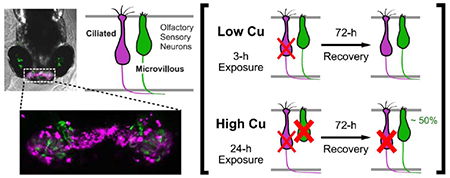
Abstract
Fish rely heavily on their sense of smell to maintain behaviors essential for survival, such as predator detection and avoidance, prey selection, social behavior, imprinting, and homing to natal streams and spawning sites. Due to its direct contact with the outside environment, the peripheral olfactory system of fish is particularly susceptible to dissolved contaminants. In particular, environmental exposures to copper (Cu) can cause a rapid loss of olfactory function. In this study, confocal imaging of double-transgenic zebrafish larvae with differentially labeled ciliated and microvillous olfactory sensory neurons (OSNs) were used to examine cell death and regeneration following Cu exposure. Changes in cell morphologies were observed at varying degrees within both ciliated and microvillous OSNs, including the presence of round dense cell bodies, cell loss and fragmentation, retraction or loss of axons, disorganized cell arrangements, and loss of cells and fluorescence signal intensity, which are all indicators of cell death after Cu exposure. A marked loss of ciliated OSNs relative to microvillous OSNs occurred after exposure to low Cu concentrations for 3 hours, with some regeneration observed after 72 hours. At higher Cu concentrations and 24-h exposures, ciliated and microvillous OSNs were damaged with increased severity of injury with longer Cu exposures. Interestingly, microvillous, but not ciliated OSNs, regenerated rapidly within the 72-h time period of recovery after death from Cu exposure, suggesting that microvillous OSNs may be replaced in lieu of ciliated OSNs. An increase in bromodeoxyuridine labeling was observed 24 h after Cu-induced OSN death, suggesting that increased proliferation of the olfactory stem cells replaced the damaged OSNs. Olfactory behavioral analyses supported our imaging studies and revealed both initial loss and restoration of olfactory function after Cu exposures. In summary, our studies indicate that following zebrafish OSN damage by Cu, regeneration of microvillous OSNs may occur exceeding ciliated OSNs, likely via increased proliferation of the cellular reservoir of neuronal OSC precursors. Transgenic zebrafish are a valuable tool to study metal olfactory injury and recovery and to characterize sensitive olfactory neuron populations in fish exposed to environmental pollutants.
Keywords: copper, zebrafish, olfactory sensory neurons, transgenics
Graphical abstract
INTRODUCTION
Fish and other aquatic vertebrates require a functional olfactory system to detect chemical cues from their environment in order to maintain behaviors essential for survival, such as predator avoidance, prey selection, kin identification, homing, and mate selection (Cooper et al., 1976; Dittman and Quinn, 1996; Hara, 1992; Quinn, 2011; Tierney et al., 2010; Sutterlin and Gray, 1973). Teleosts, which include commercially-relevant fish species such as salmon, trout, sablefish, pollock, and rockfish, have a well-developed and sensitive olfactory system that is able to detect dilute waterborne odorants, with some species of salmonids able to sense amino acids in the nanomolar range (Bandoh et al., 2011; Yamamoto et al., 2013). However, exposures to certain pollutants at concentrations measured in surface waters can decrease the ability of salmon to detect predators and prey (Baldwin et al., 2003, McIntyr et al., 2012; Tierney et al., 2010). For example, juvenile coho exposed to low levels of dissolved copper (1-20 ug/L) had impaired olfaction (Baldwin et al., 2003) and no longer exhibited alarm responses in the presence of a predator (McIntyr et al., 2012). Accordingly, the disruption of olfactory function by low-dose chemical exposures has potential far-reaching ramifications for fish populations, and several aquatic species receiving ecologically-relevant exposures to certain metals and pesticides have shown disruption of physiological processes that determine survival and reproductive success (Tierney et al., 2010). Despite the ecological importance of these sublethal toxicities in aquatic species, the mechanisms of olfactory injury are poorly understood.
Fish olfactory organs consist of an olfactory bulb as well as a pair of olfactory rosettes, the latter of which are located in cavities positioned dorsally on either side of the rostrum. The olfactory sensory neurons (OSNs) and their support cells are located apically in the olfactory sensory epithelium (OSE) on the surface of the rosettes, while immature neurons and olfactory stem cells (OSCs) are situated more basally (Hansen and Zielinski, 2005). Functionally, OSNs are chemoreceptors responsible for detecting odorants and transmitting signals to glomeruli within the olfactory bulb in the central nervous system. These OSNs detect a broad array of odorant molecules, including prostaglandins, bile acids, and amino acids, which bind to membrane-associated receptor proteins in the apical cilia or microvilli of the OSNs (Hara, 1994). Because the OSNs are in direct contact with the external environment, protected only by a mucosal lining secreted by neighboring goblet cells, they are particularly vulnerable to dissolved waterborne toxicants (Tierney et al., 2010). New OSNs are constantly being generated to replace the mature OSNs that die (Mackay-Sim and Kittel, 1991), a process that maintains sensory function following injury to certain environmental chemicals (Beites et al, 2005; Sakamoto et al., 2011)
Currently, four classes of OSNs have been identified in teleosts, with the ciliated and microvillous OSNs comprising the two major groups. These OSNs differ in morphology, location within the OSE, cell-specific gene expression, and receptivity to odorants (Hansen and Zielinski, 2005, Sato et al., 2005). Ciliated OSNs express OR-type odorant receptors and olfactory marker protein (OMP) among others (Hansen et al., 2004) and are located in the deep layer of the OSE with axons that project primarily to the dorsal and medial regions of the olfactory bulb (Hansen et al., 2003, 2004; Sato et al., 2005, 2007). Microvillous OSNs express vomeronasal receptor family 2 (V2R) type receptors and transient receptor potential channel C2 (TRPC2); these are located in the OSE superficial layer and project axons to the lateral region of the olfactory bulb (Hansen et al., 2003, 2004; Sato et al., 2005, 2007). Additionally, these two OSN populations differ in their detection of odorant classes due to differences in their olfactory signaling pathways. Ciliated OSNs have a cAMP-mediated pathway and preferentially detect bile acids such as taurochlolic acid (TCA), while microvillous OSNs have an IP3-based pathway and preferentially detect amino acids (Dew et al., 2014; Døving et al., 2011; Hansen et al., 2003; Kolmakov et al., 2009; Michel et al., 2003; Rolen et al., 2003). Our laboratory and others have demonstrated that ciliated and microvillous OSNs can differ in sensitivity to metal ions, which may further vary with developmental stage and across fish species (Dew et al., 2014; Heffern et al., 2018; Hentig and Byrd-Jacobs, 2016; Wang et al., 2013; Williams et al., 2016).
Of the trace metals, copper (Cu) is arguably the most well-studied and potent of the metal olfactory toxicants in teleosts (Shaw and Handy, 2011; Tierney et al., 2010) and a common pollutant in surface waters. Typical anthropogenic sources of Cu include vehicle emissions, pesticides, and industrial runoff (Davis and Shokouhian, 2001; Good, 1993). Previous studies have shown that even short-term exposures to Cu at environmental concentrations can decrease the ability of salmonids to detect predators (Baldwin et al., 2003, 2011; McIntyre et al., 2012). Accordingly, copper is a highly relevant model environmental pollutant to probe the mechanisms of fish olfactory injury and recovery. In this study, we leveraged the genetic and imaging capabilities of zebrafish to investigate Cu-induced injury and recovery of ciliated and microvillous OSN populations. The similarities in zebrafish morphology and neuroanatomy to other teleosts make this model particularly attractive for studies of olfaction and olfactory neurogenesis (Blechinger et al., 2007; Byrd and Brunjes, 1995, 1998; Lindsay and Vogt, 2004).
Similar to other vertebrates, zebrafish olfaction is functional at early stages of development (4 days postfertilization, dpf) and mediates behavioral and survival strategies in the developing larvae (Lindsay and Vogt, 2004). These attributes make zebrafish an excellent model system to study Cu olfactory injury (Lazzari et al., 2017; Tilton et al., 2008, 2011) and to investigate the cellular targets and recovery from metal exposures. In the present study, we analyzed Cu-induced injury and recovery of the two major OSN populations using confocal imaging of double-transgenic (Tg) zebrafish larvae (Lakhina et al., 2012; Miyasaka et al., 2005; Sato et al., 2005, 2007) that express both red fluorescent protein (RFP) in the ciliated OSNs under the control of the OMP promoter (OMP:RFP), and Venus in the microvillous OSNs driven by the TRPC2 promoter (TRPC2:Venus) (Lakhina et al., 2012; Miyasaka et al., 2005; Sato et al., 2005, 2007). These Tg[OMP:RFP/ TRPC2:Venus] zebrafish have been used in imaging studies of OSN development and axonal guidance (Lakhina et al., 2012; Miyasaka et al., 2005; Sato et al., 2005, 2007), and allowed us to observe both types of OSNs simultaneously in the same fish. From our imaging experiments, we observed differential effects of Cu exposure on the two types of OSNs that were dependent on both the concentration and duration of Cu exposure. We also assayed for cell proliferation after Cu-induced damage and behavioral changes to correlate our imaging results with loss and recovery of olfactory function.
