Summary
BACKGROUND
Despite progress in single food oral immunotherapy (OIT), there is little evidence concerning the safety and efficacy of treating individuals with multiple food (multifood) allergies. We conducted a pilot study testing whether anti-IgE (omalizumab) combined with multifood OIT benefitted multifood allergic patients.
METHODS
In this blinded, phase 2 clinical trial conducted at Stanford University, 48 participants, aged 4-15 years, with multifood allergies validated by double-blind, placebo-controlled food challenges (DBPCFCs) to their offending foods were block randomized (3:1) to receive multifood OIT to 2-5 foods, together with omalizumab (n=36) or placebo (n=12). Omalizumab or placebo was administered subcutaneously for 16 weeks with OIT starting at week 8; omalizumab or placebo was stopped 20 weeks before exit DBPCFCs (week 36) to determine the primary endpoint: the proportion of participants who passed DBPCFCs to at least 2 of their offending foods. This completed trial is registered with ClinicalTrials.gov, .
FINDINGS
At week 36, a significantly greater proportion of the omalizumab (30/36, 83%) vs. placebo (4/12, 33%) participants passed DBPCFCs to 2 g protein for ≥ 2 of their offending foods (odds ratio (OR): 10, 95% confidence interval (CI): 1·8, 58·3, P=0·004). The same individuals also tolerated 4 g protein of ≥ 2 foods (secondary endpoint, P=0·004). A greater proportion of omalizumab (13/17, 77%) vs. placebo (0/5, 0%) participants passed a DBPCFC to 2 g protein for ≥ 4 of their offending foods (OR: 33, 95% CI: 1·9, ∞, P=0·01). All participants completed the study. There were no serious or severe (≥ grade 3) adverse events.
INTERPRETATION
In multifood allergic patients, omalizumab improves the efficacy of multifood OIT and enables safe and rapid desensitization.
FUNDING
NIH U19 AADCRC and Opportunity Fund, Sean N. Parker Center for Allergy and Asthma Research at Stanford University, Simons Foundation, Myra Reinhard Foundation, FARE Center of Excellence, Department of Pathology, and Department of Pediatrics, Stanford University.
INTRODUCTION
Approximately 30% of food allergic individuals have multiple food allergies (multifood allergies).4 The development of more efficacious food allergy treatments is particularly important for such patients as the co-occurrence of multifood allergies increases risks for accidental ingestions and near fatal or fatal anaphylaxis.4 Although many studies have evaluated the efficacy of oral immunotherapy (OIT) for single foods, studies evaluating OIT to multiple foods (i.e., multifood OIT) have been limited due to efficacy and safety concerns.3,5–8
Omalizumab (Xolair), a recombinant DNA-derived humanized IgG1 monoclonal antibody that selectively binds human immunoglobulin E (IgE), inhibits binding of IgE to high-affinity IgE receptors (FcεRIs) on the surface of mast cells and basophils and downregulates their FcεRI expression, and reduces blood levels of free IgE.9,10 Recent studies showed that food allergy desensitization can be achieved relatively rapidly when single OIT is combined with omalizumab in single food-allergic participants.11–14 A phase 1 safety study reported increased initial dose thresholds of food protein during rapid milk OIT with initial omalizumab dosing (for 16 weeks out of a total 40 week OIT period).1 A subsequent study demonstrated that combining omalizumab with milk OIT significantly improved safety outcomes in the omalizumab vs. placebo arm.11 Additionally, significant improvements in efficacy in the omalizumab arm were reported in a phase 2 clinical trial implementing rapid peanut OIT with initial omalizumab dosing.12
Because multifood allergic individuals could benefit from treatment and omalizumab potentially mitigates their risk for IgE-mediated allergic reactions, we designed a randomized, placebo-controlled phase 2 study to determine whether, compared with multifood OIT alone, initial use of omalizumab (beginning 8 weeks before starting multifood OIT and stopped at 16 weeks) improves the efficacy of multifood OIT as determined by a double-blind, placebo-controlled food challenge (DBPCFC) at 36 weeks.
METHODS
Study design
This randomized, double-blind, placebo-controlled, phase 2 clinical trial was conducted at the Sean N. Parker Center for Allergy and Asthma Research at Stanford University from March 2015 to August 2016 with Stanford IRB approval under IND 14831 (). Eligible participants were randomized to receive omalizumab (n = 36; dosed per product insert) or placebo (n = 12) for 16 weeks, after which such treatment was discontinued. Multifood OIT consisting of equal parts of each of up to 5 offending foods (up to 2 g of each food) was initiated, up-dosed, and administered from weeks 8 through 36. Cashew, walnut, hazelnut, almond, sesame, cow’s milk, hen’s egg, peanut, soy, and/or wheat were included as foods in this study, so that multifood can refer to, e.g., several tree nuts. An independent Data and Safety Monitoring Board (DSMB) provided by the NIH (DAIT DSMB) and an independent NIH Medical Monitor monitored the study.
Participants
We enrolled participants 4-15 years old who had food challenge-proven allergies to more than one food. Inclusion criteria included a positive skin prick test (SPT) of ≥ 6 mm (wheal, above the negative control) and/or a food-specific IgE > 4 kU/L for each food, and a positive DBPCFC at ≤ 500 mg food protein. Exclusion criteria included eosinophilic esophagitis or severe asthma. The participant population met all inclusion and exclusion criteria as per the protocol (see appendix). Participants were recruited from referrals into a single study site (i.e. Stanford) and from a waitlist of eligible individuals that was selected randomly for screening. Stanford IRB approved the study. Written informed consent was obtained.
Randomization and masking
There were 2 groups randomly assigned 3:1 to omalizumab vs placebo, using a computer and block randomization. Randomization to receive omalizumab, or placebo, was blocked and stratified by sex and prepared by a blinded biostatistician. A 3:1 randomization between omalizumab and placebo was chosen to improve expected compliance, while also providing large enough sample sizes for the statistical analysis. Sample size considerations are described in the appendix (p 1). Unblinding of randomization assignments or during DBPCFCs was specified to take place only in the event of a complication or if the PI and/or DSMB determined that unblinding was necessary. During the course of the study, no unblinding was needed. Upon completion of each participant’s end-of study visit and confirmation of data entry lock, study staff submitted a formal request for participant-specific unblinding. This formal request was sent to the Stanford Investigational Pharmacist at the time of unblinding.
