppressed physiologic 18F-FDG uptake in the diet group (67%) than in a 12-h fast–alone group (52%) (56). Despite these studies, the optimum amount of fat or carbohydrate in these dietary manipulations has not been clearly defined or standardized, though one study did specify that the evening meal before the PET study should include more than 35 g of fat and less than 5 g of carbohydrates (57). A strategy of adding a high-fat beverage to the high-fat, low-carbohydrate diet just before administering the 18F-FDG has also been explored but does not appear to have additive benefit (57–60). On the basis of our clinical experience and the available literature, nonspecific myocardial uptake may be observed in up to 20% of patients despite various dietary preparations (52).
Table 3 provides dietary recommendations for patients before undergoing 18F-FDG PET for CS.
TABLE 3.
Recommendations for Patient Preparation Before Performing 18F-FDG PET for CS (52)
| Category | Recommendation |
| Consume | Meat fried in oil or butter without breading or broiled (chicken, turkey, bacon, meat-only sausage, hamburgers, steak, fish) |
| Eggs (prepared without milk or cheese) | |
| Oil (an option for patients who are vegan or are unable to eat and have enteral access) and butter | |
| Clear liquids (water, tea, coffee, diet sodas, etc.) | |
| Acceptable | Fasting for 18 h or longer if patient cannot eat and has no enteral access or if patient has dietary restrictions preventing consumption of advised diet |
| Avoid | Vegetables, beans, nuts, fruits, and juices |
| Bread, grain, rice, pasta, and all baked goods | |
| Sweetened, grilled, or cured meats or meat with carbohydrate-containing additives (some sausages, ham, sweetened bacon) | |
| Dairy products (milk, cheese, etc.) aside from butter | |
| Candy, gum, lozenges, sugar, and sucralose (Splenda; Heartland Food Products Group) | |
| Alcoholic beverages, soda, and sports drinks | |
| Mayonnaise, ketchup, tartar sauce, mustard, and other condiments | |
| Dextrose-containing intravenous medications |
Intravenous Heparin
Intravenous unfractionated heparin (UFH) induces lipolysis and increases serum free fatty acid levels. Indeed, in healthy volunteers, very low doses of UFH, 10–15 U/kg, at 45 min and 15 min, produced lipolysis and increased plasma free fatty acid levels without significantly prolonging the partial thromboplastin time (61). For this reason, intravenous UFH has been used alone or in combination with fasting or dietary manipulation to suppress physiologic 18F-FDG uptake. Protocols vary among institutions, but the most published protocol is a single 50 IU/kg intravenous bolus of UFH approximately 15 min before 18F-FDG administration (52,62). However, despite data supporting increased plasma free fatty acid levels with UFH administration, the utility of heparin in the suppression of myocardial glucose uptake is unclear (62–64).
Combined Approaches
A combination of approaches to suppress physiologic myocardial 18F-FDG uptake has been used in a few studies, with the results favoring a prolonged fast alone and a combination of a high-fat or low-carbohydrate diet with extended fasting. Manabe et al. showed that a minimum of 18 h of fasting combined with a low-carbohydrate diet significantly suppressed diffuse left ventricular 18F-FDG uptake when compared with a 6-h fast without a low-carbohydrate diet; both groups received intravenous UFH (50 IU/kg) 15 min before 18F-FDG injection (62). The same study also showed that intravenous UFH and a fast of more than 18 h was associated with a significantly lower rate of physiologic myocardial 18F-FDG uptake (22%) than a fast of 18 h without UFH (38%) or a fast of less than 18 h without UFH (69%) (62). Morooka et al. also showed that UFH did not reduce physiologic 18F-FDG uptake but that a 16-h fast alone was superior to a 12-h fast with intravenous UFH (50 IU/kg) and independently predicted lower physiologic myocardial 18F-FDG uptake in healthy volunteers (65). This finding again implies that a longer fast is more important than heparin administration.
Other Considerations
Intracellular calcium is known to increase glucose uptake, and calcium channel blockade has reduced myocardial 18F-FDG uptake in a mouse model (66). One study explored the use of calcium channel blockade with verapamil to improve myocardial suppression, but verapamil had no clear benefit over other preparations (58). Performing 18F-FDG PET on hospitalized patients can be challenging. Special care must be taken to avoid inadvertent carbohydrate intake and dextrose in intravenous fluids and medications (e.g., intravenous UFH infusions, antibiotic infusions, peritoneal dialysis fluids, intravenous antiarrhythmic infusions, or total parenteral nutrition).
Expert Panel Recommendations
Patients and laboratories should log the exact preparation used for the test to help ensure compliance and consistency in preparing a CS patient for serial PET studies (see also “Serial PET Studies” below).
Fasting/Dietary Options
On the basis of the available literature, we recommend two possible options for preparing CS patients to undergo 18F-FDG PET. The preferred option is for the patient to consume at least two high-fat (>35 g), low-carbohydrate (<3 g) (52) meals the day before the study and then fast for at least 4–12 h. An alternative option (especially for patients who cannot follow the dietary recommendation) is for the patient to fast for more than 18 h before the study.
Adjunctive Heparin
The use of heparin as an adjunct to dietary preparation can be considered, but its role in the suppression of myocardial glucose uptake is unclear and its impact on suppression of physiologic myocardial glucose uptake may be lower than originally thought. If heparin is used, the most common protocol is a single 50 IU/kg intravenous bolus of UFH approximately 15 min before 18F-FDG administration.
Diabetic Patients
The optimal preparation for diabetic patients has not been defined, and we currently recommend use of the same dietary approaches as for nondiabetic patients, as long as patient safety is not compromised. Patients with type 1 diabetes should continue basal insulin, avoiding or minimizing rapid-acting insulin if safe to do so. The use of sliding-scale rapid-acting insulin may be necessary the day before the study but should be avoided the day of the study. Patients with type 2 diabetes should not take oral agents or noninsulin injections while fasting or on the morning of the test. Insulin should also be avoided or reduced as much as possible if safe to do so. In view of the limited data on preparing diabetic patients, the approach must currently be individualized. Further studies are needed to help determine the best approach for suppression of physiologic myocardial 18F-FDG uptake in all patients.
CARDIAC PET ACQUISITION
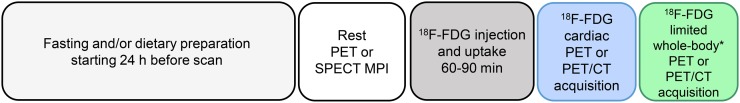
Figure 1 illustrates a typical PET protocol for assessment of CS. Two sets of images should be obtained at rest to differentiate the spectrum of CS: myocardial perfusion images acquired with either 13N-ammonia or 82Rb, and cardiac 18F-FDG images acquired according to the guideline of the SNMMI, ASNC, and Society of Cardiovascular Computed Tomography (67).
FIGURE 1.
Typical PET protocol for assessment of CS. *Include at minimum chest, liver, and spleen if there is clinical suspicion of extraCS or no recent PET extraCS evaluation. MPI = myocardial perfusion imaging.
If PET perfusion imaging is not available, SPECT with either 99mTc-labeled tracers or 201Tl may serve as a substitute, preferably performed with attenuation correction. Acquisition of gated perfusion images is highly recommended, as the presence of global and regional left ventricular systolic dysfunction has important diagnostic and prognostic implications. After the resting perfusion acquisition, intravenous 18F-FDG is administered, followed by a 60- or 90-min (preferred) uptake period and a nongated emission acquisition. The duration of the dedicated cardiac 18F-FDG acquisition ranges from 10 to 30 min, depending on the scanner and image acquisition mode (2- vs. 3-dimensional), the counting rate, and the tracer dose (67).
If there is clinical suspicion of extraCS or if there has been no recent PET study for extraCS, a limited whole-body PET study using the same 18F-FDG injection should be performed in addition to the dedicated cardiac 18F-FDG study and at minimum should include the chest, liver, and spleen. The noncardiac scan can be used to assess for extracardiac uptake for diagnosis, prognosis, and identification of possible biopsy sites. In addition, awareness of any extraCS may be helpful when deciding on the role of systemic immunosuppressive therapy.
