Abstract
Interleukin-13 (IL-13) receptor α2 (IL-13Rα2), a high-affinity IL-13 binding subunit and a tumor antigen, is amplified in a variety of human tumor cell lines and tumors in vivo. By cDNA microarray, we have shown that gene transfer of human and rat adrenomedullin (AM) up-regulates IL-13Rα2 in a human prostate tumor cell line. Here, we show that IL-13Rα2 mRNA and protein are also up-regulated in PC-3 prostate tumor cells by recombinant AM (rAM) and human synthetic AM peptide in a dose-dependent manner in vitro and in vivo in mouse prostate tumor model. The 8- to 10-fold up-regulation of IL-13Rα2 by rAM or AM peptide in prostate tumor cells in vitro and in vivo increased their sensitivity to IL-13PE cytotoxin consisting of IL-13 and a truncated form of Pseudomonas exotoxin. Immunodeficient mice with established prostate tumors transfected with AM or treated with AM peptide showed reduction in tumor size by intratumoral administration of IL-13PE in a dose-dependent manner. At the highest dose (three 100 μg/kg/d every alternate day), >70% reduction of tumor size was observed compared with controls (P ≤ 0.01). These results indicate that two completely unrelated hormones (AM and IL-13) are closely related to each other and that we have identified a novel role of AM in sensitizing certain types of prostate tumors to IL-13R–directed therapeutic agent.
Introduction
Interleukin-13 (IL-13) is a pleiotropic immune regulatory cytokine and the receptors for this cytokine are overexpressed on a variety of human cancer cell lines and primary tumors derived from renal cell carcinoma, malignant glioma, ovarian carcinoma, acquired immunodeficiency syndrome–associated Kaposi’s sarcoma, and head and neck tumors (1–7). IL-13 receptors (IL-13R) are composed of two prominent proteins termed IL-13Rα1 (also known as IL-13Rα′) and IL-13α2 (also known as IL-13Rα; refs. 8,9). IL-13 binds to IL-13Rα1 chain with low affinity, whereas it binds to IL-13Rα2 chain with high affinity. After binding to IL-13Rα1 chain, IL-13 recruits the predominant IL-4 binding protein (IL-4Rα) for signaling (1, 2, 9–11). IL-13Rα2 chain was shown to neither recruit any chain nor mediate signal transduction (12, 13). However, this chain is internalized after binding to ligand (10). Recent studies have shown that IL-13Rα2 mediates signaling through activator protein-1 pathway in murine macrophage cell line (14).
To target IL-13Rs, recombinant fusion proteins composed of IL-13 and a truncated form of a bacterial toxin, Pseudomonas exotoxin (IL-13PE38QQR and IL-13PE38), have been developed (15, 16). This immunotoxin is highly cytotoxic to tumor cells that express high levels of this receptor (5, 15, 17–21). Based on these studies, four phase I/II clinical trials were completed to determine the safety and tolerability of this agent in patients with recurrent glioblastoma multiforme (22).
Previous studies have shown the potential role of adrenomedullin (AM) in prostate carcinoma by generating a stably transfected human prostate carcinoma PC-3 cell line (23). AM, a potent hypotensive 52–amino acid amidated peptide, is a pluripotent factor isolated from renal pheochromocytoma tumors (24). Later studies identified the presence of AM in both human- and rat-derived prostate tissues, prostate carcinoma, and prostate cancer cell lines (25). AM is a tumor promotion factor (26, 27) and causes vasodilatation and bronchodilation, control hormone secretion, and renal homeostasis and regulates cell growth in cells derived from benign and malignant tumors (26, 28, 29).
To study the molecular mechanism of growth control in human prostate carcinoma by AM, we did cDNA microarray analysis of a PC-3 cell line transfected with AM. We identified ~ 100 genes that were up-regulated and involved in regulating cell cycle arrest, apoptosis, cytoskeleton, cell adhesion, the extracellular matrix, immune function, and transcription (30). Interestingly, we observed that IL-13Rα2 mRNA and protein were highly up-regulated (4- to 25-fold) as a result of AM gene transfer. However, the mechanism through which IL-13α2 is up-regulated is not known. It is also not known whether AM could up-regulate IL-13Rα2 in vivo and sensitize tumor to IL-13PE immunotoxin. It will be equally of interest to know if a synthetic peptide for AM could up-regulate IL-13Rα2 chain in vitro and in vitro.
In the present study, we have investigated the effect of purified recombinant AM (rAM), synthetic AM peptide, or gene transfection of AM on IL-13Rα2 in prostate cancer cells in vitro and in vivo in a mouse model. The biological function of up-regulated IL-13Rα2 protein was examined in a xenograft model of human prostate cancer. AM gene–transfected prostate carcinoma cells formed tumors in athymic nude mice, which were subsequently treated with IL-13PE immunotoxin, and the tumor growth was measured. A dramatic decrease in tumor size was observed.
Materials and Methods
Cell culture and reagents.
PC-3 prostate cancer cell line was purchased from the American Type Culture Collection and maintained in RPMI 1640 containing 10% fetal bovine serum (Biowhittaker), 1 mmol/L HEPES, 1 mmol/L nonessential amino acids, 100 units/mL penicillin, and 100 μg/mL streptomycin (Biowhittaker).
rAM and AM peptide.
rAM was expressed from a clone of the PC-3 cell line after stable transfection of full-length AM (see Supplementary Materials and Methods for details). Synthetic AM (1–52) peptide was purchased from Bachem Labs and used after its reconstitution in sterile protease-free water.
Western blot analysis for AM detection.
