Abstract
Signal transduction systems configured around a core phosphotransfer step between a histidine kinase and a cognate response regulator protein occur in organisms from all domains of life. These systems, termed two-component systems, constitute the majority of multi-component signaling pathways in Bacteria but are less prevalent in Archaea and Eukarya. The core signaling domains are modular, allowing versatility in configuration of components into single-step phosphotransfer and multi-step phosphorelay pathways, the former being predominant in bacteria and the latter in eukaryotes. Two-component systems regulate key cellular regulatory processes that provide adaptive responses to environmental stimuli and are of interest for the development of antimicrobial therapeutics, biotechnology applications, and biosensor engineering. In bacteria, two-component systems have been found to mediate responses to an extremely broad array of extracellular and intracellular chemical and physical stimuli, whereas in archaea and eukaryotes, the use of two-component systems is more limited. This review summarizes recent advances in exploring the repertoire of sensor histidine kinases in the Archaea and Eukarya domains of life.
Keywords: two-component system, histidine kinase, sensor, evolution, signal transduction, phosphorylation
Introduction
Protein phosphorylation is one of the most extensively used modifications in signal transduction pathways in both prokaryotic and eukaryotic cells. Prominent families of enzymes that perform protein phosphorylation encompass serine/threonine kinases, tyrosine kinases, and histidine kinases (HKs). Although HKs dominate prokaryotic signaling pathways, they are less prevalent in eukaryotes 1, 2. A distinct class of mammalian HKs, specifically nucleoside diphosphate kinases, function together with associated phosphatases to catalyze reversible histidine phosphorylation of proteins, and the roles of such modifications in cellular regulation are beginning to be uncovered 3– 7. However, the large family of HKs that is prevalent in prokaryotes is absent from animals. Historically, a small number of eukaryotic HKs have been studied in plants, yeasts, filamentous fungi, and slime molds. Recent studies have expanded the characterization of HKs in other eukaryotic lineages and archaea, allowing a broader assessment of the types of signaling systems mediated by HKs and their phylogenetic distribution and evolution. HKs are central to regulatory systems that impact agriculture, the environment, and both beneficial and pathogenic interactions of microbes with humans and other animals. Their great diversity, versatility, and broad distribution, as well as the specificity of HK communication with cognate downstream components, make them attractive targets for therapeutics 8– 12 and biotechnological interventions 13– 16 and also as building blocks for engineered biosensor systems 17– 21.
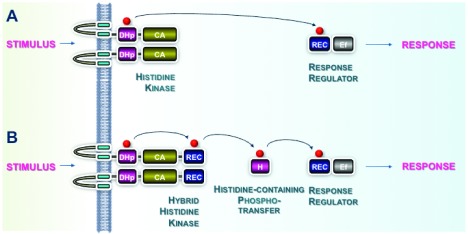
HKs occur primarily within pathways designated as “two-component systems” (TCSs) 22, 23 ( Figure 1). TCSs correspond to cell signaling circuitries that permit organisms, either unicellular or multicellular, to sense and respond to a broad palette of environmental changes. From a mechanistic perspective, these transduction pathways rely on the sequential transfer of a phosphoryl group on conserved histidine or aspartate residues (or both) located in several families of proteins. In prokaryotes, TCSs are usually restricted to communication between two functional modules (that is, phosphoryl transfer between HKs and response regulators [RRs]) ( Figure 1A). In canonical prokaryotic systems, the perception of a stimulus regulates the opposing autophosphorylation and phosphatase activities of the HK, which thus acts as a primary sensor. The phosphoryl group is transferred to a RR that effects the response. In many prokaryotic TCSs (~65%), the RR is a transcription factor that directly regulates the expression of a set of genes required for an adaptive response to the stimulus 24, 25.
Figure 1. Two-component system phosphotransfer schemes.
( A) A typical phosphotransfer pathway, as is usually found in prokaryotes. The perception of a stimulus by extracytoplasmic domains of the histidine kinase (HK) regulates its activities. The HK autophosphorylates at a conserved histidine residue (H) using ATP bound to the catalytic ATPase domain (containing conserved motifs N, G1, F, and G2). The phosphoryl group (P) is transferred to a conserved aspartate residue (D) located within the cognate response regulator (RR). ( B) An example of a multi-step phosphorelay, as often occurs in eukaryotes. The HK is termed “hybrid” because an additional aspartate-containing domain is fused to the ATPase domain. The phosphorelay involves multiple phosphoryl transfer steps. The first is an intramolecular transfer between the conserved histidine (H) and a conserved aspartate residue (D) located within the C terminus of the sensor HK. Subsequently, the phosphoryl group is transferred to a histidine-containing phosphotransfer protein and finally to a cognate RR. Conserved domains of the two-component system (TCS) proteins are shown in green, gold, and blue. Variable sensor domains of the HK and effector domains (Ef) of the RR that adapt the systems to a wide range of input stimuli and output responses are shown in gray.
In contrast, classic eukaryotic TCSs usually involve more complex multi-step phosphorelays 26, 27 but, as in prokaryotes, also begin by the perception of an input stimulus by a sensor HK, specifically a “hybrid” HK ( Figure 1B). Signal perception modulates autophosphorylation of the HK on a conserved histidine residue prior to transfer to a conserved aspartate residue in a C-terminal domain of the HK. The phosphoryl group is then transmitted to a conserved histidine residue of a small shuttle protein of about 150 amino acid residues (histidine-containing phosphotransfer protein, HPt; Pfam 28 ID PF01627) and finally to a conserved aspartate residue of a protein belonging to the RR family. The phosphorylation state of this RR orchestrates subsequent molecular events underlying the response to the input signal, either by directly regulating transcription or by interfacing with other conventional eukaryotic signaling strategies such as mitogen-activated protein kinase cascades or cAMP signaling that control the output response. As a result of specific evolutionary paths in which the various eukaryotic lineages have engaged, the canonical TCS pathway described above appears to have degenerated in several clades in which these cell signaling systems have been described.
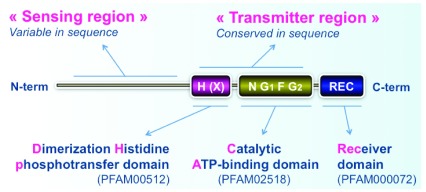
The canonical structure of HKs is composed of a set of variable and conserved domains 29 ( Figure 2) that couple the sensing of a wide range of chemical or physical stimuli to the phosphotransfer pathway. Here, we review recent advances in the characterization of HKs in archaea and eukaryotes with an emphasis on what they sense and what the main output processes that this prominent family of sensors regulates are. Some emerging trends dealing with their distribution among the various lineages and their evolution are also considered.
