Abstract
Purpose:
The purpose of this study is to twofold: 1) to determine the age when a child with spina bifida (SB) will most likely transition from caregiver clean intermittent catheterization (CIC) to self-CIC; and 2) identify factors associated with self-CIC in children older than that age.
Methods:
This is a retrospective, single-institution cohort study of individuals with SB. Data were collected prospectively as part of the National Spina Bifida Patient Registry. For Aim 1, we identified all individuals who perform self-CIC and who had a documented transition from caregiver-CIC. We then determined the age of transition to self-CIC. For Aim 2, we compared individuals over age 10 years (age cutoff determined by Aim 1) who use self-CIC to those who use caregiver-CIC to determine what variables were associated with self-CIC.
Results:
From our SB population, 206 individuals used self-CIC. Of these, 64 patients had documented ages of transitions from caregiver- to self-CIC. 46 (71.9%) and 56 (87.5%) patients had transitioned to self-CIC by 10 and 14 years, respectively. For Aim 2, we used age 10 as a cutoff, based on the findings from Aim 1, and found that 287/696 patients were ≥10 years and using CIC. Factors independently associated with lower likelihood of self-CIC were thoracic spinal lesions (odds ratio (OR) 0.45) and Medicaid insurance (OR 0.24).
Conclusions:
The ages at self-CIC transition vary, but most patients transition by age 10. Thoracic-level spinal lesions and Medicaid insurance are associated with lower odds of self-CIC.
Keywords: spina bifida, adult, pediatric, clean intermittent catheterization, urologic management
1. Introduction
Spina bifida (SB) is the most common non-chromosomal birth defect, with a prevalence of 30 per 100,000 live births (1). There are an estimated 166,000 individuals with SB currently living in the United States (2–3). SB is associated with multi-organ disability, including Chiari II malformation, paraplegia, and neuropathic bowel and bladder (4–5).
Effective bladder management is critical to prevent renal damage and promote social dryness (6–12). While 90% of individuals with SB are born with normal renal function, 50% will deteriorate without treatment (6). The deranged bladder dynamics may result in recurrent upper urinary tract infections (UTI) and/or renal scarring leading to end stage renal disease (ESRD) if untreated (12–16). These sequelae can be significantly reduced with adequate bladder management (4, 9–11, 15–16).
Since its introduction by Lapides (1972), clean intermittent catheterization (CIC) remains an important part of management for many in this population (17). CIC is a method of urinary elimination by which a sterile catheter is inserted into either a native or surgically-created urethra to drain the bladder. This process is repeated at scheduled intervals throughout the day by caregivers or patients in lieu of spontaneous voiding or chronic indwelling catheterization. Previous studies have found that urinary continence significantly improves self-concept in children compared to incontinence (18). Urinary incontinence has also been cited as a major cause of lower psychological well-being, but has not been found to consistently reduce health-related quality of life (HR-QOL) scores among SB populations (19). Urinary-related QOL (UR-QOL) may be significantly related to HR-QOL but no single factor that improves UR-QOL has been consistently identified (20). Both social dependence and lack of compliance may impair effective bladder management in individuals with SB (21–22). We hypothesize that these individuals are more likely to perform self-CIC as they approach adolescence and that those who are still dependent on caregiver-CIC in adolescence are likely to have male gender due to anatomic differences, lower ambulation status, and higher spinal lesion levels. We also hypothesized that after 10 years old, age would not play a significant role in CIC independence. This study has two distinct aims to address these hypotheses. Aim 1 is to determine the age at which individuals using CIC are likely to transition from caregiver- to self-CIC. Aim 2 is to identify factors that are associated with and contribute to CIC independence.
2. Materials and Methods
Research Setting:
All patients with SB, including both open myelomeningocele (MMC) and other closed spinal dysraphisms (meningocele, spinal lipoma, split cord malformation, etc.) are seen at the authors’ multidisciplinary SB clinics. Those individuals under 21 years are seen in the pediatric SB clinic, and those over 21 years are seen in the adult SB clinic. All urologic management is performed by two pediatric and two adult urologists. Patients followed in either clinic are enrolled in the Centers for Disease Control (CDC) National Spina Bifida Patient Registry (NSBPR). A standardized questionnaire is used at the patient’s enrollment into the NSBPR to gather baseline demographic, diagnostic, and selected procedural information. An annual visit form is completed at enrollment and at each subsequent clinic visit; this form gathers updated clinical and demographic data to be entered into the NSBPR. Data entry involves a web-based electronic medical record (EMR) which provides a standardized method for data collection and management for all participating institutions. A subset of the data is de-identified, provided to the CDC, and available for query to researchers from all participating institutions (following an established protocol for data access). In addition, all data remain available locally through the institution’s research EMR. For the present study, only NSBPR data from the authors’ institutions were collected and analyzed. For data points that may change with time (specifically self- versus caregiver-catheterization), the status at the most recent clinic follow up visit was recorded. The authors’ pediatric and adult clinics enroll more than 99% of all SB patients into the NSBPR, providing a robust research cohort with little missing data.
