Abstract
Chimeric antigen receptor (CAR)–expressing T cells induce durable remissions in patients with relapsed/refractory B cell malignancies. CARs are synthetic constructs that, when introduced into mature T cells, confer a second, non–major histocompatibility complex–restricted specificity in addition to the endogenous T cell receptor (TCR). The implications of TCR activation on CAR T cell efficacy has not been well defined. Using an immunocompetent, syngeneic murine model of CD19-targeted CAR T cell therapy for pre–B cell acute lymphoblastic leukemia in which the CAR is introduced into T cells with known TCR specificity, we demonstrate loss of CD8 CAR T cell efficacy associated with T cell exhaustion and apoptosis when TCR antigen is present. CD4 CAR T cells demonstrate equivalent cytotoxicity to CD8 CAR T cells and, in contrast, retain in vivo efficacy despite TCR stimulation. Gene expression profiles confirm increased exhaustion and apoptosis of CD8 CAR T cells upon dual receptor stimulation compared to CD4 CAR T cells and indicate inherent differences between CD4 and CD8 CAR T cells in the use of T cell–associated signaling pathways. These results provide insights into important aspects of CAR T cell immune biology and indicate opportunities to rationally design CAR constructs to optimize clinical efficacy.
INTRODUCTION
Current, multiagent chemotherapeutic regimens cure 85% of pediatric patients diagnosed with acute lymphoblastic leukemia (ALL) but carry a poor prognosis in adults, and relapsed and/or refractory ALL remains a challenge in all patients (1–3). Adoptive transfer of T cells genetically modified to express chimeric antigen receptors (CARs) targeting CD19 induces complete remissions in 70 to 90% of patients with relapsed and refractory pre–B cell ALL (4–6) and demonstrates impressive responses in B cell lymphomas (7–10). A remarkably small dose of infused CAR T cells can eradicate large disease burden, demonstrating the importance of in vivo CAR T cell expansion for efficacy (5, 6). Early trials have also indicated that CAR persistence will be important to consistently sustain durable remissions (5, 11, 12). The requirements for robust CAR T cell expansion and persistence have largely been assumed to mimic those required for T cell receptor (TCR)–stimulated T cells despite the artificial nature of the CAR construct.
Structurally, CARs consist of an extracellular antigen-binding domain (typically derived from a monoclonal antibody) directly coupled to CD3 zeta signaling domains and costimulatory domain(s) (13, 14). CAR expression allows for redirection of T cell specificity toward an antigen expressed on the surface of a tumor cell, independent of the major histocompatibility complex (MHC). CAR T cells currently being used in clinical trials retain endogenous TCRs, including trials in which CAR T cells were generated from T cell populations with known viral reactivity via the endogenous TCR (15–17). In one such trial, viral reactivation resulted in enhanced expansion of CD19-specific CAR T cells, but this was not correlated with improved clearance of CD19+ leukemia or endogenous B cells (7). Such unexpected findings underscore the need to better understand the in vivo biology of the dual antigen specificity of CAR T cells and the subsequent implications for efficacy.
Preclinical studies on CAR T cells have typically focused on human T cells in murine xenograft models, complicating interpretation of the in vivo biology of CAR T cells due to lack of an intact immune environment, including human MHC molecules, and the development of xenogeneic graft-versus-host disease (GVHD) that impedes long-term monitoring. To overcome these limitations, we used a syngeneic murine model of pre–B cell ALL, taking advantage of TCR transgenic mice to evaluate the impact of endogenous TCR stimulation on the activity of CD4+ and CD8+ CAR T cells in vivo. Using this model, we demonstrate that concomitant activation of the CAR and TCR significantly diminishes the in vivo efficacy of CAR8 cells, which is associated with increased markers of exhaustion and apoptosis. Conversely, CAR4 cells maintain the ability to clear ALL in vivo and persist in the presence of both TCR and CAR antigens. These findings illustrate the importance of understanding the unique biology of CAR T cells and provide rational approaches to enhance clinical efficacy.
RESULTS
CAR4 and CAR8 cells demonstrate comparable in vitro and in vivo efficacy and mediate durable remissions in ALL
TCR-mediated xenogeneic GVHD and host immunodeficiency impair the systematic evaluation of CAR T cell biology in xenograft models. We used a clinically relevant syngeneic murine CD19+ pre–B cell ALL model (18, 19) that leads to rapid lethality in immunocompetent mice, is resistant to 500–centigray (cGy) radiation (fig. S1, A and B), and can be eradicated by T cells transduced with a second-generation CAR with a CD28 costimulatory domain targeting murine CD19 (18). Purified CD4+ and CD8+ T cells transduced with this murine CD19 CAR (CAR4 and CAR8 cells, respectively) demonstrate equivalent in vitro cytotoxicity against CD19+ ALL (Fig. 1A). Upon in vitro coculture with CD19+ ALL, CAR4 cells produce significantly more interferon-γ (IFN-γ) (P = 0.0014), interleukin-2 (IL-2) (P < 0.0001), tumor necrosis factor–α (TNF-α) (P <0.0001), IL-6 (P < 0.0001), and IL-4 (P < 0.0001) than CAR8 cells. In contrast, CAR8 cells generate higher levels of the immunosuppressive cytokine IL-10 (P < 0.0001) (Fig. 1B).
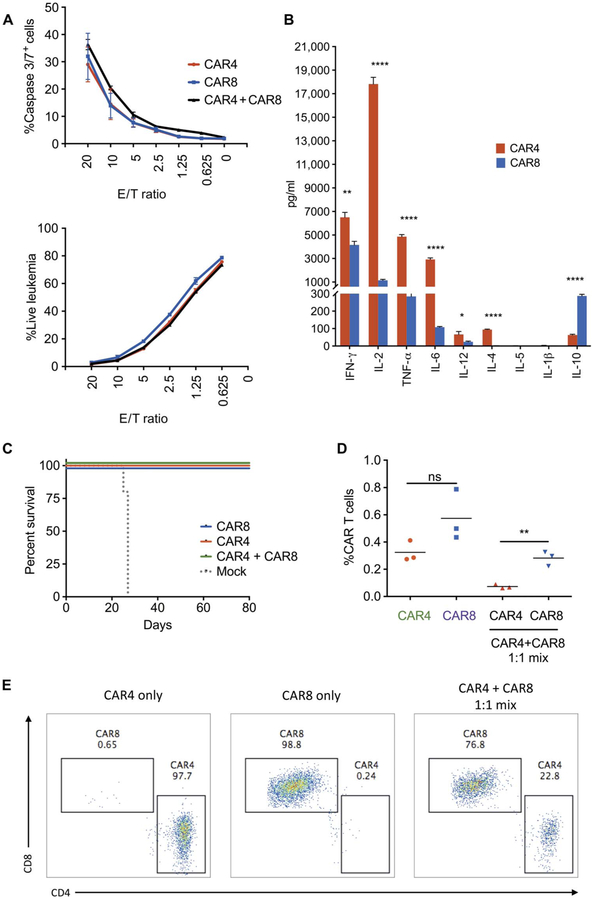
Fig. 1. CAR4 and CAR8 cells demonstrate in vitro and in vivo antileukemic efficacy.
(A) In vitro killing of CD19+ murine ALL after 4 hours of coincubation, as measured by caspase 3/7 expression and loss of viable leukemia cells. E/T ratio, effector-to-target ratio. (B) Cytokine production of three biological replicates of CAR4 and CAR8 cells after 4 hours of coincubation with CD19+ ALL (error, SD; *P < 0.05, **P < 0.01, ****P < 0.001). (C) Survival analysis of syngeneic mice after infusion of 1 × 106 CAR4 or CAR8 or 5 × 105 CAR4 + 5 × 105 CAR8 cells 4 days after CD19+ ALL inoculation (n = 5 per group; P < 0.0001, Mantel-Cox test). (D and E) Persistence of murine CAR4 and CAR8 cells in the bone marrow of syngeneic recipients 14 days after infusion of CAR4, CAR8, or mixed cells [n = 3; **P < 0.01, one-way analysis of variance (ANOVA) performed for all comparisons and log-rank Mantel-Cox test for survival analysis]. ns, not significant.
At 4 days after leukemia inoculation, coinfusion of a 1:1 mixture of CAR4 and CAR8 cells effectively mediates in vivo tumor clearance and survival (Fig. 1C). CAR4 and CAR8 cells infused alone are equally efficacious against ALL at curative doses (Fig. 1C) and equipotent at delaying leukemia progression at noncurative doses (fig. S1C). However, purified CAR8 cells show enhanced expansion at day +7 compared to purified CAR4 cells. In addition, after 1:1 CAR4/CAR8 coadministration, CAR8 cells expand preferentially to CAR4 (Fig. 1, D and E). Together, CAR4 cells show comparable antileukemic activity to CAR8 cells and produce higher amounts of immune-stimulatory cytokines, but expansion is diminished in the presence of CAR8 cells.
