Abstract
The pulsatility of GnRH release is essential for reproductive function. The key events in reproductive function, such as puberty onset and ovulatory cycles, are regulated by the frequency and amplitude modulation of pulsatile GnRH release. Abnormal patterns of GnRH pulsatility are seen in association with disease states, such as polycystic ovarian syndrome and anorexia nervosa. Recent studies with physiological, track-tracing, optogenetic and electrophysiological recording experiments indicate that a group of kisspeptin neurons in the arcuate nucleus (ARC) of the hypothalamus are responsible for pulsatile GnRH release. Thus, the kisspeptin neuron in the ARC has been called the “GnRH pulse-generator.” However, a few pieces of evidence do not quite fit into this concept. This article reviews some old works and discusses unresolved issues on the mechanism of GnRH pulse generation.
Keywords: Pulsatile GnRH release, kisspeptin, neuropeptide Y, neurokinin B, norepinephrine, GABA
Introduction:
In the early 1970’s the GnRH molecule was isolated and sequenced from the pig and sheep hypothalamus (Matsuo et al., 1971; Amoss et al., 1971). At about the same time Knobil’s group reported that the anterior pituitary releases LH in a pulsatile manner (Dierschke et al., 1970; Gay and Sheth, 1972). Subsequently, the same group reported that pulsatile administration, not continuous infusion, of GnRH in female monkeys with the lesioned basal hypothalamus or immature hypothalamus can maintain normal reproductive function (Belchetz et al., 1978; Knobil et al., 1980) and initiates puberty (Wildt et al., 1980). Knobil’s group further extended their findings to show that a steady-state pulsatile GnRH release from the hypothalamus is only necessary for the regulation of the ovulatory cycle (Knobil et al., 1980). However, Spies and his colleagues demonstrated that increase in the frequency, amplitude and baseline GnRH release from the hypothalamus as the consequence of the estradiol feedback action is also important for reproductive function (Spies and Norman, 1975; Levine et al., 1985; Pau et al., 1993). Since then, the mechanism by which pulsatile GnRH release is generated became one of the most fascinating topics in reproductive neuroendocrinology. Oscillatory activity of multiple neurons synchronizing periodically in the brain is not uncommon. However, the interval of oscillatory GnRH release occurring on the order of hours is much longer than other brain oscillations, such as neuronal oscillations detected by EEG or recurrent thalamo-cortical resonance that occur on the order of milliseconds to seconds. Oscillatory activity among multiple neurons requires cell-cell connections, but mammalian GnRH neurons are widely scattered in the preoptic area and basal hypothalamus. How do GnRH neurons communicate with each other? Is there any source that drives the GnRH neuronal oscillation? How do gonadal steroids modulate GnRH neuronal oscillators? There are several excellent articles on this topic (Constantin, 2017; Herbison, 2018; Nestor et al., 2018). This short review article discusses current views and unresolved questions regarding GnRH pulse generation.
Historical perspectives:
A series of studies led by Knobil’s group in the 1970’s indicate that pulsatile release of GnRH from the hypothalamus drives pulsatile release of LH and FSH in monkeys (see Knobil, 1980). To assess GnRH neuronal activity in the hypothalamus the group further adapted to recording multiple unit activity (MUA). Unlike single unit activity recording, MUA is recorded by electrodes with low impedance (~50kΩ). As such, electrical activity recorded through this method represents differential potentials from the field, rather than activity of a single neuron. It turned out, however, that this method was quite a useful approach for monitoring synchronous activity from multiple GnRH neurons or a group of neurons associated with pulsatile GnRH release, as an abrupt increase in electrical activity occurs in perfect unison with an LH pulse in ovariectomized rats and monkeys (Kawakami et al., 1982; Wilson et al., 1984). Not surprisingly, Knobil’s group was not able to confirm that the tip of MUA electrodes was in close proximity to GnRH neurons or their fibers (Silverman et al., 1986). A quarter century later, another group reported that GnRH pulse-generating activity by MUA is readily recorded when electrodes are located in or near the arcuate nucleus (ARC), in close proximity to kisspeptin neurons in goats (Wakabayashi et al., 2010).
Studies of deafferentation of the medial basal hypothalamus (MBH) and discrete lesions within hypothalamus have identified that pulsatile release of GnRH requires the presence of the MBH, more specifically, the ARC in rats, monkeys and sheep (Blake and Sawyer, 1974; Plant et al., 1978; Pau et al., 1982). Although GnRH neuroterminals and other surrounding cells in the median eminence (ME) may have the capacity to generate periodical release of neurohormones, as fragments of the rat ME release GnRH in a pulsatile manner (Rasmussen, 1993), as discussed later, neurons in the ARC generate periodical activity with a frequency similar to that seen from GnRH/LH release in vivo.
Endogenous capacity of the GnRH neuron for oscillatory peptide release
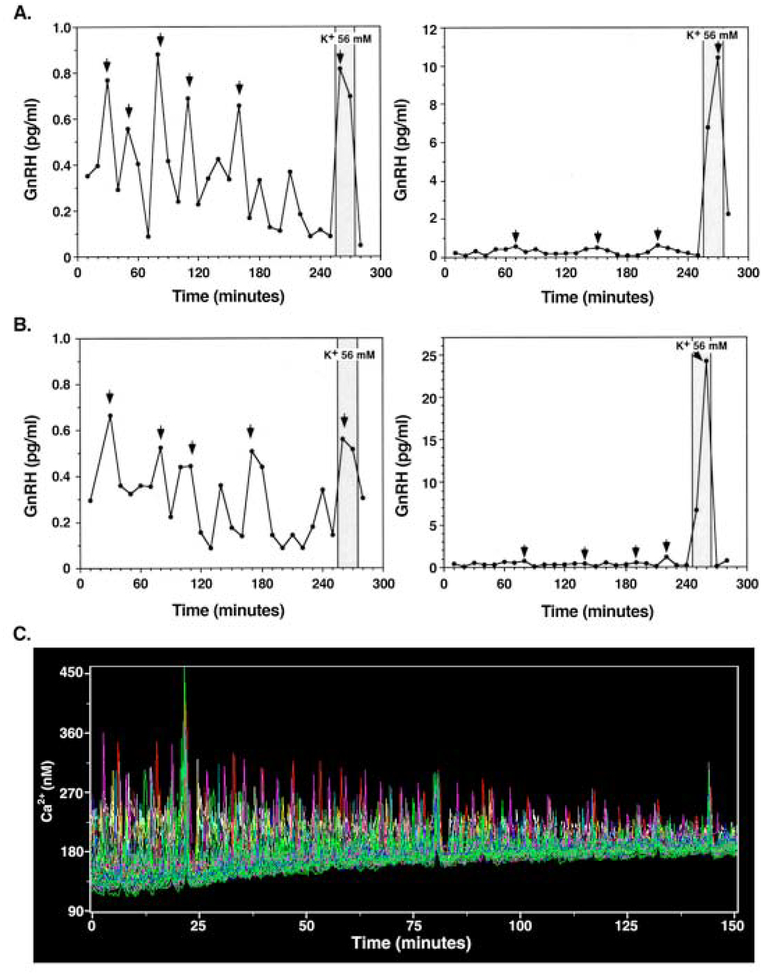
GnRH neurons themselves have the capacity to release the decapeptide in an oscillatory manner. Immortalized GT1 GnRH secreting cells and primary GnRH neurons derived from embryonic olfactory placode release the GnRH peptide in pulsatile manner with species-specific intervals (Figs. 1A and 1B, Weiner et al., 1992; Terasawa et al., 1999a; Duittoz et al., 2000; Funabashi et al., 2000; Moore et al., 2002). Primate and murine GnRH neurons also exhibit periodical synchronized intracellular calcium oscillations (Fig. 1C, Terasawa et al., 1999b; Temple et al., 2003) and increased firing activity or oscillatory calcium activity in response to estradiol, ATP, and kisspeptin (Abe and Terasawa, 2001; Abe et al., 2008; Noel et al., 2009; Constantin et al., 2009). Importantly, pulsatile release of GnRH requires full maturation of GnRH neurons (Constantin et al., 2009; Kurian et al., 2011). Primary GnRH neuronal cultures derived from the fetal olfactory placode contain non-neuronal cells, such as fibroblast and epithelial cells, but there are very little other types of neurons and glia (Terasawa et al., 1993; Fueshko and Wray, 1993). Although periodically synchronized intracellular calcium signaling among GnRH neurons occurring at ~60 min intervals in vitro (Fig. 1C) is speculated in association with the decapeptide release, this has not been directly confirmed.