For Western blot analysis, 10 μL of cell-free supernatants obtained from parental, mock-transfected (vector alone), and AM gene–transfected (AM stable transfected) PC-3 were boiled for 5 min in SDS sample buffer [2% SDS, 50 mmol/L Tris-Cl (pH 6.8), 0.1% bromphenol blue, 10% glycerol], electrophoresed, transferred on polyvinylidene difluoride membrane (Bio-Rad), and immunoreacted with anti-AM antibody (Bachem Labs) in TBS-Tween 20 buffer for 2 h at room temperature. Immunoreactive bands were visualized by enhanced chemiluminescence (Amersham).
Recombinant cytokines and toxins.
Recombinant chimeric fusion protein IL-13PE38 (IL-13PE immunotoxin or IL-13PE) was expressed in Escherichia coli and purified to >95% homogeneity in our laboratory as described previously (15, 16). Recombinant IL-4 and IL-13 were also produced as described (31). Recombinant IL-2 was a kind gift from Chiron Corp.
RNA extraction and reverse transcription-PCR.
Total RNA was extracted using the RNeasy RNA extraction kit (Qiagen) as per the manufacturer’s instructions. The optimal reverse transcription-PCR (RT-PCR) conditions for IL-13Rα2 chain (32) and AM and the primers used in the amplification technique have been described previously (23). Real-time RT-PCR analysis for IL-13Rα2 and β-actin was done using a Bio-Rad Real-time iQ5 after designing gene-specific primers. The relative fluorescence intensity was determined by dividing the intensity of IL-13Rα2 mRNA band by the intensity of β-actin band and expressed as a ratio of relative fluorescence units (RFU; refs. 15, 30). The experiments were done in quadruplicate.
IL-13R binding studies.
Recombinant human IL-13 was labeled with 125I (Amersham Research Products) using IODO-GEN reagent (Pierce) according to the manufacturer’s instructions. The specific activity of the radiolabeled cytokine was estimated to range between 40 and 120 μCi/μg of protein. Binding experiments were done as described elsewhere (11).
Immunofluorescence assay.
Twenty thousand cells were cultured in the presence or absence of rAM in a chambered glass slide (Lab-Tek, Nalge Nunc International) for 48 h under similar conditions as described earlier (6). Additional three washes with PBS were included to remove culture medium that contained AM or mock supernatant. This step minimized the possibility of interference of exogenous AM in the assay. In some experiments, PC-3 cells were preincubated with neutralizing rabbit anti-AM antibody (1:100 dilution; a generous gift from Dr. Ouafik, IFR Jean Roche, Marseille, France) for 1 h and subsequently cultured in the presence of rAM or AM peptide for an additional 48 h to evaluate the specificity of AM in up-regulation of IL-13Rα2. For controls, PC-3 cells were incubated with an equal quantity of rabbit serum in parallel. Indirect immunofluorescence assay (IFA) was done for IL-13Rα2 and AM protein expression as described above.
Cytotoxicity assay.
The cytotoxicity of IL-13PE was determined as described previously (17). For blocking experiments, the tumor cells were preincubated with IL-13, IL-4, or IL-2 (2 μg/mL) for 45 min before addition of IL-13PE. The cells were maintained for 5 more days and then viable cell population was measured by trypan blue exclusion dye technique.
Tumor xenograft studies.
PC-3 cells transfected with AM- or mock-transfected or control (5 ×106) were injected s.c. in the right flank of male athymic nude mice. When PC-3 control tumor xenografts reached a size of 20 ± 4 mm2 within 1 wk, 100 ng AM peptide was injected intratumorally to avoid any systemic adverse side effects every alternate day three times in three sides of the tumor. Forty-eight hours after the last treatment, the animals were sacrificed and tumors were excised and preserved for detection of IL-13Rα2 mRNA by real-time RT-PCR and IL-13Rα2 protein by immunohistochemical (IHC) techniques.
To determine the sensitivity of PC-3 tumors to IL-13PE after up-regulation of IL-13Rα2 by AM transfection, IL-13PE (50 and 100 μg/kg) or PBS/0.2% human serum albumin (vehicle) was injected intratumorally when the tumor size has reached 20 ± 3.2 mm2. Animals with vector only–transfected PC-3 cells served as controls. Treated and control animals were followed for 5 wk, at which point the experiment was terminated due to large tumor burden in control groups. The average values of three independent experiments were calculated and expressed as mean ± SD.
Statistical analysis.
An unpaired two-tailed t test was used to detect significant difference between experimental and corresponding control groups using JMP software package (Brooks/Cole-Thompson Learning). P values of ≤0.05 were considered significant.
Results
Expression of AM mRNA and immunoreactive protein in stable transfected PC-3 tumor cells.
The PC-3 cell line stably transfected with AM gene transcribed mRNA and translated and secreted AM protein in the cell-free supernatant. Real-time PCR showed a significant increase in mRNA expression (P < 0.01) and protein (see Supplementary Fig. S1A and B). The AM protein from two different AM clones immunoreacted with a highly specific antibody and showed a single band of ~6 kDa in the Western blot analysis. Cell-free supernatants from either PC-3 parental or vector only–transfected PC-3 cells (mock transfected) did not show a positive band for AM. As the band intensities for AM protein at a seeding density of 0.5 × 106 cells per flask in four independent experiments were identical for clone AM1, we used this seeding density in all of our experiments to maintain consistent AM expression.
AM enhances IL-13Rα2 expression in vitro and in vivo.