CAR4 but not CAR8 cells retain in vivo activity in the presence of both TCR and CAR antigens
The introduction of a CAR generates a T cell with dual antigen specificity, and the retained TCR specificity has been used to boost expansion of viral-specific CAR T cells in clinical trials (7, 16), but the effects of dual activation of a CAR T cell on antitumor efficacy has not been evaluated in immunocompetent preclinical models. We generated CAR4 and CAR8 cells with defined endogenous TCR specificity for male minor histocompatibility antigen, HY (fig. S2, A and B). As shown in Fig. 2A, HY-specific CAR4 and CAR8 cells produce IL-2 and IFN-γ upon TCR and CAR stimulation, and as with TCR polyclonal CAR T cells, both HY-specific CAR4 and CAR8 cells degranulate when exposed to CD19+ leukemia (Fig. 2B).
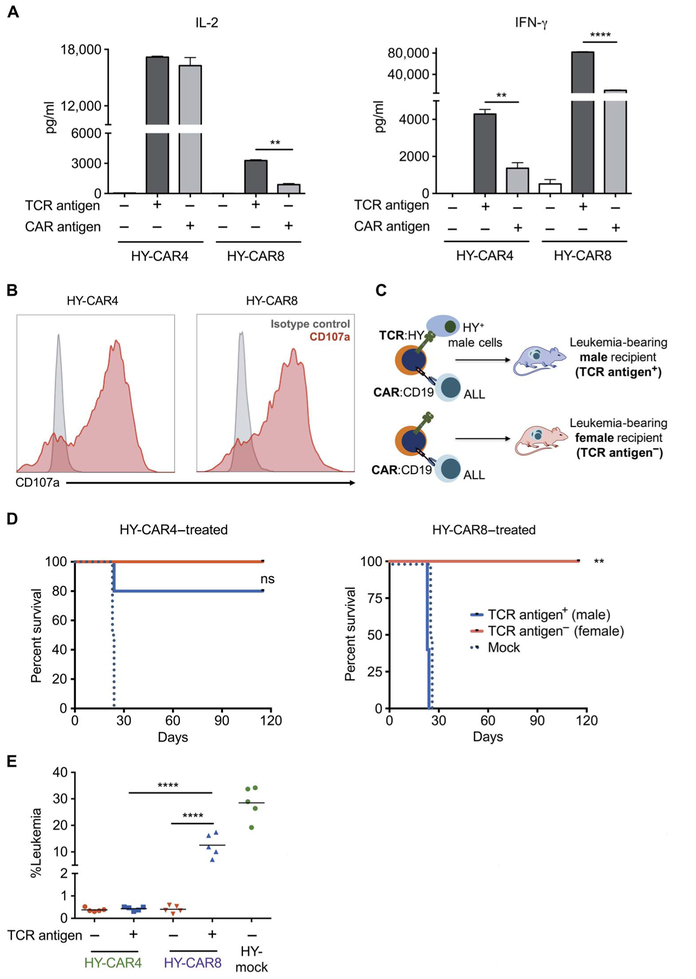
Fig. 2. Exposure of both TCR and CAR antigens diminishes efficacy of CAR8 but not CAR4 cells.
(A) IL-2 and IFN-γ production by HY-CAR4 and HY-CAR8cells after10hoursofincubationwithCD19KOfemalesplenocytes (CD19−/HY−), CD19KO male splenocytes (CD19−/HY+), or wild-type (WT) female splenocytes (CD19+/HY−) (**P < 0.001, ****P < 0.0001). (B) CD107a expression (red) and isotype control (gray) on HY-CAR T cells after 4 hours of incubation with CD19+ E2aPbx ALL. (C) HY-CAR4 or HY-CAR8 cells were administered on day 4 to E2aPbx-bearing syngeneic male (HY+) and female (HY−) recipients. (D) Survival of female (TCR antigen−) and male (TCR antigen+) recipients after treatment with 1 × 106 HY-specific CAR4 or CAR8 cells (n = 5 per group; **P < 0.01). (E) Leukemia burden in the bone marrow 7 days after infusion of mice treated with HY-CAR4 or HY-CAR8 cells (****P < 0.001, one-way ANOVA performed for all comparisons and log-rank Mantel-Cox test for survival analysis).
To determine the impact of endogenous TCR signaling on the efficacy of CAR T cells in vivo, we adoptively transferred HY-specific CAR4 and CAR8 cells from female donors into leukemia-bearing male (HY+) and female (HY−) mice (Fig. 2C). HY-specific CAR4 cells eradicate leukemia in both female and male recipients, with greater than 100 days of survival. In contrast, HY-specific CAR8 cells clear leukemia in female recipients but fail to prolong survival relative to mock (activated and nontransduced) T cells in HY+ recipients (Fig. 2D). Progressive CD19+ leukemia was confirmed in male recipients treated with HY-specific CAR8 cells at day +7 (Fig. 2E) and day +14 (fig. S2, C and D). Together, these data demonstrate that CAR T cells retain the ability to respond to antigen through endogenous TCR, but signaling through TCR impairs the ability of CAR8 cells to eliminate leukemia in vivo, exposing inherent biologic differences between CAR4 and CAR8 cells.
The presence of TCR antigen reduces CAR8 cell numbers and is associate with increased apoptosis and phenotypic exhaustion
To evaluate the mechanism of CD19+ leukemia progression in HY+ recipients of CAR8 cells, we analyzed surface CAR expression on adoptively transferred CAR T cells (CD45.2+) on days +7 and +14. The surface expression of the CAR was reduced in CAR8 cells when the TCR antigen was present. Similarly, the presence of TCR antigen resulted in decreased CAR expression in CAR4 cells that effectively cleared leukemia and protected against early relapse (fig. S3), indicating that loss of CAR expression does not explain poor efficacy of CAR8 in the presence of TCR antigen.
We next measured the impact of TCR antigen on expansion of CAR4 and CAR8 cells. Enhanced HY-specific CAR4 expansion was seen in leukemia-bearing HY+ recipients compared to HY− recipients, whereas the presence of TCR antigen resulted in reduced expansion in the percentage of HY-specific CAR8 cells (Fig. 3A). Furthermore, HY-specific CAR8 cells contained significantly higher amounts of cleaved caspase 3/7 in HY+ recipients compared to HY− recipients (P <0.0001) (Fig. 3B). In contrast, there was no change in cleaved caspase 3/7 in HY-specific CAR4 cells with the addition of TCR signaling (Fig. 3C). These results suggest that simultaneous CAR and TCR stimulation limits expansion of CAR8 cells through increased apoptosis.
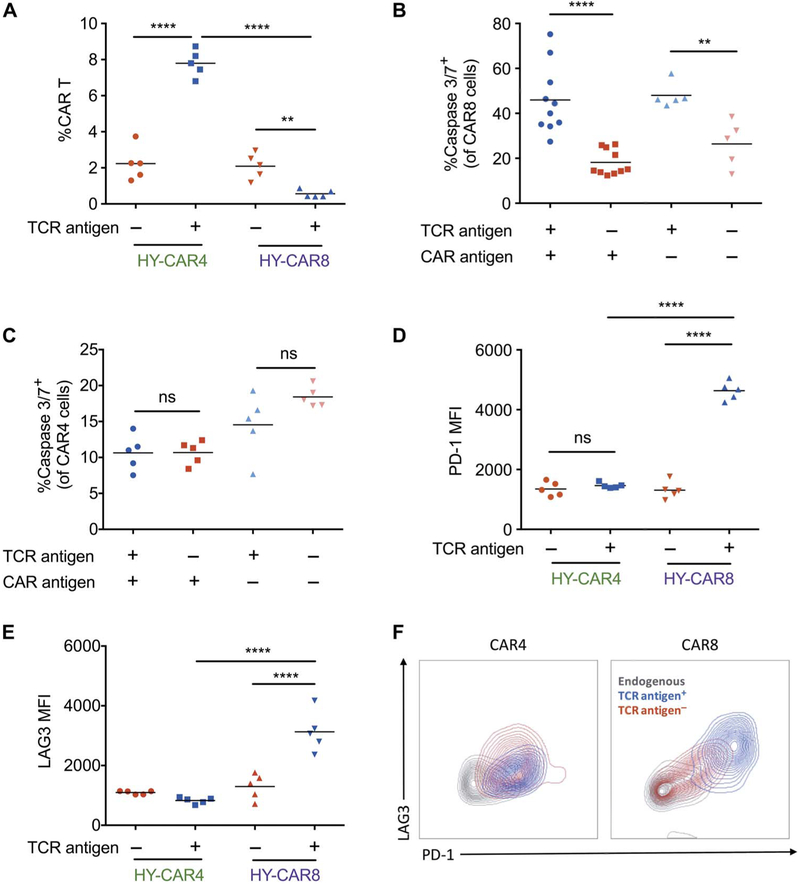
Fig. 3. Increased expression of exhaustion markers and apoptosis markers on CAR8 cells in the presence of TCR antigen.
