Abstract
Per- and polyfluoroalkyl substances (PFASs) are widely used anthropogenic chemicals. The PFAS class includes almost 5000 registered compounds, but analytical methods are lacking for most PFASs. The total oxidizable precursor (TOP) assay was developed to indirectly quantify unknown PFASs that are precursors to commonly measured perfluoroalkyl acids. To understand the behavior of recently identified per- and polyfluoroalkyl ether acids (PFEAs), including fluorinated replacements and manufacturing byproducts, we determined the fate of 15 PFEAs in the TOP assay. Ten perfluoroalkyl ether acids and a chlorinated polyfluoroalkyl ether acid (F-53B) were stable in the TOP assay and represent terminal products that are likely as persistent as historically used PFASs. Adding perfluoroalkyl ether acids and F-53B to the target analyte list for the TOP assay is recommended to capture a higher percentage of the total PFAS concentration in environmental samples. In contrast, polyfluoroalkyl ether acids with a -O-CFH- moiety were oxidized, typically to products that could not be identified by liquid chromatography and high-resolution mass spectrometry. Application of the TOP assay in its proposed enhanced form revealed high levels of PFEAs, the presence of precursors that form perfluoroalkyl carboxylic acids, and the absence of precursors that form PFEAs in surface water impacted by PFAS-containing wastewater discharges.
Graphical Abstract
INTRODUCTION
Per- and polyfluoroalkyl substances (PFASs) are extensively used anthropogenic chemicals. The unique properties of PFASs have led to a variety of applications, including their use in firefighting foams, stain repellents, paper coatings, and fluoropolymer production.1–7 To date, almost 5000 PFASs have been registered.8 Two long-chain PFASs, perfluorooctanesulfonic acid (PFOS) and perfluorooctanoic acid (PFOA), were historically produced in large quantities, but a manufacturing shift toward short-chain PFASs and fluorinated alternatives started in the early 2000s as a result of links between long-chain PFAS exposure and adverse health outcomes.4,9–12
Several per- and polyfluoroalkyl ether acids (PFEAs) have been produced to replace PFOS and PFOA. For example, ammonium 4,8-dioxa-3H-perfluorononanoic acid [ADONA (Table 1)] was developed to replace the ammonium salt of PFOA as an emulsifier in the manufacture of fluoropolymers.13 It was detected downstream of a fluoropolymer manufacturing plant in Germany in 2008.14,15 As an alternative to PFOS, 6:2 chlorinated polyfluoroalkyl ether sulfonic acid [6:2 Cl-PFESA, trade name F-53B (Table 1)] is widely used in Chinese chromium-plating facilities.16 After decades of F-53B usage in China, it has been detected in Chinese surface water, sewage sludge, wildlife, and humans.17–21 The ammonium salt of hexafluoropropylene oxide-dimer acid [HFPO-DA (Table 1)] with the trade name “GenX” has been used as a processing aid to replace the ammonium salt of PFOA in fluoropolymer manufacturing processes since 2010.14 The presence of GenX was reported in river water downstream of fluorochemical plants in China,22 Germany,22 The Netherlands,23 and the United States (U.S.).4,24 The U.S. study further showed that manufacturing byproducts, which are generated during the production of fluoropolymer building blocks, were discharged into an important drinking water supply. These byproducts can be broadly classified as (1) perfluoroalkyl monoether carboxylic acids (monoether PFECAs), (2) perfluoroalkyl multiether carboxylic acids (multiether PFECAs), and (3) polyfluoroalkyl ether acids (Table 1, Table S1, and Figure S1).4,24–26
Table 1.
Examples of Per- and Polyfluoroalkyl Ether Acids (PFEAs) Targeted in This Study
| Compound | Formula | CAS# | Molecular structure |
|---|---|---|---|
| Perfluoroalkyl mono-ether carboxylic acids (mono-ether PFECAs) | |||
| Perfluoro-2-methoxyacetic acid (PFMOAA) | C3HF5O3 | 674-13-5 | 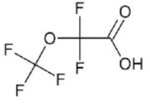 |
| Perfluoro-2-propoxypropanoic acid (PFPrOPrA) = Hexafluoropropylene oxide-dimer acid (HFPO-DA) = parent acid of “GenX” | C6HF11O3 | 13252-13-6 | 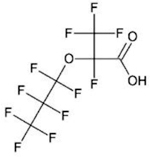 |
| Perfluoroalkyl multi-ether carboxylic acids (multi-ether PFECAs) | |||
| Perfluoro(3,5-dioxahexanoic) acid (PF02HxA) | C4HF7O4 | 39492-88-1 | 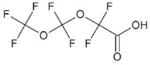 |
| Perfluoro(3,5,7,9-tetraoxadecanoic) acid (PF04DA) | C6HF1106 | 39492-90-5 | |
| Polyfluoroalkyl ether acids | |||
| Ethanesulfomc acid, 2-[1-[difluoro(1,2,2,2-tetrafluoroethoxy)methyl]-1,2,2,2-tetrafluoroethoxy]-1,1,2,2-tetrafluoro- = “Nation byproduct 2” | C7H2F14SO5 | 749836-20-2 | 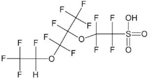 |
| 4,8-dioxa-3H-perfluorononanoic acid = parent acid of “ADONA” | C7H2F12O4 | 919005-14-4 | 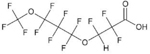 |
| 9-chlorohexadecafluoro-3-oxanone-1 –sulfonic acid (9C1-PF30NS, main component of F-53B) | C8HF166S04C1 | 756426-58-1 | 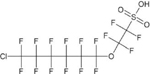 |