Figure 1:
GnRH neurons derived from monkey embyro in cultures exhibit release the GnRH peptide in a pulsatile manner (A and B) and individual GnRH neurons exhibit intracellular calcium [Ca2+]i oscillations, which periodically synchronize at ~60 min intervals (C). Two examples each of in vitro GnRH release from cultures obtained from the olfactory placode (A) or the migratory pathway (B) are shown. GnRH release was pulsatile. GnRH peaks, revealed by PULSAR algorithm, are indicated by arrows. All GnRH peaks revealed by PULSAR algorithm are above the assay sensitivity level. Cultures were also exposed to high KCl (56 mM). Note that GnRH release patterns in cultures of the olfactory placode and migratory pathways were similar. C. An example of the synchronization of [Ca2+]i oscillations in 50 GnRH neurons for the period of 152 min. Each color represents the activity of an individual cell. Note that synchronization occurred at 22, 81, and 143 min after time 0, which gives 59 and 61 min intervals between synchronizations. The amplitude of the synchronized pulses is larger than normal pulses, and the post-excitatory suppressions are seen right after the synchronized pulse. The gradual decrease of signal is caused by photobleaching. Reproduced from Terasawa, et al., Endocrinology 140, 1432–1441, 1999, and Terasawa, et al., J. Neurosci., 19, 5898–5909, 1999 with permission pending.
Direct in vivo measurements of GnRH release:
To discuss pulsatile GnRH release in vivo, a direct measurement of GnRH is essential. This was first achieved by collecting the portal blood under anesthesia or acute condition, as the portal circulation is located in the bottom of the brain, where accessibility is limited. With this method, the simultaneous measurement of hypothalamic GnRH and circulating LH is possible and the method has been successively applied to studies in rats and sheep (Sarkar et al., 1976, Clarke and Cummins, 1982, Caraty et al., 1982; Moenter et al., 1992). Importantly, in most cases, each elevated GnRH release is followed by an elevated LH release. Of note, this is the basis for measuring circulating LH levels as a surrogate for GnRH release in various animal models including humans.
Alternative methods for measuring GnRH release in unanesthetized monkeys were developed in this laboratory. First, we established a push-pull perfusion method in rhesus monkeys (Terasawa, 1994) by adapting the method reported by Ramirez’s group in rats and sheep (Levine and Ramirez, 1982; Levine et al., 1982). A fine double lumen stainless steel cannula is inserted into the pituitary stalk and median eminence (S-ME), where artificial cerebrospinal fluid (aCSF) is continuously infused through an inner cannula, while perfusates are collected through an outer cannula in fractions. This is an extremely powerful method for assessing events in the S-ME, where not only GnRH terminals, but also many other neuroterminals from neurotransmitter and neuromodulator neurons are present. This method allowed us to assess release patterns of multiple neurochemicals/neuromodulators in the S-ME with a fine time resolution, and to apply agonists/antagonists for various neuromoulators to examine the responsiveness of the GnRH neuronal system. However, we do not use this approach any longer, as the push-pull perfusion method requires animal chairing for a minimum of 3 days. Subsequently, we developed a microdialysis method (Frost et al., 2008). With this method, we use a double lumen fine stainless steel cannula with a semipermeable membrane attached to the tip of the outer cannula. Again, aCSF is infused into the inner cannula at an ultra-slow speed, and effluxes (dialysates) are collected from the S-ME in fractions. Again, this method allows us to assess the release pattern of neurohormones in the S-ME and observe the responses to agonists/antagonists for various neuromodulators. In general, microdialysate (efflux) concentrations of neurochemical substances per 10-min fraction are not as high as in 10-min samples collected by the push-pull perfusion method. As such, with the microdialysis method often we have to sacrifice the time resolution to 20 min or longer fractions, especially when we measure multiple neurochemicals/neuromodulators in the S-ME. Note that even though our microdialysis probe consists of 5 mm length, an active portion of the probe through which neuropeptides and neuromodulators are collected, appears to be limited to the tip of the probe (1–2 mm) located in the S-ME. This speculation is based on our observations that GnRH concentration map with microdialysis in the S-ME (Kenealy et al., 2013) and push pull perfusion (Gearing and Terasawa, 1988) are very similar. Perhaps, with the microdialysis method the concentration of efflux depends on chemical equilibrium between the fluid inside the probe and the extracellular tissue space, such that only the efflux from the ME, not from the hypothalamus per se, reflects measured neurotransmitters/neuropeptide values.
Findings in this laboratory before discovery of kisspeptin:
Taking advantage of our ability to assess two neurochemicals in the S-ME simultaneously, we have examined synchronous release of GnRH with other neuropeptides and neurotransmitters for many years. Primarily, we focused on endogenous neurochemicals, in which agonists and antagonists have been reported to modify pulsatile release of LH/GnRH. Among them are norepinephrine (NE), neuropeptide Y (NPY), kisspeptin, gamma amino butyric acid (GABA), and glutamate. As described below, pulses of kisspeptin, NPY, and NE had a high incidence of synchronization with GnRH pulses (Terasawa et al., 1988; Woller et al., 1992, Keen et al., 2008). However, we were not able to find any correlated changes between GnRH and GABA or glutamate (see Terasawa et al., 1999).
Neuropeptide Y (NPY):
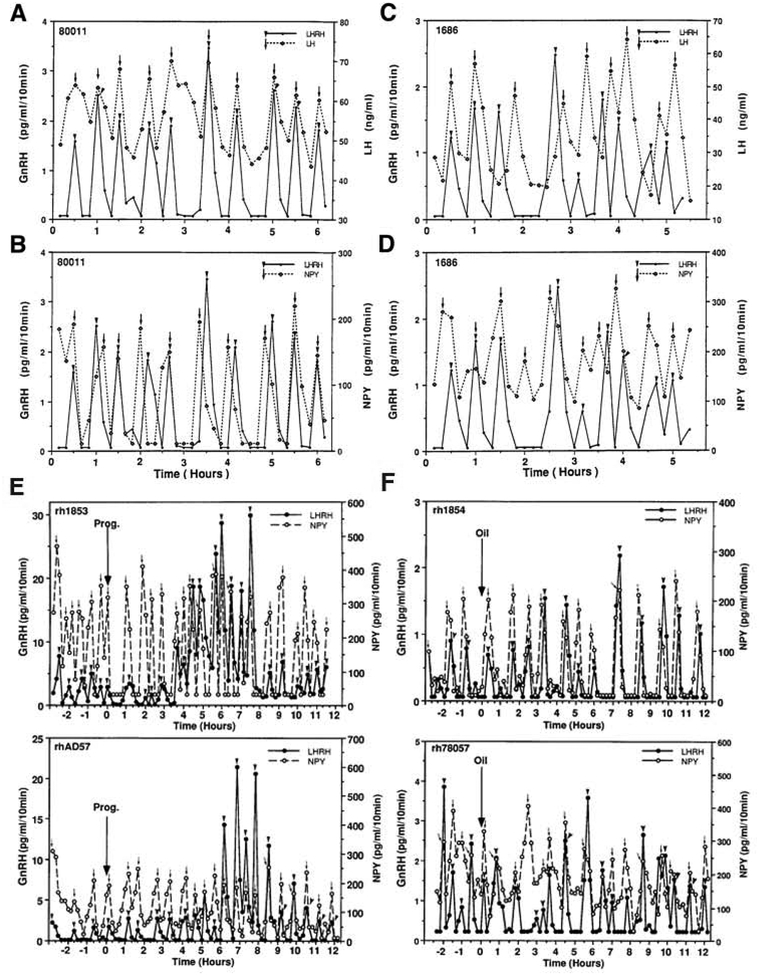
NPY is one of the first peptides found that modulates LH/GnRH release (see Kalra and Crowley, 1992). Initially, we found that NPY infusion into the S-ME stimulates GnRH release in a dose responsive manner in ovariectomized (OVX) adult female rhesus monkeys (Woller and Terasawa, 1991). Subsequently, we observed that infusion of an NPY antibody into the S-ME suppressed GnRH pulses in a dose responsive manner in OVX females (Woller et al. 1992). Accordingly, we speculated that NPY release might be pulsatile and correlated to GnRH release. We measured GnRH and NPY in the same samples collected from the S-ME at 10-min intervals. We also collected plasma samples at the same time for LH measurement. Indeed, we found that NPY release is pulsatile and NPY peaks occur with GnRH peaks, or 10 min preceding GnRH peaks (Figs. 2B and 2D, Woller et al., 1992). Subsequent calculations indicate that on average the NPY peak occurs ~5-min before GnRH peaks, which are followed by LH peaks with ~5 min delay (Fig. 2A and 2C, Woller et al., 1992). Together, it appears that the NPY pulse is necessary for the pulsatility of GnRH release. In fact, signaling by NPY pulses to GnRH neurons appears to be quite important as the interface between energy balance and reproductive function. NPY neurons are negatively and positively regulated by leptin from fat tissues and ghrelin from the stomach, respectively (see Hill and Elias, 2018). As such, reproductive function will not be maintained under negative energy balance.
