Highlights
-
•
Limited research has assessed sex-related differences in fetal brain connectivity.
-
•
Functional connectivity (FC) data were collected from 118 human fetuses.
-
•
16 distinct fetal FC networks were identified using a community detection algorithm.
-
•
Sex-related changes in fetal FC were examined using enrichment analysis.
-
•
We confirm for the first time that network FC differs with sex in utero.
Keywords: Connectivity, Gestational age, MRI, Prenatal, Resting-state, Sex
Abstract
Sex-related differences in brain and behavior are apparent across the life course, but the exact set of processes that guide their emergence in utero remains a topic of vigorous scientific inquiry. Here, we evaluate sex and gestational age (GA)-related change in functional connectivity (FC) within and between brain wide networks. Using resting-state functional magnetic resonance imaging we examined FC in 118 human fetuses between 25.9 and 39.6 weeks GA (70 male; 48 female). Infomap was applied to the functional connectome to identify discrete prenatal brain networks in utero. A consensus procedure produced an optimal model comprised of 16 distinct fetal neural networks distributed throughout the cortex and subcortical regions. We used enrichment analysis to assess network-level clustering of strong FC-GA correlations separately in each sex group, and to identify network pairs exhibiting distinct patterns of GA-related change in FC between males and females. We discovered both within and between network FC-GA associations that varied with sex. Specifically, associations between GA and posterior cingulate-temporal pole and fronto-cerebellar FC were observed in females only, whereas the association between GA and increased intracerebellar FC was stronger in males. These observations confirm that sexual dimorphism in functional brain systems emerges during human gestation.
1. Introduction
Healthy brain development in utero is imperative for achieving optimal long-term neurobehavioral outcomes. Processes that interfere with normative prenatal development, such as toxin exposure, injury, and infection, can have dramatic consequences (Adams Waldorf and McAdams, 2013; Lanphear et al., 2005; Slopen et al., 2015), and current evidence suggests that it is by acting on formation of brain circuitry in utero that these exposures exert their pernicious effects (Thompson et al., 2009). Furthermore, developmental disorders are increasingly conceptualized as disorders of the brain’s functional and structural connectome and it has been suggested that a better understanding of neurodevelopmental processes in the womb may bring us closer to understanding the developmental origins of disease (Buss et al., 2017; Di Martino et al., 2014). However, current models of healthy gestational brain development lack sex-specific effects. Though sex-related variation in hormone levels are detectable by the 8th week of gestation (Hines, 2010), in utero sexual dimorphism in the development of functional brain networks has yet to be described.
Elegant work spanning decades has born considerable insight into the timing and order within which structures of the brain mature (Huang and Vasung, 2014; Kostovic and Jovanov-Milosevic, 2006). In recent years, however, methodological advances have led to a surge in studies attempting to map development of human functional brain systems before birth using resting-state functional connectivity (FC) MRI (Anderson and Thomason, 2013; M. I. van den Heuvel and Thomason, 2016). Studies that have assessed FC in human preterm or fetal brain development have described overall network properties and have characterized changes in functional connectivity associated with advancing post-conceptual age. These studies have demonstrated that during early brain development: i) cross-hemispheric, cortico-subcortical, and long-range connectivity increase, ii) connections develop in a medial to lateral gradient, and iii) efficiency and modularity increase (Doria et al., 2010; Fan et al., 2011; Fransson et al., 2011; Gao et al., 2015a,b; Gao et al., 2011; Grayson and Fair, 2017; Huang et al., 2015; Smyser et al., 2016, 2011; Thomason et al., 2014, 2013; Thomason et al., 2015; M. P. van den Heuvel et al., 2015). However, sex-related variations in functional network formation in utero have yet to be elucidated.
Sexual dimorphism in brain structure and functional connectivity are robustly reported findings across the lifespan (Caviness et al., 1996; Koolschijn and Crone, 2013; Lenroot et al., 2007; Ruigrok et al., 2014; Satterthwaite et al., 2015; Tiemeier et al., 2010). However, limited research has assessed sexual dimorphism in the brain before birth. One prior study assessing sexual dimorphism in utero did not observe any brain volumetric differences between 20–32 weeks (Scott et al., 2011). However, ultrasound studies demonstrate that males have a larger head circumference than females as early as the 2nd trimester (i.e. 14–27 weeks) (Broere-Brown et al., 2016; Melamed et al., 2013). Further, studies in newborns and infants have shown that males and females vary in brain volume and cortical thickness (Choe et al., 2013; Gilmore et al., 2007; Knickmeyer et al., 2014), implicating a likely emergence of sexual-dimorphism in the brain before birth. Gao and colleagues assessed brain functional connectivity development at birth, at age 1 year, and age 2 years and observed greater age dependent increase in frontoparietal connectivity in males than females (Gao et al., 2015b). No other differences in network connectivity were observed between males and females. Similarly, Deoni and colleagues observed no differences in developmental trajectories for cortical growth or myelination of brain regions between male and female brains between the ages of 1–6 years (Deoni et al., 2015). Taken together, changes in hormone levels early in gestation, followed by differences in head circumference observed in utero, and differences in brain structure apparent at birth point towards sex differences in the development of fetal functional brain connectivity. However, differences in brain connectivity between male and female fetuses in utero have not been previously described.
In the present paper we examined the neurodevelopment of human fetal functional connectivity in utero and associated sexual dimorphism in functional network development. While there are many available tools to examine sex differences in functional connectivity, we chose to utilize statistical tools known as enrichment analysis. Enrichment analysis encompasses data-driven statistical approaches first utilized in massively univariate genome wide association studies (Subramanian et al., 2005), and more recently applied to functional connectome-wide association studies in children age 1–2 years (Eggebrecht et al., 2017; Marrus et al., 2017) and 12 years (Wheelock et al., 2018). We used these innovative statistical approaches to characterize how the functional connectome varies in utero with respect to sex and GA. Our aims were to i) define functional brain networks of the developing fetus, ii) compare network development across gestation between sexes, and iii) examine network development associated with GA. We hypothesized that fetus FC would form ‘proto networks’ with even greater network fragmentation observed than in neonatal brain networks or networks at age 1–2 years (Gao et al., 2015b; Smyser et al., 2010). Further, we hypothesized that increasing GA would positively correlate with increases in long-range network development. Finally, we hypothesized that male and female fetuses would differ in network development across GA.
2. Materials and methods
2.1. Participants
Participants were recruited during routine obstetric appointments into a study of longitudinal fetal brain development. Inclusion criteria included no contraindications for MRI, maternal age >18 years, and singleton pregnancies. In total, 166 pregnant women were recruited and underwent MRI between 25 and 39 weeks gestation. Gestational age was determined by a study physician (E.H.-A.) by ultrasound examination within 1 week of MRI testing. For a subset of participants, women were selectively scanned between the 26–30 t h week of gestation and were scanned again between 35–37 weeks gestation +/− 1–2 weeks. All study participants provided written informed consent prior to participation. All study procedures were approved by the Wayne State University Human Investigation Committee. Fetal cases were subsequently excluded from analysis if birth records were inaccessible (N = 5), if born preterm and/or low birth weight (<37 weeks, <2500 g; N = 35), 11 of which were also exposed to pregnancy complications, including preterm premature rupture of membranes (N = 7) and intrauterine growth restriction (N = 4). One case was born with a congenital central nervous system abnormality and was thus excluded from analyses. After preliminary exclusion, our sample consisted of 125 pregnant women. Seven of these cases were subsequently excluded due to high fetal motion during fMRI, leaving a final sample of 118. Functional connectivity analyses from 105 of these fetuses have been reported previously (Thomason et al., 2014, 2013; Thomason et al., 2017; M. I. van den Heuvel et al., 2018).
