Abstract
Objectives:
Cardiogenic shock is a highly morbid condition in which inadequate end-organ perfusion leads to death if untreated. Peripheral venoarterial extracorporeal membrane oxygenation is increasingly used to restore systemic perfusion despite limited understanding of how to optimally titrate support. This review provides insights into the physiologic basis of extracorporeal membrane oxygenation support and presents an approach to extracorporeal membrane oxygenation management in the cardiogenic shock patient.
Data Sources, Study Selection, and Data Extraction:
Data were obtained from a PubMed search of the most recent medical literature identified from MeSH terms: extracorporeal membrane oxygenation, cardiogenic shock, percutaneous mechanical circulatory support, and heart failure. Articles included original articles, case reports, and review articles.
Data Synthesis:
Current evidence detailing the use of extracorporeal membrane oxygenation to support patients in cardiogenic shock is limited to isolated case reports and single institution case series focused on patient outcomes but lacking in detailed approaches to extracorporeal membrane oxygenation management. Unlike medical therapy, in which dosages are either prescribed or carefully titrated to specific variables, extracorporeal membrane oxygenation is a mechanical support therapy requiring ongoing titration but without widely accepted variables to guide treatment. Similar to mechanical ventilation, extracorporeal membrane oxygenation can provide substantial benefit or induce significant harm. The widespread use and present lack of data to guide extracorporeal membrane oxygenation support demands that intensivists adopt a physiologically-based approach to management of the cardiogenic shock patient on extracorporeal membrane oxygenation.
Conclusions:
Extracorporeal membrane oxygenation is a powerful mechanical circulatory support modality capable of rapidly restoring systemic perfusion yet lacking in defined approaches to management. Adopting a management approach based physiologic principles provides a basis for care.
Keywords: cardiogenic shock, extracorporeal membrane oxygenation, heart failure, mechanical circulatory support
Cardiogenic shock is a highly morbid condition in which impaired heart function leads to inadequate end-organ perfusion followed by multisystem failure and death if untreated (1–5). Mortality rates remain close to 50% despite prompt medical therapy with preload and afterload optimization and contractility augmentation (6–8). High mortality has motivated the development of mechanical means of maintaining perfusion (9–11). The intra-aortic balloon pump (IABP) was the first widely available mechanical support device yet only modestly increases cardiac output indirectly with recent clinical trials finding no significant benefit over medical treatment (12–14). These findings have led to the rapid adoption of new mechanical circulatory support (MCS) devices capable of expanded support despite lack of clinical validation (15). Extracorporeal membrane oxygenation (ECMO) has been adapted to serve as one such MCS modality and is increasingly used to provide early mechanical support for patients in cardiogenic shock (16).
NO COMMON APPROACH TO ECMO MANAGEMENT
Although initially introduced to clinical practice in the 1970s, widespread use of ECMO to support medical patients in cardiogenic shock is a relatively new phenomenon (17, 18). Its recency as a MCS modality is evident in a medical literature containing insightful single institution case series reporting patient outcomes (19–24), but without clinical trials comparing approaches to ECMO management. The lack of established approaches to initiation and titration of ECMO support makes judgment of the efficacy and utility of this modality especially difficult.
Unlike medical therapy in which medication doses and dosage variables are strictly prescribed, there are no universally accepted variables for guiding ECMO support. Approaches to care vary widely between institutions and practitioners; optimal mechanical support relies on clinicians to make frequent patient observations and integrate a multitude of continuous and discrete monitoring assessments. Clinicians use this information to adjust the ECMO circuit, other support devices, and medical therapy based on their judgment and experience but not to any widely accepted guidelines.
Current application of ECMO as an MCS device mirrors the early use of mechanical ventilators to support patients in respiratory failure (25). The introduction of ventilators from the operating room to the other clinical environments initially relied on physician judgment to determine support. Decades of animal research and bedside experience demonstrated both the therapeutic benefit of mechanical ventilation and its potential to cause harm (26, 27). Synthesis of these findings led to clinical trials that elucidated the physiologic impact of mechanical ventilation and harmonized practice patterns to improve patient outcomes (28, 29).
Present-day intensivists face similar uncertainty in how to titrate support for ECMO as was confronted with the introduction of mechanical ventilation. Lacking extensive animal data to inform practice, current use of ECMO demands that the intensivist instead applies physiologic principles to determine and guide support. This review summarizes the physiologic basis that underlies MCS and presents a management approach for cardiogenic shock patients supported by ECMO.
THE MODERN ECMO CIRCUIT
ECMO is constructed from the core components of the cardiopulmonary bypass circuit modified for prolonged use in the ICU (Fig. 1) (18). The modern ECMO circuit consists of 1) venous withdrawal cannula; 2) a mechanical pump; 3) a gas exchange device termed an oxygenator; and 4) arterial return cannula (30). Terminology associated with vascular cannula is a common source of confusion depending on whether circuit or patient is the frame of reference for direction of blood flow. Cannula are variably described as being the inflow/outflow to the circuit or drainage/return to the patient. For this review, the patient will serve as the frame of reference, and cannula will be referred to as the “venous withdrawal” or “arterial return” in an effort to maintain clarity.
Figure 1.
Schematic of peripheral venoarterial extracorporeal membrane oxygenation circuit with a diffuse perfusion cannula to provide blood flow to the distal leg.
