Abstract
Varicella zoster virus (VZV) results in chicken pox and herpes zoster. Female rats show a higher level of herpes zoster associated pain than males, consistent with human studies. In this study, we addressed the novel hypothesis that sex difference in herpes zoster associated pain is due, in part, to estradiol modulating activity in the thalamus. To test this hypothesis a high and low physiological dose of estradiol was administered to castrated and ovariectomized rats and the affective pain response was measured after injection of VZV into the whisker pad. Thalamic infusion of the estrogen receptor antagonist ICI 182,780 concomitant with a high dose of estradiol addressed the role of estradiol binding to its receptor to effect pain. Phosphorylated extracellular signal-regulated protein kinase (pERK) positive cells were measured in excitatory (glutaminase positive) and inhibitory (glutamate decarboxylase 67 positive) cells of the lateral thalamic region. Our results show that a high dose of estradiol significantly reduced the pain response in both males and females. pERK significantly increased in excitatory cells after treatment with a low dose of estradiol and increased in inhibitory cells after treatment with a high dose of estradiol. Administration of ICI 182,780 significantly increased the pain response, reduced expression of GABA related genes in the thalamic region and significantly reduced the number of inhibitory cells expressing pERK. The results suggest that estradiol attenuates herpes zoster pain by increasing the activity of inhibitory neurons within the thalamus and that this reduction includes an estrogen receptor dependent mechanism.
Keywords: orofacial, herpes zoster, shingles, pain, estrogen, thalamus
Introduction
Infection with varicella zoster virus (VZV) results in chicken pox and after this initial infection the virus remains latent in sensory nerve ganglia (Mahalingam et al., 1992) until reactivation results in a painful disorder termed herpes zoster or shingles (Donahue et al., 1995; Jung et al., 2004). Nearly one in every three persons develops herpes zoster during their life-time (Harpaz et al., 2008) and although vaccination is effective, still about 20% of infected patients develop chronic pain (Harpaz et al., 2008). Unfortunately, pain resulting from herpes zoster severely effects the quality of life for many patients (Johnson et al., 2010).
Females report more zoster associate pain than men (Alvarez et al., 2007; Hillebrand et al., 2015), a potential explanation for this sex difference is the sex steroid estradiol (Craft et al., 2004; Craft, 2007; Aloisi and Sorda, 2011; Palmeira et al., 2011; Amandusson and Blomqvist, 2013; Pieretti et al., 2016). Previous studies have suggested that estradiol enhances nociception, whereas progesterone attenuates nociception (Frye et al., 1993; Ren et al., 2000; Okamoto et al., 2003; Stoffel et al., 2003). In an inflammatory temporomandibular joint (TMJ) model, rats given a low diestrus level of estradiol had a greater nociceptive response in comparison to rats given a high proestrus dose of estradiol (Kramer and Bellinger, 2009). In our VZV induced pain model males responded with less pain than females (Stinson et al., 2017). Further, studies in females indicated that during proestrus when estradiol is high there was a reduced response to VZV induced pain versus diestrus when estradiol was low (Stinson et al., 2017).
Estradiol effects neuronal activity within the thalamus indicating sex hormones control neuronal function (Ueyama et al., 2006) and sex steroids like estrogen can increase activity in the thalamus of stressed rats (Ueyama et al., 2006). Sex steroids alter the function of thalamic GABA pathways to affect orofacial nociception in animal models (Puri et al., 2012; Tashiro et al., 2014). Moreover, estradiol alters GABAergic gene expression in the thalamic region resulting in an altered pain responses (Umorin et al., 2016). Sex steroids also influence the excitability of neurons by altering GABA amino decarboxylase (GAD) and GABAA receptor subunits expression (Juptner et al., 1991; Noriega et al., 2010).
Previous work in our lab has demonstrated that males respond with less pain than females (Stinson et al., 2017) and that thalamic GABAergic signaling could have a role in controlling orofacial pain (Umorin et al., 2016; Stinson et al., 2017) but the role of estradiol in VZV associated pain has not been studied. Thus, it was hypothesized that estradiol attenuates herpes zoster induced pain by modulating GABAergic signaling in the thalamus. To address this question low and high physiological levels of estradiol were administered to male and female rats after injecting VZV into the whisker pad. pERK activity within GAD 67 and glutaminase stained cells were quantitated within the thalamus to characterize the role of inhibitory and excitatory neurons within the thalamus. Thalamic infusion of an estrogen receptor antagonist ICI 182,780 was completed to identify if estradiol was inducing its action on behavior through the estrogen receptor.
Materials and Methods
Animal husbandry
This study was carried out in accordance with the recommendations of Institutional Animal Care and Use Committee Guidebook and Texas A&M University College of Dentistry Institutional Animal Care and Use Committee. The animal protocol was approved by the Texas A&M University College of Dentistry Institutional Animal Care and Use Committee. Adult male Sprague-Dawley rats (300g) and female Sprague-Dawley rats (260g) from Envigo (Huntingdon, UK) were kept on a 12:12 light/dark cycle. A total of 160 rats were used in these studies (Table 1). The rats were given food and water ad libitum.
Table 1:
Experimental outline for testing VZV pain
| Experiment | Question | Whisker pad injection | Estradiol Treatment | Behavioral test (number of animals) | Molecular test (number of animals) | |
|---|---|---|---|---|---|---|
| Experiment # 1: Estrogen treatment of ovariectomized females | What is estradiol’s effect on VZV pain in females | control | none | Affective pain (n=10) | ||
| control | low | Affective pain (n=10) | ICC (n=5) | ELISA (n=5) | ||
| control | high | Affective pain (n=10) | ICC (n=5) | ELISA (n=5) | ||
| VZV | none | Affective pain (n=10) | ||||
| VZV | low | Affective pain (n=10) | ICC (n=5) | ELISA (n=5) | ||
| VZV | high | Affective pain (n=10) | ICC (n=5) | ELISA (n=5) | ||
| Experiment #2: Estrogen treatment of castrated males | What is estradiol’s effect on VZV pain in males | control | none | Affective pain (n=10) | ||
| control | low | Affective pain (n=10) | ICC (n=5) | ELISA (n=5) | ||
| control | high | Affective pain (n=10) | ICC (n=5) | ELISA (n=5) | ||
| VZV | none | Affective pain (n=10) | ||||
| VZV | low | Affective pain (n=10) | ICC (n=5) | ELISA (n=5) | ||
| VZV | high | Affective pain (n=10) | ICC (n=5) | ELISA (n=5) | ||
| Experiment #3: Treatment of females with antagonist ICI 182,780 | Does the estrogen receptor within the thalamus have a role in VZV pain? | control | high + vehicle | Affective pain (n=10) | ICC (n=5) | RT-PCR (n=5) |
| control | high + ICI 182,780 | Affective pain (n=10) | ICC (n=5) | RT-PCR (n=5) | ||
| VZV | high + vehicle | Affective pain (n=10) | ICC (n=5) | RT-PCR (n=5) | ||
| VZV | high + ICI 182,780 | Affective pain (n=10) | ICC (n=5) | RT-PCR (n=5) | ||
Abbreviations: varicella zoster virus (VZV); immunocytochemistry (ICC); real time polymerase chain reaction (RT-PCR); Enzyme-linked immunosorbent assay (ELISA).
Treatment and Experimental groups
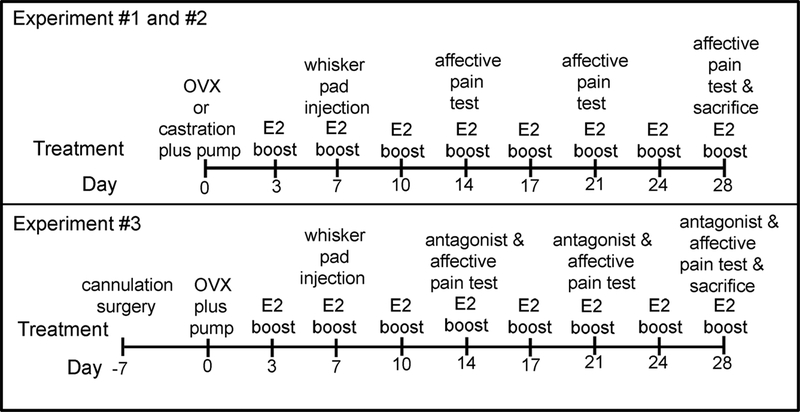
Table 1 summarizes the three experiments and each treatment group, details are within the following methods sections. Briefly, rats were ovariectomized or castrated and then an osmotic pump was placed beneath the skin that dispensed estradiol or vehicle. After pump placement, the rats were given a sub-cutaneous injection of estradiol or vehicle every 3 or 4 days (Fig. 1). One week following pump placement surgery the animals were divided so that half received a whisker pad injection of 100 μl of MeWo cells infected with VZV (>50,000–60,000 pfu/μl) or the same volume of control MeWo cells (human skin cell line) lacking virus. In experiment #1 and #2 behavioral testing was completed 90 minutes following sub-cutaneous injection of estradiol or vehicle (Fig. 1). In experiment #3 the rats were cannulated and then one week later ovariectomization and pump placement surgery was completed (Fig. 1). Then one week later the whisker pad was injected. On the day of testing the animals were infused with ICI 182,780 or vehicle followed by a subcutaneous injection of estradiol and 90 minutes after administration the behavioral tests were completed. Following three weeks of testing the animals were sacrificed for molecular studies.