MATERIALS AND METHODS
Zebrafish lines and maintenance
All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Washington. Adult zebrafish were housed in re-circulating aquaria maintained at 28 ± 0.5 °C on a 14 h light/10 h dark cycle. Fish received 2% of their body weight in flake food per day, and were supplemented with live Artemia at least once daily. Source water from city municipal water was passed through a reverse osmosis filtration system and adjusted to 1000 ± 100 μS/cm salinity (pH 7.2) using Instant Ocean® salt and Na2HCO3. Embryos produced by natural spawning from paired matings were raised in E3 embryo media (EM; 5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, and 0.33 mM MgSO4, pH 7.2–7.4) and maintained at a density of 50–60 per 100 mm2 dish. Larvae were fed live rotifers beginning at 4 dpf.
Transgenic OMP:RFP and TRPC2:Venus zebrafish were gifts from the Yoshihara lab from the RIKEN Brain Science Institute (Osaka, Japan; (Lakhina et al., 2012; Sato et al., 2005, 2007)) and maintained as single (OMP:RFP only) or double-transgenic (OMP:RFP/TRPC2:Venus) lines. To minimize interference of melanocytes in the imaging of the OSE, adults containing both transgenes were crossed into zebrafish homozygous for the roy orbison (roy) mutation (Ren et al., 2002), and intercrossed to generate lines homozygous for roy and both OMP:RFP/TRPC2:Venus transgenes. Screening of transgenic larvae were performed on 4-7 dpf larvae under a Nikon fluorescent dissecting microscope (Melville, NY). Those containing the transgenes were raised to adulthood and further screened for homozygosity through F1 screening from pairwise outcrossing with non-transgenic fish. Outbred Ekkwill (EKW) wildtype fish were used for all behavioral assays.
Copper exposures
Copper chloride salts (>95% pure) were obtained from Sigma-Aldrich (St. Louis, MO) and used to make 1.0 mM stock solutions. Copper concentrations of stocks were verified via ICP-MS (University of Washington Environmental Health Laboratory, based on EPA 6020a Rev.1 2007) and were similar to intended concentrations. The stock solution was diluted to final concentrations (0.25, 1, 5, and 10 μM Cu, corresponding to 16 ppb, 64 ppb, 318 ppb, and 635 ppb Cu) with EM on day of exposure and added to a 100 mm petri dish prior to the transfer of 5 dpf larval zebrafish (50 fish per 25 ml solution). Zebrafish larvae were exposed to Cu for either 3 or 24 hours prior to rinsing and removal into fresh EM to recover. Feeding of rotifers was reinstituted after 24 hours of recovery. At different times after Cu exposure, 15-17 fish were euthanized and fixed in 4% paraformaldehyde (PFA; in phosphate buffered saline, PBS) overnight at 4°C. Samples were washed several times with additional PBS and stored in the dark at 4°C. Confocal imaging was performed within two weeks of treatment.
Cell proliferation assay
Bromodeoxyuridine (BrdU) incorporation was performed at different times following 3-h Cu exposure (5 μM Cu or EM control) to investigate cell proliferation during recovery. Two separate sets of larval zebrafish were used and exposures were performed at two different times within day 5 of zebrafish development (5 dpf). Separate Cu exposures were necessary due to the 14 h light/10 h dark cycle of the fish facility where the BrdU incubations took place. BrdU incorporation for one set included recovery times of 6, 24, and 48 hours posttreatment (hpt), while 12 hpt and 18 hpt was performed on the second set. Larvae were incubated in EM containing 10 mM bromodeoxyuridine (BrdU; Sigma) with 1% dimethyl sulfoxide (DMSO) for 1 h at 28.5°C prior to euthanasia and fixation in 4% PFA for 2 h at room temperature or overnight at 4°C. After fixation, samples were washed several times in PBS and stored at 4°C before being processed for immunohistochemistry.
BrdU immunohistochemistry procedures followed those described by Ma et al. (2008) with minor modifications. Fixed samples were washed three times in PBDT (PBS, 1% DMSO, and 0.1% Tween-20) and dehydrated in 100% methanol for 1 h at 20°C. Samples were then rehydrated in a graded methanol series (75%, 50%, 25%; 20 min each), washed in PBDT, and incubated in 10g/ml proteinase K (in PBDT) for 20 min at RT. After rinsing three times in PBDT, samples were refixed in 4% PFA solution for 20 min, washed again in PBDT (20 min), and incubated in 1N hydrochloric acid (in PBDT) for 1 h. Following three additional rinses in PBDT, samples were incubated in 10% block solution (10% normal goat serum in PBDT) for 1 h at RT before primary and secondary antibody incubation per immunohistochemistry protocol. Rat anti-BrdU (Abeam, Cambridge, MA) primary antibodies were used at 1:100 dilution and Alexa 647-conjugated goat anti-rat (Thermo Fisher Scientific, USA) secondary antibodies were used at 1:500 dilution (both in 10% block solution). Samples were stored in PBS in the dark at 4°C until confocal imaging within one week.
Confocal imaging and analysis
Fixed larval transgenic zebrafish were mounted in 3% methyl cellulose/EM solution in 4-well coverglass-bottom chamber slides (Thermo Fisher Scientific). In order to image the OSE on an inverted confocal microscope, fish were positioned and oriented upside-down with their dorsal rostrum placed against the bottom cover glass. For live imaging of 6 dpf Tg[OMP:RFP/TRPC2:Venus] zebrafish, larvae were anesthetized with 0.016% MS-222 (Tricaine, methane suphonate pH 7), mounted in 6% methyl cellulose, and covered in EM containing MS-222 immediately prior to each imaging session (Renaud et al., 2011). Confocal z-stacks covering both OSEs were collected on a Zeiss LSM 510 inverted Axiovert 200 motorized laser scanning microscope and processed using ImageJ/Fiji (National Institutes of Health, USA). Two-color single scans were used to image the double-transgenics, while three-color scans (excitation at 488 nm, 543 nm, and 643 nm) were performed for the BrdU imaging experiments. All images shown in this paper are stacked projections of optical sections. Final image montages and figures were assembled using Adobe Photoshop CS5 and Affinity Designer (Serif Ltd., Nottingham, UK), with brightness/contrast adjusted for optimal visualization of cell morphology. Original unadjusted confocal image files were used for semi-quantitative fluorescence intensity analysis.
Analyses of relative fluorescence intensity in the OMP:RFP/TRPC2:Venus double transgenics were performed using ImageJ/Fiji and used as an indirect method of approximating relative numbers of OSNs within the OSE. Sum intensity z-stack projections were analyzed for each OSE image (n = 4-5 fish per condition). To eliminate background, a minimum threshold for each channel (red for OMP:RFP; green for TRPC2:Venus) was set and the thresholded area and intensity values were measured. OMP:RFP florescence was bright with little background, while background autofluoresence was visible in the green 488 nm TRPC2:Venus channel. Thus, the minimum florescence threshold was set higher for image analysis of TRPC2:Venus than for OMP:RFP in order to eliminate the background autofluoresence, resulting in lower values (measured as arbitrary light units, ALUs) for TRPC2:Venus. Analysis was confined to regions of interest (ROIs) drawn around the OSEs, thus eliminating any additional artifacts from the images (such as reflective pigment cells). ROIs were drawn for each individual OSE, and the total fluorescence intensity was averaged between the two OSEs for each fish. Mean fluorescence intensities were calculated for each condition and graphed as ALUs or normalized to the age-matched non-exposed (0 μM Cu) controls for each fluorophore/channel. Data were analyzed using Microsoft Excel and GraphPad Prism.
Fluorescence intensities of BrdU-labeled cells were analyzed using maximum intensity z-stack projections for 15 sections of each optical image stack (n = 3-4 fish per time and condition) that encompassed the entire OSE. TRPC2:Venus was difficult to clearly detect as the background in the 488 nm channel was relatively high, but OMP:RFP could be clearly distinguished over the background and served as a suitable landmark for the location of the OSEs. An intensity and size threshold was applied to the z-stack projections for the 643 nm BrdU channel in order to measure the intensity of nuclear BrdU labeling and to eliminate non-specific background fluoresence. Total intensities were calculated and normalized to the area of imaging, which was calculated using a low threshold applied to the OMP:RFP channel that captured the overall area of the dorsal rostrum containing the OSEs and the surrounding cells. BrdU fluorescence intensities were graphed as ALUs for both control and Cu-exposed fish. Fold change in BrdU fluorescence intensity was calculated by dividing the ALU values of the Cu-exposed fish with its corresponding age-matched control.
Behavior assays
We used the larval zebrafish behavior assay described in Heffern et al. (2018), which is a modification of the method by Shamchuk et al. (2017, 2018). The assay was used to observe olfactory behavior in larval zebrafish in response to the aversive odorants taurocholic acid (TCA) following Cu exposure and recovery. We have shown that the use of odorant TCA, which preferentially targets ciliated OSNs, yielded more consistent results in 5 dpf zebrafish larvae than other odorants such as L-cysteine, which largely targets microvillous OSNs (Heffern et al., 2018), For each experimental trial, 10 larval zebrafish were placed into the middle zone of a clear acrylic trough (10.5 cm × 3.5 cm × 1.7 cm) with removable dividers (Supplemental Figure 3A). The trough contained 15 ml EM and was set on top of a leveled light box for stable illumination. Following a 10-min acclimation period, 30 μl of odorant (EM or 0.4 M TCA) was added to either the left or right zone (randomized) prior to mixing. After a 5-min odorant equilibration period, the dividers were removed, and video recording of behavior began after one minute using an overhead camera. Approximately 10,000 frames were recorded (at 20 frames per second) prior to cessation of recordings and the fish euthanized.