To support medical patients in cardiogenic shock, the most common vascular access consists of a venous cannula inserted percutaneously or via vascular cutdown into the femoral vein and advanced to the cava-atrial junction. Blood is withdrawn through the venous withdrawal cannula by a mechanical centrifugal pump and then forced through the oxygenator to return oxygenated blood to the patient through an arterial return cannula inserted into the femoral artery and advanced to the common iliac artery or distal aorta. The venous withdrawal cannula is typically 50–60 cm in length, 21F to 25F in diameter, and multistage to achieve adequate entrainment of venous blood. The arterial return cannula is typically 15F to 21F in diameter, approximately 18 cm in length, and single stage. This circuit configuration is termed peripheral venoarterial ECMO and can be deployed at the bedside by skilled providers. In this review, ECMO will refer to peripheral venoarterial ECMO used to support the patient in cardiogenic shock.
DETERMINANTS OF ECMO SUPPORT
Level of direct support is determined by the amount of flow the ECMO circuit can provide. Factors that determine flow are 1) negative pressure at which there is venous collapse; 2) pump speed; 3) afterload to the pump including circuit resistance, arterial cannula, and patient vascular resistance; 4) patient volume status; and 5) heart function. Venous cannula selection impacts the ability of the ECMO circuit to provide support by affecting the negative pressure created at a given pump speed that causes venous collapse and cessation of flow if the pressure generated is more negative than a critical threshold. Similarly, arterial cannula selection alters the afterload the pump must overcome to provide flow. For incompressible, Newtonian fluids experiencing laminar flow, the relationship between cannula resistance and the pressure drop (dP) across the cannula is provided by Hagen-Pouiselle’s Law:
in which ΔP is the pressure difference across the cannula, is μ the fluid viscosity, L is the length of the cannula, Q is the volumetric flow rate, and r is the cannula radius. From the Hagen-Pouiselle Law, it follows that a smaller arterial cannula radius creates more resistance and less flow from a given driving pressure. A larger radius for a venous cannula permits more entrainment of blood at the same withdrawal pressure gradient and prevents venous collapse by necessitating a less negative vessel pressure. Despite the comparative advantages of larger cannula size, vessel anatomy ultimately limits the diameter of the cannula due to risk of vascular injury and possible rupture during dilation or insertion of an oversized cannula (31). Large arterial cannula also frequently partially or totally occlude the femoral artery creating the risk of leg ischemia (32). Distal perfusion catheters, typically 6F to 8F in diameter, are typically inserted into the superficial femoral artery by cutdown or percutaneous approach. The distal perfusion catheters are connected to the arterial cannula to provide the leg with oxygenated blood and are a commonly used method to maintain limb perfusion (33).
Modern ECMO circuits rely on centrifugal pumps with the flow delivered at a given revolution per minute (RPM) being a function of afterload (34). Blood enters the pump perpendicularly at the center of a rotating impeller. The centrifugal acceleration from the spinning impeller radially accelerates blood to the pump periphery where it exits via a planar outflow port. Movement of blood from the eye of the impeller creates a vacuum that draws more blood into the pump. Kinetic energy from pump rotational velocity is used to create a pressure head that overcomes resistance to flow as blood exits the pump. The pump drops pressure upstream (venous withdrawal) and increases pressure downstream (arterial return) to overcome resistance from the oxygenator, circuit tubing, arterial cannula, and patient’s vasculature. These resistance sources act to reduce flow and limit the degree of ECMO support. Flow is generated by the pressure gradient created from blood acceleration to the impeller edge. Rotational speeds do not correspond to a set flow rate and are sensitive to both circuit configuration and surrounding pressures. An example of this is that unlike a positive displacement pump, the centrifugal pump will generate minimal to no flow if not properly primed.
Because of the pump configuration, the circuit’s ability to entrain and pump blood also depends on the patient’s circulating blood volume and residual heart function. Suction events, termed circuit “chugging,” occur during vena cava collapse around the venous withdrawal cannula due to excess negative dP. Common causes of chugging include hypovolemia, large variations in intrathoracic pressure that induce changes in venous return, and mechanical compression of the vascular cannula. Administration of IV fluids or blood products, improvement of patient-ventilatory synchrony or reduction in patient coughing, and ensuring that cannula are not kinked or compressed are common remedies, respectively. Reduction in ECMO pump RPMs is an additional intervention that will reduce blood entrainment in the circuit and may lead to reduced chugging at the expense of a decreased circuit flow. Heart function contributes to this dynamic as the right ventricle (RV) competes against the ECMO circuit for venous blood return while the contractility of the left ventricle (LV) affects the systemic arterial pressure and ECMO circuit afterload. The combination of all of these factors alters the degree of support provided by the ECMO circuit.
CONTROL OF THE ECMO CIRCUIT
Following initiation, the ECMO circuit has limited variables to adjust consisting of 1) pump speed; 2) sweep gas flow rate through the oxygenator; and 3) sweep gas composition. As described, higher pump RPMs generate more blood kinetic energy translating into increased flow while also dropping negative pump inlet pressure and raising outlet pressure. Hemolysis is a well-described complication of centrifugal pumps that increases nonlinearly with higher RPMs and limits up-titration of pump speed (35, 36). CO2 removal by the ECMO circuit is dependent on the oxygenator design, total blood flow through the circuit, and the flow rate of gas through the oxygenator (37). Sweep gas flow rate is titrated to the target CO2 partial pressure; typically, 35–45 mm Hg to maintain normocapnia. High flow rates of the sweep gas can induce air bubble formation in the blood, with the threshold dependent on the specific oxygenator, and thereby limits the amount of CO2 removal (38). Sweep gas composition is typically either room air containing 21% oxygen (oxygen), 100% oxygen, or a blended combination of both, with the oxygen fraction chosen to maintain physiologic oxygen tension post-oxygenator. The efficiency of modern oxygenators is such that minimal sweep gas flow rates are needed to obtain adequate oxygen delivery with post-oxygenator oxygen tensions, a function of the sweep gas composition. Degradation of oxygenator performance with prolonged use may impair gas diffusion and require higher sweep rates for adequate CO2 clearance and higher oxygen tension to achieve adequate oxygen delivery to meet goal variables. Clot formation is the most common cause of degraded oxygenator performance and may require frequent exchange in patients unable to tolerate anticoagulation. Although multiple factors contribute to oxygenator durability, typical lifespan ranges from a few to several days while significantly longer periods, such as 4 to 6 weeks, are atypical but not uncommon.