Figure 1.
Schematic of experimental timeline. In Experiment #1 and #2 male and female Sprague Dawley rats were castrated or ovariectomized (OVX) and osmotic pumps were placed dispensing either vehicle or a low tonic dose of 17β-estradiol benzoate. An injection of 17β-estradiol benzoate (E2 boost) was administered 90 minutes before affective pain testing. The thalamic region was cannulated in Experiment #3. In Experiment #3 antagonist ICI 182,780 was infused and then the E2 boost was administered 90 minutes before pain testing.
Ovariectomization
Rats were anesthetized with 2% isoflurane in 2 liters of air per minute. The anesthetized female was placed in ventral recumbency, shaved and the dorsal surgical area swabbed with surgical scrub. Using sterile technique 10 to 15 mm skin incisions were made dorsally and bilaterally to the spine. The incision was midway between the caudal edge of the ribcage and the base of the tail. The ovary and the oviduct were exteriorized through the muscle wall incision. A sterile silk ligature was placed around the oviduct. Each ovary and part of the oviduct was removed with a single cut, the remaining tissue was replaced into the peritoneal cavity and the skin stapled.
Castration
Rats were anesthetized with 2% isoflurane in 2 liters of air per minute. The scrotal area was shaved and cleaned with betadine solution. While under anesthesia the rat was turned onto its back the testes were pushed into the scrotum and a small incision made on the scrotal sac. A testis was pushed close to the incision and another incision was made on the mesorchium sheath. The testis along with the epididymis were pushed out of the mesorchium sheath and scrotum then clamped with a hemostat behind the epididymis so that the deferent duct and associated blood vessels were crushed and then the testis removed. Sometimes a suture was placed behind the crushed tissue in order to control bleeding but was found to be unnecessary in most cases. Any excess tissue that was not removed was pushed back into the scrotal sac then the procedure repeated for the other side. The scrotum was not sutured but the edges of the incision lightly pinched together so to approximate and left to drain if needed.
Pump placement and estradiol treatment
While under anesthesia, the rat was turned onto its stomach and a small incision was cut into the skin at the neck. This surgery occurred simultaneously to ovariectomization or castration. Then using mayo scissors a pocket was made under the skin with blunt dissection and an osmotic pump inserted. The incision was closed using 9 mm wound clips. This pump was a 28-day Alzet mini-osmotic pump (Durect Corporation, Cupertino, CA) already dispensing 6 μl/day of the vehicle polyethylene glycol (no E2) or 750 ng/6μl/day of 17β-estradiol benzoate (Sigma, St. Louis). In addition, the rats receiving pumps dispensing 17β-estradiol were injected subcutaneously with 2.5 μg of 17β-estradiol benzoate in 0.1 ml of sesame seed oil every 3 or 4 days (high E2) or just the sesame seed oil (low E2). This dose of estradiol produces a diestrus (low E2) and proestrus (high E2) dose of estradiol in female rats (Kramer and Bellinger, 2009).
Thalamic guide cannula surgery
In experiment #3 (300 grams) rats underwent surgery to place a bilateral guide cannula into the thalamus one week after arrival to the vivarium. Rats were anesthetized with 2% isoflurane with an air flow of 2 liters per minute. Using sterile technique guide cannulas (C313G Plastics One, Roanoke, VA) were placed bilaterally into the thalamus stereotaxically using the coordinates 3.6 mm posterior of Bregma, 3.0 mm from midline at a depth of 5.5 mm from the top of the skull. The guide cannulas were closed with obturators.
Post-Surgical Treatment
Post-surgery the animals received nalbuphine (2mg/kg) subcutaneously and were placed under a radiant heat source. Following arousal the rats were returned to the home cage.
Thalamic Infusion protocol
For thalamic infusion the obturators were removed and an injection syringe (that projected 0.5 mm below the guide cannula) was inserted to complete bilateral infusions of 500 nanoliters (50 nanoliters/min) containing 10 μM (Sawada et al., 2000a; Sawada et al., 2000b) ICI 182,780 (Bio-Techne, Minneapolis, MN) or of the same volume vehicle DMSO (DMSO). After infusion, the injector was left in place for 5 minutes, removed and the obturator replaced. Previous reports demonstrated that 500 nanoliters injected at these coordinates spreads to the posterior thalamic nucleus (Po), ventral posteromedial (VPM) and ventral posterolateral thalamic nuclei (VPL) and the reticular thalamic region (Kramer et al., 2017). Previous work has shown that the vehicle DMSO does not lead to a decrease in neuronal cell numbers (Kramer et al., 2018).
Behavioral testing
Place Escape/Avoidance Paradigm (PEAP) testing was performed during the morning of the light phase to determine pain. To accomplish this, the rats were placed in a 30 cm × 30 cm × 30 cm acrylic box where half the box was covered in black cloth. This test chamber was modeled from the PEAP test performed by the Fuchs’s laboratory (LaBuda and Fuchs, 2000). This assay was used to measure the motivation/affective aspect of pain (LaBuda and Fuchs, 2000; Baastrup et al., 2011). The PEAP test is based on the assumption that if animals escape and/or avoid a noxious stimulus, then the stimulus is aversive to the animal. Rodents being nocturnal in nature preferred to stay on the dark side when placed into the test chamber. After placing the rat in the test chamber, the rat was immediately poked with a 60-gram filament every 15 seconds on the injected side if the rat was on the dark side and on the non-injected side if it was on the light side. Because VZV was injected into the whisker pad the target region for the poking was the area below the eye and caudal to the whisker pad. This region is innervated by the second branch of trigeminal ganglion (DaSilva and DosSantos, 2012), the nerve infected by VZV injection of the whisker pad. The time spent on the dark side of the box was recorded in 5 minute bins and testing was performed for a total of 30 minutes. Testing was performed once a week. Testing was completed for three weeks (Fig. 1). Thus, the theory behind the test is that if the rat is experiencing VZV induced pain when poked in the sensitive area it will not stay on it preferred dark side but will move to the non-preferred light side and stay there to avoid the poke. Values were given as a mean and standard error of the mean (SEM) for the ten animals in each treatment group (Table 1).
Thalamic tissue punches
Fresh thalamic tissue (5–10 mg) was collected within 1 hour of post behavioral testing in the third week. Animals were euthanized by exposure to CO2 followed by decapitation. The brain was removed using a rongeur and placed on a brain slicer (Zivic, Pittsburgh, PA). After cooling 2 mm thick sections were cut between Bregma −3 to −5. These sections were placed on glass slides and kept on dry ice. Lateral thalamic tissue was collected with punches 2 mm in diameter centered on the injection site, punches included the Po, VPM, VPL and reticular thalamus. Tissue was stored in liquid nitrogen until use.
Real time PCR
RNA was isolated from thalamic tissue punches obtained from five randomly selected animals per group in experiment #3 (see Table 1). RNA extraction was performed using the RNA Lipid Tissue Kit from Qiagen (Valencia, CA). The RNA concentration in the resulting sample was determined on a Nanodrop2000. A one-step reverse transcription PCR reaction was performed on BioRAD C1000 Thermal Cycler using the SYBR-Green 1-Step RT-PCR kit and primers from Qiagen (Table 2). The thermal protocol was 30 min @ 50 °C for the reverse transcription reaction, 15 min @ 95 °C for DNA pol activation and 40x (15 s @ 94 °C melting, 30 s @ 56 °C annealing, 30 s @ 72 °C extension). A melting curve was obtained thereafter for quality assurance. Sample amount was adjusted according to total RNA concentration to obtain 20 ng of total RNA per well in the final reaction mix. All reactions were run in triplicate. PCR runs that did not exhibit a proper amplification profile were discarded. For each sample, the threshold Ct value for GAPDH was subtracted from the Ct of value for the genes in Table 2 to give a ΔCt. The mean ΔCt from the VZV/high E2 group was subtracted from the VZV/high E2 + ICI 182,780 group to give a ΔΔCt. To get the fold change the −ΔΔCt was raised to the second power (2−ΔΔCt).
