Abstract
Increased levels of oxidatively induced DNA damage have been reported in various cases of human pathogenesis like age-related and chronic diseases. Advances in experimental carcinogenesis associate high oxidative stress with genome instability and oncogenic transformation. Cancer biomarkers are helpful for early tumor diagnostics, prediction of tumor development, and analysis of individual tumors’ response to therapy as well as recurrence. The repair resistant oxidatively induced clustered DNA lesions (OCDLs) could serve as a common indicator of oxidative stress in human malignant cells or tissues. To test this hypothesis, we assessed the levels of endogenous OCDLs in several human tumor and adjacent normal tissues from patients with liver, ovary, kidney, breast and colon cancer. These tumor tissues have already been shown to accumulate higher endogenous levels of γ-H2AX foci. For the detection of clustered DNA lesions we used the human repair enzymes APE1, OGG1 and NTH1 as well as the Escherichia coli homologue Endonuclease III. In the majority of cases we detected higher levels of OCDLs in tumor vs. normal tissues but not always with a statistically significant difference and not with uniform tissue dependence. These data suggest for the first time the importance of endogenous non-DSB clusters in human cancer and their potential use as cancer biomarkers.
Keywords: Oxidative stress, Clustered DNA damage, Human tumors, Cancer biomarkers
1. Introduction
Cellular DNA is constantly exposed to various cytotoxic and DNA damaging agents, endogenous and exogenous to the cell. DNA damage has been categorized as being of spontaneous origin (endogenous) or environmentally induced (exogenous). Endogenous sources of DNA oxidation consist of reactive lipid peroxidation products and reactive oxygen species (ROS) formed as by-products of normal cellular metabolism [1]. Aggregated DNA changes in genes (mutations) can activate proto-oncogenes or inactivate tumor-suppressor genes. The genetic instability driving tumorigenesis is fuelled by DNA damage and by errors performed by our genome maintenance machinery (i.e., DNA repair system). Numerous studies suggest the important role of oxidative DNA damage and its repair in cancer [2,3]. Higher levels of endogenous oxidative stress (reactive oxygen species, ROS), DNA damage and/or defective DNA repair have been reported in different malignancies and tumors [4–9]. In accordance with this, several human syndromes – like Xeroderma Pigmentosum (XP) or Ataxia telangiectasia (AT) – with defective genome maintenance (i.e., some type of DNA repair deficiency), show an increased predilection for developing cancer [10].
Of the different repair pathways, base excision repair (BER) is generally believed to constitute the primary defense against lesions formed by endogenous DNA-damaging agents or low levels of exogenous sources like radiotherapy, occupational ionizing radiation or environmental chemicals [11]. The resulting pool of oxidative DNA damage can contain a wide variety of different DNA lesions [single strand breaks (SSBs), oxidized bases and apurinic-apyrimidinic (abasic, AP) sites] [12]. Persistent basal DNA damage may reflect higher exposure to DNA damaging agents and/or deficient DNA repair. Higher levels of endogenous oxidative DNA damage lesions (like 8-OH-dG, 8-OH-Ade) have been reported in a variety of tumor tissues, e.g., breast, colon/rectum, cervix, liver, kidney, ovary, stomach and lung, compared with controls as reviewed in [4,6]. These findings suggest that the accumulation of DNA damage may contribute to tissue transformation and carcinogenesis.
The last decade, a significant amount of literature has been devoted to the study of clustered DNA lesions, first introduced by Ward as locally multiply damaged sites (LMDS) [13] and recently reviewed in [14]. Oxidative clustered DNA lesions (OCDLs), i.e., two or more bistranded DNA lesions within a short DNA fragment of 1–10 base pairs, including AP sites, oxidized purines/pyrimidines or SSBs, are hypothesized to be repair resistant which can produce cytotoxic and mutagenic effects [15–18], as well as substantial chromosomal instability [19]. Since clustered DNA damage can also be readily induced by either low doses of ionizing radiation and chemicals like bleomycin [20] or endogenously as a by-product of oxidative metabolism [21], they are considered to be highly significant biological lesions. In addition, endogenous OCDLs are inducible in human cell lines of different tissue origin, pointing to the possible role of endogenous OCDL accumulation in the promotion of mutagenesis and genomic instability [22–24]. Based on the possible accumulation of endogenous OCDLs in human cells and tissues, an intriguing question arrives, whether there is a way to manipulate the level of endogenous clusters? Interestingly, recent studies by Bennett et al. have shown that the levels of endogenous clusters were decreased by growing human cells in the presence of selenium [23]. Selenium, a well-accepted antioxidant, has also been shown to have a protective role against genetic damage and cancer (for a review see [25]).
