Abstract
Purpose
Our purpose was to test glycyrrhizin (GLY) effects and ciprofloxacin interactions on multidrug resistant (MDR) isolates of Pseudomonas aeruginosa in vitro and in vivo in a mouse model of keratitis.
Methods
A Hardy-disk tested antibiotic sensitivity of isolates MDR9 (nonocular) and B1045 (ocular). GLY MIC (both isolates) and ciprofloxacin was determined spectrophotometrically. A live/dead assay using confocal microscopy and plate count, tested GLY effects on bacterial permeabilization/viability. Proteomics profiled bacterial efflux pumps (MDR9 vs. PAO1); RT-PCR comparatively tested GLY effects on their mRNA expression levels. The activity of efflux pumps was tested using ethidium bromide (EB); and scanning electron microscopy (SEM) visualized the effects of GLY treatment of bacteria. A combination of GLY and ciprofloxacin was tested in C57BL/6 mice (begun 18 hours after infection) and disease scored, photographed and MPO and plate counts done.
Results
MDR9 was resistant to 6/12 and B1045 to 7/12 antibiotics (both to ciprofloxacin). MIC GLY for MDR9 was 40 mg/mL and 15 mg/mL for B1045. Ciprofloxacin MIC (32 μg/mL) was reduced 2-fold to 16 μg/mL when ciprofloxacin and GLY were combined. GLY altered bacterial membrane permeability and reduced viability. Proteomics revealed increased efflux pumps in MDR9 versus PAO1; GLY reduced their mRNA expression levels and EB suggested decreased activity. In C57BL/6 mice, treatment with GLY and ciprofloxacin versus ciprofloxacin, significantly reduced clinical scores, plate count, and MPO.
Conclusions
GLY decreases MDR by: altering bacterial parameters, including viability and efflux pump activity. In vivo, it increases the effectiveness of ciprofloxacin, reducing ocular disease, plate count, and MPO activity.
Keywords: glycyrrhizin, multi-drug resistant, pseudomonas aeruginosa
Pseudomonas aeruginosa (PA) keratitis is treated by topical antibiotics that reduce bacterial burden, yet tissue damage occurs as a result of a poorly controlled host–immune response.1,2 Additionally, emergence of antibiotic-resistant bacteria poses serious challenges for the effective management of keratitis3 and thus, it is urgent and timely to develop alternative treatments. Resistance to antimicrobials has been observed since the first antibiotics were discovered and many genes that confer drug resistance upon some strains of bacteria predate antibiotics by millennia. However, resistance has increasingly become problematic globally due to overuse of antimicrobials, increasing the rate of resistance, development, and spread. Lack of new drugs to challenge these new “supermicrobes” exacerbates the problem. Besides health care issues, there are economic consequences, as more than 2 million infections a year are caused by bacteria that are resistant to at least first-line antibiotics,4 costing the United States health system $20 billion each year.5 PA, an opportunistic pathogen, causes 51,000 healthcare-associated infections/year in the United States; 13%4 are multidrug resistant (MDR) and more difficult to treat.
Nonantibiotic approaches to prevent and treat infections are being tested, and could provide alternatives to antibiotics no longer effective against MDR-PA strains. Glycyrrhizin (GLY) is a glycoconjugated triterpene extracted from licorice root (Glycyrrhiza glabra) with numerous effects.6 It has shown efficacy in animal models of corneal infection,7,8 sepsis,9 colitis,10 lung,11 and brain12 injury, and is used in the clinical management of chronic hepatitis.13 Anti-inflammatory properties of GLY are attributed to its 18β glycyrrhetic acid moiety, which binds high mobility group box 1 (HMGB1), dampening inflammation.14,15 GLY reduces proinflammatory mediators and has direct antimicrobial effects, reducing plate count after corneal infection with a non-MDR clinical (keratitis) PA isolate, KEI 1025 by ∼4.5 logs.7 In addition, GLY has been shown to enhance the bioavailability of drugs, including antibiotics,16,17 making them more effective at lower concentrations. Lower bacterial membrane permeability, overexpression of efflux pumps, biofilm, and antibiotic inactivating enzymes are major contributors to intrinsic multidrug resistance in PA.18 Thus, the objectives of this study are to test GLY (with/without ciprofloxacin), and its effects on several of these contributors to resistance in MDR PA isolates from systemic and ocular sources. We also tested whether GLY can improve the effects of ciprofloxacin in a murine model of keratitis.
Materials and Methods
HardyDisk
PA isolates MDR9 (isolated from sputum; Detroit Medical Center, Detroit, MI, USA) and B1045 (clinical keratitis isolate provided by Regis Kowalski, PhD, Charles T. Campbell Ophthalmic Microbiology Laboratory, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA) were obtained, subcultured, and stored at −70°C. Antibiotic sensitivity was tested in vitro by agar diffusion (Kirby-Bauer) using a HardyDisk antimicrobial sensitivity test (Hardy Diagnostics, Santa Maria, CA, USA).19
GLY Minimum Inhibitory Concentration (MIC)
PA isolates (MDR9 and B1045) were grown in peptone tryptic soy broth (PTSB) medium at 37°C in a rotary shaker water bath at 150 rpm for 18 hours to an optical density (measured at 540 nm) between 1.3 and 1.8. Bacteria were pelleted by centrifugation at 5500g for 10 minutes, washed, repelleted, and resuspended in sterile saline to a concentration of 1.5 × 108 colony forming units (cfu)/mL using the 0.5 McFarland standard. Serial dilutions of GLY (0–60 mg/mL for MDR9 and 0–15 mg/mL for B1045, 5-mL/tube; Sigma-Aldrich Corp., St. Louis, MO, USA) were prepared in PTSB and a 10 μL aliquot of the diluted bacterial culture was added to each tube. Tubes were incubated for 18 hours in a MaxQ 4000 shaker (Thermo Fisher Scientific, Waltham, MA, USA) at 125 rpm and 37°C. After washing to remove GLY and resuspending the bacterial pellet in fresh PTSB, bacterial growth was determined spectrophotometrically at 540 nm. The MIC was assigned to the concentration of GLY that resulted in an absorbance reading of zero.20 MIC ciprofloxacin (1–32 μg/mL) for MDR9 was measured with and without GLY (20 mg/mL) similarly.
Membrane Permeability
The ability of GLY to permeabilize the outer membrane of the MDR9 isolate was tested in vitro using a commercial assay (Live/Dead BacLight Bacterial Viability; Molecular Probes, Waltham, MA, USA) and effects assessed by confocal microscopy, viable bacterial plate count, and scanning electron microscopy (SEM).
Confocal Microscopy
MDR9 was grown as described above for MIC using GLY at 0, 10, or 20 mg/mL. After incubation for 18 hours as described above for MIC, a 1-mL aliquot from each dilution was removed, washed, and resuspended in 1-mL sterile saline. A total of 5 μL of MDR9 samples stained with a 1:1 combination of Syto 9 (live/green) and propidium iodide (PI, red/dead) dyes was placed on a slide and coverslipped. Samples were photographed using a Leica TCS-SP8 (Leica Microsystems, Buffalo Grove, IL, USA) and the average percentage of live (green) bacteria was quantitated as described.20,21 Live (green) or dead/permeabilized (red) bacteria were counted in four 40-μm2 areas of four representative micrographs (n = 16 areas/group), averaged and expressed as percent total live bacteria.20
Plate Count
A modified time kill assay was used to test bacterial viability. For this MDR9 was grown as described above for MIC (18 hours) and similarly repeated for 6 hours with GLY (0, 10, and 20 mg/mL). Selected dilutions were plated in triplicate on Pseudomonas isolation agar plates (Becton-Dickinson, Franklin Lakes, NJ, USA), incubated overnight at 37°C and the number of bacterial colonies counted. Results are reported as log10 cfu + SEM.22
Scanning EM
Scanning EM was done essentially as described before.23 In brief, MDR9 was grown overnight, washed, and resuspended to a concentration of 1.5 × 108 cfu using the MacFarland standard. Tubes containing 5 mL PTSB (0 mg/mL GLY) or 20 mg/mL GLY were inoculated with 10 μL of the bacterial suspension, incubated 18 hours at 37°C with gentle shaking. We applied 10 μL of each suspension to a 22 × 22 mm coverslip-coated with fibronectin/collagen/albumin coating mix (Athena Enzyme Systems, Baltimore, MD, USA) and allowed to adhere for 1 hour. Coverslips were fixed in 2% OsO4, 2.5% glutaraldehyde in 0.1 M phosphate buffer, and 0.2M Sorensons's phosphate buffer for 1.5 hours; fixative was replaced with fresh and incubated for another 1.5 hours. Coverslips were the rinsed in graded ethanols (50%–100%), critical point dried (Tousimis 831, Rockville, MD, USA) and gold-coated (TedPella, Redding, CA, USA) for observation using a SEM (JEOL JSM-7600F, Peabody, MA, USA and Tokyo, Japan).