AM peptide was added to tumor cell cultures at various concentrations every alternate day and incubated for 3 days to examine the effect of synthetic AM peptide on IL-13Rα2 mRNA expression. By real-time RT-PCR studies, IL-13Rα2 mRNA expression was increased in a dose-dependent manner and approximately 7- to 8-fold increased expression was observed in treated cells compared with untreated PC-3 cells (P < 0.01; Fig. 1A). Similar to mRNA, protein for IL-13Rα2 was also increased by AM peptide treatment (Fig. 1B).
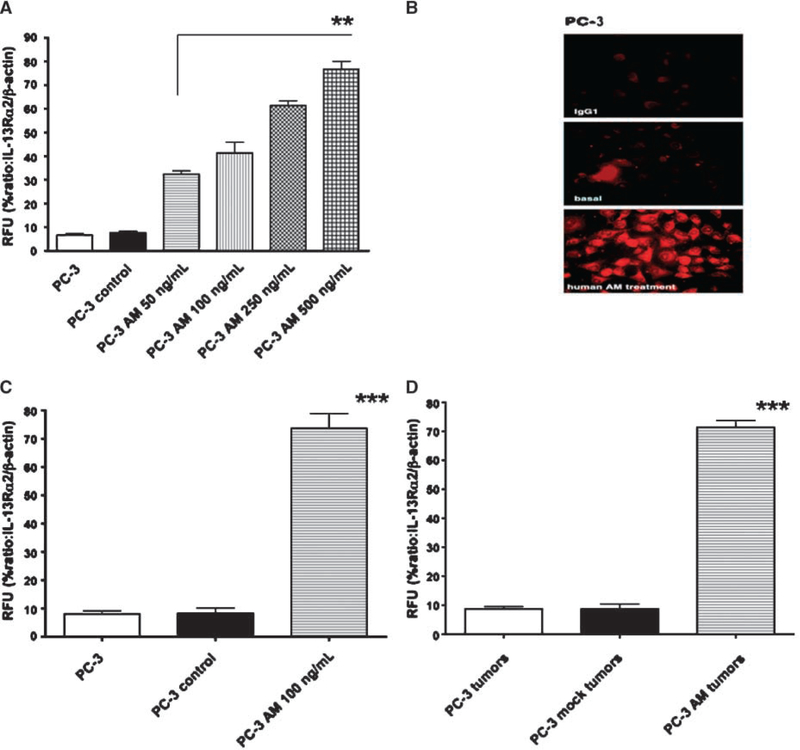
Figure 1.
Up-regulation of IL-13Rα2 mRNA and protein by AM in vitro and in vivo. A, total RNA from PC-3 or PC-3 control or PC-3 cells treated with various concentrations (50–500 ng/mL) of AM peptide was analyzed by real-time RT-PCR. RFU of IL-13Rα2 and β-actin was calculated and normalized from three independent experiments. The data are statistically significant (**, P < 0.01). B, AM peptide–treated PC-3 cells were immunostained by IFA for IL-13Rα2 protein detection. IL-13Rα2 mRNA expression by real-time PCR was evaluated in PC-3 xenograft tumors treated with AM peptide from three independent experiments (C) and in AM-transfected PC-3 tumors compared with PC-3 or PC-3 mock–transfected tumors (D). The results are statistically significant (***, P < 0.001).
For in vivo regulation, PC-3 tumor cells were injected s.c. in immunodeficient animals. Eighteen days later, some tumors received 100 ng AM peptide intratumorally, whereas some tumors were injected with excipient. As shown in Fig. 1C, AM significantly up-regulated IL-13Rα2 mRNA expression. Similar to in vitro study, AM peptide up-regulated IL-13Rα2 mRNA expression by about 7- to 8-fold (P < 0.001). Expression of β-actin mRNA remained uniform and unchanged in this tumor cell line, which indicated that AM did not alter the transcription of all genes.
To examine whether gene-transfected tumor cells maintained expression of IL-13Rα2 mRNA in vivo when these cells were s.c. injected to develop tumors, we determined mRNA expression in PC-3 AM and PC-3 mock tumors by real-time RT-PCR. As shown in Fig. 1D, PC-3 AM explant tumors maintained significantly high level expression of IL-13Rα2 mRNA 35 days after implantation (P < 0.001). PC-3 control or mock-transfected tumors did not show any up-regulation of receptor mRNA.
AM-transfected and control tumor cells were also analyzed for protein expression for AM and IL-13Rα2. As shown in Fig. 2A, PC-3 cells showed an intense immunofluorescence staining for IL-13Rα2 in green and AM protein in red in a stable PC-3 cell line transfected with AM. Approximately 90% of the cells were positive for AM and 82% were for IL-13Rα2 in a stable transfected PC-3 cell line. Similar to real-time RT-PCR results, mock-transfected cells showed a basal level for AM and IL-13Rα2. When two images were merged, IL-13Rα2 and AM showed a yellow immunofluorescence in 68% of AM-transfected cells (Fig. 2A), indicating that both red and green immunofluorescent proteins (AM and IL-13Rα2) were colocalized. The remaining cells may have a minimal overlap of the two colors or these proteins coexpressed at levels below the detection limit of the assay. These results indicate that prostate tumor cells express IL-13Rα2 protein at a basal level and AM treatment up-regulates its transcription and translation in treated cells.