Table 2.
PCR primer pairs for RT-PCR
| Gene | Qiagen Catalog # | Qiagen ID |
|---|---|---|
| GAPDH | QT00199633 | Gapd |
| GAD 67 | QT00194600 | Gad1 |
| GAD 65 | QT00190778 | Gad2 |
| VGAT | QT00378413 | Viaat |
Enzyme-linked immunosorbent assay (ELISA)
Thalamic tissue punches from five randomly selected animals in experiments #1 and #2 (Table 1) were stored in liquid nitrogen until analysis. Tissue was placed in 250 μl of T-Per tissue protein extraction reagent containing Halt Protease Inhibitor and ground (Thermo Scientific, Rockford, IL). Ground samples were frozen and thawed, followed by centrifugation and decanting of the supernatant. Quantitation of vesicular GABA transporter (VGAT) in the supernatant was completed on duplicate 100 μl samples of supernatant using an SLC32A1 (VGAT) ELISA following the manufacturer’s directions (Cusabio, catalog #CSB-EL021578MO). Total protein was determined in each sample using a BCA protein assay (Thermo Scientific, Waltham, WA). Values represent the pg of VGAT per μg of total protein. No significant difference was observed between the right and left thalamic tissues and the values were combined for each animal.
Immuno-fluorescent staining
Five animals were randomly selected from the ten animals in experiments #1, #2 and #3 (Table 1) for perfusion, sectioning and staining. Rats were injected with 100 mg/kg ketamine and 10 mg/kg xylazine. After injection the animals were perfused with 9% sucrose followed by 4% paraformaldehyde. Fixed tissues were stored in 25% sucrose, frozen, cryo-sectioned and the 32 μm sections placed on Histobond slides (VWR international, Radnor, PA). The tissue was post-fixed for 5 minutes in 4% paraformaldehyde, rinsed and then blocked for 2 hours at room temperature with a PBS solution containing 5% normal goat serum (Sigma-Aldrich, St. Louis, MO) and 0.3% Triton-X 100. The slides were then incubated in a primary antibody solution overnight at 4°C. The primary antibody consisted of a mixture of the GAD 67 (GAD 1) antibody (Millipore clone 1G10.2, MAB5406) at a 1:500 dilution and rabbit pERK antibody (phosphorylated extracellular signal-regulated kinase, Cell Signaling Technology, Boston, MA, #4695) at a 1:150 dilution. Or a mixture of the knock-out validated glutaminase monoclonal rabbit antibody (abcam, ab156876) and the pERK mouse monoclonal antibody (Santa Cruz, sc-7383) both at a 1:150 dilution. Or a mixture of the GAD 67 and glutaminase antibodies at a 1:150 dilution. The primary antibody was diluted with PBS, 5% BSA and 0.3% Triton X-100. After incubation in primary antibody the slides were then rinsed three times in PBS and Triton-X 100 for a total of 45 minutes and placed for 2 hours in secondary antibody and PBS and 0.3% Triton X-100. Secondary antibodies (1:500 dilution) included a mixture of goat anti-mouse 568 and goat anti-rabbit 488 (Invitrogen, Carlsbad, CA) or goat anti-mouse 488 and goat anti-rabbit 568. After rinsing the slides three times in PBS and 0.3% Triton X-100 for a total of 45 min, the slides were mounted with Fluoromount-G mounting medium containing Hoechst 33342 stain (Electron Microscopy Sciences, Hatfield, PA). The fluorescent signal was imaged using a Nikon fluorescent microscope and NIS-Elements imaging software and a Photometrics CoolSnap K4 CCD camera (Roper Scientific, Inc, Duluth, GA). Controls eliminating the primary antibody showed no signal (data not shown).
Cell counts were completed by a blinded reviewer. Every other section was selected for staining. Typically three sections were counted for each animal. The slides were analyzed using Image J software, the average background for the slides within a treatment group was subtracted from the image and a fluorescent signal associated with a cell nucleus was counted as a positive cell. Counts were completed for the number of GAD 67, glutaminase, GAD 67/pERK and/or glutaminase/pERK stained cells within a 0.125 mm2 field. Counts were completed within the ventral thalamic nuclei and cell counts from the two fields on each section were then averaged. This average count for the three sections was averaged for each animal. Values were given as a mean and standard error of the mean (SEM) for the five animals in each treatment group (Table 1).
Statistics
PEAP data was analyzed with the Kruskal-Wallis test, significant main effects were followed with Dunn’s post-hoc testing. PCR, ELISA and cell count data were analyzed with the non-parametric Mann-Whitney test (Prizm 5.04, GraphPad Software, La Jolla, CA).
Results
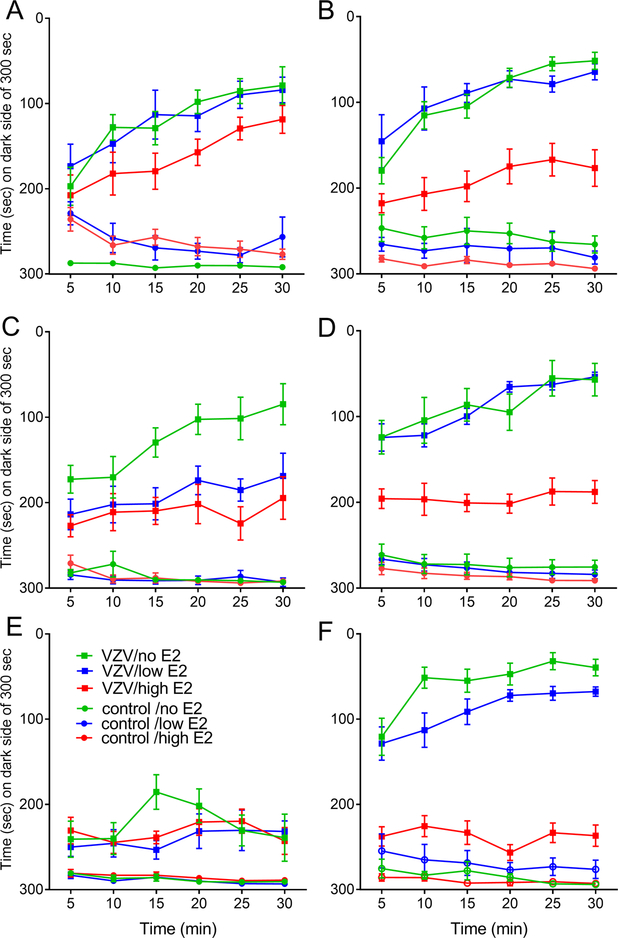
In week one, VZV injection and estradiol treatment significantly affected the pain response of male (χ5=227.5, p<0.0001) and female (χ5=285.1, p<0.0001) rats. This significant effect continued in weeks two (male χ5=195.8, p<0.0001; female χ5=306, p<0.0001) and three (male χ5=188.1, p<0.0001; female χ5=288.2, p<0.0001). Injection of VZV significantly increased the pain response versus controls in week one (p<0.0001), two (p<0.0001) and three (p<0.0001), compare the VZV to the control groups (Fig. 2A–F). In week one a high dose of estradiol significantly reduced the pain response in females, compare the VZV/high E2 group to the VZV/low E2 group (p=0.002) and compare the VZV/high E2 group to the VZV/no E2 group (p<0.0001) in Fig. 2B. Estradiol had no significant effect in males during week one (Fig. 2A). In week two a high dose of estradiol significantly reduced (p=0.03) the pain response in males, compare the VZV/high E2 group to the VZV/no E2 group (Fig. 2C). In females estradiol significantly reduced the pain response when comparing the VZV/high E2 group to the VZV/low E2 group (p<0.0001) and the VZV/no E2 group (p<0.0001), see Fig 2D. During week three a high dose of estradiol significantly reduced the pain response in females, compare the VZV/high E2 group to the VZV/low E2 group (p<0.0001) and to the VZV/no E2 group (p<0.0001) in Fig. 2F. Estradiol had no significant effect in males in week three (Fig. 2E).
Figure 2.