Analytical methods and reference standards are lacking for the vast majority of PFASs.27 For example, U.S. EPA Method 537.1 targets only 18 PFASs.28 Approaches for quantifying total PFAS concentrations in environmental samples are therefore needed.27 The total oxidizable precursor (TOP) assay was developed to indirectly quantify the concentration of perfluoroalkyl acid (PFAA) precursors, i.e., PFASs, that may degrade in the environment to terminal products such as perfluoroalkyl carboxylic and sulfonic acids (PFCAs and PFSAs, respectively).29 To date, the TOP assay has been applied to characterize wastewater,30,31 urban runoff,29,32 precipitation,33 groundwater,34–36 aqueous film-forming foams,37 paper, and textiles.38,39 However, no information about the fate of PFEAs in the TOP assay is available. It is unclear whether PFEAs are PFAA precursors in the TOP assay or whether some PFEAs are terminal products that need to be added to the analyte list for the TOP assay. Similarly, it is unknown whether the TOP assay can uncover the presence of PFEAs and/or PFEA precursors in environmental samples. The aims of this research were, therefore, (1) to determine the fate of 15 PFEAs in the TOP assay, (2) to identify possible new terminal products of the TOP assay, and (3) to apply the TOP assay to surface water impacted by a wastewater discharge containing PFEAs.
MATERIALS AND METHODS
Materials
Twenty-five PFASs in three classes (i.e., 7 PFCAs, 3 PFSAs, and 15 PFEAs) were targeted on the basis of the availability of analytical standards and the significance to the Cape Fear River (CFR) watershed in North Carolina (Table S1 and Figure S1). Analytical standards and isotopically labeled internal standards were obtained from Wellington Laboratories (Guelph, ON), Fluoryx Laboratories (Carson City, NV), The Chemours Company (Wilmington, DE), and SynQuest Laboratories (Alachua, FL) (see Table S1 for details). All other chemicals and solvents were purchased from Fisher Scientific (Hampton, NH) and Sigma-Aldrich (St. Louis, MO).
Sample Collection
CFR water samples were collected at the William O. Huske Lock and Dam (HLD) immediately downstream from a fluorochemical manufacturing site in August 2014 and April 2018, as well as at a drinking water intake (Lock and Dam #1 or LD1) located approximately 140 km downstream from the manufacturer in July 2015 (Figure S2). River discharges on the sampling dates are depicted in Figure S3. At each site, samples were collected in 20 L high density polyethylene (HDPE) carboys and stored at 4 °C until use. Details of water sample collection have been described previously.4
Total Oxidizable Precursor (TOP) Assay
TOP assays were conducted according to a previously developed protocol.29,35 For experiments using individual PFEAs, an aliquot of a 5 ng/μL individual PFEA stock solution in methanol was added to each HDPE bottle and evaporated to dryness under nitrogen. The PFEA was subsequently dissolved in 125 mL of deionized water to yield a starting concentration of ∼1000 ng/L, and the solution was preheated for 30 min at 60 °C. For experiments involving CFR water, 125 mL aliquots were added directly to HDPE bottles. To initiate precursor oxidation, potassium persulfate (5 mM) and sodium hydroxide (150 mM) were added. Samples were heated in a temperature controlled water shaker bath for 6 h at 85 °C. The pH of each sample was measured to confirm a pH of >12 was maintained during the TOP assay. At elevated temperatures, persulfate is converted to sulfate radicals (SO4•−), which, at elevated pH values, rapidly react to form hydroxyl radicals (•OH).29,35 Upon reaction, samples were cooled, neutralized to pH 5−929 with nitric acid,40 and stored at 4 °C until offline solid phase extraction (SPE) and liquid chromatography−high-resolution mass spectrometry (LC−HRMS) analysis. For each sample, PFAS concentrations were measured before and after the TOP assay. TOP assay method validation and control experiments are described in the Supporting Information (Texts S1 and S2).
Analytical Methods
SPE using Oasis WAX Plus cartridges and LC−MS analysis were carried out as previously described.24,41 While sample preparation by SPE may have led to low bias for or the loss of some oxidation products (e.g., neutral products), SPE was used to avoid the injection of large quantities of salt from the TOP assay into the LC instrument. All PFAS concentrations were determined using authentic standards. When possible, an isotope dilution approach was used, in which the analyte response was normalized to that of an isotopically labeled analogue.25 For other PFASs, the analyte response was normalized to that of an isotopically labeled PFAS with an LC retention time similar to that of the analyte (Table S1). Details of the analytical methods, including approaches to identifying reaction products by LC−HRMS, are provided in the Supporting Information (Texts S3–S7).