Figure 2:
Correlated release of GnRH in the stalk median eminence and LH release in general circulation (A, C) and synchronous release of GnRH and NPY in the stalk median eminence (B, D) from an orchidectomized male monkey (left) and an ovariectomized female monkey (right). Arrowheads indicate LHRH pulses and arrows indicate LH or NPY pulses detected by PULSAR. Note that in male monkey, all GnRH peaks are synchronized with LH pulses (A), and 9 of 10 NPY peaks either precede by one point or coincide with corresponding GnRH peaks (C). Similarly, in female monkey, GnRH and LH pulses and NPY and GnRH pulses are highly correlated as GnRH peaks either precede or coincide with LH peaks (B), and NPY and GnRH pulses are also highly correlated (D). Effects of progesterone (E) or oil (F, control for progesterone) on the release of GnRH (solid line) and NPY (broken line) measured in aliquots of the same perfusate samples from the S-ME of two ovariectomized female monkeys. Progesterone or oil was injected at time zero in animals treated with EB (30 μg) 24 h earlier. Arrowheads and arrows indicate pulses of GnRH and NPY, respectively, detected by Pulsar. Note that in E the pulse frequency was increased by progesterone in both GnRH and NPY release, maintaining the synchronous release of both neuropeptides. In contrast, the pulse amplitude of GnRH release increased dramatically 3–9 h after progesterone treatment, while the pulse amplitude of NPY remained unaltered. In F, oil injection altered neither the pulse frequency nor pulse amplitude. Again, the synchronous release of GnRH and NPY was unaltered. Reproduced from Woller et al., Endocrinology. 130, 2333–2342, 1992, and Woller and Terasawa, Endocrinology 135, 1679–1686, 1994, with permission pending.
Because in a previous series of studies we found that the treatment of OVX female monkey with a small dose of estradiol (E2, 10–30 μg) plus progesterone (2.5mg) results in LH/GnRH surges with a peak latency of 6–9h (Terasawa et al., 1984; Yeoman and Terasawa, 1984), we examined whether the steroid hormone-induced modification of GnRH pulsatility affects pulsatility of NPY release. In the study, the dose of E2 is very small, such that it suppresses LH levels in OVX females, but does not result in an LH surge. Progesterone treatment 24 h after E2, however, induces an LH surge (Terasawa et al., 1984). The results from simultaneous measurements of GnRH and NPY in push-pull perfusates indicate that a progesterone-induced LH surge is accompanied by increases in the mean release of GnRH and NPY. Moreover, the progesterone-induced mean GnRH increase is a consequence of increases in both pulse amplitude and pulse frequency, whereas the progesterone-induced mean NPY increase is accelerated pulse frequency only, not the pulse amplitude. Oil treatment after EB administration (control for progesterone) does not alter any parameter of GnRH and NPY pulses. Importantly, the NPY and GnRH pulses occur in a highly correlated manner, regardless of whether monkeys were treated with EB + progesterone or EB alone, and again NPY pulses preceded GnRH pulses by ~5 min (Figs. 2E and 2F, Woller et al., 1994). These observations indicate the presence of two layers of mechanisms for coincidental GnRH and NPY pulse-generation. The first layer consists of the pulse-generating source that is directly or indirectly connected with GnRH and NPY neurons and it determines the pulse frequency. This structure can be sensitive to gonadal steroids, as the pulse frequency of GnRH and NPY is accelerated simultaneously. Obviously, nature and neuronal substrates of this structure are yet to be identified. The second layer is kisspeptin neurons containing estrogen receptor alpha (ERα), through which estradiol action (and perhaps progesterone action through progesterone receptors) is transduced to GnRH neurons with a great amplification. Previously, we have shown the significant amplification of kisspeptin signaling to GnRH neurons by gonadal steroids (Guerriero et al., 2012, Garcia et al., 2017, 2018; Terasawa et al., 2018). As to kisspeptin neurons, I will discuss more in a later section.
Note that there is confusion as to whether NPY is stimulatory or inhibitory to GnRH/LH release. While in female monkeys we consistently reported that direct infusion of NPY into the ME stimulates GnRH release regardless of the presence or absence of circulating estradiol (Woller and Terasawa, 1991; Woller et al., 1992, Woller and Terasawa, 1992), initially Spies and collaborators reported that iv injection of NPY suppresses LH release (Kaynard et al., 1990), but later the they found that direct infusion of NPY into the ME stimulated GnRH release, especially with a small elevation of circulating estradiol (Pau et al., 1995). We believe that this is an issue of site-dependent NPY action on GnRH release. GnRH neurons express both inhibitory Y1 receptors as well as stimulatory Y4 receptors (Roa and Herbison. 2012) and whichever predominant NPY receptor subtype is activated, GnRH neurons would be inhibited or stimulated.
Norepinephrine (NE):
Catecholamines--dopamine, NE and epinephrine (E)--were the first neurotransmitters found to modify LH release (Sawyer et al, 1949). Moreover, α1 adrenergic, but not α2 or β adrenergic, input from NE/E signaling has been shown to alter LH and GnRH pulsatility and GnRH pulse generating activity (Bhattacharya et al., 1972, Kaufman et al., 1985; Gearing et al, 1991a, 1991b, 1991c). Thus, we have examined 1) whether NE release in the S-ME is pulsatile, and if so, 2) whether pulsatile GnRH release is correlated to release of NE and its metabolites in the same perfusate samples collected by the push-pull perfusion method from the S-ME. Results indicate that NE release is pulsatile and each GnRH pulse is correlated with every other NE pulse: The frequency of NE was 2 pulses/h, whereas GnRH was 1 pulse/h (Terasawa et al., 1988). The NE precursor, dopamine, and their metabolites dihydrophenyl glycol (DOPEG), methoxy hydroxyphenyl glycol (MOPEG), dihydroxyphenyl acetic acid (DOPAC) and homovanillic acid (HVA), however, are not consistently correlated to GnRH pulses (see Terasawa et al., 1988). Periodical increases in circulating NE at ~90-min intervals in rhesus monkeys have been reported (Levin et al., 1978).