CARDIAC PET IMAGE INTERPRETATION
Qualifications and Preliminary Steps
The interpreting physician should have experience with 18F-FDG imaging of the heart, including metabolic manipulations that suppress normal physiologic uptake of glucose by the myocardium in order to accentuate uptake of 18F-FDG by inflammatory cells, as well as experience in myocardial perfusion imaging. For studies with limited whole-body PET images, readers experienced in hybrid whole-body PET should interpret the images, either collaboratively or separately, given the frequent presence and implications of extracardiac 18F-FDG–avid structures. Before the PET images are examined for CS, the following steps should be undertaken.
Review of Records
A comprehensive review of the patient’s history and other diagnostic studies should be performed, along with confirmation of appropriate metabolic preparation of the patient for evaluation of CS.
Reconstruction of Images
The resting myocardial perfusion and cardiac 18F-FDG images should be reconstructed with attenuation correction and reorientation into the standard cardiac planes (short axis, vertical long axis, and horizontal long axis) for interpretation.
Coregistration of Images
Proper alignment and coregistration between the transmission and emission data should be ensured for high-quality data (67). Misregistration between transmission and emission images is an important cause of false-positive perfusion abnormalities. To avoid this pitfall, proper alignment between the two sets of data should be ensured.
Assessment of Image Quality
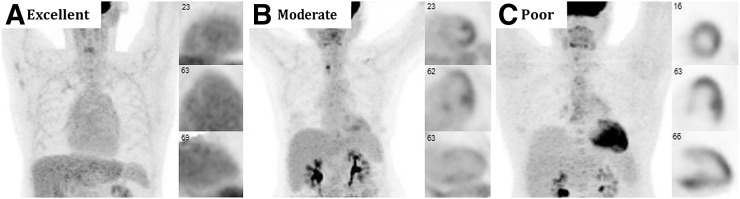
The 18F-FDG images should be assessed for adequate myocardial suppression, defined as either no visible myocardial 18F-FDG uptake or, in some instances, 18F-FDG uptake lower than that of the blood pool (Fig. 2) (52).
FIGURE 2.
Cardiac 18F-FDG PET images demonstrating variable suppression in 3 patients without cardiac disease: excellent myocardial suppression with blood-pool activity that exceeds that of myocardium (A), moderate myocardial suppression with diffuse low-level myocardial 18F-FDG uptake and nonspecific focally increased uptake in papillary muscles and lateral wall (B), and poor myocardial suppression with diffuse 18F-FDG uptake throughout heart (C). (Reprinted with permission of (52).)
Visual Interpretation
After proper alignment between the transmission and emission images has been ensured and the images have been assessed for adequate myocardial suppression (Fig. 2), the attenuation-corrected cardiac images should also be reviewed in the standard views. A normalized display is generally used, whereby the intensity of each image (perfusion and 18F-FDG) is normalized to the maximum counts per pixel of the image. However, whereas normalization is useful for imaging of relative defects, such as myocardial perfusion imaging, it may pose challenges for hot-spot imaging such as 18F-FDG imaging for CS. In particular, normalization can lead to artifactual accentuation of areas of mild 18F-FDG uptake when displayed in a normalized fashion. Other challenges to the use of a cardiac imaging display for 18F-FDG PET in CS include difficulties in judging the magnitude of treatment response and the proper orientation of focal uptake when normal myocardial surface outlines are poorly defined. The main benefit to the use of a traditional nuclear cardiology display is the ability to display perfusion and 18F-FDG images simultaneously and integrate their interpretation. Another benefit to the traditional nuclear cardiology display is the ability to assess the gated PET or SPECT myocardial perfusion images for left ventricular volume, wall motion, and systolic function.
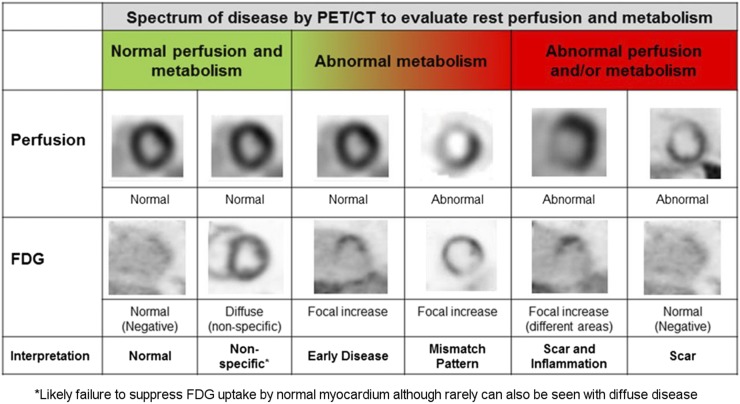
Using the normalized approach, the PET perfusion and 18F-FDG images are interpreted simultaneously for CS as shown in Figure 3 (47,68). A normal PET examination for CS will show complete suppression of 18F-FDG from the myocardium and normal resting myocardial perfusion (Fig. 3, first column). Incomplete suppression of 18F-FDG from normal myocardium, as might occur because of inadequate patient preparation, may be accompanied by a pattern of diffuse homogeneous 18F-FDG uptake (Fig. 3, second column).
FIGURE 3.
PET perfusion and 18F-FDG imaging patterns for CS. 18F-FDG PET and myocardial perfusion imaging patterns are displayed in traditional cardiac format. (Adapted with permission of (47).)
Cases of possible inflammation may also demonstrate patchy nonhomogeneous uptake of 18F-FDG or focal-on-diffuse 18F-FDG uptake. However, unlike homogeneous 18F-FDG uptake due to failure to suppress 18F-FDG from normal myocardium, pathologic 18F-FDG uptake is more likely to be associated with perfusion defects (Fig. 4A). In the presence of active inflammation, focal areas of 18F-FDG uptake will be present without (Fig. 3, third column) or with (Fig. 3, fourth and fifth columns) perfusion defects. Although some have described 18F-FDG uptake without a resting perfusion defect as representing early CS, there is no agreement or evidence that CS progresses in a linear fashion, and not all patients who have inflammation develop scarring.
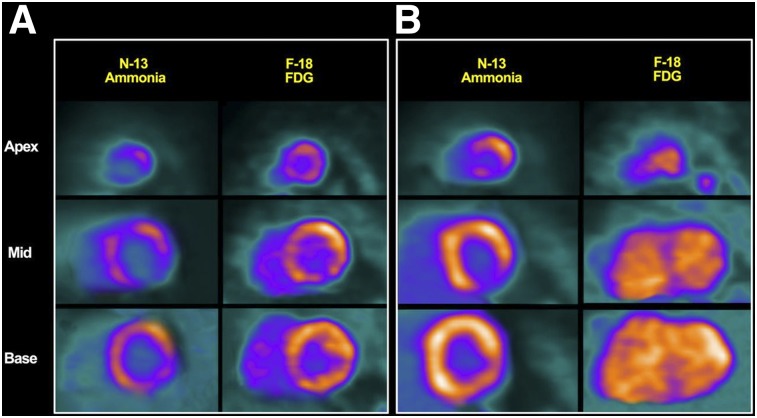
FIGURE 4.
Cardiac PET short-axis views. (A) Severely decreased 13N-ammonia uptake with corresponding 18F-FDG uptake in most of left ventricle, consistent with CS with active inflammation. Also present are scattered areas lacking 13N-ammonia uptake, with no significant 18F-FDG uptake in apex or mid-inferolateral segment, compatible with possible fibrosis. (B) After treatment with immunosuppressive medications, patient improved clinically. With exception of apical inferolateral segments, 13N-ammonia uptake increased throughout left ventricle compared with baseline, and there was myocardial 18F-FDG uptake only in basal anterolateral region. Findings are compatible with response to treatment. (Reprinted with permission of (99).)
Among patients who have focal 18F-FDG uptake without a perfusion defect, it is important to consider the location of the uptake. Focal and homogeneous 18F-FDG uptake along the lateral wall without a perfusion defect is often a nonspecific finding. On the other hand, when multiple noncontiguous focal areas of uptake are present (i.e., simultaneously involving the basal anteroseptum, the basal inferior wall, and the basal lateral wall), the uptake is more likely to be pathologic.
Resting perfusion defects can either be due to compression of the microvasculature by inflammation or be due to scarring. In the case of compression by inflammation, a mismatch between perfusion (defect) and 18F-FDG (uptake) is observed (Fig. 3, fourth column, and Fig. 4A). On the other hand, in the case of scarring/fibrosis, a resting perfusion defect without 18F-FDG uptake is present (Fig. 3, last column). Inflammation and scarring/fibrosis may coexist in the same patient and may lead to several patterns of perfusion and metabolism (Fig. 3, fifth column).
