Abstract
Objectives:
Epithelial-mesenchymal transition (EMT) plays an important role in the progression, metastasis and chemoresistance of pancreatic duct adenocarcinoma (PDAC); however, the expression of EMT markers and their clinical significance in PDAC patients who received neoadjuvant therapy (NAT) are unclear.
Methods:
We examined the expression of EMT markers, including zinc finger E-box-binding homeobox 1 (Zeb-1), E-cadherin, and vimentin by immunohistochemistry in 120 PDAC patients who received NAT and pancreatectomy from 1999 to 2007. The results were correlated with clinicopathologic parameters and survival.
Results:
Among 120 cases, 45 (37.5%) and 14 (11.7%) were positive for Zeb-1 and vimentin respectively, and 25 (20.8 %) were E-cadherin-low. The median (standard deviation) overall survival (OS) and disease-free survival (DFS) were 35.3 (2.8) and 15.9 (3.6) months, respectively, in vimentin-negative group compared to 16.1 (1.1) (P = 0.03) and 7.0 (1.1) months (P = 0.02) in vimentin-positive group. In multivariate analysis, vimentin expression was an independent predictor of shorter DFS (hazard ratio, 2.50; 95% confidence interval, 1.31–4.78; P = 0.016) and OS (hazard ratio, 2.55; 95% confidence interval, 1.33–4.89; P = 0.01).
Conclusion:
Epithelial-mesenchymal transition markers are frequently expressed in treated PDAC. Vimentin expression is a prognostic biomarker for survival in PDAC patients who received NAT.
Keywords: pancreatic ductal adenocarcinoma, epithelial-mesenchymal transition, vimentin, Zeb-1, E-cadherin, survival
INTRODUCTION
Pancreatic duct adenocarcinoma (PDAC) is the fourth leading cause of cancer-related death in the United States with a median survival of less than one year and has the lowest 5-year survival rate of 8% among common human malignancies.1 The poor prognosis for patients with PDAC is mainly due to its aggressive behavior, frequent early metastasis, and resistance to conventional chemotherapy and radiation therapy.2 Neoadjuvant therapy (NAT) has been increasingly used for treatment of patients with potentially resectable PDAC. Previous studies showed the survival benefit and other clinical advantages of NAT for patients with borderline resectable and locally advanced PDAC.3,4 NAT has been reported to reduce tumor volume/down-stage the tumor to improve resectability for some patients with borderline resectable or locally advanced disease3,5 and to increase rates of margin-negative (R0) resection.6,7 In addition, NAT can provide early treatment of occult micrometastasis and an observation period to evaluate tumor response to therapy and tumor behavior. As such it may help identify patients most likely to benefit from surgical resection.3
Epithelial-mesenchymal transition (EMT) is characterized by the downregulation of the epithelial adhesion molecule E-cadherin and upregulation of mesenchymal molecules such as vimentin, N-cadherin, and fibronectin etc.2,8,9 Several EMT-inducing transcription factors, including Twist, Snail, Slug, Zinc finger E-box-binding homeobox 1 (Zeb-1), and Zeb-2, have been shown to regulate the EMT process via suppression of epithelial marker expression and/or induction mesenchymal marker expression.10,11 Increasing evidence suggests that EMT plays an important role in pancreatic cancer progression, metastasis and chemotherapy resistance.2,12,13 Our previous studies have shown that Zeb-1, an EMT-inducing transcription factor, is a major mediator of drug resistance in pancreatic cancer cell lines.12
Expression of EMT markers is associated with poor prognosis and clinical outcome in carcinomas of several tissue origins, including breast cancer, non-small cell lung cancer, gastric cancer, endometrial carcinoma and PDAC.14-18 In patients with PDAC, EMT phenotype correlated with serum carbohydrate antigen 19-9 levels, positive peritoneal washing by cytology, portal vein invasion, lymph node metastasis, and shorter survival.18 Expression of EMT markers has also been reported to be associated with lymph node metastasis and shorter survival in patients with PDAC arising in intraductal papillary mucinous neoplasms.19 Overexpression of vimentin, an EMT marker, has been correlated with tumor budding in PDAC.20 Zeb-1, an important EMT regulator, has been found to be expressed only in malignant intraductal papillary mucinous neoplasms.19 However, the expression of EMT markers and their clinical significance in patients with PDAC who received NAT has not been investigated. In this study, we examined the expression of EMT markers, including Zeb-1, E-cadherin, and vimentin, in 120 patients who underwent pancreaticoduodenectomy (PD) after receiving NAT. The results were correlated with clinicopathologic parameters, disease-free survival (DFS) and overall survival (OS). Our results show that expression of EMT markers is an important predictor of patient survival and support the function of EMT in tumor resistance to NAT of PDAC.