(A) HY-CAR4 and HY-CAR8 expansion in the bone marrow of leukemia-bearing HY+ or HY− mice (n = 5) 7 days after CAR T cell infusion. (B and C) Percent caspase 3/7+ CAR8 and CAR4 cells at 72 hours after infusion. (D and E) Mean fluorescence intensity (MFI) of PD-1 and LAG3 on HY-specific CAR T cells in male (HY+) and female (HY−) recipients day +7 after infusion (**P < 0.01, ****P < 0.0001). (F) Overlaid zebra plots of PD-1 and LAG3 expression of endogenous T cells or HY-CAR4 or HY-CAR8 cells in the presence or absence of HY antigen (one-way ANOVA performed for all comparisons).
Finally, we assessed whether exhaustion contributes to poor leukemic clearance by CAR8 cells in the presence of TCR stimulation. In leukemia-bearing female recipients, HY-specific CAR4 and CAR8 cells expressed higher levels of PD-1 and LAG3 upon CAR stimulation compared to endogenous T cells (CD45.1+) (fig. S4). The presence of TCR antigen in male recipient mice did not further increase the level of PD-1 or LAG3 on HY-specific CAR4 cells. However, the presence of both TCR and CAR antigen drove increased expression of PD-1 and LAG3 on HY-specific CAR8 in the same mice (Fig. 3, D to F). Collectively, these results indicate that both quantitative and qualitative defects contribute to the failure of CAR8 cells in eradicating leukemia when simultaneously stimulated through the TCR.
Restriction of TCR antigen to hematopoietic tissues does not prevent CAR8 cell exhaustion and failure to clear leukemia
We previously demonstrated that restriction of TCR antigen to hematopoietic-derived cells reverses impaired TCR-mediated leukemic clearance with broad TCR antigen expression (19). Thus, we examined the impact of restricted TCR antigen expression on CAR-mediated antileukemic responses. Female/male hematopoietic chimeras were generated as detailed in Fig. 4A and in Materials and Methods. Chimeras were injected with leukemia 21 days later and treated with HY-specific CAR4, CAR8, or mock T cells. As with globally expressing HY+ recipients (Figs. 2 and 3), CAR8 cells failed to clear leukemia even when HY antigen is restricted to the hematopoietic compartment, whereas CAR4 cell–treated mice remained leukemia-free past day 50 irrespective of the compartment in which HY was expressed (F→M and M→F) (Fig. 4, A to E).
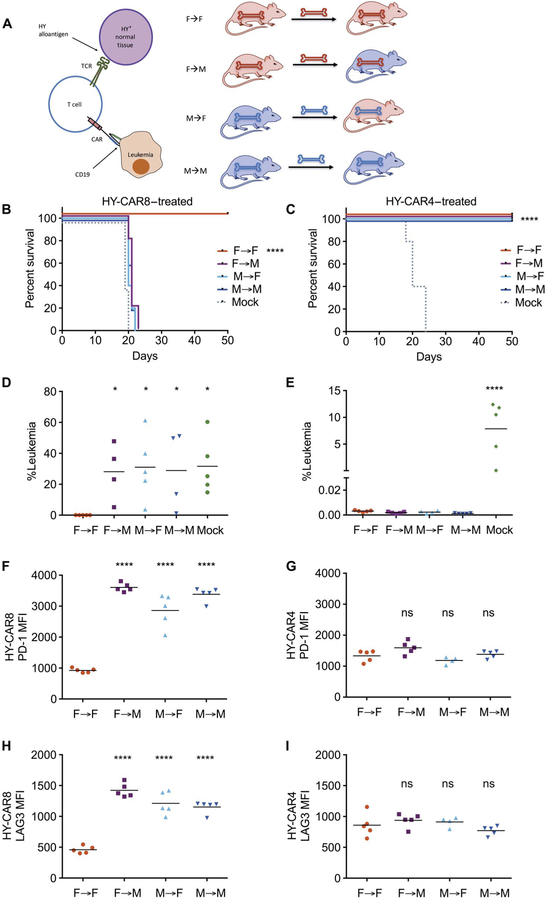
Fig. 4. Restriction of TCR antigen to hematopoietic tissues does not prevent CAR8 exhaustion and failure of leukemia clearance.
(A) Female/male hematopoietic chimeras were generated by CD3-depleted bone marrow transplantation 21 days before injection of 1 × 106 E2aPbx followed by lymphodepletion on day −1 and treatment with 1 × 106 CD19 CAR T cells or mock-transduced T cells on day 0. (B and C) Survival of HY-CAR8–or HY-CAR4–treated mice. (D and E) Leukemia burden in the bone marrow 7 days after infusion of CAR T cells. (F and G) PD-1 expression and (H and I) LAG3 expression on HY-CAR8 and HY-CAR4 7 days after infusion [for (B) to (I): *P < 0.05, ****P < 0.0001 (relative to the F→F group), one-way ANOVA performed for all comparisons and log-rank Mantel-Cox test for survival analysis].
Transfer of CAR8 cells into M→F chimeras resulted in a marked expression of PD-1 and LAG3, indicating that hematopoietic restriction of TCR antigen does not prevent CAR8 cell exhaustion. CAR4 cells did not express elevated PD-1 or LAG3 irrespective of where HY antigen was expressed (Fig. 4, F to I). Elimination of leukemia and loss of the endogenous B cell compartment were observed in F→F HY− recipients of both CAR4 and CAR8 cells (fig. S5). Incomplete eradication of leukemia but partial clearance of CD19+ endogenous B cells was observed in a subset of CAR8 cell–treated mice with hematopoietic expression of HY antigen. In contrast, CAR4 cells eliminated both leukemia and endogenous B cells regardless of the presence or location of the HY antigen in the host, again demonstrating the inherent resistance of CAR4 cells to TCR-mediated dysfunction.
TCR antigen–mediated CAR8 cell exhaustion and lack of leukemic clearance also occur in the OT1-TCR transgenic model
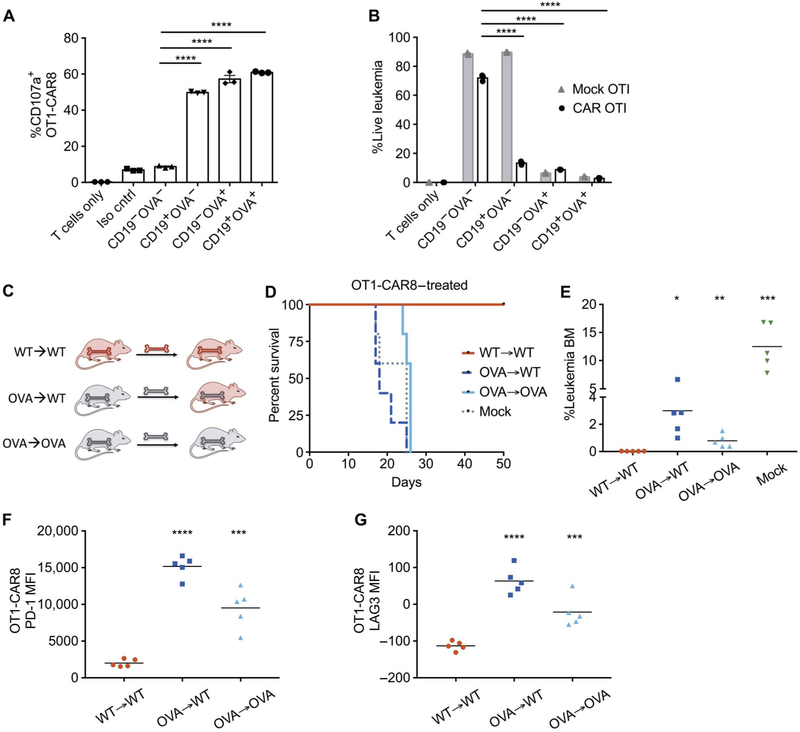
To verify TCR-mediated dysfunction in CAR8 cells in a separate TCR transgenic model, we generated CAR8 cells from Rag1−/− OT1 transgenic mice with TCR specificity against the ovalbumin (OVA) peptide SIINFEKL in the context of H2-Kb presentation. OT1-CAR8 cells demonstrated in vitro degranulation and killing through activation of the endogenous TCR, CAR, or both receptors (CD19+, CD19−OVA+, and CD19+OVA+ ALL; Fig. 5, A and B). As with the HY model, hematopoietic chimeras were generated using transgenic mice ubiquitously expressing the OVA protein as described in Materials and Methods and shown in Fig. 5C. OT1 CAR8 cells were functional in the absence of OVA in the recipient, but in accordance with the data from the HY model, bone marrow expression of both CAR and TCR antigens (CD19+OVA+) resulted in leukemia-associated mortality after treatment with CAR8 cells (Fig. 5, D and E). Furthermore, OT1-CAR8 cells expressed higher PD-1 and LAG3 in leukemia-bearing, OVA-expressing recipients compared to wild-type recipients with CD19+ leukemia, suggesting exhaustion of OT1-CAR8 cells as the mechanism of treatment failure (Fig. 5, F and G). Thus, restricted hematopoietic expression of TCR antigen does not prevent CAR8 dysfunction in multiple TCR transgenic systems, supporting that this finding is generalizable to CD8 CAR T cells.