Procedures
All participants were screened using published, standardized procedures of SPTs, specific IgE testing, and DBPCFCs as detailed in the protocol. DBPCFCs were done using standardized, validated, staged doses and were called positive if objective symptoms were diagnosed by trained personnel. Participants underwent DBPCFCs for their offending foods on separate days. Additional inclusion criteria included a positive skin prick test (SPT) result (≥ 6 mm wheal diameter) and/or an ImmunoCAP IgE level > 4 kU/L. Of the offending foods, the ones included in their multifood OIT were the foods for which a participant had a significant allergic reaction to a ≤ 500 mg cumulative dose of food protein in the separate DBPCFCs conducted for each food. Participants could have up to five foods included in their multifood OIT regimen. For those participants with allergies to greater than the five foods offered during multifood OIT, the foods chosen for the multifood OIT regimen were those which, on screening, were associated with a lower cumulative tolerated dose (CTD) in the DBPCFC, a relatively higher specific IgE, and/or a larger SPT wheal diameter compared to other food allergens. In addition, for those participants enrolled who had both cashew and pistachio or walnut and pecan food allergy, only cashew and/or walnut was included in the multifood OIT. This way, at the end of the study and depending on the individual, we could test for the possible ability of cashew OIT to desensitize to both cashew and pistachio or of walnut OIT to desensitize to both walnut and pecan. Adverse events and drug relatedness were attributed by a trained physician. Dosing of omalizumab (Xolair) was given according to manufacturer’s instructions and the product insert. Follow up intervals were approximately every 2-4 weeks and assessments were done which included skin tests, blood tests, physical examinations, diary reviews, adverse events (AEs), dose escalations, food challenges, and/or spirometry.
Outcomes
Our primary endpoint, defining success, was passing a DBPCFC at 36 weeks (i.e., no clinical reactivity to 2 g protein) for any 2 foods in that participant’s OIT. Failure was defined as not reaching the primary endpoint. The failures were further divided into treatment or desensitization failures. Treatment failure was defined by (a) failure to reach 5 mg of food protein (total) during the initial dose escalation day (IDED) (week 8), or (b) failure to reach 300 mg of total protein by week 16, or (c) as determined by the PI or medical monitor. Desensitization failure was defined as (a) a participant who ingested less than 2,000 mg (2 g) of each offending food at week 34, and therefore would not be able to undergo the week 36 DBPCFCs, or (b) a participant who had severe reactions at least 4 weeks prior to week 36, or (c) who demonstrated clinical reactivity (≥ Grade 1) at the DBPCFC to 2,000 mg of each or each but one of the foods included in their multifood OIT at week 36.
All study failures were followed until the end of the study at the specified study visits but no longer underwent DBPCFCs. Treatment failures (n = 11) were offered open-label omalizumab starting in week 17 (see appendix p 4).
Secondary endpoints were the proportion of food allergic participants who passed a DBPCFC to 4,000 mg each of 2 foods at week 36; the proportion of food allergic participants who passed a DBPCFC to 2,000 mg each of 3, 4, or 5 foods, respectively, at week 36; the proportion of food allergic participants who successfully completed the build-up phase of OIT to the highest dose (2,000 mg of each protein) with only mild (Grade 1) symptoms; and the proportion of food allergic participants who successfully underwent the build-up and maintenance phases of OIT with only mild symptoms. The remaining secondary endpoints listed in the protocol could not be analyzed since most participants did not agree to further food challenge escalation past 4 g of each food due to the length of time that would have been needed (over 8 hours total).
Safety outcomes were determined by CTCAE v4.03 criteria and documented per regulatory guidelines. We measured SPT wheal sizes and allergen-specific IgE and IgG4 levels, and evaluated relationships to safety and efficacy outcomes and changes overtime. Cross-desensitization (operationally defined herein as desensitization against a related allergenic food to which the subject is allergic but which was not included in the multifood OIT) also was evaluated.
Statistical Analysis
All analyses were conducted according to the intention-to-treat (ITT) principle. The primary analysis involved a central Fisher’s exact test to compare, between arms, the proportion of subjects achieving the primary endpoint. The two-sided test was conducted at the 0·05 level of significance. We secondarily applied an exact conditional test to compare the primary endpoint between arms after adjusting for the number of foods in OIT per participant. Our primary objective, as stated in our protocol, was to evaluate efficacy between treatment and control groups at 36 weeks. All other hypotheses tested were considered secondary.
Secondary analyses included statistical tests such as the Wilcoxon rank sum test, Kruskal-Wallis rank sum test, log-rank test, and/or linear mixed-effects models which were applied for endpoint and biomarker (i.e., IgE, IgG4, SPT) comparisons between study arms and between baseline and week 36.
Odds ratios (OR) were estimated based on unconditional maximum likelihood estimation. In cases with zero cells in 2-by-2 tables, a continuity correction was utilized to obtain the OR.15 Exact confidence intervals (CI) and central Fisher’s Exact two-sided P values were calculated using the R package exact2x2.16 Boxplots or violin plots were used to display the distribution of continuous variables with each value plotted as individual dots. The dots for the wheal diameters, IgE, IgG4 and IgG4/IgE levels per individual were connected by a line between the violin plots for baseline and week 36. To depict the time to maintenance dose, we used a Kaplan-Meier-like approach and the hazard ratio (reached maintenance in the omalizumab group relative to the placebo group) was estimated using a Cox proportional hazards regression model. Comparisons of biomarkers and clinical outcomes were adjusted for multiple comparisons by controlling the false discovery rate (FDR) to be no more than 0·05 using the Benjamini and Hochberg approach across 115 tests.
The safety analysis reported the number and proportion of subjects experiencing AEs by study period, treatment arm, and AE type. The safety analysis performed for table 3 was similar to that previously published.12
Table 3:
Safety Summary (Median per-participant percentage of doses where an adverse event occurred by week and treatment arm: Participant-level analysis)
 |
 |
Note: AEs in weeks 1-7 are related to oinalizumab/placebo only. All other AEs reported in the table are related to multifood OIT. GI = Gastrointestinal, Resp = Respiratory
Study drag administered (placebo or omalizumab)
multifood OIT administered.
P value shown when < 0.05
CTCAE v.4.03 grade where 1, 2, 3 is grade 1, 2, and 3, respectively. There were no grade 3 events.
General indicates skin reactions at injection site.
Other indicates anxiety or eye reactions.
Median per person percentage of doses at which an adverse event occurred.
IDED for participants on open-label omalizumab + OIT included.
Omalizumab in open-label was only administered in week 17 up to week 32.
Based on Wilcoxon Rank Sum test comparing ‘Any AE’ between omalizumab and Placebo
Statistical analyses were performed using R software (version 3.4.1)17 and SAS (version 9.4)18. Most figures were created using the ggplot2 R package.19
Role of the funding source
The main funder of the study was the NIH. Members of the NIH study team played a role in writing the clinical protocol and the manuscript. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
RESULTS
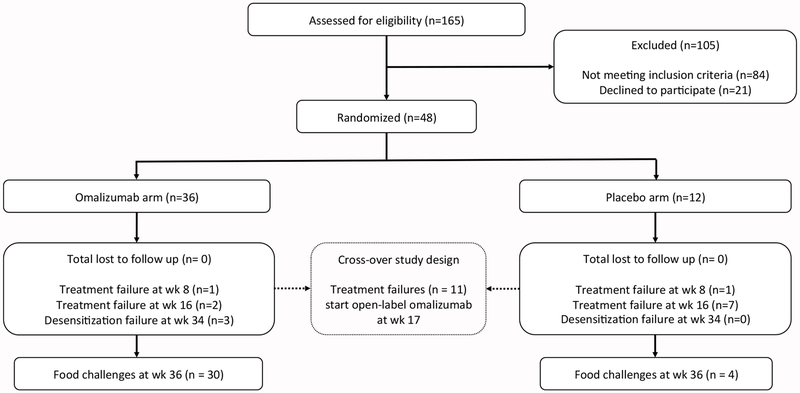
One hundred sixty-five participants were assessed for eligibility, of which 84 did not meet the inclusion criteria and 21 declined to participate (figure 1). We enrolled 48 eligible participants, aged 4-15 years, and randomized 36 to the group receiving omalizumab and 12 to the group receiving placebo. All enrolled participants finished the study and were included in the assessment for the primary endpoint. The 48 enrolled participants had similar demographic and immunologic characteristics (table 1, appendix p 18). The mean number (standard deviation) of foods in OIT per omalizumab or placebo participant was 3·4(1·1) or 3·1 (1·1), respectively, and included cashew, walnut, hazelnut, almond, sesame, cow’s milk, hen’s egg, peanut, soy, and/or wheat, depending on the participant’s enrollment eligibility criteria. At baseline DBPCFC, the median (1st and 3rd quartile) mg of protein CTD per food was 2·5 (0·0, 5·0) vs. 12·5 (4·4, 60·0) for the omalizumab vs. placebo arm, respectively (table 1).