Proteomics
MDR9 and PAO1 (reference strain) were grown in 25 mL of PTSB at 37°C for 18 hours, as described before, and pellets (1.5 mL of four separate cultures from each bacterial isolate) analyzed by the WSU Proteomics Core as reported before.19 In brief, each pellet was heated at 95°C for 5 minutes in 1% lithium dodecyl sulfate (LiDS) to extract proteins. Extracts were passed through a coarse filter and total protein concentration assayed [bicinchoninic acid (BCA)] assay. Equal protein from each extract was reduced with dithiothreitol (DTT), alkylated with iodoacetamide and digested with trypsin overnight. Digestion conditions were <0.1% LiDS, 10% acetonitrile, 80 mM Tris pH 7.5. Digested samples were run on a mass spectrometer (Thermo Fusion; Thermo Fisher Scientific). MS1 spectra were collected at 120K resolution followed by higher energy collisional dissociation (HCD) and ion trap analysis of MS2 spectra for the most abundant features. These spectra were searched against the Uniprot P. aeruginosa proteome (ID = UP000042235, 6563 entries) using MaxQuant. Resulting intensities for each protein were normalized to the same median intensity. Fold change in normalized protein intensities (average intensity of MDR9/average intensity of PAO1) accompanied with a P value calculated based on a moderated t-test, was used to test differences in protein expression.
Real-Time RT-PCR
MDR9 or B1045 pellets (prepared as described above) were treated with GLY (20 mg/mL for MDR9, 3.75 mg/mL for B1045) for 3 hours. For each pellet (1.5 mL of each bacterial culture/pellet) total RNA was isolated (RNA STAT-60; Tel-Test, Friendswood, TX, USA) per the manufacturer's instructions and as reported before.19,20 A total of 1 μg of each RNA sample was reverse transcribed using Moloney-murine leukemia virus (M-MLV) reverse transcriptase (Invitrogen, Carlsbad, CA, USA) to produce a cDNA template PCR. cDNA products for each were diluted 1:25 with DEPC-treated water and a 2-μl aliquot of diluted cDNA was used for the real-time RT-PCR reaction. SYBR green/fluorescein PCR master mix (Bio-Rad Laboratories, Richmond, CA, USA) and primer concentrations of 10 μM were used in a total 10-μL volume. After a preprogrammed hot start cycle (3 minutes at 95°C), the parameters used for PCR amplification were: 15 seconds at 95°C and 60 seconds at 60°C with the cycles repeated 45 times. Levels of resistance-nodulation-division (RND) efflux pump genes MexA, MexB, MexC, MexD, MexE, MexF, MexX, MexY, OprM, OprN, and OprJ were tested by real-time RT-PCR (CFX Connect real-time PCR detection system; Bio-Rad Laboratories). The fold-differences in gene expression were calculated relative to housekeeping genes; rpoD9 and expressed as the relative mRNA concentration ± SEM. Primer pair sequences used are shown in the Table.
Table.
Nucleotide Sequence of the Specific Primers Used for PCR Amplification
|
Gene |
Nucleotide Sequence |
Primer |
GenBank |
| rpoD | 5′- CAT CCG CAT GAT CAA CGA CA -3′ | F | AAG03965.1 |
| 5′- GAT CGA TAT AGC CGC TGA GG -3′ | R | ||
| MexA | 5′- ACC TAC GAG GCC GAC TAC CAG A -3′ | F | AAG03814.1 |
| 5′- GTT GGT CAC CAG GGC GCC TTC -3′ | R | ||
| MexB | 5′- CCT GAC ACC AGG AAG CTT GAA -3′ | F | AAG03815.1 |
| 5′- CAT CAC CAG GAA CAC GAG GAG G -3′ | R | ||
| MexC | 5′- GTA CCG GCG TCA TGC AGG GTT -3′ | F | AAG07987.1 |
| 5′- GGT TGT CTT TGA GAT CCA TGC -3′ | R | ||
| MexD | 5′- CTA CCC TGG TGA AAC AGC -3′ | F | AAG07986.1 |
| 5′- AGC AGG TAC ATC ACC ATC A -3′ | R | ||
| MexE | 5′- ATC CCA CTT CTC CTG GCG CT-3′ | F | AGG05881.1 |
| 5′- GGT CGC CTT TCT TCA CCA GT -3′ | R | ||
| MexF | 5′- AGG CCT CGC CCG ACC TGA CCA TG -3′ | F | AGG05882.1 |
| 5′- CTC GCG GAT GGC GTT GAC CAC GT -3′ | R | ||
| MexX | 5′- TGA AGG CGG CCC TGG ACA TCA GC -3′ | F | AGG05407.1 |
| 5′- GAT CTG CTC GAC GCG GGT CAG CG -3′ | R | ||
| MexY | 5′- CCG CTA CAA CGG CTA TCC CT- 3′ | F | AGG05406.1 |
| 5′- AGC GGG ATC GAC CAG CTT TC -3′ | R | ||
| OprM | 5′- GAT CCC CGA CTA CCA GCG CCC CG-3′ | F | AGG03816.1 |
| 5′- ATG CGG TAC TGC GCC CGG AAG GC -3′ | R | ||
| OprN | 5′- CAA CCG GGA GTG ACC GAG GAC CG -3′ | F | AAG05883.1 |
| 5′- TGC TCA GGG CAA TCT TCT CGC GC -3′ | R | ||
| OprJ | 5′- GTT CCG GGC CTG AAT GCC GCT GC -3′ | F | AAG07656.1 |
| 5′- TCG CGG CTG ACC AGG GTC TGA CG -3′ | R |
F, forward; R, reverse.
Ethidium Bromide
Divided petri dishes were prepared with Pseudomonas isolation agar containing 0.5 mg/L ethidium bromide (EB).24 Sections were inoculated with 30 μL of diluted cultures including: PAO1 (positive control for EB retention), untreated (no GLY) MDR9, and treated (18 hours with 20 mg/mL GLY). After overnight incubation at 37°C, plates were photographed (100-ms exposure) on an imager (FluorChem E; Protein Simple, Santa Clara, CA, USA) using a UV light source and orange filter. Using ImageJ (http://imagej.nih.gov/ij/; provided in the public domain by the National Institutes of Health, Bethesda, MD, USA), bacterial colonies were encircled using the oval brush selection tool for an area of .002 (count 156). The histogram was analyzed and the mean gray value for 10 colonies recorded. Background (a location without colonies), was similarly determined and subtracted from the mean gray value for each colony measured. Data are shown as mean + SEM.