Fig. 5. CAR8 failure in an OT1 TCR transgenic T cell after exposure to OVA.
(A) CD107a expression on OT1-CAR T cells generated from Rag1−/− OT1 spleens at 4 hours of coincubation with E2aPbx expressing CD19 and/or OVA antigens. Iso cntrl, isotype control. (B) Percent viable E2aPbx cells expressing varying combinations of the CD19 and OVA antigens after 3 days of coculture with OT1-CAR or mock-OT1 T cells. (C) OVA-expressing hematopoietic chimeras were generated by CD3-depleted bone marrow transplant 21 days before inoculation with 1 × 106 E2aPbx, with lymphodepletion and 1 × 106 CD19 OT1-CAR8 cell treatment on day 0. (D) Survival of OVA chimeric mice treated with OT1-CAR8 T cells. (E) Leukemia burden of OT1-CAR8–treated mice 7 days after infusion. BM, bone marrow. (F and G) Analysis of infused OT1-CAR8 cells from the bone marrow 7 days after infusion. (F) PD-1 expression and (G) LAG3 expression on OT1-CAR8 cell [for (E) to (G): *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, relative to the WT→WT group, one-way ANOVA performed for all comparisons and log-rank Mantel-Cox test for survival analysis].
Persistent CAR4 cells are susceptible to the inhibitory effects of chronic TCR stimulation
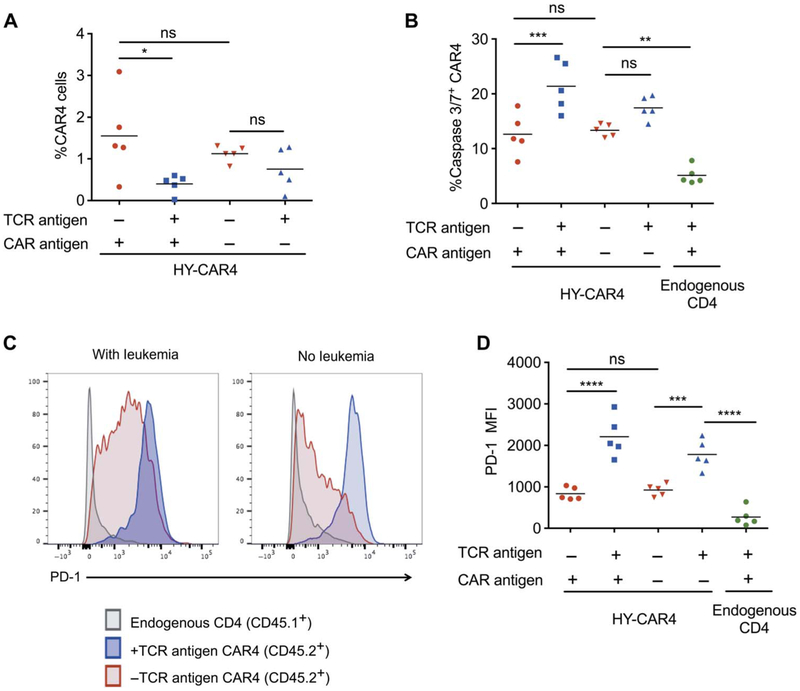
We next assessed the impact of chronic TCR antigen presence on CAR4 cell persistence after complete eradication of leukemia. The percentage of HY-specific CAR4 cells in HY+ recipients 80 days after adoptive transfer was significantly lower than that in HY− recipients (P = 0.018) (Fig. 6A). In addition, persistent CAR4 cells contained increased cleaved caspase 3/7 (P = 0.0006) (Fig. 6B) and significantly higher PD-1 in male recipients (P < 0.0001) (HY+) at day 80 compared to cells in female recipients (HY−) (Fig. 6, C and D). These results indicated that, despite efficient initial leukemic clearance and expansion, continuous exposure to TCR antigen negatively affects the long-term persistence of CAR4 cells in vivo, suggesting long-term surveillance against leukemia may be impaired.
Fig. 6. Persistence of CAR4 cells is reduced after sustained TCR engagement.
(A) Percentage of CAR4 cells in the bone marrow 80 days after infusion into leukemia-free (CAR antigen−) or leukemia-bearing (CAR antigen+) male (HY+) and female (HY−) recipients (n = 5, *P < 0.05). (B) Percentage of caspase 3/7+ CAR4 cells (CD45.2+) in the bone marrow at day 80 compared to endogenous CD4 T cells (CD45.1+) (**P < 0.01, ***P < 0.001). (C and D) PD-1 expression of CAR4 cells and endogenous CD4 T cells in the same recipient at day 80 after infusion into leukemia-free or leukemia-bearing male (HY+) and female (HY−) recipients (***P < 0.001, ****P < 0.0001, one-way ANOVA performed for all comparisons).
Disparate effects of TCR stimulation on CAR8 and CAR4 cells are associated with distinctive CAR- and TCR-driven gene expression profiles
To better understand the differences in the in vivo biology of CAR4 and CAR8 cells in the context of TCR stimulation, we compared the gene expression profiles of HY-specific CAR4 and HY-specific CAR8 cells 10 hours after CAR stimulation (cultured with CD3-depleted female splenocytes containing CD19+ B cells), TCR stimulation (cultured with CD3-depleted male splenocytes from CD19-deficient mice), or CAR + TCR stimulation (cultured with CD3-depleted male splenocytes containing CD19+ B cells) in vitro (fig. S6A). CAR and TCR stimulation in both CAR4 and CAR8 cells was confirmed by the production of IL-2 and IL-10 (fig. S6B).
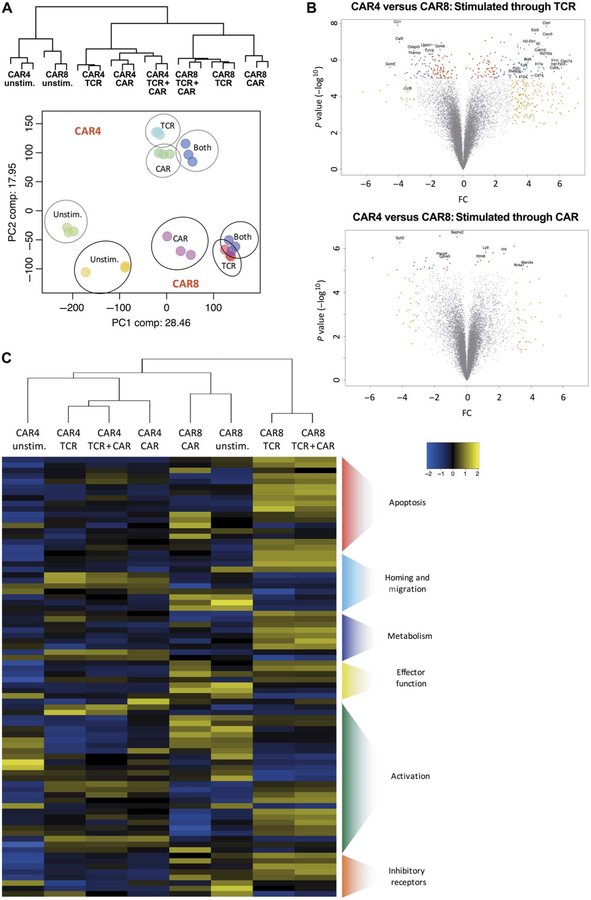
As expected, the principal components analysis demonstrated that significant gene expression differences exist between CD4 and CD8 T cells when stimulated through the endogenous TCR. Despite the expression of an identical CAR, CD19 stimulation resulted in distinct gene expression patterns between CAR4 and CAR8 cells (Fig. 7A), but fewer differentially expressed genes upon CAR stimulation compared to TCR stimulation (Fig. 7B). Furthermore, CAR8 cells stimulated through both the endogenous TCR and CAR expressed a very similar profile to CAR8 cells stimulated through the endogenous TCR alone, suggesting that the TCR signal may dominate the CAR signal in CAR8 cells. Conversely, CAR4 cells stimulated through both receptors expressed a profile more similar to CAR stimulation (Fig. 7A).
Fig. 7. Stimulation through CAR, TCR, or both receptors induces unique gene expression signatures in CAR4 and CAR8 cells.
(A) Dendrogram and principal components analysis of global transcriptional profile of three biological replicates of CAR4 and CAR8 cells evaluated 10 hours after stimulation through either TCR, CAR, or both receptors with CD3-depleted splenocytes as detailed in fig. S6. (B) Volcano plots of CAR4 versus CAR8 cells stimulated through both TCR or CAR, respectively [−log P value versus fold change (FC)]. (C) Heat map of z score values of apoptotic, homing and migration, metabolism, effector, activation, and inhibitory receptor gene expression (20) of CAR4 and CAR8 cells stimulated through CAR, TCR, or both receptors.