MATERIALS AND METHODS
Patient Population
The institutional review board of the University of Texas MD Anderson Cancer Center approved this study. One hundred and twenty patients who received NAT and underwent surgical resection at our institution from 1999 to 2007 were included. The patients’ clinicopathologic and follow-up data were retrieved from a database that is prospectively maintained by the Department of Surgical Oncology and verified by reviewing patients’ medical records and the U.S. Social Security Index. There were 73 men (60.8%) and 47 women (39.2%) with a median age at diagnosis of 63.9 years (range, 42.1–84.2 years). The follow-up duration ranged from 9.4 to 205.0 months. Two pathologists (M.W. and J.S.E.) independently reviewed pathology for histologic type, tumor differentiation, tumor regression grade, margin status, lymph node status, and post-treatment pathologic stage which was grouped using the American Joint Committee on Cancer Staging Manual, 8th edition.21
Tissue Microarray Construction
Tissue microarrays (TMAs) were constructed using a tissue microarrayer (Beecher Instrumetns, Sun Prairie, Wis) as described previously.22 A gastrointestinal pathologist reviewed the matched hematoxylin and eosin (H & E) stained slides of each case to identify the representative tumor regions. For each patient, three 1.0 mm cores from the representative tumor regions were included in the TMAs.
Immunohistochemical Analysis
Immunohistochemical stains were performed on 4-μm unstained sections from the tissue microarray blocks using primary antibody against Zeb-1 (1:300, Novus Biologicals, Centennial, Colo), E-cadherin (1:1000, clone HECD-1, Life Technologies, Carlsbad, Calif), and vimentin (1:900, clone V9; Dako, Carpinteria, Calif). The tissue sections were deparaffinized and placed in a 100°C steamer containing 20 mmol citrate buffer (pH 6.0) for 5 minutes for antigen retrieval. The tissue section was incubated with primary antibody at 35°C for 15 minutes, and then quenched with hydrogen peroxide at 35°C for 15 minutes to block endogenous peroxide activity. After immersed in polymer enhancer for 8 minutes, the sections were incubated with secondary polyhorseradish peroxidase anti-mouse/anti-rabbit immunoglobulin G at 35°C for 8 minutes. 3, 3’-diaminobenzidine was used as a chromogen, and hematoxylin used for counterstaining.
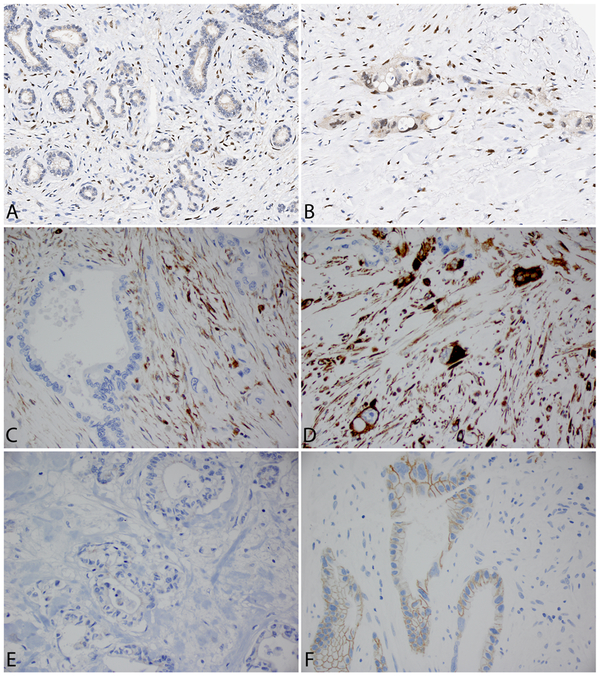
Immunohistochemically stained TMA slides were scanned at 20× magnification using a ScanScope Aperio AT Turbo whole slide scanner (Leica Biosystems, Buffalo Grove, Ill). The immunohitochemical stains of Zeb-1, E-cadherin, and vimentin, were evaluated and graded independently by two pathologists (J.S.E. and M.W.). The average score from three cores of each case were used for statistical analysis. The staining for Zeb-1 was categorized as positive (≥10% nuclear staining in tumor cells) and negative (<10% nuclear staining in tumor cells); the staining for E-cadherin was categorized as low (negative or <50% membranous staining) or high (≥50% membranous staining); the staining for vimentin was categorized as negative (<10% cytoplasmic staining) or positive (≥10% cytoplasmic staining) (Fig. 1).
