Abstract
DNA diversity varies across the genome of many species. Variation in diversity across a genome might arise from regional variation in the mutation rate, variation in the intensity and mode of natural selection, and regional variation in the recombination rate. We show that both noncoding and nonsynonymous diversity are positively correlated to a measure of the mutation rate and the recombination rate and negatively correlated to the density of conserved sequences in 50 kb windows across the genomes of humans and nonhuman homininae. Interestingly, we find that although noncoding diversity is equally affected by these three genomic variables, nonsynonymous diversity is mostly dominated by the density of conserved sequences. The positive correlation between diversity and our measure of the mutation rate seems to be largely a direct consequence of regions with higher mutation rates having more diversity. However, the positive correlation with recombination rate and the negative correlation with the density of conserved sequences suggest that selection at linked sites also affect levels of diversity. This is supported by the observation that the ratio of the number of nonsynonymous to noncoding polymorphisms is negatively correlated to a measure of the effective population size across the genome. We show these patterns persist even when we restrict our analysis to GC-conservative mutations, demonstrating that the patterns are not driven by GC biased gene conversion. In conclusion, our comparative analyses describe how recombination rate, gene density, and mutation rate interact to produce the patterns of DNA diversity that we observe along the hominine genomes.
Keywords: recombination rate, gene density, genetic diversity, purifying selection, great apes
Introduction
The level of genetic variation is known to vary across the genome of many species and this depends on genomic characteristics such as recombination, gene density and mutation rate. This was first demonstrated by Begun and Aquadro (1992) who showed that putatively neutral genetic diversity was correlated to the rate of recombination across the genome of Drosophila melanogaster. This has subsequently been observed in species as diverse as humans and tomatoes (reviewed by Cutter and Payseur 2013).
Variation in diversity across the genome of a given species might arise from variation in the mutation rate, selection, and recombination rate. The mutation rate can affect the level of diversity both directly and indirectly. Directly, the level of genetic diversity is expected to depend upon the rate of mutational input; the higher the mutation rate, the more diversity there is expected to be. It can also have an indirect effect by increasing the frequency of selection at linked sites, which is described below. Natural selection can also affect the level of genetic diversity both directly and indirectly. Direct selection tends to either decrease or increase diversity at the sites at which it is acting, depending on whether the selection is either negative or positive, particularly if there is balancing selection. However, in general, selection tends to act indirectly reducing diversity at linked sites through the processes of genetic hitch-hiking (HH) (Smith and Haigh 1974) and background selection (BGS) (Charlesworth et al. 1993). Genetic HH also has the effect of moving a locus away from a state of quasi-equilibrium; after a selective sweep, deleterious genetic variation approaches its equilibrium value more rapidly than neutral variation, leading to a disproportionate amount of the diversity being deleterious (Gordo and Dionisio 2005; Pennings et al. 2014; Do et al. 2015; Brandvain and Wright 2016; Castellano et al. 2018). Finally, recombination also affects the level of genetic diversity both directly and indirectly. In humans and all organisms that have been studied, gene conversion appears to be GC-biased (gBGC) (Eyre-Walker 1993; Marais et al. 2001; Galtier et al. 2001; Marais 2003; Duret and Galtier 2009; Pessia et al. 2012). This is counter to the pattern of mutation, which is AT-biased. In such a system in which gBGC and mutation are in opposite direction, an increase in gBGC (from no gBGC) is expected to slightly increase the levels of diversity before reducing them, when gBGC becomes strong (McVean and Charlesworth 1999). However, cross overs induced by recombination tend to act indirectly by reducing the effect of linked selection across loci increasing the local levels of genetic diversity (Berry and Barbadilla 2000).
If selection at linked sites is pervasive and acts genome-wide, this should be visible as correlations between DNA diversity and factors affecting the intensity of selection at linked sites, such as recombination rate, gene density, and mutation rate. In this work, we return to the question of whether selection at linked sites and mutation rate variation has an effect on levels of DNA sequence diversity and the efficiency of purifying selection along the autosomes of humans and our closest living relatives, the homininae subfamily: Humans, bonobos, chimpanzees, and gorillas. The role that mutation, selection, and recombination rate play in determining the levels of genetic diversity and the efficiency of natural selection across the nonhuman homininae genomes remains unresolved. In humans, it was observed many years ago that levels of diversity at putatively neutral sites are correlated to the rate of recombination (Lercher and Hurst 2002; Hellmann et al. 2005). Since the rate of substitution is also correlated to the rate of recombination it seemed likely that at least part of the correlation between diversity and the rate of recombination was due to a mutagenic effect of recombination. There is now good evidence that recombination is mutagenic in humans (Pratto et al. 2014; Francioli et al. 2015; Arbeithuber et al. 2015; Halldorsson et al. 2019) and recent analyses of the correlation between diversity and the rate of mutation, as inferred from rates of de novo mutations (DNMs) in human trios, suggests that much, but not all, of the variation in diversity across the human genome can be explained by variation in the rate of mutation at the 100 kb and 1 MB scale (Smith et al. 2018). However, several lines of evidence suggest that selection at linked sites may also affect neutral and selected diversity across the human genome. First, it has been observed that levels of diversity are negatively correlated to gene density (Payseur and Nachman 2002). Second, levels of noncoding diversity are lower near functional DNA elements in humans and nonhuman primates (McVicker et al. 2009; Enard et al. 2014; Nam et al. 2017). Third, the rate of nonsynonymous to synonymous substitution is positively correlated to gene (and exon) density (Bullaughey et al. 2008). Fourth, Hussin et al. (2015) showed that exons in regions of low recombination are significantly enriched for deleterious and disease-associated variants consistent with variation in the intensity of selection at linked sites generating variation in the efficiency of purifying selection along the genome.
In our study, we find that the three genomic variables: Recombination rate, mutation rate, and the density of conserved sites are correlated to each other. We show that the levels of both putatively neutral noncoding and putatively selected nonsynonymous variation are correlated to those genomic variables in most homininae, but that the relative importance of each genomic variable is different for noncoding and nonsynonymous polymorphisms. Interestingly, we also find evidence that indicates variation in the efficiency of negative selection likely generated by interference among deleterious mutations in the genome of all the homininae. Finally, we find little impact of gBGC in our analyses and conclusions when interrogating only GC-conservative mutations.
Materials and Methods
Population Genomic Data
SNP calls from the autosomes were retrieved from (Prado-Martinez et al. 2013) for five great ape populations: Homo sapiens, Pan paniscus, Pan troglodytes ellioti, Pan troglodytes verus, and Gorilla gorilla gorilla. Hereafter, we refer to these species as humans, bonobos, Nigeria-Cameroon chimpanzees, western chimpanzees and gorillas, respectively. We analyzed eight chromosomes per position in all populations (see details below). In Prado-Martinez et al. (2013) all reads were mapped to the human reference genome (hg18), but we used lift-over to hg19/GRCh37.75 coordinates to take advantage of more recent functional annotations (see below). To avoid errors introduced by mismapping due to paralogous variants and repetitive sequences, we also restrict all analyses to a set of sites with a unique mapping to the human genome (Cagan et al. 2016). Additionally, we require positions to have at least 5-fold coverage in all individuals per species. Only the resulting set of sites are used in further analyses (supplementary table 1, Supplementary Material online). The final list of analyzed positions is available upon request.
