Abstract
Growing evidence from studies with children and animal models suggests that elevated levels of manganese during early development lead to lasting cognitive and fine motor deficits. This study was performed to assess presynaptic biogenic amine function in forebrain of adult Long-Evans rats exposed orally to 0, 25, or 50 mg Mn/kg/day over postnatal day 1–21 or continuously from birth to the end of the study (approximately postnatal day 500). Intracerebral microdialysis in awake rats quantified evoked outflow of biogenic amines in the right medial prefrontal cortex and left striatum. Results indicated that brain manganese levels in the early life exposed groups (postnatal day 24) largely returned to control levels by postnatal day 66, whereas levels in the lifelong exposed groups remained elevated 10%–20% compared with controls at the same ages. Manganese exposure restricted to the early postnatal period caused lasting reductions in cortical potassium-stimulated extracellular norepinephrine, dopamine, and serotonin, and reductions in striatal extracellular dopamine. Lifelong manganese exposure produced similar effects with the addition of significant decreases in cortical dopamine that were not evident in the early postnatal exposed groups. These results indicate that early postnatal manganese exposure produces persistent deficits in cortical and striatal biogenic amine function. Given that these same animals exhibited lasting impairments in attention and fine motor function, these findings suggest that reductions in catecholaminergic activity are a primary factor underlying the behavioral effects caused by manganese, and indicate that children exposed to elevated levels of manganese during early development are at the greatest risk for neuronal deficiencies that persist into adulthood.
Keywords: manganese, dopamine, norepinephrine, microdialysis, medial prefrontal cortex, striatum
Epidemiological studies have long reported associations between increased manganese (Mn) exposure during early postnatal development and behavioral, cognitive, and fine motor dysfunction in children, including attention deficit hyperactivity disorder-like impairments in attention, impulse control, and cognitive flexibility (Barlow, 1983; Bouchard et al., 2007; Lucchini et al., 2012; Takser et al., 2003; Wasserman et al., 2006; Woolf et al., 2002). Collectively, these studies suggest that early life Mn exposure may impair multiple behavioral functions in children and adolescents. These reports have heightened concerns about the vulnerability of young children to elevated Mn due to their increased absorption and retention of the metal compared with that of adults and the susceptibility of their developing brains to environmental Mn exposure (Dorner et al., 1989; Keen et al., 1986; Kostial et al., 1978; Lai et al., 1984; Lonnerdal, 1997; Pennington and Young, 1991; Zlotkin and Buchanan, 1986). Infants are particularly at risk of increased Mn from contaminated well water and soy-based infant formulas that may contain as much as 300-fold higher Mn levels than are present in breast milk (Aschner and Aschner, 2005; Ericson et al., 2007; Keen et al., 1986; Ljung and Vahter, 2007).
The results of animal studies have supported concerns that elevated Mn exposure early in postnatal life can lead to lasting behavioral and cognitive impairment. Investigations in rodents have noted significant impacts of preweaning Mn exposure in paradigms assessing locomotor activity, passive avoidance, acoustic startle, and negative geotaxis (Brenneman et al., 1999; Chandra et al., 1979; Dorman et al., 2000; Kern et al., 2010; Kern and Smith, 2011; Pappas et al., 1997; Reichel et al., 2006; Tran et al., 2002). Importantly, multiple recent investigations from our group, conducted on the same animals described in the present report, have demonstrated that early postnatal Mn exposure produces lasting impairment in fine motor function as well as in focused and selective attention (Beaudin et al., 2013, 2017). Moreover, the indirect catecholamine agonist methylphenidate (MPH) fully alleviated the impairment in fine motor function (Beaudin et al., 2015). These findings provide the first evidence for a causal relationship between elevated Mn levels early in postnatal life and lasting impairment in functional domains that have been associated with elevated Mn exposure in children, and additionally they suggest that reduced catecholaminergic activity may underlie the impaired cognitive and motor function.
The early developmental period represents a time when the organism is particularly vulnerable to chemical exposure because it coincides with the development of dopamine (DA) pathways in the medial prefrontal cortex (mPFC) and striatum (STR), brain regions that are instrumental in the regulation of executive functions including attention, working memory, and planning (Arnsten, 2006; Goto and Grace, 2005; Leo et al., 2003; Packard and Knowlton, 2002). Although past efforts have sought to assess synaptic DA function after Mn exposure spanning the preweaning period, the findings have not been consistent. For example, several studies have reported that Mn exposure during this period produces a lasting decrease in DA transporter (DAT) expression and DA uptake in STR and nucleus accumbens (McDougall et al., 2008; Reichel et al., 2006), whereas other studies using a similar Mn exposure regimen reported no lasting effects on DAT levels (Kern and Smith, 2011). Similarly, DA D2 receptor forms were upregulated in STR, but D2-specific binding was reduced in PFC, when both were assessed in young adulthood (postnatal day [PND] 90) (McDougall et al., 2011). In contrast, Kern and Smith (2011) reported increases in nucleus accumbens D1 and PFC D2 receptors at PND 107 but no changes in STR. Kern et al. (2010) also found downregulation of D1 receptors and reduced DAT protein in STR and accumbens at PND 24 resulting from preweaning Mn exposure, but these changes did not persist into adulthood (Kern and Smith, 2011). The complex pattern of behavioral, cognitive, and motor dysfunction associated with developmental Mn neurotoxicity suggests that both mesocortical and nigrostriatal DA systems may be affected. Despite the fact that alterations in executive functions may be caused also by changes in noradrenergic and/or serotonergic transmission in mPFC, these systems have received less attention with respect to developmental Mn exposure.
Here, we investigated whether neuronal changes caused by early postnatal Mn exposure persist into adulthood after cessation of exposure, and whether lifelong Mn exposure exacerbates the effects of early postnatal exposure alone, using microdialysis of biogenic amine neurotransmitters from the mPFC and STR of adult rats. Results in the current investigation indicate that postnatal Mn exposure caused lasting reductions in the depolarization-induced responses of extracellular biogenic amines in STR and mPFC relative to controls. The results also indicated that continued Mn exposure throughout life did not substantially exacerbate the effects of the early postnatal exposure.
MATERIALS AND METHODS
Animals
Fifty-two adult Long-Evans male rats were utilized for dual probe microdialysis testing. All subjects were born into the study over a 2-day period from 27 nulliparous pregnant rats (Charles River, USA). Twelve-24 h after parturition (designated PND 1) litters were sexed, weighed, and culled to 8 pups per litter composed of 5–6 males and the remainder females. Only one male per litter was assigned to a particular treatment condition. Dams and weaned pups were fed Harlan Teklad rodent chow no. 2018 (reported by the manufacturer to contain 118 mg Mn/kg) through the end of behavioral testing and were housed in polycarbonate cages at a constant temperature of 21 ± 2°C. At PND 22, all pups were weaned and pair-housed with an animal of the same treatment group and maintained on a reversed 10:14-h light/dark cycle. Dams and female offspring were not continued further in the study. All animal care and treatments were approved by the Institutional Animal Care and Use Committee and adhered to NIH guidelines set forth in the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011).
Rats were food restricted to 90%–95% of free-feeding weights starting on PND 45 in preparation for an extended series of behavioral tests to assess associative learning, attention, and fine motor function (Beaudin et al., 2013, 2015, 2017). At the conclusion of behavioral testing (PND 269–338), rats were shipped simultaneously overnight to the University of Illinois College of Medicine Peoria (UICOMP) for neurochemical measures and brain tissue collection. At this site, rats were fed Purina LabDiet 5058 (containing 120 mg Mn/kg) ad libitum and maintained on a 12:12-h light/dark cycle with the lights on at 0600 h.
Manganese exposure protocol
Neonates were orally exposed to 0, 25, or 50 mg Mn/kg/day from PND 1–21 (designated “early life” exposure), or from PND 1 to end of the study (designated “lifelong” exposure). For PND 1–21, Mn dosing at the University of California – Santa Cruz (UCSC), a 225 mg Mn/ml stock solution was prepared by dissolving MnCl2·4H2O with Milli-Q water; aliquots of the stock solution were diluted to a final concentration in 2.5% (w/v) noncaloric natural sweetener stevia to facilitate oral dosing of the neonates. Doses were delivered once a day directly into the oral cavity of the pups in a volume of approximately 20–25 µl/dose via a micropipette fitted with flexible extended polyethylene tips (Thermo-Fisher Scientific, Chicago, Illinois). The stevia-sweetened dosing solution was gradually deposited on the back of the animal’s tongue to elicit a licking response, and the rats would swallow all of the small volume without notable distress. Control rats received only the stevia vehicle. Daily oral Mn exposure postweaning (PND 22 to the end of the study at UICOMP) was via the animal’s drinking water at UCSC and continuing after shipment to UICOMP. For this purpose, a 50 mg Mn/ml stock solution was prepared fresh every 2–3 weeks as above and diluted with ultrapure water to final concentrations in the range of 347–1003 µg Mn/ml in polycarbonate carboys to maintain Mn intake at the target dose levels based on fluid intake in individual rats. The water bottles were refilled with fresh solution 1–2 times per week. Drinking water Mn concentrations were adjusted to maintain target daily oral Mn intake levels of 25 or 50 mg/kg/day based on measured water intake rates. Rates of drinking water intake were not measurably different between any of the treatment groups throughout the study.