Figure 2. Canonical structure of histidine kinases (HKs).
HKs are composed of a set of variable and conserved domains. The first region corresponds to a highly variable, typically N-terminal sequence that determines which stimulus is perceived by the HK. This region is referred to as the “sensing domain”. The central “transmitter region” is composed of two conserved domains: a dimerization histidine phosphotransfer (DHp) domain (His kinase A, HisKA; Pfam ID PF00512, or other subfamily such as HisKA_2, HisKa_3) and a catalytic ATP-binding (CA) domain (histidine kinase-like ATPase catalytic, HATPase_c; Pfam ID PF02518). The DHp domain includes an H-box, usually containing the phosphorylatable histidine, and an X-box. The CA subdomain includes four distinct sequence motifs: the N-, G1-, F-, and G2-boxes. In contrast to prokaryotic HKs, most eukaryotic HKs contain an additional C-terminal RR receiver (REC) domain (Response_reg; Pfam ID PF00072) that includes a phosphorylatable aspartate residue. Thus, eukaryotic HKs are generally called “hybrid HKs” 26.
HKs in Archaea
TCSs are relatively rare in archaea and are not uniformly distributed across archaeal phyla 30– 32. The majority of archaeal TCSs have been identified in Euryarchaeota and Thaumarchaeota, but the possibility exists that greater distribution will be revealed as more archaeal genomes are sequenced. Several notable features differ between archaeal and bacterial TCSs 32. Archaeal genomes typically contain fewer TCSs and typically have an HK-to-RR ratio greater than 1, suggesting that multiple HKs might feed into a single RR or that HKs might be paired with alternative downstream components. Unlike bacterial HKs that are mostly transmembrane proteins (estimated at 73 to 88%), 62% of archaeal HKs lack identifiable transmembrane regions and are presumed to be cytoplasmic. Interestingly, previous analyses of bacterial and archaeal chemoreceptors have shown a similar bias for cytoplasmic sensing in archaea 33. Correspondingly, although extracellular Cache—calcium channels and chemotaxis receptors, also previously identified as PAS (period circadian protein-Aryl hydrocarbon receptor nuclear translocator protein-single-minded protein), PAS-like, PhoQ-DcuS-CitA (PDC), PDC-like, and PDC/PAS 34– 36 domains—are the most abundant sensor domains in bacterial HKs, intracellular PAS and GAF (cGMP-specific phosphodiesterases-adenylyl cyclases-FhlA) domains are predominant in archaeal HKs, and 72% of them contain one or more PAS or GAF domains (or both) 32. In addition to Cache domains, less populated sensor domain families include MEDS (methanogen/methylotroph, DcmR sensory domain; Pfam ID PF14417), PocR (Pfam ID PF10114, HisKA_7TM (Pfam ID PF16927), and HisKA_4TM (Pfam ID PF16926), the latter being distinct to haloarchaea.
Other than chemotaxis 37, 38 and phototaxis 39, 40 systems, few archaeal TCS pathways have been characterized. Two recently studied systems, similar to conventional TCSs, are the LtrK/LtrR TCS that mediates temperature-dependent gene regulation in an Antarctic methanogen 41 and a TCS comprised of HK FilI and RRs FilR1 and FilR2 that regulates transcription of methanogenesis genes in response to unknown stimuli in Methanosaeta harundinacea 42. Two less conventional HKs, each containing multiple PAS and GAF domains, have recently been characterized from Methanosarcina acetivorans 43, 44. MsmS is a heme-based redox/dimethyl sulfide sensor, and RdmS is a thiol-based redox sensor; both regulate genes involved in methyl sulfide metabolism. Autophosphorylation of both HKs is redox-dependent, although neither contains a phosphorylatable histidine and MsmS phosphorylation has been shown to occur at tyrosine. Furthermore, the identified downstream regulators lack RR receiver (REC) domains, indicating that these HKs function in signaling systems distinct from TCSs.
The outputs of archaeal TCSs are currently as unexplored as the inputs. However, unlike archaeal HK sensor domains that belong to families common to bacterial counterparts such as PAS and GAF, albeit with different prevalence, archaeal RR effector domains do not correspond to the major families found in bacterial RRs 32. Most notable is the low abundance of recognizable DNA-binding domains (6%). The fraction of archaeal RRs consisting solely of REC domains (39%) is almost double that observed in bacteria. Other major families include dimerization histidine phosphotransfer (DHp) domains (that is, HisKA), PAS n, GAF, PAS-GAF, chemotaxis CheB, HalX (with a predicted helix-turn-helix, possibly DNA-binding), and various enzyme domains as well as several novel domain families.
HKs in Eukarya
Historically, plants were the first eukaryotic kingdom in which HKs were identified and functions of plant HKs have been informed primarily in the last decades by descriptions of TCSs in the model plant Arabidopsis thaliana 45– 48. Along with these pioneering works in plants, some groups of HKs were progressively characterized in other eukaryotic lineages such as Amoebozoa (mainly the slime mold Dictyostelium discoideum) 49– 52 and Fungi (mainly the yeasts Saccharomyces cerevisiae, Candida albicans, and Cryptococcus neoformans and the filamentous fungi Neurospora crassa and Aspergillus fumigatus) 53, 54. To date, what the biological roles in the remaining eukaryotic phyla are and what HKs sense are still largely obscure 55. In the following section, we will briefly describe major groups of eukaryotic HKs, notably their structures, phylogenetic distribution, and their roles in hormone perception, stress adaptation, and developmental programs.
The first group of eukaryotic HKs characterized is now well documented to be dedicated to the perception of and response to the plant hormone (phytohormone) ethylene ( Table 1) 56. Ethylene is a gas that regulates many aspects of plant development such as seed germination, leaf senescence, and fruit ripening but also orchestrates plant defenses to pathogens (viruses, protists, bacteria, fungi, worms, and insects) 57. From a structural point of view, it is important to highlight that ethylene sensing through HK ethylene receptors (ETRs) occurs by the interaction of the gaseous molecule with the ethylene-binding domain (EtBD) located at the N terminus of the receptors. The EtBD consists of three hydrophobic transmembrane helices (indicated by three asterisks in Table 1) containing seven conserved amino acids required for ethylene binding 58. For a long time, typical ETRs were believed to be restricted to land plants and cyanobacteria 59. Surprisingly, these considerations have now been called into question through the identification in recent years of genes encoding ETR homologs in many other eukaryotic lineages, including green and brown algae, free-living amoebae, photosynthetic diatoms, zooxanthellae that are symbiotically associated with coral reefs, early diverging fungi, filamentous marine protists ( Labyrinthulomycetes), and even the unicellular model animal ancestor Capsaspora owczarzaki 60– 64. Interestingly, an EtDB coupled to a phytochrome domain has recently been identified in a cyanobacterial HK, integrating both light and ethylene responses 65, 66. These discoveries thus provide progressively strong arguments leading to the hypothesis that ethylene, more than strictly a plant hormone, would undoubtedly be one of the oldest molecules of intra- and inter-species communication that appeared on Earth, orchestrating not only developmental programs but also biotic interactions between many organisms 67.
