Abstract
Lysyl oxidase-like 2 (LOXL2) is associated with invasiveness and metastasis in breast cancer. We analyzed the prognostic impact of LOXL2 for breast cancer patients and investigated the role of LOXL2 in breast cancer cell lines. Immunohistochemical study of LOXL2 expression was done in samples from 309 patients. Survival analysis was performed using log-rank test and Cox regression hazard model. After identification of LOXL2 expression in breast cancer cell lines, we performed matrigel invasion and wound-healing assays with LOXL2-silenced cell lines. In the human study, LOXL2 was expressed in 16.2 % of patients. Comparing the LOXL2-positive versus negative groups, there was a significantly higher proportion of estrogen receptor-negative patients (54.0 vs. 37.0 %, respectively; p = 0.029) and triple-negative patients (34.0 vs. 18.0 %; p = 0.022) in the positive group. In multivariate analysis for overall survival and metastasis-free survival, positive LOXL2 was demonstrated as a poor prognostic factor (HR 2.27 and 2.10, respectively). In vitro study indicated that LOXL2 silencing induces a mesenchymal–epithelial transition-like process in basal cell lines (MDA-MB-231 and BT549) associated with decreased invasive and migratory properties. These clinical and preclinical data confirm that higher LOXL2 expression is associated with invasiveness of basal-like breast cancer cells and lower survival of breast cancer patients. Our results suggest the clinical value of LOXL2 as a therapeutic target in breast cancer.
Keywords: Breast cancer, LOXL2, Invasiveness, Triple negative
Introduction
Breast cancer is the most common cancer in women, with approximately 1.5 million new cases diagnosed annually worldwide, a lifetime risk of up to 12 %, and a risk of death of up to 5 % in Western countries [1]. Survival for women with breast cancer has improved, and mortality rates are decreasing by approximately 2.3 % annually [2]. However, conquering breast cancer has been hampered by metastatic disease, despite successful systemic therapies including targeted therapies, such as anti-estrogen therapy and human epidermal growth factor receptor-2 (HER2) targeting therapy [3, 4]. To improve outcomes of metastatic breast cancer, we sought to uncover modes of progression and use that knowledge to develop novel targeted therapies for metastatic disease. Among the molecules that contribute to cancer progression and metastasis, lysyl oxidase-like 2 (LOXL2) is notable for management of metastatic disease.
LOXL2 is a member of the lysil oxidase (LOX) family, composed of five homologs (LOX and LOXL1–4) [5–8] that are secreted, copper-dependent amine oxidases. LOX and LOX 1–4 are extracellular matrix-modifying enzymes that catalyze the crosslinking of collagens and elastin [9]. LOXL2 has been reported to play a crucial role in metastasis of various malignancies [10–13]. Increased LOXL2 expression leads to tumor progression and metastasis, probably by promoting tumor cell invasion and remodeling of the tumor microenvironment [14–19]. Peinado et al. [20] reported that LOXL2 mediates induction of epithelial–mesenchymal transition (EMT) by repression of E-cadherin, indicating a contribution of LOXL2 to tumor progression.
It has been proposed that LOXL2 is associated with aggressive tumors [14, 17, 18, 20, 21], and upregulation of LOXL2 in various tumor cells has been shown to promote their invasiveness in vitro and in vivo [16–20]. In breast cancer, the paucity of clinical data regarding LOXL2 has been noted. Therefore, clinical research to investigate a prognostic impact of LOXL2 in cancer patients is worthy of being explored.
In this study, we aimed to confirm the role of LOXL2 to increase invasiveness of breast cancer cells and evaluate a survival impact of LOXL2 in breast cancer patients. To accomplish this end, we assayed human breast tumors for LOXL2 expression by immunohistochemistry (IHC) and elucidated a prognostic significance of LOXL2 for breast cancer patients. Moreover, we performed in vitro study showing an association between the invasiveness of breast cancer cell lines and LOXL2 expression.
Methods
Patients
We prospectively collected tumor tissues from specimens of surgically resected breast carcinoma at the Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea, between January 1996 and December 2004. Among a total study population of 386, the exclusion criteria were as follows: unreadable LOXL2 expression (n = 29), pure in situ carcinoma (n = 19), metastatic disease (n = 9), patient receiving neoadjuvant chemotherapy (n = 13), bilateral breast cancers (n = 3), and non-epithelial origin breast cancer, such as phyllodes tumor (n = 2). Invasive carcinomas that did not present invasive focus upon review of archival H&E-stained slides were also excluded since they represented only intraductal components (n = 2). As a result, 309 patients were enrolled for analysis.
Clinical data of the patients, including age, tumor size, histologic grade, lymph node status, and expression status of estrogen receptor (ER), progesterone receptor (PgR), and HER2, were retrieved from the database. TNM disease stage was classified according to the American Joint Committee on Cancer, 7th Edition. The modified Scarf–Bl–Richardson grading system was used for tumor grading. With regard to biomarker assays, before February 1999, ER status was determined using the ligand binding assay, and tumors were considered ER-positive with a score greater than 10 fmol/mg [22]. After February 1999, the IHC method for ER staining was introduced and it replaced the biochemical method. HER2-positive was defined as a tumor with 3-positive on IHC exam, or amplification on fluorescence in situ hybridization (FISH) test or silver in situ hybridization (SISH) test. A tumor that is ER-negative, PgR-negative, and HER2-negative was defined as triple-negative. The institutional review board of Gangnam Severance Hospital, Yonsei University, Seoul, Korea, approved the study in accordance with good clinical practice guidelines and the Declaration of Helsinki (3–2011-0191).