Gene set pathway analysis demonstrated a difference in apoptotic genes (P < 0.001 cutoff on gene list) between CAR4 and CAR8 cells, supporting the differences in cleaved caspase 3/7 seen in vivo (Fig. 3). As predicted, CAR8 cells demonstrated a significant elevation of proapoptotic genes (Casp3, Bik, Bak1, and Bok) after stimulation through the TCR or both CAR and TCR when compared to CAR4 cells undergoing the same stimulation. In addition to apoptotic genes, CAR8 cells had increased expression of inhibitory receptor genes compared to CAR4 cells, including PD-1 and LAG3, consistent with flow cytometry from in vivo studies (Fig. 3). Further pathway analysis indicated additional differences downstream of CAR and TCR stimulation in CAR8 and CAR4 cells including genes involved in T cell activation (Gzma, Eomes, Ras2, Irf4, Jun, Fos, Tox, and Stat1), homing and migration (CCR12, Cxcl10, and CCR7), and metabolism (Art3, Gpd2, and Clic4) (Fig. 7C and fig. S7) (20). These results suggest that other pathways besides exhaustion and apoptosis could be targeted to improve expansion, persistence, and functionality of CAR T cells.
DISCUSSION
T cells expressing CARs targeting CD19 have demonstrated potent activity in patients with relapsed or refractory B lymphoid malignancies (5, 6, 21) with high rates of remission in multiple clinical trials. However, about 10 to 20% of patients with ALL and 50% of patients with lymphoma will not enter remission. Furthermore, longer follow-up has demonstrated that remissions are not durable in a substantial proportion of patients without further consolidative therapy (11, 12, 22). Because the infused dose of CAR T cells is remarkably small, exponential expansion is required for response, suggesting lack of initial remission induction stems from CAR T cell failure. In addition, relapses of CD19-expressing leukemia in patients who achieve initial remission after CAR T cell treatment have been correlated with loss of CAR T cells as demonstrated by the return of normal B cells (5). Thus, a better understanding of factors that affect in vivo CAR T cell expansion and persistence has the potential to improve response rates and remission durability.
Many of the fundamental principles of T cell biology have been defined in syngeneic murine models that incorporate genetically modified mice, allowing for the elucidation of T cell behavior in a complex host environment. However, differences in CAR affinity, receptor density, and the linkage of costimulatory and CD3 zeta signaling likely lead to differences in CAR signaling relative to the endogenous TCR. A number of studies suggest this (17, 23) but are limited because they were performed in vitro or relied on the study of human T cells in immuno-deficient mice where xenogeneic TCR-MHC interactions may confound results and preclude longer-term studies. CAR T cells are endowed with dual specificity via the CAR binding domain and the endogenous TCR. Activation of the endogenous TCR has been hypothesized to improve expansion of CAR T cells (15–17), but the biological consequences of dual receptor activation of CAR T cells have not been well defined. Thus, it is likely that the biology of CAR T cells cannot be fully extrapolated from that of T cells activated through their native TCR.
Using a syngeneic murine model in which TCR reactivity and the expression of cognate TCR antigen could be precisely controlled, we studied the in vivo expansion, persistence, and function of CD19-targeted CAR T cells in hosts bearing pre–B cell ALL. We demonstrated the extensive and dichotomous impact of simultaneous CAR and TCR activation in CAR T cells on the early expansion, survival, and efficacy against progressive leukemia of CD8 versus CD4 CAR T cells. In these studies, simultaneous activation of the CAR and TCR completely abolished the efficacy of CAR8 cells in leukemia-infiltrated bone marrow, which was associated with reduced CAR8 cell numbers. Further, TCR-activated CAR8 cells were more prone to apoptosis and expressed T cell exhaustion markers known to negatively affect the effectiveness of immunotherapy. Previous studies demonstrated up-regulation of exhaustion markers on alloreactive CAR T cells upon transfer into an allogeneic host with concomitant abrogation of GVHD upon CAR signaling (24). The results of our study complement these findings by demonstrating that activation of the TCR, by a well-described minor alloantigen, leads to mitigation of the CAR-mediated clearance of established leukemia, and this effect is limited to the CD8 T cell compartment, a variable left untested in previous studies. These data suggest that dual receptor stimulation may lead to hyperactivation of CAR8 cells with subsequent deletion, similar to previous studies on CD8 T cells in an allogeneic setting (18, 25) and reminiscent of deletional tolerance in the setting of strong TCR activation (26, 27). In contrast to CAR8 cells, CAR4 cells were resistant to the negative impact of TCR stimulation on early expansion and activity, which allowed for successful clearance of leukemia. However, reduced persistence of CAR4 cells was observed after leukemic clearance in the presence of persistent TCR antigen, consistent with models of chronic TCR antigen exposure (28–31).
Because CD4 T cells are restricted to antigens presented on MHC class II molecules largely expressed on hematopoietic cells, one possibility is that CAR4 cells receive protective signals (membrane-associated costimulatoryand/orcytokine-mediated)fromprofessionalantigen-presenting cells (APCs), whereas CAR8 cells are not obligated to receive such signals due to ubiquitous MHC class I expression. However, in bone marrow chimeras, in which the TCR antigen was restricted solely to cells derived from the hematopoietic lineage, CAR8 cells remained dysfunctional and unable to clear leukemia. Thus, we favor cell-intrinsic differences between how CAR signaling is processed in CD4 and CD8 T cells. We observed significant differences in the gene expression patterns of CAR T cells solely based on the activation of the CAR and/or TCR. When stimulating through both receptors at the same time, CAR8 cells adopted a gene expression profile that was more closely related to that seen with TCR stimulation alone, whereas CAR4 cells demonstrated a gene expression profile more similar to that of CAR stimulation alone, suggesting differences in which signal dominates. Specifically, we found that CAR8 cells stimulated through the TCR with or without CAR differentially expressed proapoptotic genes as well as genes associated with T cell exhaustion, metabolism, and homing relative to CAR4 cells. These data have implications for the design of CAR signaling domains to optimize CAR T cell expansion and persistence.
A limitation of these studies is the extent to which findings in a murine syngeneic model can be directly translated to humans. Nonetheless, the use of a syngeneic murine model, in contrast to human xenograft systems, allowed the use of an immunocompetent host, removed xeno-reactivity, and enabled the use of two well-validated TCRtransgenic systems to study complex T cell biology (19, 32). Concurrent involvement of the endogenous TCR mimics viral-specific or donor-derived CAR T cells in the clinic (7, 33), where a significant percentage of CAR T cells infused could have contact with both TCR and CAR antigens simultaneously. Our model mimicked the effects of TCR activation in a CAR system in the context of tumor clearance with a clinically relevant leukemia target (CD19) on pre–B cell ALL and non–cross-reactive TCRs, allowing for the precise study of CAR-mediated T cell behavior in the presence of TCR or CAR antigens.
Clinical trials have established that CAR therapy can be very effective at inducing remissions in patients with CD19+ cancers (5, 6). Extending durability of remissions in most of the patients will be an important next step in the clinical development of CAR T cells for B cell malignancies. Initial experience with other targets and tumors has indicated that there will be substantial challenges in the induction of remission (34). Although improved target selection may overcome some of these challenges, approaches to optimize CAR T cell biology will also be needed. It is currently assumed that persistence of CAR T cells and generation of immunologic memory will be critical to establishing long-term remissions needed for cure. This work exposes important aspects of CAR T cell biology, with broad implications regarding activation and persistence in the setting of simultaneous CAR and TCR engagement. There is also enthusiasm to develop “off-the-shelf” CAR T cell products, which eliminates the TCR to avoid complications of alloreactivity. Although our data suggest that there may be a benefit to such a strategy for enhancing expansion, function, and early persistence of CAR8 cells, our study also cautions that the biology of TCR and CAR cross-talk is complex and does not exclude the potential positive roles of the endogenous TCR in CAR T cell signaling and/or survival when the TCR antigen is not ubiquitously present (35). Finally, trials have been conducted using defined mixtures of CD8 and CD4 T cells, based on data inferred from T cell biology, to generate CAR products that have demonstrated success in CD19+ leukemias and lymphomas (36–38). Because clinical trials progressively evaluate the production of CARs from sources with less TCR heterogeneity (that is, viral-reactive, allogeneic, or after bone marrow transplant), the likelihood of simultaneous TCR and CAR activation within the CAR T cell population will increase, and our data predict that the composition of the CAR T cell product will become more biologically relevant. In such scenarios where there is an increased chance of TCR activation of the CAR T cells, our findings would suggest the use or addition of CD4-derived CAR products. Our results have the potential to inform future strategies to enhance the therapeutic potential of CAR T cell therapy through CAR design and product composition.