Table 1. Some important groups of eukaryotic histidine kinases (HKs), their known input signals, and their output responses.
| HK group | Structure | Presence in
eukaryotes |
Input signal | Output response | References |
|---|---|---|---|---|---|
|
Ethylene
receptors |
|
Plants, Algae,
Fungi, Amoebae, |
Ethylene | Plants: seed germination,
leaf senescence, fruit ripening, defenses to pathogens |
Ju
et al., 2015
60
Hérivaux et al., 2017 61 Kabbara et al., 2019 64 |
| CHASE-HK |
|
Plants, Algae,
Fungi, Amoebae, |
Cytokinins | Plants: cell division,
embryogenesis, vascular tissue development |
Kaltenegger
et al., 2018
74
Hérivaux et al., 2017 61 |
|
AHK1/Fungal
group VI |
|
Plants, Algae, Fungi | Osmostress Oxidant
stress |
Plants: seed desiccation,
vegetative stress tolerances Fungi: osmotic and oxidant adaptation |
Defosse
et al., 2015
53
Nongpiur et al., 2019 87 |
| Phytochromes |
|
Plants, Algae,
Fungi Amoebae, |
Red/far red light | Plants: phototropism
Fungi: vegetative growth, sexual reproduction |
Rensing
et al., 2016
88
Yu and Fischer, 2019 89 |
| CKI1 |
|
Plants | Cytokinins? | Development of female
gametophyte |
Yuan
et al., 2016
92
Liu et al., 2017 93 Yuan et al., 2018 94 |
| CKI2/AHK5 |
|
Plants | ? | Stress-induced stomatal
closure, salt sensitivity, and resistance against microbial infection |
Pham
et al., 2012
95
Mira-Rodado et al., 2012 96 Bauer et al., 2013 97 |
| Fungal group III |
|
Fungi, Amoebae | Osmostress | Fungi: oxidant adaptation,
development, virulence |
Defosse
et al., 2015
53
Hérivaux et al., 2016 54 Kabbara et al., 2019 |
| Fungal group X |
|
Fungi, Algae,
Amoebae |
Oxidant stress ? | Fungi: oxidant adaptation,
development, virulence |
Defosse
et al., 2015
53
Hérivaux et al., 2016 54 Kabbara et al., 2019 64 |
KEY
![]() :Dimerization
histidine
phosphotransfer domain
:Dimerization
histidine
phosphotransfer domain
![]() :Catalytic
ATP-binding domain
:Catalytic
ATP-binding domain
![]() :Rec eiver domain
:Rec eiver domain
![]() : cGMP-specific phosphodiesterases-
Adenylyl cyclases-
FhlA domain
: cGMP-specific phosphodiesterases-
Adenylyl cyclases-
FhlA domain
![]() :Ethylene
Binding
Domain
:Ethylene
Binding
Domain
![]() :Cyclases/
Histidine kinases
Associated
Sensing
Extracellular
:Cyclases/
Histidine kinases
Associated
Sensing
Extracellular
![]() :Trans
membrane
Region
:Trans
membrane
Region
![]() :Calcium channels and
Chemotaxis receptors domain
:Calcium channels and
Chemotaxis receptors domain
![]() :Period circadian protein-
Aryl hydrocarbon receptor nuclear translocator protein-
Single-minded protein
:Period circadian protein-
Aryl hydrocarbon receptor nuclear translocator protein-
Single-minded protein
![]() :Phytochrome domain
:Phytochrome domain
![]() :Histidine kinases-
Adenylate cyclases-
Methyl accepting proteins and
Phosphatases
:Histidine kinases-
Adenylate cyclases-
Methyl accepting proteins and
Phosphatases
![]() :Serine/
Threonine
kinase
related
domain
:Serine/
Threonine
kinase
related
domain
A second well-known eukaryotic HK group encompasses CHASE (cyclases/histidine kinases associated sensing extracellular) 68, 69 domain-containing HKs (CHASE-HKs) ( Table 1). To date, most of the members belonging to this group have been characterized in plants as cytokinin receptors 62, 70. Cytokinins correspond to another family of prominent phytohormones involved in many developmental processes in plants, including cell division, embryogenesis, vascular tissue development, and root architecture 71. The hormone is perceived by this type of transmembrane HK through the N-terminal region that comprises an extracellular loop 70, 72. More precisely, some crucial residues have been identified within the CHASE sequence to be essential for the binding of the hormones 70, 72, 73. As initially postulated for ethylene, cytokinin signal transduction pathways were presumed to be found exclusively in plants 74. However, these hormones have recently been the subject of very interesting advances that suggest a broader occurrence in the tree of life, notably in eubacteria 75 and eukaryotic microorganisms 62. It has been experimentally shown that a bacterial CHASE-HK senses cytokinin, highlighting the importance of HK-cytokinin interactions in inter-kingdom communication 75. In addition, several genes encoding CHASE-HKs were unearthed in the past two years by browsing the genomes of various non-plant eukaryotic clades 61– 64. These include, for example, some early diverging fungi, brown algae, and diatoms. Although these latter homologs have not been functionally characterized to date, the phylogenetic distribution of CHASE-HKs within the various eukaryotic clades suggests an unexpected and broad involvement of cytokinins and their HK receptors in the regulation of various physiological processes of eukaryotic organisms and interspecies interactions 62.