Video recordings of the behavioral trials were analyzed using ImageJ. In each behavioral trial, the movement of all 10 zebrafish larvae over time was treated collectively as a single mass. This analysis in the behavioral assay minimizes the variation in swimming behavior amongst individuals by observing the behavioral response of a group of 10 fish instead of individuals (Heffern et al., 2018; Shamchuck et al., 2017, 2018). To reduce background and eliminate stationary fish, every 100th frame was averaged to create a background image that was subtracted from each frame. Individual frames were summed resulting in a videogram representing 5 minutes of fish movement (Supplemental Figure 3B). For each videogram, the shift distance was determined as the distance between the mean center of mass and the center of the trough. Displacement values greater or less than the 1-99 percentiles for each age group were excluded from the data set to eliminate anomalous outliers. Percentage responses were calculated by dividing the shift distances by the mean aversive response of unexposed control larvae to TCA for each age group. Data in the ensuing figures and tables are presented as either mean mass shift ± standard error of the mean (SE) or % response ± SE.
Statistical analyses
GraphPad Prism Ver 7.0 (Graph Pad Software Inc, San Diego, CA) was used for all statistical analysis. Normality of imaging and behavioral data was confirmed using D’Agostino-Pearson omnibus test and the effects of Cu concentrations on OSN loss and regeneration was determined using one-way ANOVAs followed by Fischer’s LSD test or Tukeys’s hsd post hoc test for multiple comparisons. The effect of Cu treatment on behavioral response was analyzed using a two-way ANOVA followed by an uncorrected Fisher’s LSD test. No heterogeneity was present in these data sets. All statistical data were considered significant at p ≤ 0.05.
RESULTS
Cu-induced OSN damage in larval zebrafish is both dose and time-dependent
To visualize injury and recovery of OSNs from Cu exposure, we used double-transgenic zebrafish with differentially labeled ciliated (OMP.RFP) and microvillous (TRPC2:Venus) OSNs (Fig. 1A-E; Sato et al., 2005, 2007). As observed, the confocal imaging of 5-9 dpf larvae revealed two separate OSN populations with non-overlapping signal. Cells exhibited organized clusters with axons extending medially (Fig. 1C-E). In general, more cells expressing OMP:RFP than TRPC2:Venus were observed due to a greater number of ciliated than microvillous OSNs present in the OSE at these ages (Fig. 1C-F; Sato 2005 and 2007). Semi-quantitative image analysis of fluorescence, used as a proxy for estimating the number of cells present for each OSN population within the OSE, showed a minor, albeit significant (p < 0.001) decrease in both OMP:RFP and TRPC2:Venus fluorescence between 5 and 9 dpf (Fig. 1F) in unexposed control fish, likely due to changes and maturation of the olfactory system during this time (Sato et al., 2005, 2007). Higher background signal was observed in the TRPC2:Venus (488 nm) channel due to autofluoresence of the larval body and reflective pigment cells in the eyes. To eliminate background signal, a higher minimal threshold was set for TRPC2:Venus than for OMP:RFP in our semi-quantitative image analyses. The lower overall fluorescence intensity measured for TRPC2:Venus was due to the higher threshold level as well as the presence of fewer microvillous OSNs (Fig. 1F).
Figure 1:
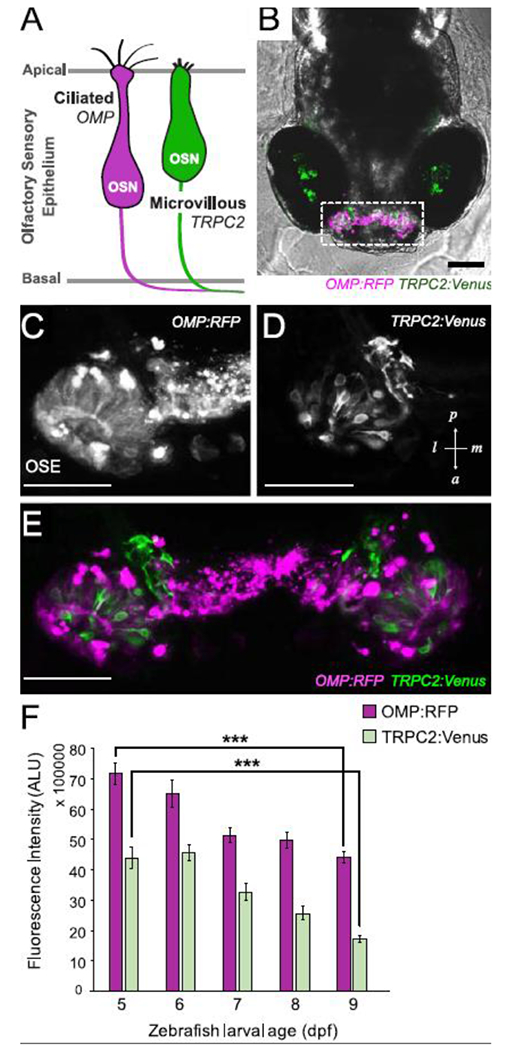
Two populations of olfactory sensory neurons (OSNs) are differentially labeled in double-transgenic zebrafish. A. Schematic of the two predominant OSNs in zebrafish olfactory sensory epithelium. B-E. In vivo confocal images of a double transgenic zebrafish larva (6 dpf, B) expressing OMP:RFP (C) in ciliated OSNs and TRPC2:Venus (D) in microvillous OSNs within the olfactory sensory epithelium (OSE; E). Images are stacked projections of optical sections. Scale bars = 100 μM (B); 50 μM (C-E). F. Semi-quantitative measurement of fluorescence intensity of OMP:RFP and TRPC2:Venus in OSE during early larval development between 5-9 dpf (measured in arbitrary light units, ALU) showing decrease in OSNs over time. Error bars = ± SE (standard error); n = 4-5 fish per time point. Asterisks indicate statistical significant change from 5 dpf larvae at ***p<0.001 significance levels.
Exposure of 5 dpf larvae to acute 3-h exposures of 0.25-10 μM Cu resulted in cellular changes within the OSE that are indicative of OSN injury. Confocal imaging of the 5-6 dpf Tg[OMP:RFP/TRPC2:Venus] larvae revealed morphological changes within both ciliated and microvillous OSNs, such as condensation of cell bodies and cell fragmentation in addition to loss of cells and fluorescence signal intensity (Fig. 2). Ciliated OSNs exhibited damage at all Cu concentrations tested, with dense OMP:RFP cell bodies, cell fragmentation, and significant 30%-50% losses of OMP:RFP fluorescence observed (Fig. 2A,C; p<0.001) after 3-h Cu exposure. Microvillous OSNs on the other hand, showed evidence of injury at higher Cu concentrations after 3-h exposure, with fewer cells observed in addition to some fragmentation and condensation of cell bodies (Fig. 2A). Image analysis showed a significant 30-42% reduction in TRPC2:Venus fluorescence after 5-10 μM Cu exposures (Fig. 2C; p<0.05). Conversely, little cell damage or loss of TRPC2:Venus fluorescence was observed at lower Cu concentrations (Fig. 2A,C). The differences in percentage of OSN signal loss between OMP:RFP and TRPC2:Venus populations were statistically significant only at the lowest Cu concentration tested (0.25 μM; p<0.01; Fig. 2C), suggesting that ciliated OSNs were likely more sensitive to the short (3-h) low-dose Cu exposure than microvillous OSNs.
Figure 2:
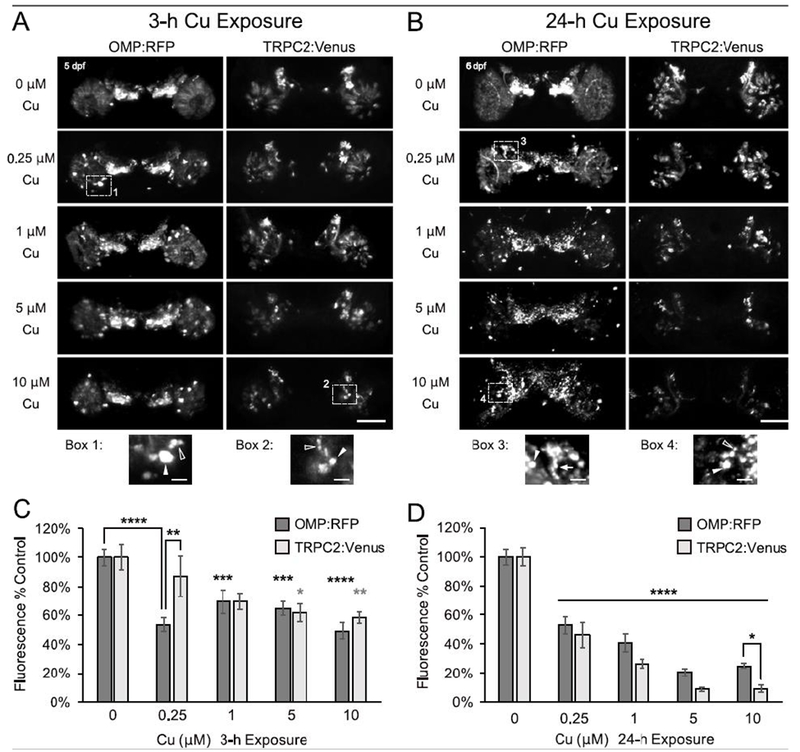
Severity of OSN damage by Cu is dose-dependent and time-dependent. A-B. Representative confocal images (stacked projections) of larval zebrafish OSE show changes in ciliated (OMP:RFP) and microvillous (TRPC2:Venus) OSNs following either a 3-h (A) or 24-h (B) exposure to Cu at 5 dpf. Magnification of numbered dotted boxes are shown below and highlight some of the observed morphological OSN changes such as dense cell bodies (white arrowheads), cell fragmentation (open arrowheads), and axonal retraction (white arrow). Scale bars = 50 μM and 10 μM (boxes). C-D. Semi-quantitative analysis of OMP:RFP and TRPC2:Venus fluorescence after (C) 3-h and (D) 24-h Cu exposure. Fluorescence intensity is normalized to age-matched non-exposed (0 μM Cu) controls. Error bars = ± SE; n=4-5 fish per condition. Asterisks indicate statistical significant change from 0 μM Cu control larvae, unless otherwise indicated (black asterisks= OMP:RFP; gray asterisks = TRPC2:Venus), at *p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001 significance levels.).