Role of kisspeptin neurons as a source of GnRH pulse generation:
Discovery of genetic mutations in the kisspeptin receptor, KISS1R (GPR54), and its ligand kisspeptin resulting in delayed puberty and infertility in humans and mice (Seminara et al., 2003: de Roux et al., 2003) and a subsequent finding that mutations of the genes encoding neurokinin B (NKB) and its receptor NK3R also induce hypogonadotropic hypogonadism (Topaloglu et al., 2009) have made exponential progress in our understanding of the regulation of GnRH release. The presence of kisspeptin neurons and its receptors in the hypothalamus is essential for GnRH pulsatility, as the absence of KISS1R in human and deletion of Kiss1 or Kiss1r in mice results in the absence of pulsatile LH release (Seminara et al., 2003, Tenenbaum-Rakover et al., 2007; Lapatto et al., 2007, Steyn et al., 2013). An elegant study with GCaMP fiber photometric approaches by Herbison’s group further indicates that individual kisspeptin neurons in the ARC of mice exhibit periodical increases in intracellular calcium [Ca2+]i and a group of ARC kisspeptin neurons exhibit synchronous activity of [Ca2+]i at an interval of 15–20 min, coinciding with LH pulses in gonadectomized mice (Clarkson et al., 2017). Together with additional experiments using channelrhodopsin and archaerhodopsin transfection in kisspeptin-Cre mice that allows stimulation and inhibition of selective ARC kisspeptin neurons, respectively, the authors conclude that activation of ARC kisspeptin neurons is necessary and sufficient for generation of LH (and presumably GnRH) pulses (Clarkson et al., 2017). A similar conclusion is drawn by Kelly’s group by optogenetic stimulation and recording of synchronized firing activity of kisspeptin neurons in the ARC and GnRH neurons in the preoptic area (Qiu et al., 2016). Therefore, a set of kisspeptin neurons represents the most prominent machinery responsible for GnRH pulse generation.
Because 100% of kisspeptin neurons in the ARC co-express NKB and dynorphin in several species including rodents and ruminants, the concept that KNDy (Kisspeptin-NKB-Dynorphin) is important for GnRH pulsatility (see Goodman et al., 2014) arose. The co-localization of kisspeptin with NKB and dynorphin in the same ARC neurons, however, may not be essential for postulating that these 3 neuropeptides are involved in pulse-generation. In primates co-localization of these 3 neuropeptides in the same neuron is far less than 100 % (Ramaswamy et al., 2010; Hrabovszky et al., 2012), yet the 3 types of neurons may form a functional network in the MBH. In fact, pulsatility can be modified at the level of the neuroterminal in the median ME, as discussed in a later section. A series of developmental studies in this lab (Garcia et al., 2017; 2018) further indicate that kisspeptin and NKB signaling undergoes maturational changes with a quite different time course, such that application of “kisspeptin and NKB signaling in the single cell concept” is not easy to apply.
A recent report by Lippincott et al. (2019) indicates that a family of hypogonadotropic hypogonadism patients due to mutation in TAC3 responded to kisspeptin challenges exhibiting increases in LH release and infusion of the opioid antagonist naloxone resulted in unambiguous pulsatile LH release. A similar finding was also made with NKB deficient mice (Lippincott et al., 2019). It is possible that during maturational process, the absence of NKB neurons is compensated by other another mechanism. Nevertheless, the findings of Lippincott et al (2019) clearly suggest that GnRH pulsatility can occur in the absence of NKB neurons, and that the mechanism for GnRH pulse generation consists of more than a single layer.
Concept that the brain has a basic rhythmic activity:
There is evidence showing that the mechanism governing pulsatile GnRH release is not completely independent of activity in the rest of the brain. First, it has been shown that in human subjects at the pubertal age the occurrence of slow-wave (deep) sleep, not rapid eye movement (REM) sleep or light sleep stages, is associated with the onset of LH pulses (Shaw et al., 2012). Moreover, fragmentations of deep sleep by loud auditory stimuli in pubertal children do not influence the relationship between slow-wave sleep and onset of LH pulses (Shaw et al., 2015), indicating there is a common mechanism between GnRH pulse-generation and sleep rhythm.
Second, Rasmussen and Malven (1981) have reported in ovariectomized ewes housed in a chamber that LH pulses assessed at 5-min intervals are highly correlated with rest-activity cycles. Those authors have monitored behavioral activities by phonocartridge detecting chamber vibrations caused by foot movements or abrupt postural changes of ewes. Temporal relationships between rhythms in motor activity and plasma LH levels are examined by cross-spectral analysis. Both activity and plasma LH levels fluctuate at ~36 min intervals and the occurrences of rhythmicity for LH and for activity were highly correlated either in phase or 180° out of phase (Rasmussen and Malven, 1981).
Based on recurring REM sleep cycles at every 1–2 h intervals, the concept that “basic rest-activity cycle (BRAC)” has been proposed by Kleitman (1963). For example, various physiological functions, such as sleep wakefulness (Kleitman, 1982), eating and drinking behaviors (Blessing et al., 2012), and thermo regulation (Blessing, 2018), recur at 1–2 h intervals. Because pulsatile GnRH release occurs at 30–90 min intervals, though periodicity varies among species, developmental ages, and physiological conditions, such as circulating gonadal steroid hormone levels, the possible relationship between GnRH pulse-generation and BRAC has been previously discussed (Rasmussen, 1986). More than thirty years later, however, we know nothing about the source or neural substrates involved in BRAC in the brain.
Pulsatility at the median eminence:
It has been reported that pulsatile release of GnRH is observed in rat ME fragments or retrochiasmatic-MBH tissues, in which GnRH perikarya are missing (Rasmussen, 1993; Bourguignon et al., 1997). In our own studies, synchronization of GnRH pulses with kisspeptin, NPY and NE described above is seen in a restricted area of the ME. Because in the primate hypothalamus a small number of GnRH, kisspeptin, and NPY neuroperikarya are present in the ME (Goldsmith et al., 1983, Ramaswamy et al., 2008; McDonald and Calka, 1994), a contribution of cell bodies cannot be excluded. Nevertheless, it is quite likely that GnRH neurofibers in the ME release the decapeptide in a pulsatile manner. We have previously shown that not only neuroterminals and cell bodies, but also dendrites of monkey GnRH neurons are equipped for decapeptide neurosecretion (Fuenzalida et al., 2011). Moreover, Herbison and colleagues have termed GnRH terminal fibers in the ME as “Dendron,” because they possess the dual properties of dendrites receiving a substantial amount of synaptic innervation and axons conducting action potentials (Herde et al, 2013). Importantly, we have consistently shown that pulsatile GnRH release is modified by local application of agonists and antagonists for neuromodulators/neurotransmitters, such as NPY, kisspeptin, NKB, GABA, glutamate, and NE.
Conclusions and unresolved issues:
As described earlier, Herbison and colleagues have clearly shown that kisspeptin neurons are a major cell group modifying GnRH pulse generation. However, there are several phenomena where it might be premature to call the kisspeptin neuron, the “pulse generator”. First, NPY pulses occur synchronously with GnRH pulses, regardless of the presence of absence of gonadal steroids (Woller and Terasawa, 1991, 1994) and modification of pulsatile NPY release by antiserum to NPY alters GnRH pulses (Woller et al., 1992). Although we have not done simultaneous measurements of kisspeptin and NPY in the same sample, we can assume that synchronous release of kisspeptin and NPY occurs in the monkey S-ME, as kisspeptin pulses accompany GnRH pulses (Keen et al., 2008). Does this mean a common mechanism of pulse-generation among neurons in the ARC? Or, does rhythmic activity of kisspeptin neurons lead to NPY rhythmicity, or vice versa? Do other neurons in the ARC, such as POMC neurons, also release their peptides in a pulsatile manner? Because innervations between NPY/Agouti-Related Peptide (AgRP) neurons and kisspeptin (Padilla et al., 2017; Fu and van den Pol, 2010) or POMC and kisspeptin neurons (Manfredi-Lozano et al., 2016; Fu and van den Pol, 2010) are present, coordinated activity within the ARC is quite possible. Furthermore, similar to kisspeptin signaling to GnRH neurons (Kirilov et al., 2013; Yip et al., 2019), NPY and POMC neurons could directly influence activity of GnRH neurons (Li et al., 1999; Israel et al., 2012; Roa and Herbison, 2012).
