Abstract
Purpose
To review outcomes following microwave ablation (MWA) of colorectal cancer pulmonary metastases and assess predictors of oncologic outcomes.
Methods
Technical success, primary and secondary technique efficacy rates were evaluated for 50 patients with 90 colorectal cancer pulmonary metastases at immediate, 4–8 weeks post-MWA and subsequent follow-up CT and/or 18F-FDG PET/CT. Local tumor progression (LTP) rate, LTP-free survival (LTPFS), cancer-specific and overall survivals were assessed. Complications were recorded according to SIR classification.
Results
Median follow-up was 25.6 months. Median tumor size was 1 cm (0.3–3.2 cm). Technical success, primary and secondary technique efficacy rates were 99, 90 and 92%, respectively. LTP rate was 10%. One-, 2- and 3-year LTPFS were: 93, 86 and 86%, respectively, with median LTPFS not reached. Median overall survival was 58.6 months, and median cancer-specific survival (CSS) was not reached. One-, 2- and 3-year overall and CSS were 94% and 98, 82 and 90%, 61 and 70%, respectively. On univariate analysis, minimal ablation margin (p < 0.001) and tumor size (p = 0.001) predicted LTPFS, with no LTP for minimal margin ≥ 5 mm and/or tumor size < 1 cm. Pleural-based metastases were associated with increased LTP risk (p = 0.002, SHR = 7.7). Pre-MWA CEA level > 10 ng/ml (p = 0.046) and ≥ 3 prior chemotherapy lines predicted decreased CSS (p = 0.02). There was no 90-day death. Major complications rate was 13%.
Conclusions
MWA with minimal ablation margin ≥ 5 mm is essential for local control of colorectal cancer pulmonary metastases. Pleural-based metastases and larger tumor size were associated with higher risk of LTP. CEA level and pre-MWA chemotherapy impacted CSS.
Keywords: Lung ablation, Pulmonary metastases, Microwave ablation, Thermal ablation, Colorectal cancer
Introduction
Approximately 5% of men and 4% of women develop colorectal cancer (CRC) during their lifetime, with 20% of patients having distant metastases at initial diagnosis [1, 2]. Lung is the second most common metastatic site with 10–15% lung metastases incidence [3, 4]. Five-year survival of patients with distant disease is roughly 12% [5]. Complete metastatectomy and/or ablation increase 5-year survival to 27–68% [6-9]. However, only minority of patients with CRC pulmonary metastases are surgical candidates due to comorbidities or compromised pulmonary function [10]. In addition, recurrences after lung metastasectomy are common (20–68%) and subsequent surgery is challenging due to limited pulmonary reserve [11-14].
Key ablation advantages compared to surgery include the ability to preserve pulmonary parenchyma and lung function and retreat new and recurrent metastases that are common in this population [15-25]. Ablation is commonly used in the treatment of patients with limited number of relatively small tumors. Preferably < 3 tumors in each hemithorax with no, limited or at least controlled extrapulmonary disease are generally accepted pulmonary ablation eligibility criteria [26-29].
Microwave ablation (MWA) has at least theoretical potential to overcome known limitations of radiofrequency ablation (RFA) [20, 21, 25-28, 30, 31], such as the diminished thermal conductivity of aerated lung and the “heat sink phenomenon” (due to flow in nearby vessels or airway) that can impact the ability to create large and more uniform ablation zones with adequate margins and in shorter time, as demonstrated in animal studies [32-35]. Consequently, higher rates of complete ablation and sustained tumor control could be expected when using MWA [33-35].
This study assessed oncologic outcomes and complications of MWA in the management of patients with colorectal pulmonary metastases as well as factors affecting these outcomes.
Methods
Study Population
IRB waiver of approval was obtained for this retrospective review of our prospectively created and maintained HIPAA registered and compliant lung tumor ablation database. All patients with colorectal pulmonary metastases undergoing image-guided MWA between March 2011 and May 2016 were included in the study.
MWA inclusion criteria were: limited number of metastases (up to 6) in each hemithorax and relatively small tumor size (up to 3.5 cm) with no, limited or at least controlled extrapulmonary disease. Central tumor location was not an exclusion criterion if ablation could be performed safely without risk of damaging adjacent structures. The decision to treat metastasis with ablation was made after multidisciplinary discussion.
Ablation Procedure, Imaging Follow-Up and Definitions
Operators’ experience, pre-ablation imaging and biopsy timing, image guidance, percutaneous entry route, MWA system and electrode choice, prophylactic antibiotics, anesthesia type, bilateral lung metastases and pneumothorax management, thermal monitoring, immediate post-procedure imaging, minimal ablation margin measurement (Fig. 1) and imaging follow-up are described in Table 1. All study definitions are described in Table 2.