In this study we assessed the levels of spontaneous OCDLs detected using different repair enzymes as damage probes, in malignant tumors of different origins, as well as in adjacent normal tissues from cancer patients of different ages. The specific tissues have already been shown to have an increased level of DNA DSBs revealed by the formation of γ-H2AX foci [26]. Our results suggest in most cases for tumor tissues that an increased level of non-DSB clustered DNA lesions can be associated with higher oxidative stress in these tissues.
2. Materials and methods
2.1. Human tissues
Twenty-six human biopsies were obtained from the Cooperative Human Tissue Network (CHTN), Mid-Atlantic Division (Charlottesville, VA) and Pediatric Division (Columbus, OH) with IRB approval. Colon tubular adenocarcinoma, breast invasive ductal adenocarcinoma, ovary adenocarcinoma, hepatoblastoma, Wilms kidney tumor (nephroblastoma), and normal adjacent tissues were removed from cancer patients (age range was from 1 year to 88 years) at the time of surgery as the “excess” tissues not essential for routine diagnosis. Patients’ confidentiality was maintained.
2.2. Isolation of human DNA and measurement of OCDLs
The “High Pure PCR Template Kit” (Roche, Indianapolis, IN) was used for isolation of DNA from the tissues as previously described [22]. For the detection and measurement of oxidatively induced clustered DNA lesions, constant-field gel electrophoresis was used along with quantitative electronic imaging, and number average length analysis (NALA). Human repair enzymes APE1, OGG1, and NTH1 were used as enzymatic probes along with E. coli EndoIII. The human NTH1 was a kind gift from Dr. Roy (Georgetown University), the remaining enzymes were purchased from New England Biolabs, MA (APE1- M0282L; E. coli EndoIII- M0268L; OGG1- M0241S, lot 1).
For the detection of bistranded oxidative clustered DNA lesions, 40 ng of isolated DNA was mixed with 7 μl of the appropriate enzyme reaction buffer and left on ice for 30 min. As reviewed in Hada and Georgakilas [27], OGG1 is expected to detect the majority of oxypurine clusters (containing at least one oxidized purine) and some abasic sites while NTH1 or EndoIII is expected to detect the majority of oxypyrimidine clusters (containing at least one oxidized pyrimidine) and some limited number of abasic sites. Using APE1 as a damage probe, we expect to detect the majority of abasic sites (Table 1). The enzyme buffers used were: APE1 buffer (50 mM potassium acetate, 20 mM Tris acetate, 10 mM magnesium acetate, pH 7.9), OGG1 buffer (50 mM NaCl, 10 mM MgCl2, 10 mM Tris–HCl, pH 7.9), E. coli NTH1 buffer (20 mM Tris-HCl, 1 mM EDTA, pH 8.0), and human NTH1 buffer(50 mM Hepes, 75 mM NaCl, pH 7.9) [28]. For each enzyme-treated sample, a corresponding non-enzyme containing sample was also run as a control following the same steps but without the addition of the enzyme. Next, 1 μl of 10× Dithiothreitol (DTT, 10 mM, Sigma) was added and the mixture was vortexed. After an incubation time of 10 min on ice (4° C), 1 μl of the respective enzyme was added (1 unit for APE1 and E. coli EndoIII, 0.53 units for OGG1 and 30 ng for human NTH1). These were mixed and further incubated on ice for 15 min. Samples were then transferred to 37 °C for 1 h. The reaction was terminated by the addition of 5 μl of ice-cold native stop solution [50% glycerol (v/v), 100 mM EDTA, 0.025% bromophenol blue (w/v), 0.025% xylene blue (w/v)] and incubation on ice for 2 h. DNA samples were loaded and left to equilibrate for 10 min on the wells after which neutral constant field gel electrophoresis was performed [0.9% agarose (w/v; Molecular Biology, Biorad), 0.25× TBE, pH 7.6, 3.125 V/cm, 3h]. The gel was stained with Ethidium Bromide and destained overnight. As size standards on every gel λ-Hind III Digest (Fisher Scientific) was included. The marker preparation comprised of addition of 0.75 μl marker DNA (0.5 μg/ml) to 4 μl native stop solution and 5 μl TE buffer (10 mM Tris–HCl, 1 mM EDTA, pH 7.5), mixing and incubating at 55 °C for 3 min and then on ice until the gel was ready to be loaded.
Table 1.