The presence of 18F-FDG uptake alone by the myocardium is not specific to CS. For instance, patients with coronary artery disease who have hibernating myocardium (i.e., reduced perfusion due to chronic ischemia) may have 18F-FDG uptake. Other inflammatory myopathies, such as some subtypes of active myocarditis or systemic rheumatologic conditions with cardiac involvement may also be associated with increased 18F-FDG uptake. Therefore, it is important to consider such conditions when using 18F-FDG PET to establish the diagnosis of CS. At the same time, the absence of 18F-FDG uptake cannot be used to rule out the presence of previous CS, especially if a perfusion abnormality is present, as this finding should be interpreted as a sign of CS with no active myocardial inflammation or scarring from another etiology.
In individuals with an ICD, 18F-FDG images with and without CT attenuation correction should be reviewed and interpreted to avoid misinterpreting focal 18F-FDG uptake around ICD leads in the attenuation-corrected images as CS (69).
The presence of defects in one or both of the perfusion or 18F-FDG images is important diagnostically, prognostically, and therapeutically (46,68,70) and will be discussed in detail in the section “Diagnostic and Prognostic Performance of Cardiac PET for CS.” In brief, the combination of both abnormal perfusion and abnormal 18F-FDG uptake appears to have the worst outcome (46,70). Although not validated in a randomized controlled trial, using 18F-FDG positivity as one criterion for immunosuppressive treatment of CS appears to be a reasonable approach based on the available literature and is discussed in more detail in the section “Management of CS.”
Quantitative Analysis
In addition to the qualitative visual interpretation reviewed above, quantitative techniques may be valuable, in particular for determining the severity and amount of inflammation before treatment (as more extensive inflammation may provide a stronger impetus for immunosuppressive therapies) or for assessing the response to therapy. Quantitative techniques for this purpose use SUVmax, the concentration of radioactive tracer in a region (Bq/mL) corrected by the injected dose and the patient’s weight (68).
Several SUV-based quantitative metrics have been described for interpreting 18F-FDG PET images for CS (Table 4). Although no data support use of one method over another, data do suggest that for quantifying treatment response, quantitative SUV metrics perform better than visual assessment of normalized images (50,70–72). To date, quantitative measures have not been correlated with clinical outcomes in large-scale studies. Furthermore, there is no SUV threshold that can distinguish CS from normal myocardium. The strengths and weaknesses of visual assessment and quantitative assessment are summarized in Table 5.
TABLE 4.
Quantitative Metrics Useful in 18F-FDG PET Interpretation in CS
| Metric | Definition | Notes |
| SUVmax | SUVmax in myocardium | Defines peak inflammatory activity (70) |
| SUVmean | SUVmean in 17 segments | (94) |
| SUVtotal | Sum of SUVs in heart (segments) | (68) |
| Heart–to–blood pool ratio | Cardiac SUVmax–to–aortic SUVmax ratio | Corrects for background blood pool (95) |
| Coefficient of variance | SD of uptake divided by average uptake in 17 segments | Measures heterogeneity of inflammatory activity (96) |
| Volume (and intensity) of 18F-FDG–positive voxels | Volume (or volume × mean activity) of 18F-FDG–positive myocardium above SUV threshold | Various SUV thresholds have been proposed (50,70) |
TABLE 5.
Comparison of Visual vs. Quantitative 18F-FDG PET for CS
| Parameter | Visual assessment | Quantitative assessment |
| Method | Is qualitative and based on visual assessment of dedicated cardiac images and whole-body images | Requires dedicated workstation to calculate SUVmax and volume of inflammation |
| Advantages | Is rapid | Is more reproducible |
| Pitfalls | Is subject to normalization | Has no single best technique for quantifying inflammation; has an unknown optimal threshold for determining SUV volume; uses techniques that may be performed differently in different institutions; does not always consider variability of activity in blood or other tissues |
| Recommendation | Evaluate whole-body images, as these are less subject to differences in normalization than dedicated cardiac images | Assess both severity (SUVmax) and extent (volume of 18F-FDG uptake) of inflammation |
Serial PET Studies
When serial imaging is performed, the comparison can be done both visually and quantitatively. All serial 18F-FDG studies for CS should be performed in a similar fashion, with the same dietary or fasting preparation, the same dose of injected activity, and the same interval from 18F-FDG injection to image acquisition. Serial whole-body images should also be compared, because relying solely on the cardiac images may lead to errors due to differences in normalization (71). In addition to the visual comparison, it is important to compare the serial studies quantitatively for intensity of inflammation (by comparing SUVmax) and amount of inflammation (by comparing the volume of myocardium that has 18F-FDG uptake above a prespecified threshold) (71). Although there is no definite threshold, a minimal change is unlikely to be significant. A change is more likely to be significant when both the intensity (SUVmax) and the amount (the volume of inflammation above a prespecified threshold) change in the same direction and by at least 20% (71). However, these changes in quantitative metrics have not been correlated with alterations in disease progression, clinical parameters, or prognosis.
EXTRACARDIAC PET AND INTERPRETATION IN THE CONTEXT OF CS
Although patients may present for evaluation of CS as an isolated cardiac abnormality, sarcoidosis is a systemic disease and most CS patients have extracardiac involvement. The lung is the most common site of involvement (∼90% of patients), and the thoracic lymph nodes are frequently affected, with typical manifestations being bilateral hilar and mediastinal lymphadenopathy (73). Extrathoracic sarcoidosis involving the skin, peripheral lymph nodes, eyes, liver, or spleen may be seen in a smaller proportion of patients (74). Identification of extraCS is critically important in the diagnostic evaluation of patients with suspected CS (30). Because many of the common sites of extraCS may be within the field of view of the cardiac scans or the limited whole-body scans, review of these images can lead to identification and biopsy of previously unrecognized extraCS (75). Further, it is important to differentiate sarcoidosis-related lesions from other pathologic 18F-FDG activity (such as pulmonary infections or cancer). These factors underscore the importance of evaluating for extraCS when evaluating for CS on PET images, as well as the importance of collaboration between nuclear cardiologists and nuclear medicine experts.
The chest or limited whole-body emission acquisitions can be reconstructed and reviewed according to the published procedure guideline for oncology PET (76). The registered and aligned CT, PET, and fusion images can be displayed on a standard workstation in the axial, coronal, and sagittal planes and as a rotating maximum-intensity-projection image. PET images with and without attenuation correction should also be reviewed. Prolonged fasting or dietary preparation is not expected to significantly affect 18F-FDG uptake in extracardiac inflammatory lesions, although identification of structures adjacent to the heart may be affected by intense cardiac uptake.
Lung sarcoidosis usually shows patchy or focal 18F-FDG uptake with or without an apparent CT correlation (Fig. 5) (77). In sarcoidosis, it is also not uncommon to find small pulmonary nodules without uptake. In a normal or enlarged node in the mediastinum or hilum, any focal increase of 18F-FDG uptake higher than the surrounding mediastinal activity is suggestive of nodal spread. Typical thoracic nodal involvement includes the upper paratracheal nodes, the right and left hilar nodes, and the subcarinal nodes (77).
FIGURE 5.
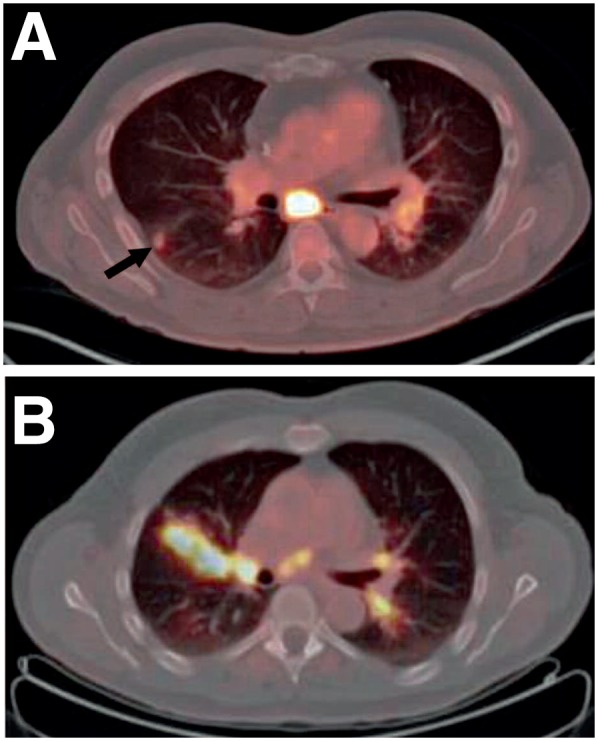
(A) A 51-y-old man with sarcoidosis. Axial fused PET/CT images showed intense 18F-FDG uptake at subcarinal nodes and left hilar lymphadenopathy. Hypermetabolic right lung nodule was also noted (arrow). (B) A 65-y-old man with sarcoidosis. Axial fused PET/CT images showed increased 18F-FDG uptake in right upper lobe associated with interstitial nodules having a perilymphatic/peribronchovascular distribution. Increased uptake at mediastinal nodes and bilateral hilar lymphadenopathy were also noted. (Reprinted with permission of (77).)