Tissue microarray blocks, IHC staining, and in situ hybridization
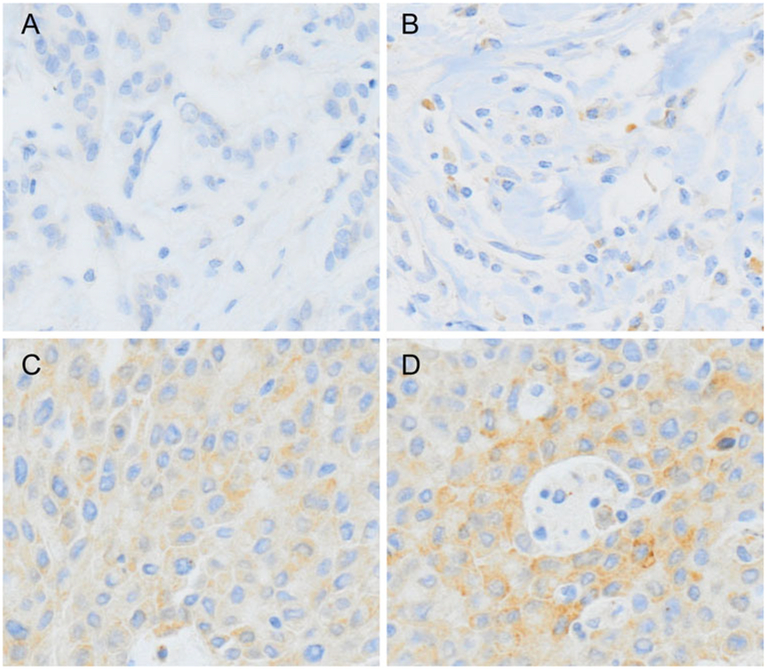
Formalin-fixed, paraffin-embedded tissue blocks were arrayed using an Accu Max Array tissue-arraying instrument (Petagen, Inc.; Seoul, Korea). Briefly, representative areas of each tumor were selected and marked on the H&E slide by breast pathologists. The designated zone of each donor block was punched with a tissue cylinder 3 mm in diameter, and the sample was transferred to a recipient block in a grid pattern. IHC staining was carried out in the tissue microarray blocks. Paraffin-embedded sections were prepared at 4 μm thickness followed by the standard H&E staining. Additional sections were manually deparaffinized in xylene and rehydrated in a series of graded ethanol solutions. After deparaffinization and rehydration, the sections were treated with a 3 % (v/v) hydrogen peroxide solution for 10 min to block endogenous peroxidase and pretreated for antigen retrieval in Epitomic retrieval solution2, pH 6.0, from Leica Biosystems (Melbourne, Australia) at 100 °C for 20 min. After incubation with primary antibodies against LOXL2 (Origene, Rockville, MD) at 1:1000 dilution, and CDH1 (BD Biosciences; Sparks, MD), at 1:500 dilution, the sections were subjected to staining using the automated Leica Bond-max immunostainer (Leica Biosystems) according to the manufacturer’s instructions. Stained tissue images were captured on a Nikon TE2000 inverted microscope with IP Lab software (BD Biosciences Clontech; Palo Alto, CA). Slides were counterstained with Harris hematoxylin. Normal breast tissue entrapped within the block and appropriate control tissues were used as positive controls. Archival H&E-stained slides for each case were reviewed by three pathologists who are experts in breast pathology. For interpretation of the IHC stain results, the IHC tests for LOXL2 and CDH1 were categorized as negative (0), “1 +,” “2+,” or “3+” in high-power fields (400× magnification) according to the intensity of cytoplasmic staining (Fig. 1) in every case. LOXL2- or CDH1-positive was assigned for scores “2+” and “3+.” The interpretation of IHC results was carried out blindly, without any information regarding clinical parameters or outcome. In FISH or SISH tests, as recommended by the ASCO/CAP guideline [23], an absolute HER2 gene copy number >6, or HER2 gene/chromosome 17 copy number ratio higher than 2.2, was considered HER2-positive.
Fig. 1.
Immunohistochemical analysis of LOXL2. LOXL2 expression was evaluated at high-power field (×400 magnification) by two experienced pathologists. a Negative for LOXL2. b One positive for LOXL2. c Two positive for LOXL2. d Three positive for LOXL2
Cell lines
Human breast cancer cell lines MDA-MB-231, HBL100, BT549, HS578T, MCF7, MDA-MB-361, BT474, SK-BR3, and T47D were purchased from the American Type Culture Collection (ATCC; Manassas, VA) and grown in accordance with ATCC recommendations. MCF7, MDA-MB-361, BT474, and T47D cells were characterized as ER-positive/PgR-positive luminal mammary carcinoma. SK-BR3 cells were characterized as HER2-positive mammary carcinoma. MDA-MB-231, BT549, HBL100, and HS578T cells were characterized as basal-like mammary carcinoma.
Construction of siRNA and transfection
For knockdown LOXL2 mRNA, the following sequences were used: siLOXL2, 5′-GAAGGAGACAUCCAGAAGATT-3′. As a negative control, we used an siRNA targeting green fluorescence protein: 5′-GGUGUGCUGUUUGGAGGUCTT-3′. Cells were transfected with the siRNAs at 50 % confluence using the transfection reagent Oligofectamine (Invitrogen, Carlsbad, CA), according to the manufacturer’s instructions.
Reverse transcription polymerase chain reaction (RT-PCR) and Western blot analysis
RNA was extracted using the Trizol regent (Invitrogen, Carlsbad, CA), and cDNA was synthesized using the MMLV enzyme (Invitrogen). PCR reaction was performed at 95 °C for 10 min and in 25 cycles at 95 °C for 15 s, 62 °C for 30 s, and 72 °C for 30 s on a GeneAmp PCR System 9700 (Applied Biosystems, Foster City, CA). Primers used for RT-PCR were LOXL2 (F: 5′-AACGAGGCGACCCTTGCAGC-3′ and R: 5′-GGGTGCGCTTGCGGTAGGTT-3′); Snail, (F: 5′-AATCGGAAGCCTAACTACAGCGAG-3′ and R: 5′-CTTTCCCACTGTCCTCATCTGACA-3′); Snai2, (F: 5′-CATGCCTGTCATACCACAAC-3′ and R: 5′-GGTGTCAGATGGAGGAGGG-3′); CDH1, (F: 5′-GACGCGGACGATGATGTGAAC-3′ and R: 5′-TTGTACGTGGTGGGATTGAAGA-3′); EpCAM, (F: 5′-GAATGGCTCAAAACTTGGGA-3′ and R: 5′-ACGCGTTGTGATCTCCTTCT-3′); SPARC, (F: 5′-GCTCCACCTGGACTACATCG-3′ and R: 5′-GGAGAGGTACCCGTCAATGG-3′) and GAPDH, (F: 5′-CGGGAAGCTTGTGATCAATGG-3′ and R: 5′-GGCAGTGATGGCATGGACTG-3′). For Western blot, cell lysates were prepared in RIPA buffer (Sigma, St Louis, MO) supplemented with protease inhibitors. Protein samples were separated by 10 % sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes. The membranes were blocked and probed with primary antibody against LOXL2 (Origene; Rockville, MD), Snail and Snai2 (Cell Signaling; Danvers, MA), EpCAM (Abcam, Cambridge, UK), SPARC (R&D System, Minneapolies, MN), pFAK and FAK (Santa Cruz, CA, USA), pSRC (Millipore, Billerica, MA, USA), SRC (Santa Cruz, CA, USA), CDH1 (BD Biosciences; Sparks, MD), and ß-actin (Sigma, St Louis, MO).