Fig. 1.
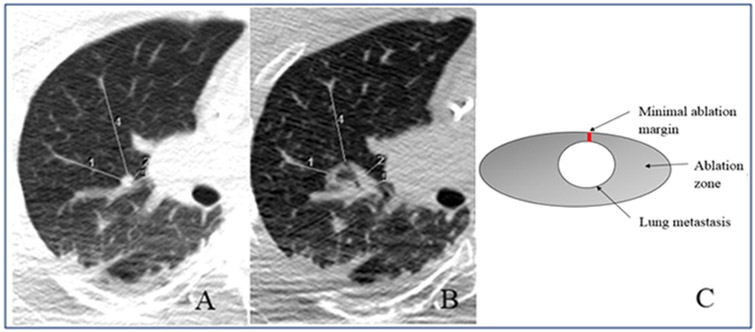
Minimal ablation margin measurement. A pre-ablation measurements: measurement (1) = 34 mm; measurement (2) = 14 mm; measurement (3) = 8 mm; measurement (4) = 50 mm. B Post-ablation measurements: measurement (1) = 19 mm; measurement (2) = 12 mm; measurement (3) = 3 mm; measurement (4) = 37 mm. After subtracting corresponding measurements at each location [1-4], the respective ablation margins were 15, 2, 5 and 13 mm. Therefore, minimal ablation margin for this ablation zone was 2 mm (insufficient). C Minimal ablation margin scheme
Table 1.
Ablation procedure descriptions
| Nr. | Parameter | Description |
|---|---|---|
| 1. | Operator’s experience | Majority of tumors (64%) were treated by two very experienced operators in lung ablation. Both operators started to perform lung thermal ablation (RFA) since year 1999, with MWA introduction since the year 2009. Importantly, since the year 2014 all the lung lesions have been treated solely with MW ablation in our institution, contributing to increased experience in this technique for all the operators |
| 2. | Lesion biopsy timing | It was chosen based on the need to know tumor pathology in disease management discussions (such as in patients with history of smoking second primary tumor was a reasonable possibility), as well as based on technical factors (such as the feasibility to biopsy the tumor without significant risk of bleeding that could obscure the target for MW ablation) |
| 3. | Pre-procedure imaging | All patients underwent pre-ablation cross-sectional CT and/or 18F-FDG PET/CT for tumor assessment, restaging and pre-procedural planning |
| 4. | Intra-procedural image guidance | Included conventional CT in all patients, with additional CT fluoroscopy and/or split-dose 18F-FDG PET/CT [39] at operator’s discretion |
| 5. | Percutaneous entry route | Chosen based on tumor size, location and proximity to adjacent structures |
| 6. | MW system/electrode number | Based on operator’s preference. Ablation protocol was completed according to each manufacturer recommendations for the desired ablation size |
| 7. | Prophylactic antibiotics | Cefazolin (1 g IV × 1) or, in case of severe allergy, Clindamycin (900 mg IV × 1) combined with Gentamicin (1.5 mg/kg IV × 1) were administered once within 1 h before the procedure |
| 8. | Anesthesia type | All MWAs were performed under general anesthesia |
| 9. | Number of ablation sessions | When metastases involved single lung, MWA was completed in a single session. In patients with bilateral tumors, contralateral lung MWA was performed at least 3 weeks later to allow recovery of the initially treated lung. In cases of persistent symptoms or persistent pneumothorax, the contralateral ablation was delayed until symptoms resolution |
| 10. | Thermal monitoring | Used with thermometer tip positioned at: (1) desired ablation margin; (2) at structure that needed be protected from the heat (chest wall, pleural surface); and (3) in the ablation zone to confirm lethal temperatures |
| 11. | Immediate post-procedure imaging | Non-contrast-enhanced CT scan was obtained to depict the ablation zone as an area of ground glass opacity covering the target tumor with a minimal ablation margin of at least 5 mm uniformly around target metastasis |
| 12. | Minimal ablation margin (MM) measurement | It was measured on immediate post-procedure non-contrast-enhanced CT (Fig. 1). MM was defined as the shortest distance between the ablated metastasis and the edge of the ablation zone. In confluent ablation zones and cases of bleeding obscuring the ablation zone the MM was not measured |
| 13. | Detection and management of pneumothorax | After ablation, chest X-rays were obtained for at least 2 h to detect pneumothorax prior to patients’ release from the post-anesthesia care unit. Asymptomatic patients without pneumothorax and with no requirements for intravenous analgesia were discharged home on the same day. Patients with small asymptomatic pneumothoraces were admitted for overnight observation. Patients with larger, enlarging and/or symptomatic pneumothoraces were treated with thoracostomy and admitted to the hospital |
MWA microwave ablation
Table 2.