At each clinic visit, the patients and caregivers meet with the clinic coordinator who has reviewed the patient’s chart for any medical or surgical interventions since their last encounter. All information is reviewed with the patient and family to ensure accuracy and completeness of the record. Additionally, all urologic management medications and interventions are reviewed and updated in the NSBPR. When available, the age at CIC initiation and age at transition to self-CIC, if applicable, are recorded. CIC dependence was collected at last follow-up.
At all visits, patients meet with their urology provider and clinic care support staff to ensure compliance with CIC. All patients are screened for UTIs and undergo renal ultrasound to assess for hydronephrosis, urinary tract stones, and other urinary abnormalities which suggest non-adherence to the CIC regimen. Prior to initiating CIC, all caregivers and patients are educated on the importance of the regimen and must adequately demonstrate the functional and mechanical capacity to perform CIC. At all visits, patients and caregivers on CIC are counseled on the importance of transition to independence. The concept of self-CIC is introduced to all patients of toilet-training age and emphasized at all subsequent clinic visits. Patients originally caregiver-dependent for CIC must successfully demonstrate self-CIC in clinic to be considered “independent.”
Study Design:
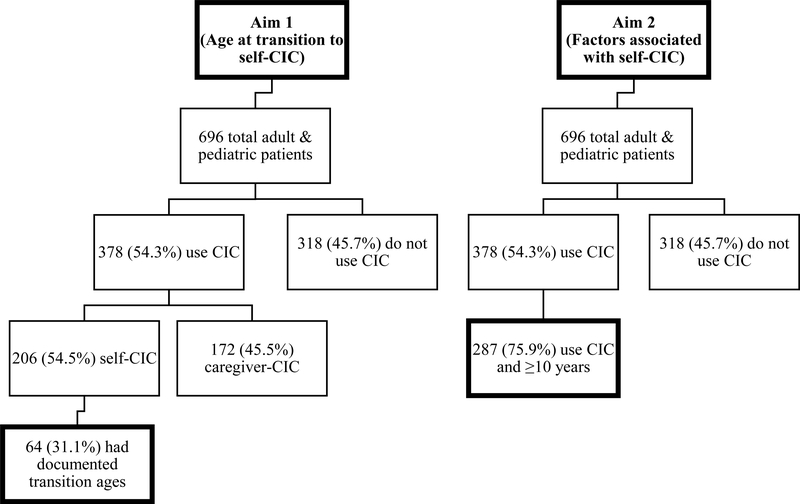
After institutional review board approval (protocol X090218005), the authors conducted a retrospective review of all pediatric and adult patients at their institutions included in the NSBPR since its implementation in 2009 until 2016. All patients performing CIC at any age were included in this study. Aim 1 of our study was to determine the age at which the majority of individuals with SB can be expected to transition to self-CIC. Figure 1 details the patient selection and population for determining the age at transition to self-CIC. For Aim 1, patients were excluded if they did not use CIC, did not have a documented age when beginning self-CIC, or began CIC independently without ever being caregiver-dependent. Sixty-four individuals met these inclusion criteria. Because these 64 patients represent only a fraction of total patients who use self-CIC (64/206), we compared the clinical and demographic characteristics between the 64 patients with documented transition ages and the remaining 142 patients who self-CIC but do not have recorded transition ages. The only significant difference between the groups via Pearson Chi-square analysis was community ambulation status. In the 64 patients with known ages at transition, 49/64 (76.5%) were community ambulators compared to 91/142 (64.1%) in the patients without documented ages of transition.
Figure 1.
Patient selection for Aim 1 (left; age at transition to self-CIC; 64 patients) and Aim 2 (right; factors associated with CIC independence; 287 patients).
Aim 2 was to determine the social and disease-specific factors associated with self-CIC in patients >10 years at last follow-up. For Aim 2, patients were excluded if they did not CIC at last follow-up or were <10 years. The a priori strategy was to inspect the results from Aim 1 and determine if there was an age by which the authors could expect the majority of patients to have transitioned to self-catheterization. The primary outcome in Aim 2 was if patients were on caregiver- or self-CIC at last follow-up. Figure 1 details the patient selection and population for Aim 2.