Wound-healing assay
For wound-healing assays, an IBIDI culture insert (IBIDI, Martinsried, Germany) consisting of two reservoirs separated by a 500-μm-thick wall was placed into one well of the six-well plate and an equal number of control and LOXL2 silenced breast cancer cell (70 μl; 1 × 105 cells/ml) were added into the two reservoirs of the same insert and incubated. After 24 h, the insert was gently removed creating a gap of ~500 μm. The cells were allowed to migrate for 24 h. Images were taken (0 and 24 h) under an Olympus IX70 inverted microscope equipped with a digital camera (Olympus France; Rungis, France) to assess the ability of the cells to migrate into the wound area.
Matrigel invasion chamber assay
The invasive potential of breast cancer cells was assessed in vitro in matrigel-coated invasion chambers (Corning; NY) in accordance with the manufacturer’s instructions. Briefly, BT549 and MDA-MB-231 cells in log growth phase were serum-starved for 24 h prior to seeding, detached by brief trypsinization and resuspended in medium containing the appropriate treatment. The matrigel invasion inserts were rehydrated and prepared as described in the manufacturer’s instructions. Cells in serum-free medium at a density 1 × 105 cells/well on the top of gelatin-coated polycarbonate filters (8 μm pore size) suspended in a membrane invasion culture system chamber; the chamber underneath the membrane contained complete medium. The cells were incubated in a CO2 incubator at 37 °C for 5 h, after which the non-invasive cells were removed from the upper surface of the membrane, and the invasive cells on the under surface of the membrane were fixed and stained with hematoxylin–eosin (H&E; Sigma-Aldrich). These experiments were done in triplicate and performed a minimum of three times. The number of invading cells was counted under fluorescence microscope in five random high-power fields.
Statistical methods
Age is presented in the study as mean value with standard deviation. Discrete variables were compared by the Chi square test. Overall survival (OS) time was measured from the date of the first curative surgery to the date of the last follow-up or death from any cause during follow-up. Disease-free survival (DFS) time was measured from the date of the first curative surgery to the date of the first locoregional recurrence or distant metastasis, or death without any type of relapse; metastasis-free survival (MFS) time was calculated to the date of the first distant metastasis. Survival curves based on the Kaplan–Meier method were compared using a log-rank test. For multivariate analysis, a Cox proportional hazard text was applied. We determined the variables for multivariate analysis that showed a statistical significance in univariate analysis for OS or MFS. The software used to perform these analyses was SPSS version 18 (SPSS; Chicago, IL). Statistical significance was defined by a p-value of <0.05 or a 95 % confidence interval (CI) that did not include 1.
Results
Patient characteristics based on LOXL2 expression
Among the 309 patients, 16.2 % (50 patients) were positive for LOXL2. Baseline characteristics were compared between LOXL2-positive and -negative patients (Table 1). The LOXL2-positive patients showed a higher rate of ER-negative tumors (p = 0.029). However, LOXL2 expression was not related to histologic grade (p = 0.735) or CDH1 expression (p = 0.385).
Table 1.
Patient characteristics based on LOXL2 expression
| LOXL2-positive (n = 50) | LOXL2-negative (n = 259) | p-value | |
|---|---|---|---|
| Age | 46.4 ± 10 | 46.2 ± 11 | 0.884 |
| Tumor size | 0.356 | ||
| >2 cm | 38 (76) | 180 (69) | |
| ≤2 cm | 12 (24) | 79 (31) | |
| Lymph node status | 0.760 | ||
| Metastasis | 25 (50) | 136 (53) | |
| No metastasis | 25 (50) | 123 (47) | |
| Stage | 0.448 | ||
| I | 8 (16) | 60 (23) | |
| II | 31 (62) | 138 (53) | |
| III | 11 (22) | 61 (24) | |
| Histologic gradea | 0.735 | ||
| I, II | 31 (61) | 165 (69) | |
| III | 17 (33) | 76 (31) | |
| ER | |||
| Positive | 23 (46) | 162 (63) | 0.029 |
| Negative | 27 (54) | 97 (37) | |
| PR | |||
| Positive | 26 (52) | 169 (63) | 0.075 |
| Negative | 24 (48) | 90 (37) | |
| HER2a,b | 0.625 | ||
| Positive | 9 (18) | 37 (14) | |
| Negative | 39 (78) | 196 (76) | |
| CDH1a | 0.385 | ||
| Positive | 34 (71) | 191 (77) | |
| Negative | 14 (29) | 58 (23) | |
| Subtypea,c | 0.022 | ||
| TN | 17 (34) | 47 (18) | |
| Non-TN | 31 (62) | 186 (72) |
ER Estrogen receptor, PR Progesterone receptor, HER2 Human epidermal growth factor receptor-2, TN Triple-negative
Data with missing values
HER2 positive was defined by three positive on IHC exam or amplification on FISH
Triple-negative was defined as a tumor with estrogen receptor-negative, progesterone receptor-negative, human epidermal growth factor receptor-2-negative
In the 281 patients with known HER2 status, the rate of positive LOXL2 was higher in triple-negative tumors than non-triple-negative tumors (26.6 % vs. 14.3 %, respectively; p = 0.022). Clinical characteristics associated with advanced breast cancer, such as tumor size, lymphatic metastasis, and tumor stage, were not related to LOXL2 positivity (Table 1). In addition, there was no statistical difference between the other parameters analyzed between the groups.