Estradiol effected the pain response in male and female rats. Male and female Sprague Dawley rats were castrated or ovariectomized and osmotic pumps placed dispensing either vehicle (no E2) or a low tonic dose of 17β-estradiol benzoate. The animals receiving this low tonic dose were split so that half received an injection of vehicle (low E2) and half an injection of 17β-estradiol benzoate every 3rd or 4th day (high E2). One week after placement of the pump the whisker pad was injected with varicella zoster virus (VZV) or control. One week after injection pain testing was performed once a week for three weeks, testing was completed 90 minutes after injection. Panels A and B show data for week one, panels C and D for week two, and panels E and F for week three. Panels A, C and E show the data for males and panels B, D and F shows the data for females. There were 10 animals per group. Values are the mean and SEM.
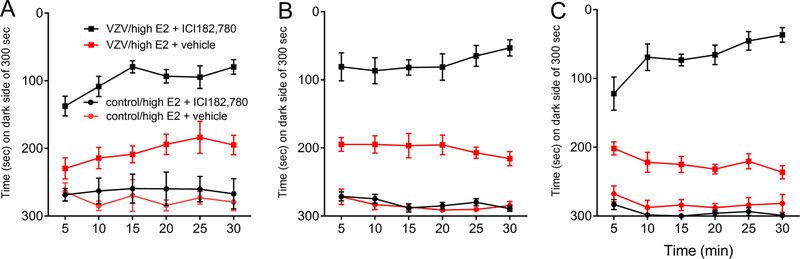
Treatment with antagonist resulted in a significant effect during week one (χ3=134.1, p<0.0001), week two (χ3=170.8, p<0.0001) and week three (χ3=140.0, p<0.0001). VZV injection significantly increased (p<0.0001) the affective pain response in week one (Fig. 3A), in week two (Fig. 3B) and week three (Fig. 3C). Infusing estrogen receptor antagonist ICI 182,780 into the thalamus significantly increased the VZV induced pain response in week one (p<0.0001), two (p=0.0002) and three (p<0.0001), compare VZV/high E2 + ICI 182,780 group and VZV/high E2 group + vehicle (Fig. 3A, B and C, respectively.
Figure 3.
Thalamic infusion of the estrogen receptor antagonist ICI 182,780 increased the affective pain response in rats treated with high physiological concentration of estradiol. The thalamus of female rats were cannulated and one week later female ovariectomized rats were implanted with osmotic pumps releasing estradiol. Rats were given a boost of estradiol every 3 or 4 days (high E2). One week after placement of the pump the whisker pad was injected with varicella zoster virus (VZV) or control. One week after virus injection the thalamus of the rats were infused with ICI 182,780 or vehicle (DMSO) and the estradiol injection given, 90 minutes later the affective pain response was measured. Pain was measured once a week for three weeks; panel A is week one data, panel B is week two data and panel C is week three data. There were 10 animals per group. Values are the mean and SEM.
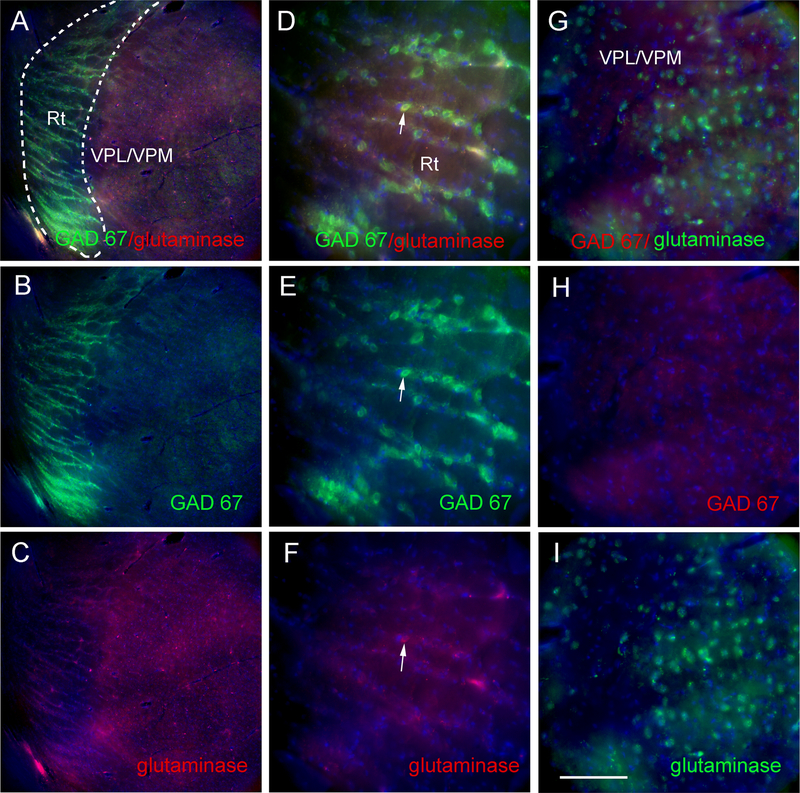
GAD 67 stained cells were localized to the reticular thalamic region (Fig. 4A and B) but glutaminase stained cells were located primarily within the VPM/VPL (Fig. 4C) with occasional cells in the reticular thalamus (Fig. 4D–F, arrow). Higher magnification of the VPM/VPL show many glutaminase positive cells (Fig. 4G and I) but few GAD 67 positive cells (Fig. 4G and H).
Figure 4.
GAD 67 and glutaminase staining of the reticular thalamic (Rt) region, the ventral posteromedial (VPM) region and the ventral posterolateral (VPL) region. Images are representative of a rat injected with VZV and treated with a high concentration of estradiol. In panels A-C a low magnification image of the thalamic region is shown after staining for GAD67 and glutaminase. Dotted line in Panel A outlines the Rt region on the brain slice. In panels D-F a higher magnification of the Rt region. In panels G-I a higher magnification image of the VPM/VPL region. Arrow indicates cell that cocolocalizes with GAD67 and glutaminase. Nuclei were stained with Hoechst 33342 (blue). Bar = 50 micrometers.
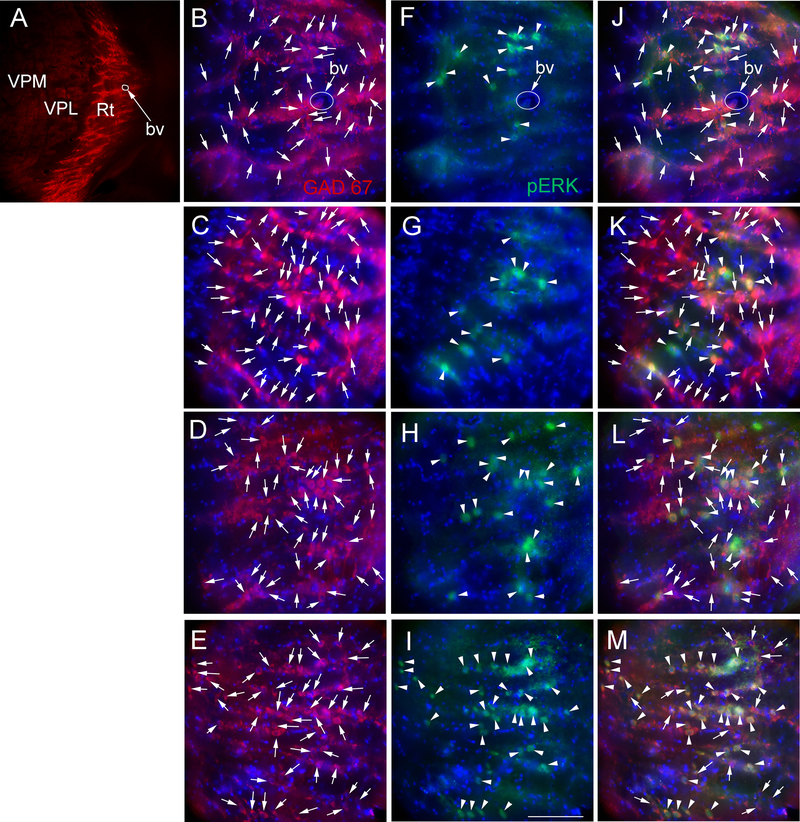
Consistent with Figure 4 a low magnification image from a control rat treated with a low concentration of estradiol shows GAD 67 staining primarily within in the reticular thalamic region (red, Fig. 5A). Enlargement shows many individual cells (Fig. 5B, arrows). Comparing the number of GAD 67 positive cells in the control/low estradiol treated group (Fig. 5B) to the control/high estradiol group (Fig. 5C), the VZV/low estradiol group (Fig. 5D), and the VZV/high estradiol group (Fig. 5E) no observable difference in the number of stained cells was detected. In contrast, the control/low estradiol treated group (Fig. 5F) and control/high estradiol treated group (Fig. 5G) had a lower number of pERK positive cells (green cells, arrowheads) as compared to the VZV/low estradiol group (Fig. 5H) and the VZV/high estradiol group (Fig. 5I). Moreover, the number of GAD 67 cells co-localizing with pERK (yellow cells, arrowheads) was greater in the VZV treated groups (Fig. 5L and M) versus the controls (Fig. 5J and K)
Figure 5.