Study definitions
| Nr. | Term | Definition |
|---|---|---|
| 1. | Technical success rate | Percentage of target tumors, successfully treated per ablation protocol, resulting in the creation of an ablation zone covering the target tumor, depicted on the immediate post-ablation imaging and on first follow-up imaging (at 4–8 weeks post-MWA), which served as the new baseline for future comparisons |
| 2. | Residual tumor | Enlargement of the ablation zone or nodular enhancement/increased focal FDG uptake (SUVmax ≥ 2.5) at or within 1 cm from the ablation zone on first post-MWA imaging (at 4–8 weeks) |
| 3. | Local tumor progression | Enlargement of the ablation zone or nodular enhancement/increased focal FDG uptake (SUVmax ≥ 2.5) at or within 1 cm from the ablation zone. LTP was defined at the earliest on the second imaging (at 9–16 weeks) post-MWA for those tumors successfully ablated as depicted at the first (4–8 weeks) post-MWA imaging |
| 4. | Primary technique efficacy rate | Percentage of target tumors successfully eradicated following the initial MWA during the entire study follow-up period [40] |
| 5. | LTP-free survival (LTPFS) | Time period, calculated from the ablation date until LTP or until the last available CT and/or 18F-FDG PET/CT |
| 6. | Secondary technique efficacy rate | Percentage of target tumors successfully eradicated following repeat ablation(s) to treat residual disease or LTP |
| 7. | Assisted LTPFS | Cumulative time interval from the initial MWA until the latest follow-up, including all ablations to treat the initial tumor and all subsequent LTP(s) |
MWA microwave ablation, LTP local tumor progression
All patients with LTP were assessed for repeat thermal ablation eligibility.
Study Objectives
Primary objectives included technical success, primary and secondary technique efficacy rates, LTP rate, LTP-free survival (LTPFS), assisted LTPFS, overall survival (OS) and cancer- specific survival as well as identification of factors, associated with LTPFS and cancer-specific survival.
Secondary study objectives included assessment of side effects and complication according to SIR classification [27, 36].
Factors Affecting Oncologic Outcomes
Tumor-related (tumor size and location) and procedure-related factors (minimal ablation margin) were analyzed as potential predictors of LTPFS.
Patient-related factors (lungs as first metastatic disease site) and prior therapies (prior lung or liver surgery, prior chemotherapy ± target therapy lines) were analyzed as potential predictors of cancer-specific survival.
Statistical Analysis
Statistical analysis methodology is described in Table 3.
Table 3.
Statistical analysis methods used in the study
| Kaplan–Meier method | Used to evaluate LTPFS, overall and cancer-specific survivals |
| Compete risk model, adjusted for clustering (tumor-based) | Analyzed LTPFS predictors (tumor size, minimal margin size and location) to account for multiple patients that underwent ablation for more than one tumor. Death was considered a competing event in this analysis |
| Fisher exact test | Used to analyze categorical variables |
| Other | Sample proportion confidence intervals were calculated using Wilson method with continuity correction |
| Software used | STATA 12.1 |
| Significance level | p < 0.05 was considered significant |
Results
Patient Population
Fifty patients with 90 colorectal lung metastases were treated in 60 MWA sessions. Twenty-eight (56%) of patients were women, and 22 (44%) were men with a mean age of 58.5 ± 13.2 years. Median follow-up for survivors was 25.6 months (range 12.8–80.6 months), and 29/50 (58%) of patients were followed for more than 2 years (Figs. 2, 3). At the time of initial diagnosis, 21/50 (42%) of patients had AJCC stage IV disease. Thirteen out of fifty (26%) of patients underwent MWA for disease progression after lung metastatectomy. Patient and tumor characteristics, biopsy timing as well as pre-MWA therapies are described in Tables 4 and 5.