FIGURE 1.
Representative micrographs show the expression of Zeb-1, vimentin and E-cadherin in residual tumors after neoadjuvant therapy. Negative (A) and positive (B) staining for Zeb-1; negative (C) and positive (D) staining for vimentin; low (E-cadherin-low, E) and high expression (E-cadherin-high, F) for E-cadherin (original magnifications: 400×).
Statistical Analysis
All statistics analyses were performed using SPSS (Version 22; IBM SPSS Inc, Armonk, NY). Chi-Square test was applied to analyze the correlations between clinicopathologic parameters and EMT markers as well as the correlations among Zeb-1, E-cadherin, and vimentin. Overall survival was defined as the time from the date of PDAC diagnosis to the date of death or the date of last follow-up if death did not occur. Disease-free survival duration was defined as the time from the date of surgery to the date of first recurrence in patients who had recurrence or to the date of last follow-up in patients without recurrence. Kaplan-Meier method was used to construct survival curves of OS and DFS, and log-rank test was used to evaluate statistical significance of differences in survival. Univariate and multivariate Cox regression analyses were used to assess the prognostic significance of clinicopathologic variables. The presence of a statistically significant difference was denoted by two sided P value of less than 0.05.
RESULTS
The Expression of Vimentin, E-cadherin, and Zeb-1 in Treated PDAC Samples
Among the 120 cases examined, 45 (37.5%) were positive for Zeb-1, 14 (11.7%) were positive for vimentin, and 25 (20.8%) were E-cadherin-low. Representative micrographs showing expression of Zeb-1, vimentin, E-cadherin are shown in Figure 1. There was a negative correlation between the expression of E-cadherin and vimentin. Among 25 patients whose tumor had low expression of E-cadherin, 6 patients were positive for vimentin. In contrast, among 95 cases whose tumor had high expression of E-cadherin, only 8 patients were positive for vimentin (P = 0.03). No correlation between the expression of Zeb-1 and the expression of E-cadherin or vimentin was found (P > 0.05).
Clinicopathological Correlation of Vimentin, E-Cadherin, and Zeb-1 Expression
Association between patients’ clinicopathological features and E-cadherin, vimentin and Zeb-1 are shown in Table 1. E-cadherin-low and vimentin-positive expression correlated with poor differentiation. Poorly differentiated histology was present in 10 of 14 (71.4%) patients whose tumors were vimentin positive compared to 32.1% (34/106) in patients whose tumors were negative for vimentin (P = 0.004). Among the 25 cases that were E-cadherin-low, 14 (56%) were poorly differentiated compared to 31.5% (30/95) of poorly differentiated histology in patients whose tumors were E-cadherin-high (P = 0.02). Interestingly, E-cadherin-high was also correlated with higher tumor stage (P = 0.04). No correlation between the expression of vimentin and E-cadherin with other clinicopathologic parameters was found (P > 0.05). There was no correlation between Zeb-1 expression and any clinicopathologic parameters (P > 0.05, Table 1).