Figure 1:
Consort Diagram demonstrating the phase 2 randomized placebo-controlled trial design (Omalizumab vs. Placebo arms).
Table 1:
Demographics and immunological characteristics of the intent-to-treat population at baseline
| Characteristics | Randomized Omalizumab (n = 36) | Randomized Placebo (n = 12) |
|---|---|---|
| Age in years | 8 (7·0, 10·3) | 7 (6·0, 8·0) |
| Sex male | 18 (50%) | 6 (50%) |
| Ethnicity Hispanic | 2 (6%) | 1 (8%) |
| History of comorbid conditions: | ||
| Asthma | 16 (44%) | 7 (58%) |
| Atopic Dermatitis | 28 (78%) | 8 (67%) |
| Allergic Rhinitis | 26 (72%) | 9 (75%) |
| Age in years at diagnosis of food allergy* | 1·1 (1·0, 2·1) | 1·7 (1·0, 2·6) |
| Years since diagnosis of food allergy* | 6·3 (4·6, 8·2) | 5·2 (4·4, 6·6) |
| Number of foods in OIT | 3·4(1·1) | 3·1(1·1) |
| Participants with … | ||
| 2 foods in OIT | 10 (28%) | 5 (42%) |
| 3 foods in OIT | 9 (25%) | 2 (17%) |
| 4 foods in OIT | 10 (28%) | 4 (33%) |
| 5 foods in OIT | 7 (19%) | 1 (8%) |
| Median CTD in DBPCFC across participant’s foods in mg^ | 2·5 (0·0, 5·0) | 12·5 (4·4, 60·0) |
| Total IgE in kU/L | 408·0(227·5, 869·1) | 450·0 (253·3, 585·5) |
| Highest specific IgE across participant’s foods in kU/L^ | 63·5 (20·9, 90·0) | 24·2(5·8,61·7) |
| Median specific IgE across participant’s foods in kU/L^ | 13·6(6·7, 30·6) | 7·1 (3·0, 15·4) |
| Median specific IgG4 across participant’s foods in mg/L^ | 0·5 (0·2, 3·6) | 0·7 (0·2, 2·5) |
| Median SPT wheal diameter across participant’s foods in mm^ | 12·1 (9·4, 16·8) | 12·0(8·1, 16·1) |
Data are n (%) or median (1st, 3rd quartile). Number of foods in OIT is in mean (SD). IQR = Interquartile range, CTD = cumulative tolerated dose.
The age of diagnosis per participant is determined by the median of the ages of diagnosis of the different allergies.
Only values of foods in participant’s OIT were included.
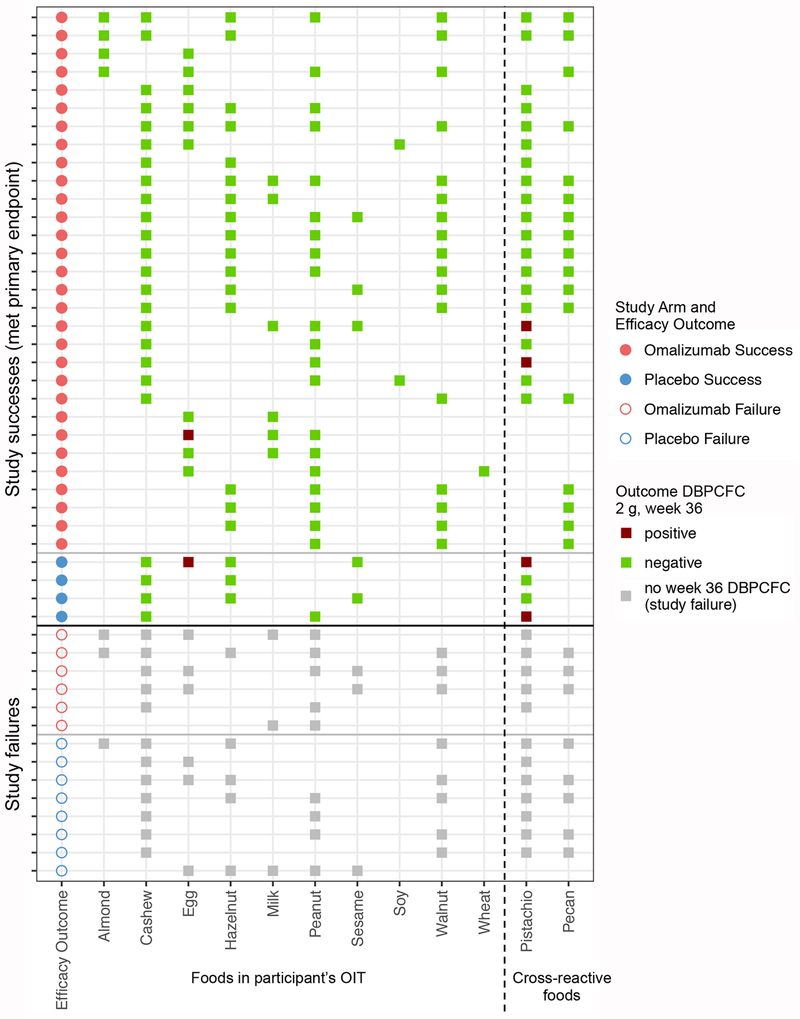
The combinations of foods in the separate multifood OIT of individual participants were substantially diverse (as presented in Andorf, et al.20). The distribution of foods in the multifood OIT of participants per arm is shown in figure 2. To highlight the study endpoints visually, the successes in reaching up to 2 g of food during the DBPCFCs at week 36 are shown for each food in that participant’s multifood OIT.
Figure 2: Overview of foods in each participant’s multifood OIT.
Overview of foods in each participant’s multifood OIT (all shown foods had a positive DBPCFC at baseline for that participant), grouped by study arm (red for omalizumab arm, blue for placebo arm) and primary endpoint outcome. The outcome of the 2 g DBPCFC for each food in week 36 is shown in green when negative (i.e., the participant passed the DBPCFC for that food) and in dark red when positive (i.e., the participant failed the DBPCFC for that food). The failures didn’t undergo food challenges in week 36 and the foods in their multifood OIT are marked by gray boxes.