Mice Infection and Clinical Score
We purchased 8-week-old female C57BL/6 mice from the Jackson Laboratory (Bar Harbor, ME, USA) and housed them in accordance with the National Institutes of Health guidelines. Mice were treated humanely and in compliance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research and the Institutional Animal Care and Use Committee of Wayne State University (IACUC-18-04-0595, June 26, 2018 to June 25, 2021). Mice (n = 5/group/time/treatment) were anesthetized with anhydrous ethyl ether, placed beneath a stereoscopic microscope (Stereomaster; Olympus Corp., New York, NY, USA) at ×40 magnification and the left cornea wounded (three 1-mm incisions) using a sterile 25-5/8–gauge needle. MDR9, 1 × 108 cfu/μL of P. aeruginosa—prepared as described before5—was topically applied (5 μL) to the cornea. Disease was graded25: 0, clear/slight opacity, partially or fully covering the pupil; +1, slight opacity, covering the anterior segment; +2, dense opacity, partially or fully covering the pupil; +3, dense opacity, covering the anterior segment; and + 4, corneal perforation. Each mouse was scored at 1, 3, and 5 days postinfection (p.i.) for statistical comparison and photographed (5 days p.i.) with a slit lamp to illustrate disease.
GLY and Ciprofloxacin
Mice (n = 5/group/time/treatment) were infected with strain MDR9 as described above. Beginning at 18 hours p.i., corneas were treated topically with GLY (100 μg/dose) and/or ciprofloxacin (7.5 μg/dose; Rising Pharmaceuticals, Allendale, NJ, USA) twice daily through 4 days p.i. Controls received similar treatment with GLY and/or PBS.7
Quantification of Viable Bacteria
MDR9-infected corneas from PBS versus GLY, ciprofloxacin alone, and GLY plus ciprofloxacin-treated C57BL/6 mice (n = 5/group/time/treatment) were harvested on 3 and 5 days p.i. Each cornea was homogenized in 1 mL sterile saline containing 0.25% BSA and 100 μL was serially diluted 1:10 in sterile saline containing 0.25% BSA. Selected dilutions were plated in triplicate on Pseudomonas isolation agar plates (Becton-Dickinson, Franklin Lakes, NJ, USA) and incubated overnight at 37°C. Bacterial colonies were manually counted and reported as the actual number of counted colonies +SEM.
Myeloperoxidase (MPO) Assay
This assay was used to enumerate neutrophils in the cornea of PBS, GLY, ciprofloxacin, and ciprofloxacin plus GLY-treated mice (n = 5/group/time/treatment) infected with MDR9. Individual corneas were removed at 5 days p.i. and homogenized in 1 mL of 50 mM phosphate buffer (pH 6.0) containing 0.5% hexadecyl trimethyl-ammonium (Sigma-Aldrich Corp.). Samples were freeze-thawed four times, centrifuged, and 100 μL of the supernatant added to 2.9 mL of 50 mM phosphate buffer containing o-dianisidine dihydrochloride (16.7 mg/mL; Sigma-Aldrich Corp.) and hydrogen peroxide (0.0005%). The change in absorbency was monitored at 460 nm for 4 minutes at 30-second intervals. The slope of the line was determined for each sample and used to calculate units of MPO/cornea. One unit of MPO activity equals ∼2 × 105 neutrophils.26
Statistical Analysis
A Student's t-test was used to determine significance of real-time RT-PCR data. For the proteomics data, a moderated t-test, with a 10% false discovery rate (FDR) between groups was used. A 1-way ANOVA was used to analyze confocal microscopy, EB efflux pump (mean colony fluorescence), and the ciprofloxacin plus GLY data and was followed by the Bonferroni's multiple comparison test. The difference in clinical scores between two groups at each time was analyzed by the Mann-Whitney U test. Data were considered significant at P < 0.05. All experiments were repeated once to ensure reproducibility and data from a representative experiment are shown as mean ± SEM.
Results
Antibiotic Susceptibility
Zones of growth inhibition were measured (data not shown) for each isolate after 18 hours incubation and the resistance phenotype determined by comparing test values to a reference list from the Clinical and Laboratory Standards Institute (CLSI). MDR9 is resistant to 6 of 12 antibiotics tested: levofloxacin, imipenem, ciprofloxacin, meropenem, piperacillin/tazobactam, sulfamethoxazole/trimethoprim and has intermediate resistance to aztreonam. It is sensitive to 5 of 12 antibiotics: amikacin, ceftazidime, cefepime, gentamicin, and tobramycin. B1045 is resistant to 7 of 12 of the antibiotics tested: levofloxacin, gentamicin, imipenem, ciprofloxacin, meropenem, tobramycin, and sulfamethoxazole/trimethoprim. It is sensitive to 4 of 12 antibiotics tested: amikacin, ceftazidime, cefepime, and piperacillin/tazobactam.
MIC Assays
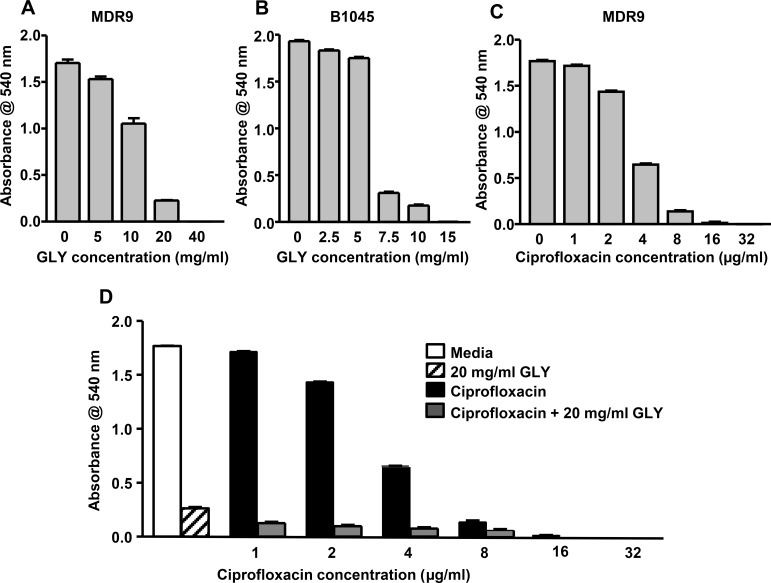
MIC GLY showed reduced growth for each isolate and was concentration dependent. MDR9 growth was completely inhibited at 40 mg/mL (48 micromolar; Fig. 1A) and B1045 at 15 mg/mL (17 micromolar; Fig. 1B). MIC ciprofloxacin was 32 μg/mL (95 nanomolar) for MDR9 (Fig. 1C). Combining ciprofloxacin with GLY (20 mg/mL, 24 micromolar), completely inhibited growth at 16 μg/mL (47 nanomolar) ciprofloxacin, reducing MIC by 2-fold compared to ciprofloxacin alone (Fig. 1D).
Figure 1.
MIC of GLY and ciprofloxacin. Treatment of either isolate with GLY (A, B) or MDR9 with ciprofloxacin (C) inhibited visible bacterial growth in a concentration dependent manner. Visible growth was completely inhibited by GLY at 40 mg/mL (MDR9), 15 mg/mL (B1045) and by ciprofloxacin at 32 μg/mL. GLY reduced MIC of ciprofloxacin for MDR9 2-fold (D).