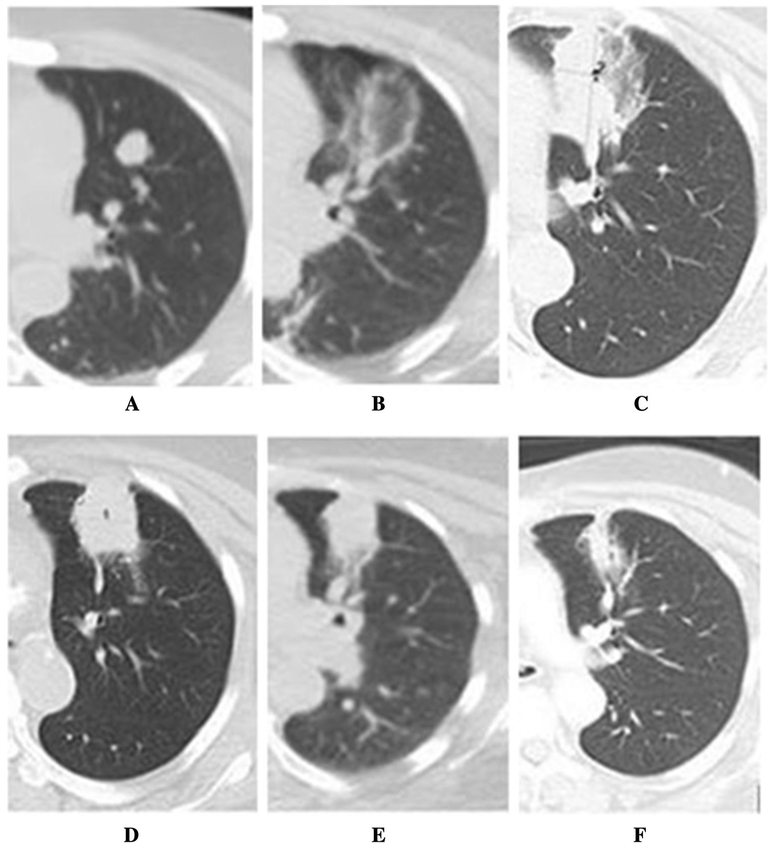
Fig. 2.
Lung MWA ablation zone changes in 72-year old woman with metastatic colorectal cancer over 28-month follow-up period on cross-sectional CT, showing gradual constriction of ablation zone and no evidence of local tumor progression. After initial diagnosis, the patient underwent right hemicolectomy, adjuvant chemotherapy with 5-FU and leucovorin and was off-treatment with no evidence of disease for 18 months. A Pre-ablation enlarging lung nodule (from 0.9 to 1.6 cm) with rising CEA (from 5.8 to 11.6 ng/ml): lesion was considered metastatic, not biopsy-proven. Due to significant comorbidities (end-stage kidney insufficiency, coronary artery disease, diabetes, arterial hypertension and history of stroke), lung ablation was preferred to surgery. B Immediate post-MWA CT with ablation zone as ground glass opacity measuring 3.2 × 3.0 cm. C First post-MWA scan at 5 weeks, served as a new baseline for future comparisons. D Follow-up scan at 17 weeks. E Follow-up scan at 15.5 months; F follow-up scan at 28 months with constricted ablation zone and no evidence of local tumor progression. The patient was off-treatment after MWA with no evidence of disease elsewhere for 2 years, when the patient developed solitary biopsy-proven liver metastasis, treated with liver segmentectomy. The patient then was off-treatment with no evidence of disease elsewhere throughout the last follow-up
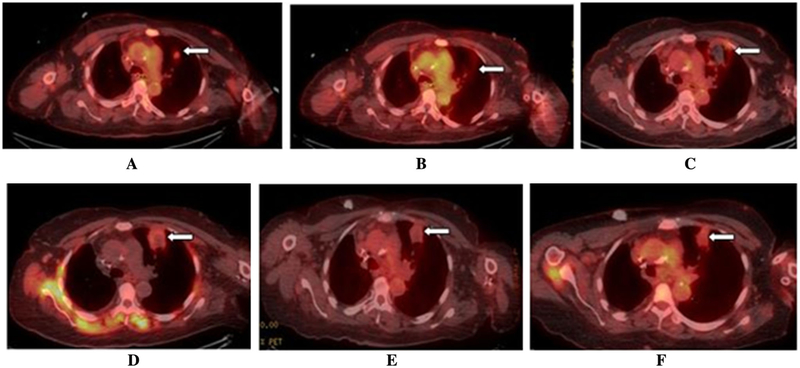
Fig. 3.