Primary endpoint results indicate a greater likelihood of passing a DBPCFC to 2 g each of at least 2 offending foods at week 36 in the omalizumab vs. the placebo arm. In the ITT analysis, 83% of participants in the omalizumab (30/36) vs. 33% in the placebo (4/12) arm passed the week 36 DBPCFCs to 2 g of each of 2 foods in their customized multifood OIT (P = 0·004, OR: 10·0, 95% CI: 1·8, 58·3, table 2, figure 3). Table 2 shows that there continued to be significant differences in rates of passing at least 2 challenges between the two arms even after stratifying by number of foods in participants’ multifood OIT (P = 0·002). Only one successful participant according to the primary endpoint in each study arm failed one of the DBPCFCs at week 36, in both cases to egg (figure 2).
Table 2:
Efficacy outcomes for primary endpoint and major secondary endpoints
| Omalizumab (n=36) | Placebo (n=12) | |||
|---|---|---|---|---|
| n of participants who achieved that endpoint / n of participants with that number of foods in that group (%) | ||||
| #foods in OIT | Success: Tolerated 2 g of ≥ 2 foods (Primary endpoint) | Odds ratio* (95% CI) | P value | |
| Total | 30/36 (83%) | 4/12 (33%) | 10·0 (1·8, 58·3) | 0·004 |
| 2 | 8/10 (80%) | 2/5 (40%) | 0·002 | |
| 3 | 9/9(100%) | 1/2 (50%) | ||
| 4 | 9/10 (90%) | 1/4 (25%) | ||
| 5 | 4/7 (57%) | 0/1 (0%) | ||
| Tolerated 2 g of ≥ 3 foods (Secondary endpoint) | Odds ratio (95% CI) | P-value | ||
| Total | 21/26 (81%) | 2/7 (29%) | 10·5(1·2,128·6) | 0·032 |
| 3 | 8/9 (89%) | 1/2 (50%) | 0·01 | |
| 4 | 9/10 (90%) | 1/4 (25%) | ||
| 5 | 4/7 (57%) | 0/1 (0%) | ||
| Tolerated 2 g of ≥ 4 foods (Secondary endpoint) | Odds ratio (95% CI) | P-value | ||
| Total | 13/17 (77%) | 0/5 (0%) | 33(1·9, ∞) | 0·01 |
| 4 | 9/10 (90%) | 0/4 (0%) | 0·003 | |
| 5 | 4/7 (57%) | 0/1 (0%) | ||
| Tolerated 2 g of = 5 foods (Secondary endpoint) | Odds ratio (95% CI) | P-value | ||
| 5 (Total) | 4/7 (57%) | 0/1 (0%) | 3·9 (0·03, ∞) | 1 |
Odds ratios (OR) were estimated based on unconditional maximum likelihood estimation. In cases with zero cells in 2-by-2 tables, a continuity correction was utilized to obtain the OR.15
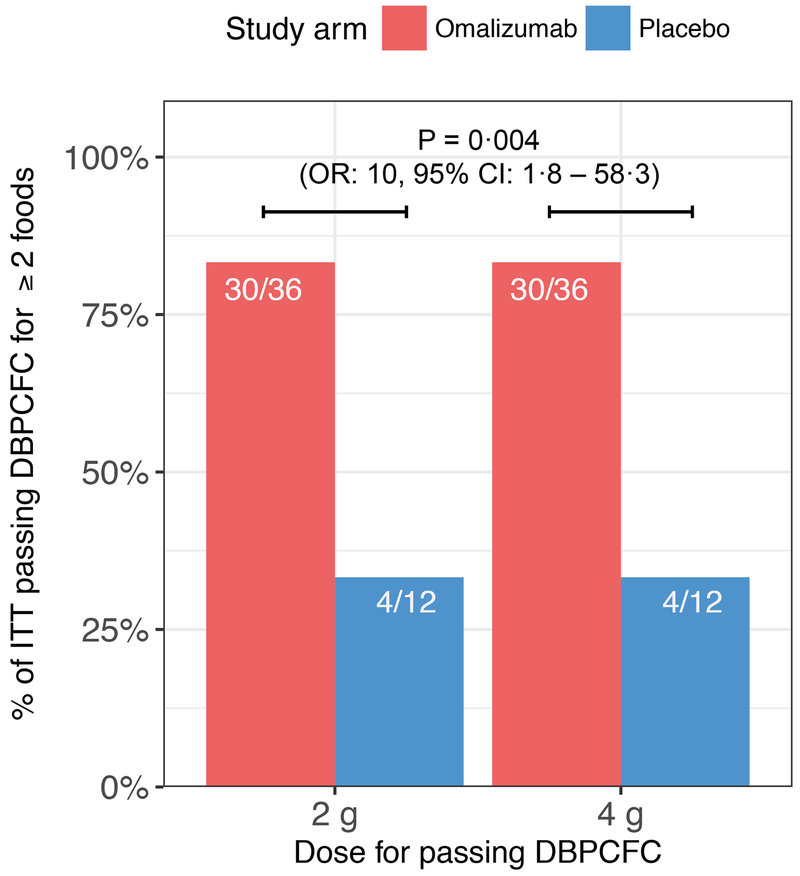
Figure 3: Percentage of participants per study arm who tolerated 2 g (primary endpoint) or 4 g (secondary endpoint) in DBPCFCs to at least 2 foods at week 36.
Every participant who passed the primary endpoint also passed this secondary endpoint. Significantly more participants in the omalizumab arm (83%) passed either endpoint than in the placebo arm (33%) [P = 0·004, OR: 10, 95% CI: 1·8 – 58·3].
Every participant who tolerated 2 g for at least 2 foods in the week 36 DBPCFCs also tolerated 4 g for atleast 2 foods in the same challenges (P = 0·004, OR: 10·0, 95% CI: 1·8, 58·3, figure 3). The secondary efficacy endpoints of passing DBPCFCs to 2 g of each of 3, 4 or 5 foods were achieved significantly more often in the omalizumab arm (table 2). In the omalizumab arm, 26 participants had at least 3 foods in their multifood OIT and 21 of these tolerated 2 g of at least 3 foods at week 36 (81% of the 26 participants). By contrast, only 2 of the 7 participants (29%) receiving placebo with at least 3 foods in their customized multifood OIT tolerated 2 g of at least 3 foods. This resulted in an odds ratio of 10·5 (P = 0·032, 95% CF 1·2, 128·6) between passing 3 challenges in the omalizumab versus placebo arm (table 2). These differences in rates of passing challenges between the study arms were significant (P = 0·01) even after stratifying by number of foods (3, 4 or 5 foods) in their OIT. Table 2 also shows the same test for participants passing at least 4 food challenges (including only those participants who had at least 4 foods in their OIT) and passing all 5 food challenges for the 5 foods in their multifood OIT.