Second, recent work in OVX sheep by Clarke et al. (2018) showing that suppression of LH pulses by icv infusion of an NKB antagonist is restored by a systemic, small dose of continuous, not pulsatile, infusion of kisspeptin-10. In that paper, the authors conclude that kisspeptin-10 augments excitability of GnRH neurons and kisspeptin signaling is downstream of NKB signaling, such that the ability to generate pulses of decapeptide release in GnRH neurons is essential. Then a question arises: Is this an analogous situation to the mammalian circadian rhythms where each cell/tissue posses endogenous ~24 h rhythm, yet the neuron in the suprachiasmatic nucleus dictates overall circadian rhythmicity?
Third, while acute ablation of kisspeptin neurons in the ARC with a neurotoxin in adult animals disrupts GnRH secretory activity, as LH levels are low and unaltered by OVX or OVX+E2 (Mittelman-Smith et al., 2012), prenatal deletion of Kiss1 by transgenic approach does not interfere with GnRH secretory activity, as it accelerates puberty onset (vaginal opening) and LH levels at puberty are higher than control animals (Mayer and Boehm, 2011, Dubois et al., 2015). Although in those studies pulsatility of GnRH release is not assessed and in both situations steroid feedback mechanisms to GnRH neurons are impaired, prenatal deletion of kisspeptin neurons may allow activation of alternative compensatory pathways for the regulation of the GnRH neurosecretory system. The neuronal substrate of compensatory signaling in this situation is presently unknown.
Fourth and most importantly, are there any common genes regulating ~60 min periodicity among the ARC neurons, equivalent to clock genes in neurons of the suprachiasmatic nucleus? It has been shown that a subset of kisspeptin and NPY neurons arises from POMC progenitor cells (Sanz et al., 2015). Collectively, even though kisspeptin neurons are a primary cell component regulating pulsatile GnRH release in normal condition, there must be multiple layers to the GnRH regulatory mechanism in the hypothalamus. The multiple layers of regulatory mechanisms for GnRH pulsatility protect from a failure to reproduce, hence preserving each species in nature.
Highlights:
Pulsatile release of GnRH is indispensable for reproductive function. This article reviews current concept and unresolved issues on the mechanism of GnRH pulse generation.
Acknowledgements:
The author would like to express her sincere appreciation to her past and current colleagues and trainees for contributions to the work presented in this article. This work is supported by NIH project grants, R01HD011355, R01HD015433, R21HD077447, R21HD092009, and R01HD089495 for ET and NIH center grant, P51OD011106 for the Wisconsin National Primate Research Center.
Funding: Supported by NIH grants: R01HD015433, R01HD089495, R01HD011355, and R21 HD092009. The work was made possible by support (OD011106) for the Wisconsin National Primate Research Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The author declares no competing financial interests.
References:
- Abe H, Terasawa E, 2005. Firing pattern and rapid modulation of activity by estrogen in primate luteinizing hormone releasing hormone-1 neurons. Endocrinology 146, 4312–4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe H, Keen KL, Terasawa E, 2008. Rapid action of estrogens on intracellular calcium oscillations in primate luteinizing hormone-releasing hormone-1 neurons. Endocrinology 149, 1155–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoss M, Burgus R, Blackwell R, Vale W, Fellows R, Guillemin R, 1971. Purification, amino acid composition and N-terminus of the hypothalamic luteinizing hormone releasing factor (LRF) of ovine origin. Biochem. Biophys. Res. Commun 44, 205–210. [DOI] [PubMed] [Google Scholar]
- Belchetz PE, Plant TM, Nakai Y, Keogh EJ, Knobil E, 1978. Hypophysial responses to continuous and intermittent delivery of hypothalamic gonadotropin-releasing hormone. Science 202, 631–633. [DOI] [PubMed] [Google Scholar]
- Bhattacharya AN, Dierschke DJ, Yamaji T, Knobil E, 1972. The pharmacologic blockade of the circhoral mode of LH secretion in the ovariectomized rhesus monkey. Endocrinology 90, 778–786. [DOI] [PubMed] [Google Scholar]
- Blake CA, Sawyer CH, 1974. Effects of hypothalamic deafferentation on the pulsatile rhythm in plasma concentrations of luteinizing hormone in ovariectomized rats. Endocrinology 94, 730–736. [DOI] [PubMed] [Google Scholar]
- Blessing W, Mohammed M, Ootsuka Y, 2012. Heating and eating: brown adipose tissue thermogenesis precedes food ingestion as part of the ultradian basic rest-activity cycle in rats. Physiol. Behav 105, 966–974. [DOI] [PubMed] [Google Scholar]
- Blessing WW, 2018. Thermoregulation and the ultradian basic rest-activity cycle. Handb. Clin. Neurol 156, 367–375. [DOI] [PubMed] [Google Scholar]
- Bourguignon JP, Gérard A, Purnelle G, Czajkowski V, Yamanaka C, Lemaître M, Rigo JM, Moonen G, Franchimont P, 1997. Duality of glutamatergic and GABAergic control of pulsatile GnRH secretion by rat hypothalamic explants: I. Effects of antisense oligodeoxynucleotides using explants including or excluding the preoptic area. J. Neuroendocrinol 9, 183–191. [DOI] [PubMed] [Google Scholar]
- Campbell RE, Gaidamaka G, Han SK, Herbison AE, 2009. Dendro-dendritic bundling and shared synapses between gonadotropin-releasing hormone neurons. Proc. Natl. Acad. Sci. U.S.A 106, 10835–10840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraty A, Orgeur P, Thiery JC, 1982. Demonstration of the pulsatile secretion of LH-RH into hypophysial portal blood of ewes using an original technic for multiple samples. C R Seances Acad Sci III 295, 103–106. [PubMed] [Google Scholar]
- Clarke IJ, Cummins JT, 1982. The temporal relationship between gonadotropin releasing hormone (GnRH) and luteinizing hormone (LH) secretion in ovariectomized ewes. Endocrinology 111, 1737–1739. [DOI] [PubMed] [Google Scholar]
- Clarke IJ, Li Q, Henry BA, Millar RP, 2018. Continuous kisspeptin restores luteinizing hormone pulsatility following cessation by a neurokinin B antagonist in female sheep. Endocrinology 159, 639–646. [DOI] [PubMed] [Google Scholar]
- Clarkson J, Han SY, Piet R, McLennan T, Kane GM, Ng J, Porteous RW, Kim JS, Colledge WH, Iremonger KJ, Herbison AE, 2017. Definition of the hypothalamic GnRH pulse generator in mice. Proc. Natl. Acad. Sci. U.S.A 114, E10216–E10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantin S 2017. Progress and challenges in the search for the mechanisms of pulsatile gonadotropin-releasing hormone secretion. Front. Endocrinol. (Lausanne) 8, 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantin S, Caraty A, Wray S, Duittoz AH, 2009. Development of gonadotropin-releasing hormone-1 secretion in mouse nasal explants. Endocrinology 150, 3221–3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantin S, Caligioni CS, Stojilkovic S, Wray S, 2009. Kisspeptin-10 facilitates a plasma membrane-driven calcium oscillator in gonadotropin-releasing hormone-1 neurons. Endocrinology 150, 1400–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Roux N, Genin E, Carel J-C, Matsuda F, Chaussain J-L, Milgrom E, 2003. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc. Natl. Acad. Sci. U.S.A 100, 10972–10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierschke DJ, Bhattacharya AN, Atkinson LE, Knobil E, 1970. Circhoral oscillations of plasma LH levels in the ovariectomized rhesus monkey. Endocrinology 87, 850–853. [DOI] [PubMed] [Google Scholar]