The environmental relevance of this Mn exposure regimen is based on the fact that the 50 mg Mn/kg/day exposure protocol increases preweaning Mn intake approximately 700-fold relative to that consumed from lactation alone. This approximates the approximately 500-fold increase in Mn exposure experienced by infants and young children exposed to Mn-contaminated water or soy-based formulas (or both) and is compared with Mn ingestion from human breast milk (Keen et al., 1981; Kern et al., 2010; Ljung and Vahter, 2007). Chronic oral exposure to the same 25 or 50 mg Mn/kg/day was maintained postweaning via drinking water, because children and adults may continue to suffer chronic elevated Mn exposures from a variety of environmental sources—eg, contaminated well water and dust (eg, Bouchard et al., 2011).
Blood and brain manganese determination
Blood and brain Mn concentrations were determined in rats at the completion of microdialysis testing (N = 11–21/group) and in littermates of the study rats that were sacrificed at PND 24 and PND 66. Methods for tissue collection at these younger ages are reported in Beaudin et al. (2013). Determination of these exposure indices was important to elucidate the dietary (Control group) and exogenous sources of Mn (exposed groups). For the present microdialysis study, rats were anesthetized by brief inhalation of isoflurane, and whole blood (2–3 ml) collected via cardiac puncture with syringes and vacutainers wetted with EDTA (to prevent coagulation) and stored at −25°C for analysis. Whole brain was subsequently removed in a cryostat maintained at −2°C, and the hindbrain regions of both hemispheres collected and stored at −80°C for Mn determination at the University of California at Santa Cruz (forebrain regions were dedicated to other outcome measures). Tissues were processed for Mn concentrations using trace metal clean techniques, as previously described (Kern et al., 2010; Smith et al., 1992). Briefly, aliquots of whole blood were digested overnight at room temperature with 16 M HNO3 (Optima grade, Thermo-Fisher Scientific), followed by addition of H2O2 and Milli-Q water. Digestates were centrifuged (15 000 × g for 15 min) and the supernatant collected for Mn analysis. Concentrations were expressed as ng Mn/ml whole blood. For brain Mn determinations, aliquots of homogenized hindbrain tissue (approximately 200 mg wet weight) were dried, then digested with hot 16 M HNO3, evaporated, and redissolved in 1 M HNO3 for analyses. Rhodium was added to sample aliquots as an internal standard. Mn levels were determined using a Thermo Element XR inductively coupled plasma-mass spectrometer, measuring masses of 55Mn and 103Rh. Concentrations were expressed as µg/g dry tissue. External standardization for Mn used certified SPEX standards (Spex Industries, Inc, Edison, New Jersey). National Institute of Standards and Technology SRM 1577b (bovine liver) was used to evaluate procedural accuracy. The analytical detection limits for Mn in blood and brain were 0.04 and 0.015 ng/ml, respectively.
Intracerebral microdialysis
Rats were anesthetized with ketamine (80–90 mg/kg, i.p.) and xylazine (2 mg/kg, i.p.) supplemented with 2% isoflurane in O2, mounted in a stereotaxic frame with flat skull surface, and plastic guide cannulae implanted into the right mPFC (from bregma, AP +2.7 mm, ML +1.4 mm, and DV −2.0 mm to the skull surface at a 12° angle to the vertical plane, lateral to medial approach) and the left caudate (from bregma, AP +0.3 mm, ML +2.6 mm, and DV −3.2 mm to the skull surface; Paxinos and Watson, 1997). Cannulae were secured with dental acrylic and machine screws. Meloxicam (2–4 mg/kg, s.c.) was administered for postoperative pain.
CMA12 Elite dialysis probes (CMA Microdialysis, Kista, Sweden) with 4-mm active lengths of polyarylethersulfone membrane (concentric tube design, OD = 0.5 mm, MW cutoff = 20 kDa) were inserted into each cannula 3–5 days later, and the awake animal immediately placed into a plexiglas chamber which allowed freedom of movement. The order of microdialysis testing was balanced by treatment group, and the mean age of rats at the time of microdialysis testing was 567 days and did not differ across treatment groups (analysis of variance [ANOVA] F[4, 47] = 0.32, p = .87). Test sessions were conducted in STR and mPFC on consecutive days in each animal. These probe locations resulted in areas of dialyzed tissue that essentially comprised the dorsal to ventral extents of the central STR and mPFC regions. The probe inlet was connected by FEP tubing to a syringe pump through a liquid switch and dual channel quartz-lined liquid swivel (Instech Labs, Plymouth Meeting, Pennsylvania). The probe outlet was connected to the swivel by the same tubing and to a collection vial in a fraction collector maintained at 4°C (CMA 170, CMA Microdialysis). Between test sessions (1 test session/probe), the dialysis system was flushed extensively with high purity water.
Experimental design
A modified Ringer’s solution (in mM: Na+ 145, K+ 4.0, Ca+2 1.3, Cl− 152, all reagents from Sigma Chemical, St Louis, Missouri, or Thermo-Fisher Scientific, Fair Lawn, New Jersey) was perfused through the probes during baseline sample collection. Two and one-half hours after probe insertion to permit stabilization, baseline extracellular fluid concentrations of norepinephrine (NE), DA, and 5-hydroxytryptamine (5-HT) were assessed by four 30-min (STR) or two 40-min (mPFC) collections prior to switching for 60 min (STR) or 100 min (mPFC) to modified Ringer’s with 120 mM K+ (K+ replaced Na+ to maintain isotonicity). Flow was maintained at 2.0 µl/min, and sample fractions were collected in tubes containing 5 µl of 0.1 M HCl and stored at −25°C until analysis. Desipramine (100 µM, Enzo Life Sciences, Farmingdale, New York) was added to the perfusion medium in test sessions in mPFC to inhibit reuptake of NE and DA and produce measurable amounts of the transmitters. Under these flow conditions, extraction efficiency of the perfusate from the dialysis probe is approximately 15%, indicating that the maximal extracellular fluid K+ concentration produced in vivo is in the range of 18 mM. Employing a reuptake inhibitor in the perfusion medium in order to elicit a quantifiable effect of K+ stimulation on extracellular NE/DA in mPFC has been utilized by others (eg, Bymaster et al., 2002; Higashino et al., 2014). During the period of elevated extracellular fluid transmitter concentrations (80 min in STR, 160 min in mPFC), 20-min (STR) or 40-min (mPFC) sample collections were made followed by two 30-min (STR) or one additional 40-min (mPFC) collection(s) for the return to baseline concentrations.
Probe placement was verified in rats briefly anesthetized with isoflurane by repeated insertion through the guide cannulae of used microdialysis probes dipped in hematoxylin (0.5% w/v) to stain the probe tracks. The harvested brain tissue was fixed in 4% paraformaldehyde and 100-µm coronal sections were cut on a vibratome (Technical Products International, St Louis, Missouri) and examined under a microscope at low power magnification. Figure 1 displays the identified probe tracks for all study rats superimposed on brain atlas plates at the appropriate stereotaxic coordinates.
Figure 1.
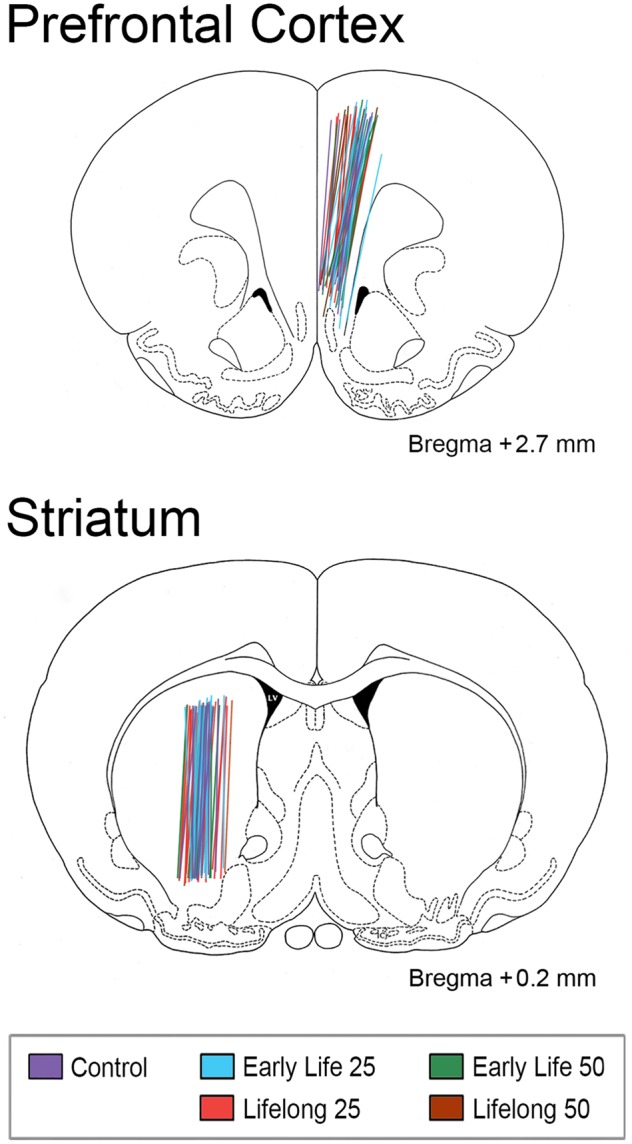
Brain atlas images and coordinates (Paxinos and Watson, 1997) depicting the central axes and active lengths (4 mm) of each microdialysis probe implanted in right medial prefrontal cortex (mPFC) and left striatum (STR) delineated by experimental groups (see legend). It is clear that probe implantation sites across experimental groups were not distinguishable.