GAD 67 and pERK stained cells within the lateral thalamic region of female rats treated with estradiol. Panel A shows a representative low magnification image of the ventral posteromedial (VPM), ventral posterolateral (VPL) and reticular thalamic nuclei (Rt) region from a rat in the control/low E2 treatment group. Panels B, F and J are enlarged images from panel A, note the blood vessel (bv) for orientation. Panels C, G and K are representative of a female from the control/high E2 treatment group. Panels D, H and L are representative of a female rat from the VZV/low E2 treatment group. Panels E, I and M are representative of a female rat from the VZV/high E2 treatment group. GAD 67 stained cells (red) in panels B-E are indicated by arrows and pERK stained cells (green) in panels F-I are indicated by arrowheads. Cells that co-localize for GAD 67 and pERK (yellow) are indicated by arrowheads in panels J-M. GAD 67 stained cells that do not co-localize with pERK in panels J-M are indicated by arrows. Nuclei were stained with Hoechst 33342 (blue). Bar = 50 micrometers.
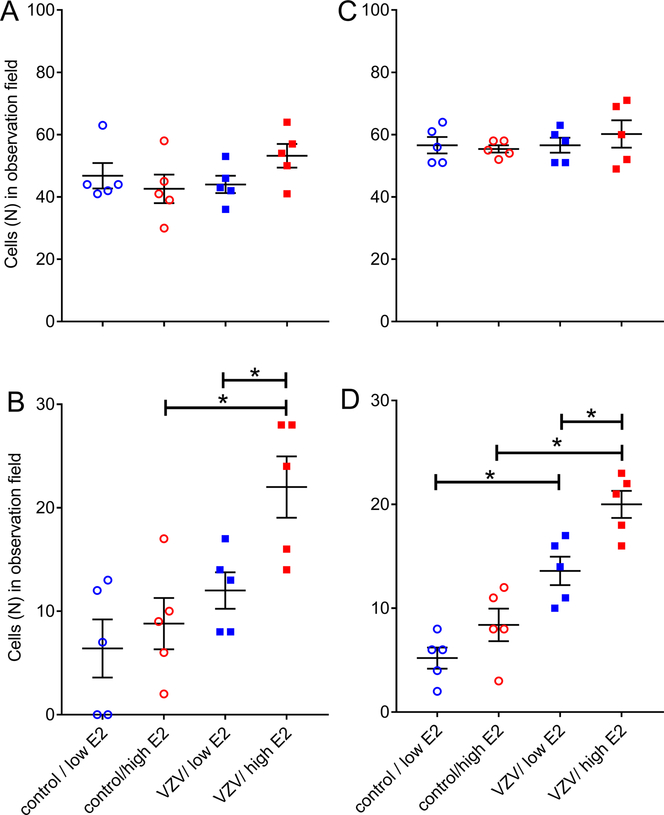
Estradiol treatment did not significantly change the number of GAD 67 positive cells in the thalamus of females or males (Fig. 6A and C, respectively). The number of cells staining for both GAD 67 and pERK was significantly greater in the VZV infected female (U5=2.5, p=0.039) and male (U5=1.5, p=0.024) rats treated with high estradiol (Fig. 6B and D, compare VZV low E2 group with VZV/high E2 group). VZV injection also increased the number of cells staining for both GAD 67 and pERK in female (U5=2.0, p=0.031) and male (U5=0, p=0.0079) rats treated with a high dose of estradiol (Fig. 6B and D).
Figure 6:
Cell counts within the reticular thalamic region of females (panels A and B) and males (Panels C and D) for GAD 67 (panels A and C) or GAD67 and pERK (panels B and D) stained cells. Rats whisker pads were injected with VZV or control while being treated with low and high levels of estradiol. Values are the mean and SEM. The asterisks indicate a significant difference of p<0.05. There were five animals in each treatment group and each dot represents the number of cells for an animal.
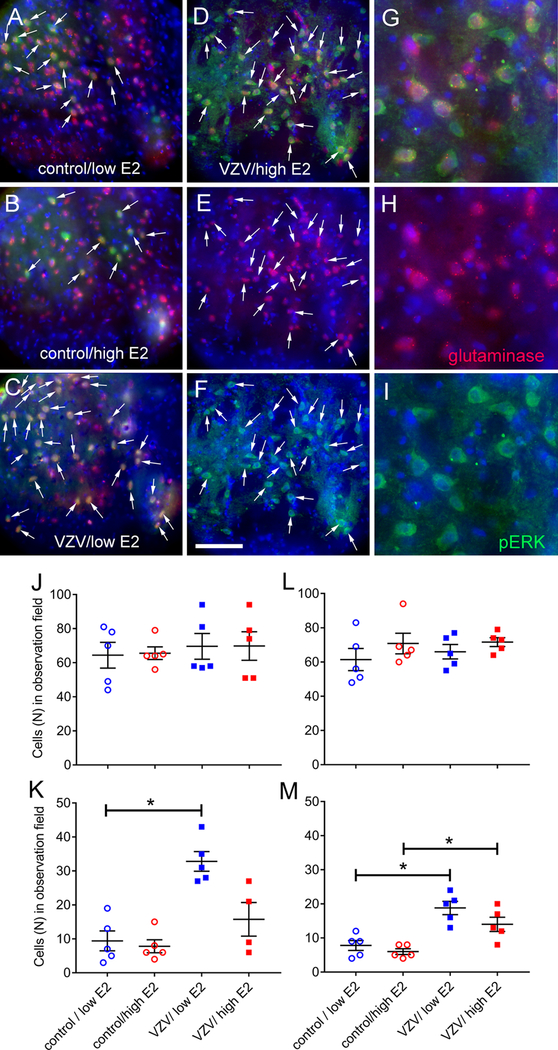
pERK co-localized with glutaminase staining within the VPM/VPL but the number of cells staining for pERK and glutaminase was lower in the controls (Fig. 7A and B, arrows) in comparison to the VZV injected rats (Fig C and D). Panels D through I are from the same VZV injected rat treated with a high concentration of estradiol showing both low and high magnification images of the positive cells. Counts of the glutaminase positive cells indicated no significant change after injection of VZV or after treatment with estradiol (Fig. 7J and L). In both male and female rats injected with VZV, a low concentration of estradiol increased the number of cells staining for glutaminase and pERK (Fig. 7K and M). VZV injection increased the number of cells staining for both glutaminase and pERK in female (U5=0, p=0.0079) and male (U5=0, p=0.0079) rats treated with a low dose of estradiol (Fig. 7K and M).
Figure 7.
Glutaminase and pERK staining from ventral posteromedial (VPM) region. Panel A is a control rat treated with a low concentration of estradiol, panel B is a control rat treated with a high concentration of estradiol and panel C is rat injected with VZV and treated with a low concentration of estradiol. Images D-I are representative of a rat injected with VZV and treated with a high concentration of estradiol. In panels A-F show low magnification images of the thalamic region after staining for glutaminase (red) and/or pERK (green). Arrow indicates cells that co-colocalize with glutaminase and pERK. In panels G-I a higher magnification image of the VPM region. Nuclei were stained with Hoechst 33342 (blue). Bar = 50 micrometers. Histograms shows cell counts within the ventral posteromedial thalamic region of females (panels J and K) and males (Panels L and M). Cells stained for glutaminase (panels J and L) or glutaminase and pERK (panels K and M) were counted and each dot represents the number of cells for an animal. Values are the mean and SEM. The asterisks indicate a significant difference of p<0.05. There were five animals in each treatment group.
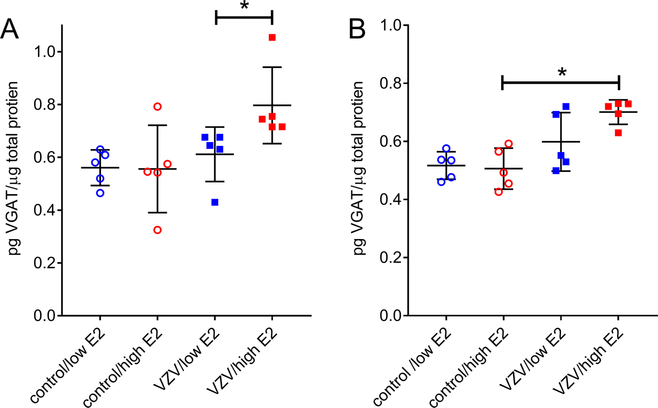
The amount of VGAT protein increased significantly (U5=4, p=0.030) after treating female rats with a high amount of estradiol, compare the VZV/low E2 group to the VZV/high E2 group (Fig. 8A). Injection of VZV significantly increased (U5=1, p=0.0031) VGAT in male rats, compare the control/high E2 group to the VZV/high E2 group (Fig. 8B).