Statistical Analysis:
For both Aim 1 and Aim 2, the authors collected the following demographic information: gender, race, ethnicity, primary diagnosis, and Medicaid status. In addition to demographics, the authors collected the following variables for patients included for Aim 2: level of spinal lesion, ambulation status, wheelchair use, anti-muscarinic use, age at last follow-up, body mass index (BMI), history and number of ventriculoperitoneal (VP) shunts, and history and number of bladder augmentations.
Statistical analyses of the data were performed via IBM SPSS Version 25 software (25). For Aim 2, univariate logistic regression analysis was performed for all variables with the primary outcome of caregiver- or self-CIC. To better assess independent predictors, those factors with univariate significance of p-value <0.10 underwent subsequent forced entry multivariable logistic regression modeled via the same primary outcome.
3. Results
Age at Transition (Aim 1):
Of a total 696 patients included in the NSBPR, 493 (70.8%) and 203 (29.2%) are followed in the pediatric and adult clinics, respectively. Of 696 patients, 378 (54.3%) use CIC for bladder management. Of these 378 patients, 206 (54.5%) were included in the self-CIC group. A total of 64/206 (31.1%) patients had documented ages at transition from caregiver- to self-CIC and are the focus of Aim 1. The general data and clinical characteristics of the selected patients for Aim 1 are detailed in Table 1.
Table 1.
Demographic distribution and clinical characteristics of patients in Aim 1
| Variable | Number of Patients (N=64, %) |
|---|---|
| Median age (years, IQR) | 18.4 (14.5–20.9) |
| Gender | |
| Male | 29 (45.3) |
| Female | 35 (54.7) |
| Race | |
| White/Caucasian | 51 (79.7) |
| African American | 10 (15.6) |
| Asian | 2 (3.1) |
| Multiracial | 1 (1.6) |
| Ethnicity | |
| Non-Hispanic | 60 (93.8) |
| Hispanic or Latino | 4 (6.2) |
| Primary Diagnosis | |
| Myelomeningocele | 58 (90.6) |
| Lipoma | 5 (7.8) |
| Meningocele | 1 (1.6) |
| Medicaid Insurance | |
| Yes | 37 (57.8) |
| No | 26 (40.6) |
| Unknown | 1 (1.6) |
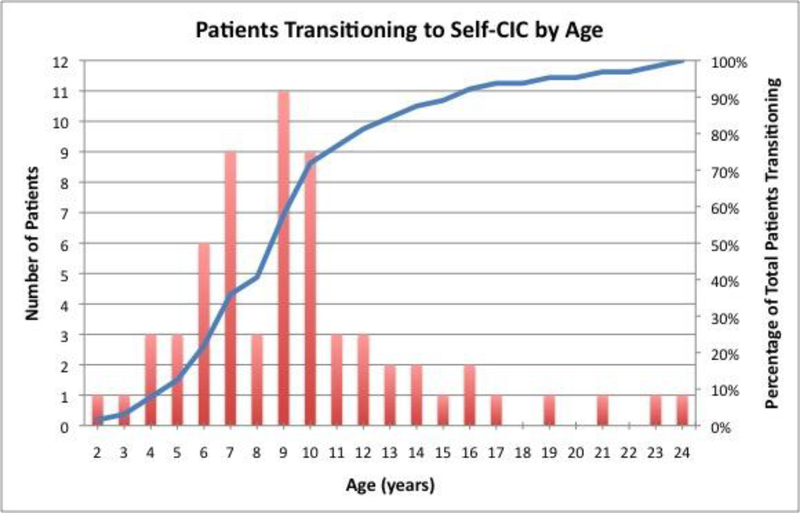
The median age at last follow-up for the 64 included patients was 18.3 years (interquartile range (IQR) 14.5–20.9 years). The median follow-up after attaining CIC independence was 7.0 years (IQR 5.3–10.6 years). The ages at transition to self-CIC were collected, and the frequencies of transition by ages are depicted in Figure 2. Based on these data, 8/64 (12.5%) patients transitioned to self-CIC at or before 5 years of age. By 10 and 14 years, 46/64 (71.9%) and 56/64 (87.5%) patients were independent in CIC, respectively. The median and mean ages of transition were 9 and 9.7 years, respectively. The greatest frequency of transition occurred at 9 years with 11/64 (17.2%) patients beginning self-CIC. Visual inspection of the data (Figure 2) shows a marked decline in self-CIC transition after age 10 years. Therefore, we defined >10 years as the expected age when transition should occur. We did not use measures of central tendency (mean, median, or mode) to select the age cutoff, as these values would lead to an age above which approximately half of the individuals would have transitioned. Our purpose was to select an age above which the majority had transitioned.
Figure 2.
Number of patients transitioning to self-CIC per year (columns) and total percent of 64 patients having transitioned (line).