Regarding evaluation of extrathoracic sarcoidosis, salivary gland uptake greater than that in the normal nasopharynx is suggestive and should be mentioned in the report. Splenic involvement generally appears as diffuse uptake greater than liver uptake or a pattern of focal-on-diffuse uptake (77). Skin sarcoidosis usually is seen as focal uptake corresponding to cutaneous or subcutaneous nodules, but it is not uncommon for small skin nodules or plaques to show no uptake. The bones should also be evaluated for possible marrow involvement. Extrathoracic nodal sarcoidosis can involve any groups of lymph nodes, including cervical, supraclavicular, axillary, abdominal, pelvic, and inguinal. Focal or extensive uptake in the muscles is suggestive of sarcoidosis in a patient with a history of CS.
Multisystem involvement is common in sarcoidosis. Thus, any abnormal 18F-FDG uptake in the nodes or organs in the context of CS should be reviewed after the cardiac PET evaluation has been completed. The SUV of the index node or organ should be measured and reported so that the treatment response can be evaluated on follow-up imaging (77). Overall, the sensitivity of PET for systemic sarcoidosis is 80%–100% (78).
The sensitivity of EMB can be improved significantly if the procedure is repeated under the guidance of cardiac PET findings (75). CS can also be diagnosed when extraCS is histologically proven and other findings of CS, such as on compatible cardiac MRI or PET scans, are present (30). In fact, the HRS criteria prefer extracardiac tissue biopsy over EMB because of the safety and higher yield of the former (30). The mediastinal nodes constitute one of the most common extracardiac sites of sarcoidosis (79) and are usually the preferred targets for biopsy through mediastinoscopy or bronchoscopy with endobronchial ultrasound guidance (75).
DIAGNOSTIC AND PROGNOSTIC PERFORMANCE OF CARDIAC PET FOR CS
Published Sensitivity and Specificity
Although several studies have attempted to determine the accuracy of cardiac PET for diagnosing CS, these studies were severely limited by the use of the JMHW criteria as the reference standard. A metaanalysis (37) that included 7 of the 8 studies in Table 6, representing 164 patients, calculated a pooled sensitivity of 89% and a pooled specificity of 78%; however, these estimates are biased as the lower specificity of PET in some studies may reflect the fact that this test is more sensitive than the JMHW criteria for identifying CS. Likewise, the lower sensitivity of PET in some studies may reflect the reduced specificity of the JMHW criteria. The aforementioned studies were also limited by being single-center and retrospective.
TABLE 6.
Published Sensitivity and Specificity of PET for CS (99)
| Study | Patients (n) | Protocol | Sensitivity | Specificity |
| Yamagishi (97) | 17 | PET, >5-h fast | 82% | NA |
| Okumura (68) | 22 | PET, >12-h fast | 100% | 91% |
| Ishimaru (49) | 32 | PET, >12-h fast | 100% | 82% |
| Ohira (98) | 21 | PET, >6-h fast, heparin | 88% | 39% |
| Langah (95) | 76 | PET, >18-h fast | 85% | 90% |
| Tahara (96) | 24 | PET, >12-h fast | 100% | 46%–97% |
| Youssef (37) | 24 | PET, >12-h fast | 79% | 70% |
| Blankstein (46) | 118 | PET, >3-h fast, high-fat, low-carbohydrate diet | 71% | 45% |
NA = not applicable.
Published Prognostic Literature
Recent data have emerged supporting the prognostic value of various PET findings. Blankstein et al. evaluated 118 patients referred for cardiac PET with known or suspected CS (46). Over a mean follow-up of 1.5 y, individuals with abnormal myocardial perfusion (i.e., scarring or compression of the microvasculature) and abnormal metabolism (i.e., focal inflammation) had a 4-fold increase in the annual rate of ventricular tachycardia or death compared with patients who had normal imaging results (Fig. 6) (46). These findings persisted even after the JMHW criteria and LVEF had been accounted for. Although inflammation of the right ventricle was rare, individuals who had evidence of focal 18F-FDG uptake involving the right ventricle had an extremely high event rate. On the other hand, the presence or absence of active extraCS was not associated with adverse events. Further supporting the prognostic value of 18F-FDG PET, Ahmadian et al. evaluated 31 patients with suspected CS, of whom 9 experienced events over a follow-up of 1.2 y (70). The authors found that most adverse cardiac events occurred in individuals with abnormal 18F-FDG uptake. These studies were also limited by their retrospective, single-center design.
FIGURE 6.
Survival free of death or ventricular tachycardia (VT) stratified by cardiac PET results. Outcome was worst in group with both abnormal perfusion and abnormal 18F-FDG uptake. Even after accounting for JMHW criteria, presence of extraCS, and LVEF, abnormal cardiac PET still identified patients at higher risk of death/ventricular tachycardia. (Reprinted with permission of (46).)
Assessment of Treatment Response
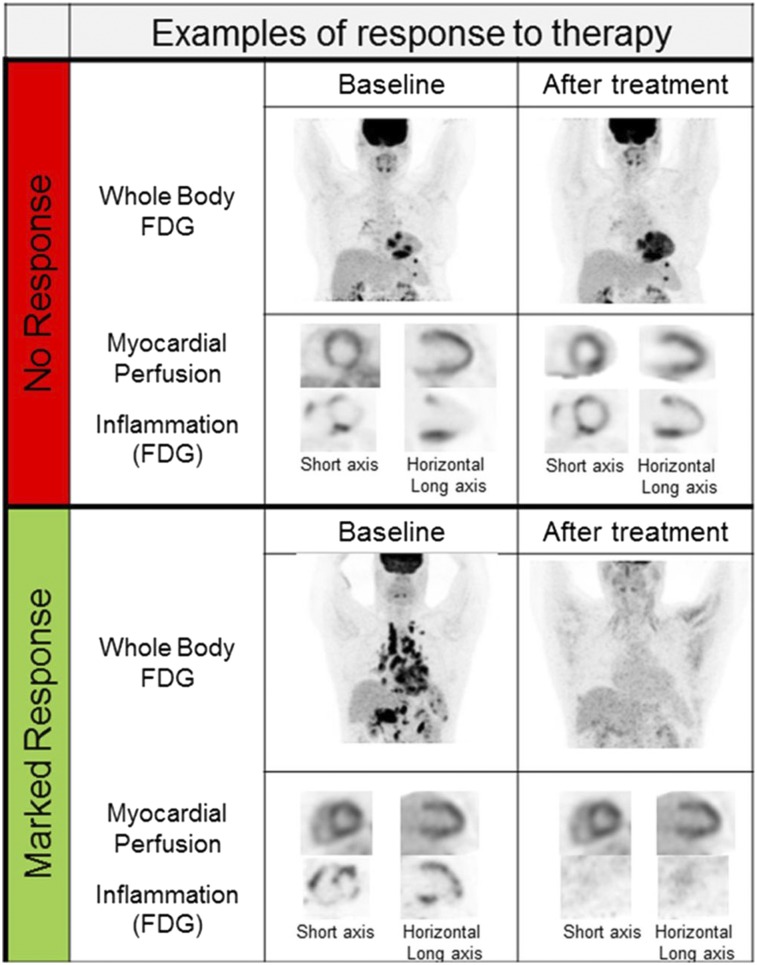
PET with 18F-FDG is often useful for evaluating patients’ variable response to immunosuppressive therapies, as assessed by imaging and clinically (Figs. 4 and 7). Although some patients may experience complete resolution of inflammation, others may demonstrate no significant change or, rarely, interval worsening. Realizing that there are no data indicating the ideal drug, dose, or duration of therapy, and given the toxic side-effect profiles of all antiinflammatory agents, imaging may allow clinicians to choose the agents to which patients may respond, while limiting the duration of therapy, or to consider alternative agents when no significant benefit is observed. Nevertheless, data showing the advantages of PET-guided therapy are limited. Osborne et al. examined 23 patients who underwent serial PET examinations during treatment for CS (50). Supporting the potential role of 18F-FDG imaging in following response to therapy, the study showed that a quantitative reduction in the intensity (SUVmax) or amount (volume of inflammation above a prespecified SUV threshold) was associated with an improvement in LVEF. However, even when complete resolution of inflammation can be visualized by 18F-FDG PET, continuation of therapy at a lower dose may have a role in preventing recurrence of disease. It is unknown whether treatment or a change in 18F-FDG uptake is associated with a reduction in event rates, or whether this reduction is significant enough to warrant delaying or avoiding ICD therapy in patients who have inflammation before the development of significant scarring or left ventricular dysfunction.