A third group of eukaryotic HKs involves transmembrane receptors that have been reported to be involved mostly in osmosensing 47, 49. The first members of this group were characterized at the beginning of the 1990s in yeast (referred to as fungal group VI of HKs) and a few years later in plants (referred to as AHK1) ( Table 1). In Saccharomyces cells, these receptors are known to allow the yeast to respond and adapt to osmotic and (to a lesser extent) oxidant stresses 53, 76. In Arabidopsis, the TCS controlled by AHK1 was reported to perceive water stress and to initiate histidine-to-aspartate phosphotransfer circuitry for seed desiccation and vegetative stress tolerances 47, 77– 87. From a structural perspective, these fungal and plant osmosensors include two large hydrophobic transmembrane helices that border a roughly 300–amino acid extracellular loop predicted to fold mostly into large helices and small sheets, recently identified as a Cache domain 36. Pioneering studies on this type of receptor demonstrated that expression of plant AHK1 genes can complement the lack of the unique and essential fungal group VI HK gene in yeast, indicating that plant and yeast putative osmosensors have common functional features and origins 47, 78. Interestingly, however, recent genome-wide analyses suggested that these structural and functional similarities rely on an evolutionary convergence process rather than a common archetypal system inherited in both fungi and plants 64.
Another well-known group of HK-type receptors widely found in many clades of the eukaryotic domain is phytochromes 88, 89. Phytochromes consist of photo-switchable red/far-red photoreceptors that likely evolved in cyanobacteria prior to being transferred to some eukaryotic lineages 90, 91 ( Table 1). In both plants and fungi, they have been demonstrated to be involved in a wide range of physiological processes 88, 89. Importantly, phytochrome sequences from early diverging plants, as fungal phytochromes, display all conserved amino acid residues for HK activity. In contrast, phytochromes from higher plants commonly contain HK-like domains that instead display Ser/Thr kinase activity, suggesting a structural evolution of these receptors in flowering plants toward other non-TCS output domains 98. Some recent genome-wide analyses—and, in some cases, functional characterization studies—demonstrated that phytochromes also occur in green and brown algae, diatoms, and amoebae 63.
There are also other important groups of eukaryotic HKs that have been deeply studied in recent years. These include, for instance, two plant-specific groups: CKI1, which is involved in female gametophyte development 92– 94, and the CKI2/AHK5, which was recently shown to govern the stress-induced stomatal closure, salt sensitivity, and resistance against microbial infection in Arabidopsis 95– 97 ( Table 1). Their precise input signals remain unknown. Finally, recent classification of HKs in fungi revealed that these sensing proteins could be categorized into 16 groups 64; among these, groups III and X seem to play important roles in stress adaptation, morphogenesis, and virulence 53, 54 ( Table 1).
Conclusions
Sequence information is available for a large number of HK sensor domains, enabling identification of abundantly populated fold families. A relatively small number of common sensor domains appear across all domains of life, although their abundance is strongly skewed in different organisms. Unfortunately, sequences and fold families often provide little information about ligands or physical stimuli (or both) detected by individual domains if experiences with bacterial HKs are generalizable.
Although studies of bacterial sensing have focused on a small number of structural folds such as Cache and four-helix bundle domains 99, it is sobering to note that the largest class of bacterial HKs, the prototypical HKs with periplasmic sensing domains, contain 50 to 300 residue-sensing domains that bear no sequence similarity to domains of known folds or functions 100. Even when folds are identifiable, a similar fold can bind many different types of ligands and conversely the same ligand can be bound by domains of different folds in different proteins. Furthermore, even in extensively studied bacterial TCSs where a general stimulus such as cell wall stress is known, the exact molecule or physical parameter sensed by the HK often remains undetermined. Even for some extensively characterized Escherichia coli TCSs, where investigative tools include robust genetics and atomic-resolution three-dimensional structures, specific stimuli remain unidentified. The problem of identifying input stimuli becomes even more complex when multiple sensor kinases or heterodimeric kinases (or both) are integrated into pathways such as the LadS/GacS/RetS/PA1611 system in which interactions among four HKs regulate biofilm formation in Pseudomonas aeruginosa 101– 103. In this system, the phosphorelay between HKs LadS and GacS is inhibited by RetS-GacS heterodimer formation which is further regulated by interactions between RetS and hybrid HK PA1611.
Remarkably, in plants, most TCSs are characterized with regard to the input stimuli and output responses. This is not true for TCSs of fungi and amoeba, and even less is known about TCSs of archaea. Although genomic analyses are a powerful tool for initial identification, experimental strategies will likely be required to drive discovery of system inputs. Recently, a previously uncharacterized Shewanella oneidensis HK was found to sense pH in a high-throughput screen of seven different S. oneidensis TCSs, using engineered RRs with S. oneidensis REC domains linked to a heterologous DNA-binding domain paired with a cognate reporter gene in E. coli 16. To the extent that heterologous proteins are functional, synthetic biology approaches such as this promise to provide a powerful strategy for identification of sensory inputs.
Two-component signaling provides a versatile molecular mechanism for stimulus-response coupling, and TCS protein architecture potentially allows an almost limitless range of inputs and outputs. Indeed, enough of the more than 300,000 TCSs 28 have been characterized to conclude that bacteria use His-Asp phosphotransfer for almost all categories of signal transduction needs. This does not appear to occur in other domains of life where regulatory systems involving Ser/Thr and Tyr phosphorylation abound. Given the great diversity of sensing and responses in bacterial TCSs, it is curious that archaeal and eukaryotic TCSs appear to have been evolved for a narrower range of functions.
