Abstract
The phenomenon of multidrug resistance in cancer is often associated with the overexpression of the ABC (ATP-binding cassette) transporters Pgp (P-glycoprotein) (ABCB1), MRP1 (multidrug resistance-associated protein 1) (ABCC1) and ABCG2 [BCRP (breast cancer resistance protein)]. Since the discovery of Pgp over 35 years ago, studies have convincingly linked ABC transporter expression to poor outcome in several cancer types, leading to the development of transporter inhibitors. Three generations of inhibitors later, we are still no closer to validating the ‘Pgp hypothesis’, the idea that increased chemotherapy efficacy can be achieved by inhibition of transporter-mediated efflux. In this chapter, we highlight the difficulties and past failures encountered in the development of clinical inhibitors of ABC transporters. We discuss the challenges that remain in our effort to exploit decades of work on ABC transporters in oncology. In learning from past mistakes, it is hoped that ABC transporters can be developed as targets for clinical intervention.
Introduction
Despite recent developments in anticancer drug discovery, various obstacles hinder successful cancer treatment. One such complication is the phenomenon of multidrug resistance (MDR), which is similar to the well-studied occurrence of antibiotic resistance in micro-organisms. Cellular resistance can be linked to the original genetic make-up of cancer cells, but may also develop in response to exposure to anticancer agents during treatment.
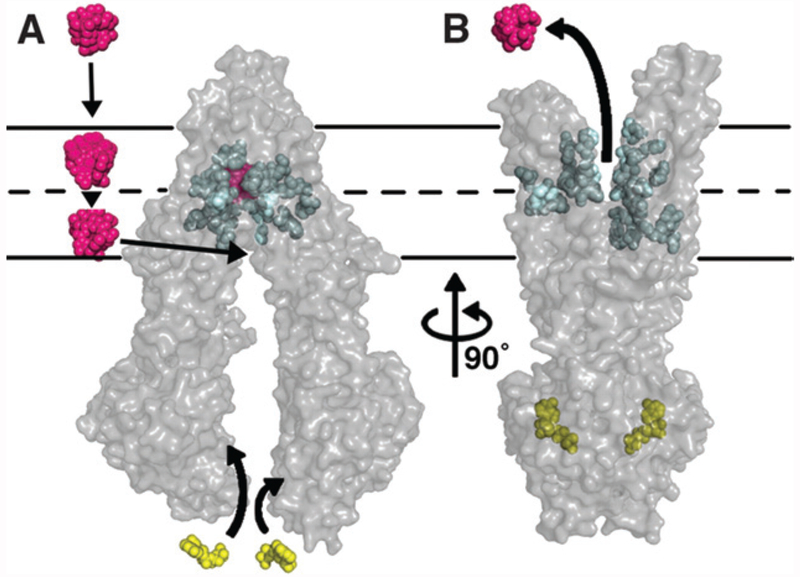
One intensively studied mechanism of MDR relies on the efflux of cytotoxic drugs from cancer cells by ABC (ATP-binding cassette) transporters. ABC transporters are energy-dependent transporters that normally function in the detoxification and protection of normal cells from xenobiotics. The substrates of ABC transporters include a wide range of structurally unrelated compounds that include numerous anticancer drugs. Although there are a number of ABC transporters that have been identified as potential transporters of anticancer drugs, three have received the most attention in the laboratory and in clinical oncology: Pgp (P-glycoprotein) (ABCB1/MDR1), MRP1 (multidrug-resistance protein 1) (ABCC1) and ABCG2 [BCRP (breast cancer resistance protein)/MXR (mitoxantrone-resistance protein)]. A model of Pgp based on the elucidated crystal structure [1] is shown in Figure 1. Whereas these transporters have been shown to confer resistance in in vitro and in vivo model systems, proof that they are responsible for a significant fraction of drug resistance in clinical oncology is lacking. The prevailing strategy used over the last decade to evaluate the contribution of the transporters to clinical drug resistance has been to investigate the efficacy of anticancer therapy in combination with ABC transporter inhibitors. Three generations of ABC transporter inhibitors have been developed and tested for clinical application. Unfortunately, these clinical trials have had minimal success. It is important to examine and understand the failure of these trials if ABC transporters are to be developed as therapeutic targets in clinical oncology.
Figure 1. Substrate transport by Pgp.
(A) The substrate, shown in magenta, enters the membrane and diffuses through a portal in the transporter. (B) Once in the drug-binding pocket, coloured blue, ATP, shown in yellow, binds to the nucleotide-binding domains and causes a conformational change, leaving the drug-binding pocket facing the extracellular space. ATP then binds again to reset the transporter to its original conformation shown in (A). From [1]: Aller, S., Yu, J., Ward, A., Weng, Y., Chittaboina, S., Zhuo, R., Harrell, P., Trinh, Y., Zhang, Q., Urbatsch, I. and Chang, G. (2009) Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science 323, 1718–1722. Reprinted with permission from AAAS.
Localization and expression
ABC transporters are endogenously expressed in a wide range of human tissues. Both Pgp and ABCG2 appear to play a major role in cellular protection from cytotoxins and xenobiotics. Pgp and ABCG2 are highly expressed in pharmacological barriers, including the brain microvessel endothelium, syncytiotrophoblasts of the placental chorionic villus, interstitial cells of the testes and haemopoietic stem cells in the bone marrow [2,3]. In addition to these barrier sites, Pgp is expressed on the canalicular surface of hepatocytes in the liver, in the epithelial cells of the proximal convoluted tubule in the kidney, the apical surface of gastrointestinal epithelial cells, the cortex and medulla of the adrenal glands, myoepithelium and in cells of the immune system [4]. ABCG2 is localized in the hepatocytes of the liver, zona reticularis layer of the adrenal glands, alveolar pneumocytes of the lung, prostate epithelium, uterine endocervical cells, cortical tubules of the kidney, islet and acinar cells in the pancreas, epithelial cells of the gastrointestinal tract and ducts and lobules of the mammary glands [5–7]. MRP1 is known to transport metabolic by-products, including glucuronide, glutathione and sulfate conjugates [8]. Key localizations of MRP1 also suggest protection at blood-normal tissue barriers, including testicular tubules, the choroid plexus, where it contributes to the blood-CSF (cerebrospinal fluid) barrier, and in bone marrow precursor cells [9,10].
In addition to their role in normal physiology, ABC transporters are highly expressed in multiple tumour types. In breast cancer, sarcoma and certain leukaemias, increased expression of Pgp was observed in recurrent or relapsed disease compared with expression at diagnosis [11]. In AML (acute myelogenous leukaemia), approximately 50% of clinical samples show Pgp expression with increasing levels in recurrent leukaemia cells, and expression has been repeatedly linked with poor outcome [12–16]. Although still under debate, numerous studies have reported increased Pgp expression following chemotherapy in tumours of the breast, ovaries, bladder, CNS (central nervous system) and cervix [2]. Pgp expression in these cancers is generally correlated with poor clinical outcome [14,17–19].
Pgp is by far the best characterized among the three ABC transporters, whereas MRP1 and other members of the ABC family are less well studied. MRP1 has not been found to be a significant factor in drug resistance in AML [12,20]. Its prognostic value in CLL (chronic lymphocytic leukaemia), lung cancer and breast cancer remains indeterminate [21–24]. To date, few studies have shown expression changes in MRP1 following treatment or have correlated expression with clinical outcome.
Association of ABCG2 with clinical outcome has also been inconclusive. Data concerning the expression of ABCG2 in AML are inconsistent, with high levels of expression being reported in some studies and lower levels in others [25–28]. The association of ABCG2 with response in AML is also debated, although some studies conclude that higher ABCG2 expression is associated with poor response to chemotherapy [15,29–31]. Notably, co-expression of Pgp and ABCG2 were linked with a lower complete response rate, worse event-free survival and worse overall survival in three studies of patients with AML[14,20]. In a study that did associate Pgp with poor outcome in breast cancer, there was no significant impact of ABCG2 expression [17]. In lung cancer, analysis of biopsy specimens from NSCLC (non-small-cell lung carcinoma) treated with cisplatin-based chemotherapy found that ABCG2 expression was associated with shorter survival, although there was no impact on response rate [32]. The same group found that ABCG2 expression in SCLC impaired response and progression free survival during platinum-based treatment [33]. Both associations were found despite the fact that platinum is not a substrate for transport.
Data such as those obtained from the lung cancer studies raise the possibility that other explanations may exist for the poor clinical outcome linked with transporter overexpression. One hypothesis is that cancer stem cells, existing as a separate and identifiable compartment of the tumour, are responsible for drug resistance. The model predicts a small subpopulation of drug-resistant pluripotent cells that is long-lived, quiescent, evades initial treatment and leads to the relapse of a drug-resistant tumour [34]. In this model, ABCG2, and Pgp in some cases, serves only as a marker for the stem cell and is responsible for the Hoechst-dim ‘side population’ phenotype, serving as a drug-resistance mechanism only for those stem cells. The stem cells are then responsible for repopulating a tumour following therapy, and drug resistance is due to this repopulation. Increasing numbers of these cells in a tumour could be linked with a poor outcome unrelated to the ability to extrude chemotherapy. Although the stem cell model is as yet unproven, it has led to the documentation of functional ABCG2 in putative cancer stem cells.