FIGURE 7.
Examples of using 18F-FDG PET to assess response to therapy. (Top) Patient with no response to treatment. (Bottom) Patient with marked response of both extraCS and CS to treatment. (Reprinted with permission of (47).)
MANAGEMENT OF CS
Immunologic Therapy
Corticosteroids are a principal treatment for CS, although there are neither prospective data nor randomized controlled trials to guide the timing, intensity, or duration of treatment or the use of cardiac PET in management (80,81). In a recent systematic review of 10 publications reporting outcomes after corticosteroid therapy, corticosteroids appeared to be beneficial for recovery of atrioventricular nodal function (81). However, clear conclusions about other outcomes could not be drawn. The authors noted that “there is a clear need for large multicentre prospective registries and trials in this patient population.”
Individual retrospective studies in the systematic review have addressed other outcomes but were limited by their small sample size. One study of 20 patients with heart block and normal cardiac function compared steroid-treated (n = 7) and non–steroid-treated (n = 13) patients and showed a marked decline in LVEF in the untreated group compared with the treated group (LVEF, 37.6% vs. 62.1%) (82). Ventricular tachycardia occurred in only 1 of 7 treated patients (14.3%) during the follow-up period but was present in 8 of 13 untreated patients (61.5%). There were no deaths in the treated group, but 2 patients in the untreated group died (82). In another retrospective study, 39 patients received steroid therapy (initial dose, 1 mg/kg/d), and 13 received additional immunosuppressive treatment (83). Thirty-four (87%) showed improvement, with 21 showing complete resolution of clinical or laboratory findings during long-term follow-up. In another retrospective study, 95 patients treated with steroids demonstrated a 5-y survival rate of 75% and a 10-y survival rate of 61% (23). Survival curves did not significantly differ between patients treated with an initial prednisone dose of more than 30 mg daily and those treated with 30 mg or less.
Currently, there is a lack of consensus on steroid dosing, duration of therapy, and use of additional immunosuppressive agents in CS patients (80). Initiation of corticosteroid therapy in CS patients with recent and clinically significant symptoms is common practice, but treatment of asymptomatic or minimally symptomatic patients is more controversial. On the basis of observational studies, steroid therapy in patients with established CS and active inflammation ideally should be initiated before left ventricular systolic function declines (50), or when it is mildly reduced. Patients should be followed closely for relapse after discontinuation of corticosteroid treatment. Some experts advocate 6–12 mo of therapy, whereas others recommend consideration of lifelong treatment because of reports of relapse or sudden death (83,84).
Other immunosuppressive therapies, such as methotrexate, azathioprine, mycophenolate mofetil, or infliximab, have been used with some success for systemic sarcoidosis (85–91), but data regarding their use in CS are quite limited.
Pacemaker or ICD Therapy
There is a high rate of recurrence of ventricular tachycardia or sudden death with antiarrhythmic drug therapy in CS patients, even when therapy is guided by electrophysiologic testing (92). For this reason, an ICD is recommended in CS patients who have sustained ventricular tachycardia or ventricular fibrillation. Other indications for ICD placement in CS patients include prior cardiac arrest or an LVEF of 35% or less despite optimal medical therapy (30). ICD implantation may also be useful when there is an indication for permanent pacing, probable cardiogenic syncope, or inducible ventricular tachycardia (30). In CS patients with an LVEF of 36%–49%, or a right ventricular ejection fraction of less than 40% despite optimal UFH therapy and immunosuppression for active inflammation, ICD implantation may be considered (30). In CS patients who are asymptomatic and have a normal LVEF, ICD implantation is not routinely performed. However, these patients should be closely followed for symptoms or deterioration of ventricular function (30). Nevertheless, a high event rate can be observed in CS even with a normal LVEF. In fact, once one has accounted for abnormal findings on 18F-FDG PET or MRI, the association between ejection fraction and subsequent events is no longer significant. Murtagh et al. recently showed, among 205 patients with preserved ejection fraction, that the presence of late gadolinium enhancement on MR images was associated with an increased risk of death or ventricular tachycardia (93). The role of ICD implantation in CS patients who have normal LVEF but abnormal imaging findings should be further evaluated.
Pacemaker implantation is frequently indicated in CS patients with high-grade atrioventricular block, even if transient (30). It seems reasonable to implant either a single-chamber or a dual-chamber ICD, rather than a pacemaker system, in these patients (30).
SUMMARY OF PET USE IN CS
CS remains an underdiagnosed condition. The prognosis of CS is variable but may be further compromised if the CS is untreated or symptomatic. Diagnosis is challenging, and given the low yield of EMB, there is no useful gold standard. Cardiac 18F-FDG PET is now included as part of the diagnostic algorithm for CS in the HRS criteria and is increasingly being used for detecting cardiac involvement and assessing the presence and severity of myocardial inflammation. Cardiac 18F-FDG PET studies for CS should combine both perfusion imaging and 18F-FDG imaging to differentiate the patterns of disease. An important consideration before performing cardiac PET for CS is to exclude the presence of significant coronary artery disease, prior myocardial infarction, resting ischemia, or hibernating myocardium. Proper patient preparation is critical for successful 18F-FDG PET for CS and may include prolonged fasting, dietary manipulation, and possibly intravenous heparin administration to suppress physiologic myocardial glucose uptake in the assessment of intramyocardial inflammation. Myocardial perfusion and cardiac 18F-FDG PET images should be interpreted in the context of the patient’s clinical presentation and other imaging studies. Both visual and quantitative interpretation should be performed. In addition to cardiac 18F-FDG imaging, limited whole-body 18F-FDG imaging is highly encouraged to assess for extracardiac uptake. The diagnostic performance of cardiac 18F-FDG PET for identifying CS has been established, but the precision with which diagnostic accuracy can be estimated is limited by lack of an adequate reference standard and by referral bias. Limited prognostic studies have demonstrated that patients with abnormal perfusion and focal inflammation, and those with focal right ventricular 18F-FDG uptake, have an adverse outcome. Despite the emerging data reviewed in this document, we acknowledge the clear need for additional studies to define the role of PET in the diagnosis and management of CS.
DISCLOSURE
Timothy M. Bateman has received grant/research support from GE Healthcare, Astellas Pharma, Lantheus Medical Imaging, and Jubilant DraxImage; holds stock from CVIT; and has received honoraria from Bracco, Lantheus Medical Imaging, and Jubilant DraxImage. Salvador Borges-Neto has received grant/research support from GE Healthcare. Manuel D. Cerqueira is a consultant for Astellas and is on its speaker’s bureau. Edward J. Miller is a consultant for GE Healthcare and Alnylam Pharmaceuticals and has received grant/research support from Bracco. David R. Moller is a consultant for Novartis and Dicerna Pharmaceuticals and has received grant/research support from Sarcoidosis Diagnostic Testing LLC. Venkatesh Murthy has received grant/research support from SNMMI and INVIA Medical Imaging Solutions and holds stock from General Electric, Mallinckrodt Pharmaceuticals, and Cardinal Health. Prem Soman is a consultant for Alnylam Pharmaceuticals and has received grant/research support from Astellas Pharma. No other potential conflict of interest relevant to this document was reported.