Genome Annotation and Identification of Putatively Neutral Noncoding Sites
Genomes were annotated using the SnpEff and SnpSift software (Cingolani et al. 2012) (version 4.3 m, last accessed June 2017) and the human database GRCh37.75. We extracted 0-fold degenerate sites from the codon information provided by SnpEff (4-fold, 2-fold, and 3-fold degenerate sites are discarded). We assume that the degeneracy and gene annotations are identical across species. In order to obtain putatively neutral non-coding (NC) sites, we applied stringent filters: 1) We kept sites annotated only as intronic or intergenic (splicing sites, UTRs, coding, or transcribed noncoding genes are discarded). 2) We removed GERP elements (Davydov et al. 2010) and positions with a PhastCons score > 5% in primates and/or in 100 vertebrate species (Siepel et al. 2005). In this way, we removed conserved sites at different phylogenetic depths. Note that GERP elements were calculated in a multiple species alignment of the human genome to 33 other mammalian species (the most distant mammalian species is Platypus), whereas the PhastCons scores used here are based on a multiple species alignment of the human genome to 9 other primates (the most distant is bushbaby) and a multiple species alignment of the human genome to 99 other vertebrate species (the most distant is zebrafish). 3) Some sites might have become functional very recently. Thus, we removed DNase I hypersensitivity sites across multiple human tissues (Song et al. 2011) (downloaded from: http://ftp.ebi.ac.uk/pub/databases/ensembl/encode/integration_data_jan2011/byDataType/openchrom/jan2011/combined_peaks/). 4) Predicted transcription factor binding sites detected in humans were also excluded (Cingolani et al. 2012). 5) Hypermutable CpG sites in humans and the rest of species were excluded to remove variation in mutation rate due to variation in GC-content. This last filter was also applied to coding sites and mutation rate estimates (see below).
Genomic Windows and Statistics
We split the autosomes in nonoverlapping windows of 50 kb, and for each window we estimate: 1) diversity at putatively neutral NC sites, 2) 0-fold degenerate (N) sites, 3) GC-conservative substitution rate at NC sites (our main proxy of the mutation rate), 4) the density of conserved sites, 5) the rate of recombination (RR, mainly crossing overs), and 6) the rate of DNMs (an alternative proxy of the mutation rate).
Recombination Maps and the Density of Conserved Sites
Population recombination rate estimates for nonhuman great apes are retrieved from Stevison et al. (2016) and Auton et al. (2012) and human population recombination rates from HapMap (Myers et al. 2005). These recombination maps are LD-based representing both crossing over and gene conversion events. However, as gene conversion tracts tend to be very small in humans (50–150 bp) (Jeffreys and May 2004; Padhukasahasram and Rannala 2013), these maps measure mainly crossing over events. Blocks for each nonhuman genome that are syntenic with human were identified as in Stevison et al. (2016). To estimate the density of conserved sites we used as before GERP elements (Davydov et al. 2010) and PhastCons scores (Siepel et al. 2005), but this time we labeled and count all GERP elements and/or positions with a PhastCons score > 50% in primates and/or positions with a PhastCons score > 50% in 100 vertebrate species in a given 50 kb window. We discarded the number of unsequenced nucleotides in the human reference genome to estimate the density of conserved sites.
Polymorphism and Mutation Rate Estimates
For a fair comparison between species, we downsampled our population genomic data to eight haploid chromosomes per position. Positions called in <8 chromosomes were excluded. For each window we counted the total number of analyzable polymorphic sites (LP, N and LP, NC) and the number of segregating sites (SN and SNC) to get the Watterson estimator (θ, Watterson 1975) for NC and N sites, respectively. We did this for all point mutations and for GC-conservative point mutations.
For divergence estimates, we counted the total number of analyzable divergent NC sites (LD, NC) from a multiple species alignment between one randomly sampled Nigeria-Cameroon chimpanzee, western chimpanzee, bonobo, gorilla, and human chromosome. This multiple species alignment is generated from Prado-Martinez et al. (2013) original VCF file. Then, to estimate our proxy of the mutation rate (dNC) in each window, and given that there are few GC-conservative substitutions per window, we sum all GC-conservative substitutions (DNC) occurring in the homininae tree and divide it by LD, NC. Thus, our proxy of the mutation rate is the same for all species and it is unaffected by gBGC. Given that species specific substitution rates at 50 kb are strongly correlated between species pairs (supplementary table 2, Supplementary Material online) we believe this is a reasonable approach to gain statistical power without losing resolution. For some validation analyses, we also used the rate of DNMs per window in humans taking our DNMs from the studies of Jónsson et al. (2017), Wong et al. (2016), and Francioli et al. (2015) as an alternative proxy of the regional mutation rate. We only considered nonCpG DNMs but this time we considered both GC-conservative and nonGC-conservative DNMs.
Hypergeometric Sampling and Grouping
We are interested in the effect of the mutation rate, the rate of recombination and the density of conserved sites on the efficiency of negative selection across the homininae genomes. We consider first the log(θN/θNC) and its relationship to the mutation rate, the rate of recombination and the density of conserved sites, and then its relationship to a measure of the local effective population size, log(θNC/dNC). log(θN/θNC) and log(θNC/dNC) are undefined if θN, θNC, or dNC are zero, we, therefore, combined data across windows in the following manner. We split the number of noncoding polymorphisms (SNC) in each window into three parts using a hypergeometric distribution (see supplementary fig. 1, Supplementary Material online for details). We used θNC, 1 to rank and bin windows into 50 groups. We then used θNC, 2 as our unbiased measure of the noncoding diversity and used θNC, 3 to estimate the ratio θN/θNC. We applied two methods to combine data across windows. In both methods we split SNC into three statistically independent estimates using a hypergeometric distribution. In the first method, we include all windows that have NC sites, irrespective of whether they have coding sites. In the second method, we only include windows with coding sites. The second method yields ∼43% of the data-points of the first method due to the requirement that windows have both coding and NC sites. The rationale for using two methods is that, for the noncoding analyses, conserved NC sites might be a source of selection at linked sites. We present results from method 1 for noncoding results and from method 2 for nonsynonymous results. There are some outlier regions with very high θNC and dNC values. These have a disproportionate influence over the statistics. We hypothesize that those high diversity regions might overlap with genes under balancing selection and/or low complexity repetitive regions. We excluded all windows overlapping with the MHC locus and other top candidate genes under balancing selection in great apes retrieved from (Azevedo et al. 2015). To remove low complexity regions we analyzed positions outside the DAC blacklisted regions (from: https://genome.ucsc.edu/cgi-bin/hgFileUi?db=hg19&g=wgEncodeMapability). This combined filtering resulted in the removal of all outlier regions and the exclusion of ∼3% of the windows and 10% of the sites.