Substrates and inhibitors of ABC transporters
ABC transporter substrates include a diverse array of compounds, many of them structurally unrelated. In general, Pgp transports large hydrophobic compounds, whereas MRP1 and ABCG2 transport both hydrophobic drugs and large anionic compounds [3]. This wide range of substrates originally led to speculation that the transporters could be responsible for significant MDR in cancer cells. The list of substrates is striking not only in the number of anticancer agents, but also in the number of non-oncologic compounds. This highlights the potential role of ABC transporters in protection against xenobiotics and in pharmacology. A comprehensive but not exhaustive list of ABC transporter substrates found among anticancer drugs is provided in Table 1. This chapter focuses on the three transporters most studied clinically: Pgp, MRP1 and ABCG2. It is entirely possible that other ABC transporters may be clinically relevant, but the association has yet to be discovered. Transporters in the ABCC subfamily, for example, generally efflux methotrexate and other anionic substrates such as SN-38 [2], but clinical information is lacking.
Table 1. Clinically relevant substrates of the MDR-related ABC transporters.
| Class | Substrate | ABCB1 | ABCC1 | ABCG2 |
|---|---|---|---|---|
| Vinca alkaloids | Vinblastine | ✓ | ✓ | |
| Vincristine | ✓ | ✓ | ||
| Vinorelbine | ✓ | ✓ | ||
| Anthracyclines | Daunorubicin | ✓ | ✓ | ✓ |
| Doxorubicin | ✓ | ✓ | ✓ | |
| Epirubicin | ✓ | ✓ | ✓ | |
| Epipodophyllotoxins | Etoposide | ✓ | ✓ | ✓ |
| Teniposide | ✓ | ✓ | ||
| Taxanes | Docetaxel | ✓ | ||
| Paclitaxel | ✓ | |||
| Kinase inhibitors | Dasatinib | ✓ | ✓ | ✓ |
| Erlotinib | ✓ | ✓ | ||
| Gefitinib | ✓ | ✓ | ||
| Imatinib (Gleevec) | ✓ | ✓ | ✓ | |
| Lapatinib | ✓ | ✓ | ||
| Nilotinib | ✓ | ✓ | ✓ | |
| Sorafenib | ✓ | ✓ | ||
| Sunitinib | ✓ | ✓ | ||
| Vandetanib | ✓ | ✓ | ||
| Campthotecins | Irinotecan (CPT-11) | ✓ | ✓ | ✓ |
| SN-38 | ✓ | ✓ | ✓ | |
| Arsenite | ✓ | |||
| AZT | ✓ | |||
| Bisantrene | ✓ | ✓ | ||
| Cisplatin | ✓ | |||
| Colchicine | ✓ | ✓ | ||
| Digoxin | ✓ | |||
| Flavopiridol | ✓ | |||
| Methotrexate | ✓ | ✓ | ✓ | |
| Mitoxantrone | ✓ | ✓ | ||
| Saquinivir | ✓ |
Attempts to overcome MDR by preventing anticancer drug efflux have led to the development of a number of ABC transporter inhibitors. It has not been difficult to identify inhibitors due to the broad range of compounds that interact with the transporters; a partial list is provided in Table 2.
Table 2. Inhibitors of MDR-related ABC transporters.
| Substrate | ABCB1 | ABCC1 | ABCG2 |
|---|---|---|---|
| Amiodarone | ✓ | ||
| Cyclosporine A | ✓ | ✓ | ✓ |
| Nifedipine | ✓ | ||
| Quinidine | ✓ | ✓ | |
| Quinine | ✓ | ✓ | |
| Verapamil | ✓ | ✓ | |
| Biricodar (VX-710) | ✓ | ✓ | ✓ |
| Dexniguldipine | ✓ | ||
| Dofequidar (MS209) | ✓ | ✓ | |
| S9788 | ✓ | ||
| Valspodar (PSC-33) | ✓ | ||
| CBT-I | ✓ | ✓ | |
| Elacridar (GF120918) | ✓ | ✓ | |
| Laniquidar (Rl01 933) | ✓ | ||
| LY475776 | ✓ | ✓ | |
| ONT-093 | ✓ | ||
| Tariquidar (XR-9576) | ✓ | ✓ | |
| Zosuquidar (LY335979) | ✓ | ||
| Curcumin | ✓ | ✓ | |
| Flavonoids | ✓ | ||
| FTC | ✓ | ||
| Mitotane | ✓ | ||
| MK57I | ✓ |
Clinical trials
It was hypothesized that inhibition of ABC drug transporter activity during cancer therapy could sensitize drug-resistant tumours and/or improve the initial activity of anticancer agents. Laboratory models showed promise for the clinical application of Pgp inhibitors to circumvent drug resistance. Unfortunately, translation of this knowledge to clinical application was unexpectedly difficult. Oncology drugs are generally developed in three steps, termed phases, which delineate how far along the drugs are in clinical development. Earliest trials, Phase I studies, are dose-finding studies. The goal is to define a safe and potentially effective dose to take to the next phase. Typically, these trials are carried out in a standardized sequential dose escalation until a pre-defined level of toxicity is observed that suggests that further escalation would be harmful to patients. Phase II studies seek to find some sign of efficacy in a given patient population. Phase III studies then compare two treatments. In drug-resistance reversal trials, where a modulator of resistance is being tested, it is critical that the activity of the modulator be tested in a Phase III trial design. Given that modulators seldom have intrinsic activity, a modulator is studied in combination with an anticancer agent that has been proven safe in the Phase I or II setting and then is compared in the Phase III setting against the anticancer agent alone. As described below, many Phase II trials of modulators were considered promising and then the results not borne out in Phase III trials. A list of clinical trials examining ABC transporter inhibitors is provided in Table 3. These inhibitors have been classified into three generations: the first indicating compounds that were already U.S. FDA (Food and Drug Administration)-approved for other medical uses; the second comprising compounds developed specifically as Pgp inhibitors, some with notable pharmacokinetic interactions; and the third including compounds intentionally developed, but lacking major pharmacokinetic interactions [2].
Table 3. Phase III drug resistance inhibitor trials.
ADE, cytarabine, daunorubicin and etoposide; MDS, myelodysplastic syndrome; OS, overall survival; PFS, progression-free survival; RAEB-t, refractory anaemia with excess blasts in transformation; VAD, vincristine, adriamycin and dexamethasone.
| Generation | Modulator | Cancer type | Anticancer drug(s) | Outcome | Reference |
|---|---|---|---|---|---|
| First | Cyclosporin | Relapsed and refractory AML | ADE | No benefit | [95] |
| Cyclosporin | Poor risk AML, RAEB-t | Daunorubicin, cytarabine | Improved OS in cyclosporin A group | [37] | |
| Cyclosporin | Myeloma | VAD | No benefit | [96] | |
| Second | Valspodar (PSC-833) | AML | Daunorubicin, cytarabine | No benefit | [97] |
| Valspodar (PSC-833) | Untreated AML | ADE | Terminated early due to toxicity | [45] | |
| Valspodar (PSC-833) | Untreated AML | ADE | No OS advantage age >45 years, OS benefit age <45 years | [48] | |
| Valspodar (PSC-833) | Ovarian | Carboplatin, paclitaxel | No benefit | [98] | |
| Valspodar (PSC-833) | Refractory AML, high-risk MDS | Mitoxantrone, etoposide, cytarabine | No benefit | [99] | |
| Valspodar (PSC-833) | NSCLC | Carboplatin, paclitaxel | Terminated early due to toxicity | Novartis | |
| Valspodar (PSC-833) | Multiple myeloma | VAD | No benefit; increased toxicity with valspodar | [44] | |
| VX-710 (Biricodar, Incel™) | Lung | Doxorubicin, vincristine | Unknown | http://clinicaltrials.gov | |
| Third | CBT-1 | NSLC | Paclitaxel, carboplatin | Ongoing | http://clinicaltrials.gov |
| Dofequidar (MS-209) | Breast | Cyclophosphamide, doxorubicin, fluorouracil | Improved PFS in subset | [100] | |
| XR-9576 (Tariquidar) | NSCLC | Vinorelbine | Terminated early due to toxicity | http://clinicaltrials.gov | |
| XR-9576 (Tariquidar) | NSCLC | Paclitaxel, carboplatin | Terminated early due to toxicity | http://clinicaltrials.gov | |
| Zosuquidar (LY335979) | AML, MDS | Daunorubicin, cytarabine | Ongoing | http://clinicaltrials.gov |
First generation
The first-generation MDR inhibitors included verapamil, quinidine and cyclosporin A. Clinical trials generally found these drugs ineffective and/or toxic at doses required to inhibit ABC transporter function [35]. However, several promising trials generated optimism for the use of ABC transporter inhibitors in clinical oncology. For example, quinine was shown to increase complete remission and survival rates in Pgp-positive myelodysplastic syndromes treated with intensive chemotherapy [36]. Also, cyclosporin A combined with daunorubicin and cytarabine in patients with poor risk AML revealed a statistically significant improvement in overall survival during a 2-year follow-up from the Phase III clinical trials [37]. Addition of dexverapamil to EPOCH chemotherapy (a regimen comprising etoposide, doxorubicin, vincristine, cyclophosphamide and prednisone) in a cross-over design resulted in an increased response rate in lymphoma patients [38]. These findings motivated investigators to develop more potent inhibitors of drug efflux. Interestingly, limited confirmation of the cyclosporin A trial was reported in two sequential Phase II trials in which the addition of cyclosporin A in the second trial again improved relapse-free survival [39].