There were no serious AEs (SAEs, defined as per ICH/CFR FDA-GCP guidelines) nor any severe AEs (grade 3 AE according to the NCI-CTCAE system, table 3). We designed the protocol to take advantage of the potential safety enhancement afforded by anti-IgE blockade. During weeks 8-16, the period that allowed a straightforward assessment of drug benefit on OIT safety, those on omalizumab experienced a lower rate of AE-associated OIT doses than those on placebo, while AEs were similar between the arms at other times. Specifically, those in the omalizumab arm had a significantly (P = 0·008) lower median per-participant percentage of OIT doses associated with any AEs (27% vs. 68% in omalizumab vs. placebo arms; table 3). Gastrointestinal and respiratory AEs have been associated with early terminations, severe reactions, and non-compliance in OIT studies.4–6 Those on omalizumab experienced significantly lower values for OIT doses associated with gastrointestinal (22% vs. 54%, P = 0·04) or respiratory (0% vs. 1%, P = 0·02) AEs (table 3). After week 16, when the treatment failures started open-label omalizumab with OIT, all participants were still followed for safety but the treatment failures are listed separately from the non-treatment failures (hence, there was a total of 33 participants in the omalizumab arm and 4 participants in the placebo arm). AEs did not increase in the omalizumab arm amongst these 33 participants during the period of omalizumab withdrawal (after week 16) (table 3). There were no statistically significant differences between arms in safety outcomes in our secondary endpoints (see appendix p 19). Analysis of treatment for AEs (see appendix p 20) and injectable epinephrine use for each participant is detailed in the supplementary materials. We found no significant associations between safety parameters and various participant characteristics (see appendix p 21) nor between percent of OIT doses associated with AEs and success outcomes (see appendix p 5). Additional results can be found in the appendix.
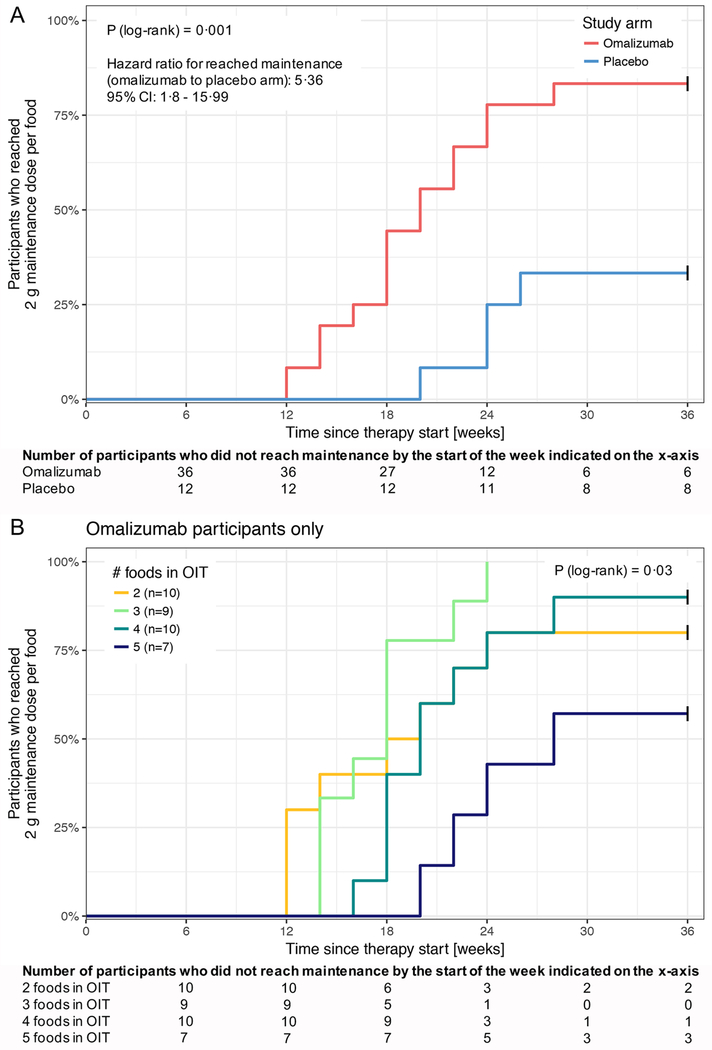
The time to achieve the maintenance dose for each food was significantly less in the omalizumab vs. placebo group (P = 0·001, FDR adjusted P = 0·006, hazard ratio (HR) for reaching maintenance (omalizumab to placebo arm): 5·36, 95% CI: 1·8, 15·99, figure 4A). For omalizumab participants, there was a trend toward a delay in achieving the maintenance dose per food with increased number of foods in the OIT (P = 0·03, FDR adjusted P = 0·1, figures 4B and appendix p 6).
Figure 4: Time since starting therapy (i.e., starting omalizumab or placebo) to reach a 2 g maintenance dose per food.
(A) The time since starting therapy (i.e., starting omalizumab or placebo) for participants in each study arm to reach a 2 g maintenance dose per food. Study failures (6 in the omalizumab arm, 8 in the placebo arm) who never reached 2 g maintenance are censored (marked by vertical black tick marks) at week 36. Participants receiving omalizumab reached the 2 g maintenance dose per food faster (P = 0·001) than the participants on placebo.
(B) The time since starting therapy for participants in the omalizumab arm to reach a 2 g maintenance dose per food, stratified by the number of foods in that participant’s multifood OIT. Study failures are censored (marked by vertical black tick marks) at week 36. Participants with lower numbers of foods in their OIT show a trend of reaching the 2 g maintenance dose per food faster (P = 0·03).
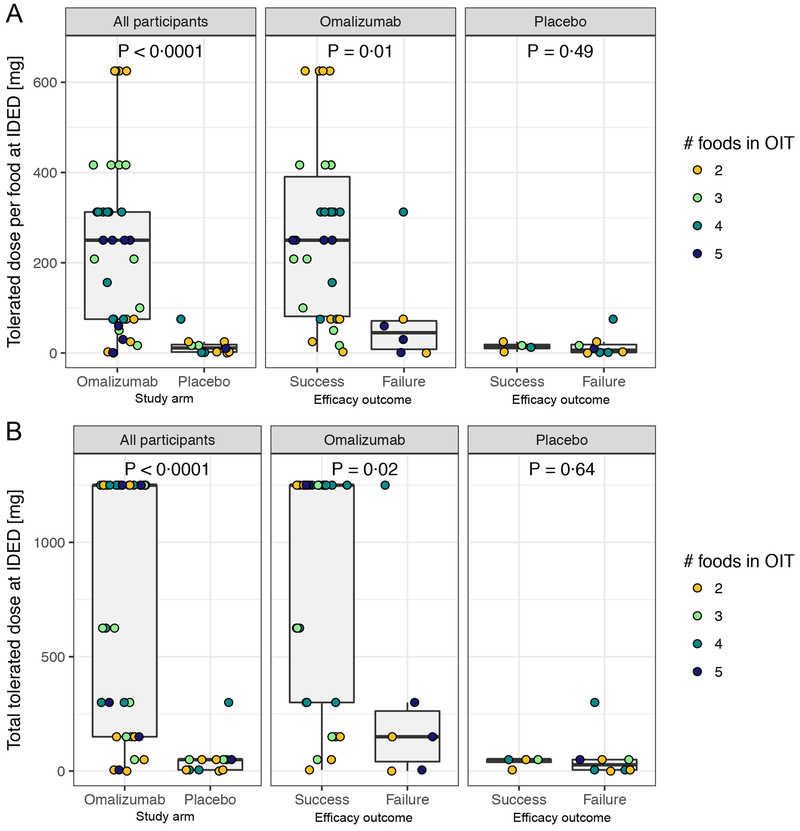
The median tolerated dose on the initial dose escalation day (IDED) at week 8 was significantly greater for omalizumab than for placebo participants (250 mg per food [1,250 mg median total dose, the maximum tested] vs. 11·25 mg median dose per food [50 mg median total dose], P < 0·0001, FDR adjusted P < 0·0001) (figure 5 A, B). Study successes (i.e., achieved the primary endpoint) in the omalizumab arm showed a greater tolerated dose per food at IDED than study failures (P = 0·01, FDR adjusted P = 0·06, figure 5A); this difference was not detected for the placebo arm (P = 0·49, FDR adjusted P = 0·69, figure 5A). The same outcomes hold true for the total tolerated dose at IDED (figure 5B).