Chromatographic analysis
For determination of the biogenic amines, collected samples were loaded into an autosampler maintained at 4°C (Waters 717Plus, Waters Chromatography Div., Milford, Massachusetts), and analyzed by isocratic liquid chromatography with electrochemical detection (LC-4C Amperometric Detector, BASi, West Lafayette, Indiana) at an oxidation potential of +700 mV. Flow rate was 1.6 ml/min. The biogenic amines and their primary metabolites were quantitated by integration of peak areas with comparison to standards of known concentrations (Sigma Chemical) included in each set of samples. Peak identification in dialysis fractions was further aided by comparisons of spiked to unspiked samples. The mobile phase consisted of 0.15 M monochloroacetate, pH 2.95, containing 1.3% acetonitrile (v/v), 1.7% tetrahydrofuran (v/v, including 250 ppm butylated hydroxytoluene as an inhibitor), 0.86 mM sodium octyl sulfate, and 0.18 mM EDTA, and was delivered to a 250× 4.6 mm, 5 µm Biophase ODS analytical column (PerkinElmer, Waltham, Massachusetts). Chromatographic peaks were quantified with EZChrom Elite software (Agilent Technologies, Pleasanton, California).
Data analysis
Blood and tissue Mn concentration data were analyzed at each age using one-way ANOVA and Dunnett’s post hoc test for pairwise comparisons to the control group. Blood and brain Mn levels at PND 66 and PND approximately 500 were compared by a two-factor ANOVA with age and Mn exposure as the main factors; values in the same exposure group were compared at the 2 ages by Sidak’s post hoc test. Microdialysis data were analyzed by an ANOVA for each neurotransmitter with Mn exposure group as the between subjects factor and time relative to K+ stimulation as the within subjects factor. Baseline extracellular fluid concentrations were examined for each transmitter or metabolite across Mn exposure groups by one-way ANOVA. Concentrations determined in individual rats were averaged at each baseline time point (2 for mPFC, 4 for STR), then collapsed across time to yield a single group baseline value. Significant main effects or Mn × time interactions for neurotransmitter concentrations were followed by Dunnett’s post hoc test to compare individual Mn exposure groups with the control group. Because the foregoing ANOVAs did not attempt to discriminate the effects of Mn dose (25 vs 50 mg/kg/day) or exposure duration (early life vs lifelong) directly, additional ANOVAs were performed only on the Mn groups at each time interval of the K+-induced responses with Mn exposure duration and dose as the main factors. These analyses were conducted in both mPFC and STR for each biogenic amine. All data analyses were performed using GraphPad Prism v.6.04 for Windows (GraphPad Software, San Diego, California) or JMP 11.0 (SAS Institute, Cary, North Carolina), and SEM was the measure of dispersion for all reported mean values.
RESULTS
Blood and Brain Mn Concentrations
Blood and brain concentrations in PND 24 rats exposed to Mn over PND 1–21 (ie, early life groups) were increased approximately 8-fold and 3-fold, respectively, in the 25 mg/kg/day group compared with controls, and by approximately 10-fold and 3.4-fold, respectively, in the 50 mg/kg/day group compared with controls (blood F[2, 40] = 20.21, p < .0001; brain F[2, 38] = 6.25, p < .01; Figure 2, data from Beaudin et al. [2017]). Similarly, in the PND approximately 500 rats blood and brain Mn levels generally remained significantly elevated but with much smaller increases in the lifelong Mn groups, but not in the early life Mn groups, compared with adult controls (blood F[4, 83] = 31.0, p < .0001; brain F[4, 72] = 15.81, p < .0001) (Figure 2). Mn values have also been reported at PND 66 using this same exposure model (blood F[4, 69] = 22.14, p < .0001; brain F[4, 70] = 16.45, p < .0001) (Beaudin et al., 2017). Overall, postnatal exposure to 25 or 50 mg Mn/kg/day increased both blood and brain Mn levels in PND 24, 66, and approximately 500 rats in a dose-dependent fashion, though levels were substantially higher in the PND 24 weanlings compared with their older adolescent and adult counterparts; blood levels in the latter 2 age groups were comparable to each other and slightly higher in all PND 66 groups than in the corresponding PND approximately 500 groups (age effect in blood Mn F[1, 152] = 103.4, p < .0001).
Figure 2.
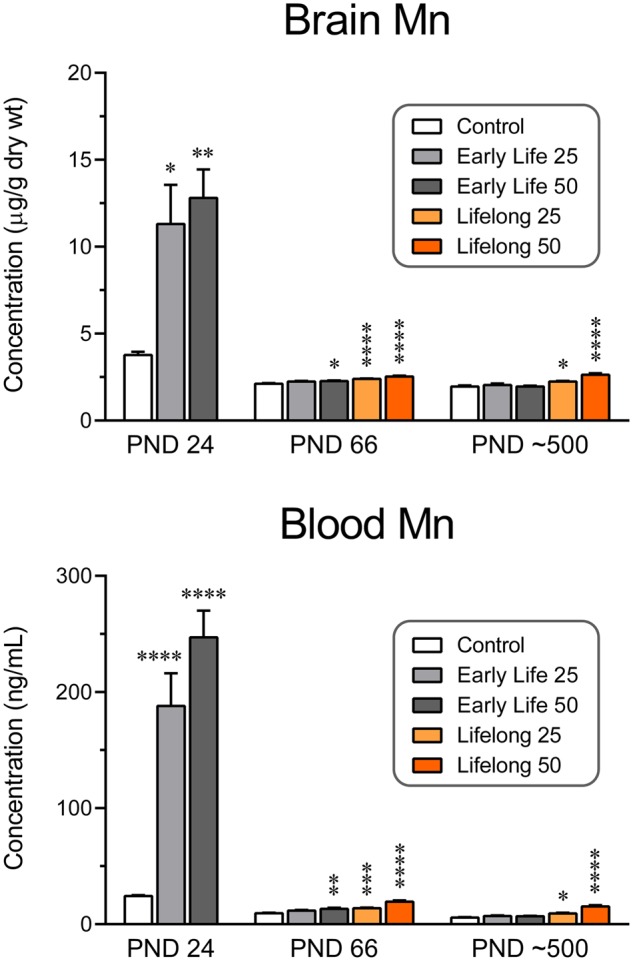
Brain (top, µg/g tissue) and blood (bottom, ng/ml) Mn concentrations in rats at 24, 66, and approximately 500 days of age. Whole brain tissue was sampled for the brain Mn determinations. Note the difference in magnitude of Mn levels as a function of age and the corresponding comparisons to control values. Group N’s for brain tissue were 11–16 at postnatal day (PND) 24, 14–17 at PND 66, and 12–17 at PND approximately 500. ****p < .0001, ***p < .001, **p < .01, and *p < .05 compared with control values at the same age. The blood Mn values of each group at 66 days of age are significantly greater than those of the same group at approximately 500 days of age by Sidak’s multiple comparisons post hoc test; this is true for brain Mn concentrations only for the Early Life 50 group. Group N’s for blood samples were 11–17 at PND 24, 13–17 at PND 66, and 15–21 at PND approximately 500.
Baseline extracellular biogenic amines
Baseline extracellular fluid concentrations of NE, DA, 5-HT, and their primary metabolites are shown in Table 1 for mPFC in the presence of 100 µM desipramine. ANOVAs were performed for each analyte examined, but no significant differences were seen between Mn treatment groups and controls for baseline levels of any transmitter or metabolite in mPFC. Similarly, baseline extracellular biogenic amines and metabolites are presented in Table 2 for STR, showing Mn treatment effects only in the DA metabolite dihydroxyphenylacetic acid (DOPAC; F[4, 46] = 3.44, p = .015), with significant reductions of 43%–45% in the early life 50 and lifelong 25 groups versus controls. No other statistically significant effects were found on baseline neurotransmitter levels.
Table 1.
Basal Concentrations in the mPFC
| Control | Early Life | Early Life | Lifelong | Lifelong | |
|---|---|---|---|---|---|
| 0 mg/kg/day | 25 mg/kg/day | 50 mg/kg/day | 25 mg/kg/day | 50 mg/kg/day | |
| NE | 1.55 ± 0.25 | 2.99 ± 0.57 | 2.46 ± 0.43 | 2.14 ± 0.36 | 2.26 ± 0.35 |
| DA | 0.70 ± 0.14 | 0.76 ± 0.14 | 1.04 ± 0.13 | 0.81 ± 0.17 | 0.98 ± 0.24 |
| DOPAC | 51.1 ± 10.5 | 40.3 ± 11.1 | 41.4 ± 11.2 | 26.1 ± 10.7 | 43.7 ± 7.6 |
| HVA | 74.2 ± 12.7 | 77.6 ± 10.9 | 74.7 ± 10.9 | 53.8 ± 9.2 | 57.6 ± 10.7 |
| 5-HT | 1.01 ± 0.24 | 1.34 ± 0.20 | 0.90 ± 0.18 | 1.37 ± 0.11 | 1.58 ± 0.36 |
| 5-HIAA | 116.0 ± 22.8 | 179.5 ± 26.1 | 140.5 ± 17.5 | 168.5 ± 14.5 | 177.4 ± 16.2 |
Values are mean nM concentrations ± SEM. N = 6–12/group, except for Control DOPAC (N = 4). The NE reuptake blocker desipramine (100 μM) was present in the perfusion fluid throughout.
Table 2.