PHYSIOLOGIC BASIS OF ECMO SUPPORT
The treatment objective of ECMO support in cardiogenic shock is to restore systemic perfusion to maintain end-organ function and patient viability. However, the therapy goals are often misunderstood. The ability and mechanisms of ECMO circuits to maintain cardiac perfusion and permit the possibility to facilitate cardiac recovery is typically not appreciated. It is only when cardiac recovery is not achievable that the goal transitions to maintaining the patient while determining candidacy for long-term interventions such as heart transplant or durable ventricular assist device (VAD) implantation (39).
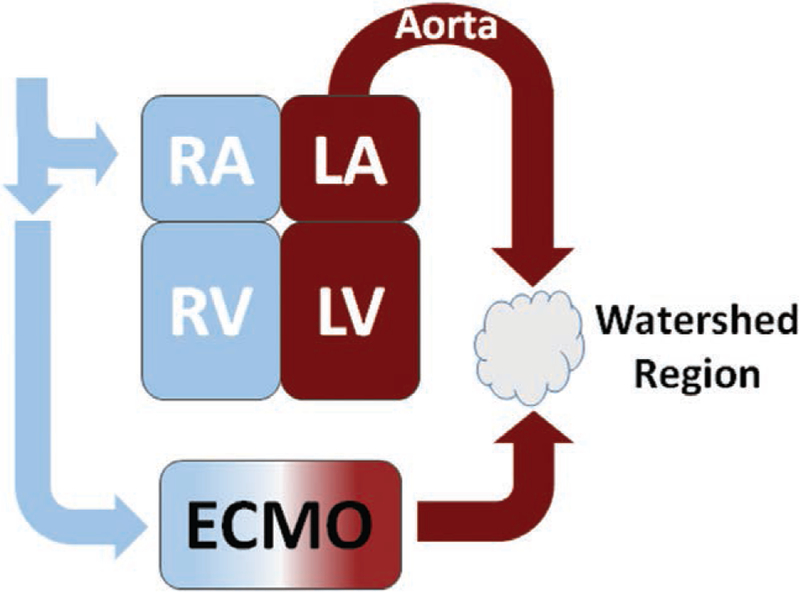
ECMO functionally introduces an external circulation coupled to a still active failing heart and intact systemic-cardiopulmonary circulation. The failing heart variably generates pulsatile antegrade blood flow that collides with continuous retrograde perfusion supplied by the ECMO circuit to generate a dynamic mixing cloud within the aorta, referred to as a watershed region (Fig. 2). Although capable of restoring arterial blood flow, the ECMO circuit profoundly disrupts physiologic ventriculo-arterial coupling and increases the load that the failing heart must overcome (40). The impact of this watershed region on end-organ perfusion is unclear and its relative size and characteristics and their dependence on the relative contributions from the ECMO circuit and the failing heart are unknown.
Figure 2.
Representation of the altered perfusion created by extracorporeal membrane oxygenation (ECMO) circuit and the disruption of ventriculo-vascular coupling and creation of watershed region resulting from antegrade perfusion generated. LA = left atrium, LV = left ventricle, RA = right atrium, RV = right ventricle.
In health, the interaction between the heart and arterial system transfers blood from the heart to the aorta efficiently while maintaining end-organ perfusion (41, 42). During cardiogenic shock, in which cardiac function is profoundly impaired, arterial responses to maintain pressure are maladaptive to maintaining total systemic perfusion and act to further decrease cardiac output (43). ECMO disrupts normal physiologic ventriculo-arterial coupling through introduction of retrograde perfusion of the arterial tree. The resultant ECMO-failing heart circulation is nonphysiologic with an unknown impact on neurovascular reflexes.
Shunting blood flow via the ECMO circuit has two simultaneous effects: 1) reduced preload with a decrease in flow through the pulmonary circulation and 2) increased afterload from retrograde perfusion of the aorta. In decompensated heart failure, reduction of preload and improved systemic perfusion is therapeutic (44). However, suboptimal titration of ECMO support may induce excessive shunting that overly reduces preload while increasing afterload to impair cardiac function. In profound heart failure, either through advanced disease or induced by excessive shunting through the ECMO circuit, the heart is unable to eject resulting in ventricular blood stasis. This can have profound negative consequence on cardiac viability and recovery. Continued drainage through the pulmonary circulation into the LV, either from preserved RV function or from the bronchial circulation, further increases intraventricular pressure in the non-ejecting LV until it approaches systemic pressure. This eliminates coronary transmural perfusion and can induce complete ischemic failure of the heart.