Extending the duration of Cu exposure to 24 hours resulted in greater loss of fluorescence in both OSN populations (Fig. 2B,D and Supplemental Fig. 1). Dense cell bodies, cell fragmentation, and axonal retraction were all observed in the OMP:RFP ciliated OSNs after 24 h Cu exposure, with few distinguishable intact cells remaining after 24 h of exposure to high Cu. Meanwhile, fewer visible cell bodies and axons of TRPC2:Venus microvillous OSNs were observed at higher Cu concentrations (compared to unexposed controls), with little to no Venus-labeled cell fragments and dense cell bodies (Fig. 2B). Both ciliated and microvillous OSNs showed a significant reduction of approximately 50% after 24-h Cu exposure to 0.25 μM Cu, with further loss of florescence observed at higher concentrations (Fig. 2D; p<0.0001). However, microvillous OSNs generally exhibited more extensive loss of fluorescence than ciliated OSNs at all Cu concentrations after 24 hours of exposure, a difference that was most notable at 10 μM Cu (Fig. 2D; p<0.05). Decreases in fluorescence of TRPC2:Venus microvillous OSNs after 24-h Cu exposure were greater than that observed after 3 h exposure at all Cu concentrations tested (Supplemental Fig. 1B; p<0.001). OMP:RFP ciliated OSNs also decreased with longer exposure (24-h) at higher Cu concentrations (1-10 μM Cu; p<0.05), but no difference was observed at the lowest Cu concentration tested (0.25 μM Cu; Supplemental Fig. 1A). Collectively, these data suggest that ciliated OSNs were more severely affected than microvillous OSNs at lower Cu concentrations and brief exposure duration, whereas both OSN types were equally affected after exposure to a higher amount of Cu, with increasing loss of fluorescence likely due to cell damage associated with exposure duration and increasing Cu concentrations.
Regeneration occurs more extensively in microvillous than ciliated OSNs
To investigate recovery and regeneration of OSNs after Cu-induced injury, Tg[OMP:RFP/TRPC2:Venus] zebrafish larvae were removed from the Cu exposure solutions after 3 or 24 hours and allowed to recover in fresh EM for 24, 48, and 72 hours prior to euthanasia and analysis. Following an acute 3-h Cu exposure, OMP:RFP ciliated OSN cell fragments and condensed cell bodies were still visible after 24 hours of recovery, but were not observed by 48 and 72 hours posttreatment (hpt; Fig. 3A and Supplemental Fig. 2A). Arrangement of ciliated OSNs also appeared to be more organized and normal by 72 h, suggesting some recovery after Cu exposure (Fig. 3A and Supplemental Fig. 2A). Semi-quantitative image analysis revealed a significant increase of OMP:RFP fluorescence after 72 h of recovery from Cu exposure at the lowest concentration tested (0.25 μM Cu; Fig 3C,E; p<0.05). Conversely, no recovery of OMP:RFP fluorescence was observed after acute 3-h exposure at higher Cu concentrations (Fig. 3C,F-H). OMP:RFP remained at a consistent level at approximately 40-50% of unexposed controls between 24 and 72 hpt, with no significant change observed over time (Fig. 3C,F-H).
Figure 3:
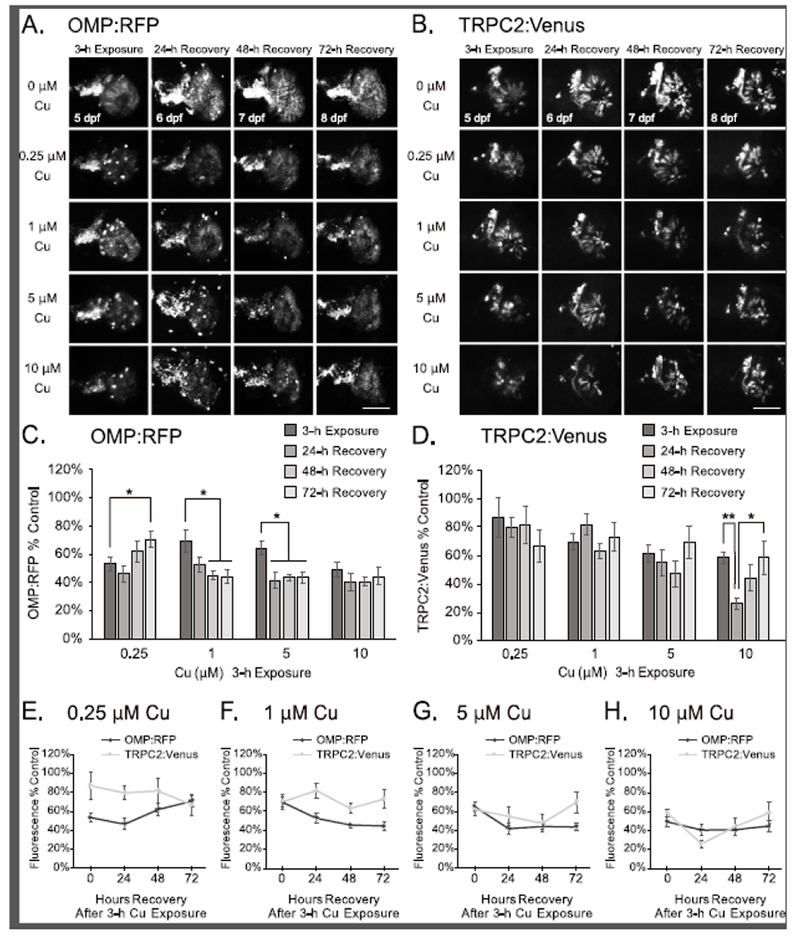
Partial OSN regeneration is observed following recovery after 3-h Cu-induced injury at 5 dpf. A-B. Representative confocal images of a single OSE from double-transgenic larval zebrafish show OMP:RFP-labeled ciliated OSNs (A) and TRPC2:Venus-labeled microvillous OSNs (B) imaging after 3h exposure of Cu at different doses after 24, 48, and 72 hours of recovery. Scale bars = 50 μM. C-D. Semi-quantitative analysis of OMP:RFP (C) and TRPC2:Venus (D) fluorescence in confocal images. E-H. Graphs comparing OMP:RFP and TRPC2:Venus fluorescence at 0.25 μM (E), 1 μM (F), 5 μM (G), and 10 μM (H) Cu exposure for 3 h (same dataset as presented in Fig. 3C-D). Fluorescence intensity is normalized to the age-matched non-exposed (0 μM Cu) controls. Error bars = ± SE; n=4-5 fish per condition. Asterisks indicate statistically significant change at *p<0.05 and **p<0.01 significance levels.
Further loss of OMP:RFP fluorescence occurred after 24-48 hours of recovery from 3-h exposure to 1 μM and 5 μM Cu, decreasing from 70% to 53% and from 65% to 41%, respectively (Fig. 3C,F-G; p<0.05). This additional loss of OMP:RFP suggests that additional damage and/or extrusion of the ciliated OSNs from the OSEs may have occurred within 24-48 hpt beyond the brief 3-h period of Cu exposure.
For microvillous OSNs, additional TRPC2:Venus cell bodies were visible by 72 hours after high 3-h Cu treatment (Fig. 3B and Supplemental Fig. 2A; 5 μM and 10 μM Cu), but semi-quantitative analyses of TRPC2:Venus fluorescence did not reveal any statistical differences between injury on 3h Cu exposure and after recovery at 72 hpt (Fig. 3D-H). Additional loss of TRPC2:Venus fluorescence occurred after 24-48 hours of recovery from high Cu exposure (Fig. 3D-H; 5 μM and 10 μM Cu). The loss of TRPC2:Venus at 24 hpt was significant only at the highest Cu concentration tested (10 μM Cu), with a decrease from 58% to 26% in observed fluorescence (Fig. 3D,H; p<0.01). Interestingly, a statistically significant recovery of this loss (from 26% to 58%; after 10 μM Cu) was observed by 72 hpt (Fig. 3D,H; p<0.05), suggesting that some recovery and/or regeneration of microvillous OSNs likely occurred between 24-72 hpt. At lower Cu concentrations, no significant changes in TRPC2:Venus microvillous OSNs were observed after acute 3-h Cu exposure (Fig. 3B,D-F). Rapid regeneration of microvillous OSNs was observed at all Cu concentrations tested following 24-h Cu exposures, with additional TRPC2:Venus cell bodies in the OSEs observed by 72 hours of recovery (Fig. 4B and Supplemental Fig. 2B). This observation of increased TRPC2:Venus cell bodies correlated with significant increases in overall TRPC2:Venus fluorescence over time (Fig. 4D-H; p<0.05). Rapid recovery of TRPC2:Venus fluorescence to near-control levels after 72 hpt (from 46% to 96%) was observed following 24-h exposure to low 0.25 μM Cu (Fig. 4B,D-E; p<0.05), suggesting that the microvillous OSNs had almost fully regenerated within the short recovery period. Significant recovery of TRPC2:Venus fluorescence also occurred after more extensive damage by 24-h Cu exposures at higher concentrations, and correlated with an increase in TRPC2:Venus OSNs observed (Fig. 4B,D,F-H; p<0.05). Recovery of TRPC2:Venus fluorescence to over 50% control levels was observed after 48 hpt following 1 μM Cu exposure (from 26%; p<0.05; Fig. 4D,F) and after 72 hpt following 5 or 10 μM Cu exposure (from 9%; p<0.0001 for 5 μM Cu; p<0.05 for 10 μM Cu; Fig. 4D,G-H). Conversely, no significant changes in OMP:RFP fluorescence were observed in ciliated OSNs during the 72-h recovery period after 24-h exposures at all Cu concentrations tested (Fig. 4A,C,E-H and Supplemental Fig. 2B).