Basal Concentrations in the STR
| Control | Early Life | Early Life | Lifelong | Lifelong | |
|---|---|---|---|---|---|
| 0 mg/kg/day | 25 mg/kg/day | 50 mg/kg/day | 25 mg/kg/day | 50 mg/kg/day | |
| NE | 0.24 ± 0.16 | 0.89 ± 0.27 | 0.19 ± 0.07 | 0.64 ± 0.29 | 1.24 ± 0.34 |
| DA | 1.24 ± 0.45 | 1.65 ± 0.45 | 1.19 ± 0.44 | 1.80 ± 0.55 | 1.68 ± 0.45 |
| DOPAC | 1725 ± 130 | 1423 ± 158 | 953 ± 176* | 979 ± 175* | 1401 ± 186 |
| HVA | 876 ± 65 | 856 ± 57 | 749 ± 61 | 765 ±75 | 861 ± 67 |
| 5-HT | 0.46 ± 0.08 | 0.62 ± 0.16 | 0.34 ± 0.10 | 0.45 ± 0.10 | 0.53 ± 0.12 |
| 5-HIAA | 234 ± 29 | 228 ± 34 | 154 ± 23 | 225 ± 17 | 198 ± 28 |
Values are mean nM concentrations ± SEM. N = 7–12/group.
p < .05 versus Control, by one-way ANOVA and Dunnett’s test.
K+-stimulated extracellular neurotransmitter
The time courses of biogenic amine outflow stimulated by perfusion with 120 mM K+ in mPFC are shown in Figure 3. For controls, peak K+-stimulated extracellular neurotransmitter was increased 5–12-fold above baseline. However, for many of the Mn groups, the peak stimulated neurotransmitter outflow was significantly blunted.
Figure 3.
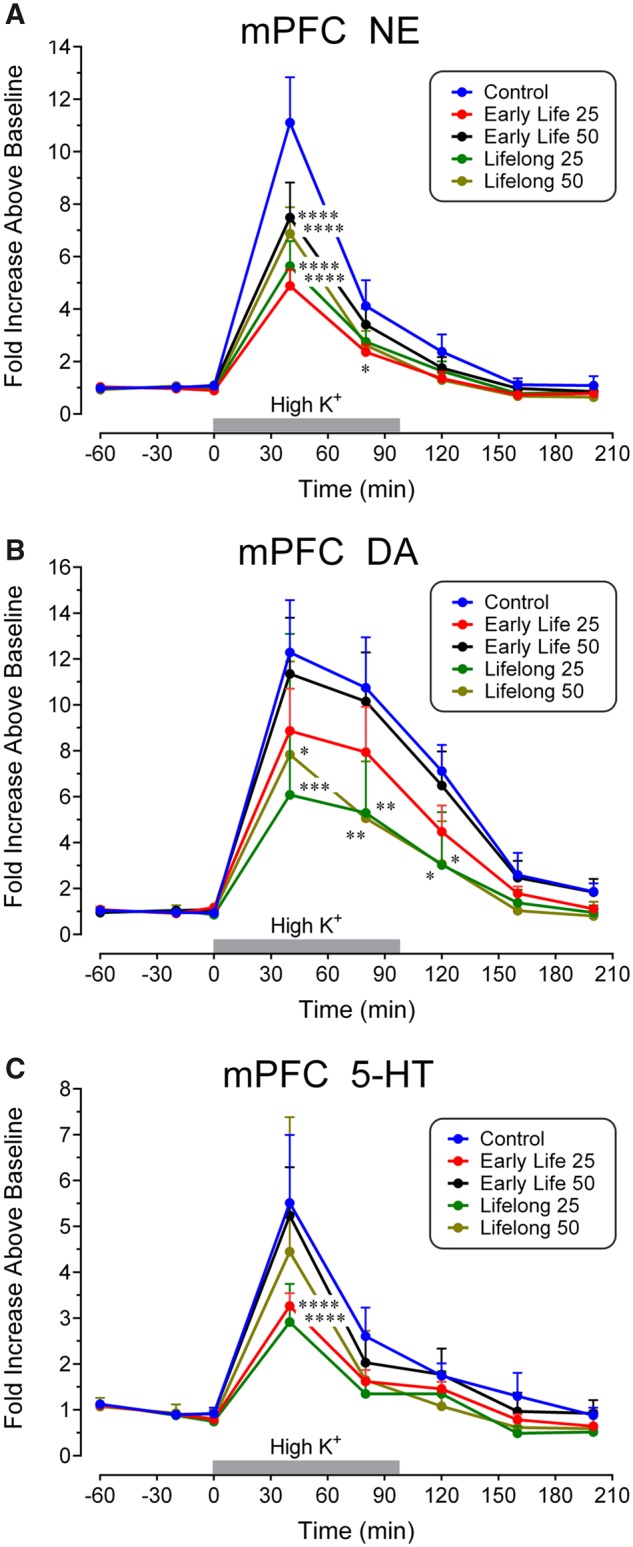
Time course of extracellular fluid concentrations of (A) norepinephrine (NE), (B) DA, and (C) 5-hydroxytryptamine (5-HT) in response to perfusion of 120 mM K+ in mPFC for the indicated time intervals (gray bars). Transmitter levels in Mn-exposed groups were decreased in the 0–120 min time interval depending on the group and biogenic amine. Data analyses uncovered significantly diminished values in the indicated Mn groups at the indicated time points. Group N’s were 7 for the Control group, 12 for the Early Life 25 group, 9 for the Early Life 50 group, and 10 for the Lifelong 25 and Lifelong 50 groups. ****p < .0001, ***p < .001, **p < .01, and *p < .05 compared with the control concentrations at the same time point.
The effects of Mn exposure were most evident for extracellular NE with peak magnitude increases of 5–7-fold over baseline (eg, 5-fold in the early life 25 group, Figure 3A), as reflected by the significant main effects of Mn Exposure: F(4, 39) = 3.73, p = .012; Time: F(7, 273) = 126.3, p < .0001; and the Mn × Time interaction: F(28, 273) = 3.03, p < .0001. For NE, Dunnett’s tests demonstrated that all Mn groups were decreased in comparison to the control group at the first time interval after initiation of high K+ (0–40 min), and extracellular NE was reduced in the early life 25 group at 40–80 min post-K+ initiation also.
Mn exposure also blunted extracellular DA concentrations in mPFC (Figure 3B), as indicated by the significant main effects of Mn Exposure: F(4, 41) = 2.65, p = .047; Time: F(7, 287) = 68.5, p < .0001; and the Mn × Time interaction: F(28, 287) = 1.56, p = .040. Subsequent Dunnett’s tests demonstrated that extracellular DA was significantly reduced in the 2 lifelong Mn exposure groups at each of the 40–120 min time points after K+ stimulation began.
In contrast, the effect of Mn exposure on extracellular 5-HT was less evident (Figure 3C), with no significant main effect of Exposure (F[4, 41] = 1.84, p = .14), though there was a main effect of Time (F[7, 287] = 74.0, p < .0001), and a Mn Exposure × Time interaction (F[28, 287] = 1.64, p = .026). Dunnett’s post hoc tests indicated that extracellular 5-HT was reduced in the early life and lifelong 25 mg/kg/day groups relative to controls at the 40 min time point after initiation of K+ stimulation.
Statistically significant Mn Exposure × Time interactions were uncovered for both HVA (F[28, 308] = 1.54, p = .043) and DOPAC (F[28, 252] = 2.52, p < .0001) in the mPFC. The time courses shown in Figure 4 for these compounds are representative of the metabolite responses to perfusion with high K+; the observed decreases in HVA and DOPAC concentrations to levels approximately 30% of baseline values generally coincide with the increase in extracellular levels of the associated transmitter (eg, DA in this instance). However, the exposure-related group distinctions are not observed until the end of K+ stimulation (80–160 min after initiation of high K+ perfusion) where both early life Mn group values are greater than those of controls, reflecting a more rapid recovery toward and/or overshoot of the initial baseline. No similar exposure-induced changes were uncovered in 5-HIAA levels.
Figure 4.
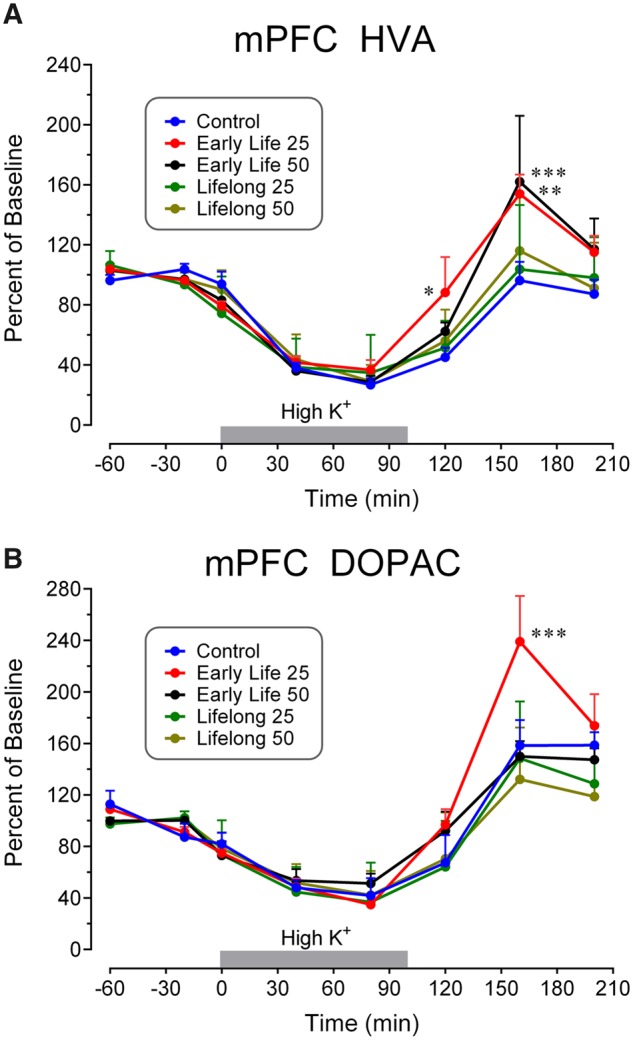
Time course of extracellular fluid concentrations of DA metabolites (A) HVA and (B) DOPAC in response to perfusion of 120 mM K+ in mPFC for the indicated time intervals (gray bars). Metabolite levels in Mn-exposed groups were increased in the time interval 80–160 min after initiation of high K+ in the Early Life groups. Note the decreases in metabolite values in all groups during high K+ administration as opposed to the increases seen with DA. Data analyses uncovered significantly enhanced values in the indicated Mn groups at the symbolized time points. Group N’s were 7 for the Control group (N = 4 for DOPAC), 12 for the Early Life 25 group, 9 for the Early Life 50 group, 10 for the Lifelong 25 group, and 11 for the Lifelong 50 group. ***p < .001, **p < .01, and *p < .05 compared with the control concentrations at the same time point.