Factors Associated with Self-CIC (Aim 2):
From Aim 1, 71.9% of the patients who use CIC transitioned to self-CIC by age 10 years. For Aim 2, the authors used 10 years as a threshold beyond which the majority of patients might be expected to self-CIC. From Figure 2, the number of patients becoming independent in CIC after age 10 years decreases in frequency. Of 696 total patients included in the NSBPR, 287 (41.2%) void via CIC and were ≥10 years at time of last follow-up. For these 287 patients, the authors compared those who utilized caregiver-CIC versus self-CIC. Table 2 contains the general data and clinical characteristics of the included 287 patients who are the focus of Aim 2.
Table 2.
Demographic distribution and clinical characteristics of patients in Aim 2
| Variable | Number of Patients (N=287, %) | Variable | Number of Patients (N=287, %) |
|---|---|---|---|
| Median Age (years, IQR) | 20.1 (15.8–26.6) | Anti-muscarinic | |
| Gender | Yes | 117 (40.8) | |
| Male | 123 (42.9) | No | 98 (34.1) |
| Female | 164 (57.1) | Unknown/Not listed | 72 (25.1) |
| Race | Wheelchair Use | ||
| White/Caucasian | 242 (84.3) | Yes | 157 (54.7) |
| African American | 35 (12.2) | No | 58 (20.2) |
| Other | 10 (3.5) | Unknown/Not listed | 72 (25.1) |
| Ethnicity | VP Shunt | ||
| Non-Hispanic | 274 (95.5) | Yes | 228 (79.4) |
| Hispanic or Latino | 13 (4.5) | No | 59 (20.6) |
| Primary Diagnosis | Bladder Augmentation | ||
| Myelomeningocele | 622 (91.3) | Yes | 75 (26.1) |
| Other1 | 25 (8.7) | No | 212 (73.9) |
| Medicaid | Median BMI kg/m2 (mean, IQR) | 23.6 (24.5, 19.5–29.3) | |
| Yes | 178 (62.0) | Number of VP Shunts | |
| No | 106 (37.0) | 1 | 202 (70.4) |
| Unknown/Not listed | 3 (1.0) | 2 | 19 (6.6) |
| Community ambulator | >22 | 7 (2.3) | |
| Yes | 167 (58.2) | Number of Bladder Augmentations | |
| No | 87 (30.3) | 1 | 72 (25.1) |
| Unknown/Not listed | 33 (11.5) | 2 | 3 (1.0) |
| Level of Lesion | |||
| Thoracic | 99 (34.5) | ||
| Lumbosacral | 155 (54.0) | ||
| Unknown/Not listed | 33 (11.5) | ||
| CIC Dependence | |||
| Self | 175 (61.0) | ||
| Caregiver | 112 (39.0) | ||
For Aim 2, the median age at last follow-up for the 287 patients included in the analysis was 20.1 years (IQR 15.8–26.6 years). In this group, 123 (42.9%) were male, and 178 (62.0%) had Medicaid insurance. The number of patients that self-CIC and the number that are caregiver-dependent are 175 (61.0%) and 112 (39.0%), respectively. Univariate logistic regression and subsequent forced entry multivariable regression analyses are shown in Table 3.
Table 3.
Factors associated with self-CIC: Univariate and forced entry multivariable regression
| Variable | Univariate Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| Odds Ratio (95% CI) | p-value | Odds Ratio (95% CI) | p-value | |
| Male gender | 2.39 (1.26–4.51) | 0.007 | 2.10 (0.97–4.59) | 0.061 |
| Female | Reference (Ref) | - | - | - |
| White race | 0.53 (0.21–1.33) | 0.18 | ||
| Non-white | Ref | - | ||
| Hispanic/Latino ethnicity | 0.57 (0.16–2.03) | 0.39 | ||
| Not Hispanic/Latino | Ref | - | ||
| MMC diagnosis | 0.15 (0.02–1.15) | 0.068 | 0.80 (0.073–8.88) | 0.86 |
| Non-MMC | Ref | - | - | - |
| Medicaid insurance | 0.19 (0.090–0.40) | <0.001 | 0.24 (0.11–0.56) | 0.001 |
| Non-Medicaid | Ref | - | - | - |
| Community ambulator | 1.44 (0.75–2.77) | 0.27 | ||
| Non-community | Ref | - | ||
| Thoracic spinal lesion | 0.27 (0.14–0.52) | <0.001 | 0.45 (0.21–0.97) | 0.042 |
| Lumbosacral | Ref | - | - | - |
| Anti-muscarinic | 0.72 (0.38–1.37) | 0.32 | ||
| None | Ref | - | ||
| Wheelchair use | 0.16 (0.053–0.46) | 0.001 | 0.32 (0.097–1.08) | 0.067 |
| None | Ref | - | - | - |
| History of VP shunt | 0.15 (0.045–0.50) | 0.002 | 0.41 (0.081–2.12) | 0.29 |
| None | Ref | - | - | - |
| History of bladder augmentation | 1.56 (0.75–3.25) | 0.24 | ||
| None | Ref | - | ||
| Age at last follow-up (years) | 0.98 (0.95–1.01) | 0.24 | ||
| BMI (kg/m2) | 0.99 (0.95–1.02) | 0.45 | ||
| Number of VP shunts | 1.80 (1.14–2.85) | 0.01 | 1.16 (0.57–2.37) | 0.68 |
| Number of bladder augmentations | 0.70 (0.36–1.39) | 0.31 | ||
By univariate analysis, gender, Medicaid insurance, thoracic level spinal lesions, wheelchair use, history of VP shunt, and number of VP shunts were significantly associated with self-CIC (p<0.05). These factors and primary diagnosis of MMC (p=0.068) were included in the multivariable regression analysis given p<0.10 via univariate analysis. In the multivariable analysis, Medicaid status (odds ratio (OR) 0.24, 95% confidence interval (CI) 0.11–0.56) and thoracic level spinal lesions (OR 0.45, 95% CI 0.21–0.97) were significantly associated with lower likelihood of self-CIC (p<0.05).
