Abstract
Objective(s).
The Gynecologic Oncology Group (GOG) examined the association between ERBB2 amplification and clinical covariates, tumor response, disease status post-chemotherapy, progression-free survival (PFS), and overall survival (OS) in epithelial ovarian cancer (EOC).
Methods.
Women with suboptimally-resected, advanced stage EOC who participated in GOG-111, a multi-center randomized phase III trial of cyclophosphamide + cisplatin versus paclitaxel + cisplatin, and provided a tumor block through the companion protocol GOG-9404 were eligible. ERBB2 amplification was examined using fluorescence in situ hybridization (FISH) with probes for ERBB2 and the centromere of chromosome 17 (CEP17).
Results.
ERBB2 amplification, defined as >2 copies of ERBB2/CEP17, was a rare event in EOC with 7% (9/133) of women exhibiting between 2.2 and 33.7 copies of ERBB2/CEP17, and was not associated with patient age, race, GOG performance status, stage, cell type, grade, measurable disease status, volume of ascites, tumor response or disease status post-chemotherapy. Women with >2 verses ≤2 copies of ERBB2/CEP17 did not have a reduced risk of disease progression (hazard ratio [HR] = 0.56; 95% confidence interval [CI] = 0.27–1.16; p = 0.120) or death (HR = 0.57; 95% CI = 0.26–1.23; p = 0.152), and ERBB2 amplification was not an independent prognostic factor for PFS or OS. ERBB2 amplification, defined as >4 copies of ERBB2/nuclei, was observed in 9% (12/133) of women with levels ranging from 4.2 to 49.2 copies of ERBB2/nuclei, and was associated with older age and volume of ascites, but not with the other clinical covariates or outcome.
Conclusion(s).
ERBB2 amplification is a rare event and has no predictive or prognostic value in suboptimally-resected, advanced stage EOC treated with platinum-based combination chemotherapy.
Keywords: ERBB2, Gene amplification, Ovary, Carcinogenesis, FISH
Introduction
Ovarian cancer is the second most common gynecologic malignancy, and the leading cause of cancer related death among the gynecologic malignancies [1,2]. It is estimated that 21,650 new cases of ovarian cancer will be diagnosed in the United States in 2008 and that 15,520 women will die from the disease. Overexpression of the ERBB2 proto-oncogene occurs in 11–30% of epithelial ovarian cancers (EOC) [3-10]. Expression of ERBB2 has traditionally been evaluated by immunohistochemistry with inconsistent prognostic results for epithelial ovarian cancer [5-9]. Some studies of EOC associate increased ERBB2 expression intensity with decreased median and overall survival [5,7]. Other studies have shown no relationship between ERBB2 expression and survival among epithelial ovarian cancer [8].
Because of these disparate results, fluorescence in situ hybridization (FISH) analysis of ERBB2 amplification has been applied to a series of EOCs in an attempt to alleviate the inherent difficulty in quantifying immunohistochemical staining [11,12]. Amplification of ERBB2 in early stage ovarian neoplasms has been reported as infrequent at around 6.7% [13]. In advanced stage EOC, FISH analysis revealed that 22% to 71% of the cases exhibited ERBB2 amplification [3,4]. Unfortunately, since their primary objective was to correlate immunohistochemical staining for ERBB2 protein with amplification by FISH analysis, the majority of these studies suffered from small sample sizes ranging from 23 to 43 women [3,4]. In addition, these studies did not evaluate clinical correlates such as progression-free survival (PFS) or overall survival (OS).
High throughput techniques such as tissue microarray (TMA) have allowed analysis of large number of specimens for ERBB2 amplification. FISH analysis by TMA of invasive ovarian cancers of all stages revealed a 24% amplification rate [14,15]. In a series of 173 invasive ovarian cancers of varying stages and histologies, only a 7.4% amplification rate was found [14]. While in a series of 103 high grade advanced stage EOC of various histologies, a 33.3% amplification rate of ERBB2 was observed [15]. Both studies also revealed a lack of correlation between ERBB2 overexpression by immunohistochemistry and ERBB2 amplification by FISH analysis. Neither study demonstrated an association between ERBB2 amplification or overexpression and PFS or OS [14,15].
To further elucidate the predictive and prognostic significance of ERBB2 amplification in EOC, the Gynecologic Oncology Group (GOG) analyzed primary tumor from 133 women with suboptimally-resected, advanced stage, EOC who participated in GOG protocol 111 (GOG-111), a multi-center phase III randomized trial of cyclophosphamide and cisplatin vs. paclitaxel and cisplatin [16] and a companion protocol GOG-9404 which collected a formalin-fixed and paraffin-embedded (FFPE) primary tumor block from women enrolled in GOG-111 for translational research.
Materials and methods
Patients
The eligibility criteria for GOG-9404 were as follows. Women with previously-untreated, suboptimally-resected, advanced stage EOC who participated in the randomized phase III treatment protocol GOG-111 [16], completed the chemotherapy portion of the protocol, provided adequate follow-up information and a had representative FFPE primary tumor block from the initial surgical staging for submission for translational research. Patients provided written informed consent consistent with federal, state and local institutional requirements for GOG-111 protocol. In addition, GOG-111 and GOG-9404 were approved by the GOG, the Cancer Therapy Evaluation Program of the National Cancer Institute and the institutional review board at each of the participating GOG institutions in accordance with assurances filed with and approved by the Department of Health and Human Services. Histologic diagnosis was confirmed centrally by the GOG Pathology Committee.