RESULTS AND DISCUSSION
Fate of Perfluoroalkyl Ether Carboxylic Acids
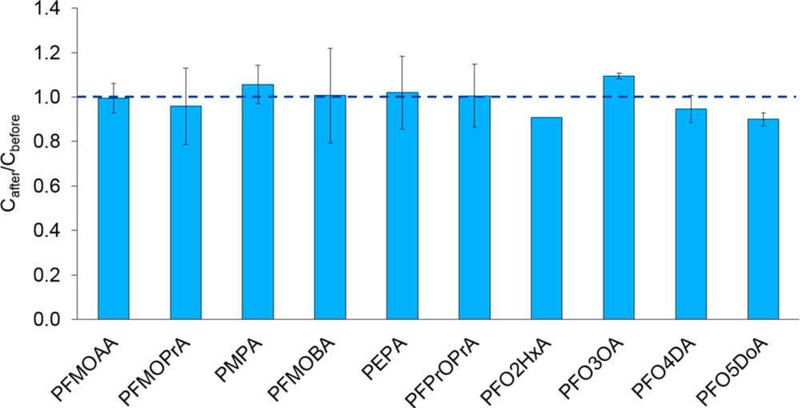
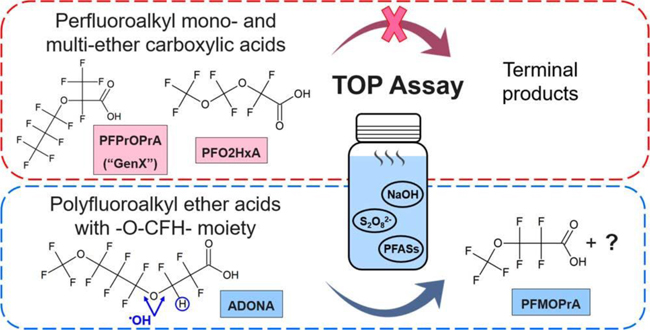
The fate of 10 perfluoroalkyl ether carboxylic acids [PFECAs, including six monoether PFECAs and four multiether PFECAs (Table S1)] in the TOP assay was evaluated. For the six monoether PFECAs, concentrations before and after oxidation showed no statistically significant difference (t test; n = 2; p < 0.05); i.e., monoether PFECAs were stable in the TOP assay and represent new terminal products (Figure 1 and Table S2). For monoether PFECAs, both linear and branched isomers (PFMOPrA and PMPA; PFMOBA and PEPA) were persistent during the TOP assay, implying that branching does not change the stability of monoether PFECAs in the TOP assay. Similarly, the four multiether PFECAs with repeating -CF2Osubunits were stable during the TOP assay (p < 0.05, except for PFO2HxA, for which the duplicate was lost), illustrating that they also represent new terminal products that are likely persistent in the environment.
Figure 1.
Fractions of perfluoroalkyl ether carboxylic acid (PFECA) concentrations remaining after oxidation relative to that measured before oxidation in the TOP assay. Averages and standard deviations of duplicate experiments are shown. See Figure S1 for compound structures.
TOP assay results indicate that PFECAs are resistant to attack by •OH, in agreement with the finding that PFECA levels did not change measurably after ozonation in a full-scale surface water treatment plant.4 Ozonation produced •OH at this plant, as evidenced by oxidation of 1,4-dioxane,42 but the oxidation conditions were less severe than those employed here. To date, no study has evaluated the reaction between •OH and PFPrOPrA (“GenX”), but a recent study indicated that GenX was not oxidized in systems involving both •OH and SO4•−43 Although one rationale behind replacing PFOA and PFOS with PFEAs was to make the replacements more labile by inserting ether linkage(s),24 the TOP assay results highlight that PFECAs are highly resistant to chemical degradation even under extreme oxidative conditions. Because the TOP assay currently employed by most research and commercial laboratories does not include PFECAs in the analyte list, it would significantly underestimate the total PFAS concentrations in samples containing PFECAs or PFECA precursors. Thus, we propose the addition of PFECAs to the analyte list for an expanded TOP assay, as they represent new terminal products (Table S4).
Fate of Polyfluoroalkyl Ether Acids
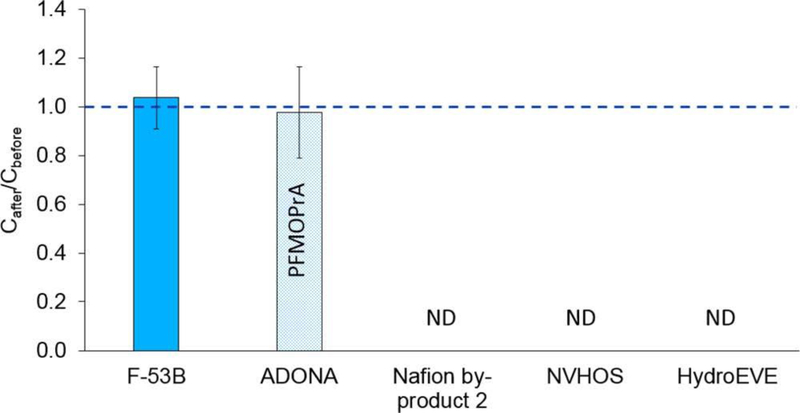
To assess the fate of polyfluoroalkyl ether acids in the TOP assay, we conducted experiments with two polyfluoroalkyl ether sulfonic acids (Nafion byproduct 2, NVHOS), two polyfluoroalkyl ether carboxylic acids (ADONA, HydroEVE), and one chlorofluoroalkyl ether sulfonic acid (F-53B) in deionized water (Table S1, Figure S1). These compounds were selected because they have been detected in the environment,14,24,26 and they allowed us to assess the effects of different structural features, such as the introduction of a C−H bond adjacent to an ether oxygen, different headgroups (sulfonic and carboxylic acids), and the presence of a C−Cl bond, on the fate of PFASs during the TOP assay.