Second generation
The second generation of inhibitors was developed to improve potency over the first-generation inhibitors. The most developed inhibitor was valspodar (PSC-833), a 10–20-fold more potent analogue of cyclosporine D [40,41]. Although valspodar fulfilled the requirement for a higher-affinity non-toxic Pgp inhibitor, the drug presented unanticipated pharmacokinetic interactions. Through concurrent inhibition of CYP3A4 (cytochrome P450 3A4), valspodar interfered with drug metabolism and elimination, thus increasing anticancer drug exposure [42,43]. The Pgp drug-binding site is similar to the drug-binding site on CYP3A4, and many anticancer agents are substrates for Pgp and also CYP3A4. Valspodar reduced CYP3A4-mediated intestinal or liver metabolism of the anticancer agents at the same time that it blocked Pgp-mediated efflux from cancer cells and normal tissues. This resulted in reduced drug metabolism that elevated drug exposure, and increased the severity and incidence of adverse effects associated with the anticancer therapy. These were often bone marrow toxicities, including neutropenia and thrombocytopenia that could be attributed to increased AUCs (areas under the concentration curves) for a chemotherapeutic agent. In order to accommodate this pharmacokinetic interaction in valspodar trials, anticancer drug doses were reduced by 25–50% [44,45]. However, because of interpatient variation in CYP3A4 metabolism, some patients were underdosed and others overdosed [46,47]. A Phase III CALGB (Cancer and Leukemia Group B) trial using valspodar in previously untreated AML patients over age 60 was closed early due to excessive mortality in the experimental arm during induction [45]. Despite this problematic result, a subset of patients with detectable leukaemic cell drug efflux had a statistically significant improvement in complete remission rates and a trend towards an improved disease-free survival [45]. Another interesting result was seen in a study with AML patients under the age of 60. The trial observed that the addition of valspodar to daunorubicin, etoposide and cytarabine showed an advantage in disease-free and overall survival in a subset of patients 45 years old or younger [48]. Unfortunately, the result has not been duplicated, and development of valspodar has since been discontinued.
Similar findings were obtained with VX-710 (Biricodar, Incel™), which has the ability to inhibit Pgp, MRP1 and ABCG2 [49]. Responses were observed in trials with sarcoma and ovarian cancer, but because of the non-randomized design, it was not possible to determine their significance [17,50,51]. Development of Biricodar also appears to have been discontinued.
Third generation
The third-generation compounds were better agents: potent, non-toxic and with minimal pharmacokinetic interaction. These new inhibitors include tariquidar (XR9576), zosuquidar (LY335979), laniquidar (R101933) and CBT-1.
Tariquidar, as well as the second-generation inhibitor elacridar, have the added benefit of extended Pgp and ABCG2 inhibition [52,53]. A Phase I study demonstrated that administration of elacridar combined with oral topotecan resulted in complete apparent oral bioavailability of topotecan [54]. Flow cytometry studies using rhodamine 123, a fluorescent substrate of Pgp, showed that the Pgp and ABCG2 antagonist tariquidar is able to inhibit Pgp-mediated rhodamine efflux for up to 48 hours after a single dose [55]. Phase I studies of tariquidar in combination with vinorelbine, paclitaxel or doxorubicin showed no significant side effects or pharmacokinetic interactions [56]. However, two large Phase III trials with tariquidar closed early due to toxicity. Both trials combined tariquidar with first-line chemotherapy for patients with NSCLC [57,58].
Zosuquidar is one of the most potent Pgp inhibitors in development. It has been evaluated in patients with AML, where zosuquidar is able to completely inhibit Pgp function, and results are awaited in these trials [59]. A previous clinical study showed a 75% response rate among 16 patients receiving zosuquidar in combination with daunorubicin and cytarabine for AML [60]. A Phase I/II trial demonstrated that administration of zosuquidar with standard chemotherapy in patients with untreated non-Hodgkin’s lymphoma had little effect on the pharmacokinetics of the anticancer drugs [61]. A similar Phase II trial tested the effects of docetaxel with zosuquidar administration in breast cancer patients. Although the trial found no significant difference in progression-free survival, overall survival or response rate, the treatment regimens were found to be safe [62].
Additional third-generation inhibitors include laniquidar and CBT-1. Laniquidar has shown promise as a potent orally active MDR inhibitor with no observed pharmacokinetic interactions. A Phase II study of laniquidar in combination with docetaxel or paclitaxel in refractory breast cancer has been conducted, but results have not yet been reported [63]. Pre-clinical studies examining CBT-1 have affirmed the drug’s ability to inhibit Pgp function at low concentrations [64]. Phase I trials testing CBT-1 with paclitaxel or doxorubicin have been completed, and the agent is now in Phase II and III trials in patients with NSCLC [65]. The initial Phase I studies demonstrated that CBT-1 had no effect on the pharmacokinetics of doxorubicin or paclitaxel [66].
Inhibitor trials revisited
To date, clinical trials using ABC transporter inhibitors have not met the expectations of the scientific community. Whereas the negative results may be explained by several factors, such as the effect of the inhibitors on pharmacokinetics, it is also possible that the hypothesis that ABC transporter inhibition will increase drug accumulation in tumours and thereby efficacy is simply incorrect. It will be difficult to be certain which conclusion is correct without further clinical and translational work. Some of the flaws in earlier trials that support the first conclusion are as follows.
The initial enthusiasm for the Pgp hypothesis led to a large number of trials and a rapid loss of optimism when these trials were unable to achieve the magnitude of benefit anticipated based on in vitro models. More realistic expectations may have allowed identification of a subset of patients with true benefit.
Clinical trials were guided by highly drug-resistant intraperitoneal murine tumour models. A recent study using a hereditary breast cancer mouse model showed that modest increases in Pgp were sufficient to cause resistance to doxorubicin [67], and to the PARP [poly(ADP-ribose) polymerase] inhibitor olaparib (AZD2281) [68]. Increased sensitivity to both drugs was shown with the addition of tariquidar. Better pre-clinical models could have aided in the selection of appropriate drug and inhibitor combinations.
A clinically validated assay for Pgp or other ABC transporters has never been established. This meant that correlative studies detecting Pgp in tumour tissue were not conducted in a standardized fashion. Since no definitive guidelines for clinical analysis existed, clinical trials differed in assay methodology, which resulted in confusing data that could not be used to interpret clinical trial results, or compared across institutions.
Patients were not selected based on tumour expression of Pgp. To conduct a trial with power to determine the true impact of ABC transporter inhibitors in MDR, it is crucial to select the subset of patients whose tumours express ABC transporters as a dominant mechanism of resistance. For example, a Phase III clinical trial used tariquidar in patients with NSCLC, despite a lack of evidence suggesting that NSCLC expresses Pgp to a significant extent [57]. Much like trastuzumab for HER2-overexpressing breast cancers, imatinib in CML (chronic myeloid leukaemia) and erlotinib for patients with lung cancers containing epidermal growth factor receptor mutations, it is not likely that a targeted therapy will succeed without presence of the target.
A corollary to the inadequate Pgp detection methods is that other transporters, both uptake and efflux, were not assessed. A diagnostic imaging test would allow identification of tumours in which Pgp was a dominant factor in drug accumulation [69]. In many tumours, ABC transporters other than Pgp are likely to be equally important in reducing drug accumulation. It is now understood that there is a large family of uptake transporters that also determine drug accumulation. The relative importance of uptake compared with efflux transporters is not known, but is likely to vary among tumours or even across degrees of differentiation. Only a tumour in which Pgp is a dominant mechanism of resistance would be expected to be sensitive to modulation.
The earlier trials did not confirm that the Pgp inhibitor under question was actually able to inhibit the ABC transporter in vivo. In time, ex vivo assays confirming Pgp inhibition by second and third-generation inhibitors in CD56+ circulating mononuclear cells were developed [70]. The radionuclide imaging agent [99mTc]sestamibi was identified as a Pgp substrate and was shown in patients to increase in tissues and tumours known to express high levels of Pgp, when administered in the presence of a Pgp inhibitor [56,71].
Most of the trials were conducted with ‘home-run’ Phase II designs in patients whose tumours did not necessarily overexpress ABC transporter, and in which it was not known whether or not an ABC transporter was a dominant mechanism of resistance. The hope was that benefit would be substantial and obvious; thus this design did not allow determination of the benefit of adding an inhibitor to the treatment regimen. Randomized studies were needed, but those that came later again failed to select patients where the dominant mechanism of resistance was transporter-mediated.
Thus the authors would conclude that the hypothesis that Pgp mediates drug resistance was never adequately tested in the clinic. The failure to document the expression of Pgp in tumours, that it conferred resistance and that the inhibitors reached the tumour to block efflux and increase drug accumulation all suggest that the negative results from the clinical trials were flawed. Indeed, pharmaceutical companies are sufficiently wary of drug transporter-mediated drug resistance that compounds are often optimized during development to make them poorer substrates for transport.
However, it is also possible that Pgp inhibitors cannot increase drug uptake in tumours. Like anticancer drugs, inhibitors access solid tumours via blood vessels and must penetrate tumour tissue to reach all cancer cells. A recent study in a xenograft model demonstrated that Pgp inhibitors increase uptake of doxorubicin only in cells close to blood vessels and have little effect on drug uptake at intermediate distances [72]. Pgp inhibition may not have a significant impact if there is impaired permeability and drug diffusion, particularly in solid tumours. Imaging studies to evaluate drug uptake in tumours are critically needed to answer this question.