Figure 4:
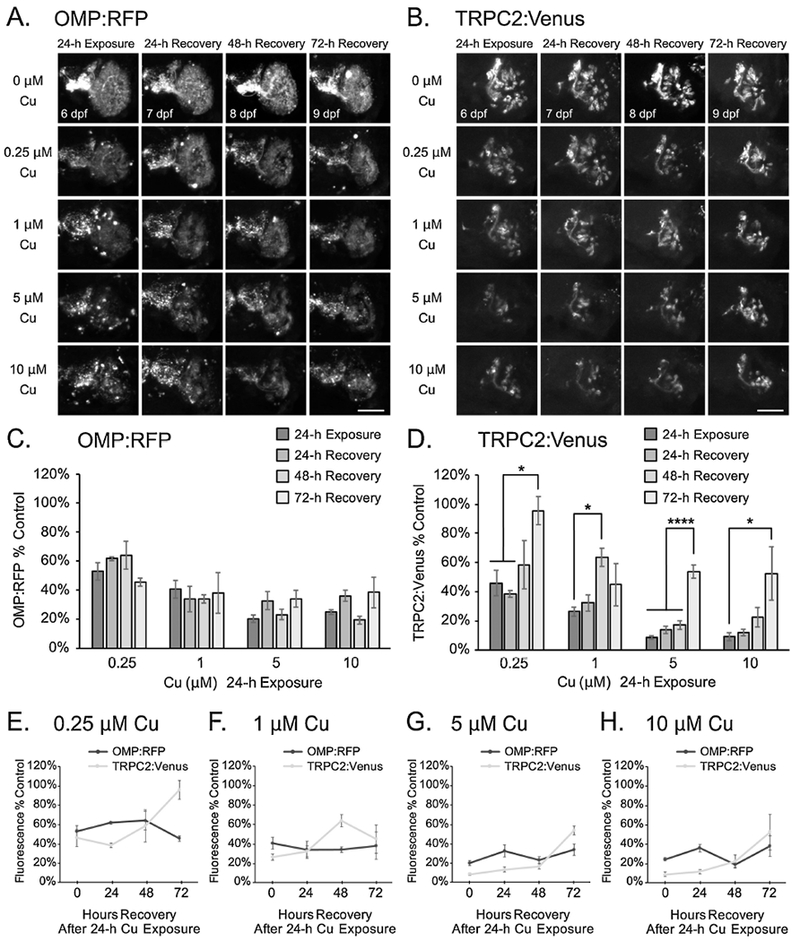
Regeneration of microvillous OSNs is observed after 24-h Cu exposure at 5 dpf. A-B. Representative confocal images of a single OSE from double-transgenic larval zebrafish show OMP:RFP-labeled ciliated OSNs (A) and TRPC2:Venus-labeled microvillous OSNs (B) after 24h Cu exposure at different doses following 24, 48, and 72 hours of recovery. C-D. Semi-quantitative analysis of OMP:RFP (C) and TRPC2:Venus (D) fluorescence in confocal images. Scale bars = 50 μM. E-H. Graphs comparing OMP:RFP and TRPC2:Venus fluorescence at 0.25 μM (E), 1 μM (F), 5 μM (G), and 10 μM (H) Cu exposure for 24 h. Fluorescence intensity is normalized to the age-matched non-exposed (0 μM Cu) controls. Error bars = ± SE; n=4-5 fish per condition. Asterisks indicate statistically significant change at *p<0.05 and ****p<0.0001 significance levels.
Collectively, our data suggest that the zebrafish microvillous OSNs regenerated rapidly within 72 hpt after prolonged 24-h exposures at all Cu concentrations, while recovery or regeneration of ciliated OSNs occured only after injury from a short (3-h) exposure to a low (0.25 μM) Cu concentration.
Increased cell proliferation occurs after Cu-induced OSN injury
Our observations of OSN regeneration after Cu exposure led us to hypothesize that increased cell proliferation occurred after OSN death to replace the damaged cells. To test this hypothesis, we performed experiments using bromodeoxyuridine (BrdU) to label proliferating S-phase cells at different times after Cu-induced OSN injury. We exposed 5 dpf Tg[OMP:RFP/TRPC2:Venus] double-transgenic fish for 3 hours to 5 μM Cu, a concentration that we had previously observed gross morphological damage to both OSN populations after acute (3-h) Cu treatment. We were unable to clearly detect TRPC2:Venus-labeled cells over the comparatively high background for the cells after BrdU processing, but were able to distinguish OMP:RFP, which served as a landmark for the location of the OSEs. BrdU-labeled cells were present in both unexposed and Cu-exposed larvae (Fig. 5A) across all times investigated. Semi-quantitative analysis of confocal images revealed a relatively constant low amount of BrdU-labeled cells within the vicinity around the OSE between 5 and 7 days of larval development (Fig 5A-B). Following 3-h Cu exposure, BrdU-labeled cells were observed clustered within the damaged OSEs after 6-24 hours of recovery (Fig. 5A), with a significant 5-fold increase over controls at 24 hpt (Fig. 5B,C; p<0.05). By 48 hpt, cell proliferation had returned to control levels. Collectively, these preliminary data suggest that injury to the OSNs by high Cu induces excess proliferation of the surrounding cells in the OSEs at approximately 24 hours following exposure in order to replace the damaged OSNs.
Figure 5:
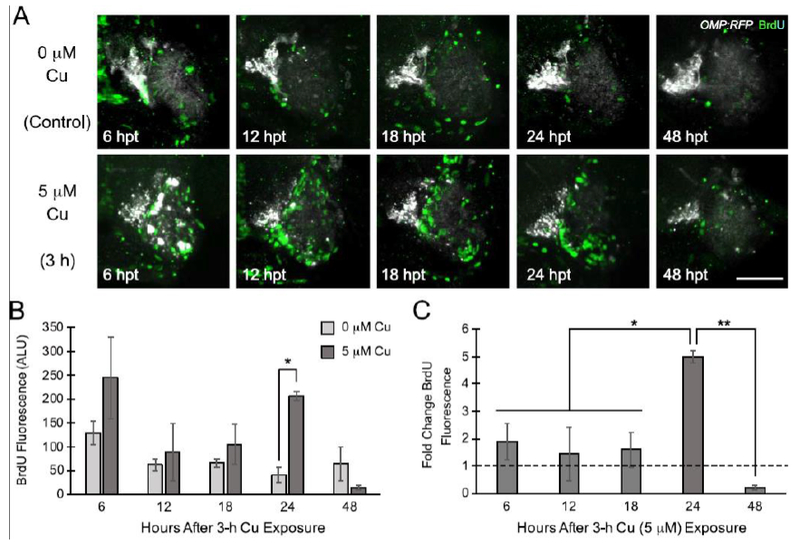
Cell proliferation increases approximately 24 hours after Cu-induced OSN injury. A. Representative confocal images of OMP:RFP transgenic fish showing BrdU-labeled cells at different times after initial Cu exposure (e.g. 5 μM Cu exposure for 3 h). Scale bar = 50 μM. B. Semi-quantitative analysis of BrdU fluorescence from confocal images normalized to the thresholded area of imaging (graphed as arbitrary light units; ALU). C. Graph showing BrdU incorporation of Cu-exposed larvae as fold-changes over the time-matched unexposed controls. Error bars = ± SE; n=3-4 fish per sample. Asterisks indicate statistically significant change at *p<0.05 and **p<0.01 significance levels.
Olfactory-mediated behavior correlates with loss and regeneration of OSNs
Because the phenotype for OSN regeneration involve recovery of olfactory function, we sought to correlate cellular changes observed with Cu-induced OSN injury and regeneration with olfactory-driven behavior. Videograms generated from the recordings of the behavioral assay (Supplemental Fig. 3A-B) were analyzed for center of mass shift distances and percent response of control to TCA as described in methods. Behavioral analysis of unexposed 6 dpf control larvae showed a significant aversive response to TCA (mean shift from center of 1.49 cm) compared to the no odorant control (Fig. 6A-B, p<0.01). After 24 hours of exposure of 5 dpf larvae to both low and high Cu concentrations (0.25 and 5 μM), a 38-42% decrease in response to TCA was observed (Fig. 6B). Statistical analyses of the Cu-exposed larval behavioral responses showed no significant difference between the TCA odorant and the no odorant control (EM; Fig. 6B), suggesting an abrogation of the olfactory-mediated response to TCA after 24-h Cu exposure.