Expected Relationship between θN/θNC and θNC
θ N and θNC are expected to be correlated through variation in the mutation rate and/or the Ne. If we assume that the distribution of fitness effects (DFE) of new deleterious mutations follows a gamma distribution, then, under free recombination, the slope (b) of the relationship between θN/θNC and θNC in a log–log scale informs us about the source of this variation (Welch et al. 2008). If there is no variation in Ne and all variation in θN and θNC is due to variation in mutation rate, then we expect b = –1. In contrast, if all the variation in θN and θNC comes from variation in the Ne, then we expect b = –β (Welch et al. 2008), where β is the shape parameter of the gamma distribution. Finally, if θN and θNC are independent we expect b = 0. Forward simulations with BGS (that is, limited recombination plus deleterious and neutral mutations) and variation in Ne among loci have shown that the shape of the deleterious DFE can be successfully estimated with the slope between log(θN/θNC) and log(θNC) after correcting for variation in the mutation rate (James et al. 2017). Castellano et al. (2018) showed that the slope is overestimated when HHs are not accounted for due to the faster recovery of the levels of deleterious variation compared with the levels of neutral variation. Recently, this relationship has been studied in >50 other species confirming that beneficial mutations must be invoked to explain the slope in large Ne species (Chen et al. 2019).
Statistical Analyses
All statistical analyses were performed within the R framework (version 3.4.4). Here, we want to explain how our dependent variable, the number of SNPs in a given window, which is discrete and overdispersed data (supplementary fig. 2, Supplementary Material online), is related to our three genomic variables. To do that, we implemented a negative binomial regression by means of the R function glm.nb from the R package MASS (Venables and Ripley 2002). We modeled the log of the expected number of noncoding or nonsynonymous SNPs as a function of the predictor variables; recombination rate, dNC, the density of conserved sites and the number of noncoding or 0-fold degenerate sites in a given 50 kb window, respectively. We can interpret the negative binomial regression coefficient as follows: For one unit of change in the predictor variable, the difference in the logs of expected counts of the response variable is expected to change by the respective regression coefficient, given the other predictor variables in the model are held constant. To assess the relative importance of each genomic variable, we reported standardized regression coefficients to make variances of dependent and independent variables equal 1. These standardized coefficients refer to how many standard deviations the log of the expected number of SNPs will change, per standard deviation increase in the predictor variable. To explain the variation in our statistic of the efficiency of negative selection, log(θN/θNC), we used a standard multiple linear regression using the R function lm. As before, the standardized regression coefficients are used to assess the relative importance of each genomic variable. Finally, to estimate the variance inflation factor (VIF) we used the R function vif from the package car (Fox and Weisberg 2011) and to perform the bivariate correlations we used the R function cor.test and the nonparametric Spearman method (Hollander et al. 2013).
Results
Genomic Determinants of Noncoding and Nonsynonymous Diversity
We are interested in how genetic variation is distributed along the genomes of the homininae and in particular, the role that selection at linked sites and mutation rate variation might play in this distribution. To see whether these patterns are consistent across all homininae, we used the data of Prado-Martinez et al. (2013) sampling eight random chromosomes per position in all five species and populations: Humans, Nigeria-Cameroon chimpanzees, western chimpanzees, bonobos, and gorillas. To allow an unbiased comparison with the other homininae we use the cosmopolitan human sample from the Great Apes Project. Supplementary table 1, Supplementary Material online shows the summary statistics of our analyzed data set. We use the same density of conserved sites and mutation rate for all species (see supplementary table 2, Supplementary Material online for the correlation of lineage specific substitution rates between pairs of species), whereas the estimates of recombination rate are population-specific and come from publicly available recombination rate maps (Myers et al. 2005; Auton et al. 2012; Stevison et al. 2016). We estimate the level of diversity at NC sites and 0-fold degenerate sites in 50 kb windows across the autosomes. We exclude NC sites that are inferred to be subject to natural selection (based on the conservation of sites across species and other potentially functional annotations such coding and noncoding genes, UTRs, DNase I hypersensitivity sites and transcription factor binding sites).
We expect the level of genetic diversity at both selected and neutral sites to depend on the mutation rate, the rate of recombination and the density of conserved sites, because each of these factors are expected to affect the diversity either directly, or indirectly. To estimate the mutation rate there are two options: Using DNMs that have been discovered by the sequencing of trios or using the divergence between species. Neither of these methods is perfect. We currently have too few DNMs to estimate the mutation rate reliably at the 50 kb scale and attempts to predict the mutation rate of DNMs based on genomic features have so far proved to be unreliable (Smith et al. 2018). The divergence between species is also not a completely satisfactory measure of the mutation rate either, for several reasons. We have used GC-conservative substitutions (i.e., A<>T and G<>C), since these are not affected by gBGC, a process known to affect substitution rates (Duret and Arndt 2008; Smith et al. 2018), and the rate of different types of mutation appear to be strongly correlated at the 100 kb and 1 MB scales, suggesting that GC-conservative mutations should therefore be a reasonable measure of the overall mutation rate (Smith et al. 2018). However, the mutation rate appears to evolve at large scales (Terekhanova et al. 2017; Smith et al. 2018), and some of the variation in the substitution rate is due to variation in the depth of the genealogy in the ancestors of the homininae (Phung et al. 2016).
The rate of recombination, the density of selected sites and our measure of the mutation rate are correlated to each other (table 1). We confirm the positive correlation between the mutation rate and the rate of recombination in humans but also, for the first time, in the other nonhuman homininae (table 1; supplementary table 3, Supplementary Material online). We also find a negative correlation between the density of conserved sites and the rate of recombination in humans (as in McVean et al. 2004; Kong et al. 2010) and the rest of homininae (table 1; supplementary table 3, Supplementary Material online). We find that this negative correlation is driven by conserved coding sites but not by conserved NC sites in agreement with the lower rate of recombination seen in exons and nearby noncoding regions (supplementary analyses, Supplementary Material online). Interestingly, there is a strong negative correlation between the density of conserved sites and our measure of the mutation rate. We investigated this further in humans using three large publicly available DNM data sets from trios (Francioli et al. 2015; Wong et al. 2016; Jónsson et al. 2017) (supplementary analyses, Supplementary Material online). We find that the density of putatively neutral DNMs is either significantly positively correlated or uncorrelated to the density of conserved sites, depending on which data set of DNMs is considered. Hence, we do not know if the negative correlation between our measure of the mutation rate, dNC, and the density of conserved sites is a consequence of covariation in the mutation rate and the density of conserved sites, or whether dNC is reduced in regions with high density of conserved sites (despite our stringent filtering, we might not be masking all sites under purifying selection).
Table 1.
Spearman Correlations between Genomic Variables at 50 kb in Humans
| d NC | DCS | |
|---|---|---|
| RR | 0.07*** | –0.04*** |
| d NC | –0.24*** |
Note.—RR, recombination rate; DCS, density of conserved sequences; dNC, GC-conservative substitution rate at noncoding sites (mutation rate proxy). ***P ≤ 0.001.