Figure 5: Tolerated dose at IDED (initial dose escalation day).
(A) Each data point represents the tolerated dose per food (total tolerated dose divided by the number of foods in the multifood OIT) for each participant stratified by study arm (left) or by study arm and primary endpoint outcome (middle and right).
(B) The total dose of food protein tolerated at IDED for each participant.
The ‘hinges’ represent the first and third quartile. The whiskers are the smallest and largest values after outliers are excluded. Outliers are defined as values greater than the 75th percentile plus 1·5 times the interquartile range (IQR), or less than 25th percentile minus 1·5 times the IQR.
To study if the treatment effect varied by age, we split participants by median age across the two arms (8 years). For participants < 8 years of age, we did not observe a statistically significant difference between arms in rates of success (P = 0·5, FDR adjusted P = 0·7, OR: 3, 95% Cl: 0·3, 30·2, table 4). In the older (≥ 8 years) participants, however, treatment of participants in the omalizumab arm was significantly more successful than that for those in the placebo arm (P = 0·007, FDR adjusted P = 0·03, OR: 42, 95% CI: 2·1, 2117·2). Based on previous publications21,22, we tested a threshold level of allergen specific IgE ≥ 15 kU/L to divide the participants into two groups based on the highest specific IgE, at baseline, to any of the foods in a participant’s OIT. For the group with ≥ 15 kU/L highest allergen-specific IgE at baseline, administration of omalizumab resulted in a significantly greater success rate than in the placebo group (P = 0·002, FDR adjusted P = 0·01, OR: 30, 95% CI: 2·5, 1417·6, table 4); no statistically significant treatment effect was seen in the group with <15 kU/L specific IgE, but the sample size was small.
Table 4:
Highest allergen-specific IgE*, and age by primary efficacy outcome
| Omalizumab | Placebo | ||||
|---|---|---|---|---|---|
| Characteristic | n successfully achieved primary endpoint / n with that characteristic (%) | OR (95% CI) | P value | FDR adjusted P value | |
| Age [years] | |||||
| <8 | 9/13 (69%) | 3/7 (43%) | 3·0 (0·3, 30·2) | 0·5 | 0·7 |
| ≥ 8 | 21/23 (91%) | 1/5 (20%) | 42·0 (2·1, 2117·2) | 0·007 | 0·028 |
| Highest baseline specific IgE [kU/L] | |||||
| < 15 | 5/6 (83%) | 3/5 (60%) | 3·3 (0·1, 234·8) | 0·85 | 0·92 |
| ≥ 15 | 25/30 (83%) | 1/7(14%) | 30·0 (2·5, 1417·6) | 0·002 | 0·011 |
Highest specific IgE value at baseline against any of the foods included in that participant’s multifood OIT.
Page 22 in the appendix shows the association of baseline and IDED characteristics with the study outcome, independent of the study arm. A trend towards a higher rate of success with an increased age could be seen (P = 0·04, FDR adjusted P = 0·12). Only a greater tolerated dose at IDED per body weight was significantly associated with study success after FDR adjustment. Furthermore, the highest of the specific IgE values at baseline to any of the foods in the participants’ OIT showed a trend to be important for the study outcome in the placebo arm (P = 0·07, FDR adjusted P = 0·19, appendix p 7), while this was not the case in the omalizumab arm. Additionally, the median of the CTDs at baseline (DBPCFCs) to all foods in the participant’s multifood OIT (appendix p 8) was not significantly different within one arm between study successes and failures, but it shows (given the small sample size) the trend that placebo failures tended to tolerate lower CTDs of allergen at baseline DBPCFCs.
In all study successes, specific IgG4/IgE ratios significantly increased between baseline and week 36 for most foods, primarily reflecting increases in IgG4 rather than changes in IgE (see appendix pp 9-11). Treatment successes for most foods were also associated with a significant decrease in SPT wheal diameters between baseline and week 36 (see appendix p 15). However, all six study successes in the omalizumab group whose multifood OIT included milk had a negative DBPCFC for milk (figure 2) but did not exhibit significant changes in either specific IgG4/IgE ratios (see appendix p 11) or SPT wheal diameters (see appendix p 15) for milk. The untreated, non-randomized controls showed no significant change in levels of specific IgE or specific IgG4, rations of specific IgG4/specific IgE, or SPT results for any food over the same time span of 36 weeks (see appendix pp 12-14, 16).
Possible cross-desensitization was tested in 24 individuals with both pistachio/cashew allergies and in 17 participants with both pecan/walnut allergies who were successfully desensitized to cashew and/or walnut. When cashew was included in their OIT, 83% passed the pistachio DBPCFC at week 36, and 100% passed the pecan week 36 DBPCFC when treated with walnut in their OIT (figures 2 and appendix p 17).
DISCUSSION
Omalizumab treatment for 16 weeks combined with multifood OIT is safe and allows for improved multifood desensitization compared to multifood OIT with placebo omalizumab, as shown by the reduced AEs during build up and increased ability to pass 2 g each of at least 2 offending foods at week 36. This phase 2 clinical trial thus illustrates the potential benefits of using omalizumab to facilitate multifood desensitization to a variety of allergens in a shortened period of time. Multiple food allergies affect a significant proportion of people with food allergies world-wide, and these multifood allergic patients can have allergic reactions after ingesting any of several different offending foods.4 Although there is currently no approved OIT for treatment of single food allergies, let alone for multiple food allergies, multiallergen immunotherapy for environmental allergies is routine.23 Attempting to treat multifood allergic individuals sequentially with single OIT to each of their offending foods would result in many years of OIT. Omalizumab has been shown to be useful in single food OIT and in shortening the time course of desensitization, as recently reviewed by Lin et al.24 In addition, our group performed a small pilot phase 1 study combining multifood OIT with omalizumab treatment.3 Therefore, we designed this randomized, controlled phase 2 study using initial omalizumab dosing to test the safety and efficacy of rapid multifood OIT.