- Dubois SL, Acosta-Martínez M, DeJoseph MR, Wolfe A, Radovick S, Boehm U, Urban JH, Levine JE, 2015. Positive, but not negative feedback actions of estradiol in adult female mice require estrogen receptor α in kisspeptin neurons. Endocrinology 156, 1111–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duittoz AH, Batailler M 2000. Pulsatile GnRH secretion from primary cultures of sheep olfactory placode explants. J. Reprod. Fertil 120, 391–396. [PubMed] [Google Scholar]
- Fuenzalida LC, Keen KL, Terasawa E, 2011. Colocalization of FM1–43, Bassoon, and GnRH-1: GnRH-1 release from cell bodies and their neuroprocesses. Endocrinology 152, 4310–4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fueshko S, Wray S, 1993. LHRH cells migrate on peripherin fibers in embryonic olfactory explant cultures: an in vitro model for neurophilic neuronal migration. Dev. Biol 166, 331–348. [DOI] [PubMed] [Google Scholar]
- Frost SI, Keen KL, Levine JE, Terasawa E, 2008. Microdialysis methods for in vivo neuropeptide measurement in the stalk-median eminence in the rhesus monkey. J, Neurosci. Methods 168, 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu LY, van den Pol AN, 2010. Kisspeptin directly excites anorexigenic proopiomelanocortin neurons but inhibits orexigenic neuropeptide Y cells by an indirect synaptic mechanism. J. Neurosci 30, 10205–10219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funabashi T, Daikoku S, Shinohara K, Kimura F, 2000. Pulsatile gonadotropin-releasing hormone (GnRH) secretion is an inherent function of GnRH neurons, as revealed by the culture of medial olfactory placode obtained from embryonic rats. Neuroendocrinology 71, 138–144. [DOI] [PubMed] [Google Scholar]
- Garcia JP, Guerriero KA, Keen KL, Kenealy BP, Seminara SB, Terasawa E, 2017. Kisspeptin and neurokinin B signaling network underlies the pubertal increase in GnRH release in female rhesus monkeys. Endocrinology 158, 3269–3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JP, Keen KL, Kenealy BP, Seminara SB, Terasawa E, 2018. Role of kisspeptin and neurokinin B signaling in male rhesus monkey puberty. Endocrinology 159, 3048–3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay VL, Sheth NA, 1972. Evidence for a periodic release of LH in castrated male and female rats. Endocrinology 90, 158–162. [DOI] [PubMed] [Google Scholar]
- Gearing M, Terasawa E, 1988. Luteinizing hormone releasing hormone (LHRH) neuroterminals mapped using the push-pull perfusion method in the rhesus monkey. Brain Res. Bull 21,117–121. [DOI] [PubMed] [Google Scholar]
- Gearing M, Terasawa E, 1991a. Suppression of luteinizing hormone release by the α1-adrenergic receptor antagonist prazosin in the ovariectomized female rhesus monkey. Am. J. Primatol 25, 23–33, 1991. [DOI] [PubMed] [Google Scholar]
- Gearing M, Terasawa E, 1991b. The alpha-1-adrenergic neuronal system is involved in the pulsatile release of luteinizing hormone-releasing hormone in the ovariectomized female rhesus monkey. Neuroendocrinology 53, 373–381. [DOI] [PubMed] [Google Scholar]
- Gearing M, Terasawa E, 1991c. Prostaglandin E2 mediates the stimulatory effect of methoxamine on in vivo luteinizing hormone-releasing hormone (LHRH) release in the ovariectomized female rhesus monkey. Brain Res 560, 276–281. [DOI] [PubMed] [Google Scholar]
- Goldsmith PC, Lambert R, Berizina LR, 1983. Gonadotropin-releasing hormone neurons and pathways in the primate hypothalamus and forebrain In: Norman RL (ed.) Neuroendocrine Aspects of Reproduction, pp. 7–45. New York, NY: Academic Press. [Google Scholar]
- Goodman RL, Coolen LM, Lehman MN, 2014. A role for neurokinin B in pulsatile GnRH secretion in the ewe. Neuroendocrinology 99, 18–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerriero KA, Keen KL, Millar RP, Terasawa E, 2012. Developmental changes in GnRH release in response to kisspeptin agonist and antagonist in female Rhesus monkeys (Macaca mulatta): Implication for the mechanism of puberty. Endocrinology 153, 825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SY, Kane G, Cheong I, Herbison AE, 2019. Characterization of GnRH pulse generator activity in male mice using GCaMP fiber photometry. Endocrinology 160, 557–567. [DOI] [PubMed] [Google Scholar]
- Herbison AE, 2018. The gonadotropin-releasing hormone pulse generator. Endocrinology 159, 3723–3736. [DOI] [PubMed] [Google Scholar]
- Herde MK, Iremonger KJ, Constantin S, Herbison AE, 2013. GnRH neurons elaborate a long-range projection with shared axonal and dendritic functions. J. Neurosci 33, 12689–12697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JW, Elias CF, 2018. Neuroanatomical framework of the metabolic control of reproduction. Physiol. Rev 98, 2349–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrabovszky E, Sipos MT, Molnár CS, Ciofi P, Borsay BÁ, Gergely P, Herczeg L, Bloom SR, Ghatei MA, Dhillo WS, Liposits Z, 2012. Low degree of overlap between kisspeptin, neurokinin B, and dynorphin immunoreactivities in the infundibular nucleus of young male human subjects challenges the KNDy neuron concept. Endocrinology 153, 4978–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel DD, Sheffer-Babila S, de Luca C, Jo YH, Liu SM, Xia Q, Spergel DJ, Dun SL, Dun NJ, Chua SC Jr., 2012. Effects of leptin and melanocortin signaling interactions on pubertal development and reproduction. Endocrinology 153, 2408–2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra SP, Crowley WR, 1992. Neuropeptide Y: a novel neuroendocrine peptide in thecontrol of pituitary hormone secretion, and its relation to luteinizing hormone. Front, Neuroendocrinol 13, 1–46. [PubMed] [Google Scholar]
- Kaufman JM, Kesner JS, Wilson RC, Knobil E, 1985. Electrophysiological manifestation of luteinizing hormone-releasing hormone pulse generator activity in the rhesus monkey: influence of alpha-adrenergic and dopaminergic blocking agent. Endocrinology 116, 1327–1333. [DOI] [PubMed] [Google Scholar]
- Kaynard AH, Pau K-YF, Hess DL, Spies HG, 1990. Third ventricular infusion of neuropeptide Y suppresses lutcinizing hormone secretion in ovariectomized rhesus macaques. Endocrinology. 127, 2437–2444. [DOI] [PubMed] [Google Scholar]
- Kawakami M, Uemura T, Hayashi R, 1982. Electrophysiological correlates of pulsatile gonadotropin release in rats. Neuroendocrinology 35, 63–67. [DOI] [PubMed] [Google Scholar]
- Keen KL, Wegner FH, Bloom SR, Ghatei MA, Terasawa E, 2008. An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology 149, 4151–4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenealy BP, Kapoor A, Guerriero KA, Keen KL, Garcia JP, Kurian JR, Ziegler TE, Terasawa E, 2013. Neuroestradiol in the hypothalamus contributes to the regulation of gonadotropin releasing hormone release. J. Neurosci 33,19051–19059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirilov M, Clarkson J, Liu X, Roa J, Campos P, Porteous R, Schütz G, Herbison AE, 2013. Dependence of fertility on kisspeptin-Gpr54 signaling at the GnRH neuron. Nat. Commun 4, 2492. [DOI] [PubMed] [Google Scholar]