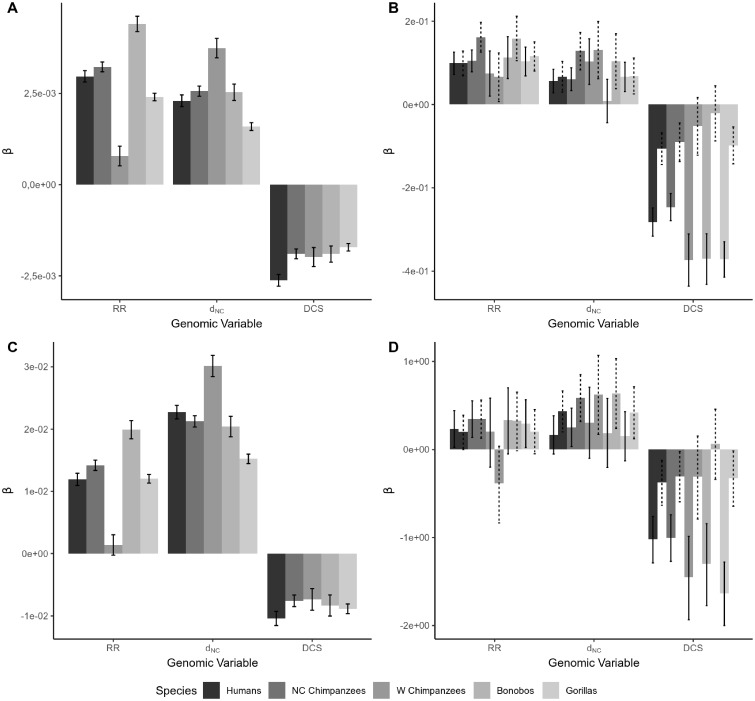
As expected, we find that noncoding diversity is positively correlated to the rate of recombination and our measure of the mutation rate, and negatively correlated to the density of selected sites if we run a bivariate analysis (supplementary figs. 3–7, Supplementary Material online). In a negative binomial multiple regressions all three genomic variables are significant (fig. 1A;supplementary table 4A, Supplementary Material online) (note that VIFs are close to 1 for each of our genomic variables suggesting that collinearity is not a problem). Standardized regression coefficients suggest that all three genomic variables are equally important in determining levels of putatively neutral diversity in humans and nonhuman homininae (fig. 1A;supplementary table 4A, Supplementary Material online). The only exception is western chimpanzees which show a weak correlation between noncoding diversity and the rate of recombination, but a stronger correlation between noncoding diversity and mutation rate.
Fig. 1.
—The standardized regression coefficients (β) in the y-axis and the three genomic variables (RR, recombination rate; dNC, mutation rate; and DCS, density of conserved sequences) in the x-axis for each species. All noncoding mutations (A), all nonsynonymous mutations with the respective matching set of (downsampled) noncoding mutations (B), GC-conservative noncoding mutations (C), GC-conservative nonsynonymous mutations with the respective matching set of (downsampled) noncoding mutations (D). The solid error bars indicate the confidence intervals 95% of the original data set and the dashed error bars represent the confidence intervals 95% of the downsampled noncoding data set for comparison.
The results for 0-fold degenerate sites are qualitatively consistent with the noncoding results; nonsynonymous diversity is positively correlated to the rate of recombination and mutation, and negatively correlated to the density of selected sites. Here, all factors remain significant in most species in the negative binomial multiple regression (fig. 1B;supplementary table 4B, Supplementary Material online). The density of conserved sites is the strongest correlate as judged by standardized regression coefficients; it has more than twice the impact on nonsynonymous diversity as the rate of recombination. Our measure of the mutation rate has slightly more than half of the impact of recombination rate. These patterns are consistent across most of the homininae. However, the results for 0-fold degenerate sites are quantitatively different from that observed for NC sites, in which diversity is equally strongly correlated to the three genomic factors. This difference between nonsynonymous and NC sites does not appear to be due to differences between the windows that have nonsynonymous sites and those that do not. If we only use windows that have nonsynonymous sites and down-sample the number of noncoding polymorphisms so that it matches the number of nonsynonymous SNPs on average we find that noncoding diversity is equally strongly correlated to each of the genomic variables and the density of conserved sites is not the dominant correlate (fig. 1B). Our results therefore suggest that there are genuine differences in the relative impact of these three genomic variables on nonsynonymous and noncoding diversity. Finally, given the collinearity between explanatory variables (table 1) we computed the VIF of the models used to generate figure 1 (and supplementary table 4, Supplementary Material online). VIF values are all close to one suggesting that multicollinearity is not an issue with this analysis.
Biased Gene Conversion
The correlation between the rate of recombination and diversity might be (directly) mediated by GC-biased gene conversion (gBGC). We repeated our analyses restricting the analysis to GC-conservative SNPs. As expected, our results are largely unaffected by this restriction; noncoding diversity remains correlated to all factors (fig. 1C;supplementary table 5A, Supplementary Material online). For nonsynonymous diversity, we again find qualitatively similar results to those using all mutations, although the correlation between nonsynonymous diversity and our estimate of the mutation is generally nonsignificant (fig. 1D;supplementary table 5B, Supplementary Material online).
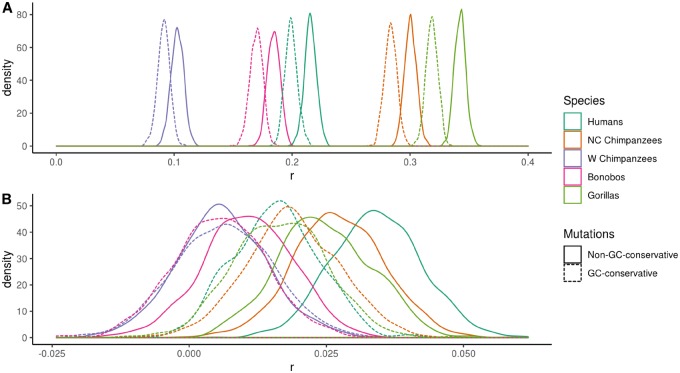
To investigate in more detail whether gBGC has an effect on the correlations between diversity and our three genomic variables, we considered the correlation between diversity and recombination rate after down-sampling the nonGC-conservative mutations to yield the same average number of mutations per 50 kb as we find in the GC-conservative data set. GC-conservative mutations represent 15–18% of all SNPs in the homininae subfamily (supplementary table 1, Supplementary Material online). Although the effect is small (fig. 2A), the correlation between θNC and the rate of recombination is greater for nonGC-conservative mutations than GC-conservative mutations in all homininae, despite the fact that there are fewer GC-conservative mutations; the consistency across the homininae is significant as judged by a sign test (P-value < 0.01). This suggests that gBGC has a significant but small effect on the correlation between recombination rate and noncoding diversity. The strength of the correlation between θN and the rate of recombination is again similar between mutation types and the difference is only significant in humans (P-value = 0.043) (fig. 2B).