Figure 6:
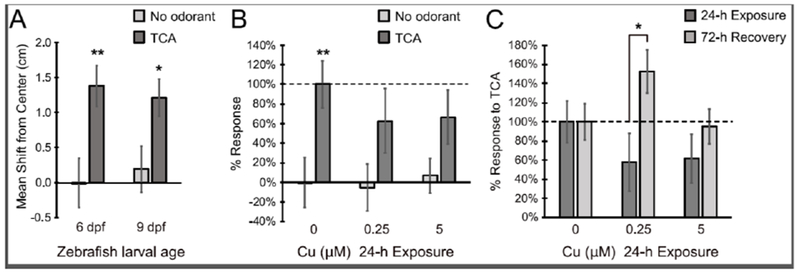
Olfactory-driven behavior assays showed loss and regeneration of olfaction after 24 h Cu exposure and following a 72 h recovery. A. Behavioral analysis of unexposed 6 dpf and 9 dpf control larvae to no odorant (embryo media) and taurocholic acid (TCA) show significant aversive response to TCA at both ages. B. Behavioral assays of 24-h Cu-exposed larvae to no odorant control and TCA show decreased aversive response to TCA with increased Cu concentration. C. Behavioral response of zebrafish larvae to TCA after Cu exposure and following 72 h of recovery in EM. Graphs are normalized to 100% TCA response of age-matched larvae. Error bars = ± SE; n=4-15 individual trials, 10 fish per trial. Asterisks indicate statistically significant change at *p<0.05 and **p<0.01 significance levels.
To correlate recovery and regeneration of OSNs after Cu-induced damage with functional recovery, behavior assays were also performed on 9 dpf larvae after 72 hours of recovery in EM following 24-h Cu exposure. At 9 dpf, the olfactory-mediated responses to TCA were similar to that of 6 dpf larvae, with a significant mean shift away from center of 1.21 cm compared to the no odorant controls (control mean shift = 0.19 cm, p<0.05; Fig. 6A). After normalizing for behavioral response differences due to age, we compared responses to TCA between the 24-h Cu-exposed larvae and those after 72 hours of recovery. A significant increase in the behavioral response to TCA from 58% to 153% (p<0.05) was observed in 9 dpf larvae after 72 hours of recovery from 0.25 μM Cu-induced injury (Fig. 6C; Supplemental Fig. 3C). Similarly, following 5 μM Cu exposure, the olfactory response to TCA increased from 62% to 95% after 72-h recovery (Fig. 6C; Supplemental Fig. 3C). Even though this increase was not statistically significant due to variation among the trials, the behavioral response to TCA after 72-h recovery was significant when compared to its corresponding no-odorant control (Supplemental Fig. 3C, p<0.05). Taken together, these behavioral experiments suggest that the ciliated OSNs were able to restore their function after 72 hours of recovery after 24-h Cu exposure.
DISCUSSION
In the current study, we used transgenic zebrafish with confocal imaging and phenotypic anchoring of olfactory behavior to better understand cellular mechanisms of Cu olfactory injury and recovery of specific neuron populations. In this regard, previous studies of toxic effects of metals on the olfactory sensory epithelium offish generally relied on histology, immunostaining, scanning electron microscopy (SEM), and behavioral studies (Dew et al., 2014; Haverroth et al., 2015; Heffern et al., 2018; Lazzari et al., 2017; Williams et al., 2016) to distinguish between the various OSN populations. However, imaging of histological and/or immunostained tissue sections can yield low cellular-level resolution and difficulty in distinguishing different cell types that are interspersed within the olfactory sensory epithelium (Lazzari et al., 2017). Conversely, high-resolution SEM allows for detailed analysis on cellular and sub-cellular levels of individual cells (Hentig and Byrd-Jacobs, 2016), but a poor ability to visualize the entire population of a particular cell type within context of the entire sensory epithelium. Use of the Tg[OMP:RFP/TRPC2:Venus] double-transgenic zebrafish provides some distinct advantages over both immunohistological and SEM imaging studies to study olfactory toxicity. Larval zebrafish are easier and cheaper to maintain relative to adults and/or juveniles of larger fish species such as salmonids, and allows us to perform larger experiments with more experimental conditions. Due to the transparency of larval zebrafish, imaging of 5-9 dpf larvae can be performed in vivo or in situ on fixed samples, thus shortening the length of time for sample processing. Because it was unnecessary to section the olfactory sensory epithelium, having the ability to visualize entire neurons within context of the entire OSE in these transgenics (from the neuronal cell bodies to their axons into the olfactory bulb; Sato et al., 2005, 2007) allowed us to visualize morphological changes that occurred in the cells as well as changes in overall fluorescence in both OSN populations in the same individuals. We observed to varying degrees within both ciliated and microvillous OSNs the presence of round dense cell bodies, cell fragmentation, retraction or loss of axons, disorganized cell arrangements, and loss of cells and fluorescence signal intensity that were all hallmarks of cell death after Cu exposure.
Since the ultimate output of olfaction is behavior, assays for olfactory-mediated behavioral responses are effective in evaluating olfactory toxicity. However, behavioral studies are often beset with a large variation in responses among individual fish and trials. In addition, other factors besides loss of olfaction can alter behavioral responses from metal toxicity. Schamchuck et al. (2018) found more inconsistent olfactory-mediated behavioral responses to the presence of nucleotides at 6 dpf and 7 dpf compared to 5 dpf zebrafish larvae, while metal-induced loss of mechanosensory lateral line hair cells had been shown to affect rheotaxis (McNeil et al., 2014, Olivari et al., 2008), thus potentially affecting swimming behavior. Our behavioral data showing a decreased response to TCA supported our imaging results that showed ciliated OSN damage by Cu. Interestingly, the sensitivity of the behavioral assay was evident through olfaction recovery when no significant increase in relative fluorescence (OMP:RFP) was observed, suggesting that functional recovery of ciliated OSNs had occurred but was not evident through confocal imaging. Therefore, the use of multiple approaches in assessing olfactory toxicity proves valuable in providing a broader picture of how the olfactory system is affected, both physically and functionally.
Of interest in the present study was the finding of differential sensitivity of OSN classes to Cu injury, indicating a preferential loss of ciliated relative to microvillous OSNs after acute exposures to low Cu. Others have observed a differing sensitivity between ciliated and microvillous OSNs with respect to chemical exposures of fish. In a recent study using adult zebrafish, Lazzari et al. (2017) observed a greater decrease and recovery in ciliated than in microvillous OSNs after 4 d exposure to a low sublethal 30 μg/L Cu. These results were consistent with those observed in other fish species such as coho salmon (Baldwin et al., 2003; Sandahl et al., 2007; Williams et al., 2016), goldfish (Kolmakov et al., 2009), yellow perch (Dew et al., 2014), and fathead minnow (Dew et al., 2014). However, it is important to point out that we observed damage to microvillous OSNs at high Cu concentrations and/or extended duration of exposure and increasing severity of cell loss in both OSN populations that correlated with cumulative Cu exposures. This dose-dependent nature of Cu toxicity on ciliated and microvillous OSNs supported data that had been reported in fathead minnows (Dew et al., 2014) in which microvillous OSNs were impaired only at the highest concentration of Cu tested (20 μg/L). Collectively, these data suggest that ciliated OSNs are more sensitive to damage by Cu, while damage to microvillous OSNs likely requires a threshold level of Cu accumulation in the OSE before cellular loss is observed.
The pattern of OSN recovery with increased Cu exposure duration was unexpected. Specifically, following OSN death from 24-hour Cu exposure, significant regeneration of microvillous OSNs occurred whereas no recovery was observed in the ciliated OSN populations. This, despite the fact that the percentages of initial OSN loss and damage were comparable (p>0.05) between the two populations across all except for the highest Cu concentration tested. Our behavioral data verified full recovery of olfaction after 72 h following a low Cu exposure, but less recovery after a high Cu exposure. Because TCA preferentially, but not exclusively, targets ciliated OSNs (Hansen et al., 2003), a functional recovery of the ciliated OSNs likely occurred, even though no significant changes in fluorescence was observed. These data suggest also that several mechanisms might have modulated the recovery pattern between the two populations of OSNs. One explanation for this difference could be that ciliated OSNs regenerated at a slower rate than microvillous OSNs. However, some recovery of ciliated OSNs was observed at the lowest Cu concentration tested. Secondly, there may exist a threshold in the number of OSNs present before the cellular regeneration process is activated. Ciliated OSNs generally outnumber microvillous OSNs (Hansen and Zielinski, 2005; Lazzari et al., 2017) in the OSE of teleosts, including both adult (Lazzari et al., 2017) and larval zebrafish (the present report and Sato et al., 2005, 2007). While both populations of OSNs were damaged by Cu in the present study, more ciliated than microvillous OSNs generally remained in the OSE after Cu toxicity. Even after exposure to high Cu concentrations, some ciliated OSNs were still visible, whereas fewer microvillous OSNs remained intact (Fig. 2A,B). It is possible that the remaining ciliated OSNs were sufficient to inhibit the regeneration process, while rapid regeneration occurred in microvillous OSNs in order to regain their threshold level. Likewise, our behavioral data indicate partial functional recovery by the ciliated OSNs, possibly due to repair of the damaged cells that remained in the OSE, rather than through regeneration of new OSNs.