ECMO shunting blood flow away from the pulmonary circulation also affects ventilation-perfusion matching (45). In health, pulmonary arterial pressure exceeds alveolar pressure to maintain pulmonary blood flow. ECMO produced shunting decreases pulmonary perfusion and may generate areas of lung with alveolar pressure exceeding pulmonary arterial pressure. This is most likely to occur with intubated patients maintained on excessive positive end-expiratory pressure with minimal blood flow through the failing heart. Recognition of these physiologic phenomenon and the profound disruption induced by ECMO support is essential to the appropriate titration of support tailored to the physiologic state of the patient.
APPROACH TO CLINICAL MONITORING OF THE ECMO PATIENT
Optimal monitoring and clinical assessment of the cardiogenic shock ECMO patient is unknown. Pulmonary artery catheters measure pulmonary arterial pressures and RV filling pressures, but due to entrainment of venous blood into the ECMO circuit are not able to provide accurate cardiac output calculated by the thermodilution method (46). Bedside assessment of systemic perfusion relies on clinical integration of measured ECMO circuit output and imperfect estimates, such as from echocardiographic calculations, of residual heart output to obtain a semiquantitative measurement of total blood flow. Serum lactate is a useful indicator of adequacy of total perfusion but is only intermittently obtained and not able to directly guide titration of support.
In the setting of lung disease, hypoxic blood from the cardiopulmonary circulation is ejected into the aorta by the failing heart where it collides with oxygenated blood from the ECMO circuit leading to a physiologic state termed “differential hypoxia.” The coronary artery ostia arise just above the aortic valve after which the right brachiocephalic artery is the first major branch off the aortic arch followed by the left common carotid and left subclavian arteries. Monitoring oxygenation of tissues supplied by the brachiocephalic artery, such as the right upper extremity or right side of the face, is required to ensure adequate cerebral oxygenation. The means of ensuring adequate oxygenation is dictated by clinical circumstance and includes pulse oximetry, arterial blood gas analysis, or tissue oximetry (47). Profound concomitant lung disease may require addition of a venous return cannula via the internal jugular vein, a circuit configuration termed veno-arterial-venous ECMO, to supply oxygenated blood to the cardiopulmonary circulation (48). Monitoring of all extremities to ensure adequate perfusion is vital, and frequent checks of the distal leg on the side of the arterial cannula are needed. Lack of arterial pulsatility makes traditional pulse checks unreliable and instead demands assessment of limb warmth and color to ensure adequate distal perfusion. Even the astute clinical observer finds this challenging which has prompted the use of tissue oximetry as a means of providing a quantitative measure of limb oxygenation. Vascular spasm of distal arteries in the limb on the side of the arterial cannula are not uncommon and have led clinicians to trial unproven therapies such as infusion of calcium channel blockers or nitrates directly into the distal perfuser in an attempt to induce vascular smooth muscle relaxation.
The ECMO circuit constitutes a significant blood-material interface that activates multiple components of circulating blood including platelets and the coagulation cascade. Circuit thrombosis is a potentially catastrophic complication of ECMO manifesting as circuit component failure or thromboemboli. Although advances in biomaterials has led to widespread use of circuit components with surface-bonded anticoagulants, systemic anticoagulation remains the mainstay therapy to reduce thrombotic complications (49, 50). Heparin infusion, titrated to activated clotting times or other measures of anticoagulation activity, such as partial thromboplastin time or anti-Xa level, is typical first-line therapy with direct thrombin inhibitors reserved for patients with clinically significant heparin allergies. Anticoagulation management in ECMO patients is frequently challenging and is often coupled with both coagulopathy and pro-thrombotic factors in the setting of shock and multi-organ dysfunction. The optimal anticoagulation monitoring approach is unknown and is an area of active investigation (51).
Clot in the oxygenator, sometimes visible as either dark clot or as white fibrin clot on the oxygenator surface, may be evident as an increasing dP across the oxygenator indicative of increased circuit resistance. Bleeding complications, including catastrophic consequences such as intracranial hemorrhage, frequently accompany use of anticoagulation in this clinical setting. Clinically significant hemolysis is an additional complication, associated with mechanical damage induced by the pump, and is monitored through serial measurement of serum concentrations of lactate dehydrogenase or plasma free hemoglobin.
APPROACH TO ECMO SUPPORT IN CARDIOGENIC SHOCK
Structuring cardiogenic shock into anatomical categories is a useful basis for consideration of how to approach and titrate ECMO support for specific presentations of cardiogenic shock.
Biventricular Failure
Cardiogenic shock resulting from global impairment of heart function is a feature of inflammatory myocarditis, decompensated heart failure, stunned myocardium following cardiac arrest, acute rejection in heart transplant patients, atrial and ventricular arrhythmias, and multivessel ischemic disease among other conditions (52). The physiologic state of the heart in this setting is one of bilaterally increased preload and low cardiac output due to impaired contractility. The emphasis of ECMO support in this case is to reduce cardiac preload while maintaining end-organ perfusion.
Total systemic perfusion consists of both the ECMO circuit and the residual output from the failing heart. At present, there is no widely available and easily deployable metric to calculate the contribution of the failing heart. Instead, the clinician must rely on intermittently obtained gross metrics of total perfusion, such as systemic lactate, markers of end-organ function—such as urine output and hepatic enzymes—and hemodynamic measures to determine if total perfusion is sufficient.
Systemic blood pressure goals are a well-studied emphasis of critical care while renewed interest in ventriculo-arterial coupling in shock has demonstrated the clinical utility of specific pressure targets (53, 54). Maintaining normotension in patients on ECMO, with a goal mean arterial pressure of 65–80, is a reasonable management approach until persuasive evidence exists to deviate from this practice. Vasopressors, such as norepinephrine and vasopressin, are useful to treat hypotension or vasoplegia while short-acting vasodilators like sodium nitroprusside are useful to control hypertension with less potential negative inotropic effects that may occur with calcium channel blockers such as nicardipine (55).