Fig. 2.
—Relative effect of gBGC on the relationship between recombination rate and noncoding diversity (A) and nonsynonymous diversity (B). Distribution of the Spearman rank correlation coefficients (r) across 1,000 bootstrap replicates for nonGC-conservative mutations (downsampled to match GC-conservative diversity) and GC-conservative mutations.
Efficiency of Negative Selection
Finally, we sought to investigate whether there is an influence of selection at linked sites on the efficiency of negative selection along the genomes by considering whether θN/θNC is correlated to the recombination rate, dNC and the density of conserved sites. If there is variation in the effects of selection at linked sites across the homininae genome then we would expect θN/θNC to be negatively correlated to recombination rate, and positively correlated to the mutation rate and the density of conserved sites. Because we have very few coding sites in each window, and hence very few nonsynonymous SNPs, we grouped windows together into 50 groups that each contains on average ∼1,800 noncoding SNPs.
The unexpected negative correlation with dNC might be due to the positive correlation between the rate of (Table 2) recombination and dNC (table 1; supplementary table 3, Supplementary Material online). In fact, none of these factors remains significant when we perform a multiple regression (supplementary table 6, Supplementary Material online). The reason may be strong correlations between recombination rate, dNC and density of conserved sites when we group windows introducing multicollinearity. Indeed, the VIF is >4.5 for each variable.
Table 2.
The Spearman Rank Correlation Coefficients of θN/θNC versus RR, dNC, and the Density of Conserved Sites (DCS) (A) for All SNPs and (B) for GC-Conservative SNPs
| Species | RR | d NC | DCS |
|---|---|---|---|
| A) | |||
| Human | –0.16 (–0.32, 0.02) | –0.19 (–0.35, 0.00) | 0.15 (–0.02, 0.32) |
| NC chimpanzee | –0.24 (–0.40, –0.08) | –0.22 (–0.39, –0.05) | 0.20 (0.02, 0.37) |
| W chimpanzee | –0.31 (–0.49, –0.13) | –0.27 (–0.44, –0.09) | 0.20 (0.03, 0.38) |
| Bonobo | –0.18 (–0.36, –0.01) | –0.18 (–0.35, –0.01) | 0.01 (–0.18, 0.20) |
| Gorilla | –0.14 (–0.32, 0.02) | –0.10 (–0.29, 0.07) | 0.16 (–0.01, 0.33) |
| B) | |||
| Human | –0.26 (–0.44, –0.07) | –0.21 (–0.39, –0.03) | 0.12 (–0.09, 0.34) |
| NC chimpanzee | –0.13 (–0.32, 0.06) | –0.14 (–0.34, 0.06) | 0.07 (–0.13, 0.28) |
| W chimpanzee | 0.01 (–0.20, 0.22) | –0.10 (–0.29, 0.11) | 0.01 (–0.20, 0.21) |
| Bonobo | –0.21 (–0.42, 0.02) | –0.19 (–0.39, 0.01) | 0.12 (–0.10, 0.34) |
| Gorilla | –0.03 (–0.23, 0.15) | –0.03 (–0.23, 0.14) | –0.08 (–0.27, 0.14) |
Note.—Given in parentheses are the 95% confidence intervals by bootstrapping.
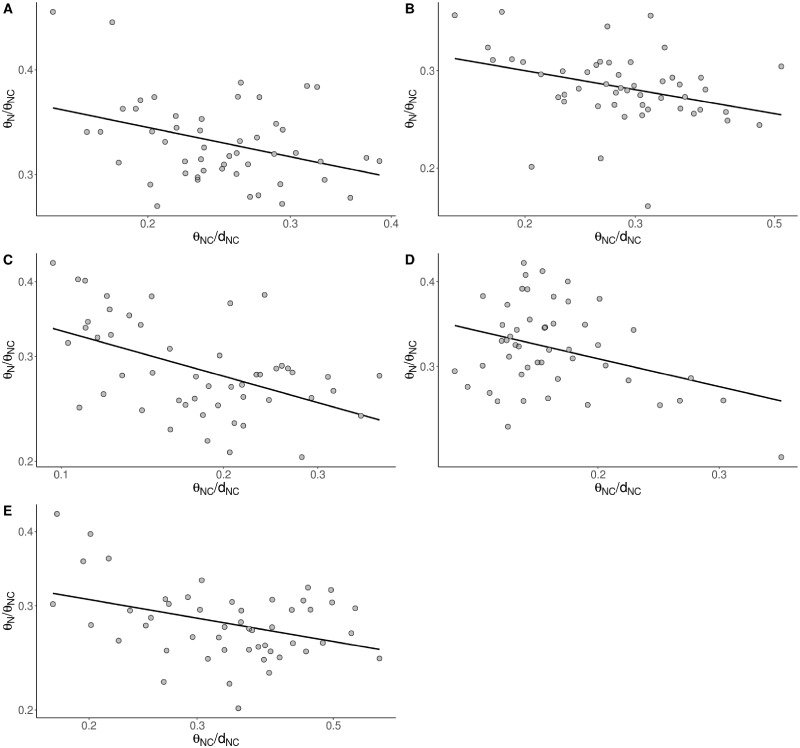
Recombination rate, our measure of the mutation rate and the density of conserved sites are likely to be crude predictors of the effects of selection at linked sites compared with the realized level of neutral diversity in a given window, after accounting for mutation rate variation. We, therefore, investigated whether θN/θNC is correlated to a measure of the effective population size (Ne) of a window, estimated by dividing the noncoding diversity by our estimate of the mutation rate: That is, θNC/dNC. We find that θN/θNC is significantly negatively correlated to our measure of the local Ne in all homininae species (fig. 3). The slope of this relationship on a log–log scale is expected to equal to the negative value of the shape parameter of the DFE, if the distribution is gamma distributed (Welch et al. 2008). The slope of this relationship in humans is –0.2 which is consistent with estimates of the distribution made from the site frequency spectrum (Eyre-Walker et al. 2006; Boyko et al. 2008; Eyre-Walker and Keightley 2009; Kim et al. 2017). The other nonhuman homininae show similar slopes (table 3) suggesting that, in these species, the shape of the DFE for new deleterious mutations is quite stable (as recently reported by Castellano et al. 2019 using the site frequency spectrum). Because of the lack of statistical power when we consider GC-conservative mutations the slope estimates become very noisy. The slope is not significantly different from 0 in the two chimpanzee populations and gorillas and not significantly different from 1 in humans and bonobos.
Fig. 3.
—Relationship between θN/θNC and θNC/dNC in a log–log scale for all mutations in (A) humans (P-value < 0.01), (B) Nigeria-Cameroon chimpanzees (P-value < 0.05), (C) western chimpanzees (P-value < 0.001), (D) bonobos (P-value < 0.05), and (E) gorillas (P-value < 0.01). Results grouping 50 kb windows into 50 bins by noncoding diversity.
Table 3.