An alternative to PFOS, F-53B, has two structural modifications, i.e., an ether linkage between C2 and C3 and a fluorine-to-chlorine substitution on C8 (Table 1).44 In the TOP assay, F-53B was persistent and represents a new terminal product that should be added to the TOP assay analyte list (Figure 2, Table S2, and Table S4). The TOP assay result is consistent with the reported stability of F-53B in advanced oxidation processes, including ultraviolet light/H2O2, ozonation, and O3/H2O2.44,45
Figure 2.
Fractions of polyfluoroalkyl ether acid concentrations remaining after oxidation relative to that measured before oxidation in the TOP assay. Averages and standard deviations of duplicate experiments are shown. In the TOP assay, ADONA was oxidized to PFMOPrA. ND means no product or intermediate was detected by LC−HRMS in the sample after oxidation.
The PFOA replacement ADONA has two ether linkages and a fluorine-to-hydrogen substitution on C3, next to the first ether bond (Table 1). In the TOP assay, ADONA was oxidized to PFMOPrA (Figure S1), a linear four-carbon PFECA and isomer of PMPA. The molar yield of PFMOPrA was 98 ± 20%, indicating complete conversion of ADONA (Figure 2 and Table S2). Formation of PFMOPrA suggests that the site of hydroxyl radical attack on the ADONA anion is the -O-CFH- moiety. This experimental observation is supported by results of quantum mechanical models, which indicate that H-abstraction of the aliphatic proton of ADONA by a hydroxyl radical is the thermodynamically favored reaction path.46 PFMOPrA represents a new terminal product of the TOP assay, further highlighting the need to add PFECAs to the TOP assay analyte list. No additional oxidation product(s) could be identified by LC−HRMS. It is unclear whether additional low-molecular weight fluorinated oxidation products were formed and/or whether a fraction of ADONA was mineralized to fluoride.47 Fluoride can be generated from some polyfluoroalkyl substances (e.g., 6:2 fluorotelomer sulfonate) in advanced oxidation processes involving hydroxyl radicals.48 In our study, fluoride production could not be measured because our starting ADONA concentration was ∼1000 ng/L. We lacked access to sufficient ADONA to complete TOP assays with ADONA concentrations that would have allowed us to quantify fluoride.
The three other polyfluoroalkyl ether acids containing the -O-CFH- moiety [Nafion byproduct 2, NVHOS, and HydroEVE (Figure S1)] were readily oxidized in the TOP assay, implying the acid headgroup (sulfonic or carboxylic acid) does not affect the fate of polyfluoroalkyl ether acids in the TOP assay (Figure 2 and Table S2). It should be noted that transformation of these compounds in the TOP assay does not suggest they are readily transformed in the environment or in (waste)water treatment, where oxidative conditions are less severe than in the TOP assay. For example, we have observed no measurable change in Nafion byproduct 2 concentrations in a full-scale surface water treatment plant that employs ozonation (unpublished data). No oxidation product(s) could be identified by LC−HRMS, suggesting transformation of these compounds to low-molecular weight organofluorine species, volatile organofluorine species, and/or fluoride.47 Because the longest perfluoroalkyl group in the studied polyfluoroalkyl ether acids with -O-CFH- moieties contains three carbon atoms (Figure S1), the longest possible PFAA formed is shorter than PFBA, the shortest PFAA that is typically quantified in the TOP assay. Hence, the results demonstrate an important weakness of the TOP assay; i.e., some compounds are oxidized to products that are not usually identified. The presence of PFASs like Nafion byproduct 2, NVHOS, and HydroEVE would be missed if analytical methods were not to target these compounds prior to oxidation.