Pattern of recurrence according to LOXL2 expression
We further investigated the relationship of recurrent pattern and LOXL2 expression (Table 2). In the 77 patients with recurrence, samples from 18 patients were LOXL2-positive. Among them, 17 patients had distant metastasis. However, a significant difference was not noted in the comparison between recurrence type and LOXL2 expression.
Table 2.
Pattern of recurrence based on LOXL2 expression
| LOXL2 positive | LOXL2 negative | p-value | |
|---|---|---|---|
| Pattern of 1st relapse (n = 77) | 0.284 | ||
| Locoregional (n = 10) | 1 | 9 | |
| Distant metastasis (n = 67) | 17 | 50 | |
| Site of 1st distant metastasis (n = 67) | 0.488 | ||
| Skeletal (n = 17) | 3 (17) | 14 (28) | |
| Visceral (n = 39) | 12 (71) | 27 (54) | |
| Combined (n = 11) | 2 (12) | 9 (18) |
LOXL2 Lysyl oxidase-like 2
In the 67 cases of distant metastasis at the first relapse, those were classified into three groups according to the site of metastasis: skeletal (n = 17), visceral (n = 39), or combined (n = 11). In the 17 patients with LOXL2-positive tumors, 12 of 17 (71 %) underwent visceral metastasis at the first relapse. The comparison between the site of distant metastasis and LOXL2 expression also showed no difference.
Negative prognostic impact of LOXL2
At a median follow-up time of 9.3 years, the 10-year OS rate was 78.8 % (95 % CI of 76.3–81.3). During the follow-up period, 62 mortalities occurred. Among the 77 recurrence cases, 10 locoregional recurrences and 67 distant metastases at the first relapse were identified.
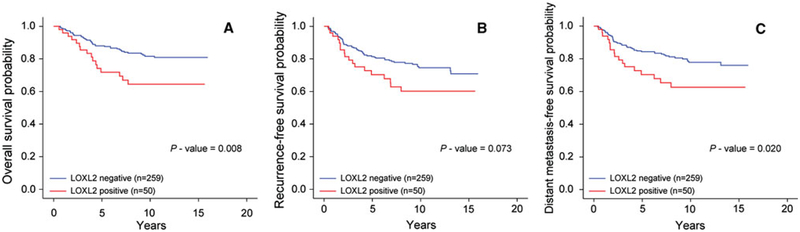
The 10-year OS rate for LOXL2-negative patients was 80.8 % (95 % CI, 78.1–83.5), and 65.3 % (95 % CI, 58.2–72.4) for LOX2-positive. In univariate analysis for OS, higher expression of LOXL2 was associated with poor outcome (p = 0.008, Fig. 2a). In univariate analysis for DFS, we did not find any significant difference according to the expression of LOXL2 (p = 0.073, Fig. 2b). However, for MFS, LOXL2 positivity was found to be a poor prognostic factor (p = 0.020, Fig. 2c). The 10-year MFS rate for LOXL2-negative patients was 77.7 %, (95 % CI, 74.6–80.6), and 63.4 % (95 % CI, 56.2–70.6) for LOX2-positive patients.
Fig. 2.
Kaplan–Meier analysis of LOXL-2 expression. Survival was compared between the LOXL2-positive and -negative groups. The p-value was calculated using log-rank test. a Overall survival (p = 0.008). b Disease-free survival (p = 0.073). c Metastasis-free survival (p = 0.020)
In multivariate analysis for OS, LOXL2 was demonstrated to be an independent prognostic factor (Table 3). Compared with LOXL2-positive tumors, the adjusted hazard ratio (HR) of LOXL2-negative tumors for OS was 2.27 (95 % CI, 1.23–4.19; p = 0.009). Additionally, in multivariate test for MFS, the adjusted HR of LOXL2-negative patients was 2.10 (95 % CI, 1.20–3.68, p = 0.010, Table 3)
Table 3.
Multivariate analysis for overall survival and metastasis-free survival
| Overall Survival | Metastasis-free Survival | |||||
|---|---|---|---|---|---|---|
| p-value (Univariate) | p-value (Multivariate) | HR (95 % CI) | p-value (Univariate) | p-value (Multivariate) | HR (95 % CI) | |
| Age | 0.011 | 0.149 | 0.001 | 0.022 | ||
| >35 | Referent | Referent | ||||
| ≤35 | 1.75 (0.92–3.31) | 2.39 (1.35–4.24) | ||||
| ER | 0.035 | 0.073 | 0.073 | n/a | n/a | |
| Positive | Referent | |||||
| Negative | 1.71 (0.94–3.11) | |||||
| Tumor size | 0.021 | 0.153 | 0.005 | 0.125 | ||
| ≤2 cm | Referent | Referent | ||||
| >2 cm | 1.44 (0.68–3.05) | 1.46 (0.74–2.88) | ||||
| LN | <0.001 | 0.001 | <0.001 | <0.001 | ||
| Negative | Referent | Referent | ||||
| Positive | 3.13 (1.60–6.11) | 3.52 (1.87–6.59) | ||||
| HG | 0.019 | 0.116 | 0.018 | 0.016 | ||
| I, II | Referent | Referent | ||||
| III | 1.74 (0.96–3.17) | 1.91 (1.14–3.19) | ||||
| LOXL2 | 0.008 | 0.039 | 0.020 | 0.038 | ||
| Negative | Referent | Referent | ||||
| Positive | 2.27 (1.23–4.19) | 2.10 (1.20–3.68) | ||||
ER Estrogen receptor, HG Histologic grade, LOXL2 Lysyl oxidase-like 2, n/a not applicable
LOXL2 is expressed in basal-like breast cell lines
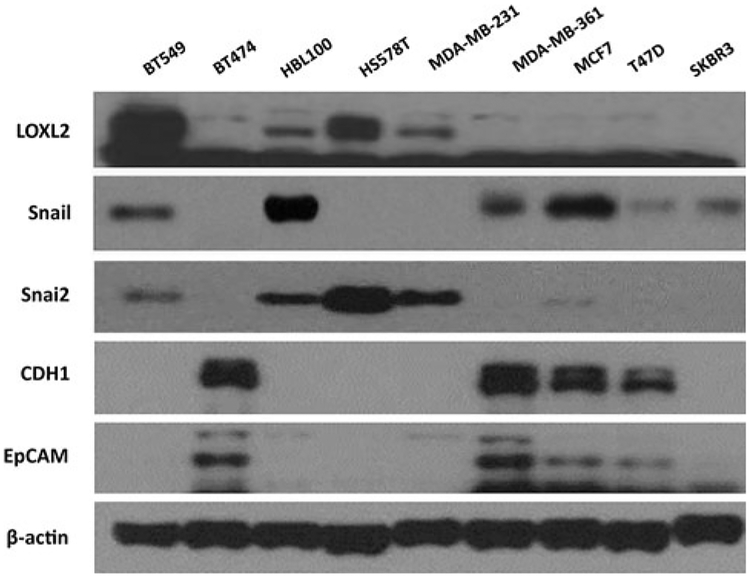
Expression of LOXL2 was analyzed in a series of breast cancer cell lines representing basal, luminal, and HER2 subtypes (Fig. 3). LOXL2 was only detected in the basal-like breast carcinoma cells. Those cells lacked expression of epithelial cell markers, such as CDH1 and EpCAM (Fig. 3), and showed a mesenchymal phenotype [24, 25].