FISH procedure
FISH was performed by Esoterix Oncology (4509 Freidrich Lane, Building 1, Suite 100, Austin, TX 78744) in FFPE primary tumor using a ERBB2 gene (17q.11.2–17q12) probe and a centromeric a-satellite probe (D17Z1) specific for chromosome17 (CEP17). The ERBB2 and CEP17 probe were simultaneously labeled with SpectrumOrange (peak excitation/emission = 559/588) and SpectrumGreen (peak excitation/emission = 509/538), respectively. The slide-mounted specimens were immersed in 70% formamide/2× SSC, pH 7.0 for 5 min at 73 °C, then dehydrated in 70% ethanol for 1 min, 85% ethanol for 1 min, and 100% ethanol for 1 min. Probe mixture was prepared as directed by Vysis' protocol. Ten ml of probe mixture was applied onto pre-warmed (45 °C) slide-section. Hybridization was carried out in a humidified box for 14–18 h at 37 °C followed by post hybridization wash with Wash buffer I (0.4× SSC/0.3% NP-40) at 72 °C for 2 min, Wash Buffer II (2× SSC/0.1%NP-40) at room temperature for 1 min. Counter stain was performed with 15 ml of DAPI (4′,6-diamidino-2-phenylindole) (a mixture of DAPI I and DAPI II, Vysis).
FISH scoring criteria
A minimum of 100 cells were counted for ERBB2 and CEP17 signals by coauthor (S.F.). Mean ERBB2 copies were normalized by number of CEP17 copies in order to confirm whether increased number of ERBB2 signal was result from gene amplification or aneusomy of chromosome 17. ERBB2/chromosome 17 copies were categorized as normal (0–2.0) and amplified (>2.0). Simultaneously, mean number of ERBB2 copies per nucleus were calculated and categorized as normal (0–4.0) and amplified (>4.0).
Platinum-based chemotherapy
Women on GOG-111 were randomly allocated to receive six cycles of cisplatin and cyclophosphamide or six cycles of paclitaxel and cisplatin every three weeks as previously reported [16].
End points
Evaluation criteria and definitions for tumor response, disease status post-chemotherapy, PFS and OS were previously reported [16,17].
Statistical methods
Data were analyzed using SPSS versions 14.0 (SPSS Inc., Chicago, IL) and SAS® version 9.1 software (SAS Institute, Inc. Cary, NC). All tests were two-sided and the level of significance was set at 0.05. Associations between categorical variables were evaluated using Fisher's exact test [18,19]. PFS and OS were estimated using the Kaplan–Meier method [20] and the logrank test was used to compare the survival distributions between groups categorized by ERBB2 amplification [21]. Unadjusted and adjusted Cox proportional hazards regression analyses were performed to model the association between ERBB2 amplification and PFS or OS [22,23]. The multivariate analyses were performed with clinical covariates added during block 1 and ERBB2 amplification added during block 2 of model development.
Results
GOG-9404 was a retrospective translational research protocol designed to examine p53 and ERBB2 in advanced stage epithelial ovarian cancer and correlations with prognostic factors and treatment outcome. The results of the associations between p53 overexpression and clinical outcome were the subject of a separate publication [17]. The characteristics of 133 women who participated in this study are summarized in Table 1 and are representative of that observed in the entire GOG-111 cohort [16]. At the time of the final analysis, seven women were alive with no evidence of the disease, five women were alive with disease progression and 121 women died. Among those who died, 97.5% of the deaths were attributed to disease progression, 0.8% were due to treatment, and 1.7% were caused by something other than disease or treatment. Median PFS and OS times for this cohort were 16.8 and 34.7 months, respectively.
Table 1.
Clinical characteristics
| Characteristics | Cases | % |
|---|---|---|
| Age (in years) median (range) 59.5 (21.7–78.6) | ||
| <50 | 36 | 27.1 |
| 50–59 | 33 | 24.8 |
| 60–69 | 46 | 34.6 |
| ≥70 | 18 | 13.5 |
| Performance | ||
| Asymptomatic | 41 | 30.8 |
| Symptomatic | 92 | 69.2 |
| Tumor stage | ||
| III | 84 | 63.2 |
| IV | 49 | 36.8 |
| Histologic cell type | ||
| Serous | 94 | 70.7 |
| Endometrioid | 12 | 9.0 |
| Mucinous | 5 | 3.8 |
| Clear cell | 3 | 2.3 |
| Other | 19 | 14.3 |
| Tumor grade | ||
| 1 Well differentiated | 6 | 4.5 |
| 2 Moderately differentiated | 58 | 43.6 |
| 3 Poorly differentiated/not specified | 69 | 51.9 |
| Gross residual disease | ||
| Measurable | 80 | 60.2 |
| Non-measurable | 53 | 39.8 |
| Ascites | ||
| <100 ml | 47 | 35.3 |
| ≥100 ml | 86 | 64.7 |
| Treatment | ||
| Cyclophosphamide + cisplatin | 68 | 51.1 |
| Paclitaxel + cisplatin | 65 | 48.9 |
ERBB2 amplification defined as >2 copies of ERBB2/CEP17
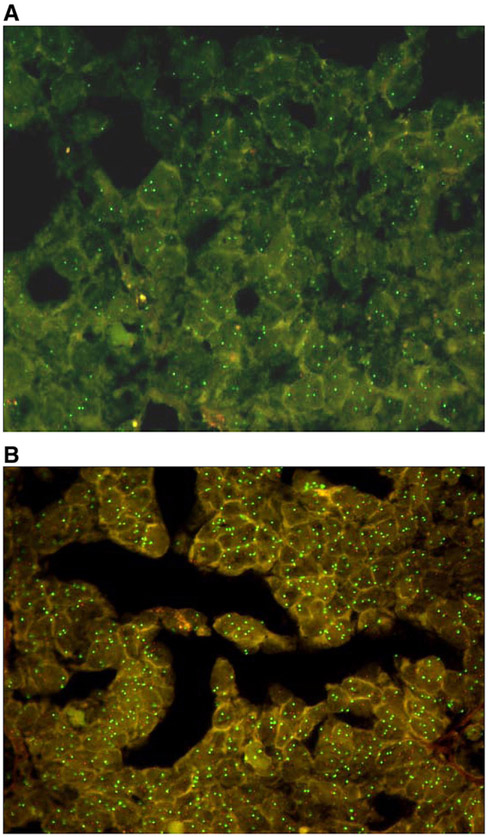
ERBB2 amplification was defined as >2 copies of ERBB2/CEP17, in accordance with previous studies in advanced epithelial ovarian cancer [15]. This was a rare event in EOC with only 7% (9/133) of women exhibiting between 2.2 and 33.7 copies of ERBB2/CEP17. Representative photomicrographs are provided that illustrate normal (Fig. 1A) and amplified ERBB2 (Fig. 1B) in the GOG-9404 cohort. There was no evidence to suggest that ERBB2 amplification, defined as >2 copies of ERBB2/CEP17, was associated with patient age, race/ethnicity, GOG performance status, stage, cell type, grade, measurable disease status or volume of ascites (Table 2), or with tumor response (Table 3), disease status post-chemotherapy (Table 3), PFS (Fig. 2A) or OS (Fig. 2B).