Application of the Expanded TOP Assay to Cape Fear River Water
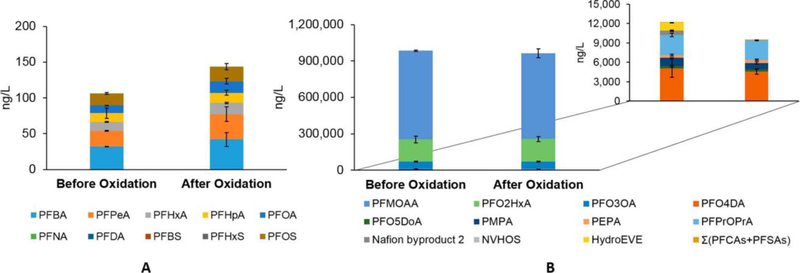
Direct measurement of legacy PFAS (PFCA and PFSA) concentrations in the water sample collected at HLD in August 2014 indicated the presence of five PFCAs and one PFSA, with their total concentrations being 89 and 17 ng/L, respectively (Figure 3A and Table S5). The dominant PFCAs were PFBA (32 ng/L) and PFPeA (22 ng/L), consistent with a previous study.4 Upon application of the TOP assay with only the traditionally analyzed PFCAs and PFSAs, the total PFCA concentration increased by 38% on a mass basis, largely as a result of the presence of precursors that form PFPeA (increased by 13 ng/L or 60%) and PFBA (increased by 10 ng/L or 31%). Precursor sources are not known; precursors could be introduced by the fluorochemical manufacturer and/or point or nonpoint sources located further upstream in the CFR watershed. When PFEAs were included in the analyte list (before TOP assay oxidation), the sum concentration of PFEAs (ΣPFEA ∼ 990000 ng/L) was nearly 4 orders of magnitude larger than that of the traditionally analyzed PFCAs and PFSAs (Figure 3B and Table S5). The dominant PFEAs were PFMOAA, PFO2HxA, and PFO3OA, consistent with a previous publication.25 The astoundingly high levels of PFEAs were attributed, in part, to a lack of complete mixing of the industrial wastewater with the bulk river water because HLD is located immediately downstream of the fluorochemical manufacturing site. By expanding the TOP assay analyte list to include PFEAs, we observed that three polyfluoroalkyl ether acids (i.e., Nafion byproduct 2, NVHOS, and HydroEVE) were oxidized during the TOP assay while the eight detected PFECAs were stable, consistent with our results for individual PFEAs. Furthermore, the expanded TOP assay results suggest that PFEA precursors were not present at significant levels in our sample. The environmental levels of PFEAs in CFR highlight the importance of adding PFEAs to the analyte list of the TOP assay and targeted LC-MS methods to capture a larger fraction of total PFASs.
Figure 3.
Application of the TOP assay (A) with traditionally analyzed PFCAs and PFSAs (ng/L) and (B) with an expanded PFEA analyte list to a water sample collected at the William O. Huske Lock and Dam (HLD) in August 2014. The inset in panel B highlights the concentrations of PFASs other than PFMOAA, PFO2HxA, and PFO3OA. Averages and standard deviations of duplicate experiments are shown. The dissolved organic carbon (DOC) concentration was 9.5 mg/L.
In the water sample collected at a drinking water intake (i.e., LD1) located approximately 140 km downstream from the manufacturer, concentrations of PFCAs and PFSAs were 120 and 45 ng/L, respectively (Figure S8 and Table S6). Higher legacy PFAS levels compared to those in the HLD sample were attributed, in part, to a lower river discharge (930 ft3/s at LD1 in July 2015 and 1600 ft3/s at HLD in August 2014). Upon application of the TOP assay, the PFCA concentration increased by 21% on a mass basis, which was mainly attributed to increases in PFPeA (33%) and PFHxA (24%). When PFEAs were included in the PFAS analyte list (before TOP assay oxidation), PFEAs dominated (ΣPFEA ∼ 130,00 ng/L), accounting for >99% of the total quantified PFAS mass concentration. Lower PFEA concentrations compared to those in HLD samples were primarily explained by the complete mixing of wastewater discharge from the fluorochemical manufacturer. As was the case for the HLD sample, PFEA precursors were not found in the LD1 sample.
Only PFMOAA (92 ng/L) was detected in the water sample collected at HLD in April 2018, and its concentration changed little in the TOP assay (Figure S9 and Table S7). Low PFAS levels were explained by (1) high streamflow (14000 ft3/s) and (2) the fluorochemical manufacturer beginning to capture PFEA-containing process wastewater starting in mid-June 2017.25
To the best of our knowledge, this is the first paper describing (1) the fate of PFEAs in the TOP assay and (2) the application of the TOP assay with 15 PFEAs added to the analyte list to impacted surface water. Results highlight that current targeted approaches (e.g., EPA Method 537.1) and a TOP assay that considers only PFCAs and PFSAs as terminal products can miss a large fraction of the total PFAS concentration in environmental samples. To more comprehensively characterize the occurrence of PFASs, we recommend a suite of complementary analytical approaches, including LC−HRMS and gas chromatography (GC)−HRMS, supplemented by “total organofluorine” methods such as the TOP assay with an expanded analyte list to include PFEAs, extractable organic fluorine (EOF), and/or adsorbable organic fluorine (AOF).27,38,49 Such approaches are needed to assess, for example, whether PFEAs and/or precursors to PFEAs occur primarily near manufacturing sites or whether they occur more widely.
Supplementary Material
Acknowledgements
This work was supported in part by the North Carolina Policy Collaborative. This document has been reviewed by the U.S. Environmental Protection Agency, Office of Research and Development, and approved for publication. The views expressed in this article are those of the authors and do not necessarily represent the views or policies of the U.S. Environmental Protection Agency.