2.2. MRI scanning
MRI data were acquired on a Siemens Verio 70-cm open-bore 3 T MR system using a 550 g abdominal 4-Channel Siemens Flex Coil (Siemens, Munich, Germany). Resting-state fMRI data were acquired using a gradient echo planar imaging sequence: TR/TE 2000/30 ms, flip angle 80°, 360 frames, axial 4 mm slice thickness, voxel size 3.4 × 3.4 × 4 mm3). Between 12–24 min of fetal resting-state fMRI data were collected per participant, and average specific absorption rate (SAR) was 0.22 W/kg, SD= 0.09.
2.3. Resting state fMRI data preprocessing
Resting state fMRI preprocessing followed previously described methods (Thomason et al., 2014, 2013; Thomason et al., 2017; M. I. van den Heuvel et al., 2018) (Supplemental Fig. S1). In brief, periods of fetal quiescence were manually identified using visual inspection with FSL image viewer. Fetal brain masks were then created separately for each low-motion epoch from a single reference image using Brainsuite (Shattuck and Leahy, 2002). Masks were then binarized and applied across frames corresponding to each previously identified low-motion period. After masking, each temporal segment was reoriented manually, realigned to the mean BOLD volume, resampled to 2 mm isotropic voxels, and normalized to a 32-week fetal brain template (Serag et al., 2012) using Statistical Parametric Mapping (SPM8) software implemented in MATLAB. Motion parameters for each manually identified quiescent period were checked to ensure only segments that consisted of at least 20 s (10 frames) of low motion (less than 2 mm translational and 3 ° rotational movement) were retained in subsequent processing steps. This level of censoring has been reported previously (Thomason et al., 2014, 2013; Thomason et al., 2017; M. I. van den Heuvel et al., 2018). Only these censored data were retained for analyses. The realigned and normalized volumes from each quiescent period were then concatenated into one run for each subject. The concatenated, normalized time series were then realigned to the mean BOLD volume and smoothed with a 4 mm FWHM Gaussian kernel in normalized template space. The six head motion parameters generated from SPM realignment of the concatenated time series (X, Y, Z translation and P, Y, R rotation) were included subsequently as motion covariates. Data quality criteria for fMRI in these analyses required volumetric time traces used in further analyses have at least 100 volumes (3.33 min) of low motion data. For these datasets, an average of 40% of data were discarded due to high fetal motion; after motion censoring, an average of 160 frames (M = 5.33, SD = 1.5 min) were retained for males and 157 frames (M = 5.23, SD = 1.3 min) were retained for females. Further preprocessing was performed in CONN functional connectivity toolbox (v14n) (Whitfield-Gabrieli and Nieto-Castanon, 2012) in MATLAB including linear detrending, nuisance regression using aCompCor of five principal components extracted from a 32-week fetal atlas white matter and CSF mask, six head motion parameters, and band-pass filtering at 0.008 to 0.09 Hz. See Supplemental Fig. S1 for depiction of processing stream and description of quality control procedures.
2.4. Derivation of putative fetal brain networks
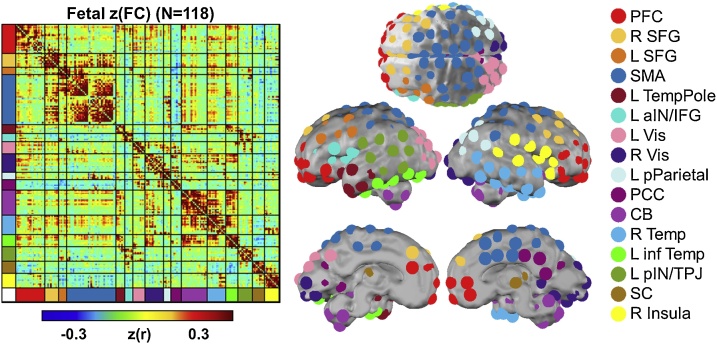
Pycluster (Craddock et al., 2012) was used to generate 197 spatially contiguous, similarly sized regions of interest (ROI) in a spatially normalized fetal atlas space, that have been previously described (van den Heuvel et al., 2018), and are available for download at www.brainnexus.com. CONN Toolbox was used to calculate functional connectivity (FC) as zero-lag Pearson correlations between these 197 ROIs for each subject in the 32-week fetal atlas space. To create a network model of the fetal brain, the complete set of unique n = 19,306 ROI-pair FC (Fisher-z) data from all participants were averaged, producing a group-wise 197 × 197 correlation matrix (Fig. 1). The set of averaged correlations across subjects was thresholded and binarized at multiple Fisher-z values, corresponding to edge density sparseness thresholds ranging from 1% to 10% of all possible surviving connections at steps of 0.1%, to generate 91 total adjacency matrices. Connections between ROI pairs separated by <10 mm were removed to minimize the effects of blurring in the spatially normalized fMRI data. The Infomap community detection algorithm (Rosvall and Bergstrom, 2008) that assigns ROIs to communities of putative networks based on maximization of within-module random walks was then applied to adjacency matrices at each threshold. Solutions for each threshold were combined using an automated consensus procedure to provide a single model of the community structure by maximizing the normalized mutual information of groups of neighboring solutions and then maximizing modularity (Eggebrecht et al., 2017). This cross-gestational age network solution (Fig. 1) facilitated network-pair-level analyses for each group.
Fig. 1.
Networks of the fetal brain. Networks were generated using functional connectivity data from 197 ROI acquired from 118 fetuses. The fetal connectome is mean Fisher - z transformed Pearson-r correlation values between every pair of ROI. The spatial location of each ROI is displayed on the surface of the brain with coloring representative of the functional network assignments. PFC, prefrontal cortex; SFG, superior frontal gyrus; SMA, somatomotor area; aIN, anterior insula; IFG, inferior frontal gyrus; Vis, Visual; pParietal, posterior parietal; PCC, posterior cingulate cortex; CB, cerebellum; inf Temp, inferior temporal; pIN, posterior insula; TPJ, temporo-parietal junction; SC, subcortical grey matter (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
2.5. Statistical analyses
2.5.1. Demographic and outcome analyses
Independent samples t-tests were used to assess differences in GA at scan in addition to comparing GA at birth, maternal prenatal stress, maternal age at scan, maternal prenatal health behavior, birth weight (g), frame count, and motion measures between males and females. Nonparametric tests of differences (i.e., Mann-Whitney U) were used for behavioral measures that were non-normally distributed based on the Shapiro-Wilk test.
2.5.2. Enrichment analyses
Enrichment analysis was performed to identify significant clustering of strong FC-GA correlations within and between network pairs, separately for males and females. These enrichment analyses were performed following previously published methods (Eggebrecht et al., 2017; Wheelock et al., 2018), and were adapted from existing large-scale genome-wide association studies (Backes et al., 2014; Khatri et al., 2012; Rivals et al., 2007). First, correlations between Fisher-z-transformed FC and GA were calculated separately in each group for each ROI-pair (n = 19,306). Due to the non-normal distribution of GA at scan, non-parametric Spearman rank correlations were used to calculate FC-GA associations. We then applied an uncorrected p-threshold of 0.05 to the resulting FC-GA correlations. Next, we used a χ2 test to assess enrichment for each within- and between-network pair. Additionally, a McNemar χ2 test was used to assess differences between male and female patterns of significant FC-GA correlations within- and between- network pairs. Significance for both χ2 enrichment and McNemar χ2 tests was determined through randomly permuting the subject pairing of FC and GA values 10,000 times (Backes et al., 2014; Eggebrecht et al., 2017). Networks that were significantly enriched for FC-GA (χ2, dof = 1, p < 0.05) for either sex and/or also significantly different in their network-pair patterns of association with GA at scan between males and females (McNemar χ2 p < 0.05) are herein considered as significant findings. All analyses and visualizations were performed in MATLAB (Release 2016a, The Mathworks, Inc. Natick, Massachusetts, United States).