Different human or Escherichia coli repair enzymes and their substrates used in the detection of oxidative clustered DNA lesions [6,49].
| Repair enzymes used as damage probes | Substrates |
|---|---|
| Human hAPE1 or E. coli Nfo protein (Endonuclease IV) | Abasic: Several types of abasic sites including oxidized abasic sites, abasic sites modified with alkoxyamines and DNA containing urea residues |
| Human OGG1 or E. coli Fpg protein (DNA glycosylase). Associated AP lyase activity | Oxypurines: Oxidized purines, FapyGua, FapyAde, C8-oxoGuanine, some abasic sites, C8-oxoAdenine and to a lesser extent, other modified purines |
| Human NTH1 protein or E. coli EndoIII (Endonuclease III). Associated AP lyase activity | Oxypyrimidines: Thymine residues damaged by ring saturation, fragmentation, or ring contraction including thymine glycol (Tg) and uracil residues (5-fo-Ura), FapyAde, 5-OH-Cyt and to a lesser extent FapyGua |
Electronic images for each gel lane were processed using QuantiScan (BioSoft, Ferguson, MO) and a densitogram was obtained. DNA standards (λ-Hind III Digest) were used to obtain the corresponding dispersion curve with Origin 6.1 (OriginLab, Northampton, MA). The number average lengths (Ln) for each sample were calculated using the equations described in Sutherland et al. [27,29]. The frequencies of OCDLs (abasic or oxidized base clusters) were measured based on Ln values and using NALA [29]. For each enzyme-treated sample (+ lane), an accompanying control sample (− lane) was prepared by following the same steps without addition of the enzyme.
2.3. Statistical analysis
Paired Student’s t-tests were used to evaluate the differences between various groups of tumor and normal tissues (p < 0.05).
3. Results and discussion
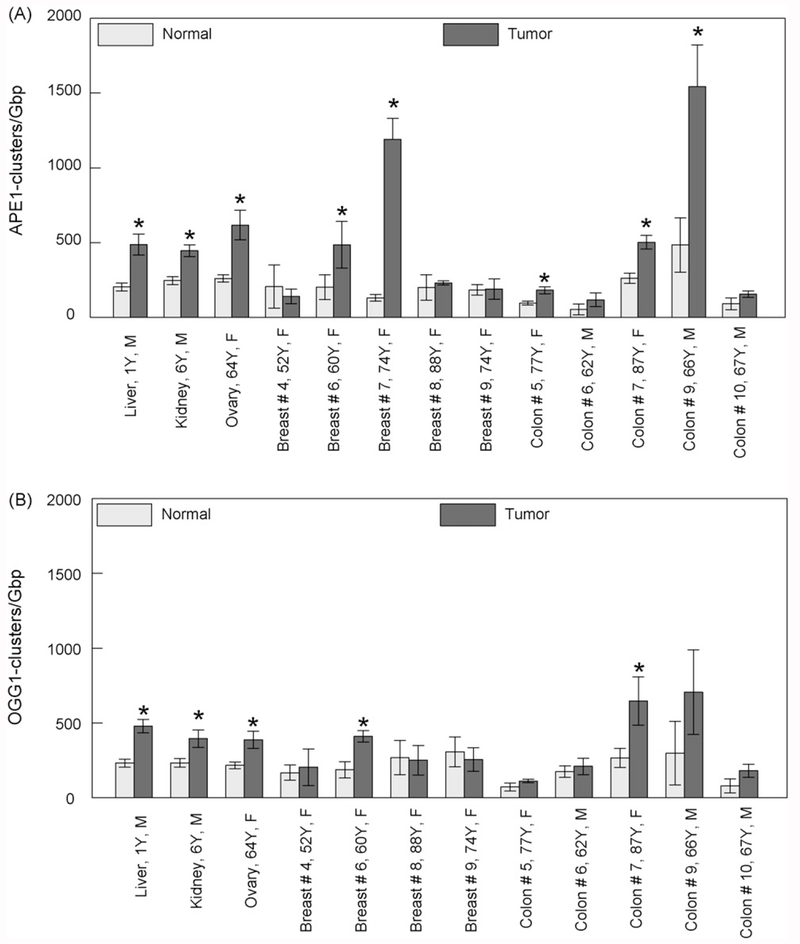
We isolated DNA from biopsies taken from cancer patients and assessed the levels of non-DSB oxidative clustered DNA lesions using various human and bacterial repair enzymes as damage probes. Fig. 1 shows a comparison between the background levels of APE1-clusters in tumors (hepatoblastoma, Wilms kidney tumor-nephroblastoma, ovary adenocarcinoma, breast invasive ductal adenocarcinoma and colon tubular adenocarcinoma), and histologically verified normal tissues adjacent to the tumors. The tissue numbering is identical to that used in Sedelnikova and Bonner (2006) to facilitate comparison [26]. Although in most cases the levels of these abasic clusters for tumor tissues are higher compared to normal tissues, statistically significant differences have been detected only for liver, kidney, ovary and some cases of breast and colon. The levels of endogenous abasic clusters in non-malignant tissues are generally very low within the range of 50–400 clusters/Giga base pair (Gbp), while tumors generally show significantly elevated values in the range of 100–1500 clusters/Gbp. Another finding is that within the same tumor localization, for example, breast or colon, we see a significant variation in the values. Comparison of the abasic clusters for tumors with other types of clusters reveals a general trend of higher values for this specific type. Fig. 1B shows the data for OGG1-clusters which are primarily oxidized purine clusters, i.e., bistranded lesions comprising of at least one oxidized purine. Of course, based on the intrinsic AP lyase activity of this enzyme towards also abasic sites (Table 1), we cannot exclude the detection of some abasic sites. Comparison between Fig. 1A and B shows a similar pattern for OGG1- and abasic clusters. Again, we detected a statistically significant difference for kidney, liver and ovary and for selected breast and colon tissues.