Membrane Permeability
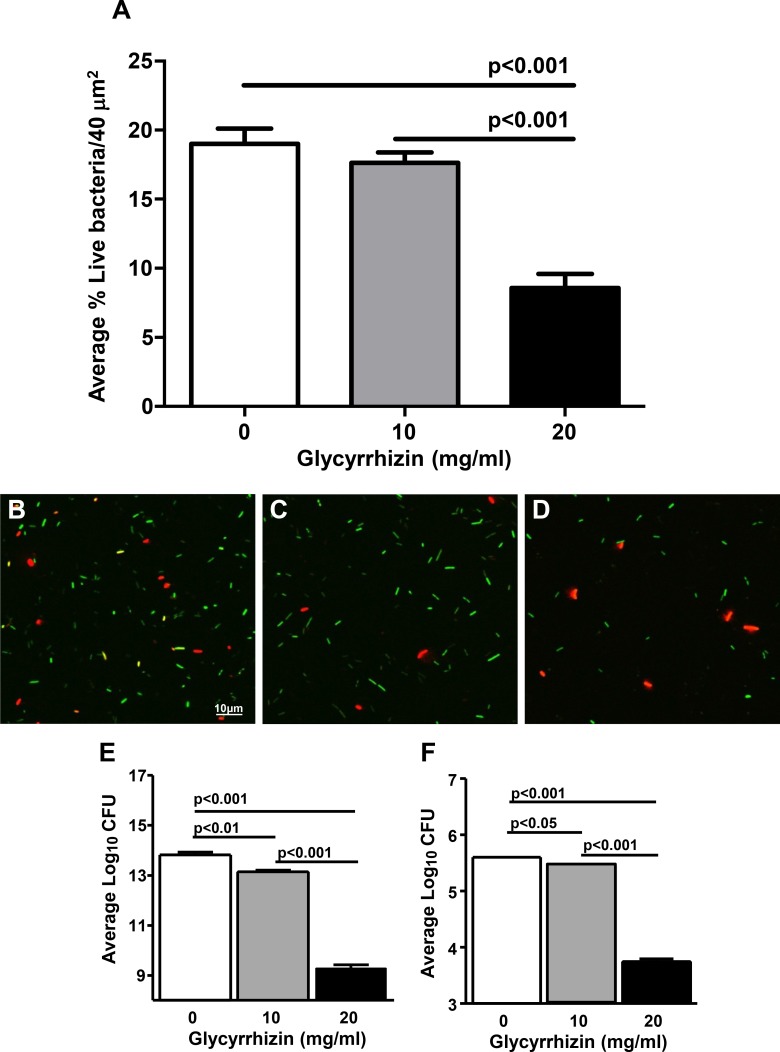
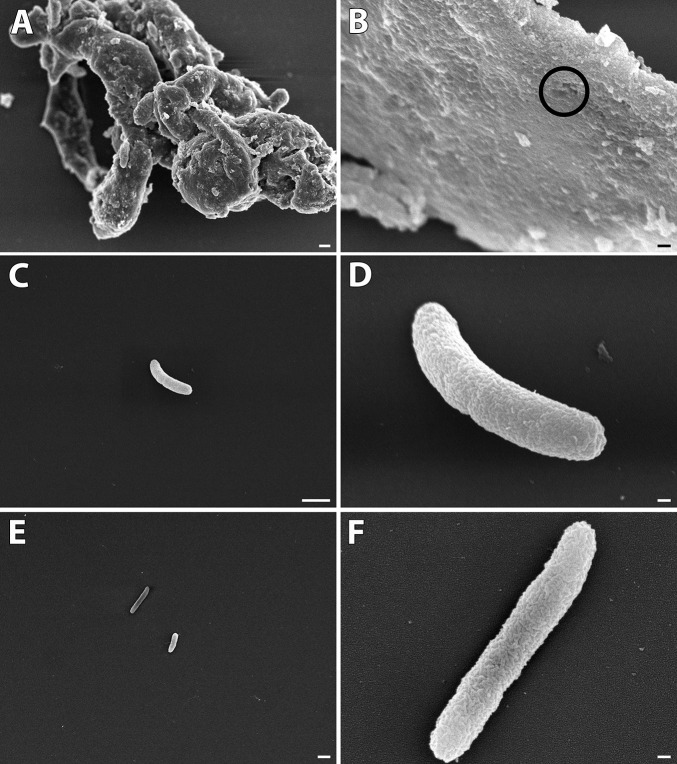
A commercial assay (Molecular Probes) was used with confocal microscopy to test membrane permeability using MDR9. Figures 2B through 2D are representative confocal images of GLY (0, 10, and 20 mg/mL) used to count permeabilized (red) versus live (green) bacteria. Figure 2A shows that at 20 mg/mL GLY, the percent of live cells was significantly decreased compared with control (no GLY) or 10 mg/mL GLY (P < 0.001 for each). To confirm these data, we quantitated the number of viable bacteria after GLY incubation (18 hours; Fig. 2E). GLY-reduced bacterial number (log10 cfu,) significantly at 10 (P < 0.01) and 20 mg/mL (P < 0.001, ∼4 logs) compared with control. A similar experiment tested shorter (6 hours) exposure to GLY. These data (Fig. 2F) also showed significantly reduced plate count (GLY at 10 [P < 0.05] and 20 mg/mL [P < 0.001, ∼2 logs]). SEM also was done similar to the experiments above (18-hour incubation) to assess further the effects of GLY exposure. Figures 3A through 3F shows bacteria treated with GLY (Figs. 3A–D) or PBS (Figs. 3E, 3F). GLY treatment resulted in clumped and swollen bacteria with irregular protuberances from their surface (Fig. 3A) or with “holes” in the bacterial membrane (Fig. 3B) for the majority of the GLY-treated bacteria. However, there were also random bacteria that appeared normal (Fig. 3C) and/or appeared to be dividing (Fig. 3D, indentation mid bacteria) suggesting their viability. PBS-treated bacteria were not swollen, nor did they cluster together, forming clumped groups, but appeared (compare Fig. 3A with Fig. 3E) and exhibited a relatively smooth outer membrane with no protuberances or “holes” (Fig. 3F).
Figure 2.
After 18 hours GLY (concentrations shown) treatment of MDR9, confocal microscopy (A–D) showed bacteria as either red/permeabilized or green/live. Viable plate count after 18 (E) or 6 (F) hours incubation with GLY showed reduced log10 cfu.
Figure 3.
Scanning EM after 18 hours GLY (20 mg/mL) in vitro. Most GLY-treated bacteria (A–D) are swollen, clumped, and show membrane “holes.” A few appeared normal (C, D) and similar to controls (E, F). Magnification: (A) 4000; (B) 45,000; (C) 10,000; (D) 45,000; (E) 4500; (F) 45,000; (A, C, E) Scale bar: 1 μm. (B, D, F) Scale bar: 100 nm.
Proteomic Analysis of MDR9 Versus PAO1
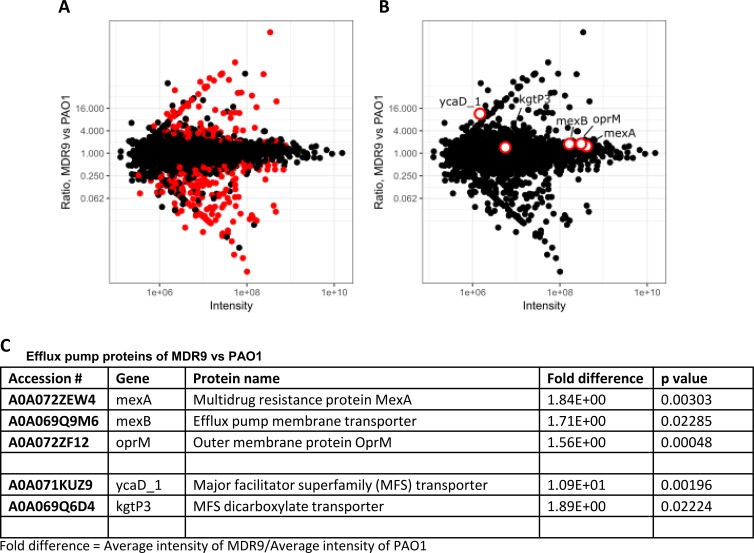
Proteomic analysis (Figs. 4A–C), identified 2213 proteins and 2199 had quantitative data. These data were normalized so each sample had the same median intensity as described before.19 A volcano plot shows 316 proteins (red dots in Fig. 4A) that were significantly different between groups (moderated t-test, 10% FDR). Another volcano plot shows efflux pump proteins (red dots, Fig. 4B, mexA, mexB, oprM, ycaD_1, and kgP3). Average intensity of MDR9/average intensity of PAO1 for these proteins is shown in Figure 4C as fold differences and P values. All are expressed significantly higher in MDR9 versus PAO.