A third possibility is that Pgp, or other ABC transporter inhibitors that reside in bone marrow cells, will not succeed in the clinic because of the lack of a therapeutic window. To the extent that transporters protect normal bone marrow cells, normal tissue sanctuaries, and are involved in drug excretion, effective inhibitors have the potential to block these vital roles and increase toxicity to patients. It has been felt that a therapeutic window would exist because transporter levels are lower in tumours than in normal cells. Further, Pgp-knockout models have generally shown only a modest impact on blood levels of cancer chemotherapeutics, presumably related to complex and redundant metabolic pathways. To date, clinical trials have suggested that a therapeutic window does exist. But this question will remain inconclusive as long as clinically effective ABC transporter inhibition has not been achieved.
Implications from pharmacology
ABC transporters have emerged as an important variable in pharmacology and drug distribution. A number of SNPs (single nucleotide polymorphisms) have been identified that may contribute to inter-individual variation in drug metabolism. It is possible that some of the increased toxicity observed in the earlier trials occurred in patients with polymorphic ABC transporters with altered folding and impaired function or lower expression levels, described for certain ABC transporter variants [73–78]. A patient whose genetics constrain expression or function of Pgp or ABCG2 would not be expected to benefit from the addition of a transport inhibitor since both normal tissues and tumours would be affected. The data showing that, in the trial with valspodar mentioned earlier, patients with efflux-positive leukaemia did not have high mortality and actually had some evidence of treatment benefit support this hypothesis [45]. Understanding the impact of inter-individual variation in pharmacogenomics could clarify patient selection in future clinical trials.
Non-traditional applications of ABC transporters as targets
As a component of the blood-brain barrier and the gastrointestinal epithelium, the clinical applications of ABC transporter inhibitors can be expanded to trials examining ways to increase CNS uptake of non-toxic drugs and improve oral drug bioavailability. This is of increased importance in diseases such as breast, lung and renal cancer, where newer targeted therapies are increasing systemic control, and more CNS metastases are emerging [79]. TKIs (tyrosine kinase inhibitors), such as erlotinib, lapatinib and sorafenib, are effective in the systemic control of these tumours, but are also substrates for the transporters [80–84]. Remarkably, murine studies show that, whereas knockout of either transporter alone has minimal impact, deletion of the orthologues for Pgp and ABCG2 results in a 22-fold increase in relative CNS uptake of dasatinib, a 40-fold enhancement of CNS uptake of lapatinib, an 8.5-fold increase for erlotinib and a 9-fold increase for sorafenib. The ability to increase CNS penetration of these agents could thus have a major impact on decreasing the occurrence of CNS disease in solid tumours sensitive to these agents. Studies to assess accumulation of radionuclide-imaging agents in the brain are in early stages and may help to identify agents that could increase the accumulation of TKIs for patients whose systemic disease has been controlled [85–88]. The mouse knockout models also show an impact of the transporters on pharmacokinetics, although to a much lesser extent. Nonetheless, the ability of transport inhibitors to increase oral bioavailability and to equalize blood levels among patients is an area for investigation that could lead to increased access to anticancer agents, particularly where infusional therapy is either too cumbersome or too costly to administer.
Future directions
The most important question is where the field should go from here, and whether this family of proteins warrants continued investigation as potential therapeutic targets in cancer. Since Pgp and ABCG2 expression continue to be linked to poor outcome, we argue that it would be a mistake to discontinue studying the role of ABC transporters in clinical oncology. Data from clinical trials being conducted with zosuquidar in AML and with CBT-1 in NSCLC are awaited. However, negative results from these trials are still subject to many of the same caveats as the older trials, if conducted without selection of patients whose tumours have been shown to have a Pgp-mediated reduction in drug accumulation.
Whereas the list of ABC transporter substrates has steadily expanded, investigation into the implications of these compounds being substrates has waned. For example, the drugs imatinib, nilotinib and dasatinib, which are used in the treatment of CML, are both Pgp and ABCG2 substrates [89,90]. Therefore drug transporters could contribute to drug resistance in CML [91]. Mutation of the target and altered uptake are also relevant mechanisms of resistance for CML, hence the need to study the individual contribution of the different mechanisms, determine which are dominant and develop strategies to overcome resistance. Whereas resistance that manifests clinically is a problem for a relatively small subset of patients with CML in chronic phase, it is an inevitable problem for patients who receive TKIs as therapy for solid tumours. In this setting, resistance is again certain to be multifactoral, and the contribution of ABC transporters is one among several mechanisms. The sanctuary site data in murine knockout models that demonstrate marked redundancy between Pgp and ABCG2 in limiting the CNS uptake of dasatinib, lapatinib, sorafenib, erlotinib and sunitinib suggest that together these transporters could markedly limit the uptake of the TKIs in solid tumours. Approaches that successfully increase accumulation of the TKIs in the CNS could reignite the question of whether systemic ABC transporter inhibition in solid tumours could be successful.
ABC transporters have received considerable attention since being discovered in putative cancer stem cells. Whether one accepts the idea that cancer cells and progeny divide stochastically, i.e. at random, or the revisionist hypothesis that cancer stem cells represent a unique subpopulation of cancer cells that persist and repopulate a tumour following each therapy-mediated reduction in cell number, the molecular pathways that produce the ‘sternness’ phenotype can be targeted. Inhibitors of Notch, Wnt and Hedgehog pathways could easily be ABC transporter substrates. Since putative cancer stem cells identified to date have high levels of ABCG2, it will be important to know whether inhibitors of these stem cell pathways are substrates. If the role of ABCG2 is to protect stem cells, then the stem cell pathway inhibitors need to circumvent drug efflux.
With the discovery of Pgp, MRP1 and ABCG2, each ABC transporter in turn was scrutinized as the crucial new transporter in MDR. As a result, research and literature focused on that one transporter rather than forming a cohesive study on multiple ABC transporters responsible for MDR. In order to avoid this bias, and to clarify tumour tissue expression of transporters in drug resistance, it is critical that transporters not be studied as individual entities in clinical samples. To understand mechanistically why drug accumulation may be limited, unbiased assays of known uptake and efflux transporters should be conducted within a single study, as they may function as a synergistic unit to reduce drug accumulation. Fortunately, advanced array technologies offer this possibility.
When Pgp inhibitors were introduced as a means of circumventing MDR, expectations for the inhibitors were very high. Early clinical trials were unable to meet these expectations and resulted in disappointment for many in the scientific community. Since then, changes have occurred in the standards of anticancer drugs as well as our perception of cancer therapy. For instance, cancer is more often viewed as a chronic disease and incremental improvements more acceptable. This concept has brought many new agents to the anticancer stage, and it may be worth examining transporter inhibitors in this current context. Indeed, AZD2281, a PARP inhibitor found to be active in BRCA1 (breast cancer early-onset 1)-deficient breast cancer, is also a substrate for Pgp-mediated drug resistance. A genetically engineered mouse model for BRCA1-associated breast cancer shows marked improvement in response and duration of response with the addition of tariquidar to AZD2281 [68].
However, clinical trials should not be conducted again until patients can be selected who have tumours in which resistance is dominated by ABC transporters. We lack a validated assay for detecting any of the transporters, i.e. an assay with known sensitivity, specificity and reproducibility for detection of Pgp, MRP1, ABCG2 or any of the transporters, in the clinical setting. The specificity of antibody-based assays has been a major problem; RNA assays appear too sensitive. More important is the development of a functional assay; indeed, unrelated to ABC transporters, determination of actual drug accumulation in solid tumours has been a neglected area of cancer research. Currently, there is no test by which a treating physician can determine whether an ineffective agent is reaching the tumour or its therapeutic target. Diagnostic imaging has the potential to determine whether Pgp or other transporters are functioning to reduce drug accumulation and whether inhibition can change drug uptake in solid tumours. A number of new PET (positron-emission tomography) imaging agents that could remedy this problem are under study [85–87,92,93]. Indeed, it can be easily argued that laboratory investigations aimed at identifying transporter inhibitors should not be conducted further until a clinical assay exists to define which tumours have transporter-dominated resistance mechanisms. It has been much easier to select new inhibitors in vitro than it has been to show that these agents increase drug accumulation in vivo. This latter question should be the focus of future research in this field.
Conclusion
“I have made some progress. But why so late and with such difficulty?”
—Paul Cezanne
Progress in science, and apparently in art, is often painstakingly slow. The effort to exploit ABC transporters to reverse drug resistance in clinical oncology has been characterized by missteps that ultimately impeded our ability to answer the question of their true role in cancer. Koch’s postulates regarding the causal role of bacteria in disease initially insisted that the micro-organism should not be found in healthy animals, but was later revised when asymptomatic carriers were discovered. In the case of ABC transporters, presence of a transporter does not define that transporter as the dominant cause of drug resistance. Thirty-five years after the discovery of Pgp, we still do not know whether or in which tumours the transporter reduces anticancer drug accumulation. This is a question that needs to be answered before the field can begin to move again. Emerging understanding of the redundancy of ABC transporters in limiting drug distribution to sanctuary sites such as the CNS has provided proof-of-concept for their ability to limit drug accumulation in solid tumours. Methods must be developed to determine whether the latter is true, and whether it matters, in patients with cancer.