The time course of DA efflux stimulated by perfusion with 120 mM K+ in STR is shown in Figure 5A. In controls, peak extracellular DA levels following K+ stimulation increased approximately 280-fold above baseline, whereas Mn treatment significantly blunted this increase in multiple groups. Analyses found a trending Mn Exposure effect (F[4, 46] = 2.13, p = .093), and a significant effect of Time (F[10, 460] = 36.6, p < .0001), as well as a significant Mn Exposure × Time interaction (F[40, 460] = 1.63, p = .010). Subsequent Dunnett’s tests revealed that extracellular DA was diminished in both early life Mn dose groups and in the 25 mg/kg/day lifelong group relative to controls 0–40 min after high K+ perfusion began.
Figure 5.
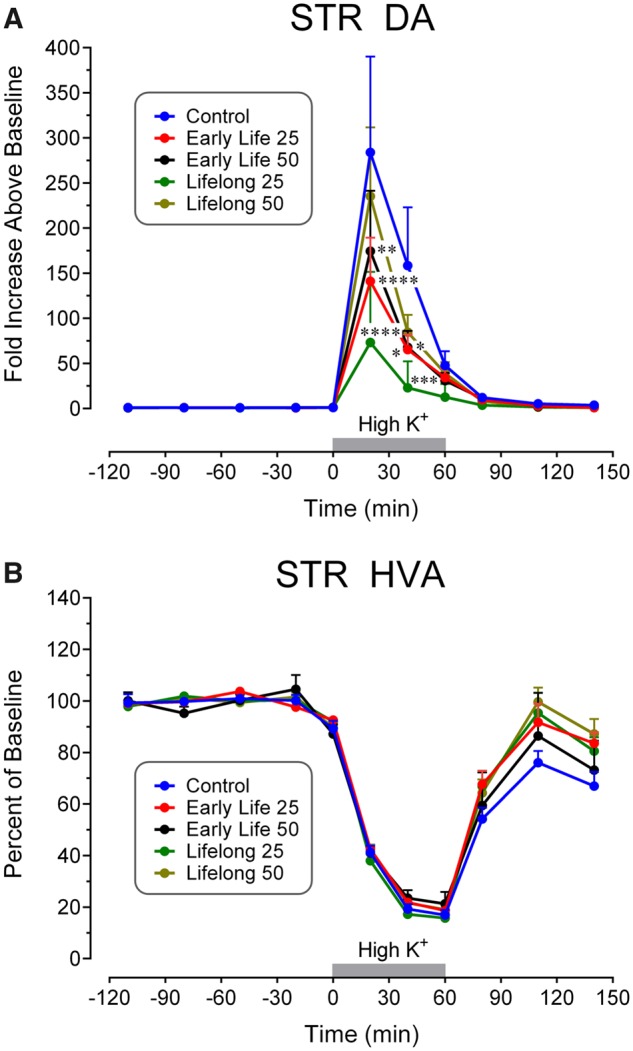
Time course of extracellular fluid concentrations of (A) DA and (B) HVA in response to perfusion of 120 mM K+ in STR for the indicated time intervals (gray bars). DA levels in Mn-exposed groups were decreased in the 0–40 min time interval depending on the group. Note the decreases in HVA values in all groups during high K+ administration as opposed to the increases seen with DA. Data analyses uncovered significantly diminished values in the indicated Mn groups at the indicated time points. Group N’s were 8 for the Control group, 12 for the Early Life 25 group, 10 for the Early Life 50 group, and 11 for the Lifelong 25 and Lifelong 50 groups. ****p < .0001, ***p < .001, **p < .01, and *p < .05 compared with the control concentrations at the same time point.
In contrast, there were no Mn exposure effects on extracellular 5-HT concentrations in the STR (Exposure: F[4, 44] = 0.50, p = .73; Exposure × Time interaction: F[40, 440] = 0.63, p = .96), whereas STR extracellular NE concentrations were too low to be reliably quantified. Furthermore, there was no statistically significant Mn Exposure × Time interaction for extracellular HVA in the STR as shown in Figure 5B (F[40, 470] = 0.93. p = .60), or for 5-HIAA. The time course for HVA is representative of the metabolite responses to perfusion with 120 mM K+—a decrease in concentration to approximately 15% of baseline values that coincides generally with the increase in extracellular levels of the associated transmitter, as noted above. A significant Mn Exposure × Time interaction was present for extracellular DOPAC in the STR (F[40, 460] = 1.49, p = .030, graph not shown) with the value in the lifelong 50 group significantly elevated compared with the control concentration at 20–50 min (Lifelong 50: 103.7% ± 25.7%, Control: 56.7% ± 3.7%) and 50–80 min (Lifelong 50: 107.2% ± 28.3%, Control: 57.8% ± 6.0%) after K+ stimulation ended (80–140 min after initiation of high K+ perfusion).
Additional ANOVAs were performed on the 4 Mn groups at each time interval of the K+-induced responses with exposure duration (early life vs lifelong) and dose (25 vs 50 mg/kg/day) as the main between subjects factors. For mPFC DA, exposure duration exhibited a significant main effect at each time interval from 40 to 160 min after initiation of K+ stimulation (0–40 min: F[1, 35] = 2.56, p = .12; 40–80 min: F[1, 35] = 5.14, p = .030; 80–120 min: F[1, 35] = 5.71, p = .022; 120–160 min: F[1, 35] = 5.15, p = .030) with the lifelong Mn groups displaying a greater decrease in response versus their early life Mn-exposed counterparts. Otherwise, Mn exposure duration or dose (ie, 25 vs 50 mg/kg/day) did not exhibit significant effects on K+-evoked responses for any other neurotransmitter in either brain region.
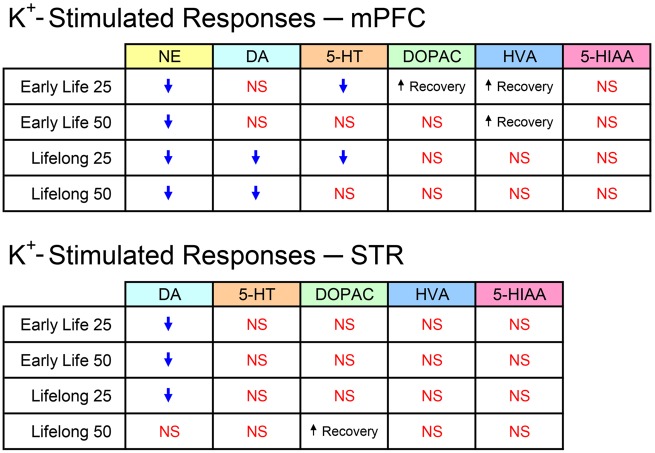
Table 3 summarizes the statistically significant exposure-related effects on K+-stimulated extracellular responses occurring in each Mn group across all analytes in both brain regions. The table also notes the more rapid rates of recovery of extracellular DA metabolites in those exposure groups and brain regions where they were observed. On the basis of the number of significant changes in all these responses, the mPFC was more affected by Mn exposure than STR.
DISCUSSION
The present study utilized a rodent model of developmental Mn exposure to elucidate lasting effects on biogenic amine function as a possible mechanism for the behavioral and cognitive dysfunction observed in the same animals. The study was performed in mPFC and STR, 2 brain areas that contribute to executive, attentional, and motor functions, domains known to be impaired in Mn-exposed children and animals (Beaudin et al., 2013, 2015, 2017; Bouchard et al., 2011; Lucchini et al., 2012). Key questions were whether early developmental Mn exposure exerts effects that persist into adulthood, and whether lifelong exposure would exacerbate the effects of early life exposure. The results revealed that preweaning Mn exposure produced lasting reductions in stimulated outflow of NE in mPFC and DA in STR. In addition, lifelong exposure at the both 25 and 50 mg/kg/day Mn doses reduced NE and DA efflux in mPFC and DA efflux at the 25 mg/kg/day Mn dose in STR. These observations emphasize the strikingly consistent effects of Mn on mPFC NE and the more selective Mn effects on STR DA, signifying that both mPFC NE and STR DA are sensitive targets of Mn exposure. Moreover, subsequent two-factor ANOVAs determined that only the DA responses in mPFC exhibited an effect of Mn exposure duration, with lifelong exposure to the 50 mg/kg/day Mn dose producing reduced DA efflux compared with the same dose restricted to the preweaning period. No other effect of Mn exposure duration or dose was present with biogenic amines in either brain region, indicating the early developmental period is particularly susceptible to elevated Mn exposure. These results provide limited evidence that lifelong Mn exposure exacerbated the effects of early life exposure, and indicate that children exposed to elevated levels of Mn during early postnatal development are at greatest risk for neuronal deficits that persist into adulthood.