Figure 4.
Proteomics: MDR9 versus PAO1. (A, B) Volcano plots; (B, C) efflux pumps significantly increased in MDR9 versus PAO1.
Real-Time RT-PCR
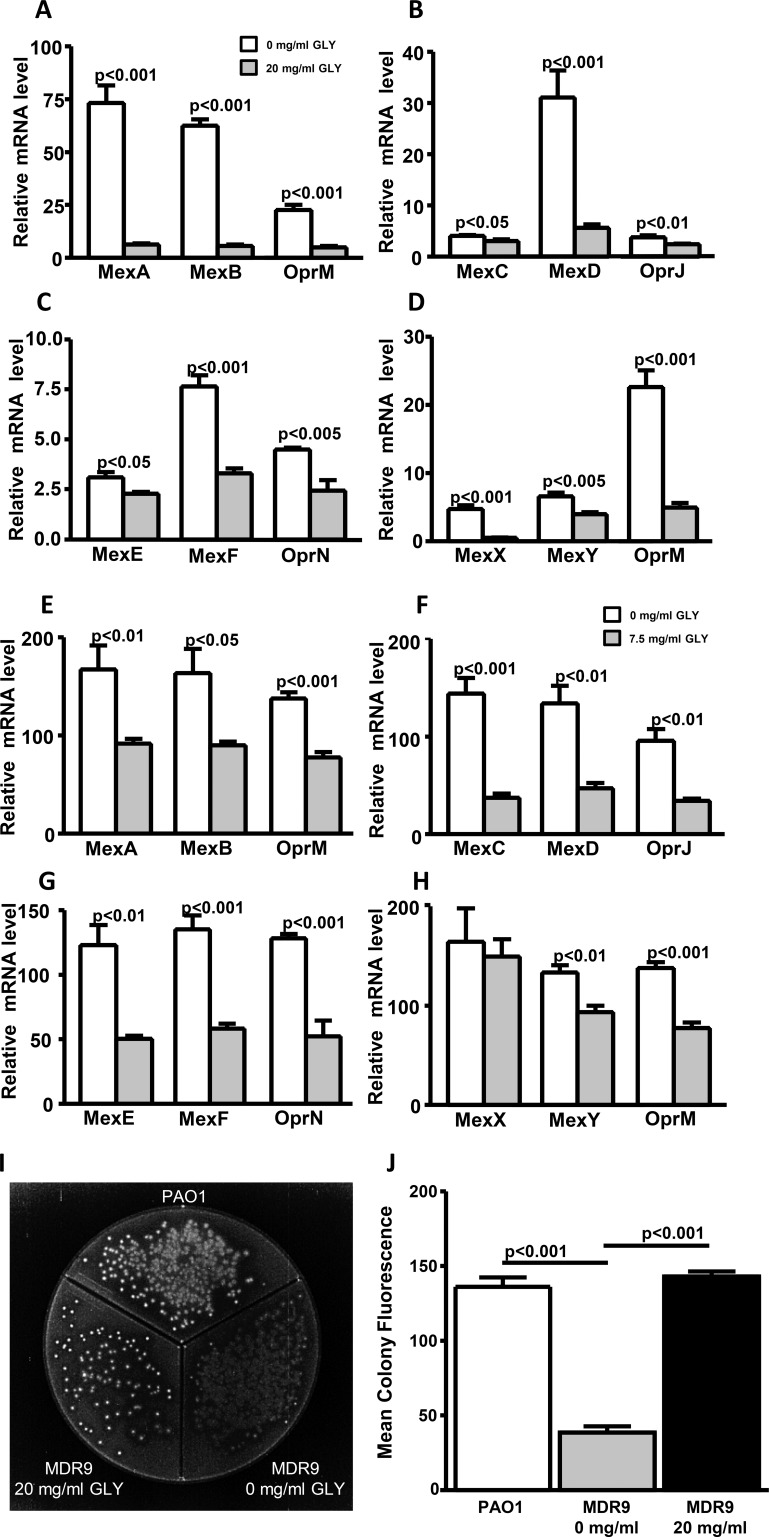
For MDR9 (Figs. 5A–D), GLY (20 mg/mL) significantly reduced mRNA expression levels of all RND (clinically relevant for antibiotic resistance) efflux pumps tested: MexA, MexB, OprM, MexD, MexF, and MexX (P < 0.001 for each), OprN and MexY (P < 0.005 for each), OprJ (P < 0.01), and MexC and MexE (P < 0.05). For B1045 (Figs. 5E–H) GLY (3.75 mg/mL) reduced mRNA expression of efflux pumps: OprM, MexC, MexF, and OprN (P < 0.001 for each), MexA, MexD, OprJ, MexE, and MexY (P < 0.01 for each), and MexB (P < 0.05). GLY had no effect on MexX (H) mRNA levels versus control.
Figure 5.
Effect of GLY (20 mg/mL or 7.5 mg/mL, 3 hours) on RND efflux pump mRNA expression for MDR9 (A–D) and B1045 (E–H). Ethidium bromide plate (I): PAO1-positive fluorescence control, MDR9 without GLY (no fluorescence) and after 18 hours treatment with 20 mg/mL GLY, fluorescence similar to control. Mean colony fluorescence (J).
To assess efflux pump activity, PAO1 (positive control) was plated on divided EB agar plates (Fig. 5I) and showed colonies with greater fluorescence intensity (retained EB) when compared with MDR9 (no GLY). GLY (20 mg/mL, 18 hours) treatment of MDR9 increased the fluorescence intensity of colonies. Mean colony fluorescence (Fig. 5J) shows significantly reduced fluorescence for MDR9 not treated with GLY compared to PAO1 (P < 0.001) and significantly increased fluorescence after GLY treatment (P < 0.001) which appeared similar to fluorescence levels seen for PAO1.