3. Results
3.1. Study cohort
Of the 118 fetal cases, 48 were female and 70 were male), with a maternal mean age at scan of 25.1 years (SD = 4.6). Additionally, for each of the 21 participants scanned twice during pregnancy, functional data from only the time point with lower motion data was selected. The median fetal age at the time of MRI was 33.6 weeks of gestation (SD = 3.9) and mean age at birth was 39.4 weeks of gestation (SD = 1.1), see Table 1. A Mann-Whitney U test was used to assess differences between males and females on variables that were non-normally distributed (GA at scan, maternal age at scan, maternal prenatal health behavior, birth weight, frame count, and motion). Relevant to the present study, males and females did not differ in GA at scan, number of retained fMRI frames, or in average XYZ and average Pitch Roll Yaw (PYR) motion, and demonstrated similar bimodal distributions in GA (p > 0.05; Fig. 2; Table 1). GA at scan did not correlate with maternal health, maternal stress, head motion, frame count, birth weight, or maternal age at time of scan (p > 0.05) (Supplemental Table S1). Finally, head motion did not correlate with GA in either the group of male or female fetuses (Supplemental Tables S2 and S3; Fig. S2).
Table 1.
Demographic Information.
| Measure | Males (M ± SD) N = 70 | Females (M ± SD) N = 48 | df | t | p |
|---|---|---|---|---|---|
| Gestational Age at Birth (weeks) | 39.5 ± 1.2 | 39.3 ± 1.0 | 116 | 1.07 | 0.29 |
| Maternal Prenatal Stress | −0.1 ± 0.7 | 0.0 ± 1.0 | 116 | −0.45 | 0.66 |
| Median | Median | U | Z | p | |
| Gestational Age at Scan (weeks) | 35.14 | 34.36 | 1492 | −1.03 | 0.30 |
| Maternal Age at Scan (years) | 23.70 | 24.68 | 1575 | −0.58 | 0.57 |
| Maternal Prenatal Health Behavior | 198.50 | 187.54 | 1545 | −0.74 | 0.46 |
| Birth weight (g) | 3348 | 3210 | 1262 | −2.29 | 0.02 |
| Frame Count | 148 | 147 | 1640 | −0.22 | 0.83 |
| XYZ Motion | 0.22 | 0.21 | 1510 | −0.93 | 0.35 |
| PYR Motion | 0.39 | 0.38 | 1504 | −0.96 | 0.34 |
PYR, pitch roll yaw.
Fig. 2.
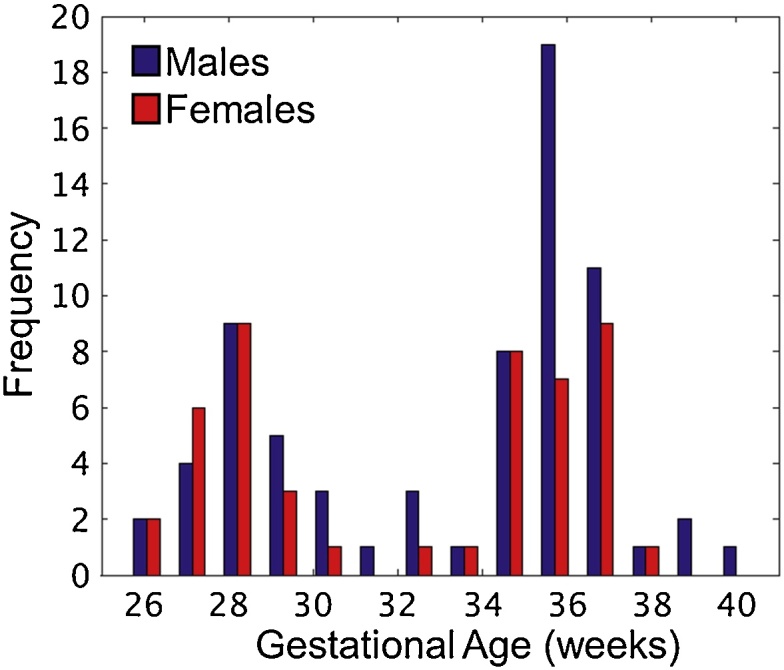
Male and female fetal gestational age in utero. Due to study sampling design across other projects (i.e. women were selectively recruited and scanned between the 26th-28th and again at 35–37 weeks), gestational age at scan demonstrated a bimodal distribution. Thus, non-parametric tests were used to assess brain-behavior interactions between sex and gestational age.
3.2. Fetal brain networks
To ensure that the FC data were not contaminated by motion-based artifact in the BOLD signal, we tested for a motion dependence on the correlation between the FC data and motion quality control (QC) measures as well as the relationship between QC-FC and Euclidian distance between ROI pairs (Ciric et al., 2017) for both male and female fetuses. A comparison of median absolute QC-FC for male (r = 0.0855) and female (r = 0.1007) fetuses demonstrated the motion preprocessing in the present paper performed as well or better than half of the motion correction techniques tested by Ciric and colleagues (i.e., Fig. 2 from Ciric et al., 2017). In contrast, QC-FC-distance dependent effects for male (r=-0.0301) and female (r=-0.0032) fetuses outperformed almost all motion correction procedures reported by Ciric and colleagues (i.e., Fig. 3 from Ciric et al., 2017), suggesting the distance between ROI and the strength of FC was not contaminated by motion (Supplemental Fig. S3). Community detection analysis was run on the 19,306 ROI pairs and generated a16 functional network consensus model (Fig. 1, Supplemental Table S4).
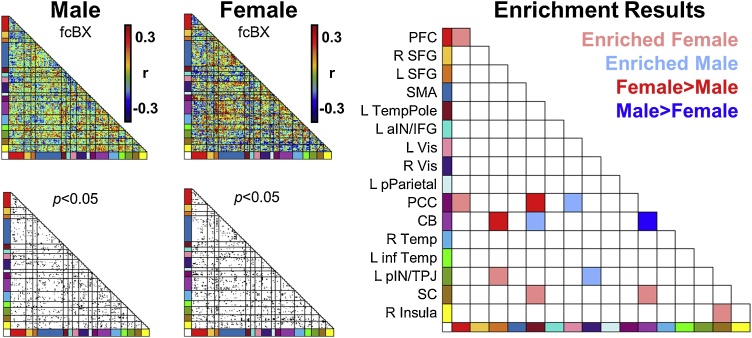
Fig. 3.