MATERIALS AND METHODS
Study design
This study was designed to evaluate the effects of CAR-mediated T cell killing of leukemia in the presence and absence of TCR antigen. We used 6- to 10-week-old female mice as CAR T cell donors and recipients of leukemia and CAR T cell treatment. We used two models of TCR transgenic mice specific for male minor histocompatibility antigen HY or OVA peptide. Mice were inoculated with murine ALL, E2aPbx. Female congenic C57BL/6 CD45.1 HY or OVA chimera mice were transplanted with CD3-depleted bone marrow from male or female congenic C57BL/6 CD45.1 mice or OVA+ mice. All groups were randomized through irradiation step and leukemia inoculation steps, and primary survival end points were determined by paralysis of animals, compliant with Institutional Animal Care and Use Committee animal protocol procedures. Differential effects on CAR T cells residing in the bone marrow were assessed by flow cytometry using well-characterized antibodies. At least three biological replicates were performed for each experiment and n > 5 for each group unless indicated otherwise in the figure legends. One-way ANOVA or unpaired Student’s t tests were performed with an α of <0.05 and a power of 0.8 to detect differences between group means. Primary data are located in table S1.
Mice and cell lines
C57BL/6 (B6) CD45.1 (H-2b congenic) mice were purchased directly from the National Cancer Institute (NCI)–Frederick Animal Production Program via The Jackson Laboratory. MataHari (B6 Rag1−/−, CD8+ TCRTg, H2-Ab–restricted, and CD45.2+) TCR transgenic mice against MHC class I–restricted HYAB peptide NAGFNSNRANSSRSS and Marilyn (B6 Rag1−/−,CD4+ TCRTg, H2-Db–restricted, and CD45.2+) TCR transgenic mice against MHC class II–restricted HYDB peptide WMHHNMDLI purchased from the National Institute of Allergy and Infectious Diseases–Taconic repository mice were previously described (32, 39). Rag1−/−/transgenic OT1 TCRs were purchased from Taconic. E2aPbx murine ALL cell line was developed previously in the laboratory with normal B6 mice with E2A:PBx translocation crossed to a CD3ε−/− mouse gifted by J. Bijl (40). The E2aPbx cell line was adapted for culture and developed into a stable cell line expressing pre–B cell ALL markers as previously described (18). CD19− E2aPbx variants were made by CRISPR (clustered regularly interspaced short palindromic repeats)/CAS9–mediated mutation of the CD19 locus. OVA-expressing E2aPbx cell lines (either CD19+ or CD19− variants) were generated by the introduction of the OVA gene by retroviral transduction. All E2aPbx variants were enriched by fluorescence-activated cell sorting (FACS) >95% to obtain the desired population and subsequently single cell–cloned. All murine cell lines and primary mouse cells were cultured in complete mouse media consisting of RPMI 1640 with 10% heat-inactivated fetal calf serum, 1% nonessential amino acids, 1% sodium pyruvate, 1% penicillin/streptomycin, 1% L-glutamine (Invitrogen), and 1% Hepes buffer (Sigma-Aldrich) and 1 ml of 2-mercaptoethanol (Sigma-Aldrich). All animals were cared for under an animal use protocol reviewed and approved by the NCI Laboratory Animal Safety Program–Animal Care and Use Committee.
Generation of murine CD19 CAR T cells
Retroviral supernatants were produced by transfection of 293GP cell line using Lipofectamine 2000 (Life Technologies) with plasmids encoding the CD19 CAR and pCL-Eco retroviral envelope DNA. Supernatants were collected 24, 48, and 72 hours after transfection. Construction of murine anti-CD19 CD28 CAR was previously described (41). T cells were extracted from murine splenocytes using a T cell enrichment column (R&D Systems). Purified (>90%) CD4 or CD8 T cell subsets were separated using untouched CD4 T Cell Isolation Kit or untouched CD8 T cell Isolation Kit (Miltenyi Biotec) before activation. Cells were then activated using anti-CD3/CD28 beads (Life Technologies) on day 1 using 3:1 beads/cell ratio with purified T cell beads then removed on day 3 after transduction. Cells were cultured with IL-2 (30 IU/ml) and IL-7 (10 ng/ml) for 5 days. Activated T cells were transduced using RetroNectin-coated (Takara) plates using combined viral supernatants on days 2 and 3. T cells were evaluated or infused on day 5. Transduction efficiencies were routinely 60 to 90% for all CAR T cells used in the experiments.
Flow cytometry
FACS analysis for surface molecule expression was performed on an LSR II Fortessa flow cytometer (BD Biosciences) and analyzed using FlowJo (FlowJo LLC). The CD19 CAR was detected using biotinylated protein L (Thermo Fisher Scientific) and a streptavidin-conjugated fluorophore (catalog no. 12–4317). The following murine monoclonal antibodies were used for flow cytometry: CD4 (clone RM4–5), CD8a (clone 53–6.7), CD45.2 (clone 104), CD45.1 (clone A20), PD-1 (clone J43), Tim3 (clone RMT3–23), Lag3 (clone C9B7W), B220 (clone RA3-6B2), CD19 (clone 1D3), CD25 (clone 3C7), and Fixable Viability Dye (catalog no. 65-0866-18) from Affymetrix, eBioscience, BioLegend, BD Pharmingen, or R&D Systems.
CD107a degranulation, in vitro leukemia clearance, and cytokine assays
CD107a degranulation assays were conducted by coincubating 5 × 104 T cells with 1 × 105 tumor cells that were previously stained with CellTrace Violet (Life Technologies) for 4 hours in the presence of 2 μM monensin and 5 μl of CD107a antibody (clone eBioH4A3, eBioscience) and then evaluated by flow cytometry. In vitro clearance of CD19+/− and OVA+/− E2aPbx cell lines was conducted by coculture of E2aPbx cells with CAR-transduced or bead-activated, nontransduced (mock) T cells (105 E2aPbx with 105 T cells) in 200 μl of the medium for 3 days. After 3 days, cocultures were evaluated for the percentages of E2aPbx and T cells by flow cytometry. Cytokine expression in CAR T cells was evaluated using a mouse V-Plex Proinflammatory Panel (MR071) performed by the core facility at NCI Frederick General Immunology/Cellular and Functional Assays Department. The supernatant was collected from CAR4 and CAR8 cells cocultured with CD3-depleted splenocytes from C57/B6 female mice. T cells were incubated with target cells at a 1:1 ratio for 10 hours before the supernatants were harvested.
Syngeneic in vivo studies
B6 CD45.1 mice were injected with 1 × 106 E2aPbx (CD45.2+) cells on day 1. Mice were then lymphodepleted by 500-cGy total body irradiation (sublethal using a 137Cs source) on day 4. On day 5, mice were retro-orbitally injected with 1 × 106 or 1 × 105 anti-CD19 CAR T cells from B6, Marilyn (CD45.2+), MataHari (CD45.2+), or Rag1−/−/OT1 mice. Mice in the survival experiments were euthanized on the basis of the protocols approved by the NCI Bethesda Animal Care and Use Committee. Takedown experiments were performed on different time points. Detection of CAR T cell was done by flow cytometry on the bone marrow or splenocytes by congenic markers (CD45.2) and protein L (Life Technologies). All data points from the bone marrow takedown experiments were biological replicates from individual mice. The bone marrow of mice was processed and ACK-lysed. Cells were then stained with flow antibodies and analyzed by flow cytometry. All experiments have been repeated at least three times in the laboratory.
T cell–depleted bone marrow transplantation
Bone marrow cells were harvested from femurs and tibias of male or female B6 CD45.1+ mice or female OVA+ mice and filtered through a 70-mm nylon filter. Cells were then ACK-lysed and T cell–depleted using CD3 microbeads (Miltenyi Biotec). Mice before transplant were lymphodepleted by 1000-cGy (137Cs source) lethal total body irradiation a day before or earlier the same day. Mice were then transplanted with 4.5 × 106 T cell–depleted bone marrow cells in serum-free, phenol red–free RPMI medium through retro-orbital injection.
In vivo caspase 3/7 apoptosis assay
Bone marrow cells of leukemia-bearing mice were analyzed ex vivo 24, 48, and 72 hours after infusion of CAR T cells. ACK-lysed bone marrow cells were stained with protein L for 1 hour followed by three separate washes. Cells were then stained with secondary antibodies and washed. Samples were then stained with Live/Dead staining and washed. Samples were then stained with CellEvent caspase 3/7 dye (catalog #C10423, Thermo Fisher Scientific) for 25 min and incubated in a 37°C incubator. Samples were then immediately analyzed using flow cytometry. All experiments have been repeated at least four times in the laboratory.