Fig. 1.
Levels of different types of endogenous non-DSB oxidatively induced DNA clusters detected in DNA isolated from tumor and control (adjacent) tissues. (A) Abasic clusters detected using human APE1 and (B) oxypurine clusters detected using human OGG1. Values, averages out of three to five independent experiments. The age and gender of the patient can be also seen for each case. Error bars, standard errors of the means (S.E.M.). *Significant statistical difference (p < 0.05).
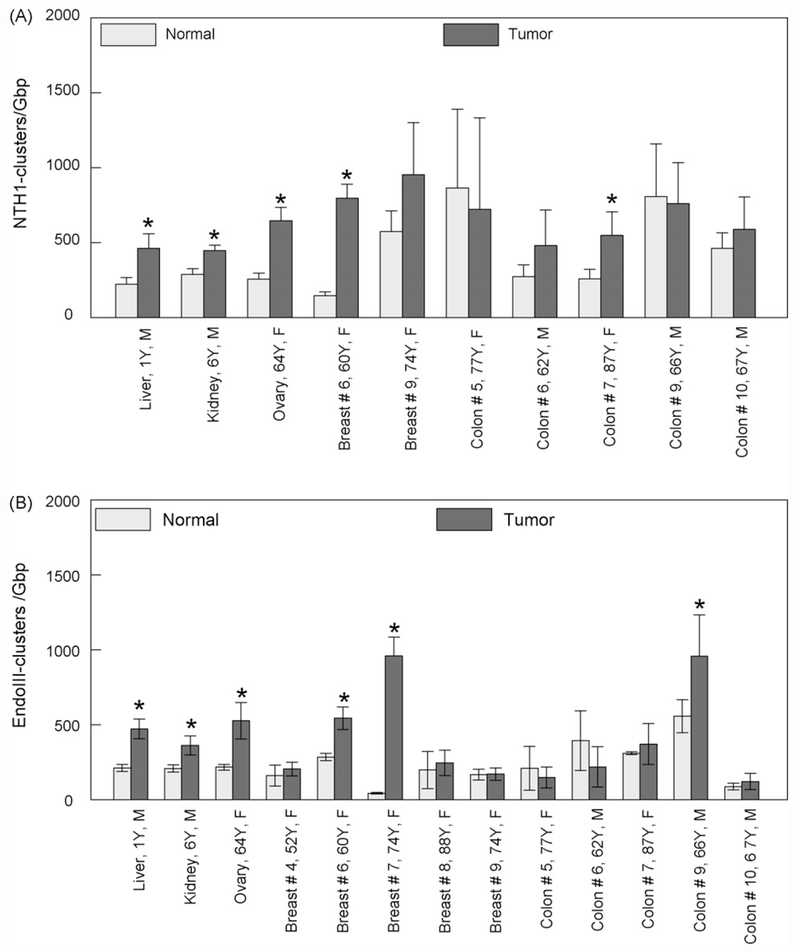
We used human NTH1 and bacterial Endonuclease III enzymes to detect oxidized pyrimidine clusters (Fig. 2). These two enzymes practically detect and cleave the same type of lesions with a tendency of the bacterial enzyme to have a stronger AP lyase activity compared to its human homologue [30] and therefore an expected higher percentage of abasic sites detected with EndoIII. In both cases, we detected a prevalence of NTH1- or EndoIII-clusters in tumor tissues compared to normal ones in the range of 250–1000 clusters/Gbp. In most cases, the levels of normal tissues are significantly lower in the range of 50–500 clusters/Gbp.