Although dopaminergic innervation of the mPFC modulates multiple executive functions, the number of DA synapses in this brain region is nonetheless much less than that of the STR, and the DAT is only sparsely expressed in the mPFC (Ciliax et al., 1995; Sesack et al., 1998). In addition, there is ample evidence that a substantial proportion of DA in mPFC neurons is released from NE terminals (Devoto et al., 2001, 2005; Devoto and Flore, 2006), effectively acting as a cotransmitter. Accordingly, electrical and chemical stimulation of the locus coeruleus results in both NE and DA release in the mPFC (Masana et al., 2011, 2012), and NE-specific transporter inhibitors also increase extracellular DA in mPFC (Kaenmaki et al., 2010). Desipramine, a NE/5-HT reuptake blocker, increases extracellular levels of both NE and DA in mPFC (Higashino et al., 2014) but does not affect striatal or accumbens DA as it has very low affinity for the DAT (Bymaster et al., 2002). Thus, to reliably quantify mPFC NE and DA in the present study, a reuptake blocker was added to the microdialysis perfusion medium to increase extracellular NE and DA levels.
The correspondence between the blood/brain Mn concentrations and the diminished evoked responses in biogenic amine efflux was not easily discriminated. As shown in Figure 3, extracellular NE levels in response to K+ stimulation were reduced in all Mn groups in mPFC (Figure 3A) compared with control values at the same time point, whereas extracellular DA was diminished in both lifelong groups (Figure 3B), and 5-HT release was decreased only in the early life and lifelong low dose Mn groups (Figure 3C, see Summary Figure 6). Figure 5A reveals reductions in extracellular STR DA in all groups, achieving significance in all but the lifelong 50 mg/kg/day group. Within an exposure duration (ie, preweaning or lifelong), the absence of a Mn dose-effect relationship may be attributed to the possibility that both doses over the preweaning period produced somewhat of a ceiling effect on neurotransmitter efflux. This suggestion is consistent with the fact that at PND 24 brain Mn levels were not measurably different between the 25 and 50 mg/kg/day groups (Beaudin et al., 2013, 2017), and with the much higher body Mn burdens produced by the Mn doses over the preweaning period versus the relatively modest increases in brain/blood Mn burden that those same daily oral doses produced during the animals’ prolonged adulthood exposure (Figure 2). Further investigation is necessary to determine how brain Mn levels and toxicokinetics are precisely related to the diminished K+-stimulated biogenic amine outflow in mPFC and STR.
Figure 6.
Summary of analytes and affected groups (↓ = decreased magnitude) in K+-stimulated responses in both brain regions. Abbreviation: NS, no significant change.
The underlying bases for the blunted evoked efflux of biogenic amines in Mn-exposed groups cannot be directly determined from this work. Reductions in neurotransmitter synthesis and vesicular storage or disruption of neurotransmitter vesicle release mechanisms could account for these observations. In work by our group previous to the study reported here, using the same early postweaning Mn exposure regimen (Beaudin et al., 2015, 2017), MPH treatment was found to alleviate the Mn deficits in fine motor function (Beaudin et al., 2015) and impulse control (Beaudin et al., 2017), but exacerbated the Mn deficit in selective attention (Beaudin et al., 2017). MPH is a DA and NE transporter blocker, and we believe these MPH findings are consistent with hypofunctioning catecholaminergic systems in the PFC as observed in the current work. Consistent with these proposed mechanisms, baseline STR extracellular DOPAC levels were reduced in all Mn groups (Table 2), achieving statistical significance in the early life 50 mg/kg/day and lifelong 25 mg/kg/day groups. However, despite significant Mn-related reductions in DA efflux in response to K+ stimulation, Mn-induced changes were not uncovered in the associated K+-stimulated efflux of DOPAC and HVA in STR or mPFC until the end of K+ perfusion, as typified by the metabolite time courses shown in Figure 4. DOPAC is formed by the enzymatic metabolism of DA by monoamine oxidase, primarily in the dopaminergic nerve endings, whereas HVA results from the combined actions of monoamine oxidase and extraneuronal catechol-O-methyltransferase. Extracellular DOPAC/HVA concentrations are typically not reported in microdialysis studies, and thus the interpretation of the metabolite rates of recovery is undetermined other than to reinforce the evidence of Mn-induced alterations in dopaminergic function.
A recent quantitative immunohistochemical analysis of mPFC protein expression in PND 100 littermates of the animals described herein may provide more definition of the mechanisms underlying these Mn-induced effects. These findings indicate that early postnatal Mn exposure leads to lasting reductions in tyrosine hydroxylase (TH), DA D1 receptors, and DA and NE transporters along with a significant increase in DA D2 receptor levels (Conley et al., submitted). This work also determined, in agreement with previous reports by our group (Kern et al., 2010; Kern and Smith, 2011), that the postnatal Mn exposure regimen (Early Life or Lifelong) had no effect on cell number in adult animals. Alternatively, or in addition, these lasting protein expression changes may be mediated via epigenetic mechanisms, given that expressions of several of these proteins (eg, TH, DA D2 receptors, and DA and NE transporters) are in part epigenetically regulated via DNA methylation (Archer et al., 2011; Day et al., 2013; Groleau et al., 2014; Hillemacher et al., 2009). This latter suggestion is supported by studies in human neuroblastoma SH-SY5Y cells showing that Mn exposure led to a hypermethylated TH promoter and downregulated TH gene transcription (Gandhi et al. 2018; Tarale et al., 2017). Prior studies have also reported that preweaning Mn exposure did not alter STR DA autoreceptor sensitivity (McDougall et al., 2013), suggesting that the reductions in stimulated DA outflow observed here may not be attributed to altered autoreceptor activity. Further, studies have reported that preweaning Mn exposure caused significant alterations in D1 (downregulated) and D2 (upregulated) receptor expression in STR and mPFC, but that only the upregulated D2 effects in the mPFC persisted into adulthood (Kern and Smith, 2011; McDougall et al., 2011). Consequently, DA receptor upregulation that might result from reduced DA efflux cannot simply account for the lasting reductions in DA outflow in the STR. Other investigators examined elevated Mn exposure during early development in rats, measuring regional brain biogenic amine concentrations (Amos-Kroohs et al., 2016; Bailey et al., 2019; Sprowles et al., 2018; Vorhees et al., 2014). Results with those endpoints cannot be directly compared with the evoked outflow in extracellular fluid produced by microdialysis, but it is apparent in those studies that NE and DA are most sensitive to elevated Mn exposure and that in general, Mn-induced alterations in brain NE/DA concentrations observed in early development do not persist into adulthood.
Additional two-way ANOVAs with the 4 Mn groups and incorporating Mn dose and exposure duration as the main factors demonstrated the absence of any main effect of dose for any neurotransmitter in either brain region. However, there was a significant main effect of exposure duration for DA in mPFC, suggesting that lifelong exposure to this higher dose was more disruptive to DA function in the mPFC than the preweaning exposure alone. These findings strongly suggest that the Mn-induced changes in biogenic amine responses largely emanate from exposure occurring during early postnatal development, and not from the elevated postweaning lifelong Mn exposure, even though the latter continued well beyond a year after weaning. This notion is consistent with the fact that blood and brain Mn levels were most elevated during the preweaning period in early development, and only marginally elevated if at all during adulthood as noted above (Figure 2). It follows then that the altered biogenic amine responses cannot be due to Mn+2 inhibition of Ca+2 influx through synaptic voltage-sensitive Ca+2 channels, as brain Mn levels were at control levels when the early life groups underwent microdialysis testing. Rather, this evidence may suggest lasting changes, possibly through epigenetic mechanisms, to biogenic amine synthesis resulting from Mn exposure during early postnatal development. TH, the rate-limiting synthetic enzyme for catecholamines, is subject to epigenetic, transcriptional, and post-transcriptional regulation (eg, Banerjee et al., 2013; Tekin et al., 2014), but the effects of developmental and/or long term exposure to Mn in vivo on this enzyme have received little attention.
Previous work has demonstrated clearly that catecholaminergic systems in mPFC are important for control of attentional processes, behavioral inhibition, working memory, arousal level, and emotional self-regulation (Arnsten, 2009; Arnsten and Pliszka, 2011; Brennan and Arnsten, 2008; Raz and Buhle, 2006). Reports of Mn-induced impairments in skilled forelimb performance and their mitigation by chronic MPH administration (Beaudin et al., 2013, 2015), as well as impaired learning and memory in a radial arm maze test (Kern et al., 2010), have been reported at Mn doses and durations identical to those used in the current work. Furthermore, prior to microdialysis testing, the same animals used in the present study were shown to have specific deficits in arousal regulation and selective attention using a series of 5-choice serial reaction time tasks (Beaudin et al., 2017). In sum, these studies found that early postnatal Mn exposure caused permanent disruption in fine motor function, selective and focused attention, and in arousal regulation. This body of evidence is consistent with the Mn-related decreases in evoked NE and DA responses in mPFC and STR observed in the current work, including specifically the diminished NE responses in mPFC shown in Figure 3A and the attentional deficits in the same Mn-exposed animals (Beaudin et al., 2017). Further, the reduced striatal DA activity observed in the present study provides a plausible mechanism for the impaired fine motor function reported in these animals (Beaudin et al., 2013), particularly in light of the ability of MPH to fully alleviate those motor deficits (Beaudin et al., 2015). DA D1 receptors in PFC have been shown to facilitate working memory (Mitrano et al., 2014), and there is evidence that α2A- and β2-adrenergic receptors in mPFC enhance cognitive function (He et al., 2014) and long term potentiation (Zhou et al., 2013), respectively. These may be the receptors and pathways that transduce the catecholamine activity diminished by Mn into the reported alterations in executive function behavior.