Associations between functional connectivity (FC), gestational age (GA), and sex. Red squares indicate network pairs that were significantly more enriched with strong rs-fMRI correlations with GA in female fetuses than male fetuses. Blue squares indicate networks exhibiting stronger enrichment of FC-GA correlations in male than female fetuses. PFC, prefrontal cortex; SFG, superior frontal gyrus; SMA, somatomotor area; aIN, anterior insula; IFG, inferior frontal gyrus; Vis, Visual; pParietal, posterior parietal; PCC, posterior cingulate cortex; CB, cerebellum; inf Temp, inferior temporal; pIN, posterior insula; TPJ, temporo-parietal junction; SC, subcortical grey matter (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
3.3. Sex effects in systems level fetal maturation
3.3.1. Stronger systems-level associations in females than males
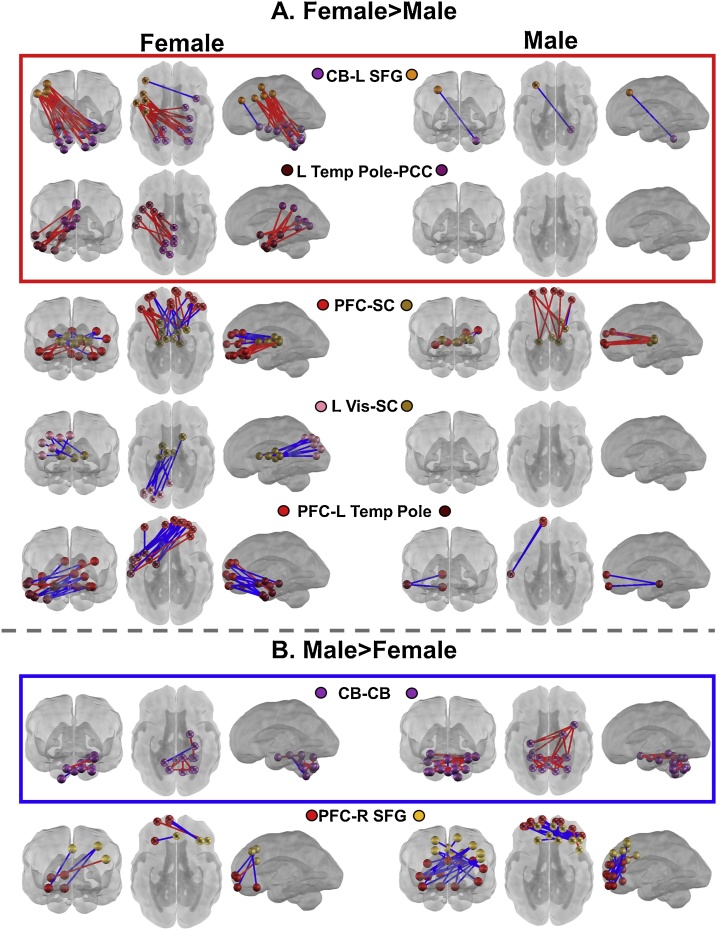
Females exhibited stronger clustering of FC-GA associations between left temporal pole (L TempPole) with posterior cingulate cortex (PCC) (McNemar χ2 = 8.1, p < 0.05) and cerebellum (CB) connections with left superior frontal gyrus (SFG) (McNemar χ2 = 17.39, p < 0.01) (Fig. 3 red squares). Specifically, older GA was associated with increased positive FC between the CB with L SFG and between L TempPole and PCC (Fig. 4A red box). Significant clustering of strong FC-GA correlations between these regions was not observed in male fetuses (Supplemental Table S5). There were three additional networks which demonstrated greater FC-GA correlations in females than males which were not significantly enriched within the female fetus group. Specifically, increased FC-GA correlations were observed between prefrontal cortex (PFC) and subcortical (SC) grey matter (McNemar χ2 = 7.26, p < 0.05), left visual (L Vis) with SC (McNemar χ2 = 7.11, p < 0.05), and PFC with L TempPole (McNemar χ2 = 11.25, p < 0.05). In total, five network pairs demonstrated significantly greater FC-GA association in female than male fetuses (Fig. 4A; Supplemental Fig. S4; Supplemental Table S6).
Fig. 4.
Between group McNemar chi-square differences in network FC-GA associations. A) Female fetuses demonstrated greater FC-GA in five network pairs than male fetuses, though only two of these network pairs were significantly enriched (chi-square) within the female fetus group (red box; same data as Fig. 3). B) Male fetuses demonstrated greater FC-GA in two networks compared to female fetuses, though only one of these networks demonstrated within-group enrichment in male fetuses (blue box; same data as Fig. 4). Blue lines represent negative FC-GA correlations, red lines represent positive FC-GA correlations. All connections between networks significant at Spearman rho p < 0.05. PFC, prefrontal cortex; SFG, superior frontal gyrus; Vis, Visual; PCC, posterior cingulate cortex; CB, cerebellum; SC, subcortical grey matter; Temp Pole, temporal pole; L, left; R, right (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
Several network pairs exhibited significant clustering in females that were not significantly different from male fetuses (McNemar χ2 p > 0.05) (pink squares Fig. 3). These network pairs included intra-network connections within prefrontal cortex (PFC) (Enrichment χ2 = 18.85, p < 0.05), PFC connections with PCC (Enrichment χ2 = 11.92, p < 0.05), left posterior insula/temporoparietal junction (pIN/TPJ) connections with superior L SFG (Enrichment χ2 = 18.85, p < 0.05), subcortical (SC) grey matter connectivity with L TempPole (Enrichment χ2 = 10.60, p < 0.05), SC connections with CB (Enrichment χ2 = 10.95, p < 0.05), and SC connections with right insula (Enrichment χ2 = 10.15, p < 0.05).
3.3.2. Stronger systems-level associations in males than females
Male fetuses exhibited an enriched association between GA and CB connectivity, while females did not (McNemar χ2 = 7, p < 0.05) (Fig. 3 dark blue square). Connectivity within the CB was positively correlated with greater GA in both male and female fetuses (Fig. 4B blue box), though the number of associations was sparser in female fetuses (Supplemental Table S5). An additional network exhibited greater FC-GA correlations in male than female fetuses, though this did not reach within-group significance for enrichment in male fetuses. Specifically, male fetuses demonstrated increased FC-GA correlations between PFC and R SFG than female fetuses (McNemar χ2 = 12.19, p < 0.01) (Fig. 4B; Supplemental Fig. S4; Supplemental Table S6).
Additional network pairs exhibited FC-GA enrichment in males, though these network associations did not significantly differ between males and females (McNemar χ2 p > 0,05) (light blue squares Fig. 3). These FC-GA associations included cerebellum to left temporal pole (Enrichment χ2 = 11.59, p < 0.05), left visual cortex to posterior cingulate cortex (PCC) (Enrichment χ2 = 12.93, p < 0.05), and right visual cortex to left posterior insula/temporo parietal junction (pIN/TPJ) (Enrichment χ2 = 9.33, p < 0.05).
3.4. Whole group FC-GA systems-level associations
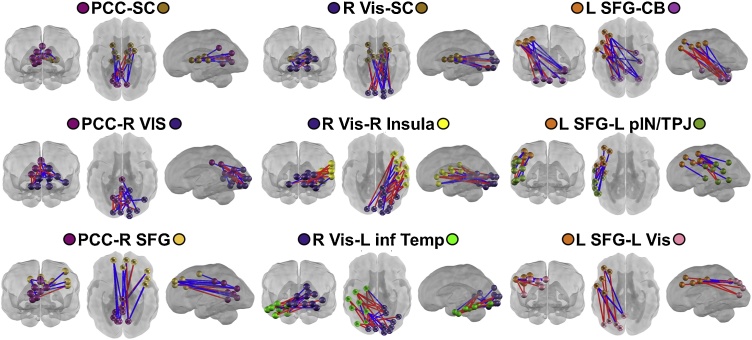
In order to understand the role of GA independent of sex, GA for the entire fetal cohort (N = 118) was correlated with FC. Changes in GA were significantly associated with FC involving eight out of sixteen functional networks (black squares Fig. 5; Supplemental Table S7). Networks showing significant age-related change include R SFG, PCC, visual, CB, pIN/TPJ, inferior temporal, SC, and insula (Fig. 5, Fig. 6). Networks with the most internetwork FC-GA associations included the right SFG, PCC, and left visual (LVis) (Fig. 6). Overlap between GA enrichment and sex-GA interaction occurred within one network pair (L SFG-CB). Enrichment of associations with GA within this network is driven by females (Fig. 3, Fig. 4; Supplemental Table S5).
Fig. 5.
Associations between gestational age (GA) and whole group (N = 118) functional connectivity (FC). GA changes associated with FC (black squares) are implicated in 8 out of 16 networks. SFG, superior frontal gyrus; SMA, somatomotor area; aIN, anterior insula; IFG, inferior frontal gyrus; Vis, Visual; pParietal, posterior parietal; PCC, posterior cingulate cortex; CB, cerebellum; inf Temp, inferior temporal; pIN, posterior insula; TPJ, temporo-parietal junction; SC, subcortical grey matter (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
Fig. 6.