Fig. 2.
Levels of different types of endogenous non-DSB oxidatively induced DNA clusters detected in DNA isolated from tumor and control (adjacent) tissues. (A) Oxypyrimidine clusters detected using human NTH1 and (B) oxypurine clusters detected using bacterial EndoIII. Values, averages out of three to five independent experiments. The age and gender of the patient can be also seen for each case. Error bars, standard errors of the means (S.E.M.). *Significant statistical difference (p < 0.05).
Sedelnikova and Bonner, 2006, have shown a higher accumulation of DSBs revealed by γ-H2AX focus formation assay for the same tumor tissues along with several malignant cell lines [26]. In our case, and for the type of non-DSB lesions that we detected, we see a similar trend, i.e., a higher accumulation of bistranded oxidative DNA lesions. Although we did not detect a significant difference in every case, the specific trend suggests that oxidatively induced clusters can accumulate at much higher levels in tumor tissues compared to control normal tissues. The nature of these clusters can be very diverse. For example, in the case of abasic APE1-clusters, two bistranded abasic sites or one abasic site opposite to a SSB can be present. In general, the origin of these detected types of complex DNA damage is the endogenous oxidative stress in the cells. Different studies suggest that in many clinical cases, including liver, colon, breast and ovary cancers, much higher levels of the specific DNA base lesion (8-OH-dG) are found in tumors or even in blood or urine from cancer patients compared to controls [4,6]. 8-OH-dG has been excessively used in many studies as a biomarker of oxidative stress. As reviewed in Toyokuni et al. [31], 8-OH-dG levels have been reported to be much higher in several tumor tissues compared to adjacent normal controls.
Substantial evidence supports the accumulation and persistence of different oxidative DNA lesions (abasic sites or oxidized bases) even in non-malignant human or animal cells and tissues at values ranging from 100 to 10,000 lesions/Gbp [7,32–34]. In addition to single base lesions, different groups have shown an accumulation of endogenous clustered DNA lesions in human cells in the range of 20–500 clusters/Gbp [22,23,35]. Previous studies have shown an accumulation of all types of clusters in human breast cancer cells MCF-7 compared to non-malignant MCF-10A coupled with a lower antioxidant capacity [22,36]. We cannot exclude the higher background levels of clusters detected for tumor tissues to be the result of a combination of factors like DNA repair and antioxidant enzyme deficiencies, lower antioxidant capacity, production of ROS or reactive nitrogen species (RNS) at higher levels in the tumor cells. One important finding of our results is the prevalence of high background levels for breast and colon tissues. One possible explanation may be the already documented higher oxidative stress detected in many cases of breast or colon cancer [37–42]. All these studies suggest a direct association between cell transformation in these tissues with DNA repair deficiencies and specifically mutations or reduced expression of BRCA1, MSH2 and other DNA repair genes. Another possible factor at least in the case of breast cancer may be an exposure to estrogen-induced free radicals and smoking [43,44].
By looking in Figs. 1 and 2 we can also detect in some cases an increase in the accumulation of clustered DNA lesions with age, and increased DNA damage in aging cells and tissues has been well documented [45]. This can partially explain the higher levels of spontaneous clusters in some tissues of the aged patients. The greater variation in the values of clusters measured in colon and breast compared to liver/kidney/ovary may be also explained by the nature of these tissues. Liver/kidney/ovary tumor biopsies might be more histologically homogeneous, and, therefore, give more even results, compared to colon and breast tumor biopsies containing cancerous epithelium and normal fibroblasts. Depending on the ratio of these cells in the analyzed samples different levels of clusters might have been detected.
In this study we report for the first time a significant accumulation of non-DSB oxidatively induced clustered DNA lesions in human tumors compared to normal tissues. Although we were not able to detect a statistically significant difference in all cases, we can conclude that clustered DNA lesions can be used at least in some cases as an indicator of endogenous oxidative stress. Our results, in general, imply that the levels of endogenous clusters can be affected by the cells’ external or internal environment and oxidative stress, and their ability to deal with DNA damage. It this sense, adjacent normal tissues may not have been an adequate control for the tumors, since they existed in the tumor environment. Tumors can emit factors that can alter an oxidative level of surrounding normal tissues [46], inducing a stress-related bystander effect [47]. This could result in accumulation of oxidative DNA damage and DSBs in bystander tissues. Generally, OCDL analysis is more sensitive compared to the DSB detection. Therefore, OCDL levels in the normal tissues used in this study are very much likely to have been affected by neighboring tumors. On the other hand, endogenous DSBs in tumors may have different origins, both oxidative stress-associated and independently formed as a consequence of genomic instability [48]. Thus, correlation between the levels of DSBs and OCDL might not be direct, but affected by tumor’s characteristics. A possible correlation between OCDL accumulation and tumor characteristics and/or developmental stage would be of a great interest and would require the more extended case analysis than the one performed in this study.