Note added in proof (received 19 July 2011)
Results from a randomized Phase III trial in older patients diagnosed with AML treated with cytarabine and daunorubicin with zosuquidar or placebo were reported recently [101]. The trial found that addition of zosuquidar to the treatment regimen did not significantly affect the complete response rate, outcome or progression-free survival.
Summary
ABC transporters are overexpressed in a variety of tumour types and are associated with poor outcome in some malignancies.
The ‘Pgp hypothesis’ conveys the notion that overexpression of Pgp or other ABC transporters confers clinical drug resistance, which could be overcome through inhibition of drug efflux mediated by the ABC transporter.
Efforts to circumvent drug resistance through ABC transporter inhibition largely failed, with Pgp inhibitors the most intensively studied. Problems encountered during early development suggest, from one perspective, that the Pgp hypothesis was never adequately tested. It is also possible that non-transporter mechanisms limiting drug delivery also limited delivery of the inhibitors, and that these non-transporter mechanisms are more crucial to adequate drug exposure.
Pharmacogenomic variation in ABC transporter expression or function could have made some individuals markedly more sensitive to the inhibitors, with loss of a therapeutic window.
Normal tissue expression may provide the most relevant strategy to exploit ABC transporters as therapeutic targets. Thus inhibition of drug efflux at the blood-brain barrier may allow increased CNS uptake and retention of anticancer agents. Inhibition of efflux in the gastrointestinal tract could allow improved oral absorption of anticancer agents.
Many novel targeted agents are substrates for ABC transporters in in vitro assays. It is not known whether Pgp or other ABC transporters are relevant in clinical resistance to targeted agents such as lapatinib, sorafenib, dasatinib or imatinib. These compounds are important candidates for strategies aimed at increasing CNS uptake.
Development of imaging agents is critical to determining whether Pgp or another ABC transporter is a dominant mechanism of drug resistance, whether there is tumour heterogeneity, and whether expression might be critical in a small subset of stem-cell-like cancer cells. Imaging anticancer agents or surrogates in vivo has the potential to tell us whether ABC transporters are rate-limiting for drug uptake, whether they are a dominant mechanism of drug resistance and whether they should re-emerge as therapeutic targets.
References
- 1.Aller S, Yu J, Ward A, Weng Y, Chittaboina S, Zhuo R, Harrell P, Trinh Y, Zhang Q,Urbatsch I and Chang G (2009) Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science 323, 1718–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boumendjel A, Boutonnat J and Robert J (2009) ABC transporters and multidrug resistance.John Wiley and Sons, Hoboken [Google Scholar]
- 3.Szakács G, Paterson J, Ludwig J, Booth-Genthe C and Gottesman M (2006) Targeting multidrug resistance in cancer. Nat. Rev. Drug Discovery 5, 219–234 [DOI] [PubMed] [Google Scholar]
- 4.Cordon-Cardo C, O’Brien JP, Boccia J, Casals D, Bertino JR and Melamed MR (1990)Expression of the multidrug resistance gene product (P-glycoprotein) in human normal and tumort issues. J. Histochem. Cytochem 38, 1277–1287 [DOI] [PubMed] [Google Scholar]
- 5.Fetsch P, Abati A, Litman T, Morisaki K, Honjo Y, Mittal K and Bates S (2006) Localization of the ABCG2 mitoxantrone resistance-associated protein in normal tissues. Cancer Lett. 235,84–92 [DOI] [PubMed] [Google Scholar]
- 6.Maliepaard M, Scheffer G, Faneyte I, van Gastelen M, Pijnenborg A, Schinkel A, van DeVijver M, Scheper R and Schellens J (2001) Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 61, 3458–3464 [PubMed] [Google Scholar]
- 7.Zhou S, Schuetz J, Bunting K, Colapietro A, Sampath J, Morris J, Lagutina I, Grosveld G, Osawa M, Nakauchi H and Sorrentino B (2001) The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat. Med 7, 1028–1034 [DOI] [PubMed] [Google Scholar]
- 8.Deeley R and Cole S (2006) Substrate recognition and transport by multidrug resistance protein 1 (ABCC1). FEBS Lett. 580, 1103–1111 [DOI] [PubMed] [Google Scholar]
- 9.Wijnholds J, Scheffer G, van der Valk M, van der Valk P, Beijnen J, Scheper R and Borst P(1998) Multidrug resistance protein 1 protects the oropharyngeal mucosal layer and the testicular tubules against drug-induced damage. J. Exp. Med 188, 797–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wijnholds J, deLange E, Scheffer G, van den Berg D, Mol C, van der Valk M, Schinkel A,Scheper R, Breimer D and Borst P (2000) Multidrug resistance protein 1 protects the choroid plexus epithelium and contributes to the blood–cerebrospinal fluid barrier. J. Clin. Invest 105,279–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polgar O and Bates S (2005) ABC transporters in the balance: is there a role in multidrug resistance? Biochem. Soc. Trans 33, 241–245 [DOI] [PubMed] [Google Scholar]
- 12.Leith C, Kopecky K, Chen I, Eijdems L, Slovak M, McConnell T, Head D, Weick J,Grever M, Appelbaum F and Willman C (1999) Frequency and clinical significance of the expression of the multidrug resistance proteins MDR1/P-glycoprotein, MRP1, and LRP in acute myeloid leukemia: a Southwest Oncology Group study. Blood 94, 1086–1099 [PubMed] [Google Scholar]
- 13.Legrand O, Simonin G, Perrot JY, Zittoun R and Marie JP (1998) Pgp and MRP activities using calcein-AM are prognostic factors in adult acute myeloid leukemia patients. Blood 91,4480–4488 [PubMed] [Google Scholar]
- 14.Wilson C, Davidson G, Martin S, Andries E, Potter J, Harvey R, Ar K, Xu Y, Kopecky K, Ankerst D et al. (2006) Gene expression profiling of adult acute myeloid leukemia identifies novel biologic clusters for risk classification and outcome prediction. Blood 108, 685–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benderra Z, Faussat AM, Sayada L, Perrot JY, Chaoui D, Marie JP and Legrand O (2004)Breast cancer resistance protein and P-glycoprotein in 149 adult acute myeloid leukemias. Clin. Cancer Res 10, 7896–7902 [DOI] [PubMed] [Google Scholar]
- 16.van den Heuvel-Eibrink M, van der Holt B, Burnett A, Knauf W, Fey M, Verhoef G,Vellenga E, Ossenkoppele G, Löwenberg B and Sonneveld P (2007) CD34-related coexpression of MDR1 and BCRP indicates a clinically resistant phenotype in patients with acute myeloid leukemia (AML) of older age. Ann. Hematol 86, 329–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burger H, Foekens J, Look M, Meijer-van Gelder M, Klijn J, Wiemer E, Stoter G andNooter K (2003) RNA expression of breast cancer resistance protein, lung resistance-related protein, multidrug resistance-associated proteins 1 and 2, and multidrug resistance gene 1 in breast cancer: correlation with chemotherapeutic response. Clin. Cancer Res 9, 827–836 [PubMed] [Google Scholar]
- 18.Trock BJ, Leonessa F and Clarke R (1997) Multidrug resistance in breast cancer: a meta-analysis of MDR1/gp170 expression and its possible functional significance. J. Natl. Cancer Inst 89, 917–931 [DOI] [PubMed] [Google Scholar]
- 19.Penson RT, Oliva E, Skates SJ, Glyptis T, Fuller AF Jr, Goodman A. and Seiden MV.(2004) Expression of multidrug resistance-1 protein inversely correlates with paclitaxel response and survival in ovarian cancer patients: a study in serial samples. Gynecol. Oncol 93,98–106 [DOI] [PubMed] [Google Scholar]
- 20.van den Heuvel-Eibrink MM, van der Holt B, Burnett AK, Knauf WU, Fey MF, Verhoef GE, Vellenga E, Ossenkoppele GJ, Lowenberg B and Sonneveld P (2007) CD34-related coexpression of MDR1 and BCRP indicates a clinically resistant phenotype in patients with acute myeloid leukemia (AML) of older age. Ann. Hematol 86, 329–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michieli M, Damiani D, Ermacora A, Masolini P, Raspadori D, Visani G, Scheper RJ and Baccarani M. (1999) P-glycoprotein, lung resistance-related protein and multidrug resistance associated protein in de novo acute non-lymphocytic leukaemias: biological and clinical implications. Br. J. Haematol 104, 328–335 [DOI] [PubMed] [Google Scholar]