Associations between whole group gestational age (GA) and functional connectivity (FC). Blue lines represent negative FC-GA correlations, red lines represent positive FC-GA correlations. All connections between networks significant at Spearman rho p < 0.05. SFG, superior frontal gyrus; SMA, somatomotor area; aIN, anterior insula; IFG, inferior frontal gyrus; Vis, Visual; pParietal, posterior parietal; PCC, posterior cingulate cortex; CB, cerebellum; inf Temp, inferior temporal; pIN, posterior insula; TPJ, temporo-parietal junction; SC, subcortical grey matter (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
4. Discussion
Characterization of human fetal brain network functional connectivity provides fundamental insight into the developmental origin of brain network architecture. While sex-related differences in brain structure and function have been reported across the lifespan, no prior research has investigated the sex-related brain development in utero. In the present study we used graph theory to identify 16 prenatal functional connectivity networks that were characteristically lacking in bilateral homotopic connectivity. We then examined sex-related changes in FC within and between networks across gestation. We observed differences between male and female fetuses in FC within and between seven networks. Further, we investigated GA-related changes, independent of sex, within and between networks. In the largest study to date, we identified variance in fetal brain network connectivity associated with GA. Cumulatively, the present study demonstrates that prenatal FC is organized into highly fragmented prenatal brain networks, and that prenatal FC varies significantly with regard to both sex and GA.
4.1. Brain networks in utero
This present study utilized graph theoretical community detection algorithms in order to identify prenatal FC brain networks. While Infomap community detection algorithms have been described previously in adults (Power et al., 2011) and in infant-toddler network models (Eggebrecht et al., 2017), this is the first time that Infomap has been used to estimate brain networks from human fetuses in utero. Research examining the developmental trajectories of the human brain suggests that, at younger ages (i.e. < 3 years of age), the functional connectivity structure relative to adults is highly fragmented and contains constituent ‘proto networks’ - precursors to adult functional network counterparts (Grayson and Fair, 2017). Consistent with prior prenatal work from our lab, we observed ‘proto networks’ in the fetal brain (Thomason et al., 2014). Unlike networks identified in neonates, infants, or toddlers (Eggebrecht et al., 2017; Gao et al., 2015a,b), the prenatal networks identified in the present study were not connected bilaterally, with the exception of the ‘supplementary motor area’ which encompassed an extensive dorsal portion of the brain. This finding of a large dorsal network, and lack of bilateral homotopic FC is consistent with prior research in prematurely born infants which suggests only weak bilateral connectivity is present prior to 38 weeks GA (Smyser et al., 2010). As with prior Infomap analyses in adults (Power et al., 2011), we observed that Infomap was capable of discerning smaller networks (i.e. 16 networks in the present study) as compared to the 5 or 6 networks previously identified using Louvain modularity (Thomason et al., 2014). Taken together, the present work adds an exciting new piece to our understanding of human brain development by demonstrating 16 unique fetal networks, precursors to infant-toddler ‘proto-networks’ (Eggebrecht et al., 2017); networks that will likely develop into their eventual adult network counterparts throughout early childhood.
4.2. Fetal brain development differences between males and females
Sexual differentiation is apparent in both neural and physiological development in the first weeks of life (Hines, 2010). One of the most consistent dimorphic findings is that male infants, children, and adults have greater whole-brain volume than females (Gilmore et al., 2007; Koolschijn and Crone, 2013). However, it is important to understand not just regional differences in structure, but also the connectivity between regions and how these connections differ early in development between males and females in utero. Specifically, males have been documented to have larger prefrontal cortex, amygdala, and hippocampi than females (Ruigrok et al., 2014). These volumetric differences may predispose individuals to certain vulnerabilities (DiPietro and Voegtline, 2017). Understanding regional differences in brain function and FC in utero may inform health outcomes and susceptibility to environmental stressors and the development of psychopathology.
In the present study, we observed developmental differences between males and females in fetal brain network FC between 25–38 weeks. We observed differences between male and female fetuses in FC within and between seven networks across GA. Females demonstrated greater change in long range FC across gestation while male fetuses demonstrated local FC-GA associations. Specifically, female fetuses demonstrated long range GA related changes in FC between subcortical and cortical regions (i.e. CB and SC connectivity with SFG, PFC, and Visual cortex). This pattern of FC-GA relationships within these network pairs was almost completely non-existent in male fetuses. Meta analyses of sex differences across the lifespan suggest that females have greater frontal gyrus, prefrontal cortex, and thalamus volume than males (Ruigrok et al., 2014), and female infants have greater volume within the dorsolateral prefrontal cortex (including the SFG) and visual cortex (Knickmeyer et al., 2014). It seems likely that these volumetric differences are mirrored by SFG FC differences observed in the present study. Further, prior studies in large samples of adults (Buckner, 2013; Buckner et al., 2011) and infants (Herzmann et al., 2018) demonstrate that the CB possesses multiple representations of the cerebral cortex. However, these prior papers did not examine gender differences in CB FC. It is possible that the SFG-CB connectivity observed in the present study supports future dimorphic functional and cognitive representations within the CB later in life.
In the present study, males demonstrated greater FC-GA associations within the CB and between dorsal and ventral regions of the PFC (i.e. PFC – R SFG) than females. While this association was stronger in males, females also demonstrated a similar pattern of FC-GA associations within the CB. Within the CB, as GA increased, intra-cerebellar FC increased, suggesting strengthening of functional connections within the cerebellum is crucial for typical brain development across gestation. The CB experiences the largest growth of any brain region following birth (Choe et al., 2013; Holland et al., 2014), and studies consistently report that males have larger cerebellum grey matter volume than females spanning from childhood to adulthood (Koolschijn and Crone, 2013; Ruigrok et al., 2014; Tiemeier et al., 2010). Taken together, the present findings suggest that greater cerebellar volume in males is mirrored by greater FC integration within the CB network in males than females during gestation. In contrast, FC within the ventral (PFC) and dorsal (R SFG) prefrontal cortex decreased as GA increased. Both groups demonstrated this FC-GA relationship within the PFC, though it was more pronounced in male fetuses. While some research would suggest that connectivity between dorsal and ventral PFC should grow stronger with age, ultimately leading to the formation of the anterior portion of the default mode network (Power et al., 2011), other research suggests distinct functionality exists in dorsal and anterior prefrontal and cingulate cortex (Bush and Posner, 2000). Perhaps these GA related changes in FC between dorsal and ventral PFC will come to support cogntive and affective processes during later development (Bush and Posner, 2000).
4.3. Functional connectivity associations with gestational age
Early in development, vast and rapid brain growth occurs. Specifically, data from preterm infants scanned between 28–38 weeks demonstrates a rapid increase in brain volume and cortical folding in regions including motor, SMA, visual, and insula cortices, while at later GA these regions slow in their growth and are surpassed by growth and folding in lateral parietal, temporal, and frontal regions (Garcia et al., 2018; Geng et al., 2017). Further, research demonstrates that gyrification of the fetal brain increases across GA, and that the rate of gyrification peaks at approximately 30 weeks (Wright et al., 2014). Determining the FC changes corresponding to these structural changes during in utero development has received less attention.
Studies of functional connectivity fMRI in infants and young children have availed further important insight into processes governing the development of FC within and between regions of the brain. Specifically, while bilateral connectivity between homotopic regions appears present in typically developing infants and toddlers (Doria et al., 2010; Fransson et al., 2011; Gao et al., 2015a,b), association networks are often fragmented into disconnected, or weakly connected, anterior and posterior segments. Limited prior research suggests that functional brain networks are further fragmented during gestation (i.e. < 40 weeks gestational age), and lack bilateral homotopic network connectivity (Smyser et al., 2010; Thomason et al., 2014, 2013; Thomason et al., 2015). However, these prior fetal and preterm imaging studies were conducted in relatively small samples (n <38) and were thus limited from well-powered age and gender comparisons. An exception comes from a recent study by our group that sought to isolate connectome “hubs” using data from 105 fetuses (M. I. van den Heuvel et al., 2018). We reported degree and betweenness centrality across the full sample, and in a secondary analysis compared consistency in identified hubs across median-split age groups. In contrast, here we examine gestational age as a continuous variable assessed in 118 human fetuses and use enrichment analyses to identify between and within network connections that show significant age-related change.