GLY and Ciprofloxacin
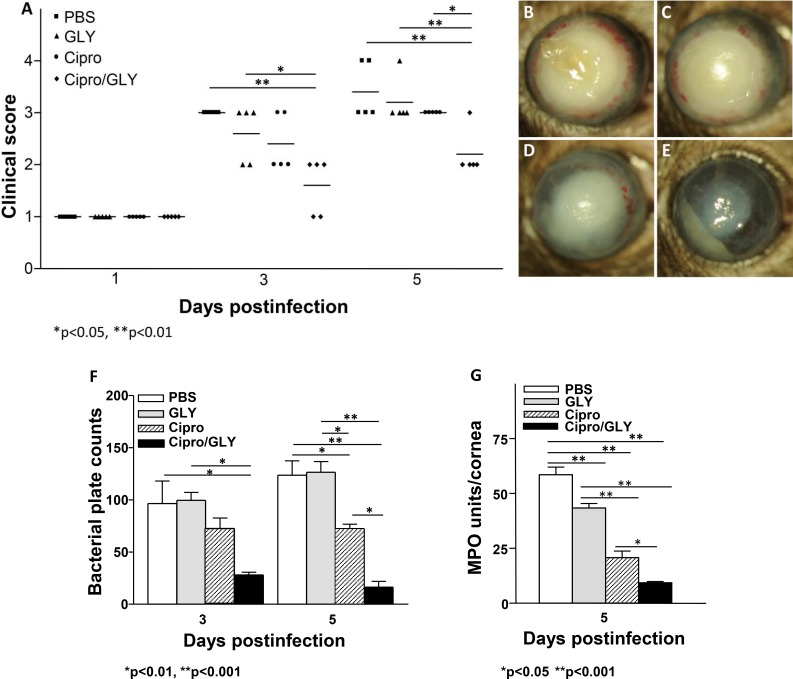
MDR9 infected mouse corneas were treated with GLY (100 μg) and/or Ciprofloxacin (7.5 μg) beginning at 18 hours p.i. (Figs. 6A–E). Clinical scores (Fig. 6A) at 3 days p.i. showed significant differences between GLY plus ciprofloxacin (P < 0.01) versus PBS or GLY alone (P < 0.05) but not between GLY and ciprofloxacin. However, at 5 days p.i., GLY plus ciprofloxacin treatment was significantly different compared with ciprofloxacin (P < 0.05), PBS (P < 0.01) or GLY (P < 0.01) alone. Photos of eyes taken with a slit lamp from PBS (Fig. 6B) and GLY treated (Fig. 6C) mice at 5 days p.i. show similar opacity, when treatment is delayed for 18 hours p.i.; ciprofloxacin-treated eyes (Fig. 6D) had slightly reduced opacity when compared to PBS- (Fig. 6B) or GLY-treated eyes (Fig. 6C). Combining GLY with ciprofloxacin (Fig. 6E) dramatically reduced opacity; hypopyon in the anterior chamber was visible. Actual bacterial plate counts (Fig. 6F) showed that GLY plus ciprofloxacin treatment significantly decreased bacterial load at 3 days p.i., compared to either PBS (P < 0.01) or GLY (P < 0.01). At 5 days p.i., actual bacterial counts were further significantly reduced in the GLY plus ciprofloxacin group compared with ciprofloxacin (P < 0.01), PBS (P < 0.001), or GLY (P < 0.001). Treatment with ciprofloxacin alone did reduce bacterial plate count compared with PBS (P < 0.01) or GLY alone (P < 0.01). However, when comparing the 2 days (Fig. 6F, 3 vs. 5 days), plate counts for PBS and GLY increased over time, ciprofloxacin alone was the same, while only the GLY plus ciprofloxacin counts were reduced (∼50%). MPO activity was significantly reduced in the GLY plus ciprofloxacin-treated group compared with all other groups (PBS = P < 0.001, GLY = P < 0.001), and ciprofloxacin alone (P < 0.05; Fig. 6G). MPO also was significantly reduced when comparing GLY (P < 0.001) and/or ciprofloxacin alone (P < 0.001) with PBS at 5 days p.i.
Figure 6.
GLY potentiates ciprofloxacin. Shown for clinical score (A) slit-lamp photo (compare ciprofloxacin + GLY [E] with PBS [B]; GLY [C] and ciprofloxacin [D]); bacterial plate count at 3 and 5 days p.i. (F), and MPO (G) at 5 days p.i.
Discussion
Over the past 20 years, increasing drug resistance in PA isolates, including those from keratitis patients4,27 has been reported. This trend in antibiotic resistant bacterial infections is of serious global concern, fueled by the lack of effective antibiotics available and few being developed.3 GLY, derived from Glycyrrhiza glabra, is antimicrobial and anti-inflammatory6; it has many additional effects6 and has shown promise against non-MDR isolates7,8,16,19,20 and our current work shows it is effective against MDR isolates. Clinically, GLY also has been successfully used to treat blepharitis28 and hepatitis patients29,30 with no toxicity.
Currently, many in vitro methods allow testing of antimicrobial efficacy31,32 but MIC is most often used. MIC is defined as the lowest concentration of a compound required to completely inhibit microbial growth over a given time. Different MIC assays (e.g., broth microdilution, disk diffusion, etc.) are used to determine antimicrobial profiles.16,20,33,34 Using broth microdilution, we found that GLY reduced growth in both MDR clinical isolates, one from sputum and one from a keratitis patient. The ocular isolate was more sensitive to GLY which was somewhat puzzling and may reflect differences in virulence factors (e.g., biofilm production, or number of efflux pumps) and requires follow up studies to determine the reason(s). In past work, we never observed different GLY MICs when testing several non-MDR clinical isolates.7,19,20 We also tested the MIC of ciprofloxacin, to which MDR9 is resistant and found an MIC of 32 μg/mL. The MIC for GLY together with ciprofloxacin showed that GLY reduced ciprofloxacin MIC 2-fold, suggesting that less antibiotic could be used for treatment. This finding could be advantageous clinically, as there is a small percentage of individuals who have adverse responses to fluoroquinolones.35 Although fluoroquinolones remain safe for most people, the drugs are widely prescribed and can severely damage human cells, including altering the host microbiome.36 In addition, the in vitro MIC assay is often a predictor of success for in vivo work.37
An assay (Molecular Probes) was used to test isolates MDR9 and B1045 with or without GLY treatment. We used two approaches to gather information regarding permeability (confocal microscopy) and viable plate count. Confocal microscopy indicated GLY was capable of increasing membrane permeabilization and this was reflected in a decreased plate count. SEM confirmed that GLY altered bacterial membrane structure and created “holes” in the bacterial membrane when compared with controls. However, GLY treatment did not affect all of the bacteria, and some appeared similar to PBS controls. These data agree with and add to recent in vitro work by Chakotiya et al.16 who tested glycyrrhetic acid and an herbal extract, reporting that they were effective in targeting several physiologic parameters (growth, membrane permeabilization, efflux activity, and biofilm formation) of one MDR isolate of PA.
In our study, a mass spectrometry–based proteomic analysis was used to comparatively analyze the proteomic profiles of MDR9 versus PAO1. Efflux pumps were among the proteins that were identified as significantly increased. Bacterial efflux pumps are proteins that are embedded in the bacterial plasma membrane. They function to recognize noxious agents that have penetrated the cell wall, and expel them.38 In this study, hyperexpression of efflux pumps of the resistance-nodulation-cell division (RND)–type chromosomally encoded by mexAB-oprM, mexCD-oprJ, mexEF-oprN, and mexXY (-oprA) were detected and have been shown to contribute to multidrug resistance phenotypes.39 PAO1, the reference strain used in our study, has 12 efflux systems of the RND type.40,41 Four of these contribute most significantly to antibiotic resistance: MexAB-OprM, MexCD-OprJ, MexEF-OprN, and MexXY-OprM.42 MexB transports β-lactams, including but not limited to β-lactamase inhibitors, carbapenems, aminoglycides, and fluoroquinolones. Fluoroquinolones like levofloxacin, ciprofloxacin, norfloxacin, and others, have been reported to be most efficiently exported by MexEF-OprN and MexCD-OprJ and with less efficiency by MexAB-OprM and MexXY-OprM (or OprA).40,41 Susceptibility of PA toward many antibiotics has been restored when four systems (MexAB-OprM, MexCD-OprJ, MexEF-OprN, and MexXY-OprM) have been deleted.42,43 In this regard, GLY decreased mRNA expression of RND efflux pumps in both isolates (exception = MexX in B1045) which is consistent with our in vivo data showing increased sensitivity of MDR9 to ciprofloxacin when combined with GLY. To test activity of MDR9 efflux pumps, EB, a common substrate, and a DNA-intercalating agent that fluoresces only when bound to DNA,44 was used. We found that higher fluorescence, indicating EB is intracellular24 was observed after treatment of MDR9 with GLY, indicating a change in efflux activity. In fact, quantitation of the data showed that with GLY, fluorescence for MDR9 was similar to the PAO1 positive control. What cannot be determined from these data is whether GLY altered uptake or retention of EB or had other deleterious effects to bacterial membranes, but the latter was provided by SEM. SEM data confirmed that bacterial membranes were perturbed, but also showed that some bacteria remained viable after GLY treatment. We hypothesize that it is this group that would be responsive to having their efflux pump systems modulated by GLY (as shown in our EB data). In this regard, uptake of compounds can be achieved by control of decreasing porins in the outer membrane and/or by increasing the effectiveness of the efflux of antibiotics, usually by increasing the number of pumps.45,46 In regard to efflux pumps, in gram-negative bacteria, the predominant form of resistance is through the efflux pump mechanism,47 leading to decreased bioavailability of antibiotic allowing the bacteria to survive, despite the noxious stimulus.48 Of course, overexpression of the main efflux pumps is one among many mechanisms, resulting in a MDR phenotype. Our mRNA data supports GLY reduction of the majority of efflux pumps (RND), but we cannot rule out that other contributors to MDR such as biofilm, production of antibiotic inactivating enzymes, etc., are involved. We also do not know if uptake was pH or temperature dependent. To resolve this, future approaches including, but not limited to flow cytometry, could be used to interrogate mechanisms involved in the change in efflux evoked by GLY.24,49
In vivo, when GLY was combined with ciprofloxacin (1/2 MIC) for treatment after infection with MDR9 (18 hours p.i.), we detected increased effectiveness (e.g., additive activity) of ciprofloxacin, including reduced corneal opacity and clinical score when compared with ciprofloxacin, GLY, or PBS. Bacterial load was decreased optimally only by this combination and MPO activity as well. GLY also has been shown in vitro (47 clinical isolates) to decrease gentamicin resistance (reduced MIC) in vancomycin resistant enterococci,50 essentially due to the saponin effect of GLY which can modify the local chemical environment of the cell allowing modification of the uptake of antibiotics. Testing comparatively the effect of saponins (e.g., GLY, β-ascein, α-hederin, and primulic acid 1) together with antibiotic might be warranted to further elucidate how GLY modifies ciprofloxacin resistance in MDR-PA.