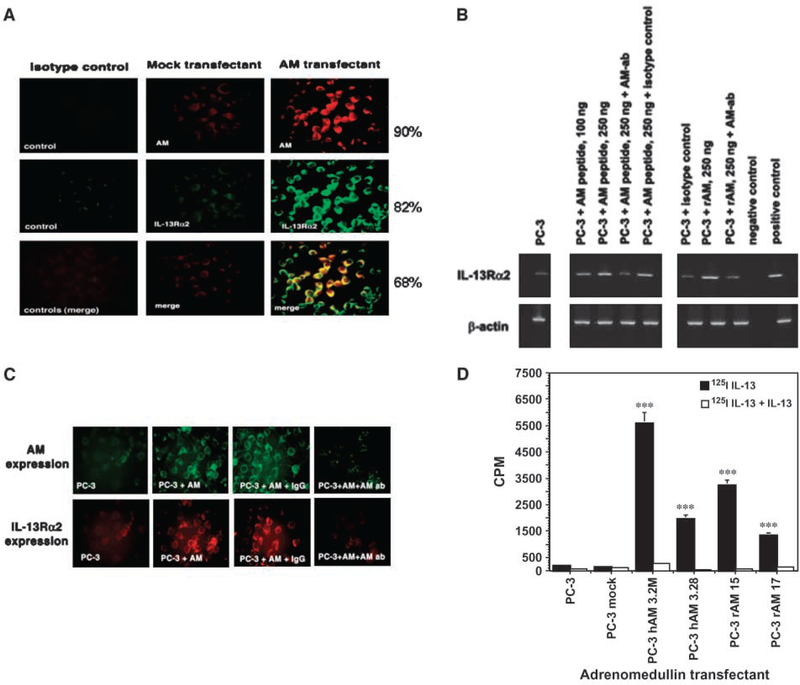
Figure 2.
Specificity of up-regulation of IL-13Rα2 by AM peptide and rAM. A, PC-3 cells were stained with either isotype control or polyclonal antibody to AM or monoclonal antibody to IL-13Rα2 as described in Materials and Methods. AM was detected using TRITC-conjugated anti-rabbit IgG to develop red fluorescence, whereas IL-13Rα2 was detected using FITC-conjugated anti-mouse IgG green fluorescence. The colocalization of both proteins in all three tumor cell lines was analyzed by merging the same field for TRITC- and FITC-immunostained red and green fluorescent tumor cells. Magnification, ×400. B, RNA was extracted from AM peptide–treated PC-3 cells in the presence of AM antibody or isotype IgG and RT-PCR analysis was done for IL-13Rα2 mRNA expression. β-Actin expression was used as a housekeeping gene. RNA from H9 cells served as negative control and PM-RCC as positive control for IL-13Rα2 expression. C, PC-3 cells were treated with AM in the presence or absence of antibody to AM or isotype IgG. The cells were stained with anti-AM and anti-IL-13Rα2 antibody. Magnification, ×400. D, receptor binding assay was done in PC-3, PC-3 mock, and AM-expressing PC-3 clones by incubating with 125I-IL-13 (black columns) or with 200× cold IL-13 (white columns) for competition. Bound CPM was measured in the cell pellet on a gamma counter. Columns, mean of duplicate determination; bars, SD. The experiment was repeated thrice. The values are statistically significant (***, P < 0.001).
To examine the specificity of IL-13Rα2 up-regulation by AM, PC-3 tumor cells were preincubated with AM neutralizing antibody or isotype control antibody for 45 minutes before treatment with AM peptide or rAM and RT-PCR assay was done for IL-13Rα2 As shown in Fig. 2B, rAM and AM peptide up-regulated IL-13Rα2 mRNA compared with control cells. Neutralizing antibody to AM decreased expression of IL-13Rα2 mRNA induced by rAM as well as AM peptide. However, isotype control antibody had no effect.
The specificity of IL-13Rα2 up-regulation in AM-treated PC-3 cells was also evaluated at protein levels (Fig. 2C). PC-3 cells were incubated with AM in the presence of either neutralizing antibody to AM or isotype IgG control. The cells were then immunostained with either AM antibody or IL-13Rα2 antibody to detect the level of expression of these two proteins. Neutralizing antibody to AM decreased not only AM protein expression but also IL-13Rα2 protein levels. In contrast, isotype control IgG did not decrease expression of AM or IL-13Rα2 protein.
Radiolabeled binding and competition study.
Based on RT-PCR and immunofluorescence results, we predicted that radiolabeled IL-13 would specifically bind to AM gene–transfected PC-3 cells. Therefore, binding studies were done using 125I-IL-13 in PC-3, PC-3 mock, and four clones of AM-transfected PC-3 cells. As shown in Fig. 2D, only four clones of PC-3 AM cells bound 125I-IL-13 at high levels (P < 0.001). Total 125I-IL-13 binding was inhibited by excess of unlabeled IL-13 indicating specificity of IL-13 binding. PC-3 control or mock-transfected cells showed no specific binding.
In vivo regulation of IL-13Rα2 by AM.
To investigate regulation of IL-13Rα2 by AM in vivo and IHC and in situ hybridization (ISH) studies were performed on paraffin-embedded tissue sections obtained from PC-3 mock–transfected and PC-3 AM–transfected tumors. PC-3 AM tumor explants showed moderate immunostaining for IL-13Rα2 compared with weakly positive staining (±) in isotype sections or PC-3 mock explants (see Supplementary Fig. S2A). Percent positive fields were found to be 58% in PC-3 AM tumors and 8% in PC-3 mock tumor sections. Isotype control sections did not show any appreciable fluorescence and served as a negative control in the IHC assay. ISH for IL-13Rα2 mRNA was a more sensitive assay and indicated that IL-13Rα2 mRNA was overexpressed in AM xenograft tumors compared with mock tumor tissues. A sense riboprobe for IL-13Rα2 gene served as a negative control in ISH experiments, which did not show any staining (see Supplementary Fig. S2B).