Real-time 18F-FDG PET/CT-guided lung MWA with ablation zone constriction and decreased metabolic uptake over 28-month follow-up period (imaging of the same patient as in Fig. 1). A Pre-ablation split-dose 18F-FDG PET/CT scan* with FDG—avid left upper lobe lung metastasis. B Immediate post-ablation 18F-FDG PET/CT scan with no metabolic uptake in the ablation zone. C 18F-FDG PET/CT scan 4 weeks after ablation. D 18F-FDG PET/CT scan 17 weeks post- ablation. E 18F-FDG PET/CT scan 15.5 months after ablation. F 18F-FDG PET/CT scan 23 months after ablation with constricted ablation zone, no metabolic uptake and no evidence of local tumor progression. *Split-dose 18F-FDG PET/CT is a technique for PET/CT-guided ablation that permits both target localization and evaluation of treatment effectiveness. During the procedure, the standard administered diagnostic FDG activity dose of approximately 12 mCi (444 MBq) is administered in two aliquots: a 4-mCi (148-MBq) target/imaging dose administered 30–60 min before ablation and an 8-mCi (296-MBq) treatment efficacy dose administered immediately after the ablation with images obtained 30 min later [43]
Table 4.
Patient characteristics
| Parameter | Incidence |
|---|---|
| Number of patients | 50 |
| Number of treated lesions | 90 |
| Number of ablations | 60 |
| Lesion size (median, cm) | 1 cm (range 0.3–3.2 cm) |
| Time from primary diagnosis to lung metastases (median, in months) | 27.9 (range 12.1–141.2) |
| Time from lung metastases diagnosis to MWA (median, in months) | 9.4 (range 0.4–106.9) |
| Time from lung resection to MWA (median, in months) | 25.5 (range 1.9–153.2) |
| Target tumor biopsy | |
| Performed within 2–6 weeks pre-MW ablation | 5/90 (6%) |
| Performed in the same setting with MW ablation | 5/90 (6%) |
| Not performed | 80/90 (78%) |
| Number of tumors ablated per procedure | |
| Median | 1 (range 1–6) |
| 1 Lesion | 40 (67%) |
| 2 Lesions | 16 (27%) |
| 3 Lesions | 6 (10%) |
| > 3 Lesions | 2 (3%) |
| AJCC stage at initial diagnosis | |
| I | 2 (4%) |
| II | 9 (18%) |
| III | 18 (36%) |
| IV | 21 (42%) |
| Liver metastases at initial diagnosis | |
| Yes | 19 (38%) |
| No | 31 (62%) |
| Pulmonary progression outside ablation zone post-MWA (in same and/or contralateral lung) | |
| Yes | 9 (18%) |
| No | 41 (82%) |
| Extrapulmonary progression of disease after MWA | |
| Yes | 32 (64%) |
| No | 18 (36%) |
Table 5.
Pre-MWA therapies received
| Pre-MWA therapy | Incidence |
|---|---|
| Prior lung surgery | |
| Yes | 13 (26%) |
| No | 37 (74%) |
| Prior lung ablation | |
| Yes | 16 (32%) |
| No | 34 (68%) |
| Multiple prior ablations (range 2–8) | 3 (6%) |
| Prior chemotherapy/target therapy received | |
| Median chemotherapy/target therapy lines received | 2 |
| 1 Line | 16 (32%) |
| 2 Lines | 21 (42%) |
| ≥ 3 Lines | 13 (26%) |
| Prior HAIP therapya | |
| Yes | 28 (56%) |
| No | 22 (44%) |
| Prior primary tumor resection | |
| Yes | 47 (94%) |
| No | 3 (6%) |
| Prior liver resection | |
| Yes | 31 (62%) |
| No | 19 (38%) |
| Prior liver ablation | |
| Yes | 16 (32%) |
| No | 34 (68%) |
| Other prior therapies for metastatic disease (radiotherapy, resection or ablation of other tumor sites, Y-90 RAEb, intraperitoneal chemotherapy) | |
| Yes | 16 (32%) |
| No | 34 (68%) |
| Received only 1 line of systemic chemotherapy + primary tumor resection ± chemoradiotherapy and no other treatments before MWA | |
| Yes | 7 (14%) |
| No | 43 (86%) |
HAIP hepatic arterial infusion pump
Y90 RAE yttrium 90 radioembolization
Technical Parameters and Technical Success Rate
Six out of fifty (12%) of patients received MWA in two separate sessions to treat bilateral lung metastases, with one patient requiring contralateral ablation delay for a week to allow persistent asymptomatic pneumothorax resolution. Ablation technical parameters are depicted in Table 6.
Table 6.