- Kleitman N, 1963. Sleep and Wakefulness. University of Chicago Press, Chicago, IL. [Google Scholar]
- Kleitman N, 1982. Basic rest-activity cycle -- 22 years later. Sleep 5, 311–317. [DOI] [PubMed] [Google Scholar]
- Knobil E,1980. The neuroendocrine control of the menstrual cycle. Recent Prog. Horm. Res 36, 53–88. [DOI] [PubMed] [Google Scholar]
- Knobil E, Plant TM, Wildt L, Belchetz PE, Marshall G, 1980. Control of the rhesus monkey menstrual cycle: permissive role of hypothalamic gonadotropin-releasing hormone. Science 207:1371–1373. [DOI] [PubMed] [Google Scholar]
- Krupa M, Vidal A, Clément F, 2013. A network model of the periodic synchronization process in the dynamics of calcium concentration in GnRH neurons. J. Math Neurosci 3, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurian JR, Keen KL, Terasawa E, 2010. Epigenetic changes coincide with in vitro primate GnRH neuronal maturation. Endocrinology 151, 5359–5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapatto R, Pallais JC, Zhang D, Chan YM, Mahan A, Cerrato F, Le WW, Hoffman GE, Seminara SB, 2007. Kiss1−/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology 148, 4927–4936. [DOI] [PubMed] [Google Scholar]
- Levin BE, Goldstein A, Natlson BH, 1978. Ultradian rhythm of plasma noradrenaline in rhesus monkeys. Nature 272, 164–166. [DOI] [PubMed] [Google Scholar]
- Levine JE, Norman RL, Gliessman PM, Oyama TT, Bangsberg DR, Spies HG, 1985. In vivo gonadotropin-releasing hormone release and serum luteinizing hormone measurements in ovariectomized, estrogen-treated rhesus macaques. Endocrinology 117, 711–721. [DOI] [PubMed] [Google Scholar]
- Levine JE, Pau KY, Ramirez VD, Jackson GL, 1982. Simultaneous measurement of luteinizing hormone-releasing hormone and luteinizing hormone release in unanesthetized, ovariectomized sheep. Endocrinology 111, 1449–1455. [DOI] [PubMed] [Google Scholar]
- Levine JE, Ramirez VD, 1982. Luteinizing hormone-releasing hormone release during the rat estrous cycle and after ovariectomy, as estimated with push-pull cannulae. Endocrinology 111, 1439–1448. [DOI] [PubMed] [Google Scholar]
- Li C, Chen P, Smith MS 1999. Morphological evidence for direct interaction between arcuate nucleus neuropeptide Y (NPY) neurons and gonadotropin-releasing hormone neurons and the possible involvement of NPY Y1 receptors. Endocrinology, 140, 5382–5390. [DOI] [PubMed] [Google Scholar]
- Lippincott MF, León S, Chan YM, Fergani C, Farooqi S, Jones CM, Arlt W, Stewart SE, Cole TR, Terasawa E, Hall J, Shaw ND, Navarro VM, Seminara SB, 2019. Hypothalamic reproductive endocrine activity independent of neurokinin B and dynorphin Signaling. J. Clin. Endocrinol. Metab (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfredi-Lozano M, Roa J, Ruiz-Pino F, Piet R, Garcia-Galiano D, Pineda R, Zamora A, Leon S, Sanchez-Garrido MA, Romero-Ruiz A, Dieguez C, Vazquez MJ, Herbison AE, Pinilla L, Tena-Sempere M, 2016. Defining a novel leptin-melanocortin-kisspeptin pathway involved in the metabolic control of puberty. Mol. Metab, 5, 844–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez de la Escalera G, Choi AL, Weiner RI, 1992. Generation and synchronization of gonadotropin-releasing hormone (GnRH) pulses: intrinsic properties of the GT1–1 GnRH neuronal cell line. Proc. Natl. Acad. Sci. U.S.A 89, 1852–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo H, Baba Y, Nair RM, Arimura A, Schally AV, 1971. Structure of the porcine LH- and FSH-releasing hormone. I. The proposed amino acid sequence. Biochem. Biophys. Res. Commun 43, 1334–1339. [DOI] [PubMed] [Google Scholar]
- Mayer C, Boehm U, 2011. Female reproductive maturation in the absence of kisspeptin/GPR54 signaling. Nat, Neurosci, 14, 704–710. [DOI] [PubMed] [Google Scholar]
- McDonald J, Calka J, 1994. Relationship between neuropeptide Y and luteinizing-hormone-releasing hormone immunoreactivities in the hypothalamus and preoptic region. Acta. Anat. (Basel) 151, 171–179. [DOI] [PubMed] [Google Scholar]
- Moenter SM, Brand RM, Midgley AR, Karsch FJ, 1992. Dynamics of gonadotropin- releasing hormone release during a pulse. Endocrinology 130, 503–510. [DOI] [PubMed] [Google Scholar]
- Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, Lai J, Ciofi P, McMullen NT, Rance NE, 2012. Arcuate kisspeptin/neurokinin B/dynorphin (KNDy) neurons mediate the estrogen suppression of gonadotropin secretion and body weight. Endocrinology 153, 2800–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JP Jr, Shang E, Wray S, 2002. In situ GABAergic modulation of synchronous gonadotropin releasing hormone-1 neuronal activity. J. Neurosci 22, 8932–8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel SD, Keen KL, Baumann DI, Filardo EJ, Terasawa E, 2009. Involvement of G protein-coupled receptor 30 (GPR30) in rapid action of estrogen in primate LHRH neurons. Mol. Endocrinol 23, 349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor CC, Bedenbaugh MN, Hileman SM, Coolen LM, Lehman MN, Goodman RL, 2018. Regulation of GnRH pulsatility in ewes. Reproduction 156, R83–R99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pau KF, Kuehl DE, Jackson GL, 1982. Effect of frontal hypothalamic deafferentation on luteinizing hormone secretion and seasonal breeding in the ewe. Biol. Reprod 27, 999–1009. [DOI] [PubMed] [Google Scholar]
- Pau KY, Berria M, Hess DL, Spies HG, 1993. Preovulatory gonadotropin-releasing hormone surge in ovarian-intact rhesus macaques..Endocrinology 133,1650–1656. [DOI] [PubMed] [Google Scholar]
- Pau KY, Berria M, Hess DL, Spies HG, 1995. Hypothalamic site-dependent effects of neuropeptide Y on gonadotropin-releasing hormone secretion in rhesus macaques. J. Neuroendocrinol 7, 63–67. [DOI] [PubMed] [Google Scholar]
- Plant TM, Krey LC, Moossy J, McCormack JT, Hess DL, Knobil E, 1978. The arcuate nucleus and the control of gonadotropin and prolactin secretion in the female rhesus monkey (Macaca mulatta). Endocrinology 102, 52–62. [DOI] [PubMed] [Google Scholar]
- Qiu J, Nestor CC, Zhang C, Padilla SL, Palmiter RD, Kelly MJ, Ronnekleiv OK, 2016. High frequency stimulation-induced peptide release synchronizes arcuate kisspeptin neurons and excites GnRH neurons. eLife 5, e16246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswamy S, Guerriero KA, Gibbs RB, Plant TM, 2008. Structural interactions between kisspeptin and GnRH neurons in the mediobasal hypothalamus of the male rhesus monkey (Macaca mulatta) as revealed by double immunofluorescence and confocal microscopy. Endocrinology 149, 387–4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM, 2010. Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology 151, 4494–4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen DD, 1986. Physiological interactions of the basic rest--activity cycle of the brain: pulsatile luteinizing hormone secretion as a model. Psychoneuroendocrinology 11, 389–405. [DOI] [PubMed] [Google Scholar]
- Rasmussen DD, 1993. Episodic gonadotropin-releasing hormone release from the rat isolated median eminence in vitro. Neuroendocrinology 58, 511–518. [DOI] [PubMed] [Google Scholar]