Enhanced sensitivity of AM-PC-3 tumor cells to IL-13PE in vitro and in vivo.
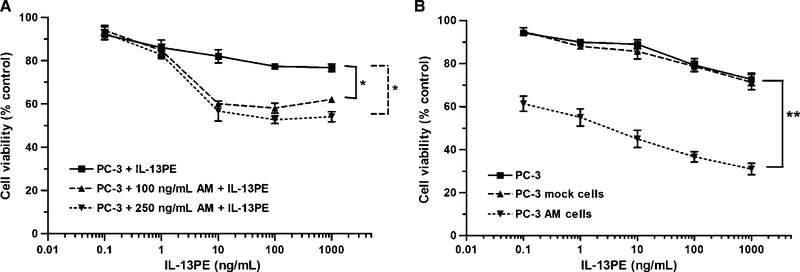
To determine the functional activity of overexpressed IL-13Rα2 protein by AM in tumor cells, we tested the cytotoxic activity of IL-13PE by measuring cell viability in control, AM-transfected, and AM peptide–treated PC-3 prostate tumor cell lines. Tumor cell lines were incubated in the presence of different concentrations of IL-13PE and cell viability was measured after 5 days in treated and untreated control tumor cells. IL-13PE was only slightly cytotoxic to PC-3 parental and mock-transfected control cell lines. However, AM peptide treatment of PC-3 cells resulted in a significant decrease in cell viability compared with untreated control cells (P < 0.05; Fig. 3A). Similarly, in AM-transfected PC-3 cells, a significant decrease in cell viability (P < 0.01) was observed by IL-13PE treatment (Fig. 3B). The IC50 (concentration of IL-13PE that decreases 50% cell viability) was ~5.0 ng/mL in PC-3 AM–transfected cells compared with PC-3 mock cells (>1,000 ng/mL; Fig. 3B). This increase in sensitivity was highly specific to IL-13PE as only IL-13 but not IL-4 or IL-2 could block the cytotoxic activity of IL-13PE in PC-3 AM–transfected cells (data not shown).
Figure 3.
Cytotoxicity of IL-13PE to AM-treated tumor cells in vitro and AM-transfected tumors in vitro. A, PC-3 cells treated with two different concentrations of AM peptide (100 and 250 ng/mL) for 48 h. Cells were then treated with various concentrations of IL-13PE for 20 h. Cell viability was measured after 5 d using trypan blue exclusion assay in hemocytometer. Points, mean percent viable cells; bars, SD. Untreated cells served as control. The experiment was repeated thrice and statistically significant differences (P < 0.05) were compared with control and AM-treated PC-3 cells. B, PC-3 control, PC-3 mock–transfected, and PC-3 AM–transfected tumor cells were incubated with various concentrations of IL-13PE. The viable cells were counted after 5d. Points, mean percent viable cells; bars, SD. Untreated cells served as 100% control. The results are statistically significant (**, P < 0.01).
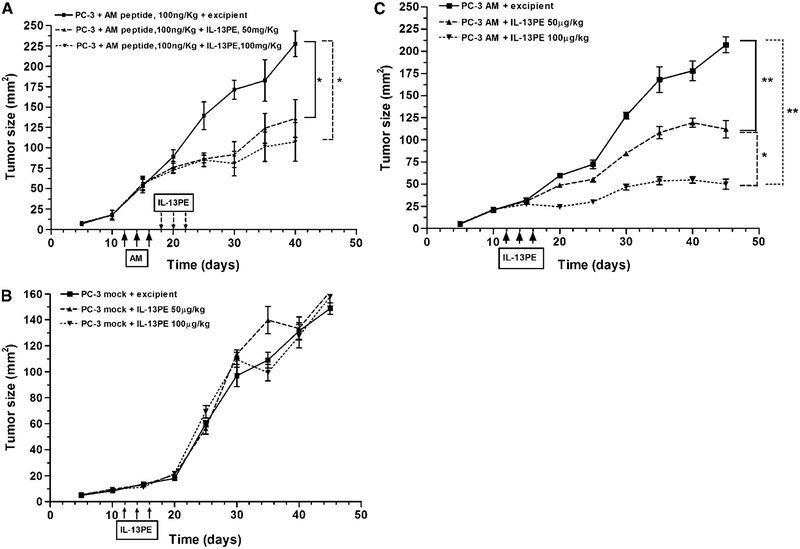
To determine the significance of up-regulated IL-13Rα2 chain in vivo in mouse model of human prostate cancer, PC-3, PC-3 mock–transfected, and AM-transfected prostate tumor cells were s.c. implanted in nude mice. Beginning day 12 of implantation, tumors were treated with 100 ng AM peptide every alternate day for 3 days, and then 2 days later, some tumors were injected with IL-13PE (50 or 100 μg/kg/d) every alternate day for 3 days. Mice bearing mock tumor or AM-transfected tumors were intratumorally injected with IL-13PE (50 or 100 μg/kg/d) every alternate day for 3 days and followed for tumor growth. As shown in Fig. 4A, both doses of IL-13PE decreased AM-treated tumor growth significantly compared with excipient treated mice (P < 0.05). In sharp contrast, IL-13PE did not have any effect on growth of mock-transfected tumors (Fig. 4B). All mice showed similar growth of tumors whether treated with 50 or 100 μg/kg/d IL-13PE or excipient control.
Figure 4.