Patients with profound cardiac failure and minimal contractility may require inotropic support to promote forward flow of blood through the heart to reduce the risk of LV blood stasis and augment total systemic perfusion. In this setting, minimal or absent systemic pulsatility is indicative of lack of aortic valve opening and raises concern for stasis in the pulmonary circulation and risk of blood stasis in the LV. Maintaining forward flow from the failing heart is a complex interplay between cardiac state as determined by preload, afterload, and residual contractility and operation of the ECMO circuit (Fig. 3). Increasing ECMO pump RPMs will divert blood flow to the ECMO circuit resulting in decreased cardiac preload and increased afterload. Measurement of pulmonary artery occlusion pressure or visualization of the LV by echocardiography provides insight into the preload state of the heart. In the setting of minimal pulsatility and inadequate preload, reduction in pump RPMs may be sufficient to restore systemic pulsatility. If preload is sufficient and afterload is within the targeted blood pressure, then patients may require inotropic support to maintain LV ejection and restore antegrade perfusion.
Figure 3.
Proposed basis of management of the extracorporeal membrane oxygenation (ECMO) patient emphasizing interconnected physiologic variables of end-organ perfusion, ventricular function, and vascular state. CO = cardiac output, LV = left ventricle, MAP = mean arterial pressure, RPM = revolution per minute.
Potential benefit from inotropes must be weighed against possible increased cardiac myocyte oxygen consumption and impaired cardiac recovery (56). Additional concerns include the effects of both inotropes and vasodilators on the pulmonary circulation with the potential to worsen ventilation-perfusion mismatch and thereby exacerbate differential hypoxemia in patients with concomitant lung disease. In the setting of using inotropes to promote forward flow from the LV, titrating to pulse pressure (the difference between maximum and minimal systemic arterial pressure) is a reasonable treatment goal. Increased doses of inotropes without achieving sufficient pulsatility or marked by the onset of tachyarrhythmias, which may also be early evidence of differential hypoxemia manifested by entrainment of hypoxemia blood into the coronary ostia, prompts consideration of venting the LV to remove static blood even when employing a strategy to limit further interventions.
Approaches to venting the LV in this setting require consideration. The threshold at which to initiate LV venting is unknown with the decision complicated by the risks of additional procedures of varying degrees of invasiveness and associated costs. LV venting can include use of an IABP to reduce afterload to promote LV ejection, direct venting of the LV via surgical placement of an apical drain, drainage of the left atrium via transeptal placement of a catheter or atrial septostomy, or placement of a percutaneous VAD, such as an Impella, across the aortic valve to induce left ventricular forward flow (57). Although additional data are required, early clinical studies suggest a survival benefit to dual mechanical support with the Impella and ECMO (58). Such an approach allows for clinician controlled forward flow through the heart and off-loading of the LV to reduce cardiac work and possibly facilitate recovery while also reducing risk of stasis and clot formation. Appropriate venting of the LV decreases intraventricular volume thereby reducing transmural pressure and myocardial oxygenation consumption while also maintaining myocardial perfusion. Although uncertain at present, this may be the physiologic basis of the observed survival benefit. If less invasive therapies are unsuccessful, transition from peripheral to central cannulation, with the return cannula surgically placed in the aortic arch, is a consideration. Although highly invasive, this cannulation approach permits antegrade perfusion of the aorta and may enable optimization of the cardiac state with improved contractility.
Left Ventricular Failure
Clinicians caring for patients with cardiogenic shock resulting from predominantly left ventricular failure, most commonly due to active ischemia or underlying ischemic cardiomyopathy, have a variety of circulatory support modalities capable of providing support (59). The challenge of titrating ECMO support to balance the needs of maintaining systemic and coronary perfusion while limiting cardiac work to prevent cardiac injury is particularly acute in the use of ECMO to support cardiogenic shock arising from isolated left ventricular failure (60). Conceptually, ECMO maintains systemic perfusion at the expense of increasing cardiac work in the presence of active ischemia and may worsen the size of the ischemic penumbra and impair cardiac recovery. Emphasis on LV unloading underlies the premise of percutaneous temporary VAD support devices, although limited clinical trials have yet to demonstrate a survival or functional benefit to this approach (61). Although selecting which device to use for which patient is presently unclear, patients in profound shock may benefit from therapies such as ECMO that are capable of rapidly restoring systemic perfusion and reversing the deleterious effects of prolonged hypoperfusion that are also coupled with methods to vent the LV to reduce cardiac myocyte injury and to promote recovery.
Right Ventricular Failure
Cardiogenic shock arising from isolated RV failure is most frequently observed following massive pulmonary embolism although is also observed in decompensated RV failure attributed to pulmonary hypertension or RV ischemia. The physiologic goal of ECMO support in this scenario is to offload the RV to restore pulsatility in the RV and permit forward flow through the pulmonary circulation. Unlike in biventricular failure or isolated LV failure, less ECMO flow is typically needed in the case of RV failure especially when treating pulmonary hypertension or pulmonary embolism (62). In these cases, modest off-loading quickly restores RV pulsatility (63). In the case of pulmonary embolism, barring immediate surgical thromboendarterectomy, maintaining adequate flow through the pulmonary circulation is paramount as this is needed to deliver anticoagulants to allow for treatment of the embolism. Inadequate forward flow through the heart may result in clot extension and catastrophic therapeutic failure.