- 22.Filipits M, Pohl G, Rudas M, Dietze O, Lax S, Grill R, Pirker R, Zielinski C, Hausmaninger H, Kubista E et al. (2005) Clinical role of multidrug resistance protein 1 expression in chemotherapy resistance in early-stage breast cancer: the Austrian Breast and Colorectal Cancer Study Group. J. Clin. Oncol 23, 1161–1168 [DOI] [PubMed] [Google Scholar]
- 23.Hsia T, Lin C, Wang J, Ho S and Kao A (2002) Relationship between chemotherapy response of small cell lung cancer and P-glycoprotein or multidrug resistance-related protein expression. Lung 180, 173–179 [DOI] [PubMed] [Google Scholar]
- 24.Li J, Li Z, Yu L, Bao Q, Wu J, Shi S and Li X (2010) Association of expression of MRP1,BCRP, LRP and ERCC1 with outcome of patients with locally advanced non-small cell lung cancer who received neoadjuvant chemotherapy. Lung Cancer 69, 116–122 [DOI] [PubMed] [Google Scholar]
- 25.Galimberti S, Guerrini F, Palumbo G, Consoli U, Fazzi R, Morabito F, Santini V and Petrini M(2004) Evaluation of BCRP and MDR-1 co-expression by quantitative molecular assessment in AML patients. Leuk. Res 28, 367–372 [DOI] [PubMed] [Google Scholar]
- 26.van der Kolk D, Vellenga E, Scheffer G, Müller M, Bates S, Scheper R and de Vries E(2002) Expression and activity of breast cancer resistance protein (BCRP) in de novo and relapsed acute myeloid leukemia. Blood 99, 3763–3770 [DOI] [PubMed] [Google Scholar]
- 27.Abbott B, Colapietro A, Barnes Y, Marini F, Andreeff M and Sorrentino B (2002) Low levels of ABCG2 expression in adult AML blast samples. Blood 100, 4594–4601 [DOI] [PubMed] [Google Scholar]
- 28.van der Pol M, Broxterman H, Pater J, Feller N, van der Maas M, Weijers G, Scheffer G, Allen J, Scheper R, van Loevezijn A et al. (2003) Function of the ABC transporters,P-glycoprotein, multidrug resistance protein and breast cancer resistance protein, in minimal residual disease in acute myeloid leukemia. Haematologica 88, 134–147 [PubMed] [Google Scholar]
- 29.Uggla B, Stahl E, Wagsater D, Paul C, Karlsson MG, Sirsjo A and Tidefelt U (2005) BCRP mRNA expression v. clinical outcome in 40 adult AML patients. Leuk. Res 29, 141–146 [DOI] [PubMed] [Google Scholar]
- 30.Suvannasankha A, Minderman H, O’Loughlin KL, Nakanishi T, Greco WR, Ross DD and Baer MR (2004) Breast cancer resistance protein (BCRP/MXR/ABCG2) in acute myeloid leukemia: discordance between expression and function. Leukemia 18, 1252–1257 [DOI] [PubMed] [Google Scholar]
- 31.Steinbach D, Sell W, Voigt A, Hermann J, Zintl F and Sauerbrey A (2002) BCRP gene expression is associated with a poor response to remission induction therapy in childhood acute myeloid leukemia. Leukemia 16, 1443–1447 [DOI] [PubMed] [Google Scholar]
- 32.Ota S, Ishii G, Goto K, Kubota K, Kim Y, Kojika M, Murata Y, Yamazaki M, Nishiwaki Y,Eguchi K and Ochiai A (2009) Immunohistochemical expression of BCRP and ERCC1 in biopsy specimen predicts survival in advanced non-small-cell lung cancer treated with cisplatin-based chemotherapy. Lung Cancer 64, 98–104 [DOI] [PubMed] [Google Scholar]
- 33.Kim Y, Ishii G, Goto K, Ota S, Kubota K, Murata Y, Mishima M, Saijo N, Nishiwaki Yand Ochiai A (2009) Expression of breast cancer resistance protein is associated with a poor clinical outcome in patients with small-cell lung cancer. Lung Cancer 65, 105–111 [DOI] [PubMed] [Google Scholar]
- 34.Dean M, Fojo T and Bates S (2005) Tumour stem cells and drug resistance. Nat. Rev. Cancer 5, 275–284 [DOI] [PubMed] [Google Scholar]
- 35.Polgar O and Bates SE (2005) ABC transporters in the balance: is there a role in multidrug resistance? Biochem. Soc. Trans 33, 241–245 [DOI] [PubMed] [Google Scholar]
- 36.Wattel E, Solary E, Hecquet B, Caillot D, Ifrah N, Brion A, Milpied N, Janvier M, Guerci A,Rochant H et al. (1999) Quinine improves results of intensive chemotherapy (IC) in myelodysplastic syndromes (MDS) expressing P-glycoprotein (PGP): updated results of a randomized study. Groupe Francais des Myelodysplasies (GFM) and Groupe GOELAMS. Adv. Exp. Med. Biol 457, 35–46 [DOI] [PubMed] [Google Scholar]
- 37.List AF, Kopecky KJ, Willman CL, Head DR, Persons DL, Slovak ML, Dorr R, Karanes C, Hynes HE, Doroshow JH et al. (2001) Benefit of cyclosporine modulation of drug resistance in patients with poor-risk acute myeloid leukemia: a Southwest Oncology Group study.Blood 98, 3212–3220 [DOI] [PubMed] [Google Scholar]
- 38.Wilson W, Bates S, Fojo A, Bryant G, Zhan Z, Regis J, Wittes R, Jaffe E, Steinberg S and Herdt J. (1995) Controlled trial of dexverapamil, a modulator of multidrug resistance, in lymphomas refractory to EPOCH chemotherapy. J. Clin. Oncol 13, 1995–2004 [DOI] [PubMed] [Google Scholar]
- 39.Chauncey T, Gundacker H, Shadman M, List A, Dakhil S, Erba H, Slovak M, Chen I,Willman C, Kopecky K and Appelbaum F (2010) Sequential phase II Southwest Oncology Group studies (S0112 and S0301) of daunorubicin and cytarabine by continuous infusion, without and with ciclosporin, in older patients with previously untreated acute myeloid leukaemia. Br. J. Haematol 148, 48–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Twentyman P and Bleehen N (1991) Resistance modification by PSC-833, a novel non-immunosuppressive cyclosporin. Eur. J. Cancer 27, 1639–1642 [DOI] [PubMed] [Google Scholar]
- 41.te Boekhorst P, van Kapel J, Schoester M and Sonneveld P (1992) Reversal of typical multidrug resistance by cyclosporin and its non-immunosuppressive analogue SDZ PSC 833 in Chinese hamster ovary cells expressing the mdr1 phenotype. Cancer Chemother. Pharmacol 30, 238–242 [DOI] [PubMed] [Google Scholar]
- 42.Bates S, Kang M, Meadows B, Bakke S, Choyke P, Merino M, Goldspiel B, Chico I,Smith T, Chen C et al. (2001) A Phase I study of infusional vinblastine in combination with the P-glycoprotein antagonist PSC 833 (valspodar). Cancer 92, 1577–1590 [DOI] [PubMed] [Google Scholar]
- 43.Wandel C, Kim R, Kajiji S, Guengerich P, Wilkinson G and Wood A (1999) P-glycoprotein and cytochrome P-450 3A inhibition: dissociation of inhibitory potencies. Cancer Res. 59, 3944–3948 [PubMed] [Google Scholar]
- 44.Friedenberg W, Rue M, Blood E, Dalton W, Shustik C, Larson R, Sonneveld P and Greipp P(2006) Phase III study of PSC-833 (valspodar) in combination with vincristine, doxorubicin, and dexamethasone (valspodar/VAD) versus VAD alone in patients with recurring or refractory multiple myeloma (E1A95): a trial of the Eastern Cooperative Oncology Group. Cancer 106,830–838 [DOI] [PubMed] [Google Scholar]
- 45.Baer MR, George SL, Dodge RK, O’Loughlin KL, Minderman H, Caligiuri MA, Anastasi J, Powell BL, Kolitz JE, Schiffer CA et al. (2002) Phase 3 study of the multidrug resistance modulator PSC-833 in previously untreated patients 60 years of age and older with acute myeloid leukemia: Cancer and Leukemia Group B Study 9720. Blood 100, 1224–1232 [PubMed] [Google Scholar]
- 46.ten Tije A, Synold T, Spicer D, Verweij J, Doroshow J and Sparreboom A (2003) Effect of valspodar on the pharmacokinetics of unbound paclitaxel. Invest. New Drugs 21, 291–298 [DOI] [PubMed] [Google Scholar]
- 47.Bates S, Bakke S, Kang M, Robey R, Zhai S, Thambi P, Chen C, Patil S, Smith T,Steinberg S et al. (2004) A phase I/II study of infusional vinblastine with the P-glycoprotein antagonist valspodar (PSC 833) in renal cell carcinoma. Clin. Cancer Res 10, 4724–4733 [DOI] [PubMed] [Google Scholar]
- 48.Kolitz JE, George SL, Dodge RK, Hurd DD, Powell BL, Allen SL, Velez-Garcia E,Moore JO, Shea TC, Hoke E et al. (2004) Dose escalation studies of cytarabine, daunorubicin, and etoposide with and without multidrug resistance modulation with PSC-833 in untreated adults with acute myeloid leukemia younger than 60 years: final induction results of Cancer and Leukemia Group B Study 9621. J. Clin. Oncol 22, 4290–4301 [DOI] [PubMed] [Google Scholar]