Antitumor activity of IL-13PE in AM-treated or AM-transfected PC-3 prostate tumors. A, nude mice were implanted with 5 × 106 PC-3 tumor cells s.c. When tumors were established, AM peptide at a fixed dose (100 ng/kg) was injected directly into tumors every alternate day for three injections. Two days after AM treatment, these tumors were then injected with two different doses of IL-13PE (50 and 100 μg/kg) every alternate day for 3 d. Tumor sizes were measured. Points, mean of six animals per group for both doses; bars, SD. *, P < 0.05. Similarly, mock tumors (B) and PC-3 AM tumors (C) were treated with IL-13PE. The differences in tumor sizes were compared for statistical significance (*, P < 0.05; **, P < 0.01) between these groups of mice. Points, mean of six animals per group; bars, SD.
IL-13PE also caused significant inhibition of AM-transfected tumor growth in a dose-dependent manner (P < 0.05 and 0.01, respectively; Fig. 4C). At the highest dose (100 μg/kg/d), the tumor size remained smaller at all time points of monitoring compared with 50 μg/kg IL-13PE dose. At day 29 after IL-13PE treatment, tumor size in treated group (38 ± 4.0 mm2) was ~75% lower compared with excipient treated tumor (220 ± 18.0 mm2; P < 0.01). PC-3 tumors in control groups increased to 220 ± 18.0 mm2 in size at day 45, at that time the experiment was terminated due to large tumor burden in control group.
Discussion
We show that exogenous AM protein or a synthetic peptide up-regulates IL-13Rα2 mRNA and protein in prostate tumor cells in vitro and in vivo in an animal model of human prostate tumor. IL-13α2 overexpression by AM was highly specific as neutralization of AM by antibody diminished IL-13Rα2 mRNA and protein levels. Interestingly, overexpression of IL-13Rα2 protein by AM sensitized tumor cells to an exclusively selective receptor–directed cytotoxic agent such as IL-13PE. IL-13PE immunotoxin mediated enhanced cytotoxicity to prostate tumor cells in vitro when treated with AM peptide or stably transfected with AM gene. This cytotoxicity was highly specific as an excess of IL-13 but not IL-4 or IL-2 neutralized IL-13PE–mediated cytotoxicity. Similar to in vitro studies, IL-13PE also mediated antitumor effects against AM peptide–treated or AM-transfected PC-3 tumors in vivo. These results provide proof of principle that AM-regulated IL-13Rα2 gene makes a functional receptor on tumor cell surface in vivo for receptor-directed therapy by IL-13PE. Partial response to this approach in >70% of AM-transfected PC-3 tumor-bearing mice may be improved by designing effective delivery of this immunotoxin in tumor.
The mechanism of up-regulation of IL-13Rα2 chain by AM in prostate tumor cells is not known. Preliminary studies indicate that AM directly interacts with IL-13Rα2 gene and that AM stabilizes IL-13Rα2 transcripts and prolongs its translation. In addition, it has been shown that human tumor cell lines that express the IL-13Rα2 chain are highly susceptible to the cytotoxic effect of IL-13PE in vitro and in vivo in animal models of human cancers (10, 19). However, tumor cell lines that expressed low levels of IL-13R were dramatically less sensitive to this targeted cytotoxin (33). We later showed that when tumor cells were transfected with IL-13Rα2 chain, they showed enhanced sensitivity to IL-13 cytotoxin compared with wild-type or mock-transfected tumor cells (34, 35). As normal immune cells, endothelial cells, or other cells do not express IL-13Rα2, IL-13 cytotoxin is slightly or not cytotoxic to these cells (17, 32). These observations suggest that IL-13Rα2 chain provides a unique target for receptor-directed cancer therapy and combination of pretreatment with AM followed by IL-13 cytotoxin therapy may lead to better antitumor activity.
It is possible that pretreatment of host with AM may also up-regulate IL-13Rα2 in normal cells, leading to toxicity to IL-13 cytotoxin administration. However, in vivo administration of AM in tumors did not cause toxicity in animals. It is to be noted that rAM or AM peptide does not seem to induce IL-13Rα2 expression in cells that do not naturally express this chain (results not shown). Most normal cells, such as B cells, T cells, monocytes, and endothelial cells, do not express basal levels of IL-13Rα2 chain (1, 2, 5). Similar to normal immune cells, other prostate cancer cell lines, such as DU145 or LNCaP, also do not express basal levels of IL-13Rα2 chain and therefore do not show up-regulation of IL-13Rα2 and sensitivity toward IL-13PE. Lack of constitutive expression of IL-13R expression in prostate cancer cell lines may represent heterogeneity in IL-13R in prostate cancer. In this regard, our results are similar to previous studies, which showed that IL-13Rα2 chain is not expressed by all tumors. Thirty percent to 72% of head and neck, ovarian, and brain tumor samples express high levels of IL-13Rα2 chain, whereas others express none or modest levels of IL-13Rα2 chain (2, 5, 6, 15, 17, 18, 21, 36). Although a large number of prostate cancer samples need to be evaluated for IL-13R expression, our results suggest testing IL-13R expression in clinical biopsy samples before enrolling subjects in clinical trials involving AM-directed and IL-13R–directed cytotoxin/immunotoxin or gene therapies. Based on the knowledge of basal level expression of IL-13R, we may be able to predict “a priori” which patients will respond to IL-13R–targeted therapy and which patients will not. Future studies will address these important issues. In addition, we are studying other tumor types in which AM can up-regulate IL-13Rα2.