PERSPECTIVES AND CONCLUSIONS
ECMO is a powerful support modality capable of rapidly maintaining systemic perfusion and restoring end-organ function for patients in cardiogenic shock. Deceptively straightforward, ECMO support requires careful titration through an understanding of the circuit fluid dynamics and its impact on physiology to optimize therapeutic benefit while limiting potentially harmful sequelae. At present, there are no clearly established guidelines for titration of support. Ongoing clinical use despite a lack of definitive evidence demands application of physiologic principles to guide support. Of major importance is ongoing research into the use of MCS to further inform clinical practice to improve patient outcomes.
TABLE 1.
Common Vasoactive Medications Used in Extracorporeal Membrane Oxygenation Patients
| Medication | Titration Variables | Considerations |
|---|---|---|
| Vasoconstrictors | ||
| Norepinephrine | MAPs > 65 | Modest inotropic effect. May worsen microvascular dysfunction. |
| Vasopressin | MAPs > 65 | Effective agent for vasoplegia. May worsen gut ischemia. |
| Vasodilators | ||
| Sodium nitroprusside | MAPs < 80 | Risks of use include cyanide toxicity and methemoglobinemia. |
| Prolonged use may lead to tachyphylaxis. | ||
| Nicardipine | MAPs < 80 | Uncertain effects on cardiac inotropy. |
| Inotropes | ||
| Dobutamine | Pulse pressure > 15 | May induce mild to moderate vasodilation. |
| Epinephrine | Pulse pressure > 15 | Vasoconstrictive effects complicate its use as a targeted inotropic agent. |
| Milrinone | Pulse pressure > 15 | May induce moderate systemic vasodilation; decreases pulmonary vascular resistance. |
MAP = mean arterial pressure.
ACKNOWLEDGMENT
The author would like to thank Dr. Brian Chang, Mr. Jon Brown, and Dr. Elazer Edelman for their thoughtful comments and careful review of this manuscript. The author would also like to thank Dr. Chang for his assistance in the design and creation of the figures.
Dr. Keller received support for article research from the National Institutes of Health (1K08HL143342-01).
REFERENCES
- 1.Shah P, Cowger JA: Cardiogenic shock. Crit Care Clin 2014; 30:391–412 [DOI] [PubMed] [Google Scholar]
- 2.Dubey L, Sharma S, Gautam M, et al. : Cardiogenic shock complicating acute myocardial infarction–a review. Acta Cardiol 2011; 66:691–699 [DOI] [PubMed] [Google Scholar]
- 3.Topalian S, Ginsberg F, Parrillo JE: Cardiogenic shock. Crit Care Med 2008; 36:S66–S74 [DOI] [PubMed] [Google Scholar]
- 4.Hollenberg SM, Kavinsky CJ, Parrillo JE: Cardiogenic shock. Ann Intern Med 1999; 131:47–59 [DOI] [PubMed] [Google Scholar]
- 5.Beyersdorf F, Buckberg GD, Acar C, et al. : Cardiogenic shock after acute coronary occlusion. Pathogenesis, early diagnosis, and treatment. Thorac Cardiovasc Surg 1989; 37:28–36 [DOI] [PubMed] [Google Scholar]
- 6.Jeger RV, Radovanovic D, Hunziker PR, et al. ; AMIS Plus Registry Investigators: Ten-year trends in the incidence and treatment of cardiogenic shock. Ann Intern Med 2008; 149:618–626 [DOI] [PubMed] [Google Scholar]
- 7.Redfors B, Angerås O, Råmunddal T, et al. : 17-year trends in incidence and prognosis of cardiogenic shock in patients with acute myocardial infarction in western Sweden. Int J Cardiol 2015; 185:256–262 [DOI] [PubMed] [Google Scholar]
- 8.Kolte D, Khera S, Aronow WS, et al. : Trends in incidence, management, and outcomes of cardiogenic shock complicating ST-elevation myocardial infarction in the United States. J Am Heart Assoc 2014; 3:e000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pitsis AA, Visouli AN: Mechanical assistance of the circulation during cardiogenic shock. Curr Opin Crit Care 2011; 17:425–438 [DOI] [PubMed] [Google Scholar]
- 10.Gilani FS, Farooqui S, Doddamani R, et al. : Percutaneous mechanical support in cardiogenic shock: A review. Clin Med Insights Cardiol 2015; 9:23–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aggarwal S, Slaughter MS: Acute myocardial infarction complicated by cardiogenic shock: Role of mechanical circulatory support. Expert Rev Cardiovasc Ther 2008; 6:1223–1235 [DOI] [PubMed] [Google Scholar]
- 12.Malliaras K, Charitos E, Diakos N, et al. : Effects of intra-aortic balloon pump counterpulsation on left ventricular mechanoenergetics in a porcine model of acute ischemic heart failure. J Cardiovasc Transl Res 2014; 7:810–820 [DOI] [PubMed] [Google Scholar]
- 13.Prondzinsky R, Unverzagt S, Russ M, et al. : Hemodynamic effects of intra-aortic balloon counterpulsation in patients with acute myocardial infarction complicated by cardiogenic shock: The prospective, randomized IABP shock trial. Shock 2012; 37:378–384 [DOI] [PubMed] [Google Scholar]
- 14.Thiele H, Zeymer U, Neumann FJ, et al. ; IABP-SHOCK II Trial Investigators: Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 2012; 367:1287–1296 [DOI] [PubMed] [Google Scholar]