In summary, established environmentally relevant preweaning and continuous lifelong Mn exposure protocols have been utilized to demonstrate persistent reductions in neurotransmission across 2 brain regions and 3 biogenic amines in adult rats. These results are consistent with previous reports—some utilizing the same animals—employing behavioral and pharmacological approaches with the same or similar exposure regimens. Subsequent work might address similar experimental measures during early development when the differences in brain Mn levels between exposed and control groups are more clearly distinct.
DECLARATION OF CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
National Institute of Environmental Health Sciences (R01 ES018990 to D.R.S.).
ACKNOWLEDGMENT
The authors would like to thank Tom Jursa for analytical assistance.
REFERENCES
- Amos-Kroohs R. M., Davenport L. L., Gutierrez A., Hufgard J. R., Vorhees C. V., Williams M. T. (2016). Developmental manganese exposure in combination with developmental stress and iron deficiency: Effects on behavior and monoamines. Neurotoxicol. Teratol. 56, 55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer T., Berman M. O., Blum K. (2011). Epigenetics in developmental disorder: ADHD and endophenotypes. J. Genet. Syndr. Gene Ther. 2, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten A. F. (2006). Fundamentals of attention-deficit/hyperactivity disorder: Circuits and pathways. J. Clin. Psychiatry 67(Suppl 8), 7–12. [PubMed] [Google Scholar]
- Arnsten A. F. T. (2009). The emerging neurobiology of attention deficit hyperactivity disorder: The key role of the prefrontal association cortex. J. Pediatr. 154, S1–S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten A. F. T., Pliszka S. R. (2011). Catecholamine influences on prefrontal cortical function: Relevance to treatment of attention deficit/hyperactivity disorder and related disorders. Pharmacol. Biochem. Behav. 99, 211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner J. L., Aschner M. (2005). Nutritional aspects of manganese homeostasis. Mol. Aspects Med. 26, 353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey R. A., Gutierrez A., Kyser T. L., Hemmerle A. M., Hufgard J. R., Seroogy K. B., Vorhees C. V., Williams M. T. (2019). Effects of preweaning manganese in combination with adult striatal dopamine lesions on monoamines, BDNF, TrkB, and cognitive function in Sprague-Dawley rats. Neurotox. Res. 35, 606–620. [DOI] [PubMed] [Google Scholar]
- Banerjee K., Akiba Y., Baker H., Cave J. W. (2013). Epigenetic control of neurotransmitter expression in olfactory bulb interneurons. Int. J. Dev. Neurosci. 31, 415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow P. J. (1983). A pilot study on the metal levels in the hair of hyperactive children. Med. Hypotheses 11, 309–318. [DOI] [PubMed] [Google Scholar]
- Beaudin S. A., Nisam S., Smith D. R. (2013). Early life versus lifelong oral manganese exposure differently impairs skilled forelimb performance in adult rats. Neurotoxicol. Teratol. 38, 36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudin S. A., Strupp B. J., Lasley S. M., Fornal C. A., Mandal S., Smith D. R. (2015). Oral methylphenidate alleviates the fine motor dysfunction caused by chronic postnatal manganese exposure in adult rats. Toxicol. Sci. 144, 318–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudin S. A., Strupp B. J., Strawderman M., Smith D. R. (2017). Early postnatal manganese exposure causes lasting impairment of selective and focused attention and arousal regulation in adult rats. Environ. Health Perspect. 125, 230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard M., Laforest F., Vandelac L., Bellinger D., Mergler D. (2007). Hair manganese and hyperactive behaviors: Pilot study of school-age children exposed through tap water. Environ. Health Perspect. 115, 122–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard M. F., Sauve S., Barbeau B., Legrand M., Brodeur M. E., Bouffard T., Limoges E., Bellinger D. C., Mergler D. (2011). Intellectual impairment in school-age children exposed to manganese from drinking water. Environ. Health Perspect. 119, 138–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan A. R., Arnsten A. F. T. (2008). Neuronal mechanisms underlying attention deficit hyperactivity disorder: The influence of arousal on prefrontal cortical function. Ann. N. Y. Acad. Sci. 1129, 236–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenneman K. A., Cattley R. C., Ali S. F., Dorman D. C. (1999). Manganese-induced developmental neurotoxicity in the CD rat: Is oxidative damage a mechanism of action? NeuroToxicology 20, 477–487. [PubMed] [Google Scholar]
- Bymaster F. P., Katner J. S., Nelson D. L., Hemrick-Luecke S. K., Threlkeld P. G., Heiligenstein J. H., Morin S. M., Gehlert D. R., Perry K. W. (2002). Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: A potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology 27, 699–711. [DOI] [PubMed] [Google Scholar]
- Chandra S. V., Shukla G. S., Saxena D. K. (1979). Manganese-induced behavioral dysfunction and its neurochemical mechanism in growing mice. J. Neurochem. 33, 1217–1221. [DOI] [PubMed] [Google Scholar]
- Ciliax B. J., Heilman C., Demchyshyn L. L., Pristupa Z. B., Ince E., Hersch S. M., Niznik H. B., Levey A. I. (1995). The dopamine transporter: immunochemical characterization and localization in brain. J. Neurosci. 15, 1714–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day J. J., Childs D., Guzman-Karlsson M. C., Kibe M., Moulden J., Song E., Tahir A., Sweatt J. D. (2013). DNA methylation regulates associative reward learning. Nat. Neurosci. 16, 1445–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoto P., Flore G. (2006). On the origin of cortical dopamine: Is it a co-transmitter in noradrenergic neurons? Curr. Neuropharmacol. 4, 115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoto P., Flore G., Pani L., Gessa G. L. (2001). Evidence for co-release of noradrenaline and dopamine from noradrenergic neurons in the cerebral cortex. Mol. Psychiatry 6, 657–664. [DOI] [PubMed] [Google Scholar]
- Devoto P., Flore G., Saba P., Fa M., Gessa G. L. (2005). Stimulation of the locus coeruleus elicits noradrenaline and dopamine release in the medial prefrontal and parietal cortex. J. Neurochem. 92, 368–374. [DOI] [PubMed] [Google Scholar]
- Dorman D. C., Struve M. F., Vitarella D., Byerly F. L., Goetz J., Miller R. (2000). Neurotoxicity of manganese chloride in neonatal and adult CD rats following subchronic (21-day) high-dose oral exposure. J. Appl. Toxicol. 20, 179–187. [DOI] [PubMed] [Google Scholar]
- Dorner K., Dziadzka S., Hohn A., Sievers E., Oldigs H. D., Schulz-Lell G., Schaub J. (1989). Longitudinal manganese and copper balances in young infants and preterm infants fed on breast-milk and adapted cow’s milk formulas. Br. J. Nutr. 61, 559–572. [DOI] [PubMed] [Google Scholar]
- Ericson J. E., Crinella F. M., Clarke-Stewart K. A., Allhusen V. D., Chan T., Robertson R. T. (2007). Prenatal manganese levels linked to childhood behavioral disinhibition. Neurotoxicol. Teratol. 29, 181–187. [DOI] [PubMed] [Google Scholar]
- Gandhi D., Sivanesan S., Kannan K. (2018). Manganese-induced neurotoxicity and alterations in gene expression in human neuroblastoma SH-SY5y cells. Biol. Trace Elem. Res. 183, 245–253. [DOI] [PubMed] [Google Scholar]
- Goto Y., Grace A. A. (2005). Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nat. Neurosci. 8, 805–812. [DOI] [PubMed] [Google Scholar]
- Groleau P., Joober R., Israel M., Zeramdini N., DeGuzman R., Steiger H. (2014). Methylation of the dopamine D2 receptor (DRD2) gene promoter in women with a bulimia-spectrum disorder: Associations with borderline personality disorder and exposure to childhood abuse. J. Psychiatr. Res. 48, 121–127. [DOI] [PubMed] [Google Scholar]
- He X. T., Yu J., Li B. M., Zhang X. H. (2014). The expression of alpha 2A-adrenoceptors in the calcium-binding protein immunoreactive interneurons in rat prefrontal cortex. Acta Physiol. Sin. 66, 537–544. [PubMed] [Google Scholar]
- Higashino K., Ago Y., Umehara M., Kita Y., Fujita K., Takuma K., Matsuda T. (2014). Effects of acute and chronic administration of venlafaxine and desipramine on extracellular monoamine levels in the mouse prefrontal cortex and striatum. Eur. J. Pharmacol. 729, 86–93. [DOI] [PubMed] [Google Scholar]
- Hillemacher T., Frieling H., Hartl T., Wilhelm J., Kornhuber J., Bleich S. (2009). Promoter specific methylation of the dopamine transporter gene is altered in alcohol dependence and associated with craving. J. Psychiatr. Res. 43, 388–392. [DOI] [PubMed] [Google Scholar]