4. Discussion
CIC remains the gold standard for bladder management in patients with spina bifida and spinal dysraphisms (17). The authors’ multidisciplinary pediatric and adult SB clinics follow a proactive approach for the bladder management. Similar to other clinics, the authors emphasize the importance of caregiver-CIC initiation and daily anticholinergic prophylaxis starting at a young age when necessary. Caregivers initially perform CIC until the patients become physically and intellectually able to execute it independently (23–24). To our knowledge, no studies have yet been performed to evaluate the transition from caregiver- to self-CIC. Previous studies have documented the importance of transitioning to self-CIC and preserving continence for both the promotion of quality of life and independence (26–27).
In a series of 44 patients, Seth et al. determined the majority (73%) of patients experienced at least one barrier to self-CIC adherence. While lack of public toilet access was cited as the most common barrier (34%), difficulty positioning for catheter insertion and problems with dexterity were present in 25% and 21% of patients, respectively. Eighteen percent of patients cited supply cost as an additional obstacle for CIC adherence (22). This study demonstrated the wide range of potential barriers to maintaining self-CIC, and these may also contribute to the initial transition to CIC independence. The data available in the present study do not allow for analysis of these social and cultural barriers.
Recently, Castillo et al. (2017) described their experience with 110 patients transitioning to independent-CIC (28). They found that patients were capable of self-CIC between 2–17 year with a mean age of 9.45 years at independence. Castillo et al. determined male gender was associated with a higher rate of CIC independence, and thoracic-level spinal lesions were associated with a lower rate of independence. In patients ≥5 years, intellectual disability and independence in dressing, bathing, and skin checks were significantly associated with increased odds of self-CIC transition. Ethnicity, race, lesion level, primary diagnosis, presence of shunt, and mean fine motor skills were not significantly different between patients who did and did not self-CIC (28).
The present study identified a slightly broader range of ages at transition from 2–24 years and found that almost 13% of the patient population was capable of independent CIC by five years. Our study found that the majority of individuals become independent of CIC between 9–14 years, which may correspond to increasing manual dexterity and desire to become more autonomous. Transition during the peri-pubertal years of 9–14 may also be linked to burgeoning social independence. In addition to establishing the average age of self-CIC, we determined which factors are associated with self-CIC. Patients with thoracic level spinal lesions had lower odds of self-CIC, which is consistent with Castillo et al. (28).
In another study, which reviewed the association of mobility and level of lesion, Schoenmakers et al. (2005) determined that individuals with lesions below L3 had greater functional independence with regard to self-care and mobility. This study also found that mobility independence (OR 5.3, 95%CI 1.6–17.4) was the most important determinant of health-related quality of life (21). It is known that individuals with higher-level spinal lesions tend to have greater functional and ambulatory impairment (29). However, in the current study cohort, the authors found that neither ambulation status nor wheelchair use was an independent predictor of CIC independence.