Fig. 1.
FISH analysis. Representative FISH analysis of ERBB2 labeled with SpectrumOrange and CEP17 labeled with SpectrumGreen illustrating a normal ratio (A), and amplification of ERBB2 (B).
Table 2.
Association between ERBB2 amplification and clinical characteristics
| >2 ERBB2/CEP17 copies |
>4 Copies of ERBB2/nuclei |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No |
Yes |
p-valuea | No |
Yes |
p-valuea | |||||
| Cases | % | Cases | % | Cases | % | Cases | % | |||
| Age (in years) | NS | 0.010 | ||||||||
| <50 | 34 | 94.4 | 2 | 5.6 | 36 | 100.0 | 0 | 0.0 | ||
| 50-59 | 32 | 97.0 | 1 | 3.0 | 30 | 90.9 | 3 | 9.1 | ||
| 60-69 | 44 | 95.7 | 2 | 4.3 | 42 | 91.3 | 4 | 8.7 | ||
| 70-79 | 14 | 77.8 | 4 | 22.2 | 13 | 72.2 | 5 | 27.8 | ||
| Race and ethnicity | NS | NS | ||||||||
| Caucasian | 112 | 94.1 | 7 | 5.9 | 109 | 91.6 | 10 | 8.4 | ||
| African American | 8 | 88.9 | 1 | 11.1 | 8 | 88.9 | 1 | 11.1 | ||
| Hispanic | 2 | 100.0 | 0 | 0.0 | 2 | 100.0 | 0 | 0.0 | ||
| Other | 2 | 66.7 | 1 | 33.32 | 2 | 66.7 | 1 | 33.3 | ||
| Performance status | NS | NS | ||||||||
| Asymptomatic | 39 | 95.1 | 2 | 4.9 | 37 | 90.2 | 4 | 9.8 | ||
| Symptomatic | 85 | 92.4 | 7 | 7.6 | 84 | 91.3 | 8 | 8.7 | ||
| Tumor stage | NS | NS | ||||||||
| III | 79 | 94.0 | 5 | 6.0 | 75 | 89.3 | 9 | 10.7 | ||
| IV | 45 | 91.8 | 4 | 8.2 | 46 | 93.9 | 3 | 6.1 | ||
| Histologic cell type | NS | NS | ||||||||
| Serous | 87 | 92.6 | 7 | 7.4 | 85 | 90.4 | 9 | 9.6 | ||
| Endometrioid | 12 | 100.0 | 0 | 0 | 11 | 91.7 | 1 | 8.3 | ||
| Mucinous | 4 | 80.0 | 1 | 20.0 | 4 | 80.0 | 1 | 20.0 | ||
| Clear cell | 3 | 100.0 | 0 | 0 | 3 | 100.0 | 0 | 0 | ||
| Other | 18 | 94.7 | 1 | 5.3 | 18 | 94.7 | 1 | 5.3 | ||
| Tumor grade | NS | NS | ||||||||
| 1 Well differentiated | 5 | 83.3 | 1 | 16.7 | 5 | 83.3 | 1 | 16.7 | ||
| 2 Moderately differentiated | 52 | 89.7 | 6 | 10.3 | 53 | 91.4 | 5 | 8.6 | ||
| 3 Poorly differentiated/not specified | 67 | 97.1 | 2 | 2.9 | 63 | 91.3 | 6 | 8.7 | ||
| Gross residual disease | NS | NS | ||||||||
| Measurable | 74 | 92.5 | 6 | 7.5 | 70 | 87.5 | 10 | 12.5 | ||
| Non-Measurable | 50 | 94.3 | 3 | 5.7 | 51 | 96.2 | 2 | 3.8 | ||
| Ascites | NS | 0.004 | ||||||||
| <100 ml | 43 | 91.5 | 4 | 8.5 | 38 | 80.9 | 9 | 19.1 | ||
| ≥100 ml | 81 | 94.2 | 5 | 5.8 | 83 | 96.5 | 3 | 3.5 | ||
NS: p-value >0.05.
Fisher's exact test.
Table 3.