As expected, the present results suggest that increasing GA is associated with widespread change in FC across the brain. A notable number of FC changes were observed involving SFG, PCC, and visual cortex connectivity with other cortical and subcortical networks. While prior research demonstrated an increase in cross-hemispheric connectivity with increasing age (Smyser et al., 2016), we did not observe a pattern of increasing cross-hemispheric connectivity in the present study. In contrast, many of the connections which changed with increasing GA appeared to be long distance anterior-posterior connections. In interpreting these differences, it is important to note that fetuses in the present study were less than 40 weeks GA, while prior work (Smyser et al., 2016) included term born infants (> 40 weeks GA). The present study suggests that bilateral homotopic connectivity does not begin to mature until the final weeks of gestation (i.e. after 38 weeks) or even until after birth.
4.4. Neurovascular coupling and estimates of functional connectivity
Assessing functional brain networks in the human fetus presents multiple significant methodological challenges, including the potential for differing hemodynamics as compared to infant or older human participants. Indeed, multiple key components of neurovascular coupling, from gene networks to local cellular contributors like astrocytes and pericytes to network-level brain connectivity, undergo significant changes during both fetal and postnatal development (Anderson and Thomason, 2013; Hillman, 2014; Parikshak et al., 2015). More generally, prenatal hemodynamics in animal model studies (Colonnese et al., 2008) have been shown to display similarity in the maturation of the hemodynamic response of human participants at equivalent ages that are imaged in this study (Arichi et al., 2012). Though this is a period of rapid development, previous foundational studies have illuminated the emergence of the spatial structure of low-frequency correlations in early life human resting state networks and their relationship to normative function and neurodevelopmental disorders (Eggebrecht et al., 2017; Emerson et al., 2016; Fransson et al., 2011; Gao et al., 2009; Lin et al., 2008; Rogers et al., 2017; Smyser et al., 2011; Thomason et al., 2013). In the present study, because we are not assessing shape or amplitude of blood flow changes, we expect that hemodynamic variation is less likely to bias inter-individual results over the age range of focus. Additionally, as with other resting state functional connectivity analyses in early development, we do not assume an explicit hemodynamic response function (HRF). This is in contrast to effective connectivity studies that aim to assess the causal influence of one brain region on another by deconvolving an assumed shape of the HRF from the fMRI time series. To utilize such HRF-dependent methods, future fetal fMRI studies may optimize model parameters through estimation of oxygenation possibly through the BOLD activity of the placenta (De Vis et al., 2015).
4.5. Limitations
Imaging and analyzing prenatal FC data presents several unique challenges (as summarized by van den Heuvel and Thomason, 2016). The ROI utilized in the present study are in fetal atlas space based on a 32-week template. The general utility of these ROI to data in other template spaces is limited. However, due to rapid and non-linear changes in fetal brain structures (e.g. the cerebellum), transformation of fetal data to adult atlas space should be performed with caution. Due to challenges inherent to fetuses moving inside the womb, standard segmentation and reorientation algorithms used in adult data could not be performed. Therefore, the BOLD volumes used in the present analysis were derived from manual segmentation and reorientation. Future analyses with this data may endeavor to automate this process with computer routines and algorithms catered to this unique dataset. Further, we acknowledge that the resolution of functional data here (3 × 3 x 4 mm and 2 mm isotropic after resampling) is fairly large considering the size of the fetal brain and raises concerns of partial voluming of anatomical structures in our parcellation strategy. We recommend other groups take resolution into consideration when developing scanning protocols and when considering parcellation strategies. Lastly, the large GA range analyzed as a continuous variable (as opposed to binning age ranges as prior studies have done) is both a strength of the study design but also a consideration for interpretation of the data. Rapid changes in cortical growth, thickness, folding, and connectivity across the 13 weeks measured in the present study might result in a different set of consensus fetal networks than a consensus network determined using fetal data from a more limited GA range.
5. Conclusions
The study of brain development in utero is imperative for understanding typical and atypical brain development trajectories and achieving optimal long-term neurobehavioral outcomes. The present study demonstrates for the first time that development of fetal brain FC varies with sex. The differential development of FC over gestation in male and female fetuses likely acts as a precursor to sex-related brain connectivity differences observed across the lifespan. Further, the fetal brain networks observed in the present study likely serve as the building blocks for nascent neonatal, toddler, and adult networks.
Funding
This work was supported by the National Institutes of Health [T32 MH100019 to MDW, K01 MH103594 to ATE, MH110793 and ES026022 to MET] and by a NARSAD Young Investigator Award to MET. This research was also supported, in part, by the Perinatology Research Branch, Division of Obstetrics and Maternal-Fetal Medicine, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS); and, in part, with federal funds from NICHD/NIH/DHHS under Contract No. HHSN275201300006C.
Disclosure statement
The authors report no conflicts of interest.
Acknowledgements
We would like to thank Dustin Scheinost for the contribution of a fetal surface mesh for visualization purposes. The authors thank Pavan Jella, Sophia Neuenfeldt, Toni Lewis, Tamara Qawasmeh, Fatimah Alismail, and Nada Alrajhi for their assistance in data acquisition and analyses. We would also like to thank the participant families who generously shared their time.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2019.100632.
Contributor Information
A.T. Eggebrecht, Email: aeggebre@wustl.edu.
M.E. Thomason, Email: Moriah.Thomason@nyulangone.org.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Adams Waldorf K.M., McAdams R.M. Influence of infection during pregnancy on fetal development. Reproduction. 2013;146(5):R151–R162. doi: 10.1530/REP-13-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson A.L., Thomason M.E. Functional plasticity before the cradle: a review of neural functional imaging in the human fetus. Neurosci. Biobehav. Rev. 2013;37(9 Pt B):2220–2232. doi: 10.1016/j.neubiorev.2013.03.013. [DOI] [PubMed] [Google Scholar]
- Arichi T., Fagiolo G., Varela M., Melendez-Calderon A., Allievi A., Merchant N. Development of BOLD signal hemodynamic responses in the human brain. Neuroimage. 2012;63(2):663–673. doi: 10.1016/j.neuroimage.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backes C., Ruhle F., Stoll M., Haas J., Frese K., Franke A. Systematic permutation testing in GWAS pathway analyses: identification of genetic networks in dilated cardiomyopathy and ulcerative colitis. BMC Genomics. 2014;15:622. doi: 10.1186/1471-2164-15-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broere-Brown Z.A., Baan E., Schalekamp-Timmermans S., Verburg B.O., Jaddoe V.W., Steegers E.A. Sex-specific differences in fetal and infant growth patterns: a prospective population-based cohort study. Biol. Sex Differ. 2016;7:65. doi: 10.1186/s13293-016-0119-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R.L. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron. 2013;80(3):807–815. doi: 10.1016/j.neuron.2013.10.044. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Krienen F.M., Castellanos A., Diaz J.C., Yeo B.T. The organization of the human cerebellum estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106(5):2322–2345. doi: 10.1152/jn.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G., Posner M.I. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 2000;4(6) doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Buss C., Entringer S., Moog N.K., Toepfer P., Fair D.A., Simhan H.N. Intergenerational transmission of maternal childhood maltreatment exposure: implications for fetal brain development. J. Am. Acad. Child Adolesc. Psychiatry. 2017;56(5):373–382. doi: 10.1016/j.jaac.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviness V.S., Jr, Kennedy D.N., Richelme C., Rademacher J., Filipek P.A. The human brain age 7-11 years: a volumetric analysis based on magnetic resonance imaging. Cereb. Cortex. 1996;6:726–736. doi: 10.1093/cercor/6.5.726. [DOI] [PubMed] [Google Scholar]