Individuals with Medicaid insurance had a lower odds of self-CIC, but the exact association between insurance status and CIC independence is difficult to identify. As a surrogate for socioeconomic status, families with Medicaid may represent disparities in resource availability or social support. However, given that Medicaid insurance provides the same monthly allocation of CIC supplies as private insurers at the authors’ institution, factors other than financial limitations may play a role in transition to CIC independence. Medicaid insurance status may also highlight disparities in health literacy and educational levels of caregivers which may contribute to CIC dependence. A 2015 multi-institutional study of SB outcomes found non-Hispanic black patients were least likely to have urinary and bowel continence and patients with private insurance were more likely to attain continence than those without private insurance (30). While our study did not find race or ethnicity to be associated with CIC independence, this may be in part to the relatively small number of non-white subjects (15.6%) in our study. The disparity between CIC independence in patients with and without private insurance most likely represents an inequality among employment, income, and/or community resources between the two groups. Further studies are needed to delineate the roles that patients and their caregivers on Medicaid insurance have in CIC independence.
Interestingly, this study did not find any modifiable risk factors independently associated with self-CIC. BMI and anti-muscarinic use were not associated with self-CIC. This suggests that urinary independence among SB patients is most influenced by socioeconomic and anatomic factors. Primary diagnosis was not significantly associated with the self-CIC transition, which suggests that disease etiology does not contribute to achievable independence, as may be expected (21). However, only 25 (8.7%) non-MMC patients were included in the analysis, which may be too small of a sample to accurately evaluate the contribution primary diagnosis has in CIC independence. These results will help the multidisciplinary spina bifida clinic better understand the limitations to self-CIC and to provide more focused patient education to those patients who are approaching an age of potential independence. Furthermore, this study will help recognize individuals with lower odds of self-CIC, and will enable clinics to allocate more resources to these patients and ultimately facilitate their functional independence.
The lack of association between age at last follow-up and self-CIC shown in this study is key for the promotion of CIC independence. Once patients cross the “threshold” of age 10 years, it becomes increasingly important to address the socioeconomic factors which may limit self-CIC instead of assuming that individuals will become independent if given more time.
5. Limitations
The authors acknowledge the limitations of this study. This is a retrospective study at a single institution, subject to its inherent design deficiencies. Data regarding the age of transition from caregiver to self-CIC were not collected prospectively, and, since the analysis, the authors have added this as an additional field to our NSBPR data collection form. Recollection bias is possible, as the information on age of transition was based on caregiver response. Regional and cultural differences could influence the results, and single center studies are prone to treatment/management biases compared to other centers. The relationships between bowel and urinary management and outcomes were not studied, both of which are interrelated on many domains of independence and social continence. Data regarding CIC through a surgical or native urethra were not consistently reported in the NSBPR. While a surgical urethra may indeed facilitate self-CIC, we are currently unable to reliably include this in our analysis. A future study could investigate the role that a catheterizable channel has in self-CIC. Several other unincluded family, caregiver or even patient related factors could influence the results. A source of bias in this study may be the small number of patients (64/206, 31%) with a known age at transition to self-CIC. These data were not initially entered into the NSBPR at our institution. It was queried separately from the annual clinic questionnaire; therefore, the data were not consistently collected for all patients who self-CIC. Given the small sample of available data, the 64 patients may not represent a completely accurate sample of the 206 patients who use self-CIC. However, in comparing the characteristics of included patients with those who did not have a documented time of transition, only community ambulation was significantly different. This may limit the generalizability of the findings, but given that all other differences were insignificant, the authors believe the findings are a reasonable representation of the population. Another potential confounder related to CIC independence may be the cognitive abilities of the patient. Data regarding cognitive function of patients and their current grade level are not currently included in the NSBPR. Additionally, the NSBPR does not contain data regarding parental education level, health literacy, or occupation, so we used insurance status as a proxy for socioeconomic status.
In spite of the limitations, this study addresses some critical aspects in understanding this patient population in more depth, with regards to functional independence for bladder management. To the authors’ knowledge, this is the first study that attempts to identify the age at transition as well the factors associated with functional independence in bladder management. The future goal is to combine the CDC data from all the participating institutions to assess both the age and factors associated with self-CIC across other high-volume centers. This future study will include a wider geographic distribution, larger sample size with different practice patterns, and will help to understand the complex nature of the disease and many interrelated variables.
6. Conclusion
Based on these results, individuals with spina bifida achieve functional independence with bladder management anytime between toddlerhood and adulthood (2 to 24 years of age). The majority of patients transition to self-CIC during the peri-pubertal period of 9–14 years. Non-modifiable factors such as gender, MMC diagnosis, wheelchair use, history and number of VP shunts were associated with lack of CIC independence by univariate analysis. Thoracic-level spinal lesions and Medicaid insurances status were independent factors associated with lower odds of self-CIC by multivariable analysis. Age at last follow-up was not significantly associated with self-CIC for patients greater than age 10 years. Further studies are needed at a multi-institutional level to better determine other factors that are associated with CIC independence.