Figure 8.
VGAT protein in the lateral thalamus of male and female rats after treatment with estradiol. Ovariectomized female (Panel A) and castrated male (Panel B) rats whisker pad was injected with VZV and then low and high levels of estradiol was administered. After three weeks following the VZV injection the thalamic tissue was isolated and the level of VGAT quantitated by ELISA. Values are the mean and SEM. The asterisks indicate a significant difference of p<0.05. There were five animals in each treatment group.
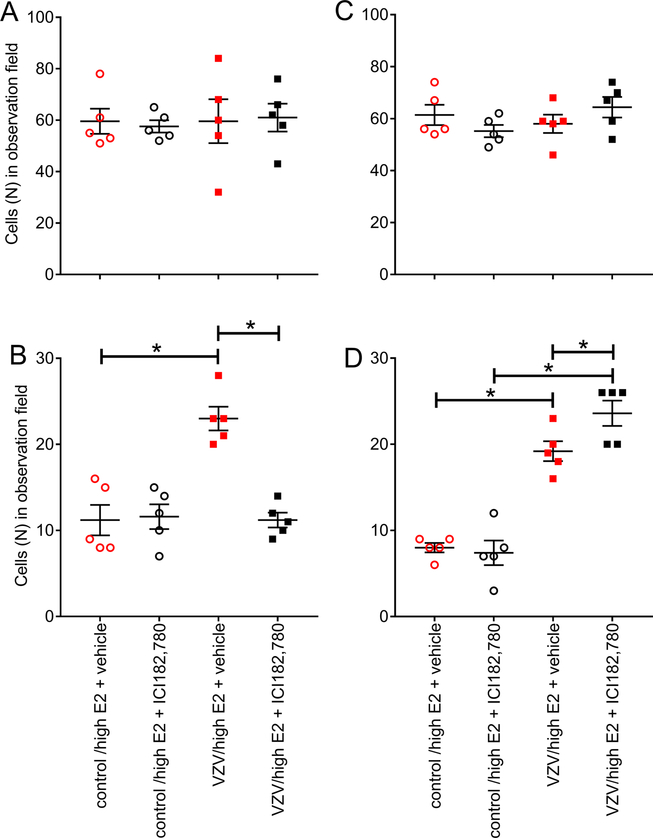
Estrogen receptor antagonist ICI 182,780 did not alter GAD 67 or glutaminase expression (Fig. 9A and C, respectively). Interestingly, the number cells staining for GAD 67 and pERK significantly decreased (U5=0, p=0.0079) after antagonist treatment, compare the VZV/high E2 + vehicle group to the VZV/high E2 + ICI182,780 group (Fig. 9B). In contrast, the number of glutaminase and pERK positive cells significantly increased (U5=0.5, p=0.023) in rats treated with antagonist (Fig. 9D).
Figure 9.
Cell counts for cells stained for GAD 67 (panel A) or pERK and GAD 67 (panel B) or glutaminase (panel C) or pERK and glutaminase (panel D). Counts were completed within the reticular thalamic region of female rats. In this experiment the animal’s whisker pads were injected with VZV or control. The thalamus was infused with estrogen receptor antagonist ICI 182 780 or vehicle before injecting a high dose of estradiol. Values are the mean and SEM. The asterisks indicate a significant difference of p<0.05. There were five animals in each treatment group.
GAD 67, GAD 65 and VGAT transcript decreased at least two fold after treatment with ICI 182,780 (VZV injected animals); GAD 67 decreased 2.4 fold, GAD 65 decreased 2.4 fold and VGAT decreased 2.7 fold.
Discussion
A high, physiological concentration of estradiol reduced VZV associated affective pain and administration of estrogen receptor antagonist ICI 182,780 increased the pain response suggesting a role for estrogen and the estrogen receptor in shingles pain. Interestingly, pERK was elevated in the excitatory cell population of the VPL/VPM after treatment with a low concentration of estradiol. pERK increased in the thalamic inhibitory cell population with the higher dose of estradiol and pain was greater with a low dose of estradiol versus the higher dose. Together the results suggest a low concentration of estradiol activated the excitatory drive in the VPM/VPL leading to increased pain and a higher concentration of estradiol recruited inhibitory transmission in the reticular thalamic region ultimately causing reduced pain. GABA genes such as VGAT increased their expression after treatment with estradiol but decreased after treatment with estrogen receptor antagonist ICI 182,780. These results suggests estradiol reduced orofacial pain by increasing inhibitory thalamic activity and that the mechanism was dependent, in part, on the estrogen receptor.
Female patients report herpes zoster pain more often and female rats have a greater pain response than males (Alvarez et al., 2007; Hillebrand et al., 2015; Stinson et al., 2017). Moreover, studies in female rats indicated that during proestrus, when the plasma concentration of estradiol is relatively high, there is a reduced response to VZV associated pain (Stinson et al., 2017). This is in contrast to diestrus when the pain response is greater and estradiol concentrations are lower (Stinson et al., 2017). Pain resulting from injection of CFA into the rat TMJ is consistent with this result, in that, administration of a high physiological level of estradiol will reduce the nociceptive response (Kramer and Bellinger, 2009).
Several nuclear estrogen receptors and membrane estrogen receptors are located in the brain suggesting estradiol can effect cellular physiology by signaling through these receptors (Perez et al., 2003; Brailoiu et al., 2007; Hazell et al., 2009). Consistent with this idea, estrogen facilitated pain transmission via membrane bound estrogen receptors by increasing cytostolic calcium and reactive oxygen species within the central nervous system (Deliu et al., 2012; Zhang et al., 2012). Administration of ICI 182,780, a nuclear estrogen receptor antagonist, partially blocked the effect of estradiol (Zhang et al., 2012), consistent with our results showing ICI 182,780 modulated the pain response. It should be pointed out that membrane estrogen receptors can also have an effect on the nociceptive response, as the membrane estrogen receptor antagonist G15 also blocked estrogens effect on the nociceptive response (Zhang et al., 2012). Moreover, 17β-estradiol, modulates expression of voltage gated sodium channels via ERα and ERβ resulting in an increase in orofacial pain (Hu et al., 2012; Bi et al., 2017). Thus, estrogen can modulate pain acting centrally through genomic or non-genomic receptors to alter gene expression, calcium levels and oxidative responses (Craft, 2007; Amandusson and Blomqvist, 2013).
Increased GABA activity can decrease excitatory activity and decrease the pain response (Osikowicz et al., 2013; Acher and Goudet, 2015). Thalamic expression of VGAT attenuates pain in the orofacial region (Kramer and Bellinger, 2009; Kramer et al., 2015) suggesting GABAergic signaling has a role in controlling orofacial pain (Umorin et al., 2016; Stinson et al., 2017). Sex steroids alter the function of GABA pathways in the central nervous system to modulate orofacial nociception in animal models (Puri et al., 2012; Tashiro et al., 2014). Estradiol can increase expression of GABA related genes in the thalamus reducing the pain response (Umorin et al., 2016). To date, the role of estradiol in VZV associated pain has not been studied. Because GABA attenuates the pain response and estradiol reduces pain we surmised that estradiol could reduce VZV associated pain by increasing GABA expression.
Changes in VGAT expression resulting from estradiol have been reported in the hypothalamic region (Ottem et al., 2004; Noriega et al., 2010) and our lab has reported changes in VGAT expression within the thalamus throughout the estrous cycle (Umorin et al., 2016). Multiple potential estrogen response elements are present in the VGAT promoter suggesting expression can be modulated by estradiol (Hudgens et al., 2009). Estradiol administration increased VGAT within the thalamus and concomitantly increased the number of GAD 67/pERK positive cells. Although estrogen receptor alpha (ERα) is not expressed at significant levels within the reticular thalamus, estrogen receptor beta (ERβ) has been observed in the rostral region of the reticular thalamus (Lein et al., 2007). The decreased pain response after estradiol treatment was reversed after ICI 182,780 treatment, a result that could be explained by estradiol signaling estrogen receptors to decrease GABA gene expression.