Microarray
Biological replicates of CAR T cells generated from two TCR transgenic mouse strains, Marilyn (CD4) and MataHari (CD8), were cocultured with CD3-depleted splenocytes harvested from C57/B6 males (CD19+, HY+), C57/B6 females (CD19+, HY−), CD19KO males (CD19−, HY+), and CD19KO females (CD19−, HY−) for 10 hours. T cells were then isolated by a mouse Pan T Cell Isolation Kit II (Miltenyi Biotec, catalog #130-095-130). The isolated RNA was performed using QIAGEN RNeasy Micro Kit (catalog #74004). The above groups plus unstimulated CAR4 or CAR8 T cells were assessed using GeneChip Mouse Genome 430 2.0 array (Affymetrix). The RNA extracts were sent to the microarray core facility in The Frederick National Laboratory for Cancer Research (FNLCR) for labeling and hybridization on microarray chips according to protocols specified by the manufacturers. This experiment was repeated at least twice in the laboratory. Microarray data, as well as the library and annotation files generated by this study, are publicly available in a MIAME (minimum information about a microarray experiment)–compliant database [www.ncbi.nlm.nih.gov/geo; Gene Expression Omnibus (GEO) accession no. GSE103632].
Statistical analysis of microarray data
All statistical analyses were performed within R/Bioconductor statistical environment (www.bioconductor.org). The CEL files were normalized with the Robust Multichip Average (RMA) methodology in R Package oligo (version 1.34.0). Each Affy probe ID in the data set was matched with the annotation file, GPL1261–14790, which is available from the National Center for Biotechnology Information (NCBI) GEO site. Differential expression P values were determined with t test function, and false discovery rate was adjusted using the multitest Bioconductor package (version 2.26.0).
Pathway analysis and heat maps
Statistically significant, differentially expressed genes were analyzed through the use of QIAGEN’s Ingenuity Pathway Analysis (IPA) (QIAGEN, IPA Fall Release, September 2015, www.qiagen.com/ingenuity). Genes with P values less than 0.001 were used as input to run the IPA core analysis, which calculated enriched canonical pathways and generated pathway figures. Heat maps were created in R using the heatmap.2 function in gplots (version 2.17.0).
Statistics
Statistical tests were performed using GraphPad Prism version 6.0 for Macintosh (GraphPad Software). Significance of survival was calculated using Kaplan-Meier survival curves by Wilcoxon signed-rank test. Significant differences between groups in in vivo T cell numbers, in vivo marker expressions, and in vitro assays were determined by one-way ANOVA [P ≥ 0.05 (not significant), *P < 0.05, **P < 0.01, ***P <0.001, ****P < 0.0001]. All data points represent biological replicates.
Supplementary Material
Fig. S1. CAR4 and CAR8 cells demonstrate comparable in vivo efficacy at low doses against murine ALL, E2aPbx.
Fig. S2. The presence of both TCR and CAR antigens diminishes efficacy of CAR8 but not CAR4 cells at 14 days.
Fig. S3. TCR stimulation induces down-regulation of CAR expression.
Fig. S4. Up-regulation of PD-1 and Lag3 expression is dependent on CAR stimulation.
Fig. S5. CAR8 cells are capable of endogenous B cell clearance despite ineffective leukemia clearance.
Fig. S6. In vitro CAR4 and CAR8 cell cytokine profiles when stimulated through TCR, CAR, or both receptors.
Fig. S7. Gene list for pathways/gene sets from CAR4 and CAR8 cells stimulated through CAR, TCR, or both receptors.
Table S1. Primary data.
Acknowledgments:
We thank J. Kochenderfer (NCI, NIH) for the murine CD19-CD28 CAR construct. We also thank J. Buckley for assistance with the murine injections, the Clinical Support Laboratory of the Frederick National Laboratory for Cancer Research for assisting in the cytokine assays, and the Laboratory of Molecular Technology Microarray Group for assisting in the microarray assays. We would also like to thank the thesis committee members L. Weiner, A. Uren, A. Wellstein, and L. Gattinoni for providing comments and reviewing the manuscript.
Funding: This work was supported by the Intramural Research Program of the NIH. M.E.K. was supported through the NIH T32 training grant through the Sydney Kimmel Comprehensive Cancer Center of Johns Hopkins University. C.T.S. was supported through the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from the Doris Duke Charitable Foundation, The American Association for Dental Research, The Howard Hughes Medical Institute, and the Colgate-Palmolive Company, as well as other private donors.
Footnotes
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: GEO accession no. GSE103632.
SUPPLEMENTARY MATERIALS
www.sciencetranslationalmedicine.org/cgi/content/full/9/417/eaag1209/DC1
REFERENCES AND NOTES
- 1.Lo Nigro L, Biology of childhood acute lymphoblastic leukemia. J. Pediatr. Hematol. Oncol 35, 245–252 (2013). [DOI] [PubMed] [Google Scholar]
- 2.Pui C-H, V Relling M, Downing JR, Acute lymphoblastic leukemia. N. Engl. J. Med 350, 1535–1548 (2004). [DOI] [PubMed] [Google Scholar]
- 3.Curran E, Stock W, How I treat acute lymphoblastic leukemia in older adolescents and young adults. Blood 125, 3702–3710 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, Chung SS, Stefanski J, Borquez-Ojeda O, Olszewska M, Qu J, Wasielewska T, He Q, Fink M, Shinglot H, Youssif M, Satter M, Wang Y, Hosey J, Quintanilla H, Halton E, Bernal Y, Bouhassira DCG, Arcila ME, Gonen M, Roboz GJ, Maslak P, Douer D, Frattini MG, Giralt S, Sadelain M, Brentjens R, Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl. Med 6, 224ra25 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, Mahnke YD, Melenhorst JJ, Rheingold SR, Shen A, Teachey DT, Levine BL, June CH, Porter DL, Grupp SA, Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med 371, 1507–1517 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee DW, Kochenderfer JN, Stetler-stevenson M, Cui YK, Delbrook C, Feldman SA, Fry TJ, Orentas R, Sabatino M, Shah NN, Steinberg SM, Stroncek D, Tschernia N, Yuan C, Zhang H, Zhang L, Rosenberg SA, Wayne AS, Mackall CL, T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: A phase 1 dose-escalation trial. Lancet 385, 517–528 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cruz CRY, Micklethwaite KP, Savoldo B, Ramos CA, Lam S, Ku S, Diouf O, Liu E, Barrett AJ, Ito S, Shpall EJ, Krance RA, Kamble RT, Carrum G, Hosing CM, Gee AP, Mei Z, Grilley BJ, Heslop HE, Rooney CM, Brenner MK, Bollard CM, Dotti G, Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: A phase 1 study. Blood 122, 2965–2973 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, Stetler-Stevenson M, Phan GQ, Hughes MS, Sherry RM, Yang JC, Kammula US, Devillier L, Carpenter R, Nathan D-AN, Morgan RA, Laurencot C, Rosenberg SA, B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor–transduced T cells. Blood 119, 2709–2720 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kochenderfer JN, Dudley ME, Kassim SH, Somerville RPT, Carpenter RO, Stetler-Stevenson M, Yang JC, Phan GQ, Hughes MS, Sherry RM, Raffeld M, Feldman S, Lu L, Li YF, Ngo LT, Goy A, Feldman T, Spaner DE, Wang ML, Chen CC, Kranick SM, Nath A, Nathan D-AN, Morton KE, Toomey MA, A Rosenberg S, Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J. Clin. Oncol 33, 540–549 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turtle CJ, Hanafi L-A, Berger C, Hudecek M, Pender B, Robinson E, Hawkins R, Chaney C, Cherian S, Chen X, Soma L, Wood B, Li D, Heimfeld S, Riddell SR, Maloney DG, Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor–modified T cells. Sci. Transl. Med 8, 355ra116 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee III DW, Stetler-Stevenson M, Yuan CM, Shah NN, Delbrook C, Yates B, Zhang H, Zhang L, Kochenderfer JN, Rosenberg SA, Fry TJ, Stroncek D, Mackall CL, in Long-Term Outcomes Following CD19 CAR T Cell Therapy for B-ALL are Superior in Patients Receiving a Fludarabine/Cyclophosphamide Preparative Regimen and Post-CAR Hematopoietic Stem Cell Transplantation (American Society of Hematology, 2016), vol. 128, p. 218. [Google Scholar]
- 12.Gardner R, Finney O, Smithers H, Leger KJ, Annesley CE, Summers C, Brown C, Mgebroff S, Lindgren C, Spratt K, Oron A, Li D, Bleakley M, Park JR, Jensen MC, in CD19CAR T Cell Products of Defined CD4:CD8 Composition and Transgene Expression Show Prolonged Persistence and Durable MRD-Negative Remission in Pediatric and Young Adult B-Cell ALL (American Society of Hematology, 2016), vol. 128, p. 219. [Google Scholar]