Association between ERBB2 amplification and clinical outcomea
| ERBB2 Amplification | Progression-free survival |
Overall survival |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted model |
Adjusted modelb |
Unadjusted model |
Adjusted modelb |
|||||||||
| HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | |
| >2 Copies ERBB2/CEP17 | ||||||||||||
| No (N = 124) | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | ||||
| Yes (N = 9) | 0.56 | 0.27–1.16 | 0.120 | 0.56 | 0.26–1.19 | 0.132c | 0.57 | 0.26–1.23 | 0.152 | 0.53 | 0.23–1.17 | 0.115d |
| >4 Copies ERBB2/nucleus | ||||||||||||
| No | ||||||||||||
| Yes | 0.90 | 0.48–1.67 | 0.730 | 0.71 | 0.36–1.39 | 0.314e | 0.99 | 0.53–1.85 | 0.980 | 0.76 | 0.38–1.49 | 0.420f |
HR: hazard ratio estimated per 0.1 U change in copies of ERBB2/CEP17 or ERBB2/nuclei; 95% CI: 95% confidence interval.
Cox regression analysis for modeling the relative risk of disease progression or death. The goodness of fit of the overall model was evaluated using the likelihood ratio test and the association between the ERBB2 gene amplification and outcome was assessed using the Wald test.
Modeling was adjusted for patient age and stratified by tumor stage (III vs. IV), histologic subtype (clear cell or mucinous vs. other histologic subtypes), tumor grade (well differentiated vs. moderately differentiated vs. poorly differentiated), measurable disease (no vs. yes), ascites (<100 ml vs. ≥100 ml) and treatment regimen (cyclophosphamide plus cisplatin vs. paclitaxel plus cisplatin).
Inclusion of ERBB2 gene amplification defined as >2 copies of ERBB2/CEP17 into the Cox model for PFS reduced the −2 log likelihood from 991.773 to 989.168 and did not add significant prognostic information over the clinical covariates added during the first stage of model development (p=0.107).
Inclusion of ERBB2 gene amplification defined as >2 copies of ERBB2/CEP17 into the Cox model for OS reduced the −2 log likelihood from 955.613 to 952.696 and did not add significant prognostic information over the clinical covariates added during the first stage of model development (p=0.088).
Inclusion of ERBB2 gene amplification defined as >4 copies of ERBB2/nuclei into the Cox model for PFS reduced the −2 log likelihood from 991.773 to 990.693 and did not add significant prognostic information over the clinical covariates added during the first stage of model development (p=0.299).
Inclusion of ERBB2 gene amplification defined as >4 copies of ERBB2/nuclei into the Cox model for OS reduced the −2 log likelihood from 955.613 to 954.929 and did not add significant prognostic information over the clinical covariates added during the first stage of model development (p=0.408).
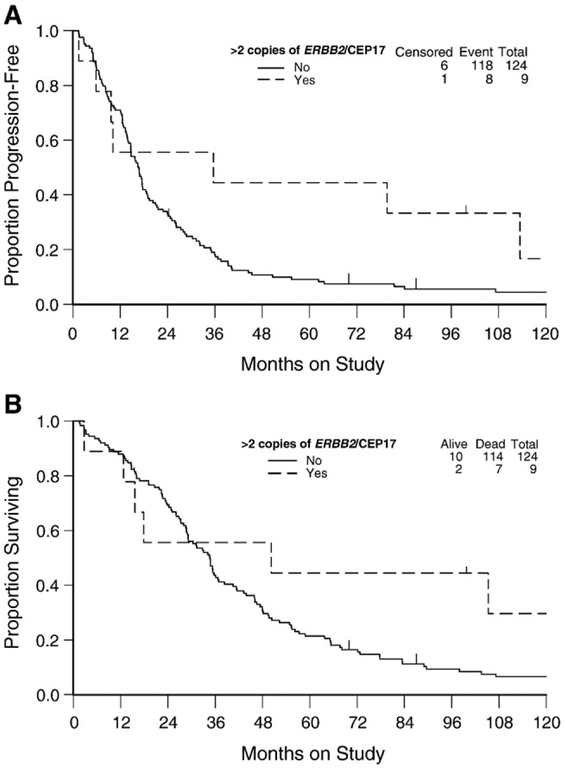
Fig. 2.
Kaplan–Meier estimate of progression-free survival (A) and overall survival (B) for women with or without ERBB2 amplification defined as >2 copies of ERBB2/CEP17. Censored indicates patients who were alive with no evidence of disease progression. Logrank test was used to evaluate the equality in progression-free survival (p = 0.115) and survival (p = 0.147) distributions between women with or without ERBB2 amplification defined as >2 copies of ERBB2/CEP17.
Women with >2 copies of ERBB2/CEP17 did not have a reduced risk of disease progression (hazard ratio [HR] = 0.56; 95% confidence interval [CI] = 0.27–1.16; p = 0.120) or death (HR = 0.57; 95% CI = 0.26–1.23; p = 0.152) compared with women with ≤2 copies of ERBB2/CEP17 (Table 4). After stratifying by patient age and adjusting for tumor stage, cell type, grade, measurable disease status, volume of ascites and treatment, ERBB2 amplification was not an independent prognostic factor for PFS (HR = 0.56; 95% CI = 0.26–1.19; p = 0.132) or OS (HR = 0.53; 95% CI = 0.23–1.17; p = 0.115).
Table 4.
Association between ERBB2 amplification and clinical response or results of second look laparotomya
| Tumor response |
Evidence of disease/progression after chemotherapy |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cases | No | Yes | p-value † | Cases | No | Yes | p-value ‡ | |||
| >2 copies ERBB2/CEP17 | NS | NS | ||||||||
| No | 72 | 19 (26.4) | 53 (73.6) | 20 PR 33 CR |
115 | 25 (21.7) | 90 (78.3) | 55+RL 35+DP |
||
| Yes | 6 | 2 (33.3) | 4 (66.7) | 3 PR 1 CR |
8 | 2 (25.0) | 6 (75.0) | 3+RL 3+DP |
||
| >4 copies ERBB2/nuclei | NS | NS | ||||||||
| No | 68 | 16 (23.5) | 52 (76.5) | 19 PR 33 CR |
112 | 26 (23.2) | 86 (76.8) | 54+RL 32+DP |
||
| Yes | 10 | 5 (50.0) | 5 (50.0) | 4 PR 1 CR |
11 | 1 (9.1) | 10 (90.9) | 4+RL 6+DP |
||
Response includes a complete response (CR) or a partial response (PR). Of the 80 women with measurable disease, only 78 were evaluated for tumor response. An additional 53 women had non-measurable disease and were not evaluable for tumor response.