Contributor Information
Collaborators: NAME OF COLLAB GROUP, Expert Content Reviewers: Timothy M. Bateman, Manuel D. Cerqueira, Vasken Dilsizian, Gary V. Heller, David R. Moller, Michael T. Osborne, Mehran M. Sadeghi, and Prem Soman
REFERENCES
- 1.Judson MA, Costabel U, Drent M, et al. The WASOG Sarcoidosis Organ Assessment Instrument: an update of a previous clinical tool. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31:19–27. [PubMed] [Google Scholar]
- 2.Dumas O, Abramovitz L, Wiley AS, Cozier YC, Camargo CA. Epidemiology of sarcoidosis in a prospective cohort study of U.S. women. Ann Am Thorac Soc. 2016;13:67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erdal BS, Clymer BD, Yildiz VO, Julian MW, Crouser ED. Unexpectedly high prevalence of sarcoidosis in a representative U.S. metropolitan population. Respir Med. 2012;106:893–899. [DOI] [PubMed] [Google Scholar]
- 4.Grunewald J, Spagnolo P, Wahlström J, Eklund A. Immunogenetics of disease-causing inflammation in sarcoidosis. Clin Rev Allergy Immunol. 2015;49:19–35. [DOI] [PubMed] [Google Scholar]
- 5.Hillerdal G, Nöu E, Osterman K, Schmekel B. Sarcoidosis: epidemiology and prognosis. Am Rev Respir Dis. 1984;130:29–32. [DOI] [PubMed] [Google Scholar]
- 6.Milman N, Selroos O. Pulmonary sarcoidosis in the Nordic countries 1950-1982: epidemiology and clinical picture. Sarcoidosis. 1990;7:50–57. [PubMed] [Google Scholar]
- 7.Rybicki BA, Major M, Popovich J, Maliank MJ. lannuzzi MC. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol. 1997;145:234–241. [DOI] [PubMed] [Google Scholar]
- 8.Ungprasert P, Carmona EM, Utz JP, Ryu JH, Crowson CS, Matteson EL. Epidemiology of sarcoidosis 1946-2013: a population-based study. Mayo Clin Proc. 2016;91:183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baughman RP, Field S, Costabel U, et al. Sarcoidosis in America: analysis based on health care use. Ann Am Thorac Soc. 2016;13:1244–1252. [DOI] [PubMed] [Google Scholar]
- 10.Morimoto T, Azuma A, Abe S, et al. Epidemiology of sarcoidosis in Japan. Eur Respir J. 2008;31:372–379. [DOI] [PubMed] [Google Scholar]
- 11.Iwai K, Sekiguti M, Hosoda Y, et al. Racial differences in cardiac sarcoidosis incidence observed at autopsy. Sarcoidosis. 1994;11:26–31. [PubMed] [Google Scholar]
- 12.Silverman KJ, Hutchins GM, Bulkley BH. Cardiac sarcoid: a clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation. 1978;58:1204–1211. [DOI] [PubMed] [Google Scholar]
- 13.Greulich S, Deluigi CC, Gloekler S, et al. CMR imaging predicts death and other adverse events in suspected cardiac sarcoidosis. JACC Cardiovasc Imaging. 2013;6:501–511. [DOI] [PubMed] [Google Scholar]
- 14.Nagai T, Kohsaka S, Okuda S, Anzai T, Asano K, Fukuda K. Incidence and prognostic significance of myocardial late gadolinium enhancement in patients with sarcoidosis without cardiac manifestation. Chest. 2014;146:1064–1072. [DOI] [PubMed] [Google Scholar]
- 15.Patel MR, Cawley PJ, Heitner JF, et al. Detection of myocardial damage in patients with sarcoidosis. Circulation. 2009;120:1969–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hulten E, Agarwal V, Cahill M, et al. Presence of late gadolinium enhancement by cardiac magnetic resonance among patients with suspected cardiac sarcoidosis is associated with adverse cardiovascular prognosis: a systematic review and meta-analysis. Circ Cardiovasc Imaging. 2016;9:e005001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lagana SM, Parwani AV, Nichols LC. Cardiac sarcoidosis: a pathology-focused review. Arch Pathol Lab Med. 2010;134:1039–1046. [DOI] [PubMed] [Google Scholar]
- 18.Tavora F, Cresswell N, Li L, Ripple M, Solomon C, Burke A. Comparison of necropsy findings in patients with sarcoidosis dying suddenly from cardiac sarcoidosis versus dying suddenly from other causes. Am J Cardiol. 2009;104:571–577. [DOI] [PubMed] [Google Scholar]
- 19.Fleming HA. Sarcoid heart disease. Br Med J (Clin Res Ed). 1986;292:1095–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsui Y, Iwai K, Tachibana T, et al. Clinicopathological study on fatal myocardial sarcoidosis. Ann N Y Acad Sci. 1976;278:455–469. [DOI] [PubMed] [Google Scholar]
- 21.Roberts WC, McAllister HA, Jr, Ferrans VJ. Sarcoidosis of the heart. Am J Med. 1977;63:86–108. [DOI] [PubMed] [Google Scholar]
- 22.Smedema J-P, Snoep G, van Kroonenburgh MPG, et al. Cardiac involvement in patients with pulmonary sarcoidosis assessed at two university medical centers in the Netherlands. Chest. 2005;128:30–35. [DOI] [PubMed] [Google Scholar]
- 23.Yazaki Y, Isobe M, Hiroe M, et al. Prognostic determinants of long-term survival in Japanese patients with cardiac sarcoidosis treated with prednisone. Am J Cardiol. 2001;88:1006–1010. [DOI] [PubMed] [Google Scholar]
- 24.Uusimaa P, Ylitalo K, Anttonen O, et al. Ventricular tachyarrhythmia as a primary presentation of sarcoidosis. Europace. 2008;10:760–766. [DOI] [PubMed] [Google Scholar]
- 25.Wyplosz B, Marijon E, Dougados J, Pouchot J. Sarcoidosis: an unusual cause of acute pericarditis. Acta Cardiol. 2010;65:83–84. [DOI] [PubMed] [Google Scholar]
- 26.Dubrey SW, Falk RH. Diagnosis and management of cardiac sarcoidosis. Prog Cardiovasc Dis. 2010;52:336–346. [DOI] [PubMed] [Google Scholar]
- 27.Ward EV, Nazari J, Edelman RR. Coronary artery vasculitis as a presentation of cardiac sarcoidosis. Circulation. 2012;125:e344–e346. [DOI] [PubMed] [Google Scholar]
- 28.Chiu C-Z, Nakatani S, Zhang G, et al. Prevention of left ventricular remodeling by long-term corticosteroid therapy in patients with cardiac sarcoidosis. Am J Cardiol. 2005;95:143–146. [DOI] [PubMed] [Google Scholar]
- 29.Diagnostic standard and guidelines for sarcoidosis [in Japanese]. Japanese J Sarcoidosis Other Granulomatous Disord. 2007;27:89–102. [Google Scholar]
- 30.Birnie DH, Sauer WH, Bogun F, et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11:1305–1323. [DOI] [PubMed] [Google Scholar]
- 31.Cooper LT, Baughman KL, Feldman AM, et al. The role of endomyocardial biopsy in the management of cardiovascular disease. Eur Heart J. 2007;28:3076–3093. [DOI] [PubMed] [Google Scholar]
- 32.Kandolin R, Lehtonen J, Graner M, et al. Diagnosing isolated cardiac sarcoidosis. J Intern Med. 2011;270:461–468. [DOI] [PubMed] [Google Scholar]
- 33.Casella M, Pizzamiglio F, Dello Russo A, et al. Feasibility of combined unipolar and bipolar voltage maps to improve sensitivity of endomyocardial biopsy. Circ Arrhythm Electrophysiol. 2015;8:625–632. [DOI] [PubMed] [Google Scholar]
- 34.Liang JJ, Hebl VB, DeSimone CV, et al. Electrogram guidance: a method to increase the precision and diagnostic yield of endomyocardial biopsy for suspected cardiac sarcoidosis and myocarditis. JACC Heart Fail. 2014;2:466–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nery PB, Keren A, Healey J, Leug E, Beanlands RS, Birnie DH. Isolated cardiac sarcoidosis: establishing the diagnosis with electroanatomic mapping-guided endomyocardial biopsy. Can J Cardiol. 2013;29:1015.e1–1015.e3. [DOI] [PubMed] [Google Scholar]