Fig. 3.
LOXL2 expression analyzed in various breast cancer cell lines. In Western blot analysis, LOXL2 was only detected in the basal-like breast carcinoma cell lines (BT549, HBL100, HS578T, and MDA-MB-231)
Silencing LOXL2 reduces invasiveness
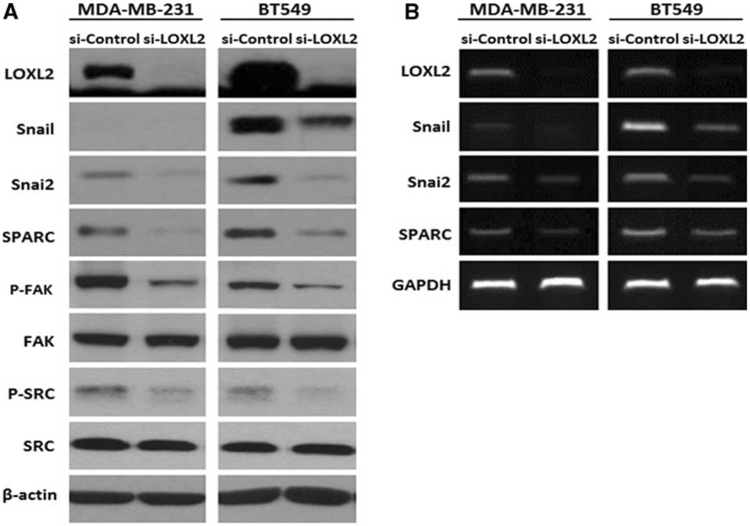
We selected MDA-MB-231 and BT549 to evaluate the role of LOXL2 in basal-like carcinoma cells, MDA-MB-231 and BT549, which showed more aggressive character. We generated stable basal breast carcinoma MDA-MB-231 and BT549 cells in which LOXL2 was silenced. The selected clones of siLOXL2 showed significantly reduced LOXL2 protein (Fig. 4a) and mRNA levels (Fig. 4b).
Fig. 4.
Analysis of LOXL2 expression in two basal-like breast cancer cell lines (MDA-MB-231 and BT549) with LOXL2 silencing. a Western analysis; Cells with LOXL2 silencing by siLOXL2 showed reduced LOXL2, Snail, Snai2, SPARC, p-FAK, and p-SRC at protein level. b RT-PCR analysis; LOXL2 silencing has an effect on reducing of LOXL2, Snail, Snail2, and SPARC at mRNA level
LOXL2 silencing did not affect the expression of CDH1 and EpCAM in MDA-MB-231-siLOXL2 and BT549-siLOXL2. Downregulation of Snail expression was detected in BT549-siLOXL2. Since Snail protein was not detected in MDA-MB-231 due to its very low transcriptional expression, only transcriptional downregulation of Snail was detected in MDA-MB-231-siLOXL2. We also observed that significant changes to molecules related to invasiveness and EMT-dependent LOXL2 silencing. Downregulation of phospho-SRC and phospho-FAK were detected in Western analyses (Fig. 4a) and downregulation of Snai2 and SPARC were detected both at mRNA and protein levels (Fig. 4a and 4b).
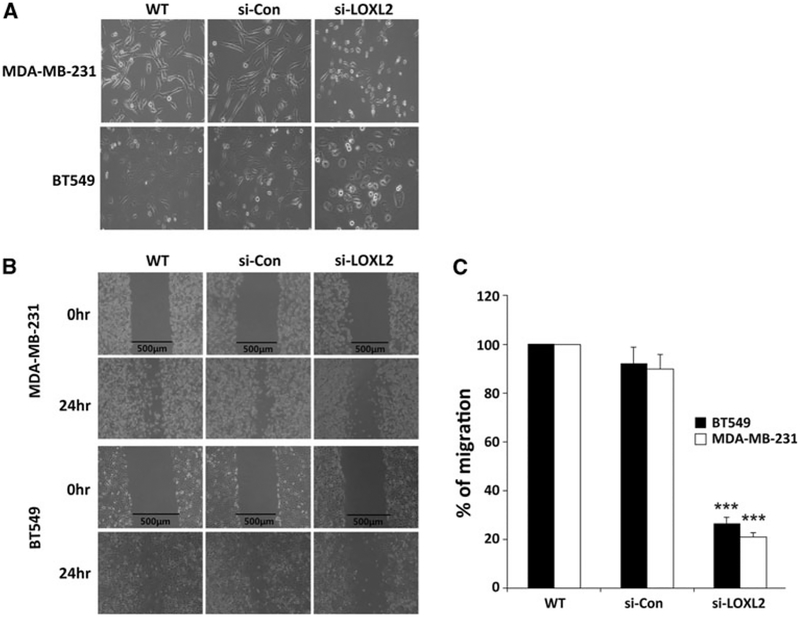
On the other hand, MDA-MB-231-siLOXL2 and BT549-siLOXL2 cells exhibited a more epithelial phenotype compared to control (Fig. 5a). Importantly, silencing of LOXL2 resulted in a marked decrease in migratory ability and motility of MDA-MB-231 and BT549 cells, as determined from wound-healing assays (Fig. 5b). Furthermore, LOXL2 silencing in MDA-MB-231 and BT549 cells resulted in a highly significant decrease in invasion capacity (p < 0.001), as measured by Matrigel invasion assays (Fig. 5c).