Ablation technical parameters
| Ablation parameter (n = 90 lesions) | Incidence |
|---|---|
| Median time of ablation per target tumor | 10 min (range 2–30 min) |
| Median power of ablation per target tumor | 59 W (20–140 W) |
| Thermal monitoring (in ablation zone center ± periphery) | 43/60 (46%) of ablations |
| MWA system used | |
| Neuwave (Neuwave, Madison, WI) | 82 (91%) |
| Medwaves (Medwaves, San Diego, Calif) | 3 (3%) |
| Emprint (Medtronic, Minneapolis, MN) | 3 (3%) |
| Microsulis Medical (Microsulis Medical Ltd, UK) | 1 (1%) |
| Amica microwave (HealthTronics) | 1 (1%) |
| Number of electrodes used | |
| 1 Electrode | 73 (81%) |
| 2 Electrodes | 11 (12%) |
| 4 Electrodes (for ablation of six lesions during procedure) | 6 (7%) |
| Image guidance | |
| Conventional CT-only | 34 (38%) |
| Additional CT fluoroscopy | 46 (51%) |
| Additional split-dose PET/CTa + CT fluoroscopy | 10 (11%) |
| Patient positioning | |
| Prone | 34 (38%) |
| Lateral | 25 (28%) |
| Supine | 20 (22%) |
| Modified prone/lateral/supine | 11 (12%) |
| Approach | |
| Posterior | 24 (27%) |
| Posterolateral | 19 (21%) |
| Anterolateral | 19 (21%) |
| Anterior | 14 (16%) |
| Lateral | 13 (14%) |
| Minimal ablation margin | |
| 0 mm (pleural-based metastases) | 14/90 (16%) |
| 0 mm (non-pleural-based metastases) | 3/90 (3%) |
| 1–4 mm | 21/90 (23%) |
| 5–9 mm | 37/90 (41%) |
| ≥ 10 mm | 11/90 (12%) |
| Not measurable | 4/90 (4%) |
| Number of overlapping ablations | |
| One | 21 (23%) |
| Two | 33 (37%) |
| Three–four | 33 (37%) |
| Unclear | 3 (3%) |
Split-dose 18F-FDG PET/CT is a technique for PET/CT-guided ablation that permits target tumor localization and evaluation of treatment effectiveness. With this technique, standard diagnostic FDG activity dose of approximately 12 mCi (444 MBq) is administered in two aliquots: a 4-mCi (148-MBq) target/imaging dose is administered 30–60 min before the ablation followed by an 8-mCi (296-MBq) administered immediately after the ablation with images obtained 30 min later. The higher dose overcomes background signal of the first lower dose and allows assessment of metabolic activity of the ablation zone and detection of residual hypermetabolic signal representing untreated residual tumor [39]
Technical success rate was 89/90 (99%): 1/90 (1%) of ablated tumors (3.2 cm in size, pleural based) maintained focal metabolic uptake on first post-MWA 18F-FDG PET/CT scan, consistent with residual tumor. Further tumor management is discussed in Sect. 7.
Primary Technique Efficacy Rate, LTP Rate and LTPFS
Median tumor size was 1 cm (range 0.3–3.2 cm). Primary technique efficacy rate was 81/90 (90%). Nine tumors progressed after MWA resulting in LTP rate of 9/90 (10%) during the entire study follow-up. LTP occurred 3.9–21.8 months after initial MWA. One-, 2- and 3-year LTPFS were: 93, 86 and 86%, respectively. Median time to LTP was not reached (Table 7).
Table 7.
Local tumor control, prognosticators of local tumor progression-free survival (LTPFS) and description of lung progression-free survival (PFS)
| A. Lung PFS (in same and/or contralateral lung) | |
| Median (inside and outside ablation zone) | 8.8 months (95% CI 7–12.7) |
| Median (outside ablation zone-only) | 10 months |
| 1-Year lung PFS | 40% (95% CI 27–53%) |
| 2- and 3-year lung PFS | 15% (95% CI 7–27%) |
| B. Local tumor control | |
| Local tumor progression (LTP) rate | 9/90 (10%), 95% CI 5–19% |
| Median LTPFS | Not reached |
| 1-Year LTPFS | 93% (95% CI 85–97%) |
| 2- and 3-year LTPFS | 86% (95% CI 75–93%) |
| C. Prognosticators of LTPFS | |
| 1. Minimal ablation margin (MM) size | p < 0.001, SHR = 0.18, 95% CI 0.08–0.42 |
| LTP rate for ablations with 0 mm MM | 6/17 (35%, 95% CI 12–58%) |