Evidence of disease/progression after chemotherapy includes women with microscopic or gross disease at reassessment laparotomy (+RL) or with clinical evidence of disease progression documented during first-line treatment (+DP). Ten women did not undergo reassessment laparotomy as specified in the protocol due to patient refusal or medical contraindications.
Fisher's exact test; NS: p-value>0.05.
ERBB2 amplification defined as >4 copies of ERBB2/nuclei
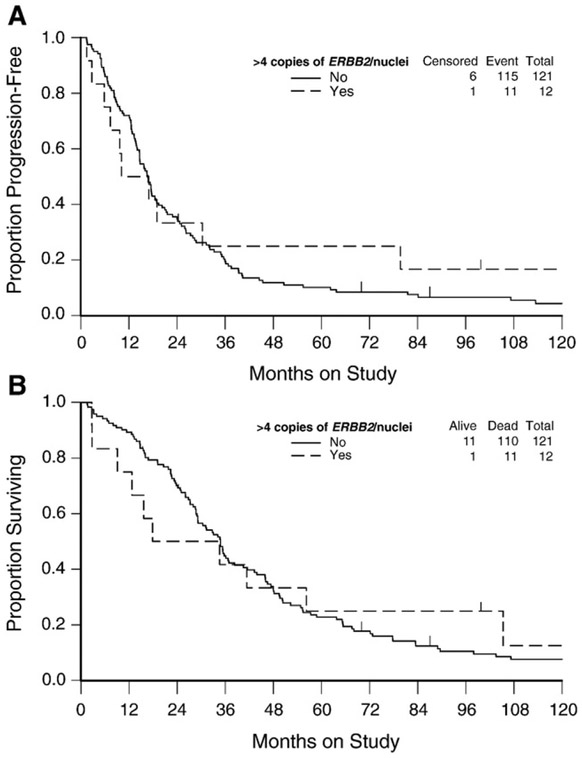
ERBB2 amplification was also defined as >4 copies of ERBB2/nuclei [15]. Using this definition, ERBB2 amplification was still a rare event in EOC with only 9% (12/133) of women with levels ranging from 4.2 to 49.2 copies of ERBB2/nuclei. There was a direct association between ERBB2 amplification and older age but not with race/ethnicity, GOG performance status, stage, cell type, grade or measurable disease status (Table 2). An inverse relationship was observed between ERBB2 amplification and >100 ml of ascites (Table 2). There was no evidence to suggest that ERBB2 amplification, defined as >4 copies of ERBB2/nuclei, was associated with tumor response (Table 3), disease status post-chemotherapy (Table 3), PFS (Fig. 3A) or OS (Fig. 3B).
Fig. 3.
Kaplan–Meier estimate of progression-free survival (A) and overall survival (B) for women with normal, borderline or amplified ERBB2/nuclei copies. Censored indicates patients who were alive with no evidence of disease progression. Logrank test was used to evaluate the equality in progression-free survival (p = 0.730) and survival (p = 0.980) distributions between women with normal and amplified ERBB2/nuclei copies.
Women with >4 copies of ERBB2/nuclei did not have a reduced risk of disease progression (HR = 0.90; 95% CI = 0.48–1.67; p = 0.730) or death (HR = 0.99; 95% CI = 0.53–1.85; p = 0.980) compared with women with ≤4 copies of ERBB2/nuclei (Table 4). After adjusting for the clinical covariates, ERBB2 amplification was not an independent prognostic factor for PFS (HR = 0.71; 95% CI = 0.36–1.39; OS (HR = 0.76; 95% CI = 0.38–1.49; p = 0.420).
Discussion
ERBB2 amplification defined as >2 copies of ERBB2/CEP17, or as >4 copies of ERBB2/nuclei, was a rare event and not associated with any of the usual clinical parameters such as race, GOG performance status, International Federation of Gynaecology and Obstetrics (FIGO) stage, histologic cell type, tumor grade, or gross residual disease. ERBB2 amplification per chromosome 17 or per nuclei was not associated with tumor response, disease status post-chemotherapy, PFS or OS. A number of studies have suggested that overexpression of ERBB2 indicates poor prognosis in ovarian, breast, endometrial, and colon cancers [5,24,27,28,9,30-35]. Tissue microarray analysis of 300 breast cancers by immunohistochemistry found that high expression of ERBB2 was associated with a poor outcome [24]. Immunohistochemistry evaluation of 670 breast cancer specimens for ERBB2 and EGFR expression revealed that the combination of epidermal growth factor receptor (EGFR) and ERBB2 expression was an independently significant factor for disease free survival and OS [29]. ERBB2 expression in endometrial cancer was associated with a more malignant phenotype and worse PFS [25,27], and was an independent prognostic factor for OS [27].
The association of ERBB2 overexpression with prognosis in EOC has been less clear. The evaluation of 73 ovarian cancers by immunohistochemistry found a decreased median survival time of 15.7 months for women with ERBB2 overexpression versus 32.8 months for women with normal ERBB2 expression [5]. ERBB2 overexpression has also been associated with a decreased total dose–response effect to cisplatin based chemotherapy in ovarian cancer [34]. Conversely, in an immunoperoxidase technique analysis of 40 early stage and 105 advanced stage ovarian cancer patients, ERBB2 overexpression occurred infrequently, and upon multivariate analysis ERBB2 expression was not a prognostic marker for OS [7,8].