TABLE 1.
Clinicopathological Correlation of Vimentin, E-cadherin, and Zeb-1
| Characteristics | Vimentin (− ), n (%) |
Vimentin (+), n (%) |
P | E-cad Low, n (%) |
E-cad High, n (%) |
P | Zeb-1 (−), n (%) |
Zeb-1 (+), n (%) |
P |
|---|---|---|---|---|---|---|---|---|---|
| Age, y | 0.15 | 0.05 | 0.71 | ||||||
| <65 | 54 (51.9) | 10 (71.4) | 9 (36.0) | 55 (57.9) | 41 (54.7) | 23 (51.1) | |||
| ≥65 | 52 (49.1) | 4 (28.6) | 16 (64.0) | 40 (42.1) | 34 (45.3) | 22 (48.9) | |||
| Sex | 0.76 | 0.41 | 0.04 | ||||||
| Female | 41 (38.7) | 6 (42.9) | 8 (32.0) | 39 (41.1) | 24 (32.0) | 23 (51.1) | |||
| Male | 65 (61.3) | 8 (57.1) | 17 (68.0) | 56 (58.9) | 51 (68.0) | 22 (48.9) | |||
| Tumor differentiation | 0.004* | 0.02* | 0.33 | ||||||
| Well-moderate | 72 (67.9) | 4 (28.6) | 11 (44.0) | 65 (68.4) | 50 (66.7) | 26 (57.8) | |||
| Poor | 34 (32.1) | 10 (71.4) | 14 (56.0) | 30 (31.6) | 25 (33.3) | 19 (42.2) | |||
| Margin | 0.52 | 0.66 | |||||||
| Negative | 94 (88.7) | 12 (85.7) | 0.75 | 23 (92.0) | 83 (87.4) | 67 (89.3) | 39 (86.7) | ||
| Positive | 12 (11.3) | 2 (14.3) | 2 (8.0) | 12 (12.6) | 8 (10.7) | 6 (13.3) | |||
| Tumor stage | 0.28 | 0.04† | 0.62 | ||||||
| pT1 (≤2.0 cm) | 24 (22.6) | 5 (35.7) | 10 (40.0) | 19 (20.0) | 17 (22.7) | 12 (26.7) | |||
| pT2 and pT3 (>2.0 cm) | 82 (77.4) | 9 (64.3) | 15 (60.0) | 76 (80.0) | 58 (77.3) | 33 (73.3) | |||
| Tumor regression grade | 0.82 | 0.14 | 0.62 | ||||||
| Cap grade 0 & 1 | 6 (5.7) | 1 (7.1) | 3 (12.0) | 4 (4.2) | 5 (6.7) | 2 (4.4) | |||
| Cap grade 2 & 3 | 100 (94.3) | 13 (92.9) | 22 (88.0) | 91 (95.8) | 70 (93.3) | 43 (95.6) | |||
| Lymph node status | 0.60 | 0.97 | 0.06 | ||||||
| Negative | 37 (34.9) | 3 (21.4) | 8 (32.0) | 32 (33.7) | 24 (32.0) | 16 (35.6) | |||
| 1–3 positive LNs | 43 (40.6) | 7 (50.0) | 11 (44.0) | 39 (41.0) | 27 (36.0) | 23 (51.1) | |||
| ≥4 positive LNs | 26 (24.5) | 4 (28.6) | 6 (24.0) | 24 (25.3) | 24 (32.0) | 6 (13.3) | |||
| Recurrence | 0.60 | 0.29 | 0.79 | ||||||
| No | 30 (28.3) | 3 (21.4) | 9 (36.0) | 24 (25.3) | 20 (26.7) | 13 (28.9) | |||
| Yes | 76 (71.7) | 11 (78.6) | 16 (64.0) | 71 (74.7) | 55 (73.3) | 32 (71.1) |
Positive expression of vimentin and E-cadherin-low correlated with poor differentiation (P = 0.004 and P = 0.02, respectively).
E-cadherin-high correlated with higher tumor stage (P = 0.04).
Survival Analysis
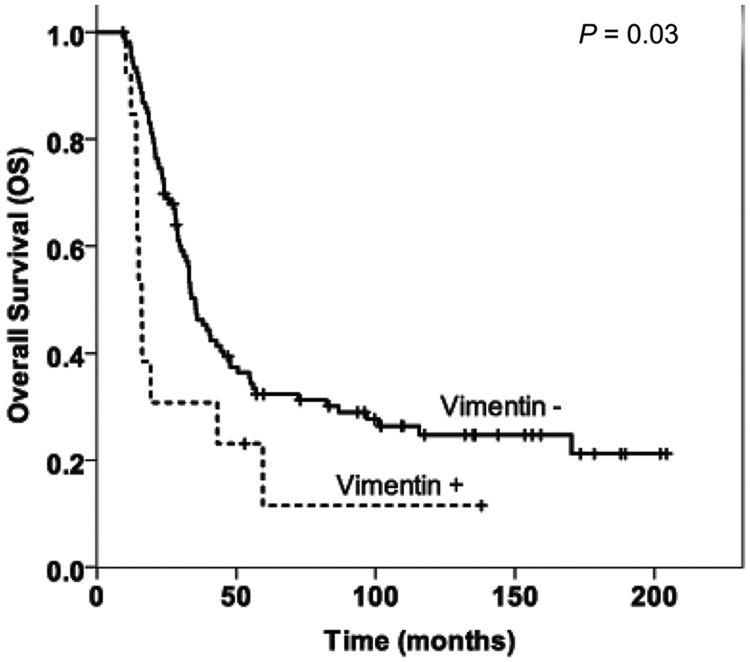
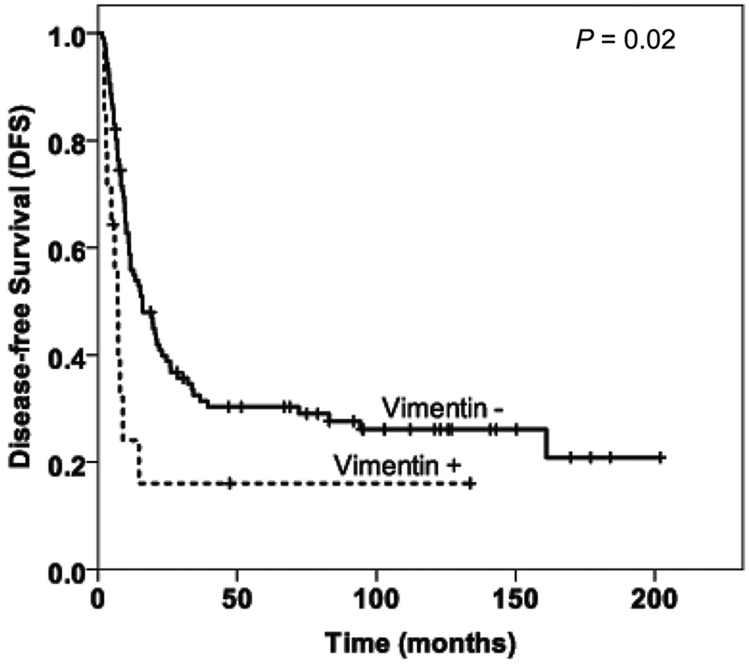