Fig. 5.
Silencing of LOXL2 suppresses the invasive potential of human breast cancer cells. a MDA-MB-231-siLOXL2 and BT549-siLOXL2 cells exhibited a more epithelial phenotype compared to si-control and WT. b Motility of MDA-MB-231 (WT, si-Control, and si-LOXL2) and BT549 (WT, si-Control, and si-LOXL2) cells was examined by wound-healing assay. Wound-healing assay was performed three times. Images were taken at 24 h. c Invasion capacity of MDA-MB-231 (WT, si-Control, and si-LOXL2) and BT549 (WT, si-Control, and si-LOXL2) cells were analyzed by a Matrigel invasion assay. Migrated tumor cells were taken at 5 h and stained with H&E. Microphotographic images were captured at ×100 magnification using an Olympus IX70 inverted microscope and the number of invading cells was counted in five random high-power fields (***p < 0.001)
Discussion
The results from this study demonstrate, for the first time, that LOXL2 is an independent prognostic marker in breast cancer patients. In the analysis, LOXL2 was demonstrated to be an independent prognostic factor for OS and MFS in breast cancer. Adjusted HRs of LOXL2 for OS were 2.27 (95 % CI, 1.23–4.19), and 2.10 (95 % CI, 1.20–3.68) for MFS. Those are comparable with conventional prognostic indicators, such as ER, histologic grade, and tumor size, or seem even more significant (Table 3).
More importantly, our data provide evidence that LOXL2 expression in breast tumor could contribute to metastasis in a clinical setting. The worse MFS found in LOXL2-positive patients supports previous findings that the extracellular activities of LOXL2 promote tumor progression or metastasis [10–13]. Our results show that the reduced MFS is connected with a decreased OS in the LOXL2-positive patients. It potentially suggests that interruption of the LOXL2-dependent activity contributing to metastasis could bring survival benefit to breast cancer patients, as well as in a preclinical condition.
In this study, LOXL2-positive tumors were found in 16.5 % of 309 breast cancer patients with stage I to III disease. Moreno-Bueno et al. reported that the rate of cytosolic/perinuclear staining of LOXL2 by IHC is 20.5 % in 195 patients [21]. Our LOXL2-positive rate is in line with that report. However, in our data, LOXL2-positive tumors were not related to higher histologic grade (p = 0.735) or lower CDH1 expression (p = 0.385). Evidence for antagonistic interaction between high LOXL2 and repressed CDH1, described in a previous report [16], was not found in our IHC data. The dynamic property of EMT might be one potential reason for this disparity. Also, in our results, LOXL2 was not associated with higher histologic tumor grade. This disparity requires further investigations of LOXL2, which, in the present study, was only evaluated by IHC.
Investigation of an association between pattern of recurrence and LOXL2 status resulted in no significant finding. In addition, high LOXL2 expression was not associated with the site of metastasis at the first relapse, despite the association between ER-negative and triple-negative tumors, which have been shown to have a tendency for visceral metastasis [26–28]. To determine whether LOXL2 promotes metastatic propensity of a tumor irrespective of its site, further investigation is warranted.
To identify the mechanistic basis for these findings that LOXL2 is associated with a poor prognosis in breast cancer patients, we also performed in vitro study for LOXL2 in breast cancer cells. In this part, we found that LOXL2 plays an integral part for promotion of invasiveness of basal-like breast cancer cells. Our in vitro study indicates that LOXL2 silencing induces a mesenchymal–epithelial transition-like process in basal cell lines that is associated with decreased invasive and migratory properties. Moreover, LOXL2 contributes positively to the activation of FAK/SRC and influences the expressions of Snail, Snai2, and SPARC, which are all related to invasiveness and EMT of breast tumor cells. These results were consistent with previous observations [21, 29, 30] and those of other groups, which have shown that ectopic expression of LOXL2 in luminal MCF7 cells induces a mesenchymal-like phenotype and migratory and invasive potential [14, 31]. The present results support the view that LOXL2 is involved in the maintenance of the mesenchymal phenotype in basal carcinoma cells.
It is noteworthy that LOXL2 was prominently expressed in basal-like carcinoma and showed a capacity to increase aggressiveness. It has been proposed in several studies that LOXL2 could be a candidate marker of basal-like carcinoma [18, 21]. LOXL2 expression was also increased in patients with the triple-negative subtype. Although this subtype was not the same as that of basal-like carcinoma, our findings of the association of LOXL2 and the triple-negative subtype are concordant with previous reports on the relationship of LOXL2 and basal-like carcinoma. The establishment of treatment strategies for triple-negative tumors has remained a challenging task because of the heterogeneity of this subtype and the absence of targeted therapies, such as endocrine therapy or HER-2-targeted treatments [26]. Therefore, our findings are clinically important in providing a novel potential target candidate for this breast cancer subtype.
Principally based on experimental evidence, LOXL2 has been proposed as a therapeutic target in cancer treatment [18, 19, 21]. The current targeting strategy for LOXL2 focuses on inhibiting enzymatic activity. LOXL2 at the protein level seems to be effectively targeted either through the use of small-molecule inhibitors that may act both intracellularly and extracellularly, or through the use of antibodies [19]. Therefore, our data also support a therapeutic approach in which intracellular LOXL2 expression could be an effective target molecule for the improvement of survival in breast cancer patients. AB0024, which is a humanized LOXL2 antibody, has already entered phase I clinical trials in patients with solid tumors (ClinicalTrioal.gov.) [32].