Abbreviations
Cache, calcium channels and chemotaxis receptors; CHASE, cyclases/histidine kinases associated sensing extracellular; EtBD, ethylene-binding domain; ETR, ethylene receptor; GAF, cGMP-specific phosphodiesterases-adenylyl cyclases-FhlA; HK, histidine kinase; PAS, period circadian protein-Aryl hydrocarbon receptor nuclear translocator protein-single-minded protein; PDC, PhoQ-DcuS-CitA; REC, receiver; RR, response regulator; TCS, two-component system
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Sean Crosson, Department of Microbiology & Molecular Genetics, Michigan State University, East Lansing, MI, USA
Jeffrey J. Tabor, Department of Bioengineering & Department of Biosciences, Rice University, Houston, TX, USA
Wei Qian, State Key Laboratory of Plant Genomics, Institute of Microbiology, Chinese Academy of Sciences, Beijing, China
Funding Statement
AMS was supported by National Institutes of Health grant R35 GM131727
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 3 approved]
References
- 1. Fuhs SR, Hunter T: pHisphorylation: The emergence of histidine phosphorylation as a reversible regulatory modification. Curr Opin Cell Biol. 2017;45:8–16. 10.1016/j.ceb.2016.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 2. Adam K, Hunter T: Histidine kinases and the missing phosphoproteome from prokaryotes to eukaryotes. Lab Invest. 2018;98(2):233–47. 10.1038/labinvest.2017.118 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 3. Cai X, Srivastava S, Surindran S, et al. : Regulation of the epithelial Ca²⁺ channel TRPV5 by reversible histidine phosphorylation mediated by NDPK-B and PHPT1. Mol Biol Cell. 2014;25(8):1244–50. 10.1091/mbc.E13-04-0180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fuhs SR, Meisenhelder J, Aslanian A, et al. : Monoclonal 1- and 3-Phosphohistidine Antibodies: New Tools to Study Histidine Phosphorylation. Cell. 2015;162(1):198–210. 10.1016/j.cell.2015.05.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Panda S, Srivastava S, Li Z, et al. : Identification of PGAM5 as a Mammalian Protein Histidine Phosphatase that Plays a Central Role to Negatively Regulate CD4+ T Cells. Mol Cell. 2016;63(3):457–69. 10.1016/j.molcel.2016.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Srivastava S, Li Z, Soomro I, et al. : Regulation of K ATP Channel Trafficking in Pancreatic β-Cells by Protein Histidine Phosphorylation. Diabetes. 2018;67(5):849–60. 10.2337/db17-1433 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 7. Hindupur SK, Colombi M, Fuhs SR, et al. : The protein histidine phosphatase LHPP is a tumour suppressor. Nature. 2018;555(7698):678–82. 10.1038/nature26140 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 8. Bem AE, Velikova N, Pellicer MT, et al. : Bacterial Histidine Kinases as Novel Antibacterial Drug Targets. ACS Chem Biol. 2014;10(1):213–24. 10.1021/cb5007135 [DOI] [PubMed] [Google Scholar]
- 9. Hussain R, Harding SE, Hughes CS, et al. : Purification of bacterial membrane sensor kinases and biophysical methods for determination of their ligand and inhibitor interactions. Biochem Soc Trans. 2016;44(3):810–23. 10.1042/BST20160023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tiwari S, Jamal SB, Hassan SS, et al. : Two-Component Signal Transduction Systems of Pathogenic Bacteria As Targets for Antimicrobial Therapy: An Overview. Front Microbiol. 2017;8:1878. 10.3389/fmicb.2017.01878 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 11. Utsumi R: Bacterial signal transduction networks via connectors and development of the inhibitors as alternative antibiotics. Biosci Biotechnol Biochem. 2017;81(9):1663–9. 10.1080/09168451.2017.1350565 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 12. Cardona ST, Choy M, Hogan AM: Essential Two-Component Systems Regulating Cell Envelope Functions: Opportunities for Novel Antibiotic Therapies. J Membr Biol. 2018;251(1):75–89. 10.1007/s00232-017-9995-5 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 13. Wolf D, Mascher T: The applied side of antimicrobial peptide-inducible promoters from Firmicutes bacteria: Expression systems and whole-cell biosensors. Appl Microbiol Biotechnol. 2016;100(11):4817–29. 10.1007/s00253-016-7519-3 [DOI] [PubMed] [Google Scholar]
- 14. Ravikumar S, Baylon MG, Park SJ, et al. : Engineered microbial biosensors based on bacterial two-component systems as synthetic biotechnology platforms in bioremediation and biorefinery. Microb Cell Fact. 2017;16(1):62. 10.1186/s12934-017-0675-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jayaraman P, Holowko MB, Yeoh JW, et al. : Repurposing a Two-Component System-Based Biosensor for the Killing of Vibrio cholerae. ACS Synth Biol. 2017;6(7):1403–15. 10.1021/acssynbio.7b00058 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 16. Schmidl SR, Ekness F, Sofjan K, et al. : Rewiring bacterial two-component systems by modular DNA-binding domain swapping. Nat Chem Biol. 2019;15(7):690–8. 10.1038/s41589-019-0286-6 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 17. Ganesh I, Ravikumar S, Yoo IK, et al. : Construction of malate-sensing Escherichia coli by introduction of a novel chimeric two-component system. Bioprocess Biosyst Eng. 2015;38(4):797–804. 10.1007/s00449-014-1321-3 [DOI] [PubMed] [Google Scholar]
- 18. Jung K, Fabiani F, Hoyer E, et al. : Bacterial transmembrane signalling systems and their engineering for biosensing. Open Biol. 2018;8(4):pii: 180023. 10.1098/rsob.180023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ravikumar S, David Y, Park SJ, et al. : A Chimeric Two-Component Regulatory System-Based Escherichia coli Biosensor Engineered to Detect Glutamate. Appl Biochem Biotechnol. 2018;186(2):335–49. 10.1007/s12010-018-2746-y [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 20. Cormann KU, Baumgart M, Bott M: Structure-Based Design of Versatile Biosensors for Small Molecules Based on the PAS Domain of a Thermophilic Histidine Kinase. ACS Synth Biol. 2018;7(12):2888–97. 10.1021/acssynbio.8b00348 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 21. Park DM, Taffet MJ: Combinatorial Sensor Design in Caulobacter crescentus for Selective Environmental Uranium Detection. ACS Synth Biol. 2019;8(4):807–17. 10.1021/acssynbio.8b00484 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 22. Stock AM, Robinson VL, Goudreau PN: Two-Component Signal Transduction. Annu Rev Biochem. 2000;69:183–215. 10.1146/annurev.biochem.69.1.183 [DOI] [PubMed] [Google Scholar]