Accumulation of the repair resistant oxidative lesions can lead eventually to mutations, pre-malignant lesions and transformation, and, therefore, require further investigation. The identification of susceptibility factors that predispose individuals to any cancer (for instance, if they are exposed to particular environmental agents) could possibly give further insights into the etiology of the malignancy and provide targets for the future development of therapeutics.
Acknowledgements
This work was supported by funds provided to Dr. Georgakilas by the Biology Department of East Carolina University, a Research/Creative Activity Grant and a College Research Award to A. Georgakilas (East Carolina University), and partly by the Intramural Research Program of the National Cancer Institute, NIH. Dr. Panayiotidis was supported by funds provided by the University of Nevada at Reno.
Footnotes
Conflict of interest statement
None.
References
- [1].Nilsen H, Krokan HE, Base excision repair in a network of defense and tolerance, Carcinogenesis 22 (2001) 987–998. [DOI] [PubMed] [Google Scholar]
- [2].Kastan MB, Bartek J, Cell-cycle checkpoints and cancer, Nature 432 (2004) 316–323. [DOI] [PubMed] [Google Scholar]
- [3].Hussain SP, Hofseth LJ, Harris CC, Radical causes of cancer, Nat. Rev. Cancer 3 (2003) 276–285. [DOI] [PubMed] [Google Scholar]
- [4].Loft S, Moller P, Oxidative DNA damage and human cancer: need for cohort studies, Antioxid. Redox Signal 8 (2006) 1021–1031. [DOI] [PubMed] [Google Scholar]
- [5].Joseph T, Kusumakumary P, Chacko P, Abraham A, Pillai MR, DNA repair gene XRCC1polymorphisms in childhood acute lymphoblastic leukemia, Cancer Lett. 217 (2005) 17–24. [DOI] [PubMed] [Google Scholar]
- [6].Cooke MS, Evans MD, Dizdaroglu M, Lunec J, Oxidative DNA damage: mechanisms, mutation, and disease, FASEB J. 17 (2003) 1195–1214. [DOI] [PubMed] [Google Scholar]
- [7].De Bont R, van Larebeke N, Endogenous DNA damage in humans: a review of quantitative data, Mutagenesis 19 (2004) 169–185. [DOI] [PubMed] [Google Scholar]
- [8].Fan R, Kumaravel TS, Jalali F, Marrano P, Squire JA, Bristow RG, Defective DNA strand break repair after DNA damage in prostate cancer cells: implications for genetic instability and prostate cancer progression, Cancer Res. 64 (2001) 8526–8533. [DOI] [PubMed] [Google Scholar]
- [9].Hannah MA, Siddiqui Y, Rostom A, Al-Ahdal MN, Chaudhary MA, Kunhi M, Evidence of DNA repair/processing defects in cultured skin fibroblasts from breast cancer patients, Cancer Res. 61 (2001) 3627–3631. [PubMed] [Google Scholar]
- [10].Hoeijmakers JH, Genome maintenance mechanisms for preventing cancer, Nature 411 (2001) 366–374. [DOI] [PubMed] [Google Scholar]
- [11].Lindahl T, Wood RD, Quality control of DNA repair, Science 286 (1999) 1897–1905. [DOI] [PubMed] [Google Scholar]
- [12].Ward JF, The complexity of DNA damage: relevance to biological consequences, Int. J. Radiat. Biol 66 (1994) 427–432. [DOI] [PubMed] [Google Scholar]
- [13].Ward JF, Some biochemical consequences of the spatial distribution of ionizing radiation produced free radicals, Radiat. Res 86 (1981) 185–195. [PubMed] [Google Scholar]
- [14].Georgakilas AG, Processing of DNA damage clusters in human cells: current status of knowledge, Mol. Biosyst 4 (2008) 30–35. [DOI] [PubMed] [Google Scholar]