- Choe M.S., Ortiz-Mantilla S., Makris N., Gregas M., Bacic J., Haehn D. Regional infant brain development: an MRI-based morphometric analysis in 3 to 13 month olds. Cereb. Cortex. 2013;23(9):2100–2117. doi: 10.1093/cercor/bhs197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciric R., Wolf D.H., Power J.D., Roalf D.R., Baum G.L., Ruparel K. Benchmarking of participant-level confound regression strategies for the control of motion artifact in studies of functional connectivity. Neuroimage. 2017;154:174–187. doi: 10.1016/j.neuroimage.2017.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonnese M.T., Phillips M.A., Constantine-Paton M., Kaila K., Jasanoff A. Development of hemodynamic responses and functional connectivity in rat somatosensory cortex. Nat. Neurosci. 2008;11(1):72–79. doi: 10.1038/nn2017. [DOI] [PubMed] [Google Scholar]
- De Vis J.B., Hendrikse J., Petersen E.T., de Vries L.S., van Bel F., Alderliesten T. Arterial spin-labelling perfusion MRI and outcome in neonates with hypoxic-ischemic encephalopathy. Eur. Radiol. 2015;25(1):113–121. doi: 10.1007/s00330-014-3352-1. [DOI] [PubMed] [Google Scholar]
- Deoni S.C., Dean D.C., 3rd, Remer J., Dirks H., O’Muircheartaigh J. Cortical maturation and myelination in healthy toddlers and young children. Neuroimage. 2015;115:147–161. doi: 10.1016/j.neuroimage.2015.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A., Fair D.A., Kelly C., Satterthwaite T.D., Castellanos F.X., Thomason M.E. Unraveling the miswired connectome: a developmental perspective. Neuron. 2014;83(6):1335–1353. doi: 10.1016/j.neuron.2014.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPietro J.A., Voegtline K.M. The gestational foundation of sex differences in development and vulnerability. Neuroscience. 2017;342:4–20. doi: 10.1016/j.neuroscience.2015.07.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doria V., Beckmann C.F., Arichi T., Merchant N., Groppo M., Turkheimer F.E. Emergence of resting state networks in the preterm human brain. Proc. Natl. Acad. Sci. U. S. A. 2010;107(46):20015–20020. doi: 10.1073/pnas.1007921107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggebrecht A.T., Elison J.T., Feczko E., Todorov A., Wolff J.J., Kandala S. Joint attention and brain functional connectivity in infants and toddlers. Cereb. Cortex. 2017;27(3):1709–1720. doi: 10.1093/cercor/bhw403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson R.W., Gao W., Lin W. Longitudinal study of the emerging functional connectivity asymmetry of primary language regions during infancy. J. Neurosci. 2016;36(42):10883–10892. doi: 10.1523/JNEUROSCI.3980-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y., Shi F., Smith J.K., Lin W., Gilmore J.H., Shen D. Brain anatomical networks in early human brain development. Neuroimage. 2011;54(3):1862–1871. doi: 10.1016/j.neuroimage.2010.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P., Aden U., Blennow M., Lagercrantz H. The functional architecture of the infant brain as revealed by resting-state fMRI. Cereb. Cortex. 2011;21(1):145–154. doi: 10.1093/cercor/bhq071. [DOI] [PubMed] [Google Scholar]
- Gao W., Zhu H., Giovanello K.S., Smith J.K., Shen D., Gilmore J.H., Lin W. Evidence on the emergence of the brain’s default network from 2-week-old to 2-year-old healthy pediatric subjects. Proc. Natl. Acad. Sci. U. S. A. 2009;106(16):6790–6795. doi: 10.1073/pnas.0811221106. 0811221106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W., Gilmore J.H., Giovanello K.S., Smith J.K., Shen D., Zhu H., Lin W. Temporal and spatial evolution of brain network topology during the first two years of life. PLoS One. 2011;6(9) doi: 10.1371/journal.pone.0025278. e25278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W., Alcauter S., Elton A., Hernandez-Castillo C.R., Smith J.K., Ramirez J., Lin W. Functional Network Development During the First Year: Relative Sequence and Socioeconomic Correlations. Cereb. Cortex. 2015;25(9):2919–2928. doi: 10.1093/cercor/bhu088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W., Alcauter S., Smith J.K., Gilmore J.H., Lin W. Development of human brain cortical network architecture during infancy. Brain Struct. Funct. 2015;220(2):1173–1186. doi: 10.1007/s00429-014-0710-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia K.E., Robinson E.C., Alexopoulos D., Dierker D.L., Glasser M.F., Coalson T.S. Dynamic patterns of cortical expansion during folding of the preterm human brain. Proc. Natl. Acad. Sci. U. S. A. 2018;115(12):3156–3161. doi: 10.1073/pnas.1715451115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng X., Li G., Lu Z., Gao W., Wang L., Shen D. Structural and maturational covariance in early childhood brain development. Cereb. Cortex. 2017;27(3):1795–1807. doi: 10.1093/cercor/bhw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore J.H., Lin W., Prastawa M.W., Looney C.B., Vetsa Y.S.K., Knickmeyer R.C. Regional gray matter growth, sexual dimorphism, and cerebral asymmetry in the neonatal brain. J. Neurosci. 2007;27(6):1255–1260. doi: 10.1523/JNEUROSCI.3339-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson D.S., Fair D.A. Development of large-scale functional networks from birth to adulthood: A guide to the neuroimaging literature. Neuroimage. 2017;160:15–31. doi: 10.1016/j.neuroimage.2017.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzmann C.S., Snyder A.Z., Kenley J.K., Rogers C.E., Shimony J.S., Smyser C.D. Cerebellar functional connectivity in term- and very preterm-born infants. Cereb. Cortex. 2018 doi: 10.1093/cercor/bhy023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman E.M. Coupling mechanism and significance of the BOLD signal: a status report. Annu. Rev. Neurosci. 2014;37:161–181. doi: 10.1146/annurev-neuro-071013-014111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines M. Sex-related variation in human behavior and the brain. Trends Cogn. Sci. 2010;14(10):448–456. doi: 10.1016/j.tics.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland D., Chang L., Ernst T.M., Curran M., Buchthal S.D., Alicata D. Structural growth trajectories and rates of change in the first 3 months of infant brain development. JAMA Neurol. 2014;71(10):1266–1274. doi: 10.1001/jamaneurol.2014.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H., Vasung L. Gaining insight of fetal brain development with diffusion MRI and histology. Int. J. Dev. Neurosci. 2014;32:11–22. doi: 10.1016/j.ijdevneu.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H., Shu N., Mishra V., Jeon T., Chalak L., Wang Z.J. Development of human brain structural networks through infancy and childhood. Cereb. Cortex. 2015;25(5):1389–1404. doi: 10.1093/cercor/bht335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatri P., Sirota M., Butte A.J. Ten years of pathway analysis: current approaches and outstanding challenges. PLoS Comput. Biol. 2012;8(2) doi: 10.1371/journal.pcbi.1002375. e1002375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knickmeyer R.C., Wang J., Zhu H., Geng X., Woolson S., Hamer R.M. Impact of sex and gonadal steroids on neonatal brain structure. Cereb. Cortex. 2014;24(10):2721–2731. doi: 10.1093/cercor/bht125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolschijn P.C., Crone E.A. Sex differences and structural brain maturation from childhood to early adulthood. Dev. Cogn. Neurosci. 