The reticular thalamic nucleus projects to regions such as the parafacicular and intralaminar nuclei (Clemente-Perez et al., 2017). Evidence suggests the parafacicular nucleus is important in motivational and effective pain (Weigel and Krauss, 2004). Thus, increased GABA cell activity in the reticular thalamus after estradiol treatment, as evidenced by more GAD 67/pERK positive cells, could have decreased the VZV pain response by acting on this pathway. Note that ICI 182,780 treatment decreased the number of GAD 67/pERK positive cells within the reticular thalamic nucleus and increased the pain response potentially by acting through the parafcicular pathway. Alternatively, estradiol could have acted at the zona incerta and ICI 182,780 could have spread to the zona incerta after infusion into the thalamus. The zona incerta contains estrogen receptors (Jakab et al., 1993; Shughrue et al., 1997). The mediodorsal thalamus receives dense GABAergic inputs from the zona incerta (Bartho et al., 2002; Erickson et al., 2004) and is heavily connected to cortical areas involved in processing the affective aspects of pain (Cornwall and Phillipson, 1988; Groenewegen, 1988). Thus, changes in estradiol signaling within the zona incerta could alter the VZV pain response through modulation of GABAergic cells.
The decrease in glutaminase/pERK positive cells after treating with a high concentration of estradiol can result from increased inhibitory input from the reticular thalamic nucleus or the zona incerta. Because GABA neurons from the reticular thalamic nucleus and zona incerta project to the VPM (Barbaresi et al., 1986; Nicolelis et al., 1992; Pinault and Deschenes, 1998; Bartho et al., 2002) increased activity within the GABA positive cells of these nuclei should increase inhibitory signaling to the VPM, thus reduce activity of these excitatory cells. Control of excitatory cells within the VPM has been shown to influence VZV associated pain in rats (Kramer et al., 2017). Further studies would identify the role these excitatory cells have in modulating the VZV associated pain.
In both male and female rats, a high physiological concentration of estradiol, at a level observed during proestrus, reduced the VZV associated pain response. This pain response correlated to increased GABA cell activity and decreased glutaminergic activity within the thalamus. Blocking the estrogen receptor attenuated estrogens effect on pain and on cellular activity. In conclusion, the results suggests estradiol modulates the pain response by altering activity within the thalamic region through an estrogen receptor dependent mechanism.
Acknowledgements:
The authors wish to thank Priscilla Hooks, Connie Tillberg and Gerald Hill for their excellent technical assistance. This study was supported by NIDCR grant DE026749 (PRKramer) and a grant from the 30, 300, 3000 Pain Research Challenge from the University of Pittsburgh Clinical and Translational Science Institute (PRKinchington). PRKinchington also acknowledges support from grant NS064022, NEI core grant EY08098 and unrestricted funds from Eye & Ear Foundation and Research to Prevent Blindness Inc. There are no conflicts of interest with this work.
References
- Acher F, Goudet C (2015), Therapeutic potential of group III metabotropic glutamate receptor ligands in pain. Curr Opin Pharmacol 20:64–72. [DOI] [PubMed] [Google Scholar]
- Aloisi AM, Sorda G (2011), Relationship of female sex hormones with pain perception: focus on estrogens. Pain Manag 1:229–238. [DOI] [PubMed] [Google Scholar]
- Alvarez FK, de Siqueira SR, Okada M, Teixeira MJ, de Siqueira JT (2007), Evaluation of the sensation in patients with trigeminal post-herpetic neuralgia. J Oral Pathol Med 36:347–350. [DOI] [PubMed] [Google Scholar]
- Amandusson A, Blomqvist A (2013), Estrogenic influences in pain processing. Front Neuroendocrinol 34:329–349. [DOI] [PubMed] [Google Scholar]
- Baastrup C, Jensen TS, Finnerup NB (2011), Pregabalin attenuates place escape/avoidance behavior in a rat model of spinal cord injury. Brain Res 1370:129–135. [DOI] [PubMed] [Google Scholar]
- Barbaresi P, Spreafico R, Frassoni C, Rustioni A (1986), GABAergic neurons are present in the dorsal column nuclei but not in the ventroposterior complex of rats. Brain Res 382:305–326. [DOI] [PubMed] [Google Scholar]
- Bartho P, Freund TF, Acsady L (2002), Selective GABAergic innervation of thalamic nuclei from zona incerta. Eur J Neurosci 16:999–1014. [DOI] [PubMed] [Google Scholar]
- Bi RY, Meng Z, Zhang P, Wang XD, Ding Y, Gan YH (2017), Estradiol upregulates voltage-gated sodium channel 1.7 in trigeminal ganglion contributing to hyperalgesia of inflamed TMJ. PLoS One 12:e0178589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brailoiu E, Dun SL, Brailoiu GC, Mizuo K, Sklar LA, Oprea TI, Prossnitz ER, Dun NJ (2007), Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J Endocrinol 193:311–321. [DOI] [PubMed] [Google Scholar]
- Clemente-Perez A, Makinson SR, Higashikubo B, Brovarney S, Cho FS, Urry A, Holden SS, Wimer M, David C, Fenno LE, Acsady L, Deisseroth K, Paz JT (2017), Distinct Thalamic Reticular Cell Types Differentially Modulate Normal and Pathological Cortical Rhythms. Cell Rep 19:2130–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwall J, Phillipson OT (1988), Afferent projections to the dorsal thalamus of the rat as shown by retrograde lectin transport--I. The mediodorsal nucleus. Neuroscience 24:1035–1049. [DOI] [PubMed] [Google Scholar]
- Craft RM (2007), Modulation of pain by estrogens. Pain 132 Suppl 1:S3–12. [DOI] [PubMed] [Google Scholar]
- Craft RM, Mogil JS, Aloisi AM (2004), Sex differences in pain and analgesia: the role of gonadal hormones. Eur J Pain 8:397–411. [DOI] [PubMed] [Google Scholar]
- DaSilva AF, DosSantos MF (2012), The role of sensory fiber demography in trigeminal and postherpetic neuralgias. J Dent Res 91:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deliu E, Brailoiu GC, Arterburn JB, Oprea TI, Benamar K, Dun NJ, Brailoiu E (2012), Mechanisms of G protein-coupled estrogen receptor-mediated spinal nociception. J Pain 13:742–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue JG, Choo PW, Manson JE, Platt R (1995), The incidence of herpes zoster. Arch Intern Med 155:1605–1609. [PubMed] [Google Scholar]
- Erickson SL, Melchitzky DS, Lewis DA (2004), Subcortical afferents to the lateral mediodorsal thalamus in cynomolgus monkeys. Neuroscience 129:675–690. [DOI] [PubMed] [Google Scholar]
- Frye CA, Cuevas CA, Kanarek RB (1993), Diet and estrous cycle influence pain sensitivity in rats. PharmacolBiochemBehav 45:255–260. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ (1988), Organization of the afferent connections of the mediodorsal thalamic nucleus in the rat, related to the mediodorsal-prefrontal topography. Neuroscience 24:379–431. [DOI] [PubMed] [Google Scholar]
- Harpaz R, Ortega-Sanchez IR, Seward JF (2008), Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 57:1–30; quiz CE32–34. [PubMed] [Google Scholar]
- Hazell GG, Yao ST, Roper JA, Prossnitz ER, O’Carroll AM, Lolait SJ (2009), Localisation of GPR30, a novel G protein-coupled oestrogen receptor, suggests multiple functions in rodent brain and peripheral tissues. J Endocrinol 202:223–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillebrand K, Bricout H, Schulze-Rath R, Schink T, Garbe E (2015), Incidence of herpes zoster and its complications in Germany, 2005–2009. J Infect 70:178–186. [DOI] [PubMed] [Google Scholar]
- Hu F, Wang Q, Wang P, Wang W, Qian W, Xiao H, Wang L (2012), 17beta-Estradiol regulates the gene expression of voltage-gated sodium channels: role of estrogen receptor alpha and estrogen receptor beta. Endocrine 41:274–280. [DOI] [PubMed] [Google Scholar]