- 23. Jacob-Dubuisson F, Mechaly A, Betton JM, et al. : Structural insights into the signalling mechanisms of two-component systems. Nat Rev Microbiol. 2018;16(10):585–93. 10.1038/s41579-018-0055-7 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 24. Galperin MY: Diversity of structure and function of response regulator output domains. Curr Opin Microbiol. 2010;13(2):150–9. 10.1016/j.mib.2010.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gao R, Bouillet S, Stock AM: Structural basis of response regulator function. Annu Rev Microbiol. 2019;73:175–197. 10.1146/annurev-micro-020518-115931 [DOI] [PubMed] [Google Scholar]
- 26. Appleby JL, Parkinson JS, Bourret RB: Signal Transduction via the Multi-Step Phosphorelay: Not Necessarily a Road Less Traveled. Cell. 1996;86(6):845–8. 10.1016/s0092-8674(00)80158-0 [DOI] [PubMed] [Google Scholar]
- 27. Alvarez AF, Barba-Ostria C, Silva-Jiménez H, et al. : Organization and mode of action of two component system signaling circuits from the various kingdoms of life. Environ Microbiol. 2016;18(10):3210–26. 10.1111/1462-2920.13397 [DOI] [PubMed] [Google Scholar]
- 28. El-Gebali S, Mistry J, Bateman A, et al. : The Pfam protein families database in 2019. Nucleic Acids Res. 2019;47(D1):D427–D432. 10.1093/nar/gky995 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 29. Grebe TW, Stock JB: The histidine protein kinase superfamily. Adv Microb Physiol. 1999;41:139–227. 10.1016/s0065-2911(08)60167-8 [DOI] [PubMed] [Google Scholar]
- 30. Wuichet K, Cantwell BJ, Zhulin IB: Evolution and phyletic distribution of two-component signal transduction systems. Curr Opin Microbiol. 2010;13(2):219–25. 10.1016/j.mib.2009.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Esser D, Hoffmann L, Pham TK, et al. : Protein phosphorylation and its role in archaeal signal transduction. FEMS Microbiol Rev. 2016;40(5):625–47. 10.1093/femsre/fuw020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Galperin MY, Makarova KS, Wolf YI, et al. : Phyletic Distribution and Lineage-Specific Domain Architectures of Archaeal Two-Component Signal Transduction Systems. J Bacteriol. 2018;200(7): pii: e00681-17. 10.1128/JB.00681-17 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 33. Ortega Á, Zhulin IB, Krell T: Sensory Repertoire of Bacterial Chemoreceptors. Microbiol Mol Biol Rev. 2017;81(4): pii: e00033-17. 10.1128/MMBR.00033-17 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 34. Anantharaman V, Aravind L: Cache - a signaling domain common to animal Ca 2+-channel subunits and a class of prokaryotic chemotaxis receptors. Trends Biochem Sci. 2000;25(11):535–7. 10.1016/s0968-0004(00)01672-8 [DOI] [PubMed] [Google Scholar]
- 35. Zhang Z, Hendrickson WA: Structural characterization of the predominant family of histidine kinase sensor domains. J Mol Biol. 2010;400(3):335–53. 10.1016/j.jmb.2010.04.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Upadhyay AA, Fleetwood AD, Adebali O, et al. : Cache Domains That are Homologous to, but Different from PAS Domains Comprise the Largest Superfamily of Extracellular Sensors in Prokaryotes. PLoS Comput Biol. 2016;12(4):e1004862. 10.1371/journal.pcbi.1004862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rudolph J, Tolliday N, Schmitt C, et al. : Phosphorylation in halobacterial signal transduction. EMBO J. 1995;14(17):4249–57. 10.1002/j.1460-2075.1995.tb00099.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Salah Ud-Din AIM, Roujeinikova A: Methyl-accepting chemotaxis proteins: a core sensing element in prokaryotes and archaea. Cell Mol Life Sci. 2017;74(18):3293–303. 10.1007/s00018-017-2514-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Klare JP, Bordignon E, Engelhard M, et al. : Transmembrane signal transduction in archaeal phototaxis: the sensory rhodopsin II-transducer complex studied by electron paramagnetic resonance spectroscopy. Eur J Cell Biol. 2011;90(9):731–9. 10.1016/j.ejcb.2011.04.013 [DOI] [PubMed] [Google Scholar]
- 40. Inoue K, Tsukamoto T, Sudo Y: Molecular and evolutionary aspects of microbial sensory rhodopsins. Biochim Biophys Acta. 2014;1837(5):562–77. 10.1016/j.bbabio.2013.05.005 [DOI] [PubMed] [Google Scholar]
- 41. Najnin T, Siddiqui KS, Taha T, et al. : Characterization of a temperature-responsive two component regulatory system from the Antarctic archaeon, Methanococcoides burtonii. Sci Rep. 2016;6: 24278. 10.1038/srep24278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li J, Zheng X, Guo X, et al. : Characterization of an archaeal two-component system that regulates methanogenesis in Methanosaeta harundinacea. PLoS One. 2014;9(4):e95502. 10.1371/journal.pone.0095502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Molitor B, Stassen M, Modi A, et al. : A heme-based redox sensor in the methanogenic archaeon Methanosarcina acetivorans. J Biol Chem. 2013;288(25):18458–72. 10.1074/jbc.M113.476267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fiege K, Frankenberg-Dinkel N: Thiol-based redox sensing in the methyltransferase associated sensor kinase RdmS in Methanosarcina acetivorans. Environ Microbiol. 2019;21(5):1597–610. 10.1111/1462-2920.14541 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 45. Chang C, Kwok SF, Bleecker AB, et al. : Arabidopsis ethylene-response gene ETR1: similarity of product to two-component regulators. Science. 1993;262(5133):539–44. 10.1126/science.8211181 [DOI] [PubMed] [Google Scholar]
- 46. Kakimoto T: CKI1, a histidine kinase homolog implicated in cytokinin signal transduction. Science. 1996;274(5289):982–5. 10.1126/science.274.5289.982 [DOI] [PubMed] [Google Scholar]
- 47. Urao T, Yakubov B, Satoh R, et al. : A transmembrane hybrid-type histidine kinase in Arabidopsis functions as an osmosensor. Plant Cell. 1999;11(9):1743–54. 10.1105/tpc.11.9.1743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Inoue T, Higuchi M, Hashimoto Y, et al. : Identification of CRE1 as a cytokinin receptor from Arabidopsis. Nature. 2001;409(6823):1060–3. 10.1038/35059117 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 49. Wang N, Shaulsky G, Escalante R, et al. : A two-component histidine kinase gene that functions in Dictyostelium development. EMBO J. 1996;15(15):3890–8. 10.1002/j.1460-2075.1996.tb00763.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schuster SC, Noegel AA, Oehme F, et al. : The hybrid histidine kinase DokA is part of the osmotic response system of Dictyostelium. EMBO J. 1996;15(15):3880–9. 10.1002/j.1460-2075.1996.tb00762.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Singleton CK, Zinda MJ, Mykytka B, et al. : The histidine kinase dhkC regulates the choice between migrating slugs and terminal differentiation in Dictyostelium discoideum. Dev Biol. 1998;203(2):345–57. 10.1006/dbio.1998.9049 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 52. Zinda MJ, Singleton CK: The hybrid histidine kinase dhkB regulates spore germination in Dictyostelium discoideum. Dev Biol. 1998;196(2):171–83. 10.1006/dbio.1998.8854 [DOI] [PubMed] [Google Scholar]