- 36.Okada DR, Bravo PE, Vita T, et al. Isolated cardiac sarcoidosis: a focused review of an under-recognized entity. J Nucl Cardiol. September 9, 2016 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Youssef G, Leung E, Mylonas I, et al. The use of 18F-FDG PET in the diagnosis of cardiac sarcoidosis: a systematic review and metaanalysis including the Ontario experience. J Nucl Med. 2012;53:241–248. [DOI] [PubMed] [Google Scholar]
- 38.Eguchi M, Tsuchihashi K, Hotta D, et al. Technetium-99m sestamibi/tetrofosmin myocardial perfusion scanning in cardiac and noncardiac sarcoidosis. Cardiology. 2000;94:193–199. [DOI] [PubMed] [Google Scholar]
- 39.Le Guludec D, Menad F, Faraggi M, Weinmann P, Battesti J-P, Valeyre D. Myocardial sarcoidosis: clinical value of technetium-99m sestamibi tomoscintigraphy. Chest. 1994;106:1675–1682. [DOI] [PubMed] [Google Scholar]
- 40.Surasi DS, Manapragada PP, Lloyd SG, Bhambhvani P. Role of multimodality imaging including thallium-201 myocardial perfusion imaging in the diagnosis and monitoring of treatment response in cardiac sarcoidosis. J Nucl Cardiol. 2014;21:849–852. [DOI] [PubMed] [Google Scholar]
- 41.Futamatsu H, Suzuki J, Adachi S, et al. Utility of gallium-67 scintigraphy for evaluation of cardiac sarcoidosis with ventricular tachycardia. Int J Cardiovasc Imaging. 2006;22:443–448. [DOI] [PubMed] [Google Scholar]
- 42.Momose M, Kadoya M, Koshikawa M, Matsushita T, Yamada A. Usefulness of 67Ga SPECT and integrated low-dose CT scanning (SPECT/CT) in the diagnosis of cardiac sarcoidosis. Ann Nucl Med. 2007;21:545–551. [DOI] [PubMed] [Google Scholar]
- 43.Nakazawa A, Ikeda K, Ito Y, et al. Usefulness of dual 67Ga and 99mTc-sestamibi single-photon-emission CT scanning in the diagnosis of cardiac sarcoidosis. Chest. 2004;126:1372–1376. [DOI] [PubMed] [Google Scholar]
- 44.Okayama K, Kurata C, Tawarahara K, Wakabayashi Y, Chida K, Sato A. Diagnostic and prognostic value of myocardial scintigraphy with thallium-201 and gallium-67 in cardiac sarcoidosis. Chest. 1995;107:330–334. [DOI] [PubMed] [Google Scholar]
- 45.Pellegrino D, Bonab AA, Dragotakes SC, Pitman JT, Mariani G, Carter EA. Inflammation and infection: imaging properties of 18F-FDG–labeled white blood cells versus 18F-FDG. J Nucl Med. 2005;46:1522–1530. [PubMed] [Google Scholar]
- 46.Blankstein R, Osborne M, Naya M, et al. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol. 2014;63:329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blankstein R, Waller AH. Evaluation of known or suspected cardiac sarcoidosis. Circ Cardiovasc Imaging. 2016;9:e000867. [DOI] [PubMed] [Google Scholar]
- 48.Tung R, Bauer B, Schelbert H, et al. Incidence of abnormal positron emission tomography in patients with unexplained cardiomyopathy and ventricular arrhythmias: the potential role of occult inflammation in arrhythmogenesis. Heart Rhythm. 2015;12:2488–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ishimaru S, Tsujino I, Takei T, et al. Focal uptake on 18F-fluoro-2-deoxyglucose positron emission tomography images indicates cardiac involvement of sarcoidosis. Eur Heart J. 2005;26:1538–1543. [DOI] [PubMed] [Google Scholar]
- 50.Osborne MT, Hulten EA, Singh A, et al. Reduction in 18F-fluorodeoxyglucose uptake on serial cardiac positron emission tomography is associated with improved left ventricular ejection fraction in patients with cardiac sarcoidosis. J Nucl Cardiol. 2014;21:166–174. [DOI] [PubMed] [Google Scholar]
- 51.Mehta D, Lubitz SA, Frankel Z, et al. Cardiac involvement in patients with sarcoidosis: diagnostic and prognostic value of outpatient testing. Chest. 2008;133:1426–1435. [DOI] [PubMed] [Google Scholar]
- 52.Osborne MT, Hulten EA, Murthy VL, et al. Patient preparation for cardiac fluorine-18 fluorodeoxyglucose positron emission tomography imaging of inflammation. J Nucl Cardiol. 2017;24:86–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taegtmeyer H. Tracing cardiac metabolism in vivo: one substrate at a time. J Nucl Med. 2010;51(suppl 1):80S–87S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Masuda A, Naya M, Manabe O, et al. Administration of unfractionated heparin with prolonged fasting could reduce physiological 18F-fluorodeoxyglucose uptake in the heart. Acta Radiol. 2016;57:661–668. [DOI] [PubMed] [Google Scholar]
- 55.Williams G, Kolodny GM. Suppression of myocardial 18F-FDG uptake by preparing patients with a high-fat, low-carbohydrate diet. AJR. 2008;190:W151–W156. [DOI] [PubMed] [Google Scholar]
- 56.Harisankar CN, Mittal BR, Agrawal KL, Abrar ML, Bhattacharya A. Utility of high fat and low carbohydrate diet in suppressing myocardial FDG uptake. J Nucl Cardiol. 2011;18:926–936. [DOI] [PubMed] [Google Scholar]
- 57.Cheng VY, Slomka PJ, Ahlen M, Thomson LE, Waxman AD, Berman DS. Impact of carbohydrate restriction with and without fatty acid loading on myocardial 18F-FDG uptake during PET: a randomized controlled trial. J Nucl Cardiol. 2010;17:286–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Demeure F, Hanin FX, Bol A, et al. A randomized trial on the optimization of 18F-FDG myocardial uptake suppression: implications for vulnerable coronary plaque imaging. J Nucl Med. 2014;55:1629–1635. [DOI] [PubMed] [Google Scholar]
- 59.Kobayashi Y, Kumita S, Fukushima Y, Ishihara K, Suda M, Sakurai M. Significant suppression of myocardial 18F-fluorodeoxyglucose uptake using 24-h carbohydrate restriction and a low-carbohydrate, high-fat diet. J Cardiol. 2013;62:314–319. [DOI] [PubMed] [Google Scholar]
- 60.Wykrzykowska J, Lehman S, Williams G, et al. Imaging of inflamed and vulnerable plaque in coronary arteries with 18F-FDG PET/CT in patients with suppression of myocardial uptake using a low-carbohydrate, high-fat preparation. J Nucl Med. 2009;50:563–568. [DOI] [PubMed] [Google Scholar]
- 61.Asmal AC, Leary WP, Thandroyen F, Botha J, Wattrus S. A dose-response study of the anticoagulant and lipolytic activities of heparin in normal subjects. Br J Clin Pharmacol. 1979;7:531–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Manabe O, Yoshinaga K, Ohira H, et al. The effects of 18-h fasting with low-carbohydrate diet preparation on suppressed physiological myocardial F-fluorodeoxyglucose (FDG) uptake and possible minimal effects of unfractionated heparin use in patients with suspected cardiac involvement sarcoidosis. J Nucl Cardiol. 2016;23:244–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gormsen LC, Christensen NL, Bendstrup E, Tolbod LP, Nielsen SS. Complete somatostatin-induced insulin suppression combined with heparin loading does not significantly suppress myocardial 18F-FDG uptake in patients with suspected cardiac sarcoidosis. J Nucl Cardiol. 2013;20:1108–1115. [DOI] [PubMed] [Google Scholar]
- 64.Bois JP, Chareonthaitawee P. Continuing evolution in preparation protocols for 18FDG PET assessment of inflammatory or malignant myocardial disease. J Nucl Cardiol. March 25, 2016 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 65.Morooka M, Moroi M, Uno K, et al. Long fasting is effective in inhibiting physiological myocardial 18F-FDG uptake and for evaluating active lesions of cardiac sarcoidosis. EJNMMI Res. 2014;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gaeta C, Fernandez Y, Pavia J, et al. Reduced myocardial 18F-FDG uptake after calcium channel blocker administration: initial observation for a potential new method to improve plaque detection. Eur J Nucl Med Mol Imaging. 2011;38:2018–2024. [DOI] [PubMed] [Google Scholar]