- Hudgens ED, Ji L, Carpenter CD, Petersen SL (2009), The gad2 promoter is a transcriptional target of estrogen receptor (ER)alpha and ER beta: a unifying hypothesis to explain diverse effects of estradiol. J Neurosci 29:8790–8797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab RL, Horvath TL, Leranth C, Harada N, Naftolin F (1993), Aromatase immunoreactivity in the rat brain: gonadectomy-sensitive hypothalamic neurons and an unresponsive “limbic ring” of the lateral septum-bed nucleus-amygdala complex. J Steroid Biochem Mol Biol 44:481–498. [DOI] [PubMed] [Google Scholar]
- Johnson RW, Bouhassira D, Kassianos G, Leplege A, Schmader KE, Weinke T (2010), The impact of herpes zoster and post-herpetic neuralgia on quality-of-life. BMC Med 8:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung BF, Johnson RW, Griffin DR, Dworkin RH (2004), Risk factors for postherpetic neuralgia in patients with herpes zoster. Neurology 62:1545–1551. [DOI] [PubMed] [Google Scholar]
- Juptner M, Jussofie A, Hiemke C (1991), Effects of ovariectomy and steroid replacement on GABAA receptor binding in female rat brain. J Steroid BiochemMol Biol 38:141–147. [DOI] [PubMed] [Google Scholar]
- Kramer P, Rao M, Stinson C, Bellinger LL, Kinchington PR, Yee MB (2018), Aromatase Derived Estradiol Within the Thalamus Modulates Pain Induced by Varicella Zoster Virus. Front Integr Neurosci 12:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer PR, Bellinger LL (2009), The effects of cycling levels of 17beta-estradiol and progesterone on the magnitude of temporomandibular joint-induced nociception. Endocrinology 150:3680–3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer PR, Stinson C, Umorin M, Deng M, Rao M, Bellinger LL, Yee MB, Kinchington PR (2017), Lateral thalamic control of nociceptive response after whisker pad injection of varicella zoster virus. Neuroscience 356:207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer PR, Umorin M, Bellinger LL (2015), Attenuation of myogenic orofacial nociception and mechanical hypersensitivity by viral mediated enkephalin overproduction in male and female rats. BMC Neurol 15:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBuda CJ, Fuchs PN (2000), A behavioral test paradigm to measure the aversive quality of inflammatory and neuropathic pain in rats. Exp Neurol 163:490–494. [DOI] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen L, Chen TM, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, Dong HW, Dougherty JG, Duncan BJ, Ebbert AJ, Eichele G, Estin LK, Faber C, Facer BA, Fields R, Fischer SR, Fliss TP, Frensley C, Gates SN, Glattfelder KJ, Halverson KR, Hart MR, Hohmann JG, Howell MP, Jeung DP, Johnson RA, Karr PT, Kawal R, Kidney JM, Knapik RH, Kuan CL, Lake JH, Laramee AR, Larsen KD, Lau C, Lemon TA, Liang AJ, Liu Y, Luong LT, Michaels J, Morgan JJ, Morgan RJ, Mortrud MT, Mosqueda NF, Ng LL, Ng R, Orta GJ, Overly CC, Pak TH, Parry SE, Pathak SD, Pearson OC, Puchalski RB, Riley ZL, Rockett HR, Rowland SA, Royall JJ, Ruiz MJ, Sarno NR, Schaffnit K, Shapovalova NV, Sivisay T, Slaughterbeck CR, Smith SC, Smith KA, Smith BI, Sodt AJ, Stewart NN, Stumpf KR, Sunkin SM, Sutram M, Tam A, Teemer CD, Thaller C, Thompson CL, Varnam LR, Visel A, Whitlock RM, Wohnoutka PE, Wolkey CK, Wong VY, Wood M, Yaylaoglu MB, Young RC, Youngstrom BL, Yuan XF, Zhang B, Zwingman TA, Jones AR (2007), Genome-wide atlas of gene expression in the adult mouse brain. Nature 445:168–176. [DOI] [PubMed] [Google Scholar]
- Mahalingam R, Wellish MC, Dueland AN, Cohrs RJ, Gilden DH (1992), Localization of herpes simplex virus and varicella zoster virus DNA in human ganglia. Ann Neurol 31:444–448. [DOI] [PubMed] [Google Scholar]
- Nicolelis MA, Chapin JK, Lin RC (1992), Somatotopic maps within the zona incerta relay parallel GABAergic somatosensory pathways to the neocortex, superior colliculus, and brainstem. Brain Res 577:134–141. [DOI] [PubMed] [Google Scholar]
- Noriega NC, Eghlidi DH, Garyfallou VT, Kohama SG, Kryger SG, Urbanski HF (2010), Influence of 17beta-estradiol and progesterone on GABAergic gene expression in the arcuate nucleus, amygdala and hippocampus of the rhesus macaque. Brain Res 1307:28–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K, Hirata H, Takeshita S, Bereiter DA (2003), Response properties of TMJ units in superficial laminae at the spinomedullary junction of female rats vary over the estrous cycle. J Neurophysiol 89:1467–1477. [DOI] [PubMed] [Google Scholar]
- Osikowicz M, Mika J, Przewlocka B (2013), The glutamatergic system as a target for neuropathic pain relief. Exp Physiol 98:372–384. [DOI] [PubMed] [Google Scholar]
- Ottem EN, Godwin JG, Krishnan S, Petersen SL (2004), Dual-phenotype GABA/glutamate neurons in adult preoptic area: sexual dimorphism and function. J Neurosci 24:8097–8105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmeira CC, Ashmawi HA, Posso Ide P (2011), Sex and pain perception and analgesia. Rev Bras Anestesiol 61:814–828. [DOI] [PubMed] [Google Scholar]
- Perez SE, Chen EY, Mufson EJ (2003), Distribution of estrogen receptor alpha and beta immunoreactive profiles in the postnatal rat brain. Brain Res Dev Brain Res 145:117–139. [DOI] [PubMed] [Google Scholar]
- Pieretti S, Di Giannuario A, Di Giovannandrea R, Marzoli F, Piccaro G, Minosi P, Aloisi AM (2016), Gender differences in pain and its relief. Ann Ist Super Sanita 52:184–189. [DOI] [PubMed] [Google Scholar]
- Pinault D, Deschenes M (1998), Projection and innervation patterns of individual thalamic reticular axons in the thalamus of the adult rat: a three-dimensional, graphic, and morphometric analysis. J Comp Neurol 391:180–203. [DOI] [PubMed] [Google Scholar]
- Puri J, Vinothini P, Reuben J, Bellinger LL, Ailing L, Peng YB, Kramer PR (2012), Reduced GABA(A) receptor alpha6 expression in the trigeminal ganglion alters inflammatory TMJ hypersensitivity. Neuroscience 213:179–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren K, F W, Dubner R, Murphy A, Hoffman GE (2000), Progesterone attenuates persistent inflammatory hyperalgesia in female rats: involvement of spinal NMDA receptor mechanisms. Brain Res 865:272–277. [DOI] [PubMed] [Google Scholar]
- Sawada H, Ibi M, Kihara T, Urushitani M, Honda K, Nakanishi M, Akaike A, Shimohama S (2000a), Mechanisms of antiapoptotic effects of estrogens in nigral dopaminergic neurons. Faseb j 14:1202–1214. [DOI] [PubMed] [Google Scholar]
- Sawada M, Alkayed NJ, Goto S, Crain BJ, Traystman RJ, Shaivitz A, Nelson RJ, Hurn PD (2000b), Estrogen receptor antagonist ICI182,780 exacerbates ischemic injury in female mouse. J Cereb Blood Flow Metab 20:112–118. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I (1997), Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol 388:507–525. [DOI] [PubMed] [Google Scholar]
- Stinson C, Deng M, Yee MB, Bellinger LL, Kinchington PR, Kramer PR (2017), Sex differences underlying orofacial varicella zoster associated pain in rats. BMC Neurol 17:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffel EC, Ulibarri CM, Craft RM (2003), Gonadal steroid hormone modulation of nociception, morphine antinociception and reproductive indices in male and female rats. Pain 103:285–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro A, Bereiter DA, Thompson R, Nishida Y (2014), GABAergic influence on temporomandibular joint-responsive spinomedullary neurons depends on estrogen status. Neuroscience 259:53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueyama T, Tanioku T, Nuta J, Kujira K, Ito T, Nakai S, Tsuruo Y (2006), Estrogen alters c-Fos response to immobilization stress in the brain of ovariectomized rats. Brain Res 1084:67–79. [DOI] [PubMed] [Google Scholar]
- Umorin M, Stinson C, Bellinger LL, Kramer PR (2016), Genes in the GABA Pathway Increase in the Lateral Thalamus of Sprague-Dawley Rats During the Proestrus/Estrus Phase. J Cell Physiol 231:1057–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel R, Krauss JK (2004), Center median-parafascicular complex and pain control. Review from a neurosurgical perspective. Stereotact Funct Neurosurg 82:115–126. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Lu N, Zhao ZQ, Zhang YQ (2012), Involvement of estrogen in rapid pain modulation in the rat spinal cord. Neurochem Res 37:2697–2705. [DOI] [PubMed] [Google Scholar]