The median follow-up duration after surgery was 26 months (range, 5–202 months) for all patients. The patients whose tumors were vimentin negative had longer DFS and OS than those whose tumors were vimentin positive (Fig. 2). The DFS durations were 15.9 (standard deviation [SD], 3.6) months in the vimentin-negative group, compared to 7.0 (SD, 1.1) months in the vimentin-positive group (P = 0.02). The median overall survival (OS) were 35.3 (SD, 2.8) months in the vimentin-negative group compared to 16.1 (SD, 1.1) months (P = 0.03) in the vimentin-positive group. There was no correlation between the expression of Zeb-1 and E-cadherin with either OS or DFS (P > 0.05, data not shown).
FIGURE 2.
Kaplan-Meier curves for overall survival (A) and disease-free survival (B) stratified by vimentin expression in treated pancreatic ductal adenocarcinoma.
In univariate analysis, positive vimentin expression and lymph node metastasis correlated significantly with shorter DFS and shorter OS (Table 2). By multivariate analysis, vimentin-positive patients had significantly shorter DFS (hazard ratio [HR], 2.50; 95% confidence interval [CI], 1.31–4.78; P = 0.016) and shorter OS (HR, 2.55; 95% CI, 1.33–4.89; P = 0.01) compared to vimentin-negative patients. In addition, lymph node metastasis and tumor regression grade were also independent prognostic factors for both DFS and OS (Table 3).
TABLE 2.
Univariate Cox Regression Analysis of DFS and OS in Relation to Clinicopatholgocial Features
| DFS |
OS |
||||
|---|---|---|---|---|---|
| Characteristics | No. Patients | HR (95% CI) | P | HR (95% CI) | P |
| Vimentin | |||||
| Negative | 106 | 1.00 (reference) | 1.00 (reference) | ||
| Positive | 14 | 2.15 (1.14–4.07) | 0.02* | 2.06 (1.09–3.89) | 0.03* |
| Age, y | 120 | 0.96 (0.94–0.98) | 0.001 | 0.97 (0.95–1.0) | 0.015 |
| Sex | |||||
| Female | 47 | 1.00 (reference) | 1.00 (reference) | ||
| Male | 73 | 0.77 (0.50–1.19) | 0.24 | 0.83 (0.54–1.28) | 0.41 |
| Tumor differentiation | |||||
| Well-moderate | 76 | 1.00 (reference) | 1.00 (reference) | ||
| Poor | 44 | 1.34 (0.87–2.077) | 0.18 | 1.30 (0.85–2.00) | 0.23 |
| Margin | |||||
| Negative | 106 | 1.00 (reference) | 1.00 (reference) | ||
| Positive | 14 | 1.50 (0.79–2.83) | 0.21 | 1.82 (0.96–3.46) | 0.67 |
| Tumor stage | |||||
| pT1 (≤2.0 cm) | 29 | 1.00 (reference) | 1.00 (reference) | ||
| pT2 and pT3 (>2.0 cm) | 91 | 1.28 (0.76–2.15) | 0.35 | 1.22 (0.73–2.05) | 0.45 |
| Tumor regression grade | |||||
| Cap grade 0 & 1 | 7 | 1.00 (reference) | 1.00 (reference) | ||
| Cap grade 2 & 3 | 113 | 2.95 (0.93–9.35) | 0.07 | 2.94 (0.93–9.34) | 0.07 |
| Lymph node status | |||||
| Negative | 40 | 1.00 (reference) | 1.00 (reference) | ||
| 1–3 positive LNs | 50 | 1.21 (0.73–2.00) | 0.47 | 1.08 (0.65–1.79) | 0.76 |
| ≥4 positive LNs | 30 | 2.25 (1.30–3.89) | 0.04† | 2.57 (1.50–4.42) | 0.001† |
Positive expression of vimentin correlated with shorter DFS and OS (P = 0.02 and P = 0.03, respectively).
