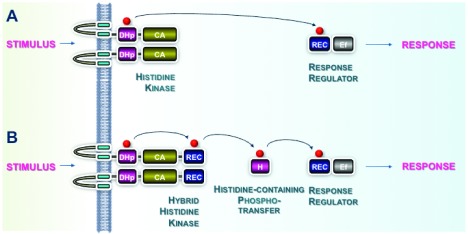
Figure 1. Two-component system phosphotransfer schemes.
( A) A typical phosphotransfer pathway, as is usually found in prokaryotes. The perception of a stimulus by extracytoplasmic domains of the histidine kinase (HK) regulates its activities. The HK autophosphorylates at a conserved histidine residue (H) using ATP bound to the catalytic ATPase domain (containing conserved motifs N, G1, F, and G2). The phosphoryl group (P) is transferred to a conserved aspartate residue (D) located within the cognate response regulator (RR). ( B) An example of a multi-step phosphorelay, as often occurs in eukaryotes. The HK is termed “hybrid” because an additional aspartate-containing domain is fused to the ATPase domain. The phosphorelay involves multiple phosphoryl transfer steps. The first is an intramolecular transfer between the conserved histidine (H) and a conserved aspartate residue (D) located within the C terminus of the sensor HK. Subsequently, the phosphoryl group is transferred to a histidine-containing phosphotransfer protein and finally to a cognate RR. Conserved domains of the two-component system (TCS) proteins are shown in green, gold, and blue. Variable sensor domains of the HK and effector domains (Ef) of the RR that adapt the systems to a wide range of input stimuli and output responses are shown in gray.