In summary, these studies provide evidence that GLY has antimicrobial and anti-inflammatory effects on MDR isolates capable of causing difficult to treat and severe keratitis in vivo. Several mechanisms appear to be involved and include: reducing MIC of ciprofloxacin, permeabilizing bacteria, inducing their swelling and clumping, reducing efflux pump activity and increasing bacterial killing when combined with ciprofloxacin in vivo.
Acknowledgments
Supported by grants R01EY016058 and P30EY004068 from the National Eye Institute, National Institutes of Health, by a Research to Prevent Blindness unrestricted grant to the Department of Ophthalmology, Kresge Eye Institute and by bridge funding from the Office of the Vice President for Research, Wayne State University.
Disclosure: L.D. Hazlett, None; S.A. Ekanayaka, None; S.A. McClellan, None; R. Francis, None
References
- 1.Gadjeva M, Nagashima J, Zaidi T, Mitchell RA, Pier GB. Inhibition of macrophage migration inhibitory factor ameliorates ocular Pseudomonas aeruginosainduced keratitis. PLoS Pathog. 2010;6:e1000826. doi: 10.1371/journal.ppat.1000826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Brien TP, Maguire MG, Fink NE, Alfonso E, McDonnell P. Efficacy of ofloxacin vs cefazolin and tobramycin in the therapy for bacterial keratitis. Report from the Bacterial Keratitis Study Research Group. Arch Ophthalmol. 1995;113:1257–1265. doi: 10.1001/archopht.1995.01100100045026. [DOI] [PubMed] [Google Scholar]
- 3.Mesaros N, Nordmann P, Plesiat P, et al. Pseudomonas aeruginosa resistance and therapeutic options at the turn of the new millennium. Clin Microbiol Infec. 2007;13:560–578. doi: 10.1111/j.1469-0691.2007.01681.x. [DOI] [PubMed] [Google Scholar]
- 4.U.S. Department of Health and Human Services. Antibiotic resistance threats in the United States, 2013. Available at: https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf.
- 5.Smith R, Coast J. The true cost of antimicrobial resistance. BMJ. 2013;346:f1493. doi: 10.1136/bmj.f1493. [DOI] [PubMed] [Google Scholar]
- 6.Asl MN, Hosseinzadeh H. Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytother Res. 2008;22:709–724. doi: 10.1002/ptr.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ekanayaka SA, McClellan SA, Barrett RP, Kharotia S, Hazlett LD. Glycyrrhizin reduces HMGB1 and bacterial load in Pseudomonas aeruginosa keratitis. Invest Ophthalmol Vis Sci. 2016;57:5799–5809. doi: 10.1167/iovs.16-20103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ekanayaka SA, McClellan SA, Barrett RP, Hazlett LD. Topical Glycyrrhizin Is Therapeutic for Pseudomonas aeruginosa Keratitis. J Ocul Pharmacol Ther. 2018;34:239–249. doi: 10.1089/jop.2017.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang W, Zhao F, Fang Y, et al. Glycyrrhizin protects against porcine endotoxemia through modulation of systemic inflammatory response. Crit Care. 2013;17:R44. doi: 10.1186/cc12558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y, Xiang J, Liu M, Wang S, Lee RJ, Ding H. Protective effects of glycyrrhizic acid by rectal treatment on a TNBS-induced rat colitis model. J Pharm Pharmacol. 2011;63:439–446. doi: 10.1111/j.2042-7158.2010.01185.x. [DOI] [PubMed] [Google Scholar]
- 11.Ni YF, Kuai JK, Lu ZF, et al. Glycyrrhizin treatment is associated with attenuation of lipopolysaccharide-induced acute lung injury by inhibiting cyclooxygenase-2 and inducible nitric oxide synthase expression. J Surg Res. 2011;165:e29–e35. doi: 10.1016/j.jss.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Gong G, Xiang L, Yuan L, et al. Protective effect of glycyrrhizin, a direct HMGB1 inhibitor, on focal cerebral ischemia/reperfusion-induced inflammation, oxidative stress, and apoptosis in rats. PLoS One. 2014;9:e89450. doi: 10.1371/journal.pone.0089450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arase Y, Ikeda K, Murashima N, et al. The long term efficacy of glycyrrhizin in chronic hepatitis C patients. Cancer. 1997;79:1494–1500. doi: 10.1002/(sici)1097-0142(19970415)79:8<1494::aid-cncr8>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 14.Cavone L, Muzzi M, Mencucci R, et al. 18beta-glycyrrhetic acid inhibits immune activation triggered by HMGB1, a pro-inflammatory protein found in the tear fluid during conjunctivitis and blepharitis. Ocul Immunol Inflamm. 2011;19:180–185. doi: 10.3109/09273948.2010.538121. [DOI] [PubMed] [Google Scholar]
- 15.Mollica L, De Marchis F, Spitaleri A, et al. Glycyrrhizin binds to high-mobility group box 1 protein and inhibits its cytokine activities. Chem Biol. 2007;14:431–441. doi: 10.1016/j.chembiol.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Chakotiya AS, Tanwar A, Narula A, Sharma RK. Alternative to antibiotics against Pseudomonas aeruginosa Effects of Glycyrrhiza glabra on membrane permeability and inhibition of efflux activity and biofilm formation in Pseudomonas aeruginosa and its in vitro time-kill activity. Microb Pathog. 2016;98:98–105. doi: 10.1016/j.micpath.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Kesarwani K, Gupta R, Mukerjee A. Bioavailability enhancers of herbal origin: an overview. Asian Pac J Trop Biomed. 2013;3:253–266. doi: 10.1016/S2221-1691(13)60060-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poole K. Pseudomonas aeruginosa resistance to the max. Front Microbiol. 2011;2:65. doi: 10.3389/fmicb.2011.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng X, Ekanayaka SA, McClellan SA, Barrett RP, Vistisen K, Hazlett LD. Effects of Glycyrrhizin on a drug resistant isolate of Pseudomonas aeruginosa. EC Ophthalmol. 2018;9:265–280. [Google Scholar]
- 20.Peng X, Ekanayaka SA, McClellan SA, Barrett RP, Vistisen K, Hazlett LD. Characterization of three ocular clinical isolates of P. aeruginosa Viability, biofilm formation, adherence, infectivity, and effects of glycyrrhizin. Pathogens. 2017;6:E52. doi: 10.3390/pathogens6040052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Senoo T, Joyce NC. Cell cycle kinetics in corneal endothelium from old and young donors. Invest Ophthalmol Vis Sci. 2000;41:660–667. [PubMed] [Google Scholar]
- 22.Barry AL, Craig WA, Nadler H, Reller LB, Sanders CC, Swenson JM. Methods for Determining Bactericidal Activity of Antimicrobial Agents: Approved Guideline. Document M26-A. Wayne, PA: Clinical Laboratory Standards Institute;; 1999. p. 19. [Google Scholar]
- 23.Esco MA, Hazlett LD, Kurpakus-Wheater M. Pseudomonas aeruginosa binds to extracellular matrix deposited by human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2002;43:3654–3659. [PubMed] [Google Scholar]