Patients with ≥4 positive lymph nodes had shorter DFS and OS (P = 0.04 and P = 0.001, respectively).
TABLE 3.
Multivariate Cox Regression Analysis of DFS and OS in Relation to Clinicopatholgocal Features
| DFS |
OS |
||||
|---|---|---|---|---|---|
| Characteristics | No. Patients | HR (95% CI) | P | HR (95% CI) | P |
| Vimentin | |||||
| Negative | 106 | 1.00 (reference) | 1.00 (reference) | ||
| Positive | 14 | 2.50 (1.31–4.78) | 0.016* | 2.55 (1.33–4.89) | 0.01* |
| Lymph node status | |||||
| Negative | 40 | 1.00 (reference) | 1.00 (reference) | ||
| 1–3 positive LNs | 50 | 1.08 (0.63–1.84) | 0.7 | 1.14 (0.68–1.89) | 0.67 |
| ≥4 positive LNs | 30 | 1.88 (1.03–3.42) | 0.04† | 3.06 (1.76–5.33) | <0.001† |
| Tumor regression grade | |||||
| Cap grade 0 & 1 | 7 | 1.00 (reference) | 1.00 (reference) | ||
| Cap grade 2 & 3 | 13 | 3.35 (1.03–10.88) | 0.05‡ | 3.82 (1.18–12.35) | 0.03‡ |
| Age, y | 120 | 0.97 (0.94–0.99) | 0.01 | 0.98 (0.96–1.01) | 0.19 |
Positive expression of vimentin was associated with shorter DFS (P = 0.016) and OS (P = 0.01).
Patients with ≥4 positive lymph nodes had significantly shorter DFS (P = 0.04) and OS (P < 0.001).
Patients with higher tumor regression grade (CAP grade 2 or 3) had shorter DFS (P = 0.05) and OS (P = 0.03).
DISCUSSION
Increasing evidences suggest that EMT plays a pivotal role in cancer progression and drug resistance. EMT has been reported to have a poor prognostic impact in patients with endometrial cancer,14 gastric cancer,16 breast cancer,17 non-small cell lung cancer,15 and untreated pancreatic adenocarcinoma.18,23,24 However, no prior studies have examined expression of EMT biomarkers and their clinical significance in patients with PDAC who received NAT and underwent surgical resection with curative intent. In this study, we examined 120 such patients and found that the level of vimentin expression was significantly correlated with shorter disease-free survival and shorter overall survival in PDAC patients who received NAT. In addition, we found that vimentin expression was an independent prognostic factor for both DFS and OS in these patients. Our results are consistent with those from previous studies, which showed that high expression of vimentin was associated with shorter overall survival in PDAC patients who underwent surgery without previously receiving NAT.24,25
Previous studies have shown that expression of E-cadherin and Zeb-1 is associated with overall survival in PDAC patients who underwent surgery without receiving NAT.24,25 In addition, loss of E-cadherin expression and overexpression of Zeb1 have been associated with worse clinicopathological features and poorer clinical outcome in other cancers.26-28 In this study, however, we did not find significant correlation between the expression of E-cadherin or Zeb-1 and either DFS or OS in PDAC patients treated with NAT. This may be in part due to the influence of NAT. One study of patients with esophageal cancer reported a significant down-regulation of E-cadherin expression and up-regulation of Zeb-1 in residual tumor after NAT compared to treatment-naive tumors.29 It is possible that neoadjuvant chemotherapy and chemoradiation therapy may also affect E-cadherin and Zeb-1 expression in PDAC. Similar to our results, Otsuki et al found that in patients with gastrointestinal tract tumors, the expression of E-cadherin was not correlated with prognosis, while vimentin expression was linked to a more aggressive phenotype and worse clinical outcome.30
In this study, we found that overexpression of vimentin and low expression of E-cadherin correlated with poorly differentiated histology in treated PDAC. Similar to our results, loss of E-cadherin expression has been correlated with poorly differentiated histology in patients with PDAC who underwent upfront surgery without NAT.31,32 Although previous studies have found that Zeb-1 to be an important regulator of E-cadherin and Zeb-1 expression and involved in chemoresestance in pancreatic cancer,8,10,12,30,33-35 our study did not identify a significant correlation between Zeb-1 expression and expression of E-Cadherin or vimentin. The strong correlation between overexpression of vimentin and low expression of E-cadherin with poorly differentiated histology support the EMT phenotype in our patients.