- Kaenmaki M., Tammimaki A., Myohanen T., Pakarinen K., Amberg C., Karayiorgou M., Gogos J. A., Mannisto P. T. (2010). Quantitative role of COMT in dopamine clearance in the prefrontal cortex of freely moving mice. J. Neurochem. 114, 1745–1755. [DOI] [PubMed] [Google Scholar]
- Keen C. L., Bell J. G., Lonnerdal B. (1986). The effect of age on manganese uptake and retention from milk and infant formulas in rats. J. Nutr. 116, 395–402. [DOI] [PubMed] [Google Scholar]
- Keen C. L., Lonnerdal B., Clegg M., Hurley L. S. (1981). Developmental changes in composition of rat milk: Trace elements, minerals, protein, carbohydrate and fat. J. Nutr. 111, 226–236. [DOI] [PubMed] [Google Scholar]
- Kern C. H., Smith D. R. (2011). Preweaning Mn exposure leads to prolonged astrocyte activation and lasting effects on the dopaminergic system in adult male rats. Synapse 65, 532–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern C. H., Stanwood G. D., Smith D. R. (2010). Preweaning manganese exposure causes hyperactivity, disinhibition, and spatial learning and memory deficits associated with altered dopamine receptor and transporter levels. Synapse 64, 363–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostial K., Kello D., Jugo S., Rabar I., Maljkovic T. (1978). Influence of age on metal metabolism and toxicity. Environ. Health Perspect. 25, 81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai J. C., Leung T. K., Lim L. (1984). Differences in the neurotoxic effects of manganese during development and aging: Some observations on brain regional neurotransmitter and non-neurotransmitter metabolism in a developmental rat model of chronic manganese encephalopathy. NeuroToxicology 5, 37–47. [PubMed] [Google Scholar]
- Leo D., Sorrentino E., Volpicelli F., Eyman M., Greco D., Viggiano D., di Porzio U., Perrone-Capano C. (2003). Altered midbrain dopaminergic neurotransmission during development in an animal model of ADHD. Neurosci. Biobehav. Rev. 27, 661–669. [DOI] [PubMed] [Google Scholar]
- Ljung K., Vahter M. (2007). Time to re-evaluate the guideline value for manganese in drinking water? Environ. Health Perspect. 115, 1533–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonnerdal B. (1997). Effects of milk and milk components on calcium, magnesium, and trace element absorption during infancy. Physiol. Rev. 77, 643–669. [DOI] [PubMed] [Google Scholar]
- Lucchini R. G., Guazzetti S., Zoni S., Donna F., Peter S., Zacco A., Salmistraro M., Bontempi E., Zimmerman N. J., Smith D. R. (2012). Tremor, olfactory and motor changes in Italian adolescents exposed to historical ferro-manganese emission. NeuroToxicology 33, 687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masana M., Bortolozzi A., Artigas F. (2011). Selective enhancement of mesocortical dopaminergic transmission by noradrenergic drugs: Therapeutic opportunities in schizophrenia. Int. J. Neuropsychopharmacol. 14, 53–68. [DOI] [PubMed] [Google Scholar]
- Masana M., Castane A., Santana N., Bortolozzi A., Artigas F. (2012). Noradrenergic antidepressants increase cortical dopamine: Potential use in augmentation strategies. Neuropharmacology 63, 675–684. [DOI] [PubMed] [Google Scholar]
- McDougall S. A., Der-Ghazarian T., Britt C. E., Varela F. A., Crawford C. A. (2011). Postnatal manganese exposure alters the expression of D2L and D2S receptor isoforms: Relationship to PKA activity and Akt levels. Synapse 65, 583–591. [DOI] [PubMed] [Google Scholar]
- McDougall S. A., Mohd-Yusof A., Kaplan G. J., Abdulla Z. I., Lee R. J., Crawford C. A. (2013). Postnatal manganese exposure does not alter dopamine autoreceptor sensitivity in adult and adolescent male rats. Eur. J. Pharmacol. 706, 4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall S. A., Reichel C. M., Farley C. M., Flesher M. M., Der-Ghazarian T., Cortez A. M., Wacan J. J., Martinez C. E., Varela F. A., Butt A. E., et al. (2008). Postnatal manganese exposure alters dopamine transporter function in adult rats: potential impact on nonassociative and associative processes. Neuroscience 154, 848–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrano D. A., Pare J. F., Smith Y., Weinshenker D. (2014). D1-dopamine and alpha1-adrenergic receptors co-localize in dendrites of the rat prefrontal cortex. Neuroscience 258, 90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. (2011). Guide for the Care and Use of Laboratory Animals, The National Academies Press, Washington, DC. [Google Scholar]
- Packard M. G., Knowlton B. J. (2002). Learning and memory functions of the Basal Ganglia. Annu. Rev. Neurosci. 25, 563–593. [DOI] [PubMed] [Google Scholar]
- Pappas B. A., Zhang D., Davidson C. M., Crowder T., Park G. A., Fortin T. (1997). Perinatal manganese exposure: Behavioral, neurochemical, and histopathological effects in the rat. Neurotoxicol. Teratol. 19, 17–25. [DOI] [PubMed] [Google Scholar]
- Paxinos G., Watson C. (1997). The Rat Brain in Stereotaxic Coordinates, Academic Press, San Diego, CA. [Google Scholar]
- Pennington J. A., Young B. E. (1991). Total diet study nutritional elements, 1982–1989. J. Am. Diet Assoc. 91, 179–183. [PubMed] [Google Scholar]
- Raz A., Buhle J. (2006). Typologies of attentional networks. Nat. Rev. Neurosci. 7, 367–379. [DOI] [PubMed] [Google Scholar]
- Reichel C. M., Wacan J. J., Farley C. M., Stanley B. J., Crawford C. A., McDougall S. A. (2006). Postnatal manganese exposure attenuates cocaine-induced locomotor activity and reduces dopamine transporters in adult male rats. Neurotoxicol. Teratol. 28, 323–332. [DOI] [PubMed] [Google Scholar]
- Sesack S. R., Hawrylak V. A., Matus C., Guido M. A., Levey A. I. (1998). Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J. Neurosci. 18, 2697–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. R., Osterloh J. D., Niemeyer S., Flegal A. R. (1992). Stable isotope labeling of lead compartments in rats with ultralow lead concentrations. Environ. Res. 57, 190–207. [DOI] [PubMed] [Google Scholar]
- Sprowles J. L. N., Amos-Kroohs R. M., Braun A. A., Sugimoto C., Vorhees C. V., Williams M. T. (2018). Developmental manganese, lead, and barren cage exposure have adverse long-term neurocognitive, behavioral and monoamine effects in Sprague-Dawley rats. Neurotoxicol. Teratol. 67, 50–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takser L., Mergler D., Hellier G., Sahuquillo J., Huel G. (2003). Manganese, monoamine metabolite levels at birth, and child psychomotor development. NeuroToxicology 24, 667–674. [DOI] [PubMed] [Google Scholar]
- Tarale P., Sivanesan S., Daiwile A. P., Stoger R., Bafana A., Naoghare P. K., Parmar D., Chakrabarti T., Kannan K. (2017). Global DNA methylation profiling of manganese-exposed human neuroblastoma SH-SY5Y cells reveals epigenetic alterations in Parkinson’s disease-associated genes. Arch. Toxicol. 91, 2629–2641. [DOI] [PubMed] [Google Scholar]
- Tekin I., Roskoski R. Jr, Carkaci-Salli N., Vrana K. E. (2014). Complex molecular regulation of tyrosine hydroxylase. J. Neural Transm. (Vienna) 121, 1451–1481. [DOI] [PubMed] [Google Scholar]
- Tran T. T., Chowanadisai W., Lonnerdal B., Le L., Parker M., Chicz-Demet A., Crinella F. M. (2002). Effects of neonatal dietary manganese exposure on brain dopamine levels and neurocognitive functions. NeuroToxicology 23, 645–651. [DOI] [PubMed] [Google Scholar]
- Vorhees C. V., Graham D. L., Amos-Kroohs R. M., Braun A. A., Grace C. E., Schaefer T. L., Skelton M. R., Erikson K. M., Aschner M., Williams M. T. (2014). Effects of developmental manganese, stress, and the combination of both on monoamines, growth, and corticosterone. Toxicol. Rep. 1, 1046–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman G. A., Liu X., Parvez F., Ahsan H., Levy D., Factor-Litvak P., Kline J., van Geen A., Slavkovich V., Lolacono N. J., et al. (2006). Water manganese exposure and children’s intellectual function in Araihazar, Bangladesh. Environ. Health Perspect. 114, 124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf A., Wright R., Amarasiriwardena C., Bellinger D. (2002). A child with chronic manganese exposure from drinking water. Environ. Health Perspect. 110, 613–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H. C., Sun Y. Y., Cai W., He X. T., Yi F., Li B. M., Zhang X. H. (2013). Activation of beta2-adrenoceptor enhances synaptic potentiation and behavioral memory via cAMP-PKA signaling in the medial prefrontal cortex of rats. Learn. Mem. 20, 274–284. [DOI] [PubMed] [Google Scholar]
- Zlotkin S. H., Buchanan B. E. (1986). Manganese intakes in intravenously fed infants—Dosage and toxicity studies. Biol. Trace Elem. Res. 9, 271. [Google Scholar]