- 15.Khera R, Cram P, Lu X, et al. : Trends in the use of percutaneous ventricular assist devices: Analysis of national inpatient sample data, 2007 through 2012. JAMA Intern Med 2015; 175:941–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paden ML, Conrad SA, Rycus PT, et al. ; ELSO Registry: Extracorporeal life support organization registry report 2012. ASAIO J 2013; 59:202–210 [DOI] [PubMed] [Google Scholar]
- 17.Bartlett R, Roloff D: Extracorporeal life support. JAMA 2000;283:904–908 [PubMed] [Google Scholar]
- 18.Bartlett RH: Historical perspectives: Extracorporeal membrane oxygenation (ECMO). Neoreviews 2005; 6:e251–e254 [Google Scholar]
- 19.Nascimbene A, Banjac I, Janowiak L, et al. : ECMO for hemodynamic support in patients with profound cardiogenic shock: Experience and outcomes from a large single center. J Am Coll Cardiol 2015; 66:B76 [Google Scholar]
- 20.Bouabdallaoui N, Demondion P, Leprince P, et al. : Short-term mechanical circulatory support for cardiogenic shock in severe peripartum cardiomyopathy: La Pitié-Salpêtrière experience. Interact Cardiovasc Thorac Surg 2017; 25:52–56 [DOI] [PubMed] [Google Scholar]
- 21.Pontailler M, Demondion P, Lebreton G, et al. : Experience with extracorporeal life support for cardiogenic shock in the older population more than 70 years of age. ASAIO J 2017; 63:279–284 [DOI] [PubMed] [Google Scholar]
- 22.Pineton de Chambrun M, Bréchot N, Lebreton G, et al. : Venoarterial extracorporeal membrane oxygenation for refractory cardiogenic shock post-cardiac arrest. Intensive Care Med 2016; 42:1999–2007 [DOI] [PubMed] [Google Scholar]
- 23.Dangers L, Bréchot N, Schmidt M, et al. : Extracorporeal membrane oxygenation for acute decompensated heart failure. Crit Care Med 2017; 45:1359–1366 [DOI] [PubMed] [Google Scholar]
- 24.Guihaire J, Dang Van S, Rouze S, et al. : Clinical outcomes in patients after extracorporeal membrane oxygenation support for post-cardiotomy cardiogenic shock: A single-centre experience of 92 cases. Interact Cardiovasc Thorac Surg 2017; 25:363–369 [DOI] [PubMed] [Google Scholar]
- 25.Kacmarek RM: The mechanical ventilator: Past, present, and future. Respir Care 2011; 56:1170–1180 [DOI] [PubMed] [Google Scholar]
- 26.Dreyfuss D, Saumon G: Ventilator-induced lung injury: Lessons from experimental studies. Am J Respir Crit Care Med 1998; 157:294–323 [DOI] [PubMed] [Google Scholar]
- 27.Slutsky AS: Lung injury caused by mechanical ventilation. Chest 1999; 116:9S–15S [DOI] [PubMed] [Google Scholar]
- 28.Brower RG, Matthay MA, Morris A, et al. ; Acute Respiratory Distress Syndrome Network: Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000; 342:1301–1308 [DOI] [PubMed] [Google Scholar]
- 29.Amato MB, Barbas CS, Medeiros DM, et al. : Effect of a protectiveventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 1998; 338:347–354 [DOI] [PubMed] [Google Scholar]
- 30.Lequier L, Horton SB, McMullan DM, et al. : Extracorporeal membrane oxygenation circuitry. Pediatr Crit Care Med 2013; 14:S7–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gokalp O, Besir Y, Eygi B, et al. : Complications of cannulation in extracorporeal membrane oxygenation. Ann Vasc Surg 2015; 29:164. [DOI] [PubMed] [Google Scholar]
- 32.Yen CC, Kao CH, Tsai CS, et al. : Identifying the risk factor and prevention of limb ischemia in extracorporeal membrane oxygenation with femoral artery cannulation. Heart Surg Forum 2018; 21:E018–E022 [DOI] [PubMed] [Google Scholar]
- 33.Juo YY, Skancke M, Sanaiha Y, et al. : Efficacy of distal perfusion cannulae in preventing limb ischemia during extracorporeal membrane oxygenation: A systematic review and meta-analysis. Artif Organs 2017; 41:E263–E273 [DOI] [PubMed] [Google Scholar]
- 34.Moazami N, Fukamachi K, Kobayashi M, et al. : Axial and centrifugal continuous-flow rotary pumps: A translation from pump mechanics to clinical practice. J Heart Lung Transplant 2013; 32:1–11 [DOI] [PubMed] [Google Scholar]
- 35.Lou S, MacLaren G, Best D, et al. : Hemolysis in pediatric patients receiving centrifugal-pump extracorporeal membrane oxygenation: Prevalence, risk factors, and outcomes. Crit Care Med 2014; 42:1213–1220 [DOI] [PubMed] [Google Scholar]
- 36.Toomasian JM, Bartlett RH: Hemolysis and ECMO pumps in the 21st Century. Perfusion 2011; 26:5–6 [DOI] [PubMed] [Google Scholar]
- 37.Epis F, Belliato M: Oxygenator performance and artificial-native lung interaction. J Thorac Dis 2018; 10:S596–S605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matte GS: It is time to update membrane oxygenator testing standards and their instructions for use. J Extra Corpor Technol 2016; 48:148–151 [PMC free article] [PubMed] [Google Scholar]
- 39.Rousse N, Juthier F, Pinçon C, et al. : ECMO as a bridge to decision: Recovery, VAD, or heart transplantation? Int J Cardiol 2015; 187:620–627 [DOI] [PubMed] [Google Scholar]