In conclusion, we have identified AM, a new class of tumor sensitizer for IL-13R–targeted anticancer therapy, in certain types of prostate cancers. IL-13R–directed therapy may be useful in prostate cancers with low expression of IL-13Rα2 after AM treatment followed by IL-13PE cytotoxin-based therapy. Finally, as recent studies have identified IL-13Rα2 chain as a tumor rejection antigen (37), AM may also be useful as a sensitizer for cancer vaccines for localized cancers.
Supplementary Material
Grant support:
Intramural Program of the NIH, Center for Cancer Research, National Cancer Institute and FDA, Center for Biologics Evaluation and Research.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
We thank Dr. Steven Bauer (Division of Cellular and Gene Therapies, Center for Biologics Evaluation and Research) for reviewing t>he manuscript and providing help in fluorescence densitometry and quantitation of RT-PCR products, Dr. Brent McCright (Division of Cellular and Gene Therapies, Center for Biologics Evaluation and Research) for reading the manuscript and helpful suggestions, and Dr. Hira L. Nakhasi (Division of Emerging Transfusion Transmitted Diseases, Center for Biologics Evaluation and Research) and members of Tumor Vaccines and Biotechnology Branch for their general help and suggestions.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Obiri NI, Debinski W, Leonard WJ, Puri RK. Receptor for interleukin 13. Interaction with interleukin 4 by a mechanism that does not involve the common γ chain shared by receptors for interleukins 2, 4, 7, 9, and 15. J Biol Chem 1995;270:8797–804. [DOI] [PubMed] [Google Scholar]
- 2.Obiri NI, Leland P, Murata T, Debinski W, Puri RK. The IL-13 receptor structure differs on various cell types and may share more than one component with IL-4 receptor. J Immunol 1997;158:756–64. [PubMed] [Google Scholar]
- 3.Obiri NI, Husain SR, Debinski W, Puri RK. Interleukin 13 inhibits growth of human renal cell carcinoma cells independently of the p140 interleukin 4 receptor chain. Clin Cancer Res 1996;2:1743–9. [PubMed] [Google Scholar]
- 4.Debinski W, Miner R, Leland P, Obiri NI, Puri RK. Receptor for interleukin (IL) 13 does not interact with IL4 but receptor for IL4 interacts with IL13 on human glioma cells. J Biol Chem 1996;271:22428–33. [DOI] [PubMed] [Google Scholar]
- 5.Husain SR, Obiri NI, Gill P, et al. Receptor for interleukin 13 on AIDS-associated Kaposi’s sarcoma cells serves as a new target for a potent Pseudomonas exotoxin-based chimeric toxin protein. Clin Cancer Res 1997;3:151–6. [PubMed] [Google Scholar]
- 6.Joshi BH, Plautz GE, Puri RK. Interleukin-13 receptor α chain: a novel tumor-associated transmembrane protein in primary explants of human malignant gliomas. Cancer Res 2000;60:1168–72. [PubMed] [Google Scholar]
- 7.Murata T, Obiri NI, Puri RK. Human ovariancarcinoma cell lines express IL-4 and IL-13 receptors: comparison between IL-4- and IL-13-induced signal transduction. Int J Cancer 1997;70:230–40. [DOI] [PubMed] [Google Scholar]
- 8.Caput D, Laurent P, Kaghad M, et al. Cloning and characterization of a specific interleukin (IL)-13 binding protein structurally related to the IL-5 receptor α chain. J Biol Chem 1996;271:16921–6. [DOI] [PubMed] [Google Scholar]
- 9.Aman MJ, Tayebi N, Obiri NI, Puri RK, Modi WS, Leonard WJ. cDNA cloning and characterization of the human interleukin 13 receptor a chain. J Biol Chem 1996;271:29265–70. [DOI] [PubMed] [Google Scholar]
- 10.Kawakami K, Husain SR, Bright RK, Puri RK. Gene transfer of interleukin 13 receptor α2 chain dramatically enhances the antitumor effect of IL-13 receptor-targeted cytotoxin in human prostate cancer xenografts. Cancer Gene Ther 2001;8:861–8. [DOI] [PubMed] [Google Scholar]
- 11.Murata T, Taguchi J, Puri RK. Interleukin-13 receptor α′ but not α chain: a functional component of interleukin-4 receptors. Blood 1998;91:3884–91. [PubMed] [Google Scholar]
- 12.Kawakami K, Taguchi J, Murata T, Puri RK. The interleukin-13 receptor α2 chain: an essential component for binding and internalization but not for interleukin-13-induced signal transduction through the STAT6 pathway. Blood 2001;97:2673–9. [DOI] [PubMed] [Google Scholar]
- 13.Murata T, Obiri NI, Puri RK. Structure of and signal transduction through interleukin-4 and interleukin-13 receptors [review]. Int J Mol Med 1998;1:551–7. [DOI] [PubMed] [Google Scholar]
- 14.Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A. IL-13 signaling through the IL-13α2 receptor is involved in induction of TGF-h1 production and fibrosis. Nat Med 2006;12:99–106. [DOI] [PubMed] [Google Scholar]
- 15.Joshi BH, Kawakami K, Leland P, Puri RK. Heterogeneity in interleukin-13 receptor expression and subunit structure in squamous cell carcinoma of head and neck: differential sensitivity to chimeric fusion proteins comprised of interleukin-13 and a mutated form of Pseudomonas exotoxin. Clin Cancer Res 2002;8: 1948–56. [PubMed] [Google Scholar]