The significant increase observed in cell proliferation surrounding the OSE following 24 hours of recovery after Cu exposure reflect the activity of neuronal precursors dividing after OSN injury and death to regenerate new replacement OSNs (Lazzari et al., 2017; Mackay-Sim and Kittel, 1991; Yanagi et al., 2004). The proliferation of basal olfactory stem cells (OSCs) and differentiation into new OSNs in the fish olfactory system typically occurs throughout growth and maintenance of the OSE, and OSNs that reach the end of their life span are replaced by newly differentiated OSNs at a basal rate (Hansen and Zielinski, 2005). In order to maintain a predetermined population size of OSNs, the mature OSNs within the OSE normally inhibit the proliferation and differentiation of the neuronal OSC precursors (Wu et al., 2003). When the OSNs are lost due to cell death and damage, the OSCs re-enter the cell cycle to proliferate in order to rapidly regenerate new OSNs (Lazarri et al., 2017; Mackay-Sim and Kittel, 1991; Yanagi et al., 2004). Studies in mice describe a similar process (Ducray et al., 2002; Leung et al., 2007). In the mammalian OSE, two types of OSCs are present: horizontal basal cells (HBCs) and globose basal cells (GBCs) (Graziadei and Graziadei, 1979). GBCs proliferate to produce neuronal precursors that differentiate into postmitotic OSNs (Bermingham-McDonogh and Reh, 2011), while HBCs are slow-cycling multipotent stem cells that remain quiescent during normal neuronal turnover or acute OSN damage (Holbrook et al., 1995; Leung et al., 2007). After injury that depletes GBCs, HBCs transiently proliferate and differentiate into all mature OSE cell types (Leung et al., 2007). In fish, however, the OSC populations are poorly characterized, with no clear distinction between the different types (such as HBCs vs. GBCs). Therefore, a possible mechanism to explain the pattern of regeneration observed after high Cu exposure in larval zebrafish in the present study could be that loss of OSNs led to rapid proliferation of these neuronal OSC precursors. Since more microvillous than ciliated OSNs were depleted in the OSE of zebrafish, these precursors likely differentiated into microvillous OSNs in lieu of ciliated OSNs. The recovery of ciliated OSNs with low Cu exposure supports this hypothesis, as under the acute low Cu exposure paradigm, microvillous OSNs were not damaged, thus only ciliated OSNs needed replacement by the proliferating precursors. Further studies would be necessary to characterize the OSC populations and OSN regeneration to fully test this hypothesis.
In conclusion, we have demonstrated the loss of ciliated and microvillous OSNs in zebrafish upon exposure to Cu, a common contaminant in surface waters. Our studies using double-transgenic zebrafish indicate that with extensive OSN damage by Cu, regeneration of microvillous OSNs may occur exceeding ciliated OSNs, likely via increased proliferation of the cellular reservoir of neuronal OSC precursors. Behavioral analyses support and extend our imaging studies to reveal functional recovery of OSNs after Cu damage that was not evident through confocal imaging. Collectively, our studies shed light on the nature of cellular injury in fish exposed to metals, and highlight the value of using transgenic zebrafish concomitantly with specific behavioral assays to study environmental chemical-mediated olfactory injury and recovery. Ultimately, these approaches help us better understand and identify sensitive olfactory neuron populations relevant to fish exposed to environmental pollutants.
Supplementary Material
Highlights.
Transgenic zebrafish enable visualization and characterization of copper olfactory injury and recovery
Copper causes differential effects on toxicity and recovery to olfactory sensory neuron populations
Olfactory sensory neuronal precursors may limit olfactory recovery by copper in zebrafish larvae
Acknowledgements
The authors would like to acknowledge and thank all the members of the Gallagher laboratory for their support and technical assistance. This work was supported by the National Institutes of Health Superfund Research Program [P42-ES004696] and the National Science Foundation (NSF 339637). Confocal imaging was conducted at the Molecular Analysis Facility, a National Nanotechnology Coordinated Infrastructure site at the University of Washington, which is supported in part by the National Science Foundation (grant ECC-1542101), the University of Washington, the Molecular Engineering and Sciences Institute, the Clean Energy Institute, and the National Institutes of Health.
Abbreviations:
- ALU:
arbitrary light units
- BrdU:
bromodeoxyuridine
- Cu:
copper
- DMSO:
dimethyl sulfoxide
- dpf:
days postfertilization
- EM:
embryo media
- GBCs:
globose basal cells
- HBCs:
horizontal basal cells
- OMP:
olfactory marker protein
- OSC:
olfactory stem cell
- OSE:
olfactory sensory epithelium
- OSN:
olfactory sensory neurons
- PBS:
phosphate buffered saline
- RFP:
red fluoresecent protein
- SE:
standard error
- SEM:
scanning electron microscopy
- TCA:
taurocholic acid
- Tg:
transgenic
- TRPC2:
transient receptor potential channel C2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Baldwin DH, Sandahl JF, Labenia JS, and Scholz NL. 2003. Sublethal effects of copper on coho salmon: impacts on nonoverlapping receptor pathways in the peripheral olfactory nervous system. Environ Toxicol Chem, 22(10), 2266–74. [DOI] [PubMed] [Google Scholar]
- Baldwin DH, Tatara CP, and Scholz NL, 2011. Copper-induced olfactory toxicity in salmon and steelhead: extrapolation across species and rearing environments. Aquat Toxicol. 101(1): 295–7. [DOI] [PubMed] [Google Scholar]
- Bandoh H, Kida I, and Ueda H 2011. Olfactory responses to natal stream water in sockeye salmon by BOLD fMRI. PLoS One, 6(1), e16051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beites CL, Kawauchi S, Crocker CE, and Calof AL (2005). Identification and molecular regulation of neural stem cells in the olfactory epithelium. Experimental cell research, 306(2), 309–316. [DOI] [PubMed] [Google Scholar]
- Bermingham-McDonogh O, and Reh TA 2011. Regulated reprogramming in the regeneration of sensory receptor cells. Neuron, 71(3), 389–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blechinger SR, Kusch RC, Haugo K, Matz C, Chivers DP, and Krone PH 2007Brief embryonic cadmium exposure induces a stress response and cell death in the developing olfactory system followed by long-term olfactory deficits in juvenile zebrafish. Toxicology and applied pharmacology, 224(1), 72–80. [DOI] [PubMed] [Google Scholar]
- Byrd CA, and Brunjes PC 1995. Organization of the olfactory system in the adult zebrafish: histological, immunohistochemical, and quantitative analysis. Journal of Comparative Neurology, 358(2), 247–259. [DOI] [PubMed] [Google Scholar]
- Byrd CA, and Brunjes PC 1998. Addition of new cells to the olfactory bulb of adult zebrafish. Annals of the New York Academy of Sciences, 855(1), 274–276. [DOI] [PubMed] [Google Scholar]
- Cooper JC, Scholz AT, Horrall RM, Hasler AD, Madison DM, 1976. Experimental confirmation of the olfactory hypothesis with homing, artificially imprinted coho salmon (Oncorhynchus kisutch). J. Fish. Board Can 33 (4), 703–710. [Google Scholar]
- Davis AP, Shokouhian M, and Ni S 2001. Loading estimates of lead, copper, cadmium, and zinc in urban runoff from specific sources. Chemosphere, 44(5), 997–1009. [DOI] [PubMed] [Google Scholar]
- Dew WA, Azizishirazi A, and Pyle GG 2014. Contaminant-specific targeting of olfactory sensory neuron classes: Connecting neuron class impairment with behavioural deficits. Chemosphere, 112, 519–525. [DOI] [PubMed] [Google Scholar]
- Dittman A, and Quinn T (1996). Homing in Pacific salmon: mechanisms and ecological basis. Journal of Experimental Biology, 199(1), 83–91. [DOI] [PubMed] [Google Scholar]
- Døving KB, Hansson KA, and Backstrom T 2011. Visualizing a set of olfactory sensory neurons responding to a bile salt. Journal of Experimental Biology, 214(1), 80–87. [DOI] [PubMed] [Google Scholar]
- Ducray A, Bondier JR, Michel G, Bon K, Propper A, and Kastner A 2002. Recovery following peripheral destruction of olfactory neurons in young and adult mice. European Journal of Neuroscience, 15(12), 1907–1917. [DOI] [PubMed] [Google Scholar]
- Good JC 1993. Roof runoff as a diffuse source of metals and aquatic toxicity in storm water. Water Science and Technology, 28(3-5), 317. [Google Scholar]
- Graziadei PP and Graziadei GA 1979. Neurogenesis and neuron regeneration in the olfactory system of mammals. I. Morphological aspects of differentiation and structural organization of the olfactory sensory neurons. J Neurocytol, 8(1): 1–18. [DOI] [PubMed] [Google Scholar]
- Hansen A, Rolen SH, Anderson K, Morita Y, Caprio J, and Finger TE, 2003Correlation between olfactory recepto cell type and function in the channel catfish. Journal of Neuroscience, 23(28), 9328–9339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen A, Anderson KT, and Finger TE 2004. Differential distribution of olfactory receptor neurons in goldfish: structural and molecular correlates. Journal of Comparative Neurology, 477(4), 347–359. [DOI] [PubMed] [Google Scholar]