- 53. Defosse TA, Sharma A, Mondal AK, et al. : Hybrid histidine kinases in pathogenic fungi. Mol Microbiol. 2015;95(6):914–24. 10.1111/mmi.12911 [DOI] [PubMed] [Google Scholar]
- 54. Hérivaux A, So YS, Gastebois A, et al. : Major Sensing Proteins in Pathogenic Fungi: The Hybrid Histidine Kinase Family. PLoS Pathog. 2016;12(7):e1005683. 10.1371/journal.ppat.1005683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Schaller GE, Shiu SH, Armitage JP: Two-component systems and their co-option for eukaryotic signal transduction. Curr Biol. 2011;21(9):R320–R330. 10.1016/j.cub.2011.02.045 [DOI] [PubMed] [Google Scholar]
- 56. Gallie DR: Ethylene receptors in plants - why so much complexity? F1000Prime Rep. 2015;7:39. 10.12703/P7-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Abeles F, Morgan P, Saltveit MJ: Ethylene in Plant Biology.2 edn, Academic Press.1992. Reference Source [Google Scholar]
- 58. Wang W, Esch JJ, Shiu SH, et al. : Identification of important regions for ethylene binding and signaling in the transmembrane domain of the ETR1 ethylene receptor of Arabidopsis. Plant Cell. 2006;18(12):3429–42. 10.1105/tpc.106.044537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mount SM, Chang C: Evidence for a plastid origin of plant ethylene receptor genes. Plant Physiol. 2002;130(1):10–4. 10.1104/pp.005397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ju C, Van de Poel B, Cooper ED, et al. : Conservation of ethylene as a plant hormone over 450 million years of evolution. Nat Plants. 2015;1:14004. 10.1038/nplants.2014.4 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 61. Hérivaux A, Dugé de Bernonville T, Roux C, et al. : The Identification of Phytohormone Receptor Homologs in Early Diverging Fungi Suggests a Role for Plant Sensing in Land Colonization by Fungi. mBio. 2017;8(1):pii: e01739-16. 10.1128/mBio.01739-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kabbara S, Schmülling T, Papon N: CHASEing Cytokinin Receptors in Plants, Bacteria, Fungi, and Beyond. Trends Plant Sci. 2018;23(3):179–81. 10.1016/j.tplants.2018.01.001 [DOI] [PubMed] [Google Scholar]
- 63. Kabbara S, Bidon B, Kilani J, et al. : Megaviruses: An involvement in phytohormone receptor gene transfer in brown algae? Gene. 2019;704:149–51. 10.1016/j.gene.2019.04.055 [DOI] [PubMed] [Google Scholar]
- 64. Kabbara S, Hérivaux A, Dugé de Bernonville T, et al. : Diversity and Evolution of Sensor Histidine Kinases in Eukaryotes. Genome Biol Evol. 2019;11(1):86–108. 10.1093/gbe/evy213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lacey RF, Binder BM: Ethylene Regulates the Physiology of the Cyanobacterium Synechocystis sp. PCC 6803 via an Ethylene Receptor. Plant Physiol. 2016;171(4):2798–809. 10.1104/pp.16.00602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lacey RF, Allen CJ, Bakshi A, et al. : Ethylene causes transcriptomic changes in Synechocystis during phototaxis. Plant Direct. 2018;2(3):e00048. 10.1002/pld3.48 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 67. Papon N, Binder BM: An Evolutionary Perspective on Ethylene Sensing in Microorganisms. Trends Microbiol. 2019;27(3):193–6. 10.1016/j.tim.2018.12.002 [DOI] [PubMed] [Google Scholar]
- 68. Anantharaman V, Aravind L: The CHASE domain: a predicted ligand-binding module in plant cytokinin receptors and other eukaryotic and bacterial receptors. Trends Biochem Sci. 2001;26(10):579–82. 10.1016/s0968-0004(01)01968-5 [DOI] [PubMed] [Google Scholar]
- 69. Mougel C, Zhulin IB: CHASE: an extracellular sensing domain common to transmembrane receptors from prokaryotes, lower eukaryotes and plants. Trends Biochem Sci. 2001;26(10):582–4. 10.1016/s0968-0004(01)01969-7 [DOI] [PubMed] [Google Scholar]
- 70. Gruhn N, Halawa M, Snel B, et al. : A subfamily of putative cytokinin receptors is revealed by an analysis of the evolution of the two-component signaling system of plants. Plant Physiol. 2014;165(1):227–37. 10.1104/pp.113.228080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kieber JJ, Schaller GE: Cytokinins. Arabidopsis Book. 2014;12:e0168. 10.1199/tab.0168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hothorn M, Dabi T, Chory J: Structural basis for cytokinin recognition by Arabidopsis thaliana histidine kinase 4. Nat Chem Biol. 2011;7(11):766–8. 10.1038/nchembio.667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Daudu D, Kisiala A, Werner Ribeiro C, et al. : Setting-up a fast and reliable cytokinin biosensor based on a plant histidine kinase receptor expressed in Saccharomyces cerevisiae. J Biotechnol. 2019;289:103–11. 10.1016/j.jbiotec.2018.11.013 [DOI] [PubMed] [Google Scholar]
- 74. Kaltenegger E, Leng S, Heyl A: The effects of repeated whole genome duplication events on the evolution of cytokinin signaling pathway. BMC Evol Biol. 2018;18(1):76. 10.1186/s12862-018-1153-x [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 75. Wang FF, Cheng ST, Wu Y, et al. : A Bacterial Receptor PcrK Senses the Plant Hormone Cytokinin to Promote Adaptation to Oxidative Stress. Cell Rep. 2017;21(10):2940–51. 10.1016/j.celrep.2017.11.017 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 76. Hérivaux A, Lavín JL, de Bernonville TD, et al. : Progressive loss of hybrid histidine kinase genes during the evolution of budding yeasts (Saccharomycotina). Curr Genet. 2018;64(4):841–51. 10.1007/s00294-017-0797-1 [DOI] [PubMed] [Google Scholar]
- 77. Chefdor F, Bénédetti H, Depierreux C, et al. : Osmotic stress sensing in Populus: components identification of a phosphorelay system. FEBS Lett. 2006;580(1):77–81. 10.1016/j.febslet.2005.11.051 [DOI] [PubMed] [Google Scholar]