It was hoped that gene amplification analysis would resolve some of these conflicting results, however associations between amplification of the ERBB2 gene and prognosis in EOC have also had disparate results. Multicolor FISH allows the simultaneous study of individual cells for amplification. FISH also allows the quantification of the amount and distribution of the oncogene signals and the number of centromeres present in each cell [4]. FISH analysis in ovarian cancer has associated ERBB2 amplification with more advanced stage [3,36,4,37]. These small studies however have found no specific clinical correlation between ERBB2 amplification and the usual clinical prognostic factors, but the lack of correlation was possibly thought to be attributable to small sample size [4,37].
With the advent of tissue microarray (TMA) technology scientists have been able to solve the small sample size dilemma through the evaluation of large numbers of specimens by FISH analysis. A FISH analysis of 79 FIGO stage I and II EOC found a 6.7% rate of ERBB2 amplification. No clinical correlation of survival was attempted in this study secondary to the limited number of informative cases in the sample set [13]. A FISH analysis of 103 advanced stage ovarian cancer specimens using a fluorescence ratio of 2.0 and 1.5 as cutoffs found ERBB2 amplification rates of 10.7% and 33.3% respectively [15]. They found that immunohistochemical expression of ERBB2 protein was not correlated with ERBB2 amplification by FISH analysis. They also found ERBB2 amplification defined as >2.0 copies of ERBB2/CEP17 was not associated with PFS [15]. This study involved women with advanced stage disease but encompassed a variety of histologies and treatment regimens. Finally this was a retrospective cohort study in which the patients identified for analysis were obtained through a registry review of the British Columbia Cancer Agency. Thus the data analyzed are subject to the accuracy of the data entered into the registry. Operative reports, chemotherapy treatment cycles, and tissue histology were not specifically reviewed to ensure accuracy of data obtained [15].
A comprehensive FISH analysis of 173 invasive ovarian cancers of all stages however, revealed that ERBB2 amplification was associated with immunohistochemical expression of ERBB2, but not with tumor stage, histologic cell type, grade or prognosis [14]. In this study, seventy-nine percent of the invasive EOC specimens analyzed were of serous histology. The treatment period however spanned 17 years from 1985 to 2002, and as a result probably included a variety of adjuvant chemotherapy regimens. Although the authors conclude that ERBB2 amplification did not correlate with FIGO stage, the authors did not specifically give the percentages of early and advanced stage cancers analyzed. They also did not clarify any inclusion of borderline or germ cell tumors, which were evaluated for ERBB2 amplification, and may have been included in the analysis [14]. The current study is truly comprehensive and solves the pitfalls of the previous studies of the predictive and prognosis associations of ERBB2 amplification in EOC by examining a large cohort (N= 133) of women who were uniformly staged and treated. Only 71% of the women with suboptimally-resected, advanced stage disease had serous adenocarcinomas which is representative of that observed for this patient population [16,38].
As previously stated the lack of correlation between ERBB2 amplification and survival in the current study contradicts evaluation of this marker in other cancers including breast and endometrial cancer [4,5,9,24-30-35]. The disparity in findings does not reflect the use of a different cut point for ERBB2 amplification between disease sites. Using the criteria established for breast cancer in 2007 [39], ERBB2 amplification defined as >2.2 copies of ERBB2/CEP17 or >4 copies of ERBB2/nuclei was a rare event in EOC occurring in 7% (9/133) or 4% (5/133) of the women in this cohort, respectively, and was not associated with any measure of clinical outcome including tumor response, disease status post-chemotherapy, PFS or OS (data not show). Moreover, our finding, is consistent with some clinical observations in ovarian cancer. In a phase II evaluation of Trastuzumab by the Gynecologic Oncology group in 837 patients with recurrent ovarian cancer, 95 tumors (11%) exhibited overexpression of ERBB2 by immunohistochemistry [10]. Treatment of 41 patients with Trastuzumab by weekly infusion revealed only a 7% response rate. They also found no evidence to suggest that tumor expression of ERBB2 was associated with tumor response, PFS or OS [10].
The current study is comprehensive and as a result, solves the pitfalls of the previous studies regarding the predictive and prognostic associations of ERBB2 amplification in EOC. We report a large FISH analysis of advanced stage, uniformly treated, suboptimally debulked, ovarian cancer patients who were treated on GOG protocol 111. To date this is the largest analysis of a homogeneously treated patient population. It should be noted however that patients randomized to the cyclophosphamide + cisplatin arm were found to have an inferior treatment when comparing progression-free and overall survival compared to the paclitaxel + cisplatin arm which was adjusted for in the statistical analysis and no difference was noted. Our analysis demonstrated that ERBB2 amplification using either definition was a rare event, was not associated with tumor response, disease status post-chemotherapy, PFS or OS, and was not associated with patient race/ethnicity, tumor stage, cell type, grade or measurable disease status. ERBB2 amplification defined as >4 copies of ERBB2/nuclei, but not as >2 copies of ERBB2/CEP17, was directly associated with older age and inversely associated with >100 ml ascites. The lack of association between ERBB2 amplification defined as >4 and PFS and OS could be due to the relatively small power of our study and the low incidence (9%) of this event. The ratio of ERBB2 copies per nuclei is likely a more accurate assessment of true ERBB2 amplification because it could be possible to amplify a complete diploid chromosome 17 many times and still have a ERBB2 to chromosome 17 ratio that is unity. FISH analysis does provide a powerful accurate method for the evaluation of oncogene amplification among tumor samples. In conclusion, ERBB2 amplification is a rare event and has no predictive or prognostic value in suboptimally-resected, advanced stage EOC treated with platinum-based combination chemotherapy.