- 24.Blair JMA, Piddock LJV. How to measure export via bacterial multidrug resistance efflux pumps. mBio. 2016;7:e00840–16. doi: 10.1128/mBio.00840-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hazlett LD, Moon MM, Strejc M, Berk RS. Evidence for N-acetylmannosamine as an ocular receptor for P. aeruginosa adherence to scarified cornea. Invest Ophthalmol Vis Sci. 1987;28:1978–1985. [PubMed] [Google Scholar]
- 26.Williams RN, Patterson CA, Eakins KE, Bhattacherjee P. Quantification of ocular inflammation: evaluation of polymorphonuclear leucocyte infiltration by measuring myeloperoxidase activity. Curr Eye Res. 1982;2:465–470. doi: 10.3109/02713688208996350. [DOI] [PubMed] [Google Scholar]
- 27.Oldenburg CE, Lalitha P, Srinivasan M, et al. Emerging moxifloxacin resistance in Pseudomonas aeruginosa keratitis isolates in South India. Ophthalmic Epidemiol. 2013;20:155–158. doi: 10.3109/09286586.2013.790978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mencucci R, Favuzza E, Menchini U. Assessment of the tolerability profile of an ophthalmic solution of 5% glycyrrhizin and copolymer Peg/Ppg on healthy volunteers and evaluation of its efficacy in the treatment of moderate to severe blepharitis. Clin Ophthalmol. 2013;7:1403–1410. doi: 10.2147/OPTH.S47657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arase Y, Ikeda K, Murashima N, et al. The long term efficacy of glycyrrhizin in chronic hepatitis C patients. Cancer. 1997;79:1494–1500. doi: 10.1002/(sici)1097-0142(19970415)79:8<1494::aid-cncr8>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 30.Davis EA, Morris DJ. Medicinal uses of licorice through the millennia: The good and plenty of it. Mol Cell Endocrinol. 1991;78:1–6. doi: 10.1016/0303-7207(91)90179-v. [DOI] [PubMed] [Google Scholar]
- 31.Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: A review. J Pharm Ana. 2016;6:71–79. doi: 10.1016/j.jpha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Neill AJ, Chopra I. Preclinical evaluation of novel antibacterial agents by microbiological and molecular techniques. Expert Opin Investig Drugs. 2004;13:1045–1063. doi: 10.1517/13543784.13.8.1045. [DOI] [PubMed] [Google Scholar]
- 33.Krausse R, Bielenberg J, Blaschek W, Ullmann U. In vitro anti-Helicobacter pylori activity of Extractum liquiritiae, glycyrrhizin and its metabolites. J Antimicrob Chemother. 2004;54:243–246. doi: 10.1093/jac/dkh287. [DOI] [PubMed] [Google Scholar]
- 34.Liu G, He YH, Zhang FF, et al. Effects of glycyrrhizic acid on the growth and acid-producing of Streptococcus mutans in vitro. Sichuan Da Xue Bao Yi Xue Ban. 2010;41:634–637. [PubMed] [Google Scholar]
- 35.Marchant J. When antibiotics turn toxic. Nature. 2018;555:431–433. doi: 10.1038/d41586-018-03267-5. [DOI] [PubMed] [Google Scholar]
- 36.Keeney KM, Yurist-Doutsch S, Arrieta MC, Finlay BB. Effects of antibiotics on human microbiota and subsequent disease. Annu Rev Microbiol. 2014;68:217–235. doi: 10.1146/annurev-micro-091313-103456. [DOI] [PubMed] [Google Scholar]
- 37.Lepak A, Castanheira M, Diekema D, Pfaller M, Andes D. Optimizing echinocandin dosing and susceptibility breakpoint determination via in vivo pharmacodynamic evaluation against Candida glabrata with and without fks mutations. Antimicrob Agents Chemother. 2012;56:5875–5882. doi: 10.1128/AAC.01102-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amaral L, Martins A, Spengler G, Molnar J. Efflux pumps of gram-negative bacteria: what they do, how they do it, with what and how to deal with them. Front Pharmacol. 2014;4:168. doi: 10.3389/fphar.2013.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dreier J, Ruggerone P. Interaction of antibacterial compounds with RND efflux pumps in Pseudomonas aeruginosa. Front Microbiol. 2015;6:660. doi: 10.3389/fmicb.2015.00660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fernandez L, Hancock RE. Adaptive and mutational resistance: role of porins and efflux pumps in drug resistance. Clin Microbiol Rev. 2012;25:661–681. doi: 10.1128/CMR.00043-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun J, Deng Z, Yan A. Bacterial multidrug efflux pumps: mechanisms, physiology and pharmacological exploitations. Biochem Biophys Res Commun. 2014;453:254–267. doi: 10.1016/j.bbrc.2014.05.090. [DOI] [PubMed] [Google Scholar]
- 42.Morita Y, Komori Y, Mima T, Kuroda T, Mizushima T, Tsuchiya T. Construction of a series of mutants lacking all of the four major mex operons for multidrug efflux pumps or possessing each one of the operons from Pseudomonas aeruginosa PAO1: MexCD-OprJ is an inducible pump. FEMS Microbiol Lett. 2001;202:139–143. doi: 10.1111/j.1574-6968.2001.tb10794.x. [DOI] [PubMed] [Google Scholar]
- 43.Kumar A, Chua KL, Schweizer HP. Method for regulated expression of single-copy efflux pump genes in a surrogate Pseudomonas aeruginosa strain: identification of the BpeEF-OprC chloramphenicol and trimethoprim efflux pump of Burkholderia pseudomallei 1026b. Antimicrob Agents Chemother. 2006;50:3460–3463. doi: 10.1128/AAC.00440-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olmsted J, Kearns DR. Mechanism of ethidium bromide fluorescence enhancement on binding to nucleic acids. Biochemistry. 1977;16:3647–3654. doi: 10.1021/bi00635a022. [DOI] [PubMed] [Google Scholar]
- 45.Nikaido H. Preventing drug access to targets: cell surface permeability barriers and active efflux in bacteria. Semin Cell Dev Biol. 2001;12:215–223. doi: 10.1006/scdb.2000.0247. [DOI] [PubMed] [Google Scholar]
- 46.Piddock LJ. Multidrug-resistance efflux pumps-not just for resistance. Nat Rev Microbiol. 2006;4:629–636. doi: 10.1038/nrmicro1464. [DOI] [PubMed] [Google Scholar]
- 47.Karmakar P, Gaitonde V. Promising recent strategies with potential clinical translational value to combat antibacterial resistant surge. Medicines. 2019;6:21. doi: 10.3390/medicines6010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davin-Regli A, Pagès JM. Regulation of efflux pumps in Enterobacteriaceae: genetic and chemical effectors. In: Zmabiles-Cuevos CF, editor. Antimicrobial Resistance in Bacteria. Norfolk, VA: Horizon Biosciences;; 2006. pp. 55–75. [Google Scholar]
- 49.Martins M, McCusker MP, Viveiros M, et al. A simple method for assessment of MDR bacteria for over-expressed efflux pumps. Open Microbiol J. 2013;7:72–82. doi: 10.2174/1874285801307010072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmidt S, Heymann K, Melzig MF, Bereswill S, Heimesaat MM. Glycyrrhizic acid decreases gentamicin-resistance in vancomycin-resistant enterococci. Planta Med. 2016;82:1540–1545. doi: 10.1055/s-0042-114781. [DOI] [PubMed] [Google Scholar]