Footnotes
Supporting Information
Tables, figures, experimental details about the TOP assay, SPE and LC−MS analysis, and PFAS quantification
References
- 1.Pan Y; Zhang H; Cui Q; Sheng N; Yeung LWY; Guo Y; Sun Y; Dai J First Report on the Occurrence and Bioaccumulation of Hexafluoropropylene Oxide Trimer Acid: An Emerging Concern. Environ. Sci. Technol 2017, 51, 9553–9560. [DOI] [PubMed] [Google Scholar]
- 2.Dombrowski PM; Kakarla P; Caldicott W; Chin Y; Sadeghi V; Bogdan D; Barajas-Rodriguez F; Chiang SY D. Technology Review and Evaluation of Different Chemical Oxidation Conditions on Treatability of PFAS. Remediation 2018, 28, 135–150. [Google Scholar]
- 3.Hatton J; Holton C; DiGuiseppi B Occurrence and Behavior of Per- and Polyfluoroalkyl Substances from Aqueous Film-Forming Foam in Groundwater Systems. Remediation 2018, 28, 89–99. [Google Scholar]
- 4.Sun M; Arevalo E; Strynar M; Lindstrom A; Richardson M; Kearns B; Pickett A; Smith C; Knappe DRU Legacy and Emerging Perfluoroalkyl Substances Are Important Drinking Water Contaminants in the Cape Fear River Watershed of North Carolina. Environ. Sci. Technol. Lett 2016, 3, 415–419. [Google Scholar]
- 5.Buck RC; Franklin J; Berger U; Conder JM; Cousins IT; de Voogt P; Jensen AA; Kannan K; Mabury SA; van Leeuwen SPJ Perfluoroalkyl and Polyfluoroalkyl Substances in the Environment: Terminology, Classification, and Origins. Integr. Environ. Assess. Manage 2011, 7, 513–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindstrom AB; Strynar MJ; Libelo EL Polyfluorinated Compounds: Past, Present, and Future. Environ. Sci. Technol 2011, 45, 7954–7961. [DOI] [PubMed] [Google Scholar]
- 7.Wang Z; Dewitt JC; Higgins CP; Cousins IT A Never-Ending Story of Per- and Polyfluoroalkyl Substances (PFASs)? Environ. Sci. Technol 2017, 51, 2508–2518. [DOI] [PubMed] [Google Scholar]
- 8.OECD. 033-066-C609-51.Pdf Ser. Risk Manag 2018, 39, 1–24. [Google Scholar]
- 9.Scheringer M; Trier X; Cousins IT; de Voogt P; Fletcher T; Wang Z; Webster TF Helsingør Statement on Poly- and Perfluorinated Alkyl Substances (PFASs). Chemosphere 2014, 114, 337–339. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z; Cousins IT; Scheringer M; Hungerbuhler K Fluorinated Alternatives to Long-Chain Perfluoroalkyl Carboxylic Acids (PFCAs), Perfluoroalkane Sulfonic Acids (PFSAs) and Their Potential Precursors. Environ. Int 2013, 60, 242–248. [DOI] [PubMed] [Google Scholar]
- 11.Wang Z; Cousins IT; Scheringer M; Hungerbuehler K Hazard Assessment of Fluorinated Alternatives to Long-Chain Perfluoroalkyl Acids (PFAAs) and Their Precursors: Status Quo, Ongoing Challenges and Possible Solutions. Environ. Int 2015, 75, 172–179. [DOI] [PubMed] [Google Scholar]
- 12.Barzen-Hanson KA; Field JA Discovery and Implications of C2 and C3 Perfluoroalkyl Sulfonates in Aqueous Film-Forming Foams and Groundwater. Environ. Sci. Technol. Lett 2015, 2, 95–99. [Google Scholar]
- 13.Gordon SC Toxicological Evaluation of Ammonium 4,8-Dioxa-3H-Perfluorononanoate, a New Emulsifier to Replace Ammonium Perfluorooctanoate in Fluoropolymer Manufacturing. Regul. Toxicol. Pharmacol 2011, 59, 64–80. [DOI] [PubMed] [Google Scholar]
- 14.Pan Y; Zhang H; Cui Q; Sheng N; Yeung LWY; Sun Y; Guo Y; Dai J Worldwide Distribution of Novel Perfluoroether Carboxylic and Sulfonic Acids in Surface Water. Environ. Sci. Technol 2018, 52, 7621–7629. [DOI] [PubMed] [Google Scholar]
- 15.Fromme H; Wockner M; Roscher E; Volkel W ADONA and Perfluoroalkylated Substances in Plasma Samples of German Blood Donors Living in South Germany. Int. J. Hyg. Environ. Health 2017, 220, 455–460. [DOI] [PubMed] [Google Scholar]
- 16.Xiao F Emerging Poly- and Perfluoroalkyl Substances in the Aquatic Environment: A Review of Current Literature. Water Res 2017, 124, 482–495. [DOI] [PubMed] [Google Scholar]
- 17.Ruan T; Lin Y; Wang T; Liu R; Jiang G Identification of Novel Polyfluorinated Ether Sulfonates as PFOS Alternatives in Municipal Sewage Sludge in China. Environ. Sci. Technol 2015, 49, 6519–6527. [DOI] [PubMed] [Google Scholar]