- 67.Dilsizian V, Bacharach SL, Beanlands R, et al. ASNC imaging guidelines/SNMMI procedure standard for positron emission tomography (PET) nuclear cardiology procedures. J Nucl Cardiol. 2016;23:1187–1226. [DOI] [PubMed] [Google Scholar]
- 68.Okumura W, Iwasaki T, Toyama T, et al. Usefulness of fasting 18F-FDG PET in identification of cardiac sarcoidosis. J Nucl Med. 2004;45:1989–1998. [PubMed] [Google Scholar]
- 69.DiFilippo FP, Brunken RC. Do implanted pacemaker leads and ICD leads cause metal-related artifact in cardiac PET/CT? J Nucl Med. 2005;46:436–443. [PubMed] [Google Scholar]
- 70.Ahmadian A, Brogan A, Berman J, et al. Quantitative interpretation of FDG PET/CT with myocardial perfusion imaging increases diagnostic information in the evaluation of cardiac sarcoidosis. J Nucl Cardiol. 2014;21:925–939. [DOI] [PubMed] [Google Scholar]
- 71.Waller AH, Blankstein R. Quantifying myocardial inflammation using F18-fluorodeoxyglucose positron emission tomography in cardiac sarcoidosis. J Nucl Cardiol. 2014;21:940–943. [DOI] [PubMed] [Google Scholar]
- 72.Ahmadian A, Pawar S, Govender P, Berman J, Ruberg FL, Miller EJ. The response of FDG uptake to immunosuppressive treatment on FDG PET/CT imaging for cardiac sarcoidosis. J Nucl Cardiol. 2017;24:413–424. [DOI] [PubMed] [Google Scholar]
- 73.Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007;357:2153–2165. [DOI] [PubMed] [Google Scholar]
- 74.Baughman RP, Teirstein A, Judson M, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164:1885–1889. [DOI] [PubMed] [Google Scholar]
- 75.Simonen P, Lehtonen J, Kandolin R, et al. F-18-fluorodeoxyglucose positron emission tomography-guided sampling of mediastinal lymph nodes in the diagnosis of cardiac sarcoidosis. Am J Cardiol. 2015;116:1581–1585. [DOI] [PubMed] [Google Scholar]
- 76.Delbeke D, Coleman RE, Guiberteau MJ, et al. Procedure guideline for tumor imaging with 18F-FDG PET/CT 1.0. J Nucl Med. 2006;47:885–895. [PubMed] [Google Scholar]
- 77.Promteangtrong C, Salavati A, Cheng G, Torigian DA, Alavi A. The role of positron emission tomography-computed tomography/magnetic resonance imaging in the management of sarcoidosis patients. Hell J Nucl Med. 2014;17:123–135. [PubMed] [Google Scholar]
- 78.Mañá J, Gamez C. Molecular imaging in sarcoidosis. Curr Opin Pulm Med. 2011;17:325–331. [DOI] [PubMed] [Google Scholar]
- 79.Teirstein AS, Machac J, Almeida O, Lu P, Padilla ML, Iannuzzi MC. Results of 188 whole-body fluorodeoxyglucose positron emission tomography scans in 137 patients with sarcoidosis. Chest. 2007;132:1949–1953. [DOI] [PubMed] [Google Scholar]
- 80.Hamzeh NY, Wamboldt FS, Weinberger HD. Management of cardiac sarcoidosis in the United States: a Delphi study. Chest. 2012;141:154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sadek MM, Yung D, Birnie DH, Beanlands RS, Nery PB. Corticosteroid therapy for cardiac sarcoidosis: a systematic review. Can J Cardiol. 2013;29:1034–1041. [DOI] [PubMed] [Google Scholar]
- 82.Kato Y, Morimoto S, Uemura A, Hiramitsu S, Ito T, Hishida H. Efficacy of corticosteroids in sarcoidosis presenting with atrioventricular block. Sarcoidosis Vasc Diffuse Lung Dis. 2003;20:133–137. [PubMed] [Google Scholar]
- 83.Chapelon-Abric C, de Zuttere D, Duhaut P, et al. Cardiac sarcoidosis: a retrospective study of 41 cases. Medicine (Baltimore). 2004;83:315–334. [DOI] [PubMed] [Google Scholar]
- 84.Bargout R, Kelly RF. Sarcoid heart disease: clinical course and treatment. Int J Cardiol. 2004;97:173–182. [DOI] [PubMed] [Google Scholar]
- 85.Baughman RP, Drent M, Kavuru M, et al. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Respir Crit Care Med. 2006;174:795–802. [DOI] [PubMed] [Google Scholar]
- 86.Baughman RP, Lower EE. The effect of corticosteroid or methotrexate therapy on lung lymphocytes and macrophages in sarcoidosis. Am Rev Respir Dis. 1990;142:1268–1271. [DOI] [PubMed] [Google Scholar]
- 87.Müller-Quernheim J, Kienast K, Held M, Pfeifer S, Costabel U. Treatment of chronic sarcoidosis with an azathioprine/prednisolone regimen. Eur Respir J. 1999;14:1117–1122. [DOI] [PubMed] [Google Scholar]
- 88.Zuber M, Defer G, Cesaro P, Degos JD. Efficacy of cyclophosphamide in sarcoid radiculomyelitis. J Neurol Neurosurg Psychiatry. 1992;55:166–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zabel P, Entzian P, Dalhoff K, Schlaak M. Pentoxifylline in treatment of sarcoidosis. Am J Respir Crit Care Med. 1997;155:1665–1669. [DOI] [PubMed] [Google Scholar]
- 90.Baughman RP, Judson MA, Teirstein A, et al. Presenting characteristics as predictors of duration of treatment in sarcoidosis. QJM. 2006;99:307–315. [DOI] [PubMed] [Google Scholar]
- 91.Moravan M, Segal BM. Treatment of CNS sarcoidosis with infliximab and mycophenolate mofetil. Neurology. 2009;72:337–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Winters SL, Cohen M, Greenberg S, et al. Sustained ventricular tachycardia associated with sarcoidosis: assessment of the underlying cardiac anatomy and the prospective utility of programmed ventricular stimulation, drug therapy and an implantable antitachycardia device. J Am Coll Cardiol. 1991;18:937–943. [DOI] [PubMed] [Google Scholar]
- 93.Murtagh G, Laffin LJ, Beshai JF, et al. Prognosis of myocardial damage in sarcoidosis patients with preserved left ventricular ejection fraction: risk stratification using cardiovascular magnetic resonance. Circ Cardiovasc Imaging. 2016;9:e003738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mc Ardle BA, Birnie DH, Klein R, et al. Is there an association between clinical presentation and the location and extent of myocardial involvement of cardiac sarcoidosis as assessed by 18F-fluorodeoxyglucose positron emission tomography? Circ Cardiovasc Imaging. 2013;6:617–626. [DOI] [PubMed] [Google Scholar]
- 95.Langah R, Spicer K, Gebregziabher M, Gordon L. Effectiveness of prolonged fasting 18f-FDG PET-CT in the detection of cardiac sarcoidosis. J Nucl Cardiol. 2009;16:801–810. [DOI] [PubMed] [Google Scholar]
- 96.Tahara N, Tahara A, Nitta Y, et al. Heterogeneous myocardial FDG uptake and the disease activity in cardiac sarcoidosis. JACC Cardiovasc Imaging. 2010;3:1219–1228. [DOI] [PubMed] [Google Scholar]
- 97.Yamagishi H, Shirai N, Takagi M, et al. Identification of cardiac sarcoidosis with 13N-NH3/18F-FDG PET. J Nucl Med. 2003;44:1030–1036. [PubMed] [Google Scholar]
- 98.Ohira H, Tsujino I, Ishimaru S, et al. Myocardial imaging with 18F-fluoro-2-deoxyglucose positron emission tomography and magnetic resonance imaging in sarcoidosis. Eur J Nucl Med Mol Imaging. 2008;35:933–941. [DOI] [PubMed] [Google Scholar]
- 99.Aggarwal NR, Snipelisky D, Young PM, Gersh BJ, Cooper LT, Chareonthaitawee P. Advances in imaging for diagnosis and management of cardiac sarcoidosis. Eur Heart J Cardiovasc Imaging. 2015;16:949–958. [DOI] [PubMed] [Google Scholar]