- Rasmussen DD, Malven PV, 1981. Relationship between rhythmic motor activity and plasma luteinizing hormone in ovariectomized sheep. Neuroendocrinology 32, 364–369. [DOI] [PubMed] [Google Scholar]
- Roa J, Herbison AE, 2012. Direct regulation of GnRH neuron excitability by arcuate nucleus POMC and NPY neuron neuropeptides in female mice. Endocrinology 153, 5587–5599. [DOI] [PubMed] [Google Scholar]
- Sanz E, Quintana A, Deem JD, Steiner RA, Palmiter RD, McKnight GS,2015. Fertility-regulating Kiss1 neurons arise from hypothalamic POMC-expressing progenitors. J. Neurosci 35, 5549–5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar DK, Chiappa SA, Fink G, Sherwood NM, 1976. Gonadotropin-releasing hormone surge in pro-oestrous rats. Nature 264, 461–463. [DOI] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS Jr., Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MB, Crowley WF Jr., Aparicio SA, Colledge WH, 2003. The GPR54 gene as a regulator of puberty. N. Engl, J. Med 349, 1614–1627. [DOI] [PubMed] [Google Scholar]
- Shaw ND, Butler JP, McKinney SM, Nelson SA, Ellenbogen JM, Hall JE, 2012. Insights into puberty: the relationship between sleep stages and pulsatile LH secretion. J. Clin. Endocrinol. Metab 97, E2055–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw ND, Butler JP, Nemati S, Kangarloo T, Ghassemi M, Malhotra A, Hall JE, 2015. Accumulated deep sleep is a powerful predictor of LH pulse onset in pubertal children. J. Clin. Endocrinol. Metab 100, 1062–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman AJ, Wilson R, Kesner JS, Knobil E, 1986. Hypothalamic localization of multiunit electrical activity associated with pulsatile LH release in the rhesus monkey. Neuroendocrinology 44, 168–171. [DOI] [PubMed] [Google Scholar]
- Steyn FJ, Wan Y, Clarkson J, Veldhuis JD, Herbison AE, Chen C, 2013. Development of a methodology for and assessment of pulsatile luteinizing hormone secretion in juvenile and adult male mice. Endocrinology 154, 4939–4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spies HG, Norman RL, 1975. Interaction of estradiol and LHRH on LH release in rhesus females: evidence for a neural site of action. Endocrinology, 97, 685–692. [DOI] [PubMed] [Google Scholar]
- Temple JL, Laing E, Sunder A, Wray S, 2004. Direct action of estradiol on gonadotropin-releasing hormone-1 neuronal activity via a transcription-dependent mechanism. J. Neurosci 24, 6326–6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenenbaum-Rakover Y, Commenges-Ducos M, Iovane A, Auma C, Admoni O, de Roux N, 2007. Neuroendocrine phenotype analysis in five patients with isolated hypogonadotropic hypogonadism due to a L102P inactivating mutation of GPR54. J. Clin. Endocrinol. Metab 92, 1137–1144. [DOI] [PubMed] [Google Scholar]
- Terasawa E, Yeoman RR, Schultz NJ, 1984. Factors influencing the progesterone-induced luteinizing hormone surge in rhesus monkeys: diurnal influences and time interval after estrogen. Biol. Reprod 31, 732–741. [DOI] [PubMed] [Google Scholar]
- Terasawa E, Krook C, Hei DL, Gearing M, Schultz NJ, Davis GA, 1988. Norepinephrine is a possible neurotransmitter stimulating pulsatile release of luteinizing hormone-releasing hormone in the rhesus monkey. Endocrinology 123, 1808–1816. [DOI] [PubMed] [Google Scholar]
- Terasawa E, 1994. In vivo measurement of pulsatile release of neuropeptides and neurotransmitters in rhesus monkeys using push-pull perfusion In: Levine JE (ed) Methods in Neuroscience: Pulsatility in Neuroendocrine System, pp 184–202. New York, Academic Press. [Google Scholar]
- Terasawa E, Keen KL, Mogi K, Claude P, 1999a. Pulsatile release of luteinizing hormone-releasing hormone (LHRH) in cultured LHRH neurons derived from the embryonic olfactory placode of the rhesus monkey. Endocrinology, 140, 1432–1441. [DOI] [PubMed] [Google Scholar]
- Terasawa E, Garcia JP, Seminara SB, Keen KL, 2018. Role of Kisspeptin and Neurokinin B in Puberty in Female Non-Human Primates. Front. Endocrinol. (Lausanne) 9, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasawa E, Schanhofer WK, Keen KL, Luchansky L, 1999b. Intracellular Ca(2+) oscillations in luteinizing hormone-releasing hormone neurons derived from the embryonic olfactory placode of the rhesus monkey. J. Neurosci 19, 5898–5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Ozbek MN, Imamoglu S, Akalin NS, Yuksel B, O’Rahilly S, Semple RK, 2009. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for neurokinin B in the central control of reproduction. Nat. Genet 41, 354–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Martinez R, Shorte SL, Boockfor FR, Frawley LS, 2001. Synchronized exocytotic bursts from gonadotropin-releasing hormone-expressing cells: dual control by intrinsic cellular pulsatility and gap junctional communication. Endocrinology 142, 2095–2101. [DOI] [PubMed] [Google Scholar]
- Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K, Steiner RA, Okamura H, 2010. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J. Neurosci 30, 3124–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner RI, Wetsel W, Goldsmith P, Martinez de la Escalera G, Windle J, Padula C, Choi A, Negro-Vilar A, Mellon P, 1992. Gonadotropin-releasing hormone neuronal cell lines. Front. Neuroendocrinol 13, 95–119. [PubMed] [Google Scholar]
- Wilson RC, Kesner JS, Kaufman JM, Uemura T, Akema T, Knobil E, 1984. Central electrophysiologic correlates of pulsatile luteinizing hormone secretion in the rhesus monkey. Neuroendocrinology 39, 256–260. [DOI] [PubMed] [Google Scholar]
- Wildt L, Marshall G, Knobil E, 1980. Experimental induction of puberty in the infantile female rhesus monkey. Science 207, 1373–1375. [DOI] [PubMed] [Google Scholar]
- Woller MJ, McDonald JK, Reboussin DM, Terasawa E, 1992. Neuropeptide Y is a neuromodulator of pulsatile luteinizing hormone-releasing hormone release in the gonadectomized rhesus monkey. Endocrinology 130, 2333–2342. [DOI] [PubMed] [Google Scholar]
- Woller MJ, Terasawa E, 1991. Infusion of neuropeptide Y into the stalk-median eminence stimulates in vivo release of luteinizing hormone-release hormone in gonadectomized rhesus monkeys. Endocrinology 128, 1144–1150. [DOI] [PubMed] [Google Scholar]
- Woller MJ, Terasawa E, 1992. Estradiol enhances the action of neuropeptide Y on in vivo luteinizing hormone-releasing hormone release in the ovariectomized rhesus monkey. Neuroendocrinology 56, 921–925. [DOI] [PubMed] [Google Scholar]
- Woller MJ, Terasawa E, 1994. Changes in pulsatile release of neuropeptide-Y and luteinizing hormone (LH)-releasing hormone during the progesterone-induced LH surge in rhesus monkeys. Endocrinology 135, 1679–1686. [DOI] [PubMed] [Google Scholar]
- Yeoman RR, Terasawa E, 1984. An increase in single unit activity of the medial basal hypothalamus occurs during the progesterone-induced luteinizing hormone surge in the female rhesus monkey. Endocrinology 115, 2445–2452. [DOI] [PubMed] [Google Scholar]
- Yip SH, Boehm U, Herbison AE, Campbell RE, 2015. Conditional viral tract tracing delineates the projections of the distinct kisspeptin neuron populations to gonadotropin-releasing hormone (GnRH) neurons in the mouse. Endocrinology 156, 2582–2594. [DOI] [PubMed] [Google Scholar]