Our study included participants with high levels of food allergen-specific IgE. This finding provides evidence that, in multi-food OIT, as with single food OIT25–30, OIT can be successful in such subjects. Specifically, we used omalizumab to induce rapid multifood OIT in participants proven to be allergic to at least two foods based on sensitization on SPT and/or specific IgE, and confirmed in DBPCFCs. Our population was screened using thresholds which would likely indicate a high level of clinical reactivity (SPT ≥ 6 mm and/or specific IgE level > 4 kU/L), and exhibited reactions at eliciting cumulative doses of offending foods of ≤ 500 mg of protein to each food at baseline DBPCFCs.
Our results showed that those in the omalizumab vs. placebo arm were significantly more likely to pass a DBPCFC of 2 g to at least two foods at week 36 (83% vs. 33%, P = 0·004, OR: 10·0, 95% CI: 1·8, 58·3, table 2, figure 3). These efficacy data are at least comparable to those reported for single food OIT without omalizumab5 and therefore would suggest that the overall effectiveness of OIT (with omalizumab) is not reduced with increased numbers of foods.
We chose 2 g for the primary endpoint because many multifood allergic patients have milk or egg allergies for which a 2 g accidental ingestion is common.31 The ability to tolerate 2 g for at least two foods was also achieved in individuals with multifood OIT containing 3, 4, or 5 offending foods (table 2). Moreover, the same individual participants who met the primary endpoint of 2 g also tolerated 4 g for at least 2 foods in the same staged food challenge at 36 weeks (figure 3). For many of these food proteins, 4 g represents a serving of that food (e.g., about one tablespoon of peanut butter) and being able to increase their threshold of food ingestion to a serving of protein is important for patients’ nutrition and overall quality of life.32 Substantially shorter times to reach maintenance dosing of 2 g of each food were achieved in omalizumab vs. placebo (as early as 12 vs. 20 weeks, figure 4A, P = 0·001), thus potentially improving the chance of adherence since many individuals drop out of clinical OIT studies in the first 3-6 months.27–29,33 This is another advantage shown by the omalizumab arm because this implies less frequent visits for updosing, a potentially important consideration in school-aged children and working adults. Our data demonstrate that OIT-induced protection was evident at least 20 weeks after stopping the 16-week course of omalizumab therapy. The time point chosen to determine primary and secondary efficacy endpoints was 36 weeks, thus allowing for several omalizumab half-lives to pass; however, we cannot formally conclude that there was no residual effect of omalizumab treatment at 36 weeks. A potential concern with using omalizumab is that participants may be at risk for allergic reactions once omalizumab treatment is stopped; however, our data showed no increased frequencies of AEs after discontinuation of omalizumab (table 3) and no increased frequency of treatment use for AEs in the omalizumab arm (see appendix p 20).
Our study revealed significant differences between the omalizumab vs. placebo arms for many of the primary and secondary endpoints. In addition, there were significant differences in safety outcomes in the omalizumab vs. placebo arms during weeks 8-16, notably decreases in gastrointestinal and respiratory symptoms. This improvement in safety with omalizumab is a critical aspect of our protocol. Indeed, we expected that omalizumab would effectively enable us to reach higher doses faster because of incremental safety improvements.3,11,12 Additionally, minimizing AEs during updosing may enhance long term compliance with OIT. Finally, we found evidence of possible cross-desensitization in individuals with pistachio/cashew allergies or pecan/walnut allergies when they only ingested one of each (cashew or walnut) in the multi OIT (figure 2), but further work will be needed to identify the mechanism(s) underlying these findings.
There are limitations of our study. The interpretation of the safety data between omalizumab- vs. placebo-treated participants after week 16 is challenging because we included the participants that were treatment failures and started open-label omalizumab at week 17 in the safety assessment. However, we anticipated that this design would encourage participant adherence, and this may have contributed to the fact that no one dropped out of the study. In this small study of limited duration, we did not perform basophil assays (which would have required additional blood for testing), nor did we measure total IgE over time. We did not collect total IgE at 36 weeks. Some current evidence indicates that total IgE is still being studied for its use in food allergy.34–36 We only measured total IgE at baseline to follow the product insert for omalizumab dosing. In future studies, we could try to examine ratios of markers to total IgE among markers to possibly predict oral food challenge outcomes. Furthermore, we did not test sustained unresponsiveness.
However, we plan to perform such studies in a future trial. Finally, we assume that omalizumab speeds up the process of desensitization by decreasing the threshold of reactivity of allergic effector cells compared to placebo. We are continuing to pursue other phase 2 trials to answer the question if the placebo participants would benefit from OIT if carried out for a longer period of time.
In conclusion, our results suggest that multifood OIT in combination with a short initial course of omalizumab (16 weeks) will permit effective desensitization to be achieved rapidly in the majority of multifood allergic participants.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed with the medical subject heading terms “multiple food allergy”, “food allergy and omalizumab”, and “oral immunotherapy” for articles published on or before October 1, 2017. We found two pilot clinical trials using omalizumab with single allergen oral immunotherapy (OIT) to either milk1 or peanut2. The only multiple allergen OIT protocol using omalizumab was our original phase 1 publication about the safety and tolerability of an oral immunotherapy protocol to multiple foods using omalizumab.3 However, our phase 1 study did not include a placebo arm.
Added value of this study
This is the first phase 2, randomized, controlled study to investigate the efficacy and safety of omalizumab and multifood allergen oral immunotherapy. Our approach addresses a crucial need for effective concomitant multifood desensitization in a highly atopic population with multiple allergies, who are at risk for near-fatal or fatal food allergic reactions.
Implications of all the available evidence
Our findings provide evidence that patients with multifood allergies can be safely and effectively desensitized to their offending foods with a combination of multifood oral immunotherapy with omalizumab treatment.
Acknowledgements
Funding for this study was provided by NIH NIAID AADCRC Grant U19AI104209, the Sean N. Parker Center for Allergy and Asthma Research at Stanford University, the Simons Foundation, the Myra Reinhard Foundation, the Food Allergy Research and Education (FARE) Center of Excellence, and the Department of Pathology and Department of Pediatrics, Stanford University. Genentech provided omalizumab and placebo omalizumab at no cost. We thank Jennifer Bollyky, Wendy Davidson, Tina Dominguez, Joy Laurenzo-Panza, David Lewis, Katherine Lloyd, Sally Mackey, Gerri O’Riordan, Marshall Plaut, Olivia Raeber, Daniel Rotrosen, Vanitha Sampath, Christy Sandborg, Alkis Togias, Mindy Tsai and Margie Woch, and the Sean N. Parker Center for Allergy and Asthma research team, for their contributions to the research and/or their critical review of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest
We declare no competing interests.