Mean Slope (b) of log(θN/θNC) ∼ log(θNC/dNC) Linear Regression for All SNPs and GC-Conservative SNPs
| Species | All Mutations | GC-Conservative Mutations |
|---|---|---|
| Human | –0.20 (–0.31, –0.08) | –0.59 (–1.09, –0.13) |
| NC chimpanzee | –0.20 (–0.33, –0.07) | –0.17 (–0.60, 0.22) |
| W chimpanzee | –0.21 (–0.31, –0.11) | –0.04 (–0.43, 0.35) |
| Bonobo | –0.31 (–0.46, –0.15) | –0.71 (–1.20, –0.18) |
| Gorilla | –0.14 (–0.24, –0.03) | –0.05 (–0.38, 0.29) |
Note.—Given in parentheses are the 95% confidence intervals by bootstrapping. Results grouping 50 kb windows into 50 bins by noncoding diversity.
Discussion
We have investigated the three genomic variables known to affect the level of genetic diversity across the genomes of humans and other homininae. We find highly significant effects of recombination rate, mutation rate and the density of conserved elements on levels of putatively neutral genetic diversity in most species, even if we restrict the analysis to GC-conservative polymorphisms, and hence remove the effects of gBGC. The positive correlation between diversity and our measure of the mutation rate is not surprising given that we expect regions of the genome with high rates of mutation to have high levels of diversity. Previous analyses have suggested that much of the variation in diversity at the 100 kb level can be explained in terms of variation in the mutation rate (Smith et al. 2018). Noncoding diversity is also positively correlated to the rate of recombination and negatively correlated to the density of conserved sites. Similarly, the level of nonsynonymous diversity for all mutations as well as for GC-conservative mutations is strongly negatively correlated to the density of conserved sites and positively correlated to the recombination rate and our measure of the mutation rate in most homininae. However, the pattern of correlation differs between the two sets of sites; whilst noncoding diversity is approximately equally strongly correlated to all three genomic variables, nonsynonymous diversity is much more strongly correlated to the density of selected sites.
The strong negative correlation between nonsynonymous diversity and the density of conserved sites may be explained if the coding mutation rate and the density of conserved sites covary along the genome. To explore this hypothesis, we compiled DNM data from three studies that have discovered large numbers of DNMs in the sequencing of trios (supplementary analyses, Supplementary Material online). We then investigated the relationship between putatively selected DNMs and the density of conserved sites. We find a significantly negative correlation between putatively selected DNMs and the density of conserved sites in one DNM data set (Jónsson et al. 2017), a significantly positive correlation in another data set (Wong et al. 2016), and no correlation in the third data set (Francioli et al. 2015). Such contradictory patterns have been observed before for other genomic variables and likely arise as a consequence of biases in the ascertainment of DNMs (Smith et al. 2018). Thus, it is not possible to conclude that the lower coding mutation rate in functionally rich regions is driving our strong negative correlation between θN and the density of conserved sites. There is a second, nonmutually exclusive interpretation to explain the importance of the density of conserved sites on the levels of nonsynonymous diversity. That is covariation of the DFE of new amino acid mutations and gene density along the genome. In other words, if genes in highly gene-dense regions are more constrained (their DFE has a lower proportion of new nearly neutral and slightly deleterious mutations and a greater proportion of new strongly deleterious mutations) than “isolated” genes, then this will explain the negative correlation between the gene density and θN without invoking selection at linked sites or a lower mutation rate in functionally rich regions. The degree to which gene density (plus the associated regulatory sequences) and the DFE covary is therefore an interesting question for further investigation. However, the significantly negative slope between log(θN/θNC) ∼ log(θNC/dNC) (see below) suggests that selection at linked sites is indeed reducing the efficiency of purifying selection across the homininae genome. A third explanation may be the lower recombination rate reported at exons bodies (McVean et al. 2004; Myers et al. 2005; Kong et al. 2010). Hill–Robertson interference (Hill and Robertson 1966) will be then very intense at coding rich regions due to the concomitant reduction of recombination.
It should be appreciated that the correlations between recombination rate, the density of conserved sites and genetic diversity may be an artifact of deficiencies in multiple regressions. Recombination is known to be mutagenic in humans (Lercher and Hurst 2002; Hellmann et al. 2005; Pratto et al. 2014; Francioli et al. 2015; Arbeithuber et al. 2015; Halldorsson et al. 2019) and since dNC is an imperfect measure of the mutation rate, this may allow the rate of recombination to remain significant in a multiple regression. Similarly, there is a negative correlation between the density of conserved sites and our measure of the mutation rate, dNC. This could be due to regions of the genome with a high density of conserved sites having lower mutation rates, or due to the fact that we have not successfully masked all sites subject to selection (i.e., regions of the genome with high density of conserved sites might have a higher density of other sites subject to recent selection, and these sites will decrease dNC). To explore this possibility, we have also investigated the relationship between the density of putatively neutral DNMs in humans and the density of conserved elements. Again, we find that the density of DNMs at putatively neutral sites is either significantly positively or uncorrelated to the density of conserved sites, depending on which data set of DNMs is considered (supplementary analyses, Supplementary Material online). As a consequence, we do not know whether the negative correlation between dNC and density of conserved sites is a consequence of variation in the mutation rate with the density of conserved sites, or that dNC is reduced in regions with a high density of conserved sites because there are sites subject to selection that we have not masked.
However, we find additional evidence that selection at linked sites affects the efficiency of purifying selection. First, we find that our measure of the efficiency of purifying selection, θN/θNC, is negatively correlated to our estimate of the rate of recombination and positively correlated to the density of conserved sites in a bivariate analysis. Unfortunately, when we group data we find that our genomic variables are strongly correlated to each other and so it is not possible to disentangle which variable is actually correlated to θN/θNC—that is, there is a problem of multicollinearity in our multiple regression. Second, we find that θN/θNC is negatively correlated to a measure of the effective population size of a window, θNC/dNC. We find that the slope of the relationship in a log–log scale is consistent with the estimated shape parameter of the DFE in great apes (assuming the distribution is gamma distributed) (Castellano et al. 2019). This is in contrast to what has been observed in D.melanogaster, in which the slope of the relationship between log(θN/θS) versus log(θS) is significantly steeper than expected given an estimate of the DFE estimated from the site frequency spectrum; a similar pattern is apparent between species (Chen et al. 2017; James et al. 2017). Castellano et al. (2018) considered a number of explanations for this and concluded that it was most likely due to genetic HH; they showed by simulation that HH increases the slope of the relationship, because deleterious genetic variation recovers more rapidly after a HH event than neutral genetic variation (Gordo and Dionisio 2005; Pennings et al. 2014; Do et al. 2015; Brandvain and Wright 2016; Chen et al. 2019). This is consistent with the high rates of adaptive evolution observed in Drosophila (Smith and Eyre-Walker 2002; Andolfatto 2005; Eyre-Walker and Keightley 2009; Enard et al. 2014). Rates of adaptive evolution seem to be lower in humans than in Drosophila (Gossmann et al. 2012; Galtier 2016; Rousselle et al. 2019) which is therefore consistent with the fact that the slope is similar to the shape parameter of the gamma distribution recently estimated across great apes (Castellano et al. 2019). However, note that Zhen et al. (2018) have reported higher rates of adaptive substitutions in the past in humans than in Drosophila when accounting for ancestral population size. Hence, it is likely that most of the current variation in θN/θNC along these genomes reflects variation in the efficiency of selection caused by selection at linked sites, mainly driven by weakly deleterious mutations.