Although there were limitations to our study, including retrospective study design with a small sample size, our findings provide preclinical and clinical evidence that the enzymatic activity of LOXL2 contributing to metastasis in an experimental setting can be translated into poor survival outcome in breast cancer patients.
In summary, results from IHC analyses of tumors demonstrate that LOXL2 is an independent marker for metastatic disease and death in patients with breast cancer. Also, our in vitro study demonstrated that LOXL2 expression promotes EMT and invasiveness of basal-like breast cancer cell lines, a finding that was compatible with previous in vitro study results. This suggests that LOXL2 could potentially be a valuable target for improvement of survival in breast cancer.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. 2007-0056092), and a research grant from National Cancer Center (1110490). Preparation of the final manuscript was done with the assistance of BioScience Writers LLC (Houston, TX). The authors acknowledge Dr. Janine T Erler for advice on studying LOXL2-siRNA. The authors thank Mr. Dong-Su Jang, Research Assistant, Department of Anatomy, Yonsei University College of Medicine, Seoul, Korea, for his help with the figures.
Contributor Information
Sung Gwe Ahn, Department of Surgery, Gangnam Severance Hospital, Yonsei University College of Medicine, 712 Eonjuro, Gangnam-gu, Seoul, Republic of Korea.
Seung Myung Dong, Research Institute and Hospital, National Cancer Center, Goyang, Gyeonggi, Republic of Korea.
Akira Oshima, Cha Research Institute, Cha University, Seoul, Republic of Korea.
Woo Ho Kim, Department of Pathology, Seoul National University College of Medicine, Seoul, Republic of Korea.
Hak Min Lee, Department of Surgery, Gangnam Severance Hospital, Yonsei University College of Medicine, 712 Eonjuro, Gangnam-gu, Seoul, Republic of Korea.
Seung Ah Lee, Department of Surgery, Eulji University College of Medicine, Seoul, Republic of Korea.
Seung-hyun Kwon, Department of Microbiology, Yonsei University Medical College, Seoul, Republic of Korea.
Ji-hae Lee, Research Institute and Hospital, National Cancer Center, Goyang, Gyeonggi, Republic of Korea.
Jae Myun Lee, Department of Microbiology, Yonsei University Medical College, Seoul, Republic of Korea; Department of Microbiology, Brain Korea 21 Project for Medical Sciences, Yonsei University Medical College, 250 Seongsanno, Seodaemun-gu, Seoul 120-752, Republic of Korea.
Joon Jeong, Department of Surgery, Gangnam Severance Hospital, Yonsei University College of Medicine, 712 Eonjuro, Gangnam-gu, Seoul, Republic of Korea.
Hy-De Lee, Department of Surgery, Gangnam Severance Hospital, Yonsei University College of Medicine, 712 Eonjuro, Gangnam-gu, Seoul, Republic of Korea.
Jeffrey E. Green, Laboratory of Cancer Biology and Genetics, National Cancer Institute, Bethesda, MD, USA
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55:74–108 [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ (2007) Cancer statistics, 2007. CA Cancer J Clin 57:43–66 [DOI] [PubMed] [Google Scholar]
- 3.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Lang I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Ruschoff J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD (2005) Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 353:1659–1672. doi: 10.1056/NEJMoa052306 [DOI] [PubMed] [Google Scholar]
- 4.Fernandez Y, Cueva J, Palomo AG, Ramos M, de Juan A, Calvo L, Garcia-Mata J, Garcia-Teijido P, Pelaez I, Garcia-Estevez L (2010) Novel therapeutic approaches to the treatment of metastatic breast cancer. Cancer Treat Rev 36:33–42. doi: 10.1016/j.ctrv.2009.10.001 [DOI] [PubMed] [Google Scholar]
- 5.Asuncion L, Fogelgren B, Fong KS, Fong SF, Kim Y, Csiszar K (2001) A novel human lysyl oxidase-like gene (LOXL4) on chromosome 10q24 has an altered scavenger receptor cysteine rich domain. Matrix Biol 20:487–491 [DOI] [PubMed] [Google Scholar]
- 6.Jourdan-Le Saux C, Tomsche A, Ujfalusi A, Jia L, Csiszar K (2001) Central nervous system, uterus, heart, and leukocyte expression of the LOXL3 gene, encoding a novel lysyl oxidase-like protein. Genomics 74:211–218 [DOI] [PubMed] [Google Scholar]
- 7.Kim Y, Boyd CD, Csiszar K (1995) A new gene with sequence and structural similarity to the gene encoding human lysyl oxidase. J Biol Chem 270:7176–7182 [DOI] [PubMed] [Google Scholar]
- 8.Saito H, Papaconstantinou J, Sato H, Goldstein S (1997) Regulation of a novel gene encoding a lysyl oxidase-related protein in cellular adhesion and senescence. J Biol Chem 272:8157–8160 [DOI] [PubMed] [Google Scholar]
- 9.Kim YM, Kim EC, Kim Y (2011) The human lysyl oxidase-like 2 protein functions as an amine oxidase toward collagen and elastin. Mol Biol Rep 38:145–149 [DOI] [PubMed] [Google Scholar]
- 10.Fong SF, Dietzsch E, Fong KS, Hollosi P, Asuncion L, He Q, Parker MI, Csiszar K (2007) Lysyl oxidase-like 2 expression is increased in colon and esophageal tumors and associated with less differentiated colon tumors. Genes Chromosom Cancer 46:644–655 [DOI] [PubMed] [Google Scholar]