Supplementary Material
Acknowledgments
This study was supported by National Cancer Institute grants of the Gynecologic Oncology Group Administrative Office (CA 27469) and the Gynecologic Oncology Group Statistical Office (CA 37517). The following Gynecologic Oncology Group (GOG) institutions participated in this study: University of Alabama School of Medicine, Abington Memorial Hospital, University of Rochester Medical Center, Walter Reed Army Medical Center, Wayne State University, Colorado Gynecologic Oncology Group P.C., University of California at Los Angeles, University of Washington, Milton S. Hershey Medical Center, Georgetown University Hospital, Wake Forest University School of Medicine, University of California Medical Center at Irvine, University of Kentucky, The Cleveland Clinic Foundation, Johns Hopkins Oncology Center, Eastern Pennsylvania Gyn/Onc Center, P.C., Cooper Hospital/University Medical Center, Columbus Cancer Council, University of Massachusetts Medical Center, University of Oklahoma, and Tacoma General Hospital, as well as the following resigned or terminated GOG member institutions: Oregon Health Sciences University, University of Southern California at Los Angeles and Stanford University Medical Center.
Abbreviations:
- AOC
advanced ovarian cancer
- FISH
fluorescence in situ hybridization
- FIGO
International Federation of Gynecologic Oncologists
- GOG
Gynecologic Oncology Group
Footnotes
The views expressed herein are those of the authors and do not reflect the official policy or opinion of the Department of Defense or the United States Army or Navy.
Conflict of interest statement
The authors declare that there are no conflicts of interest with the exception of Dr. William J. Hoskins who reports that he is CoChair NCI Gynecologic Cancer Steering Committee, Member of the Executive Board of ACOG, Investor of Chestnut Medical and his spouse is the Vice President of the ACOG.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.ygyno.2009.02.009.
References
- [1].Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin 2008;58:71–96. [DOI] [PubMed] [Google Scholar]
- [2].Young R Cancer of the ovary. 4th ed. Philadelphia, Pa: Lippincott; 1993. p. 1126–63. [Google Scholar]
- [3].Afify AM, Werness B, Mark HF. Her2 oncogene amplification in stage I and stage III ovarian papillary serous carcinoma. Exp Mol Pathol 1999;66:163–9. [DOI] [PubMed] [Google Scholar]
- [4].Young SR, Liu WH, Brock JA, Smith ST. ERBB2 and chromosome 17 centromere studies of ovarian cancer by fluorescence in situ hybridization. Genes Chromosomes Cancer 1996;16:130–7. [DOI] [PubMed] [Google Scholar]
- [5].Berchuck A, Kamel A, Whitaker R, et al. Overexpression of Her2 is associated with poor survival in advanced epithelial ovarian cancer. Cancer Res 1990;50:4087–91. [PubMed] [Google Scholar]
- [6].Kacinski BM, Mayer AG, King BL, Carter D, Chambers SK. NEU protein overexpression in benign, borderline, and malignant ovarian neoplasms. Gynecol Oncol 1992;44:245–53. [DOI] [PubMed] [Google Scholar]
- [7].Rubin SC, Finstad CL, Federici MG, Scheiner L, Lloyd KO, Hoskins WJ. Prevalence and significance of Her2 expression in early epithelial ovarian cancer. Cancer 1994;73:1456–9. [DOI] [PubMed] [Google Scholar]
- [8].Rubin SC, Finstad CL, Wong GY, Almadrones L, Plante M, Lloyd KO. Prognostic significance of Her-2 expression in advanced epithelial ovarian cancer: a multivariate analysis. Am J Obstet Gynecol 1993;168:162–9. [DOI] [PubMed] [Google Scholar]
- [9].Slamon DJ, Godolphin W, Jones LA, et al. Studies of the Her2 proto-oncogene in human breast and ovarian cancer. Science 1989;244:707–12. [DOI] [PubMed] [Google Scholar]
- [10].Bookman MA, Darcy KM, Clarke-Pearson D, Boothby RA, Horowitz IR, Campos SM. Evaluation of monoclonal humanized anti-HER2 antibody (Trastuzumab) in patients with recurrent or refractory ovarian or primary peritoneal carcinoma with overexpression of HER2: a phase II trial of the Gynecologic Oncology Group. Women's Oncol Rev 2003;3:239–40. [DOI] [PubMed] [Google Scholar]
- [11].Serrano-Olvera A, Duenas-Gonzalez A, Gallardo-Rincon D, Candelaria M, De la Garza-Salazar J. Prognostic, predictive and therapeutic implications of HER2 in invasive epithelial ovarian cancer. Cancer Treat Rev 2006;32:180–90. [DOI] [PubMed] [Google Scholar]
- [12].Mano MS, Awada A, Di Leo A, et al. Rates of topoisomerase II-alpha and HER-2 gene amplification and expression in epithelial ovarian carcinoma. Gynecol Oncol 2004;92:887–95. [DOI] [PubMed] [Google Scholar]
- [13].Wu Y, Soslow RA, Marshall DS, Leitao M, Chen B. Her-2 expression and amplification in early stage ovarian surface epithelial neoplasms. Gynecol Oncol 2004;95:570–5. [DOI] [PubMed] [Google Scholar]
- [14].Mayr D, Kanitz V, Amann G, et al. Her-2 gene amplification in ovarian tumours: a comprehensive immunohistochemical and FISH analysis on tissue microarrays. Histopathology 2006;48:149–56. [DOI] [PubMed] [Google Scholar]
- [15].Lee CH, Huntsman DG, Cheang MC, et al. Assessment of Her-1, Her-2, And Her-3 expression and Her-2 amplification in advanced stage ovarian carcinoma. Int J Gynecol Pathol 2005;24:147–52. [DOI] [PubMed] [Google Scholar]