Finally, we would like to highlight that interference among slightly deleterious mutations can potentially lead to indirect selection on recombination modifiers (Otto and Lenormand 2002; Coop and Przeworski 2007; Bullaughey et al. 2008) and that such selection may contribute to the evolution of fine-scale recombination patterns.
Conclusions
We show that noncoding and nonsynonymous diversity are positively correlated to both mutation rate and recombination rate, whereas the density of conserved sites is associated with low levels of genetic diversity in all homininae. Nonetheless, we also find genuine differences in the relative effect of these three genomic variables on the levels nonsynonymous and noncoding diversity which deserve further investigation. The positive correlation with the rate of recombination and the negative correlation with the density of conserved sites is consistent with variation in the intensity of selection at linked sites along the genome. Although the positive correlation with the mutation rate just indicates that the higher the number of new mutations per generation the higher the level of genetic diversity. We find a negative correlation between the ratio of the number of nonsynonymous to noncoding polymorphisms and a measure of the effective population size across the homininae genome which suggests pervasive interference selection, mainly, among weakly deleterious variants.
Supplementary Material
Supplementary data are available at Genome Biology and Evolution online.
Supplementary Material
Acknowledgments
We thank D. Weghorn and three anonymous reviewers for comments. We also thank Alex Cagan for sharing the set of sites with a unique mapping to the human genome with at least 5-fold coverage in all individuals per species. This study was supported by the Danish Council For Independent Research (grant number 4181-00358).
Literature Cited
- Andolfatto P. 2005. Adaptive evolution of non-coding DNA in Drosophila. Nature 437(7062):1149–1152. [DOI] [PubMed] [Google Scholar]
- Arbeithuber B, Betancourt AJ, Ebner T, Tiemann-Boege I. 2015. Crossovers are associated with mutation and biased gene conversion at recombination hotspots. Proc Natl Acad Sci USA. 112(7):2109–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auton A, et al. 2012. A fine-scale chimpanzee genetic map from population sequencing. Science 336(6078):193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo L, et al. 2015. Trans-species polymorphism in humans and the great apes is generally maintained by balancing selection that modulates the host immune response. Hum Genomics. 9:21.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begun DJ, Aquadro CF. 1992. Levels of naturally occurring DNA polymorphism correlate with recombination rates in D. melanogaster. Nature 356(6369):519–520. [DOI] [PubMed] [Google Scholar]
- Berry A, Barbadilla A. 2000. Gene conversion is a major determinant of genetic diversity at the DNA level. Evol Genet Mol Morphol. 1:102. [Google Scholar]
- Boyko AR, et al. 2008. Assessing the evolutionary impact of amino acid mutations in the human genome. PLoS Genet. 4(5):e1000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandvain Y, Wright SI. 2016. The limits of natural selection in a nonequilibrium world. Trends Genet. 32(4):201.. [DOI] [PubMed] [Google Scholar]
- Bullaughey K, Przeworski M, Coop G. 2008. No effect of recombination on the efficacy of natural selection in primates. Genome Res. 18(4):544–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagan A, et al. 2016. Natural selection in the great apes. Mol Biol Evol. 33(12):3268–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano D, James J, Eyre-Walker A. 2018. Nearly neutral evolution across the Drosophila melanogaster genome. Mol Biol Evol. 35(11):2685–2694. [DOI] [PubMed] [Google Scholar]
- Castellano D, Macià MC, Tataru P, Bataillon T, Munch K. 2019. Comparison of the full distribution of fitness effects of new amino acid mutations across great apes. Genetics doi:10.1534/genetics. 213(3):953–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth B, Morgan MT, Charlesworth D. 1993. The effect of deleterious mutations on neutral molecular variation. Genetics 134(4):1289–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Glémin S, Lascoux M. 2019. From drift to draft: how much do beneficial mutations actually contribute to predictions of Ohta’s slightly deleterious model of molecular evolution? bioRxiv 681866. doi: 10.1101/681866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Glémin S, Lascoux M. 2017. Genetic diversity and the efficacy of purifying selection across plant and animal species. Mol Biol Evol. 34(6):1417–1428. [DOI] [PubMed] [Google Scholar]
- Cingolani P, et al. 2012. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly 6(2):80–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coop G, Przeworski M. 2007. An evolutionary view of human recombination. Nat Rev Genet. 8(1):23–34. [DOI] [PubMed] [Google Scholar]
- Cutter AD, Payseur BA. 2013. Genomic signatures of selection at linked sites: unifying the disparity among species. Nat Rev Genet. 14(4):262–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davydov EV, et al. 2010. Identifying a high fraction of the human genome to be under selective constraint using GERP++. PLoS Comput Biol. 6(12):e1001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do R, et al. 2015. No evidence that selection has been less effective at removing deleterious mutations in Europeans than in Africans. Nat Genet. 47:126–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duret L, Arndt PF. 2008. The impact of recombination on nucleotide substitutions in the human genome. PLoS Genet. 4(5):e1000071.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duret L, Galtier N. 2009. Biased gene conversion and the evolution of mammalian genomic landscapes. Annu Rev Genom Hum Genet. 10(1):285–311. [DOI] [PubMed] [Google Scholar]
- Enard D, Messer PW, Petrov DA. 2014. Genome-wide signals of positive selection in human evolution. Genome Res. 24(6):885–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre-Walker A. 1993. Recombination and mammalian genome evolution. Proc Biol Sci. 252(1335):237–243. [DOI] [PubMed] [Google Scholar]
- Eyre-Walker A, Keightley PD. 2009. Estimating the rate of adaptive molecular evolution in the presence of slightly deleterious mutations and population size change. Mol Biol Evol. 26(9):2097–2108. [DOI] [PubMed] [Google Scholar]
- Eyre-Walker A, Woolfit M, Phelps T. 2006. The distribution of fitness effects of new deleterious amino acid mutations in humans. Genetics 173(2):891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J, Weisberg S. 2011. An R companion to applied regression. Thousand Oaks, CA: SAGE Publications. [Google Scholar]
- Francioli LC, et al. 2015. Genome-wide patterns and properties of de novo mutations in humans. Nat Genet. 47(7):822–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galtier N. 2016. Adaptive protein evolution in animals and the effective population size hypothesis. PLoS Genet. 12(1):e1005774.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galtier N, Piganeau G, Mouchiroud D, Duret L. 2001. GC-content evolution in mammalian genomes: the biased gene conversion hypothesis. Genetics 159(2):907–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordo I, Dionisio F. 2005. Nonequilibrium model for estimating parameters of deleterious mutations. Phys Rev E. 71(3):031907. [DOI] [PubMed] [Google Scholar]
- Gossmann TI, Keightley PD, Eyre-Walker A. 2012. The effect of variation in the effective population size on the rate of adaptive molecular evolution in eukaryotes. Genome Biol Evol. 4(5):658–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halldorsson BV, et al. 2019. Characterizing mutagenic effects of recombination through a sequence-level genetic map. Science 363(6425):eaau1043. [DOI] [PubMed] [Google Scholar]
- Hellmann I, et al. 2005. Why do human diversity levels vary at a megabase scale? Genome Res. 15(9):1222–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill WG, Robertson A. 1966. The effect of linkage on limits to artificial selection. Genet Res. 8(3):269–294. [PubMed] [Google Scholar]
- Hollander M, Wolfe DA, Chicken E. 2013. Nonparametric Statistical Methods. John Wiley & Sons.