In summary, our study showed the expression of mesenchymal markers, vimentin and Zeb-1, and loss of E-cadherin in patients with treated PDAC. Importantly, we found that positive expression of vimentin correlated with poorly differentiated histology and represented a predictor of shorter disease-free survival and overall survival in PDAC patients who received neoadjuvant therapy. Therefore, EMT markers may represent an important prognosticator for this group of patients. Our results support the important functions of EMT in the aggressive behavior and treatment resistance of pancreatic cancer.
Acknowledgments
Funding: This work was supported by the National Institutes of Health grants (1R01 CA196941).
Footnotes
Declarations of interest: none
Parts of this study were presented as a poster at Annual Meeting of American Association for Cancer Research, Chicago, IL. 2018 and published as an abstract.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Gaianigo N, Melisi D, Carbone C. EMT and Treatment Resistance in Pancreatic Cancer. Cancers (Basel). 2017;9:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mirkin KA, Hollenbeak CS, Wong J. Survival impact of neoadjuvant therapy in resected pancreatic cancer: A Prospective Cohort Study involving 18,332 patients from the National Cancer Data Base. Int J Surg. 2016;34:96–102. [DOI] [PubMed] [Google Scholar]
- 4.Shubert CR, Bergquist JR, Groeschl RT, et al. Overall survival is increased among stage III pancreatic adenocarcinoma patients receiving neoadjuvant chemotherapy compared to surgery first and adjuvant chemotherapy: An intention to treat analysis of the National Cancer Database. Surgery. 2016;160:1080–1096. [DOI] [PubMed] [Google Scholar]
- 5.Zhan HX, Xu JW, Wu D, et al. Neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of prospective studies. Cancer Med. 2017;6:1201–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pingpank JF, Hoffman JP, Ross EA, et al. Effect of preoperative chemoradiotherapy on surgical margin status of resected adenocarcinoma of the head of the pancreas. J Gastrointest Surg. 2001;5:121–130. [DOI] [PubMed] [Google Scholar]
- 7.Katz MH, Wang H, Balachandran A, et al. Effect of neoadjuvant chemoradiation and surgical technique on recurrence of localized pancreatic cancer. J Gastrointest Surg. 2012;16:68–78; discussion 78-69. [DOI] [PubMed] [Google Scholar]
- 8.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eastham AM, Spencer H, Soncin F, et al. Epithelial-mesenchymal transition events during human embryonic stem cell differentiation. Cancer Res. 2007;67:11254–11262. [DOI] [PubMed] [Google Scholar]
- 10.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. [DOI] [PubMed] [Google Scholar]
- 11.Comijn J, Berx G, Vermassen P, et al. The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol Cell. 2001;7:1267–1278. [DOI] [PubMed] [Google Scholar]
- 12.Arumugam T, Ramachandran V, Fournier KF, et al. Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer. Cancer Res. 2009;69:5820–5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krebs AM, Mitschke J, Lasierra Losada M, et al. The EMT-activator Zeb1 is a key factor for cell plasticity and promotes metastasis in pancreatic cancer. Nat Cell Biol. 2017;19:518–529. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka Y, Terai Y, Kawaguchi H, et al. Prognostic impact of EMT (epithelial-mesenchymal-transition)-related protein expression in endometrial cancer. Cancer Biol Ther. 2013;14:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahmood MQ, Ward C, Muller HK, et al. Epithelial mesenchymal transition (EMT) and non-small cell lung cancer (NSCLC): a mutual association with airway disease. Med Oncol. 2017;34:45. [DOI] [PubMed] [Google Scholar]
- 16.Murai T, Yamada S, Fuchs BC, et al. Epithelial-to-mesenchymal transition predicts prognosis in clinical gastric cancer. J Surg Oncol. 2014;109:684–689. [DOI] [PubMed] [Google Scholar]