2013;5:106–118. doi: 10.1016/j.dcn.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostovic I., Jovanov-Milosevic N. The development of cerebral connections during the first 20-45 weeks’ gestation. Semin. Fetal Neonatal Med. 2006;11(6):415–422. doi: 10.1016/j.siny.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Lanphear B.P., Vorhees C.V., Bellinger D.C. Protecting children from environmental toxins. PLoS Med. 2005;2(3):e61. doi: 10.1371/journal.pmed.0020061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot R.K., Gogtay N., Greenstein D.K., Wells E.M., Wallace G.L., Clasen L.S. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36(4):1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W., Zhu Q., Gao W., Chen Y., Toh C.H., Styner M. Functional connectivity MR imaging reveals cortical functional connectivity in the developing brain. AJNR Am. J. Neuroradiol. 2008;29(10):1883–1889. doi: 10.3174/ajnr.A1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrus N., Eggebrecht A.T., Todorov A., Elison J.T., Wolff J.J., Cole L. Walking, gross motor development, and brain functional connectivity in infants and toddlers. Cereb. Cortex. 2017;28(2):750–763. doi: 10.1093/cercor/bhx313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melamed N., Meizner I., Mashiach R., Wiznitzer A., Glezerman M., Yogev Y. Fetal sex and intrauterine growth patterns. J. Ultrasound Med. 2013;32:35–43. doi: 10.7863/jum.2013.32.1.35. [DOI] [PubMed] [Google Scholar]
- Parikshak N.N., Gandal M.J., Geschwind D.H. Systems biology and gene networks in neurodevelopmental and neurodegenerative disorders. Nat. Rev. Genet. 2015;16(8):441–458. doi: 10.1038/nrg3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Cohen A.L., Nelson S.M., Wig G.S., Barnes K.A., Church J.A. Functional network organization of the human brain. Neuron. 2011;72(4):665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivals I., Personnaz L., Taing L., Potier M.C. Enrichment or depletion of a GO category within a class of genes: which test? Bioinformatics. 2007;23(4):401–407. doi: 10.1093/bioinformatics/btl633. [DOI] [PubMed] [Google Scholar]
- Rogers C.E., Sylvester C.M., Mintz C., Kenley J.K., Shimony J.S., Barch D.M., Smyser C.D. Neonatal amygdala functional connectivity at rest in healthy and preterm infants and early internalizing symptoms. J. Am. Acad. Child Adolesc. Psychiatry. 2017;56(2):157–166. doi: 10.1016/j.jaac.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosvall M., Bergstrom C. Maps of random walks on complex networks reaveal community structure. PNAS. 2008;105:1118–1123. doi: 10.1073/pnas.0706851105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruigrok A.N., Salimi-Khorshidi G., Lai M.C., Baron-Cohen S., Lombardo M.V., Tait R.J., Suckling J. A meta-analysis of sex differences in human brain structure. Neurosci. Biobehav. Rev. 2014;39:34–50. doi: 10.1016/j.neubiorev.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite T.D., Wolf D.H., Roalf D.R., Ruparel K., Erus G., Vandekar S. Linked Sex Differences in Cognition and Functional Connectivity in Youth. Cereb. Cortex. 2015;25(9):2383–2394. doi: 10.1093/cercor/bhu036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J.A., Habas P.A., Kim K., Rajagopalan V., Hamzelou K.S., Corbett-Detig J.M. Growth trajectories of the human fetal brain tissues estimated from 3D reconstructed in utero MRI. Int. J. Dev. Neurosci. 2011;29(5):529–536. doi: 10.1016/j.ijdevneu.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serag A., Aljabar P., Ball G., Counsell S.J., Boardman J.P., Rutherford M.A. Construction of a consistent high-definition spatio-temporal atlas of the developing brain using adaptive kernel regression. Neuroimage. 2012;59(3):2255–2265. doi: 10.1016/j.neuroimage.2011.09.062. [DOI] [PubMed] [Google Scholar]
- Shattuck D.W., Leahy R.M. BrainSuite: an automated cortical surface identification tool. Med. Image Anal. 2002;6(2):129–142. doi: 10.1016/s1361-8415(02)00054-3. [DOI] [PubMed] [Google Scholar]
- Slopen N., Loucks E.B., Appleton A.A., Kawachi I., Kubzansky L.D., Non A.L. Early origins of inflammation: an examination of prenatal and childhood social adversity in a prospective cohort study. Psychoneuroendocrinology. 2015;51:403–413. doi: 10.1016/j.psyneuen.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser C.D., Inder T.E., Shimony J.S., Hill J.E., Degnan A.J., Snyder A.Z., Neil J.J. Longitudinal analysis of neural network development in preterm infants. Cereb. Cortex. 2010;20(12):2852–2862. doi: 10.1093/cercor/bhq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser C.D., Snyder A.Z., Neil J.J. Functional connectivity MRI in infants: exploration of the functional organization of the developing brain. Neuroimage. 2011;56(3):1437–1452. doi: 10.1016/j.neuroimage.2011.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser C.D., Dosenbach N.U., Smyser T.A., Snyder A.Z., Rogers C.E., Inder T.E. Prediction of brain maturity in infants using machine-learning algorithms. Neuroimage. 2016;136:1–9. doi: 10.1016/j.neuroimage.2016.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A., Tamayo P., Mootha V.K., Mukherjee S., Ebert B.L., Gillette M.A. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide epxression profiles. PNAS. 2005;102(43):15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason M.E., Dassanayake M.T., Shen S., Katkuri Y., Alexis M., Anderson A.L. Cross-hemispheric functional connectivity in the human fetal brain. Sci. Transl. Med. 2013;5(173) doi: 10.1126/scitranslmed.3004978. 173ra124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason M.E., Brown J.A., Dassanayake M.T., Shastri R., Marusak H.A., Hernandez-Andrade E. Intrinsic functional brain architecture derived from graph theoretical analysis in the human fetus. PLoS One. 2014;9(5) doi: 10.1371/journal.pone.0094423. e94423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason M.E., Grove L.E., Lozon T.A., Jr, Vila A.M., Ye Y., Nye M.J. Age-related increases in long-range connectivity in fetal functional neural connectivity networks in utero. Dev. Cogn. Neurosci. 2015;11:96–104. doi: 10.1016/j.dcn.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason M.E., Scheinost D., Manning J.H., Grove L.E., Hect J., Marshall N. Weak functional connectivity in the human fetal brain prior to preterm birth. Sci. Rep. 2017;7:39286. doi: 10.1038/srep39286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson B.L., Levitt P., Stanwood G.D. Prenatal exposure to drugs: effects on brain development and implications for policy and education. Nat. Rev. Neurosci. 2009;10(4):303–312. doi: 10.1038/nrn2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiemeier H., Lenroot R.K., Greenstein D.K., Tran L., Pierson R., Giedd J.N. Cerebellum development during childhood and adolescence: a longitudinal morphometric MRI study. Neuroimage. 2010;49(1):63–70. doi: 10.1016/j.neuroimage.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel M.I., Thomason M.E. Functional connectivity of the human brain in utero. Trends Cogn. Sci. 2016;20(12):931–939. doi: 10.1016/j.tics.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel M.P., Kersbergen K.J., de Reus M.A., Keunen K., Kahn R.S., Groenendaal F. The neonatal connectome during preterm brain development. Cereb. Cortex. 2015;25(9):3000–3013. doi: 10.1093/cercor/bhu095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel M.I., Turk E., Manning J.H., Hect J., Hernandez-Andrade E., Hassan S.S. Hubs in the human fetal brain network. Dev. Cogn. Neurosci. 2018;30:108–115. doi: 10.1016/j.dcn.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelock M.D., Austin N.C., Bora S., Eggebrecht A.T., Melzer T.R., Woodward L.J., Smyser C.D. Altered functional network connectivity relates to motor development in children born very preterm. Neuroimage. 2018;183:574–583. doi: 10.1016/j.neuroimage.2018.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Wright R., Kyriakopoulou V., Ledig C., Rutherford M.A., Hajnal J.V., Rueckert D., Aljabar P. Automatic quantification of normal cortical folding patterns from fetal brain MRI. Neuroimage. 2014;91:21–32. doi: 10.1016/j.neuroimage.2014.01.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.