- Hussin JG, et al. 2015. Recombination affects accumulation of damaging and disease-associated mutations in human populations. Nat Genet. 47. doi:10.1038/ng.3216. [DOI] [PubMed] [Google Scholar]
- James J, Castellano D, Eyre-Walker A. 2017. DNA sequence diversity and the efficiency of natural selection in animal mitochondrial DNA. Heredity 118(1):88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys AJ, May CA. 2004. Intense and highly localized gene conversion activity in human meiotic crossover hot spots. Nat Genet. 36(2):151–156. [DOI] [PubMed] [Google Scholar]
- Jónsson H, et al. 2017. Parental influence on human germline de novo mutations in 1,548 trios from Iceland. Nature 549(7673):519–522. [DOI] [PubMed] [Google Scholar]
- Kim BY, Huber CD, Lohmueller KE. 2017. Inference of the distribution of selection coefficients for new nonsynonymous mutations using large samples. Genetics 206(1):345–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong A, et al. 2010. Fine-scale recombination rate differences between sexes, populations and individuals. Nature 467(7319):1099–1103. [DOI] [PubMed] [Google Scholar]
- Lercher MJ, Hurst LD. 2002. Human SNP variability and mutation rate are higher in regions of high recombination. Trends Genet. 18(7):337–340. [DOI] [PubMed] [Google Scholar]
- Marais G. 2003. Biased gene conversion: implications for genome and sex evolution. Trends Genet. 19(6):330–338. [DOI] [PubMed] [Google Scholar]
- Marais G, Mouchiroud D, Duret L. 2001. Does recombination improve selection on codon usage? Lessons from nematode and fly complete genomes. Proc Natl Acad Sci USA. 98(10):5688–5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVean GAT, et al. 2004. The fine-scale structure of recombination rate variation in the human genome. Science 304(5670):581–584. [DOI] [PubMed] [Google Scholar]
- McVean GAT, Charlesworth B. 1999. A population genetic model for the evolution of synonymous codon usage: patterns and predictions. Genet Res. 74(2):145–158. [Google Scholar]
- McVicker G, Gordon D, Davis C, Green P. 2009. Widespread genomic signatures of natural selection in hominid evolution. PLoS Genet. 5(5):e1000471.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers S, Bottolo L, Freeman C, McVean G, Donnelly P. 2005. A fine-scale map of recombination rates and hotspots across the human genome. Science 310(5746):321–324. [DOI] [PubMed] [Google Scholar]
- Nam K, et al. 2017. Evidence that the rate of strong selective sweeps increases with population size in the great apes. Proc Natl Acad Sci USA. 114(7):1613–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto SP, Lenormand T. 2002. Resolving the paradox of sex and recombination. Nat Rev Genet. 3(4):252–261. [DOI] [PubMed] [Google Scholar]
- Padhukasahasram B, Rannala B. 2013. Meiotic gene-conversion rate and tract length variation in the human genome. Eur J Hum Genet. doi:10.1038/ejhg.2013.30. [DOI] [PubMed] [Google Scholar]
- Payseur BA, Nachman MW. 2002. Gene density and human nucleotide polymorphism. Mol Biol Evol. 19(3):336–340. [DOI] [PubMed] [Google Scholar]
- Pennings PS, Kryazhimskiy S, Wakeley J. 2014. Loss and recovery of genetic diversity in adapting populations of HIV. PLoS Genet. 10(1):e1004000.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessia E, et al. 2012. Evidence for widespread GC-biased gene conversion in eukaryotes. Genome Biol Evol. 4(7):675–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phung TN, Huber CD, Lohmueller KE. 2016. Determining the effect of natural selection on linked neutral divergence across species. PLoS Genet. 12(8):e1006199.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado-Martinez J, et al. 2013. Great ape genetic diversity and population history. Nature 499(7459):471–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratto F, et al. 2014. Recombination initiation maps of individual human genomes. Science 346(6211):1256442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousselle M, et al. 2019. Is adaptation limited by mutation? A timescale dependent effect of genetic diversity on the adaptive substitution rate in animals. bioRxiv. 643619. doi:10.1101/643619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siepel A, et al. 2005. Evolutionarily conserved elements in vertebrate, insect, worm, and yeast genomes. Genome Res. 15(8):1034–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JM, Haigh J. 1974. The hitch-hiking effect of a favourable gene. Genet Res. 23(1):23–35. [PubMed] [Google Scholar]
- Smith NGC, Eyre-Walker A. 2002. Adaptive protein evolution in Drosophila. Nature 415(6875):1022–1024. [DOI] [PubMed] [Google Scholar]
- Smith TCA, Arndt PF, Eyre-Walker A. 2018. Large scale variation in the rate of germ-line de novo mutation, base composition, divergence and diversity in humans. PLoS Genet. 14(3):e1007254.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, et al. 2011. Open chromatin defined by DNaseI and FAIRE identifies regulatory elements that shape cell-type identity. Genome Res. 21(10):1757–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevison LS, et al. 2016. The time scale of recombination rate evolution in great apes. Mol Biol Evol. 33(4):928–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terekhanova NV, Seplyarskiy VB, Soldatov RA, Bazykin GA. 2017. Evolution of local mutation rate and its determinants. Mol Biol Evol. 34(5):1100–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables WN, Ripley BD. 2002. Modern applied statistics, 4th ed. New York: Springer. [Google Scholar]
- Watterson GA. 1975. On the number of segregating sites in genetical models without recombination. Theor Popul Biol. 7(2):256–276. [DOI] [PubMed] [Google Scholar]
- Welch JJ, Eyre-Walker A, Waxman D. 2008. Divergence and polymorphism under the nearly neutral theory of molecular evolution. J Mol Evol. 67(4):418–426. [DOI] [PubMed] [Google Scholar]
- Wong WSW, et al. 2016. New observations on maternal age effect on germline de novo mutations. Nat Commun. 7:10486.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen Y, Huber CD, Davies RW, Lohmueller KE. 2018. Stronger and higher proportion of beneficial amino acid changing mutations in humans compared to mice and flies. bioRxiv 427583 doi:10.1101/427583. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.