3. Acknowledgements
2. Statement of Funding
This project was supported in part by NIH Grant 1KL2TR001419, and by the KPRI of Children’s of Alabama.
One author (TSW) of this publication is the principal investigator for Ipsen Innovation©. One author (DBJ) is principal investigator on CDC grant 1U01DD001080–01, which contains the NSBPR used in this study. One author (BGR) received support from NIH Grant 1KL2TR001419 and the Kaul Pediatric Research Institute (KPRI) of Children’s of Alabama.
Footnotes
1. Statement of Conflicts of Interest
All other authors certify that they have no financial conflict of interest relevant to the publication of the subject matter or materials in this manuscript.
4. References
- 1.Lloyd JC, Wiener JS, Gargollo PC, Inman BA, Ross SS, Routh JC. Contemporary epidemiological trends in complex congenital genitourinary anomalies. J Urol. 2013; 190(4): 1590–95. doi: 10.1016/j.juro.2013.04.034. [DOI] [PubMed] [Google Scholar]
- 2.Parker SE, Mai CT, Canfield MA, Rickard R, Wang Y, Meyer RE, et al. Updated national birth prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Res A Clin Mol Teratol. 2010; 88: 1008–16. doi: 10.1002/bdra.20735. [DOI] [PubMed] [Google Scholar]
- 3.Pal-de Bruin KM, Buitendijk SE, Hirasing RA, den Ouden AL. Prevalence of neural tube defects in births before and after promotion of periconceptional folic acid supplementation. Ned Tijdschr Geneeskd 2000; 144(36): 1732–36. doi: 10.1007/s00467-008-0780-7. [DOI] [PubMed] [Google Scholar]
- 4.Oakeshott P, Hunt GM, Poulton A, Reid F. Open spina bifida: birth findings predict long-term outcome. Arch Dis Child. 2011; 97(5): 474–76. [DOI] [PubMed] [Google Scholar]
- 5.Wen SW, Liu S, Joseph KS, Rouleau J, Allen A. Patterns of infant mortality caused by major congenital anomalies. Teratology. 2000; 61(5): 342–46. doi: . [DOI] [PubMed] [Google Scholar]
- 6.Filler G, Gharib M, Casier S, Lodige P, Ehrich JH, Dave S. Prevention of chronic kidney disease in spina bifida. Int Urol Nephrol. 2012; 44: 817–27. doi: 10.1007/s11255-010-9894-5. [DOI] [PubMed] [Google Scholar]
- 7.McGuire EJ, Woodside JR, Borden TA, Weiss RM. Prognostic value of urodynamic testing in myelodysplastic patients. J Urol. 1981; 126: 205–09. [DOI] [PubMed] [Google Scholar]
- 8.Galloway NT, Mekras JA, Helms M, Webster GD. An objective score to predict upper tract deterioration in myelodysplasia. J Urol. 1991; 145: 535–37. [DOI] [PubMed] [Google Scholar]
- 9.Woodhouse CR, Neild GH, Yu RN, Bauer S. Adult care of children from pediatric urology. J Urol. 2012; 187(4): 1164–71. doi: 10.1016/j.juro.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 10.McDonnell GV, McCann JP. Why do adults with spina bifida and hydrocephalus die? A clinic-based study. Eur J Pediatr Surg. 2000; 10 Suppl 1: 31–32. doi: 10.1055/s-2008-1072411. [DOI] [PubMed] [Google Scholar]
- 11.Veenboer PW, Bosch JL, Asbeck FW, Kort LM. Upper and lower urinary tract Outcomes in adult myelomeningocele patients: a systematic review. PLoS ONE. 2012; 7(10). doi: 10.1371/journal.pone.0048399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holzbeierlein J, Pope JC IV, Adams MC, Bruner J, Tulipan N, Brock JW III. The urodynamic profile of myelodysplasia in childhood with spinal closure during gestation. J Urol. 2000; 164 (4): 1336–39. doi: 10.1016/S0022-5347(05)67191-1. [DOI] [PubMed] [Google Scholar]
- 13.Singhal B, Mathew KM. Factors affecting mortality and morbidity in adult spina bifida. Eur J Pediatr Surg. 1999: 9(Suppl 1): 31–32. doi: 10.1055/s-2008-1072310. [DOI] [PubMed] [Google Scholar]
- 14.Greig JD, Young DG, Azmy AF. Follow-up of spina bifida children with and without upper renal tract changes at birth. Eur J Pediatr Surg. 1991; 1(1): 5–9. doi: 10.1055/s-2008-1042449. [DOI] [PubMed] [Google Scholar]