| LTP rate for ablations with 1–4 mm MM | 3/21 (14%, 95% CI 0–29%) |
| LTP rate for ablations with ≥ 5 mm MM | 0/48 (0%) |
| Cum. 1-year LTP hazard for NPB with MM < 5 mm | 14% (95% CI 4–43%) |
| Cum. 2-year LTP hazard for NPB with MM < 5 mm | 23% (95% CI 8–64%) |
| Cum. 2-year LTP hazard for NPB with MM ≥ 5 mm | 0% |
| Overall 1-year cum. LTP hazard of NPB | 4% (95% CI 1–13%) |
| Overall 2-year cum. LTP hazard of NPB | 7% (95% CI 3–22%) |
| 2. Tumor location (pleural-based (PB) vs. NPB) | p = 0.002, SHR = 7.7 |
| Cum. 1-year LTP hazard for PB metastases | 25% (95% CI 8–77%) |
| Cum. 2-year LTP hazard for PB metastases | 47% (95% CI 19–100%) |
| 3. Tumor size | p = 0.001, SHR = 5.38, 95% CI = 1.9–15.1 |
| LTP rate for tumors ≥ 1 cm in size | 19% (95% CI 8–30%) |
| LTP rate for tumors < 1 cm in size | 0/43 (0%) |
| LTP rate for tumors ≥ 1 cm with MM < 5 mm | 41% (95% CI 20–62%) |
| LTP rate for tumors ≥ 1 cm with MM≥5 mm | 0/25 (0%) |
PB pleural-based metastases, NPB non-pleural-based metastases, Cum. cumulative, SHR sub-hazard ratio
LTP occurred at ablation margin or directly adjacent to the ablation zone in 6/9 (66%) LTPs. LTP within the ablation zone occurred in 3/9 (33%) of ablations: one stable in size cavitary ablation zone filled in, forming spiculated mass with pleural tethering, micronodularities and peribronchial thickening; another ablation zone showed considerable increase in solid component centrally; third ablation zone demonstrated gradual increase in size.
New focal FDG-avidity within the ablation zone consistent with LTP occurred in 4/9 (44%) LTPs, with SUVmax ranging from 3.8 to 7.1. In all these cases, chest CT imaging also demonstrated suspicious increased ablation zone or focal nodularity adjacent to it.
Minimal Ablation Margin
Median minimal ablation margin size was 5 mm (range 0–19 mm). Measurement of minimal margin was feasible in 86/90 (96%) ablations. In 4/90 (4%) ablations, it could not be calculated due to bleeding obscuring ablation zone (in 2%) or confluent ablation zones (in 2%). Minimal margin of at least 5 mm was achieved in 48/86 (56%) of measurable ablation zones. Fourteen out of eighty-six (16%) of metastases were pleural based, and minimal margin was considered 0 mm. Minimal margin size is described in Table 6.
Prognosticators of LTPFS in Univariate Analysis
Minimal ablation margin size LTPFS was statistically significantly associated with minimal margin size (p < 0.001) (Table 7). LTP was observed only for tumors ablated with minimal margin < 5 mm, with LTP rate of 24% (Table 7).
Tumor location (pleural vs. non-pleural based) Pleural-based metastases had 7.7 times higher LTP risk, compared to non-pleural-based metastases (p = 0.002). One- and 2-year cumulative LTP hazard for non-pleural versus pleural-based metastases was 4% and 7% versus 25% and 47%, respectively (Table 7, Fig. 4). When analyzing non-pleural-based metastases alone, minimal ablation margin retained significance as a predictor of LTP on univariate analysis (p < 0.001) (Table 7, Fig. 5).
Tumor size LTPFS was statistically significantly associated with tumor size (p = 0.001): LTP was only observed after MWA of tumors ≥ 1 cm, with LTP rate of 19% (Table 7). LTP rate of tumors ≥ 1 cm in size was 41% when minimal margin was < 5 mm, compared to 0% when minimal margin was ≥ 5 mm (p = 0.0004, Fig. 6).
Fig. 4.
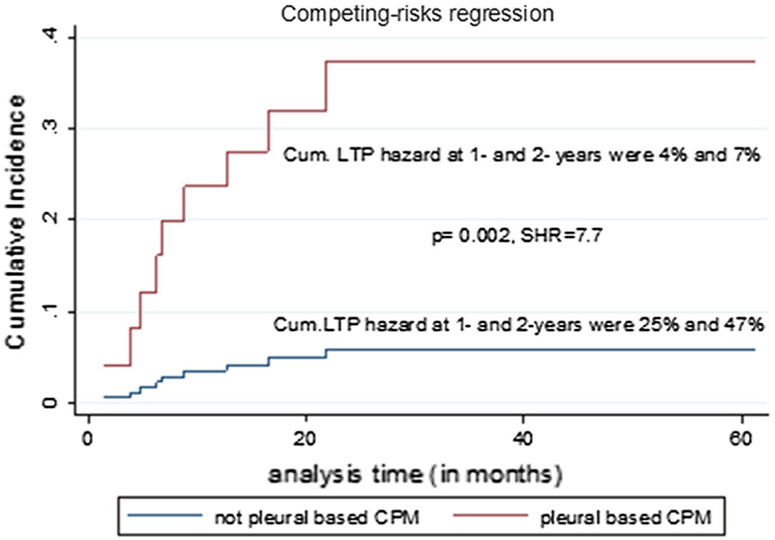
Cumulative (Cum.) local tumor progression (LTP) hazard of non-pleural-based and pleural-based colorectal cancer pulmonary metastases (CPM). The figure demonstrated more than 7 times increased LTP hazard for pleural-based metastases, compared to non-pleural based
Fig. 5.