- 16.Joshi BH, Puri RK. Optimization of expression and purification of two biologically active chimeric fusion proteins that consist of human interleukin-13 and Pseudomonas exotoxin in Escherichia coli. Protein Expr Purif 2005;39:189–98. [DOI] [PubMed] [Google Scholar]
- 17.Puri RK, Leland P, Obiri NI, et al. Targeting of interleukin-13 receptor on human renal cell carcinoma cells by a recombinant chimeric protein composed of interleukin-13 and a truncated form of Pseudomonas exotoxin A (PE38QQR). Blood 1996;87:4333–9. [PubMed] [Google Scholar]
- 18.Joshi BH, Leland P, Puri RK. Identification and characterization of interleukin-13 receptor in human medulloblastoma and targeting these receptors with interleukin-13-pseudomonas exotoxin fusion protein. Croat Med J 2003;44:455–62. [PubMed] [Google Scholar]
- 19.Husain SR, Puri RK. Interleukin-13 fusion cytotoxin as a potent targeted agent for AIDS-Kaposi’s sarcoma xenograft. Blood 2000;95:3506–13. [PubMed] [Google Scholar]
- 20.Kawakami K, Joshi BH, Puri RK. Sensitization of cancer cells to interleukin 13-pseudomonas exotoxininduced cell death by gene transfer of interleukin 13 receptor α chain. Hum Gene Ther 2000;11:1829–35. [DOI] [PubMed] [Google Scholar]
- 21.Maini A, Hillman G, Haas GP, et al. Interleukin-13 receptors on human prostate carcinoma cell lines represent a novel target for a chimeric protein composed of IL-13 and a mutated form of Pseudomonas exotoxin. J Urol 1997;158:948–53. [DOI] [PubMed] [Google Scholar]
- 22.Joshi BH, Hogaboam C, Dover P, Husain SR, Puri RK. Role of interleukin-13 in cancer, pulmonary fibrosis, and other T(H)2-type diseases. Vitam Horm 2006;74: 479–504. [DOI] [PubMed] [Google Scholar]
- 23.Abasolo I, Yang L, Haleem R, et al. Overexpression of adrenomedullin gene markedly inhibits proliferation of PC3 prostate cancer cells in vitro and in vivo. Mol Cell Endocrinol 2003;199:179–87. [DOI] [PubMed] [Google Scholar]
- 24.Kitamura K, Kangawa K, Kawamoto M, et al. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun 1993;192:553–60. [DOI] [PubMed] [Google Scholar]
- 25.Rocchi P, Boudouresque F, Zamora AJ, et al. Expression of adrenomedullin and peptide amidation activity in human prostate cancer and in human prostate cancer cell lines. Cancer Res 2001;61:1196–206. [PubMed] [Google Scholar]
- 26.Martinez A, Vos M, Guedez L, et al. The effects of adrenomedullin overexpression in breast tumor cells. J Natl Cancer Inst 2002;94:1226–37. [DOI] [PubMed] [Google Scholar]
- 27.Ouafik L, Sauze S, Boudouresque F, et al. Neutralization of adrenomedullin inhibits the growth of human glioblastoma cell lines in vitro and suppresses tumor xenograft growth in vivo. Am J Pathol 2002;160: 1279–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cuttitta F, Pio R, Garayoa M, et al. Adrenomedullin functions as an important tumor survival factor in human carcinogenesis. Microsc Res Tech 2002;57:110–9. [DOI] [PubMed] [Google Scholar]
- 29.Hinson JP, Kapas S, Smith DM. Adrenomedullin, a multifunctional regulatory peptide. Endocr Rev 2000;21: 138–67. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalez-Moreno O, Calvo A, Joshi BH, et al. Gene expression profiling identifies IL-13 receptor α2 chain as a therapeutic target in prostate tumor cells overexpressing adrenomedullin. Int J Cancer 2005;114: 870–8. [DOI] [PubMed] [Google Scholar]
- 31.Oshima Y, Joshi BH, Puri RK. Conversion of interleukin-13 into a high affinity agonist by a single amino acid substitution. J Biol Chem 2000;275:14375–80. [DOI] [PubMed] [Google Scholar]
- 32.Murata T, Obiri NI, Debinski W, Puri RK. Structure of IL-13 receptor: analysis of subunit composition in cancer and immune cells. Biochem Biophys Res Commun 1997;238:90–4. [DOI] [PubMed] [Google Scholar]
- 33.Debinski W, Obiri NI, Powers SK, Pastan I, Puri RK. Human glioma cells overexpress receptors for interleukin 13 and are extremely sensitive to a novel chimeric protein composed of interleukin 13 and pseudomonas exotoxin. Clin Cancer Res 1995;1:1253–8. [PubMed] [Google Scholar]
- 34.Kawakami K, Kawakami M, Husain SR, Puri RK. Potent antitumor activity of IL-13 cytotoxin in human pancreatic tumors engineered to express IL-13 receptor α2 chain in vivo. Gene Ther 2003;10:1116–28. [DOI] [PubMed] [Google Scholar]
- 35.Kawakami K, Kawakami M, Snoy PJ, Husain SR, Puri RK. In vivo overexpression of IL-13 receptor α2 chain inhibits tumorigenicity of human breast and pancreatic tumors in immunodeficient mice. J Exp Med 2001;194: 1743–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Debinski W, Obiri NI, Pastan I, Puri RK. A novel chimeric protein composed of interleukin 13 and Pseudomonas exotoxin is highly cytotoxic to human carcinoma cells expressing receptors for interleukin 13 and interleukin 4. J Biol Chem 1995;270:16775–80. [DOI] [PubMed] [Google Scholar]
- 37.Kawakami K, Terabe M, Kawakami M, Berzofsky JA, Puri RK. Characterization of a novel human tumor antigen interleukin-13 receptor α2 chain. Cancer Res 2006;66:4434–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.