- 40.Kass DA, Kelly RP: Ventriculo-arterial coupling: Concepts, assumptions, and applications. Ann Biomed Eng 1992; 20:41–62 [DOI] [PubMed] [Google Scholar]
- 41.London GM: The concept of ventricular/vascular coupling: Functional and structural alterations of the heart and arterial vessels go in parallel. Nephrol Dial Transplant 1998; 13:250–253 [DOI] [PubMed] [Google Scholar]
- 42.Antonini-Canterin F, Poli S, Vriz O, et al. : The ventricular-arterial coupling: From basic pathophysiology to clinical application in the echocardiography laboratory. J Cardiovasc Echogr 2013; 23:91–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sasayama S, Asanoi H: Coupling between the heart and arterial system in heart failure. Am J Med 1991; 90:14S–18S [DOI] [PubMed] [Google Scholar]
- 44.Yancy CW, Jessup M, Bozkurt B, et al. : 2013 ACCF/AHA guideline for the management of heart failure: Executive summary: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013; 128:1810–1852 [DOI] [PubMed] [Google Scholar]
- 45.Permutt S, Bromberger-Barnea B, Bane HN: Alveolar pressure, pulmonary venous pressure, and the vascular waterfall. Med Thorac 1962; 19:239–260 [DOI] [PubMed] [Google Scholar]
- 46.Douflé G, Ferguson ND: Monitoring during extracorporeal membrane oxygenation. Curr Opin Crit Care 2016; 22:230–238 [DOI] [PubMed] [Google Scholar]
- 47.Toffaletti JG, Rackley CR: Monitoring oxygen status. Adv Clin Chem 2016; 77:103–124 [DOI] [PubMed] [Google Scholar]
- 48.Pavlushkov E, Berman M, Valchanov K: Cannulation techniques for extracorporeal life support. Ann Transl Med 2017; 5:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.von Segesser LK: Heparin-bonded surfaces in extracorporeal membrane oxygenation for cardiac support. Ann Thorac Surg 1996; 61:330–335; discussion 340–341 [DOI] [PubMed] [Google Scholar]
- 50.Bembea MM, Annich G, Rycus P, et al. : Variability in anticoagulation management of patients on extracorporeal membrane oxygenation: An international survey. Pediatr Crit Care Med 2013; 14:e77–e84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Delmas C, Jacquemin A, Vardon-Bounes F, et al. : Anticoagulation monitoring under ECMO support: A comparative study between the activated coagulation time and the anti-Xa activity assay. J Intensive Care Med 2018. January 1:885066618776937 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 52.Tewelde SZ, Liu SS, Winters ME: Cardiogenic shock. Cardiol Clin 2018; 36:53–61 [DOI] [PubMed] [Google Scholar]
- 53.Guarracino F, Ferro B, Morelli A, et al. : Ventriculoarterial decoupling in human septic shock. Crit Care 2014; 18:R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guarracino F, Baldassarri R, Pinsky MR: Ventriculo-arterial decoupling in acutely altered hemodynamic states. Crit Care 2013; 17:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aroney CN, Semigran MJ, Dec GW, et al. : Inotropic effect of nicardipine in patients with heart failure: Assessment by left ventricular end-systolic pressure-volume analysis. J Am Coll Cardiol 1989; 14:1331–1338 [DOI] [PubMed] [Google Scholar]
- 56.DeWitt ES, Black KJ, Thiagarajan RR, et al. : Effects of commonly used inotropes on myocardial function and oxygen consumption under constant ventricular loading conditions. J Appl Physiol (1985) 2016; 121:7–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Camboni D, Schmid C: To vent or not on veno-arterial extracorporeal membrane oxygenation, does it improve myocardial recovery and outcome? J Thorac Dis 2017; 9:4915–4918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pappalardo F, Schulte C, Pieri M, et al. : Concomitant implantation of Impella® on top of veno-arterial extracorporeal membrane oxygenation may improve survival of patients with cardiogenic shock. Eur J Heart Fail 2017; 19:404–412 [DOI] [PubMed] [Google Scholar]
- 59.Miller PE, Solomon MA, McAreavey D: Advanced percutaneous mechanical circulatory support devices for cardiogenic shock. Crit Care Med 2017; 45:1922–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schiller P, Vikholm P, Hellgren L: Experimental venoarterial extracorporeal membrane oxygenation induces left ventricular dysfunction. ASAIO J 2016; 62:518–524 [DOI] [PubMed] [Google Scholar]
- 61.Ouweneel DM, Eriksen E, Sjauw KD, et al. : Impella CP versus intra-aortic balloon pump in acute myocardial infarction complicated by cardiogenic shock: The IMPRESS trial. J Am Coll Cardiol 2017; 69:278–28727810347 [Google Scholar]
- 62.Pasrija C, Kronfli A, George P, et al. : Utilization of veno-arterial extracorporeal membrane oxygenation for massive pulmonary embolism. Ann Thorac Surg 2018; 105:498–504 [DOI] [PubMed] [Google Scholar]
- 63.Watanabe Y, Sakakura K, Akashi N, et al. : Veno-arterial extracorporeal membrane oxygenation with conventional anticoagulation can be a best solution for shock due to massive PE. Int Heart J 2017; 58:831–834 [DOI] [PubMed] [Google Scholar]