- [16].McGuire WP, Hoskins WJ, Brady MF, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med 1996;334:1–6. [DOI] [PubMed] [Google Scholar]
- [17].Darcy KM, Brady WE, McBroom JW, et al. Associations between p53 overexpression and multiple measures of clinical outcome in high-risk, early stage or suboptimally-resected, advanced stage epithelial ovarian cancers: a Gynecologic Oncology Group study. Gynecol Oncol (GYN-08-446R1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Fisher RA. On the interpretation of χ2 from contingency tables, and the calculation of p.J R Stat Soc 1992;85(1):87–94. [Google Scholar]
- [19].Mehta CR, Patel NR. A network algorithm for performing Fisher's exact test in r × c contingency tables. J Am Stat Assoc 1983;78:427–34. [Google Scholar]
- [20].Kaplan EL, Meier P. Nonparametric estimation from incomplete observations.J Am Stat Assoc 1958;53:457–81. [Google Scholar]
- [21].Savage IR. Contributions to the theory of rank order statistics — the two sample case. Ann Math Stat 1956;27:590–615. [Google Scholar]
- [22].Cox DR. Regression models and life tables. J Royal Stat Soc 1972;34:187–220. [Google Scholar]
- [23].Hosmer DW, Lemeshow S. Applied Survival Analysis: Regression Modeling of Time to Event Data. New York, NY: John Wiley&Sons, Inc; 1999. [Google Scholar]
- [24].Camp RL, Dolled-Filhart M, King BL, Rimm DL. Quantitative analysis of breast cancer tissue microarrays shows that both high and normal levels of HER2 expression are associated with poor outcome. Cancer Res 2003;63:1445–8. [PubMed] [Google Scholar]
- [25].Cherchi PL, Marras V, Capobianco G, et al. Prognostic value of p53, c-erb-B2 and MIB-1 in endometrial carcinoma. Eur J Gynaecol Oncol 2001;22:451–3. [PubMed] [Google Scholar]
- [26].Heffner HM, Freedman AN, Asirwatham JE, Lele SB. Prognostic significance of p53, PCNA, and c-erbB-2 in endometrial endocarcinoma. Eur J Gynaecol Oncol 1999;20:8–12. [PubMed] [Google Scholar]
- [27].Hetzel DJ, Wilson TO, Keeney GL, Roche PC, Cha SS, Podratz KC. Her-2 expression: a major prognostic factor in endometrial cancer. Gynecol Oncol 1992;47:179–85. [DOI] [PubMed] [Google Scholar]
- [28].Nathanson DR, Culliford AT, Shia J, et al. HER 2/neu expression and gene amplification in colon cancer. Int J Cancer 2003;105:796–802. [DOI] [PubMed] [Google Scholar]
- [29].Tsutsui S, Ohno S, Murakami S, Kataoka A, Kinoshita J, Hachitanda Y. Prognostic value of the combination of epidermal growth factor receptor and c-erbB-2 in breast cancer. Surgery 2003;133:219–21. [DOI] [PubMed] [Google Scholar]
- [30].Zhang F, Yang Y, Smith T, et al. Correlation between HER-2 expression and response to neoadjuvant chemotherapy with 5-fluorouracil, doxorubicin, and cyclophosphamide in patients with breast carcinoma. Cancer 2003;97:1758–65. [DOI] [PubMed] [Google Scholar]
- [31].Safari B, Jones LA, el-Naggar A, Felix JC, George J, Press MF. Amplification and overexpression of Her-2 (c-erbB2) in endometrial cancers: correlation with overall survival. Cancer Res 1995;55:5693–8. [PubMed] [Google Scholar]
- [32].Rolitsky CD, Theil KS, McGaughy VR, Copeland LJ, Niemann TH. Her-2 amplification and overexpression in endometrial carcinoma. Int J Gynecol Pathol 1999;18:138–43. [DOI] [PubMed] [Google Scholar]
- [33].Schmidt M, Lewark B, Kohlschmidt N, et al. Long-term prognostic significance of Her-2 in untreated node-negative breast cancer depends on the method of testing. Breast Cancer Res 2005;7:R256–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Meden H, Marx D, Roegglen T, Schauer A, Kuhn W. Overexpression of the oncogene c-erbB-2 (HER2/neu) and response to chemotherapy in patients with ovarian cancer. Int J Gynecol Pathol 1998;17:61–5. [DOI] [PubMed] [Google Scholar]
- [35].Katsaros D, Theillet C, Zola P, et al. Concurrent abnormal expression of erbB-2, myc and ras genes is associated with poor outcome of ovarian cancer patients. Anticancer Res 1995;15:1501–10. [PubMed] [Google Scholar]
- [36].Fukushi Y, Sato S, Yokoyama Y, Kudo K, Maruyama H, Saito Y. Detection of numerical aberration in chromosome 17 and c-erbB2 gene amplification in epithelial ovarian cancer using recently established dual color FISH. Eur J Gynaecol Oncol 2001;22:23–5. [PubMed] [Google Scholar]
- [37].Wang ZR, Liu W, Smith ST, Parrish RS, Young SR. c-myc and chromosome 8 centromere studies of ovarian cancer by interphase FISH. Exp Mol Pathol 1999;66:140–8. [DOI] [PubMed] [Google Scholar]
- [38].Muggia FM, Braly PS, Brady MF, et al. Phase III randomized study of cisplatin versus paclitaxel versus cisplatin and paclitaxel in patients with suboptimal stage III or IV ovarian cancer: a gynecologic oncology group study. J Clin Oncol 2000;18(1):106–15. [DOI] [PubMed] [Google Scholar]
- [39].Wolff AC, Hammond ME, Schwartz JN, et al. American society of clinical oncology, college of american pathologists. american society of clinical oncology/college of American pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 2007;25(1):118–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.