- 13.Sadelain M, Brentjens R, Rivière I, The basic principles of chimeric antigen receptor design. Cancer Discov. 3, 388–398 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barrett DM, Singh N, Porter DL, Grupp SA, June CH, Chimeric antigen receptor therapy for cancer. Annu. Rev. Med 65, 333–347 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossig C, Bollard CM, Nuchtern JG, Rooney CM, Brenner MK, Epstein-Barr virus–specific human T lymphocytes expressing antitumor chimeric T-cell receptors: Potential for improved immunotherapy. Blood 99, 2009–2016 (2002). [DOI] [PubMed] [Google Scholar]
- 16.Pule MA, Savoldo B, Myers GD, Rossig C, V Russell H, Dotti G, Huls MH, Liu E, Gee AP, Mei Z, Yvon E, Weiss HL, Liu H, Rooney CM, Heslop HE, Brenner MK, Virus-specific T cells engineered to coexpress tumor-specific receptors: Persistence and antitumor activity in individuals with neuroblastoma. Nat. Med 14, 1264–1270 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terakura S, Yamamoto TN, Gardner RA, Turtle CJ, Jensen MC, Riddell SR, Generation of CD19-chimeric antigen receptor modified CD8+ T cells derived from virus-specific central memory T cells. Blood 119, 72–82 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacoby E, Yang Y, Qin H, Chien CD, Kochenderfer JN, Fry TJ, Murine allogeneic CD19 CAR T cells harbor potent antileukemic activity but have the potential to mediate lethal GVHD. Blood 127, 1361–1370 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shand JC, Qin H, Nasholm N, Capitini CM, Fry TJ, Minor antigen distribution predicts site-specific graft-versus-tumor activity of adoptively transferred, minor antigen-specific CD8 T cells. Biol. Blood Marrow Transplant. 20, 26–36 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wherry EJ, Ha S-J, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R, Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 27, 670–684 (2007). [DOI] [PubMed] [Google Scholar]
- 21.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, Milone MC, Levine BL, June CH, Chimeric antigen receptor–modified T cells for acute lymphoid leukemia. N. Engl. J. Med 368, 1509–1518 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maude SL, Barrett DM, Rheingold SR, Aplenc R, Teachey DT, Callahan C, Baniewicz D, White C, Talekar MK, Shaw PA, Brogdon JL, Young RM, Scholler J, Marcucci KT, Levi BL, Frey N, Porter DL, Lacey SF, Melenhorst JJ, June CH, Grupp SA, in Efficacy of Humanized CD19-Targeted Chimeric Antigen Receptor (CAR)-Modified T Cells in Children and Young Adults with Relapsed/Refractory Acute Lymphoblastic Leukemia (American Society of Hematology, 2016), vol. 128, p. 217. [Google Scholar]
- 23.Chmielewski M, Hombach AA, Abken H, Antigen-specific T-cell activation independently of the MHC: Chimeric antigen receptor-redirected T cells. Front. Immunol 4, 371 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghosh A, Smith M, James SE, Davila ML, Velardi E, Argyropoulos KV, Gunset G, Perna F, Kreines FM, Levy ER, Lieberman S, Jay HV, Tuckett AZ, Zakrzewski JL, Tan L, Young LF, Takvorian K, Dudakov JA, Jenq RR, Hanash AM, Motta ACF, Murphy GF, Liu C, Schietinger A, Sadelain M, van den Brink MRM, Donor CD19 CAR T cells exert potent graft-versus-lymphoma activity with diminished graft-versus-host activity. Nat. Med 23, 242–249 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fehr T, Takeuchi Y, Kurtz J, Wekerle T, Sykes M, Early regulation of CD8 T cell alloreactivity by CD4+CD25− T cells in recipients of anti-CD154 antibody and allogeneic BMT is followed by rapid peripheral deletion of donor-reactive CD8+ T cells, precluding a role for sustained regulation. Eur. J. Immunol 35, 2679–2690 (2005). [DOI] [PubMed] [Google Scholar]
- 26.Mueller DL, Mechanisms maintaining peripheral tolerance. Nat. Immunol 11, 21–27 (2010). [DOI] [PubMed] [Google Scholar]
- 27.Hogquist KA, Jameson SC, The self-obsession of T cells: How TCR signaling thresholds affect fate ‘decisions’ and effector function. Nat. Immunol 15, 815–823 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brooks DG, Teyton L, Oldstone MBA, McGavern DB, Intrinsic functional dysregulation of CD4 T cells occurs rapidly following persistent viral infection. J. Virol 79, 10514–10527 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brooks DG, McGavern DB, Oldstone MBA, Reprogramming of antiviral T cells prevents inactivation and restores T cell activity during persistent viral infection. J. Clin. Invest 116, 1675–1685 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crawford A, Angelosanto JM, Kao C, Doering TA, Odorizzi PM, Barnett BE, Wherry EJ, Molecular and transcriptional basis of CD4+ T cell dysfunction during chronic infection. Immunity 40, 289–302 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kahan SM, Wherry EJ, Zajac AJ, T cell exhaustion during persistent viral infections. Virology 479–480, 180–193 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perez-Diez A, Joncker NT, Choi K, Chan WFN, Anderson CC, Lantz O, Matzinger P, CD4 cells can be more efficient at tumor rejection than CD8 cells. Blood 109, 5346–5355 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kochenderfer JN, Dudley ME, Carpenter RO, Kassim SH, Rose JJ, Telford WG, Hakim FT, Halverson DC, Fowler DH, Hardy NM, Mato AR, Hickstein DD, Gea-Banacloche JC, Pavletic SZ, Sportes C, Maric I, Feldman SA, Hansen BG, Wilder JS, Blacklock-Schuver B, Jena B, Bishop MR, Rosenberg SA, Gress RE, in Donor-Derived Anti-CD19 Chimeric-Antigen-Receptor-Expressing T Cells Cause Regression of Malignancy Persisting After Allogeneic Hematopoietic Stem Cell Transplantation (American Society of Hematology, 2013), vol. 122, p. 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maus MV, June CH, Making better chimeric antigen receptors for adoptive T-cell therapy. Clin. Cancer Res. 22, 1875–1884 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bridgeman JS, Hawkins RE, Bagley S, Blaylock M, Holland M, Gilham DE, The optimal antigen response of chimeric antigen receptors harboring the CD3z transmembrane domain is dependent upon incorporation of the receptor into the endogenous TCR/CD3 complex. J. Immunol 184, 6938–6949 (2010). [DOI] [PubMed] [Google Scholar]
- 36.Turtle CJ, Hanafi L-A, Berger C, Gooley TA, Cherian S, Hudecek M, Sommermeyer D, Melville K, Pender B, Budiarto TM, Robinson E, Steevens NN, Chaney C, Soma L, Chen X, Yeung C, Wood B, Li D, Cao J, Heimfeld S, Jensen MC, Riddell SR, Maloney DG, CD19 CAR–T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J. Clin. Invest 126, 2123–2138 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gardner RA, Finney O, Annesley C, Brakke H, Summers C, Leger K, Bleakley M, Brown C, Mgebroff S, Kelly-Spratt KS, Hoglund V, Lindgren C, Oron AP, Li D, Riddell SR, Park JR, Jensen MC, Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood 129, 3322–3331 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sommermeyer D, Hudecek M, Kosasih PL, Gogishvili T, Maloney DG, Turtle CJ, Riddell SR, Chimeric antigen receptor-modified T cells derived from defined CD8+ and CD4+ subsets confer superior antitumor reactivity in vivo. Leukemia 30, 492–500 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valujskikh A, Lantz O, Celli S, Matzinger P, Heeger PS, Cross-primed CD8+ T cells mediate graft rejection via a distinct effector pathway. Nat. Immunol 3, 844–851 (2002). [DOI] [PubMed] [Google Scholar]
- 40.Bijl J, Sauvageau M, Thompson A, Sauvageau G, High incidence of proviral integrations in the Hoxa locus in a new model of E2a–PBX1-induced B-cell leukemia. Genes Dev. 19, 224–233 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kochenderfer JN, Yu Z, Frasheri D, Restifo NP, Rosenberg SA, Adoptive transfer of syngeneic T cells transduced with a chimeric antigen receptor that recognizes murine CD19 can eradicate lymphoma and normal B cells. Blood 116, 3875–3886 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. CAR4 and CAR8 cells demonstrate comparable in vivo efficacy at low doses against murine ALL, E2aPbx.
Fig. S2. The presence of both TCR and CAR antigens diminishes efficacy of CAR8 but not CAR4 cells at 14 days.
Fig. S3. TCR stimulation induces down-regulation of CAR expression.
Fig. S4. Up-regulation of PD-1 and Lag3 expression is dependent on CAR stimulation.
Fig. S5. CAR8 cells are capable of endogenous B cell clearance despite ineffective leukemia clearance.
Fig. S6. In vitro CAR4 and CAR8 cell cytokine profiles when stimulated through TCR, CAR, or both receptors.
Fig. S7. Gene list for pathways/gene sets from CAR4 and CAR8 cells stimulated through CAR, TCR, or both receptors.
Table S1. Primary data.