- [15].Georgakilas AG, Bennett PV, Wilson III DM, Sutherland BM, Processing of bistranded abasic DNA clusters in gamma-irradiated human hematopoietic cells, Nucleic Acids Res. 32 (2004) 5609–5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Malyarchuk S, Brame KL, Youngblood R, Shi R, Harrison L, Two clustered 8-oxo-7,8-dihydroguanine (8-oxodG) lesions increase the point mutation frequency of 8-oxodG, but do not result in double strand breaks or deletions in Escherichia coli, Nucleic Acids Res. 32 (2004) 5721–5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Pearson CG, Shikazono N, Thacker J, O’Neill P, Enhanced mutagenic potential of 8-oxo-7,8-dihydroguanine when present within a clustered DNA damage site, Nucleic Acids Res. 32 (2004) 263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sutherland B, Bennett PV, Sidorkina O, Laval J, DNA damage clusters induced by ionizing radiation in isolated DNA and in human cells, Proc. Natl. Acad. Sci. U.S.A 97 (2000) 103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Singleton BK, Griffin CS, Thacker J, Clustered DNA damage leads to complex genetic changes in irradiated human cells, Cancer Res. 62 (2002) 6263–6269. [PubMed] [Google Scholar]
- [20].Weinfeld M, Rasouli-Nia A, Chaudhry MA, Britten RA, Response of base excision repair enzymes to complex DNA lesions, Radiat. Res 156 (2001) 584–589. [DOI] [PubMed] [Google Scholar]
- [21].Sutherland BM, Bennett PV, Cintron NS, Guida P, Laval J, Low levels of endogenous oxidative damage cluster levels in unirradiated viral and human DNAs, Free Radic. Biol. Med 35 (2003) 495–503. [DOI] [PubMed] [Google Scholar]
- [22].Gollapalle E, Wong R, Adetolu R, Tsao D, Francisco D, Sigounas G, Georgakilas AG, Detection of oxidative clustered DNA lesions in X-irradiated mouse skin tissues and human MCF-7 breast cancer cells, Radiat. Res 167 (2007) 207–216. [DOI] [PubMed] [Google Scholar]
- [23].Bennett PV, Cuomo NL, Paul S, Tafrov ST, Sutherland BM, Endogenous DNA damage clusters in human skin, 3-D model and cultured skin cells, Free Radic. Biol. Med 39 (2005) 832–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bennett PV, Cintron NS, Gros L, Laval J, Sutherland BM, Are endogenous clustered DNA damages induced in human cells? Free Radic. Biol. Med 37 (2004) 488–499. [DOI] [PubMed] [Google Scholar]
- [25].El-Bayoumy K, The protective role of selenium on genetic damage and on cancer, Mutat. Res 475 (2001) 123–139. [DOI] [PubMed] [Google Scholar]
- [26].Sedelnikova OA, Bonner WM, GammaH2AX in cancer cells: a potential biomarker for cancer diagnostics, prediction and recurrence, Cell Cycle 5 (2006) 231–240. [DOI] [PubMed] [Google Scholar]
- [27].Hada M, Georgakilas AG, Formation of clustered DNA damage after high-LET irradiation: a review, J. Radiat. Res 49 (2008) 203–210. [DOI] [PubMed] [Google Scholar]
- [28].Liu X, Roy R, Truncation of amino-terminal tail stimulates activity of human Endonuclease III (hNTH1), J. Mol. Biol 321 (2002) 265–276. [DOI] [PubMed] [Google Scholar]
- [29].Sutherland BM, Georgakilas AG, Bennett PV, Laval J, Sutherland JC, Quantifying clustered DNA damage induction and repair by gel electrophoresis, electronic imaging and number average length analysis, Mutat. Res. (Review) 531 (2003) 93–107. [DOI] [PubMed] [Google Scholar]
- [30].Marenstein DR, Chan MK, Altaminaro A, Basu AK, Boorstein RJ, Cunningham RP, Teebor GW, Substrate specificity of human endonuclease III (hNTH1). Effect of human APE1 on hNTH1 activity, J. Biol. Chem 278 (2003) 9005–9012. [DOI] [PubMed] [Google Scholar]
- [31].Toyokuni S, Okamoto K, Yodoi J, Hiai H, Persistent oxidative stress in cancer, FEBS Lett. 358 (1995) 1–3. [DOI] [PubMed] [Google Scholar]
- [32].Nakamura J, Swenberg AJ, Endogenous apurinic/apyrimidinic sites in genomic DNA of mammalian tissues, Cancer Res. 59 (1999) 2522–2526. [PubMed] [Google Scholar]