- 49.Minderman H, O’Loughlin KL, Pendyala L and Baer MR (2004) VX-710 (biricodar) increases drug retention and enhances chemosensitivity in resistant cells overexpressing P-glycoprotein, multidrug resistance protein, and breast cancer resistance protein. Clin. Cancer Res 10, 1826–1834 [DOI] [PubMed] [Google Scholar]
- 50.Seiden M, Swenerton K, Matulonis U, Campos S, Rose P, Batist G, Ette E, Garg V, Fuller A, Harding M and Charpentier D (2002) A phase II study of the MDR inhibitor biricodar (INCEL, VX-710) and paclitaxel in women with advanced ovarian cancer refractory to paclitaxel therapy. Gynecol. Oncol 86, 302–310 [DOI] [PubMed] [Google Scholar]
- 51.Bramwell V, Morris D, Ernst D, Hings I, Blackstein M, Venner P, Ette E, Harding M,Waxman A and Demetri G (2002) Safety and efficacy of the multidrug-resistance inhibitor biricodar (VX-710) with concurrent doxorubicin in patients with anthracycline-resistant advanced soft tissue sarcoma. Clin. Cancer Res 8, 383–393 [PubMed] [Google Scholar]
- 52.de Bruin M, Miyake K, Litman T, Robey R and Bates SE (1999) Reversal of resistance by GF120918 in cell lines expressing the ABC half-transporter, MXR. Cancer Lett. 146, 117–126 [DOI] [PubMed] [Google Scholar]
- 53.Robey RW, Steadman K, Polgar O, Morisaki K, Blayney M, Mistry P and Bates SE (2004)Pheophorbide a is a specific probe for ABCG2 function and inhibition. Cancer Res. 64, 1242–1246 [DOI] [PubMed] [Google Scholar]
- 54.Kuppens I, Witteveen E, Jewell R, Radema S, Paul E, Mangum S, Beijnen J, Voest E andSchellens J (2007) A phase I, randomized, open-label, parallel-cohort, dose-finding study of elacridar (GF120918) and oral topotecan in cancer patients. Clin. Cancer Res 13, 3276–3285 [DOI] [PubMed] [Google Scholar]
- 55.Stewart A, Steiner J, Mellows G, Laguda B, Norris D and Bevan P (2000) Phase I trial of XR9576 in healthy volunteers demonstrates modulation of P-glycoprotein in CD56+ lymphocytes after oral and intravenous administration. Clin. Cancer Res 6, 4186–4191 [PubMed] [Google Scholar]
- 56.Abraham J, Edgerly M, Wilson R, Chen C, Rutt A, Bakke S, Robey R, Dwyer A,Goldspiel B, Balis F et al. (2009) A phase I study of the P-glycoprotein antagonist tariquidar in combination with vinorelbine. Clin. Cancer Res 15, 3574–3582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nobili S, Landini I, Giglioni B and Mini E (2006) Pharmacological strategies for overcoming multidrug resistance. Curr. Drug Targets 7, 861–879 [DOI] [PubMed] [Google Scholar]
- 58.Fox E and Bates S (2007) Tariquidar (XR9576): a P-glycoprotein drug efflux pump inhibitor.Expert Rev. Anticancer Ther 7, 447–459 [DOI] [PubMed] [Google Scholar]
- 59.Lancet J, Baer M, Duran G, List A, Fielding R, Marcelletti J, Multani P and Sikic B (2009) A phase I trial of continuous infusion of the multidrug resistance inhibitor zosuquidar with daunorubicin and cytarabine in acute myeloid leukemia. Leuk. Res 33, 1055–1061 [DOI] [PubMed] [Google Scholar]
- 60.Gerrard G, Payne E, Baker R, Jones D, Potter M, Prentice H, Ethell M, McCullough H,Burgess M, Mehta A and Ganeshaguru K (2004) Clinical effects and P-glycoprotein inhibition in patients with acute myeloid leukemia treated with zosuquidar trihydrochloride, daunorubicin and cytarabine. Haematologica 89, 782–790 [PubMed] [Google Scholar]
- 61.Morschhauser F, Zinzani P, Burgess M, Sloots L, Bouafia F and Dumontet C (2007) Phase I/II trial of a P-glycoprotein inhibitor, Zosuquidar.3HCl trihydrochloride (LY335979), given orally in combination with the CHOP regimen in patients with non-Hodgkin’s lymphoma. Leuk. Lymphoma 48, 708–715 [DOI] [PubMed] [Google Scholar]
- 62.Ruff P, Vorobiof D, Jordaan J, Demetriou G, Moodley S, Nosworthy A, Werner I, Raats J and Burgess L (2009) A randomized, placebo-controlled, double-blind phase 2 study of docetaxel compared to docetaxel plus zosuquidar (LY335979) in women with metastatic or locally recurrent breast cancer who have received one prior chemotherapy regimen. Cancer Chemother. Pharmacol 64, 763–768 [DOI] [PubMed] [Google Scholar]
- 63.van Zuylen L, Sparreboom A, van der Gaast A, van der Burg M, van Beurden V, Bol C,Woestenborghs R, Palmer P and Verweij J (2000) The orally administered P-glycoprotein inhibitor R101933 does not alter the plasma pharmacokinetics of docetaxel. Clin. Cancer Res 6,1365–1371 [PubMed] [Google Scholar]
- 64.Robey R, Shukla S, Finley E, Oldham R, Barnett D, Ambudkar S, Fojo T and Bates S(2008) Inhibition of P-glycoprotein (ABCB1)- and multidrug resistance-associated protein 1 (ABCC1)-mediated transport by the orally administered inhibitor, CBT-1®. Biochem. Pharmacol 75, 1302–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oldham R, Reid W, Preisler H and Barnett D (1998) A phase I and pharmacokinetic study of CBT-1 as a multidrug resistance modulator in the treatment of patients with advanced cancer. Cancer Biother. Radiopharm 13, 71–80 [DOI] [PubMed] [Google Scholar]
- 66.Oldham R, Reid W and Barnett D (2000) Phase I study of CBT-1 and Taxol in patients with Taxol resistant cancers. Cancer Biother. Radiopharm 15, 153–159 [DOI] [PubMed] [Google Scholar]
- 67.Pajic M, Iyer J, Kersbergen A, van der Burg E, Nygren A, Jonkers J, Borst P andRottenberg S (2009) Moderate increase in Mdr1a/1b expression causes in vivo resistance to doxorubicin in a mouse model for hereditary breast cancer. Cancer Res. 69, 6396–6404 [DOI] [PubMed] [Google Scholar]
- 68.Rottenberg S, Jaspers J, Kersbergen A, van der Burg E, Nygren A, Zander S, Derksen P,de Bruin M, Zevenhoven J, Lau A et al. (2008) High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc. Natl. Acad. Sci. U.S.A 105, 17079–17084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Holló Z, Homolya L, Hegedûs T, Müller M, Szakács G, Jakab K, Antal F and Sarkadi B(1998) Parallel functional and immunological detection of human multidrug resistance proteins, P-glycoprotein and MRP1. Anticancer Res. 18, 2981–2987 [PubMed] [Google Scholar]
- 70.Robey R, Bakke S, Stein W, Meadows B, Litman T, Patil S, Smith T, Fojo T and Bates S (1999) Efflux of rhodamine from CD56+ cells as a surrogate marker for reversal of P-glycoprotein-mediated drug efflux by PSC 833. Blood 93, 306–314 [PubMed] [Google Scholar]
- 71.Agrawal M, Abraham J, Balis F, Edgerly M, Stein W, Bates S, Fojo T and Chen C (2003)Increased 99mTc-sestamibi accumulation in normal liver and drug-resistant tumors after the administration of the glycoprotein inhibitor, XR9576. Clin. Cancer Res 9, 650–656 [PubMed] [Google Scholar]
- 72.Patel K and Tannock I (2009) The influence of P-glycoprotein expression and its inhibitors on the distribution of doxorubicin in breast tumors. BMC Cancer 9, 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cusatis G, Gregorc V, Li J, Spreafico A, Ingersoll R, Verweij J, Ludovini V, Villa E, Hidalgo M, Sparreboom A and Baker S (2006) Pharmacogenetics of ABCG2 and adverse reactions to gefitinib. J. Natl. Cancer Inst 98, 1739–1742 [DOI] [PubMed] [Google Scholar]
- 74.Müller P, Dally H, Klappenecker C, Edler L, Jäger B, Gerst M, Spiegelhalder B, Tuengerthal S, Fischer J, Drings P et al. (2009) Polymorphisms in ABCG2, ABCC3 and CNT1 genes and their possible impact on chemotherapy outcome of lung cancer patients. Int. J. Cancer 124, 1669–1674 [DOI] [PubMed] [Google Scholar]
- 75.Han J, Lim H, Yoo Y, Shin E, Park Y, Lee S, Lee J, Lee D, Kim H and Lee J (2007) Associations of ABCB1, ABCC2, and ABCG2 polymorphisms with irinotecan-pharmacokinetics and clinical outcome in patients with advanced non-small cell lung cancer. Cancer 110, 138–147 [DOI] [PubMed] [Google Scholar]
- 76.Hoffmeyer S, Burk O, von Richter O, Arnold H, Brockmöller J, Johne A, Cascorbi I,Gerloff T, Roots I, Eichelbaum M and Brinkmann U (2000) Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc. Natl. Acad. Sci. U.S.A 97, 3473–3478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jamroziak K, Młynarski W, Balcerczak E, Mistygacz M, Trelinska J, Mirowski M, Bodalski J and Robak T (2004) Functional C3435T polymorphism of MDR1 gene: an impact on genetic susceptibility and clinical outcome of childhood acute lymphoblastic leukemia. Eur. J. Haematol 72, 314–321 [DOI] [PubMed] [Google Scholar]