- 15.Lawrenson R, Wyndaele JJ, Vlachonikolis I, Farmer C, Glickman S. Renal failure in patients with neurogenic lower urinary tract dysfunction. Neuroepidemiology. 2001; 20(2): 138–43. doi: 54774. [DOI] [PubMed] [Google Scholar]
- 16.Müller T, Arbeiter K, Aufricht C. Renal function in meningomyelocele: risk factors, chronic renal failure, renal replacement therapy and transplantation. Curr Opin Urol. 2002; 12(6): 479–84. doi: 10.1097/01.mou.0000039446.39928.32. [DOI] [PubMed] [Google Scholar]
- 17.Lapides J, Diokno AC, Silber SM, Lowe BS. Clean, Intermittent self-catheterization in the treatment of urinary tract disease. J Urol. 1972; 107: 458–61. doi: 10.1016/S0022-5347(02)80359-7. [DOI] [PubMed] [Google Scholar]
- 18.Moore C, Kogan B, Parekh A. Impact of urinary incontinence on self-concept in children with spina bifida. J Urol. 2004; 171: 1659–62. doi: 10.1097/01.ju.0000117865.98229.e5. [DOI] [PubMed] [Google Scholar]
- 19.Lemelle J, Guillemin F, Aubert D, Guys J, Lottmann H, Lortat-Jacob S, Moriquand P, Ruffion A, Schmitt M. Quality of life and continence in patients with spina bifida. Qual Life Res. 2006; 15(9): 1481–92. doi: 10.1007/s11136-006-0032-x. [DOI] [PubMed] [Google Scholar]
- 20.Liu JS, Dong C, Casey JT, Greiman A, Mukherjee S, Kielb SJ. Quality of life related to urinary continence in adult spina bifida patients. Cent European J Urol. 2015; 68: 61–67. doi: 10.5173/ceju.2015.01.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schoenmakers MA, Uiterwaal CS, Gulmans VA, Gooskens RH, Helders PJ. Determinants of functional independence and quality of life in children with spina bifida. Clin Rehabil. 2005; 19(6): 677–85. doi: 10.1191/0269215505cr865oa. [DOI] [PubMed] [Google Scholar]
- 22.Seth J, Haslam C, Panicker J. Ensuring patient adherence to clean intermittent self-catheterization. Patient Prefer Adherence. 2014; 8: 191–98. doi: 10.2147/PPA.S49060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaefer M, Pabby A, Kelly M, Darbey M, Bauer SB. Improved bladder function after prophylactic treatment of the high risk neurogenic bladder in newborns with myelomeningocele. J Urol. 1999; 162: 1068–71. [DOI] [PubMed] [Google Scholar]
- 24.Edelstein RA, Bauer SB, Kelly MD, Darbey MM, Peters CA, Atala A, Mandell J, Colodny AH, Retik AB. The long-term urological response of neonates with myelodysplasia treated proactively with intermittent catheterization and anticholinergic therapy. J Urol. 1995; 154: 1500–04. [PubMed] [Google Scholar]
- 25.IBM Corp. Released 2016. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp. [Google Scholar]
- 26.Almodhen F, Capolicchio JP, Jednak R, et al. Postpubertal urodynamic and upper urinary tract changes in children with conservatively treated myelomeningocele. J Urol. 2007; 178: 1479–82. doi: 10.1016/j.juro.2007.05.171. [DOI] [PubMed] [Google Scholar]
- 27.Snow-Lisy DC, Yerkes EB, Cheng EY. Update on urological management of spina bifida from prenatal diagnosis to adulthood. J Urol. 2015; 194(2): 288–96. doi: 10.1016/j.juro.2015.03.107. [DOI] [PubMed] [Google Scholar]
- 28.Castillo J, Ostermaier KK, Fremion E, Collier T, Zhu H, Huang GO, Tu D, Castillo H. Urologic self-management through intermittent self-catheterization among individuals with spina bifida: a journey to self-efficacy and autonomy. J Pediatr Rehabil Med. 2017; 10(3–4): 219–226. doi: 10.3233/PRM-170447. [DOI] [PubMed] [Google Scholar]
- 29.Fletcher JM, Copeland K, Frederick JA, Blaser SE, Kramer LA, Northrup H, Dennis M. Spinal lesion level in spina bifida: a source of neural and cognitive heterogeneity . J Neurosurg Pediatr. 2005; 102(3): 268–79. doi: 10.3171/ped.2005.102.3.0268. [DOI] [PubMed] [Google Scholar]
- 30.Schechter MS, Liu T, Soe M, Swanson M, Ward E, Thibadeau J. Sociodemographic attributes and spina bifida outcomes. Pediatrics. 2015; 135(4): 957–64. doi: 10.1542/peds.2014-2576d. [DOI] [PMC free article] [PubMed] [Google Scholar]