- [33].Klungland A, Rosewell I, Hollenbach S, Larsen E, Daly G, Epe B, Seeberg E, Lindahl T, Barnes DE, Accumulation of premutagenic DNA lesions in mice defective in removal of oxidative base damage, Proc. Natl. Acad. Sci. U.S.A 96 (1999) 13300–13305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Atamna H, Cheung I, Ames BN, A method of detecting abasic sites in living cells: age-dependent changes in base excision repair, Proc. Natl. Acad. Sci. U. S. A 97 (2000) 686–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chastain II PD, Nakamura J, Swenberg J, Kaufman D, Nonrandom AP site distribution in highly proliferative cells, FASEB J. 20 (2006) 2612–2614. [DOI] [PubMed] [Google Scholar]
- [36].Francisco DC, Peddi P, Hair JM, Flood BA, Cecil AM, Kalogerinis PT, Sigounas G, Georgakilas AG, Induction and processing of complex DNA damage in human breast cancer cells MCF-7 and non-malignant MCF-10A cells, Free Radic. Biol. Med 44 (2008) 558–569. [DOI] [PubMed] [Google Scholar]
- [37].Calaf G, Russo J, Transformation of human breast epithelial cells by chemical carcinogens, Carcinogenesis 14 (1993) 483–492. [DOI] [PubMed] [Google Scholar]
- [38].Futreal PA, Liu Q, Shattuck-Eidens D, Cochran C, Harshman K, Tavtigian S, Bennett LM, Haugen-Strano A, Swensen J, Miki Y, Eddington K, McClure M, Frye C, Weaver-Feldhaus J, Ding W, Gholami Z, Soderkvist P, Terry L, Jhanwar S, Berchuck A, Inglehart JD, Marks J, Ballinger DG, Barrett JC, Skolnick MH, Kamb A, Wiseman R, BRCA1 mutations in primary breast and ovarian carcinomas, Science 266 (1994) 120–122. [DOI] [PubMed] [Google Scholar]
- [39].Helzlsouer K, Harris E, Parshad R, Perry HR, Price FM, Sanford KK, DNA repair proficiency: potential susceptibility factor for breast cancer, J. Natl. Cancer Inst 88 (1996) 754–755. [DOI] [PubMed] [Google Scholar]
- [40].Li D, Zhang W, Zhu J, Chang P, Sahin A, Singletary E, Bondy M, Hazra T, Mitra S, Lau SS, Shen J, DiGiovanni J, Oxidative DNA damage and 8-hydroxy-2-deoxyguanosine DNA glycosylase/apurinic lyase in human breast cancer, Mol. Carcinog 31 (2001) 214–223. [DOI] [PubMed] [Google Scholar]
- [41].Peltomaki P, Deficient DNA mismatch repair: a common etiologic factor for colon cancer, Hum. Mol. Genet 10 (2001) 735–740. [DOI] [PubMed] [Google Scholar]
- [42].Barciszewska A-M, Murawa D, Gawronska I, Murawa P, Nowak S, Barciszewska MZ, Analysis of 5-methylcytosine in DNA of breast and colon cancer tissues, IUBMB Life 59 (2007) 765–770. [DOI] [PubMed] [Google Scholar]
- [43].Hankinson S, Colditz G, Willett W, Towards an integrated model for breast cancer etiology: the lifelong interplay of genes, lifestyle, and hormones, Breast Cancer Res. 6 (2004) 213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Bolton JL, Thatcher GRJ, Potential mechanisms of estrogen quinone carcinogenesis, Chem. Res. Toxicol 21 (2008) 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Akbari M, Krokan H, Cytotoxicity and mutagenicity of endogenous DNA base lesions as potential cause of human aging, Mech. Ageing Dev 129 (2008) 353–365. [DOI] [PubMed] [Google Scholar]
- [46].Szatrowski TP, Nathan CF, Production of large amounts of hydrogen peroxide by human tumor cells, Cancer Res. 51 (1991) 794–798. [PubMed] [Google Scholar]
- [47].Sokolov MV, Dickey JS, Bonner WM, Sedelnikova OA, _-H2AX in bystander cells: not just a radiation-triggered event, a cellular response to stress mediated by intercellular communication, Cell Cycle 6 (2007) 2210–2212. [DOI] [PubMed] [Google Scholar]
- [48].Bartkova J, Horejsi Z, Koed K, Kramer A, Tort F, Zieger K, Guldberg P, Sehested M, Nesland JM, Lukas C, Orntoft T, Lukas J, Bartek J, DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis, Nature 434 (2005) 864–870. [DOI] [PubMed] [Google Scholar]
- [49].Sutherland BM, Bennett PV, Sidorkina O, Laval J, Clustered damages and total lesions induced in DNA by ionizing radiation: oxidized bases and strand breaks, Biochemistry 39 (2000) 8026–8031. [DOI] [PubMed] [Google Scholar]