- 78.Sparreboom A, Gelderblom H, Marsh S, Ahluwalia R, Obach R, Principe P, Twelves C,Verweij J and McLeod H (2004) Diflomotecan pharmacokinetics in relation to ABCG2 421C>Agenotype. Clin. Pharmacol. Ther 76, 38–44 [DOI] [PubMed] [Google Scholar]
- 79.Aragon-Ching J and Zujewski J (2007) CNS metastasis: an old problem in a new guise. Clin. Cancer Res 13, 1644–1647 [DOI] [PubMed] [Google Scholar]
- 80.Dohse M, Scharenberg C, Shukla S, Robey R, Volkmann T, Deeken J, Brendel C,Ambudkar S, Neubauer A and Bates S (2010) Comparison of ATP-binding cassette transporter interactions with the tyrosine kinase inhibitors imatinib, nilotinib and dasatinib. Drug Metab. Dispos 38, 1371–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Polli J, Olson K, Chism J, John-Williams L, Yeager R, Woodard S, Otto V, Castellino S and Demby V (2009) An unexpected synergist role of P-glycoprotein and breast cancer resistance protein on the central nervous system penetration of the tyrosine kinase inhibitor lapatinib (N-{3-chloro-4-[(3-fluorobenzyl)oxy]phenyl}-6-[5-({[2-(methylsulfonyl)ethyl]amino}methyl)-2-furyl]-4-quinazolinamine; GW572016). Drug Metab. Dispos 37, 439–442 [DOI] [PubMed] [Google Scholar]
- 82.Lagas J, van Waterschoot R, Sparidans R, Wagenaar E, Beijnen J and Schinkel A (2010)Breast cancer resistance protein and P-glycoprotein limit sorafenib brain accumulation. Mol. Cancer Ther 9, 319–326 [DOI] [PubMed] [Google Scholar]
- 83.Lagas J, van Waterschoot R, van Tilburg V, Hillebrand M, Lankheet N, Rosing H, Beijnen Jand Schinkel A (2009) Brain accumulation of dasatinib is restricted by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) and can be enhanced by elacridar treatment. Clin. Cancer Res 15, 2344–2351 [DOI] [PubMed] [Google Scholar]
- 84.Marchetti S, de Vries N, Buckle T, Bolijn M, van Eijndhoven M, Beijnen J, Mazzanti R, van Tellingen O and Schellens J (2008) Effect of the ATP-binding cassette drug transporters ABCB1, ABCG2, and ABCC2 on erlotinib hydrochloride (Tarceva) disposition in in vitro and in vivo pharmacokinetic studies employing Bcrp1−/−/Mdr1a/1b−/− (triple-knockout) and wild-type mice. Mol. Cancer Ther 7, 2280–2287 [DOI] [PubMed] [Google Scholar]
- 85.Bauer M, Karch R, Neumann F, Wagner C, Kletter K, Müller M, Löscher W, Zeitlinger Mand Langer O (2010) Assessment of regional differences in tariquidar-induced P-glycoprotein modulation at the human blood–brain barrier. J. Cereb. Blood Flow Metab 30, 510–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Luurtsema G, Molthoff C, Schuit R, Windhorst A, Lammertsma A and Franssen E (2005)Evaluation of (R)-[11C]verapamil as PET tracer of P-glycoprotein function in the blood–brain barrier: kinetics and metabolism in the rat. Nucl. Med. Biol 32, 87–93 [DOI] [PubMed] [Google Scholar]
- 87.Muzi M, Mankoff D, Link J, Shoner S, Collier A, Sasongko L and Unadkat J (2009) Imaging of cyclosporine inhibition of P-glycoprotein activity using 11C-verapamil in the brain: studies of healthy humans. J. Nucl. Med 50, 1267–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kreisl W, Liow J, Kimura N, Seneca N, Zoghbi S, Morse C, Herscovitch P, Pike V and Innis R, (2010) P-glycoprotein function at the blood–brain barrier in humans can be quantified with the substrate radiotracer 11C-N-desmethyl-loperamide. J. Nucl. Med 51, 559–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rumpold H, Wolf AM, Gruenewald K, Gastl G, Gunsilius E and Wolf D (2005)RNAi-mediated knockdown of P-glycoprotein using a transposon-based vector system durably restores imatinib sensitivity in imatinib-resistant CML cell lines. Exp. Hematol 33, 767–775 [DOI] [PubMed] [Google Scholar]
- 90.Brendel C, Scharenberg C, Dohse M, Robey RW, Bates SE, Shukla S, Ambudkar SV, Wang Y, Wennemuth G, Burchert A et al. (2007) Imatinib mesylate and nilotinib (AMN107) exhibit high-affinity interaction with ABCG2 on primitive hematopoietic stem cells. Leukemia 21, 1267–1275 [DOI] [PubMed] [Google Scholar]
- 91.Hegedus C, Ozvegy-Laczka C, Szakács G and Sarkadi B (2009) Interaction of ABC multidrug transporters with anticancer protein kinase inhibitors: substrates and/or inhibitors? Curr. Cancer Drug Targets 9, 252–272 [DOI] [PubMed] [Google Scholar]
- 92.Luurtsema G, Schuit R, Klok R, Verbeek J, Leysen J, Lammertsma A and Windhorst A(2009) Evaluation of [11C]laniquidar as a tracer of P-glycoprotein: radiosynthesis and biodistribution in rats. Nucl. Med. Biol 36, 643–649 [DOI] [PubMed] [Google Scholar]
- 93.Kawamura K, Konno F, Yui J, Yamasaki T, Hatori A, Yanamoto K, Wakizaka H, Takei M, Nengaki N, Fukumura T and Zhang M (2010) Synthesis and evaluation of [11C]XR9576 toassess the function of drug efflux transporters using PET. Ann. Nucl. Med 24, 403–412 [DOI] [PubMed] [Google Scholar]
- 94.Szakacs G, To KKW, Polgar O, Robey RW and Bates SE (2009) Multidrug resistance mediated by MDR-ABC transporters In Drug Resistance in Cancer Cells (Mehta K and Siddik ZH, eds), Springer, New York [Google Scholar]
- 95.Liu Yin JA, Wheatley K, Rees JK and Burnett AK (2001) Comparison of ‘sequential’ versus ’standard’ chemotherapy as re-induction treatment, with or without cyclosporine, in refractory/relapsed acute myeloid leukaemia (AML): results of the UK Medical Research Council AML-Rtrial. Br. J. Haematol 113, 713–726 [DOI] [PubMed] [Google Scholar]
- 96.Sonneveld P, Suciu S, Weijermans P, Beksac M, Neuwirtova R, Solbu G, Lokhorst H, vander Lelie J, Dohner H, Gerhartz H et al. (2001) Cyclosporin A combined with vincristine, doxorubicin and dexamethasone (VAD) compared with VAD alone in patients with advanced refractory multiple myeloma: an EORTC-HOVON randomized phase III study (06914). Br. J. Haematol 115, 895–902 [DOI] [PubMed] [Google Scholar]
- 97.van der Holt B, Lowenberg B, Burnett AK, Knauf WU, Shepherd J, Piccaluga PP,Ossenkoppele GJ, Verhoef GE, Ferrant A, Crump M et al. (2005) The value of the MDR1 reversal agent PSC-833 in addition to daunorubicin and cytarabine in the treatment of elderly patients with previously untreated acute myeloid leukemia (AML), in relation to MDR1 status at diagnosis. Blood 106, 2646–2654 [DOI] [PubMed] [Google Scholar]
- 98.Joly F, Joly F, Mangioni C, Nicoletto M, Manikhas GM, Walker J, Mietlowski W, Jones G,Wysowskyj H and Dugan M (2002) A phase 3 study of PSC 833 in combination with paclitaxel and carboplatin (PC-PSC) versus paclitaxel and carboplatin (PC) alone in patients with stage IV or suboptimally debulked stage III epithelial ovarian cancer or primary cancer of the peritoneum.Proc. Am. Soc. Clin. Oncol 21, Abstract 806 [Google Scholar]
- 99.Greenberg PL, Lee SJ, Advani R, Tallman MS, Sikic BI, Letendre L, Dugan K, Lum B,Chin DL, Dewald G et al. (2004) Mitoxantrone, etoposide, and cytarabine with or without valspodar in patients with relapsed or refractory acute myeloid leukemia and high-risk myelodysplastic syndrome: a phase III trial (E2995). J. Clin. Oncol 22, 1078–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Saeki T, Nomizu T, Toi M, Ito Y, Noguchi S, Kobayashi T, Asaga T, Minami H,Yamamoto N, Aogi K et al. (2007) Dofequidar fumarate (MS-209) in combination with cyclophosphamide, doxorubicin, and fluorouracil for patients with advanced or recurrent breast cancer. J. Clin. Oncol 25, 411–417 [DOI] [PubMed] [Google Scholar]
- 101.Cripe LD, Uno H, Paietta EM, Litzow MR, Ketterling RP, Bennett JM, Rowe JM,Lazarus HM, Luger S and Tallman MS (2010) Zosuquidar, a novel modulator of P-glycoprotein, does not improve the outcome of older patients with newly diagnosed acutem yeloid leukemia: a randomized, placebo-controlled trial of the Eastern Cooperative Oncology Group 3999. Blood 116, 4077–4085 [DOI] [PMC free article] [PubMed] [Google Scholar]