REFERENCES
- 1.Nadeau KC, Schneider LC, Hoyte L, Borras I, Umetsu DT. Rapid oral desensitization in combination with omalizumab therapy in patients with cow’s milk allergy. J Allergy Clin Immunol 2011; 127(6): 1622–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schneider LC, Rachid R, LeBovidge J, Blood E, Mittal M, Umetsu DT. A pilot study of omalizumab to facilitate rapid oral desensitization in high-risk peanut-allergic patients. J Allergy Clin Immunol 2013; 132(6): 1368–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Begin P, Dominguez T, Wilson SP, et al. Phase 1 results of safety and tolerability in a rush oral immunotherapy protocol to multiple foods using Omalizumab. Allergy Asthma Clin Immunol 2014; 10(1): 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta RS, Springston EE, Warrier MR, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics 2011; 128(1): e9–17. [DOI] [PubMed] [Google Scholar]
- 5.Burks A, Jones S, Wood R, et al. Oral immunotherapy for treatment of egg allergy in children. N Engl J Med 2012; 367(3): 233–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones SM, Pons L, Roberts JL, et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. The Journal of allergy and clinical immunology 2009; 124(2): 292–300, e1–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anagnostou K, Islam S, King Y, et al. Assessing the efficacy of oral immunotherapy for the desensitisation of peanut allergy in children (STOP II): a phase 2 randomised controlled trial. Lancet 2014; 383(9925): 1297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsiao K-C, Ponsonby A-L, Axelrad C, et al. Long-term clinical and immunological effects of probiotic and peanut oral immunotherapy after treatment cessation: 4-year follow-up of a randomised, double-blind, placebo-controlled trial. The Lancet Child & Adolescent Health 2017; 1(2): 97–105. [DOI] [PubMed] [Google Scholar]
- 9.Navines-Ferrer A, Serrano-Candelas E, Molina-Molina GJ, Martin M. IgE-Related Chronic Diseases and Anti-IgE-Based Treatments. J Immunol Res 2016; 2016: 8163803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu W, Freeland DMH, Nadeau KC. Food allergy: immune mechanisms, diagnosis and immunotherapy. Nat Rev Immunol 2016; 16(12): 751–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wood RA, Kim JS, Lindblad R, et al. A randomized, double-blind, placebo-controlled study of omalizumab combined with oral immunotherapy for the treatment of cow’s milk allergy. J Allergy Clin Immunol 2015; 137(4): 1103-10.e1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacGinnitie AJ, Rachid R, Gragg H, et al. Omalizumab facilitates rapid oral desensitization for peanut allergy. J Allergy Clin Immunol 2016; 139(3): 873–81.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martorell-Calatayud C, Michavila-Gomez A, Martorell-Aragones A, et al. Anti-IgE-assisted desensitization to egg and cow’s milk in patients refractory to conventional oral immunotherapy. Pediat Allerg Imm-Uk 2016; 27(5): 544–6. [DOI] [PubMed] [Google Scholar]
- 14.Frischmeyer-Guerrerio PA, Masilamani M, Gu W, et al. Mechanistic correlates of clinical responses to omalizumab in the setting of oral immunotherapy for milk allergy. J Allergy Clin Immunol 2017; 140(4): 1043–53 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamilton MA. Choosing the parameter for a 2 x 2 table or a 2 x 2 x 2 table analysis. Am J Epidemiol 1979; 109(3): 362–75. [DOI] [PubMed] [Google Scholar]
- 16.Fay MP. Confidence intervals that match Fisher’s exact or Blaker’s exact tests. Biostatistics 2010; 11(2): 373–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. [Google Scholar]
- 18.SAS Institute Inc. SAS software version [9.4] of the SAS system. Cary, NC, USA. [Google Scholar]
- 19.Wickham H ggplot2: Elegant Graphics for Data Analysis. New York: Springer; 2009. [Google Scholar]
- 20.Andorf S, Borres M, Block W, et al. Association of clinical reactivity with sensitization to allergen components in multifood-allergic children. J Allergy Clin Immunol Pract 2017; 5(5): 1325–34.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sampson HA, Ho DG. Relationship between food-specific IgE concentrations and the risk of positive food challenges in children and adolescents. J Allergy Clin Immunol 1997; 100(4): 444–51. [DOI] [PubMed] [Google Scholar]
- 22.Ta V, Weldon B, Yu G, Humblet O, Neale-May S, Nadeau K. Use of specific IgE and skin prick test to determine clinical reaction severity. Br J Med Med Res 2011; 1(4): 410–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts G, Pfaar O, Akdis CA, et al. EAACI Guidelines on Allergen Immunotherapy: Allergic Rhinoconjunctivitis. Allergy 2017; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 24.Lin C, Lee IT, Sampath V, et al. Combining anti-IgE with oral immunotherapy. Pediatr Allergy Immunol 2017; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 25.Vickery BP, Scurlock AM, Kulis M, et al. Sustained unresponsiveness to peanut in subjects who have completed peanut oral immunotherapy. J Allergy Clin Immunol 2014; 133(2): 468–75 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blumchen K, Ulbricht H, Staden U, et al. Oral peanut immunotherapy in children with peanut anaphylaxis. J Allergy Clin Immunol 2010; 126(1): 83–91 e1. [DOI] [PubMed] [Google Scholar]
- 27.Anagnostou K, Clark A, King Y, Islam S, Deighton J, Ewan P. Efficacy and safety of high-dose peanut oral immunotherapy with factors predicting outcome. Clin Exp Allergy 2011; 41(9): 1273–81. [DOI] [PubMed] [Google Scholar]
- 28.Vazquez-Ortiz M, Alvaro M, Piquer M, et al. Baseline specific IgE levels are useful to predict safety of oral immunotherapy in egg-allergic children. Clin Exp Allergy 2014; 44(1): 130–41. [DOI] [PubMed] [Google Scholar]
- 29.Vazquez-Ortiz M, Alvaro-Lozano M, Alsina L, et al. Safety and predictors of adverse events during oral immunotherapy for milk allergy: severity of reaction at oral challenge, specific IgE and prick test. Clin Exp Allergy 2013; 43(1): 92–102. [DOI] [PubMed] [Google Scholar]
- 30.Meglio P, Giampietro PG, Carello R, Gabriele I, Avitabile S, Galli E. Oral food desensitization in children with IgE-mediated hen’s egg allergy: a new protocol with raw hen’s egg. Pediatr Allergy Immunol 2013; 24(1): 75–83. [DOI] [PubMed] [Google Scholar]
- 31.Leonard SA. Debates in allergy medicine: baked milk and egg ingestion accelerates resolution of milk and egg allergy. World Allergy Organ J 2016; 9: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arasi S, Otani IM, Klingbeil E, et al. Two year effects of food allergen immunotherapy on quality of life in caregivers of children with food allergies. Allergy Asthma Clin Immunol 2014; 10(1): 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nowak-Wegrzyn A, Fiocchi A. Is oral immunotherapy the cure for food allergies? Curr Opin Allergy Clin Immunol 2010; 10(3): 214–9. [DOI] [PubMed] [Google Scholar]
- 34.Lock RJ, Unsworth DJ. Food allergy: which tests are worth doing and which are not? Ann Clin Biochem 2011; 48(Pt 4): 300–9. [DOI] [PubMed] [Google Scholar]
- 35.Grabenhenrich L, Lange L, Hartl M, et al. The component-specific to total IgE ratios do not improve peanut and hazelnut allergy diagnoses. J Allergy Clin Immunol 2016; 137(6): 1751–60 e8. [DOI] [PubMed] [Google Scholar]
- 36.Horimukai K, Hayashi K, Tsumura Y, et al. Total serum IgE level influences oral food challenge tests for IgE-mediated food allergies. Allergy 2015; 70(3): 334–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.