- 17.Liu F, Gu LN, Shan BE, et al. Biomarkers for EMT and MET in breast cancer: An update. Oncol Lett. 2016;12:4869–4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamada S, Fuchs BC, Fujii T, et al. Epithelial-to-mesenchymal transition predicts prognosis of pancreatic cancer. Surgery. 2013;154:946–954. [DOI] [PubMed] [Google Scholar]
- 19.Lahat G, Lubezky N, Loewenstein S, et al. Epithelial-to-mesenchymal transition (EMT) in intraductal papillary mucinous neoplasm (IPMN) is associated with high tumor grade and adverse outcomes. Ann Surg Oncol. 2014;21 Suppl 4:S750–S757. [DOI] [PubMed] [Google Scholar]
- 20.Chouat E, Zehani A, Chelly I, et al. Tumor budding is a prognostic factor linked to epithelial mesenchymal transition in pancreatic ductal adenocarcinoma. Study report and literature review. Pancreatology. 2018;18:79–84. [DOI] [PubMed] [Google Scholar]
- 21.Amin MB, Edge SB, Greene FL, et al. AJCC Cancer Staging Manual 8th ed. Chicago, IL: American College of Surgeons; 2018. [Google Scholar]
- 22.Wang H, Wang H, Zhang W, et al. Tissue microarrays: applications in neuropathology research, diagnosis, and education. Brain Pathol. 2002;12:95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurahara H, Takao S, Maemura K, et al. Epithelial-mesenchymal transition and mesenchymal-epithelial transition via regulation of ZEB-1 and ZEB-2 expression in pancreatic cancer. J Surg Oncol. 2012;105:655–661. [DOI] [PubMed] [Google Scholar]
- 24.Hong SM, Li A, Olino K, et al. Loss of E-cadherin expression and outcome among patients with resectable pancreatic adenocarcinomas. Mod Pathol. 2011;24:1237–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bronsert P, Kohler I, Timme S, et al. Prognostic significance of Zinc finger E-box binding homeobox 1 (ZEB1) expression in cancer cells and cancer-associated fibroblasts in pancreatic head cancer. Surgery. 2014;156:97–108. [DOI] [PubMed] [Google Scholar]
- 26.Endo K, Ueda T, Ueyama J, et al. Immunoreactive E-cadherin, alpha-catenin, beta-catenin, and gamma-catenin proteins in hepatocellular carcinoma: relationships with tumor grade, clinicopathologic parameters, and patients’ survival. Hum Pathol. 2000;31:558–565. [DOI] [PubMed] [Google Scholar]
- 27.Gillett CE, Miles DW, Ryder K, et al. Retention of the expression of E-cadherin and catenins is associated with shorter survival in grade III ductal carcinoma of the breast. J Pathol. 2001;193:433–441. [DOI] [PubMed] [Google Scholar]
- 28.Shimamura T, Sakamoto M, Ino Y, et al. Dysadherin overexpression in pancreatic ductal adenocarcinoma reflects tumor aggressiveness: relationship to e-cadherin expression. J Clin Oncol. 2003;21:659–667. [DOI] [PubMed] [Google Scholar]
- 29.Hara J, Miyata H, Yamasaki M, et al. Mesenchymal phenotype after chemotherapy is associated with chemoresistance and poor clinical outcome in esophageal cancer. Oncol Rep. 2014;31:589–596. [DOI] [PubMed] [Google Scholar]
- 30.Otsuki S, Inokuchi M, Enjoji M, et al. Vimentin expression is associated with decreased survival in gastric cancer. Oncol Rep. 2011;25:1235–1242. [DOI] [PubMed] [Google Scholar]
- 31.Joo YE, Rew JS, Park CS, et al. Expression of E-cadherin, alpha- and beta-catenins in patients with pancreatic adenocarcinoma. Pancreatology. 2002;2:129–137. [DOI] [PubMed] [Google Scholar]
- 32.Pignatelli M, Ansari TW, Gunter P, et al. Loss of membranous E-cadherin expression in pancreatic cancer: correlation with lymph node metastasis, high grade, and advanced stage. J Pathol. 1994;174:243–248. [DOI] [PubMed] [Google Scholar]
- 33.Wang Z, Li Y, Kong D, et al. Acquisition of epithelial-mesenchymal transition phenotype of gemcitabine-resistant pancreatic cancer cells is linked with activation of the notch signaling pathway. Cancer Res. 2009;69:2400–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wellner U, Brabletz T, Keck T. ZEB1 in Pancreatic Cancer. Cancers (Basel). 2010;2:1617–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nieto MA, Huang RY, Jackson RA, et al. Emt: 2016. Cell. 2016;166:21–45. [DOI] [PubMed] [Google Scholar]