- 18.Wang T; Vestergren R; Herzke D; Yu J; Cousins IT Levels, Isomer Profiles, and Estimated Riverine Mass Discharges of Perfluoroalkyl Acids and Fluorinated Alternatives at the Mouths of Chinese Rivers. Environ. Sci. Technol 2016, 50, 11584–11592. [DOI] [PubMed] [Google Scholar]
- 19.Cui Q; Pan Y; Zhang H; Sheng N; Wang J; Guo Y; Dai J Occurrence and Tissue Distribution of Novel Perfluoroether Carboxylic and Sulfonic Acids and Legacy Per/Polyfluoroalkyl Substances in Black-Spotted Frog (Pelophylax Nigromaculatus). Environ. Sci. Technol 2018, 52, 982–990. [DOI] [PubMed] [Google Scholar]
- 20.Shi Y; Vestergren R; Xu L; Zhou Z; Li C; Liang Y; Cai Y Human Exposure and Elimination Kinetics of Chlorinated Polyfluoroalkyl Ether Sulfonic Acids (Cl-PFESAs). Environ. Sci. Technol 2016, 50, 2396–2404. [DOI] [PubMed] [Google Scholar]
- 21.Pan Y; Zhu Y; Zheng T; Cui Q; Buka SL; Zhang B; Guo Y; Xia W; Yeung LWY; Li Y; et al. Novel Chlorinated Polyfluorinated Ether Sulfonates and Legacy Per/Polyfluoroalkyl Substances: Placental Transfer and Relationship with Serum Albumin and Glomerular Filtration Rate. Environ. Sci. Technol 2017, 51, 634–644. [DOI] [PubMed] [Google Scholar]
- 22.Heydebreck F; Tang J; Xie Z; Ebinghaus R Alternative and Legacy Perfluoroalkyl Substances: Differences between European and Chinese River/Estuary Systems. Environ. Sci. Technol 2015, 49, 8386–8395. [DOI] [PubMed] [Google Scholar]
- 23.Gebbink WA; Van Asseldonk L; Van Leeuwen SPJ Presence of Emerging Per- and Polyfluoroalkyl Substances (PFASs) in River and Drinking Water near a Fluorochemical Production Plant in the Netherlands. Environ. Sci. Technol 2017, 51, 11057–11065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strynar M; Dagnino S; McMahen R; Liang S; Lindstrom A; Andersen E; McMillan L; Thurman M; Ferrer I; Ball C Identification of Novel Perfluoroalkyl Ether Carboxylic Acids (PFECAs) and Sulfonic Acids (PFESAs) in Natural Waters Using Accurate Mass Time-of-Flight Mass Spectrometry (TOFMS). Environ. Sci. Technol 2015, 49, 11622–11630. [DOI] [PubMed] [Google Scholar]
- 25.Hopkins ZR; Sun M; DeWitt JC; Knappe DRU Recently Detected Drinking Water Contaminants: GenX and Other Per- and Polyfluoroalkyl Ether Acids. J. - Am. Water Works Assoc 2018, 110, 13–28. [Google Scholar]
- 26.McCord J; Strynar M Identification of Per- and Polyfluoroalkyl Substances in the Cape Fear River by High Resolution Mass Spectrometry and Nontargeted Screening. Environ. Sci. Technol 2019, 53, 4717–4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDonough CA; Guelfo JL; Higgins CP Measuring Total PFASs in Water: The Tradeoff between Selectivity and Inclusivity. Curr. Opin. Environ. Sci. Heal 2019, 7, 13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.U.S. Environmental Protection Agency. Method 537.1. Determination of Selected Perfluorinated Alkyl Acids in Drinking Water by Solid Phase Extraction and Liquid Chromatography/Tandem Mass Spectrometry (LC/MS/MS) Technical Report EPA/600/R-18/352; 2018; pp 1–50. [Google Scholar]
- 29.Houtz EF; Sedlak DL Oxidative Conversion as a Means of Detecting Precursors to Perfluoroalkyl Acids in Urban Runoff. Environ. Sci. Technol 2012, 46, 9342–9349. [DOI] [PubMed] [Google Scholar]
- 30.Houtz EF; Sutton R; Park JS; Sedlak M Poly- and Perfluoroalkyl Substances in Wastewater: Significance of Unknown Precursors, Manufacturing Shifts, and Likely AFFF Impacts. Water Res 2016, 95, 142–149. [DOI] [PubMed] [Google Scholar]
- 31.Houtz E; Wang M; Park JS Identification and Fate of Aqueous Film Forming Foam Derived Per- and Polyfluoroalkyl Substances in a Wastewater Treatment Plant. Environ. Sci. Technol 2018, 52, 13212–13221. [DOI] [PubMed] [Google Scholar]
- 32.Wang S; Cao X; Zhang H; Yang Y; Zhang M Oxidative Conversion of Potential Perfluoroalkyl Acid Precursors in Jiaozhou Bay and Nearby Rivers and Sewage Treatment Plant Effluent in China. Mar. Pollut. Bull 2018, 136, 481–490. [DOI] [PubMed] [Google Scholar]
- 33.Chen H; Zhang L; Li M; Yao Y; Zhao Z; Munoz G; Sun H Per- and Polyfluoroalkyl Substances (PFASs) in Precipitation from Mainland China: Contributions of Unknown Precursors and Short-Chain (C2–C3) Perfluoroalkyl Carboxylic Acids. Water Res 2019, 153, 169–177. [DOI] [PubMed] [Google Scholar]