- Hansen A, and Zielinski BS 2005. Diversity in the olfactory epithelium of bony fishes: development, lamellar arrangement, sensory neuron cell types and transduction components. Journal of neurocytology, 34(3-5), 183–208. [DOI] [PubMed] [Google Scholar]
- Hara TJ, 1992. Mechanisms of Olfaction In Fish Chemoreception. Springer, Dordrecht, pp. 150–170. [Google Scholar]
- Hara TJ 1994. The diversity of chemical stimulation in fish olfaction and gustation. Reviews in Fish Biology and Fisheries, 4(1), 1–35. [Google Scholar]
- Haverroth GM, Welang C, Mocelin RN, Postay D, Bertoncello KT, Franscescon F, … and Dalla Corte CL 2015. Copper acutely impairs behavioral function and muscle acetylcholinesterase activity in zebrafish (Danio rerio). Ecotoxicology and environmental safety, 122, 440–447. [DOI] [PubMed] [Google Scholar]
- Heffern K, Tierney K and Gallagher EP, 2018. Comparative effects of cadmium, zinc, arsenic and chromium on olfactory-mediated neurobehavior and gene expression in larval zebrafish (Danio rerio). Aquatic Toxicology. 201, 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentig JT and Byrd-Jacobs CA, 2016. Exposure to zinc sulfate results in differential effects on olfactory sensory neuron subtypes in adult zebrafish. International journal of molecular sciences, 17(9), 1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook EH, Szumowski KEM, and Schwob JE 1995. An immunochemical, ultrastructural, and developmental characterization of the horizontal basal cells of rat olfactory epithelium. Journal of Comparative Neurology, 363(1), 129–146. [DOI] [PubMed] [Google Scholar]
- Kolmakov NN, Hubbard PC, Lopes O, and Canario AV 2009. Effect of acute copper sulfate exposure on olfactory responses to amino acids and pheromones in goldfish (Carassius auratus). Environmental science and technology, 43(21), 8393–8399. [DOI] [PubMed] [Google Scholar]
- Lakhina V, Marcaccio CL, Shao X, Lush ME, Jain RA, Fujimoto E, Bonkowsky JL, Granato M and Raper JA, 2012. Netrin/DCC signaling guides olfactory sensory axons to their correct location in the olfactory bulb. Journal of Neuroscience, 32(13), 4440–4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzari M, Bettini S, Milani L, Maurizii MG, and Franceschini V 2017. Differential response of olfactory sensory neuron populations to copper ion exposure in zebrafish. Aquatic toxicology, 183, 54–62. [DOI] [PubMed] [Google Scholar]
- Leung CT, Coulombe PA, and Reed RR 2007. Contribution of olfactory neural stem cells to tissue maintenance and regeneration. Nature neuroscience, 10(6), 720. [DOI] [PubMed] [Google Scholar]
- Lindsay SM, and Vogt RG 2004. Behavioral responses of newly hatched zebrafish (Danio rerio) to amino acid chemostimulants. Chemical Senses, 29(2), 93–100. [DOI] [PubMed] [Google Scholar]
- Ma EY, Rubel EW, and Raible DW 2008. Notch signaling regulates the extent of hair cell regeneration in the zebrafish lateral line. Journal of Neuroscience, 28(9), 2261–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay-Sim A, and Kittel PW 1991. On the life span of olfactory receptor neurons. European Journal of Neuroscience, 3(3), 209–215. [DOI] [PubMed] [Google Scholar]
- McIntyre JK, Baldwin DH, Beauchamp DA, and Scholz NL. 2012. Low-level copper exposures increase visibility and vulnerability of juvenile coho salmon to cutthroat trout predators. Ecol Appl, 22(5), 1460–71. [DOI] [PubMed] [Google Scholar]
- McNeil PL, Boyle D, Henry TB, Handy RD, and Sloman KA 2014. Effects of metal nanoparticles on the lateral line system and behaviour in early life stages of zebrafish (Danio rerio). Aquatic toxicology. 152: 318–323. [DOI] [PubMed] [Google Scholar]
- Michel WC, Sanderson MJ, Olson JK, Lipschitz DL (2003) Evidence of a novel transduction pathway mediating detection of polyamines by the zebrafish olfactory system. J Exp Biol 206:1697–1706. [DOI] [PubMed] [Google Scholar]
- Miyasaka N, Sato Y, Yeo SY, Hutson LD, Chien CB, Okamoto H, and Yoshihara Y, 2005. Robo2 is required for establishment of a precise glomerular map in the zebrafish olfactory system. Development. 132(6), 1283–1293. [DOI] [PubMed] [Google Scholar]
- Olivari FA, Hernández PP, and Allende ML 2008. Acute copper exposure induces oxidative stress and cell death in lateral line hair cells of zebrafish larvae. Brain research. 1244, 1–12. [DOI] [PubMed] [Google Scholar]
- Quinn TP 2011. The behavior and ecology of Pacific salmon and trout. UBC press. [Google Scholar]
- Ren JQ, McCarthy WR, Zhang H, Adolph AR, Li L 2002. Behavioral visual responses of wild-type and hypopigmented zebrafish. Vision Res, 42: 293–299. [DOI] [PubMed] [Google Scholar]
- Renaud O, Herbomel P, and Kissa K, 2011. Studying cell behavior in whole zebrafish embryos by confocal live imaging: application to hematopoietic stem cells. Nature protocols. 6(12), 1897. [DOI] [PubMed] [Google Scholar]
- Rolen SH, Sorensen PW, Mattson D, Caprio J 2003. Polyamines as olfactory stimuli in the goldfish Carassius auratus. J Exp Biol 206:1683–1696 [DOI] [PubMed] [Google Scholar]
- Sandahl JF, Baldwin DH, Jenkins JJ, and Scholz NL 2007. A sensory system at the interface between urban stormwater runoff and salmon survival. Environmental Science and Technology, 41(8), 2998–3004. [DOI] [PubMed] [Google Scholar]
- Sakamoto M, Imayoshi I, Ohtsuka T, Yamaguchi M, Mori K, and Kageyama R (2011). Continuous neurogenesis in the adult forebrain is required for innate olfactory responses. Proceedings of the National Academy of Sciences, 108(20), 8479–8484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Miyasaka N, and Yoshihara Y 2005. Mutually exclusive glomerular innervation by two distinct types of olfactory sensory neurons revealed in transgenic zebrafish. Journal of Neuroscience, 25(20), 4889–4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Miyasaka N, and Yoshihara Y 2007. Hierarchical regulation of odorant receptor gene choice and subsequent axonal projection of olfactory sensory neurons in zebrafish. Journal of Neuroscience, 27(7), 1606–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamchuk AL, Allison WT, and Tierney KB 2017. The Importance of Olfactory and Motor Endpoints for Zebrafish Models of Neurodegenerative Disease In Animal Models for the Study of Human Disease (Second Edition), p. 525–554. [Google Scholar]
- Shamchuk AL, Blunt BJ, Lyons DD, Wang MQ, Gasheva A, Lewis CR, Tomlin K, Hazard ES, Hardiman GT and Tierney KB, 2018. Nucleobase-containing compounds evoke behavioural, olfactory, and transcriptional responses in model fishes. FACETS. 3(1), 79–102. [Google Scholar]
- Shaw BJ, and Handy RD 2011. Physiological effects of nanoparticles on fish: a comparison of nanometals versus metal ions. Environment International, 37(6), 1083–1097. [DOI] [PubMed] [Google Scholar]
- Sutterlin AM, Gray R, 1973. Chemical basis for homing of Atlantic Salmon (Salmo salar) to a hatchery. J. Fish. Board Can 30 (7), 985–989. [Google Scholar]
- Tierney KB, Baldwin DH, Hara TJ, Ross PS, Scholz NL, and Kennedy CJ 2010. Olfactory toxicity i fishes. Aquatic toxicology. 96(1), 2–26. [DOI] [PubMed] [Google Scholar]
- Tilton FA, Bammler TK, and Gallagher EP, 2011. Swimming impairment and acetylcholinesterase inhibition in zebrafish exposed to copper or chlorpyrifos separately, or as mixtures. Comparative Biochemistry and Physiology Part C: Toxicology and Pharmacology. 153(1), 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilton F, Tilton SC, Bammler TK, Beyer R, Farin F, Stapleton PL, and Gallagher EP, 2008. Transcriptional biomarkers and mechanisms of copper-induced olfactory injury in zebrafish. Environ Sci Technol 42(24), 9404–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Bammler TK, Beyer RP, and Gallagher EP, 2013. Copper-induced deregulation of microRNA expression in the zebrafish olfactory system. Environ Sci Technol 47(13), 7466–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CR, MacDonald JW, Bammler TK, Paulsen MH, Simpson CD, and Gallagher EP, 2016. From the Cover: Cadmium Exposure Differentially Alters Odorant-Driven Behaviors and Expression of Olfactory Receptors in Juvenile Coho Salmon (Oncorhynchus kisutch). Toxicological Sciences. 154(2), 267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HH, Ivkovic S, Murray RC, Jaramillo S, Lyons KM, Johnson JE, and Calof AL 2003. Autoregulation of neurogenesis by GDF11. Neuron, 37(2), 197–207. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Shibata H, and Ueda H, 2013. Olfactory homing of chum salmon to stable compositions of amino acids in natal stream water. Zoological science. 30(8), 607–612. [DOI] [PubMed] [Google Scholar]
- Yanagi S, Kudo H, Doi Y, Yamauchi K, and Ueda H 2004. Immunohistochemical demonstration of salmon olfactory glutathione S-transferase class pi (N24) in the olfactory system of lacustrine sockeye salmon during ontogenesis and cell proliferation. Anatomy and embryology, 208(3), 231–238. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