- 78. Tran LS, Urao T, Qin F, et al. : Functional analysis of AHK1/ATHK1 and cytokinin receptor histidine kinases in response to abscisic acid, drought, and salt stress in Arabidopsis. Proc Natl Acad Sci U S A. 2007;104(51):20623–8. 10.1073/pnas.0706547105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wohlbach DJ, Quirino BF, Sussman MR: Analysis of the Arabidopsis histidine kinase ATHK1 reveals a connection between vegetative osmotic stress sensing and seed maturation. Plant Cell. 2008;20(4):1101–17. 10.1105/tpc.107.055871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bertheau L, Chefdor F, Guirimand G, et al. : Identification of five B-type response regulators as members of a multistep phosphorelay system interacting with histidine-containing phosphotransfer partners of Populus osmosensor. BMC Plant Biol. 2012;12:241. 10.1186/1471-2229-12-241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Héricourt F, Chefdor F, Bertheau L, et al. : Characterization of histidine-aspartate kinase HK1 and identification of histidine phosphotransfer proteins as potential partners in a Populus multistep phosphorelay. Physiol Plant. 2013;149(2):188–99. 10.1111/ppl.12024 [DOI] [PubMed] [Google Scholar]
- 82. Kumar MN, Jane WN, Verslues PE: Role of the putative osmosensor Arabidopsis histidine kinase1 in dehydration avoidance and low-water-potential response. Plant Physiol. 2013;161(2):942–53. 10.1104/pp.112.209791 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 83. Kushwaha HR, Singla-Pareek SL, Pareek A: Putative osmosensor--OsHK3b--a histidine kinase protein from rice shows high structural conservation with its ortholog AtHK1 from Arabidopsis. J Biomol Struct Dyn. 2014;32(8):1318–32. 10.1080/07391102.2013.818576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bertheau L, Djeghdir I, Foureau E, et al. : Insights into B-type RR members as signaling partners acting downstream of HPt partners of HK1 in the osmotic stress response in Populus. Plant Physiol Biochem. 2015;94:244–52. 10.1016/j.plaphy.2015.06.006 [DOI] [PubMed] [Google Scholar]
- 85. Héricourt F, Chefdor F, Djeghdir I, et al. : Functional Divergence of Poplar Histidine-Aspartate Kinase HK1 Paralogs in Response to Osmotic Stress. Int J Mol Sci. 2016;17(12): pii: E2061. 10.3390/ijms17122061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Chefdor F, Héricourt F, Koudounas K, et al. : Highlighting type A RRs as potential regulators of the dkHK1 multi-step phosphorelay pathway in Populus. Plant Sci. 2018;277:68–78. 10.1016/j.plantsci.2018.09.010 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 87. Nongpiur RC, Singla-Pareek SL, Pareek A: The quest for ‘osmosensors’ in plants. J Exp Bot. 2019; pii: erz263. 10.1093/jxb/erz263 [DOI] [PubMed] [Google Scholar]
- 88. Rensing SA, Sheerin DJ, Hiltbrunner A: Phytochromes: More Than Meets the Eye. Trends Plant Sci. 2016;21(7):543–6. 10.1016/j.tplants.2016.05.009 [DOI] [PubMed] [Google Scholar]
- 89. Yu Z, Fischer R: Light sensing and responses in fungi. Nat Rev Microbiol. 2019;17(1):25–36. 10.1038/s41579-018-0109-x [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 90. Yeh KC, Wu SH, Murphy JT, et al. : A cyanobacterial phytochrome two-component light sensory system. Science. 1997;277(5331):1505–8. 10.1126/science.277.5331.1505 [DOI] [PubMed] [Google Scholar]
- 91. Rockwell NC, Lagarias JC: Phytochrome diversification in cyanobacteria and eukaryotic algae. Curr Opin Plant Biol. 2017;37:87–93. 10.1016/j.pbi.2017.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 92. Yuan L, Liu Z, Song X, et al. : The CKI1 Histidine Kinase Specifies the Female Gametic Precursor of the Endosperm. Dev Cell. 2016;37(1):34–46. 10.1016/j.devcel.2016.03.009 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 93. Liu Z, Yuan L, Song X, et al. : AHP2, AHP3, and AHP5 act downstream of CKI1 in Arabidopsis female gametophyte development. J Exp Bot. 2017;68(13):3365–73. 10.1093/jxb/erx181 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 94. Yuan L, Liu Z, Song X, et al. : The gymnosperm ortholog of the angiosperm central cell-specification gene CKI1 provides an essential clue to endosperm origin. New Phytol. 2018;218(4):1685–96. 10.1111/nph.15115 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 95. Pham J, Desikan R: Modulation of ROS production and hormone levels by AHK5 during abiotic and biotic stress signaling. Plant Signal Behav. 2012;7(8):893–7. 10.4161/psb.20692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Mira-Rodado V, Veerabagu M, Witthöft J, et al. : Identification of two-component system elements downstream of AHK5 in the stomatal closure response of Arabidopsis thaliana. Plant Signal Behav. 2012;7(11):1467–76. 10.4161/psb.21898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bauer J, Reiss K, Veerabagu M, et al. : Structure-function analysis of Arabidopsis thaliana histidine kinase AHK5 bound to its cognate phosphotransfer protein AHP1. Mol Plant. 2013;6(3):959–70. 10.1093/mp/sss126 [DOI] [PubMed] [Google Scholar]
- 98. Li FW, Melkonian M, Rothfels CJ, et al. : Phytochrome diversity in green plants and the origin of canonical plant phytochromes. Nat Commun. 2015;6:7852. 10.1038/ncomms8852 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 99. Cheung J, Hendrickson WA: Sensor domains of two-component regulatory systems. Curr Opin Microbiol. 2010;13(2):116–23. 10.1016/j.mib.2010.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Mascher T, Helmann JD, Unden G: Stimulus perception in bacterial signal-transducing histidine kinases. Microbiol Mol Biol Rev. 2006;70(4):910–38. 10.1128/MMBR.00020-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Goodman AL, Merighi M, Hyodo M, et al. : Direct interaction between sensor kinase proteins mediates acute and chronic disease phenotypes in a bacterial pathogen. Genes Dev. 2009;23(2):249–59. 10.1101/gad.1739009 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 102. Chambonnier G, Roux L, Redelberger D, et al. : The Hybrid Histidine Kinase LadS Forms a Multicomponent Signal Transduction System with the GacS/GacA Two-Component System in Pseudomonas aeruginosa. PLoS Genet. 2016;12(5):e1006032. 10.1371/journal.pgen.1006032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Bhagirath AY, Pydi SP, Li Y, et al. : Characterization of the Direct Interaction between Hybrid Sensor Kinases PA1611 and RetS That Controls Biofilm Formation and the Type III Secretion System in Pseudomonas aeruginosa. ACS Infect Dis. 2017;3(2):162–75. 10.1021/acsinfecdis.6b00153 [DOI] [PubMed] [Google Scholar]