- 34.Weber AK; Barber LB; Leblanc DR; Sunderland EM; Vecitis CD Geochemical and Hydrologic Factors Controlling Subsurface Transport of Poly- and Perfluoroalkyl Substances, Cape Cod, Massachusetts. Environ. Sci. Technol 2017, 51, 4269–4279. [DOI] [PubMed] [Google Scholar]
- 35.Houtz EF; Higgins CP; Field JA; Sedlak DL Persistence of Perfluoroalkyl Acid Precursors in AFFF-Impacted Groundwater and Soil. Environ. Sci. Technol 2013, 47, 8187–8195. [DOI] [PubMed] [Google Scholar]
- 36.Casson R; Chiang SYD Integrating Total Oxidizable Precursor Assay Data to Evaluate Fate and Transport of PFASs. Remediation 2018, 28, 71–87. [Google Scholar]
- 37.Mumtaz M; Bao Y; Liu L; Huang J; Cagnetta G; Yu G Per- and Polyfluoroalkyl Substances in Representative Fluorocarbon Surfactants Used in Chinese Film-Forming Foams: Levels, Profile Shift, and Environmental Implications. Environ. Sci. Technol. Lett 2019, 6, 259–264. [Google Scholar]
- 38.Robel AE; Marshall K; Dickinson M; Lunderberg D; Butt C; Peaslee G; Stapleton HM; Field JA Closing the Mass Balance on Fluorine on Papers and Textiles. Environ. Sci. Technol 2017, 51, 9022–9032. [DOI] [PubMed] [Google Scholar]
- 39.Mumtaz M; Bao Y; Li W; Kong L; Huang J; Yu G Screening of Textile Finishing Agents Available on the Chinese Market: An Important Source of per- and Polyfluoroalkyl Substances to the Environment. Front. Environ. Sci. Eng 2019, 13, 67. [Google Scholar]
- 40.Nakayama SF; Strynar MJ; Reiner JL; Delinsky AD; Lindstrom AB Determination of Perfluorinated Compounds in the Upper Mississippi River Basin. Environ. Sci. Technol 2010, 44, 4103–4109. [DOI] [PubMed] [Google Scholar]
- 41.Newton S; McMahen R; Stoeckel JA; Chislock M; Lindstrom A; Strynar M Novel Polyfluorinated Compounds Identified Using High Resolution Mass Spectrometry Downstream of Manufacturing Facilities near Decatur, Alabama. Environ. Sci. Technol 2017, 51, 1544–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knappe D; Lopez-Velandia C; Hopkins Z; Sun M Occurrence of 1,4-Dioxane in the Cape Fear River Watershed and Effectiveness of Water Treatment Options for 1,4-Dioxane Control Technical Report UNC-WRRI-478; North Carolina Water Resources Research Institute, 2016; p 97. [Google Scholar]
- 43.Bao Y; Deng S; Jiang X; Qu Y; He Y; Liu L; Chai Q; Mumtaz M; Huang J; Cagnetta G; et al. Degradation of PFOA Substitute: GenX (HFPO-DA Ammonium Salt): Oxidation with UV/Persulfate or Reduction with UV/Sulfite? Environ. Sci. Technol 2018, 52, 11728–11734. [DOI] [PubMed] [Google Scholar]
- 44.Zhang K; Cao Z; Huang J; Deng S; Wang B; Yu G Mechanochemical Destruction of Chinese PFOS Alternative F-53B. Chem. Eng. J 2016, 286, 387–393. [Google Scholar]
- 45.Wang S; Huang J; Yang Y; Hui Y; Ge Y; Larssen T; Yu G; Deng S; Wang B; Harman C First Report of a Chinese PFOS Alternative Overlooked for 30 Years: Its Toxicity, Persistence, and Presence in the Environment. Environ. Sci. Technol 2013, 47, 10163–10170. [DOI] [PubMed] [Google Scholar]
- 46.Baggioli A; Sansotera M; Navarrini W Thermodynamics of Aqueous Perfluorooctanoic Acid (PFOA) and 4,8-Dioxa-3H-Perfluorononanoic Acid (DONA) from DFT Calculations: Insights into Degradation Initiation. Chemosphere 2018, 193, 1063–1070. [DOI] [PubMed] [Google Scholar]
- 47.Larsson PA Study to Understand the Information Gap between Total Organofluorine Analysis and Total Oxidizable Precursor Assay on Polyfluoroalkyl/Perfluoroalkyl Substances (PFASs) Örebro University, 2019. [Google Scholar]
- 48.Yang X; Huang J; Zhang K; Yu G; Deng S; Wang B Stability of 6:2 Fluorotelomer Sulfonate in Advanced Oxidation Processes: Degradation Kinetics and Pathway. Environ. Sci. Pollut. Res 2014, 21, 4634–4642. [DOI] [PubMed] [Google Scholar]
- 49.Schultes L; Peaslee GF; Brockman JD; Majumdar A; McGuinness SR; Wilkinson JT; Sandblom O; Ngwenyama RA; Benskin JP Total Fluorine Measurements in Food Packaging: How Do Current Methods Perform? Environ. Sci. Technol. Lett 2019, 6, 73–78. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.