- 11.Offenberg H, Brunner N, Mansilla F, Orntoft Torben F, Birkenkamp-Demtroder K (2008) TIMP-1 expression in human colorectal cancer is associated with TGF-B1, LOXL2, INHBA1, TNF-AIP6 and TIMP-2 transcript profiles. Mol Oncol 2:233–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peng L, Ran YL, Hu H, Yu L, Liu Q, Zhou Z, Sun YM, Sun LC, Pan J, Sun LX, Zhao P, Yang ZH (2009) Secreted LOXL2 is a novel therapeutic target that promotes gastric cancer metastasis via the Src/FAK pathway. Carcinogenesis 30:1660–1669 [DOI] [PubMed] [Google Scholar]
- 13.Ruckert F, Joensson P, Saeger HD, Grutzmann R, Pilarsky C (2010) Functional analysis of LOXL2 in pancreatic carcinoma. Int J Colorectal Dis 25:303–311 [DOI] [PubMed] [Google Scholar]
- 14.Akiri G, Sabo E, Dafni H, Vadasz Z, Kartvelishvily Y, Gan N, Kessler O, Cohen T, Resnick M, Neeman M, Neufeld G (2003) Lysyl oxidase-related protein-1 promotes tumor fibrosis and tumor progression in vivo. Cancer Res 63:1657–1666 [PubMed] [Google Scholar]
- 15.Erler JT, Bennewith KL, Nicolau M, Dornhofer N, Kong C, Le QT, Chi JT, Jeffrey SS, Giaccia AJ (2006) Lysyl oxidase is essential for hypoxia-induced metastasis. Nature 440:1222–1226 [DOI] [PubMed] [Google Scholar]
- 16.Peinado H, Moreno-Bueno G, Hardisson D, Perez-Gomez E, Santos V, Mendiola M, de Diego JI, Nistal M, Quintanilla M, Portillo F, Cano A (2008) Lysyl oxidase-like 2 as a new poor prognosis marker of squamous cell carcinomas. Cancer Res 68:4541–4550. doi: 10.1158/0008-5472.can-07-6345 [DOI] [PubMed] [Google Scholar]
- 17.Barry-Hamilton V, Spangler R, Marshall D, McCauley S, Rodriguez HM, Oyasu M, Mikels A, Vaysberg M, Ghermazien H, Wai C, Garcia CA, Velayo AC, Jorgensen B, Biermann D, Tsai D, Green J, Zaffryar-Eilot S, Holzer A, Ogg S, Thai D, Neufeld G, Van Vlasselaer P, Smith V (2010) Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat Med 16:1009–1017 [DOI] [PubMed] [Google Scholar]
- 18.Barker HE, Chang J, Cox TR, Lang G, Bird D, Nicolau M, Evans HR, Gartland A, Erler JT (2011) LOXL2-mediated matrix remodeling in metastasis and mammary gland involution. Cancer Res 71:1561–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barker HE, Cox TR, Erler JT (2012) The rationale for targeting the LOX family in cancer. Nat Rev Cancer 12:540–552 [DOI] [PubMed] [Google Scholar]
- 20.Peinado H, Del Carmen Iglesias-de la Cruz M, Olmeda D, Csiszar K, Fong KS, Vega S, Nieto MA, Cano A, Portillo F (2005) A molecular role for lysyl oxidase-like 2 enzyme in snail regulation and tumor progression. EMBO J 24:3446–3458. doi: 10.1038/sj.emboj.7600781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moreno-Bueno G, Salvador F, Martin A, Floristan A, Cuevas EP, Santos V, Montes A, Morales S, Castilla MA, Rojo-Sebastian A, Martinez A, Hardisson D, Csiszar K, Portillo F, Peinado H, Palacios J, Cano A (2011) Lysyl oxidase-like 2 (LOXL2), a new regulator of cell polarity required for metastatic dissemination of basal-like breast carcinomas. EMBO Mol Med 3:528–544. doi: 10.1002/emmm.201100156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hammond ME, Hayes DF, Dowsett M et al. (2010) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch Pathol Lab Med 134:48–72 [DOI] [PubMed] [Google Scholar]
- 23.Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler TM, Hayes DF (2007) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 25:118–145 [DOI] [PubMed] [Google Scholar]
- 24.Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, Clark L, Bayani N, Coppe JP, Tong F, Speed T, Spellman PT, DeVries S, Lapuk A, Wang NJ, Kuo WL, Stilwell JL, Pinkel D, Albertson DG, Waldman FM, McCormick F, Dickson RB, Johnson MD, Lippman M, Ethier S, Gazdar A, Gray JW (2006) A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell 10:515–527. doi: 10.1016/j.ccr.2006.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomaskovic-Crook E, Thompson EW, Thiery JP (2009) Epithelial to mesenchymal transition and breast cancer. Breast Cancer Res 11:213. doi: 10.1186/bcr2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P, Narod SA (2007) Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 13:4429–4434 [DOI] [PubMed] [Google Scholar]
- 27.Alexieva-Figusch J, Van Putten WL, Blankenstein MA, Blonk-Van Der Wijst J, Klijn JG (1988) The prognostic value and relationships of patient characteristics, estrogen and progestin receptors, and site of relapse in primary breast cancer. Cancer 61:758–768 [DOI] [PubMed] [Google Scholar]
- 28.Foulkes WD, Smith IE, Reis-Filho JS (2010) Triple-negative breast cancer. N Engl J Med 363:1938–1948 [DOI] [PubMed] [Google Scholar]
- 29.Brekhman V, Neufeld G (2009) A novel asymmetric 3D in vitro assay for the study of tumor cell invasion. BMC Cancer 9:415. doi: 10.1186/1471-2407-9-415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brekhman V, Lugassie J, Zaffryar-Eilot S, Sabo E, Kessler O, Smith V, Golding H, Neufeld G (2011) Receptor activity modifying protein-3 mediates the protumorigenic activity of lysyl oxidase-like protein-2. FASEB J 25:55–65. doi: 10.1096/fj.10-162677 [DOI] [PubMed] [Google Scholar]
- 31.Hollosi P, Yakushiji JK, Fong KS, Csiszar K, Fong SF (2009) Lysyl oxidase-like 2 promotes migration in noninvasive breast cancer cells but not in normal breast epithelial cells. Int J Cancer 125:318–327. doi: 10.1002/ijc.24308 [DOI] [PubMed] [Google Scholar]
- 32.ClinicalTrioal.gov (2013) First-in-human study of AB0024 to evaluate safety and tolerability in adults with advanced solid tumors. http://www.clinicaltrials.gov/ct2/show/NCT01323933. Accessed Mar 25, 2013