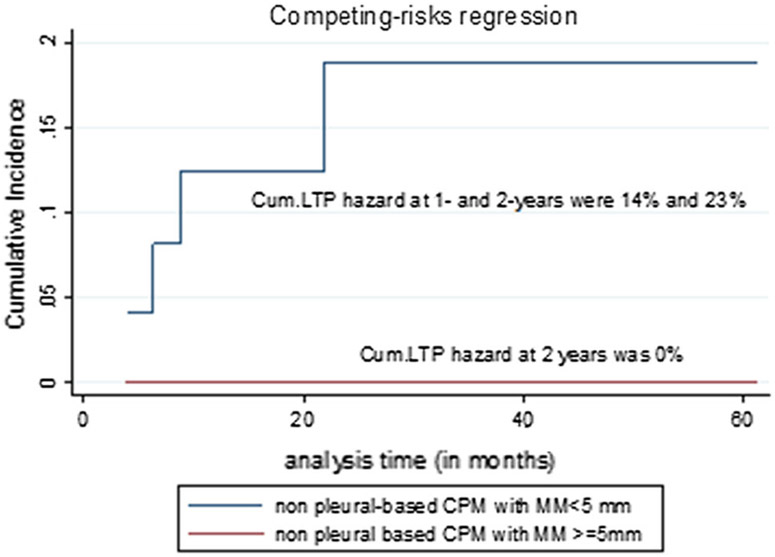
Cumulative (Cum.) local tumor progression (LTP) hazard of non-pleural-based colorectal cancer pulmonary metastases (CPM) treated with < 5 mm and ≥ 5 mm minimal ablation margin (MM). There was no LTP in non-pleural metastases, treated with ≥ 5 mm MM, compared to 23% 2-year cumulative LTP hazard for metastases treated with < 5 mm MM
Fig. 6.
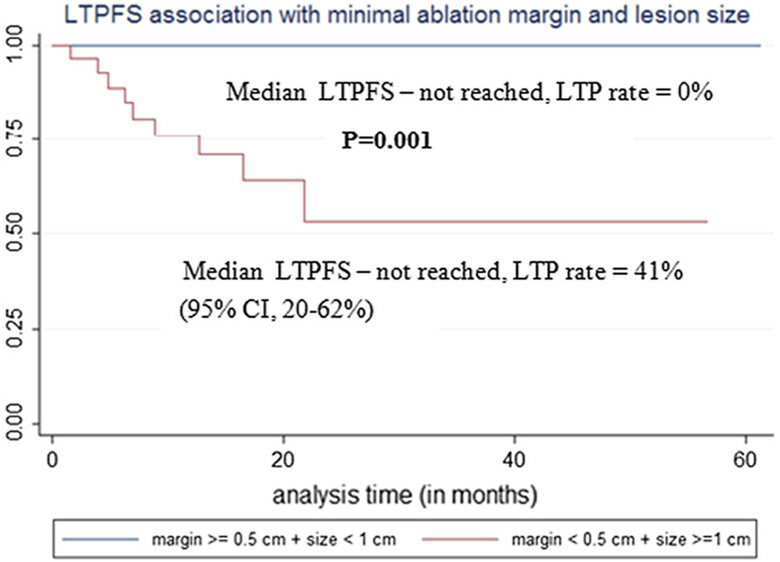
Local tumor progression-free survival (LTPFS) association with metastasis size and minimal ablation margin
Overall and Cancer-Specific Survival
Median OS was 58.6 months, and median cancer-specific survival was not reached (Table 7). Median OS for patients without LTP was 45.2 months and not reached for patients with LTP (HR = 0.63, p = 0.54).
Three out of sixteen (19%) of deaths were not cancer-related. They included sepsis and multi-organ failure in a patient with retroperitoneal abscess (4.7 months post-MWA); respiratory failure due to pneumonia (18 months post-MWA); and death of other cause 11 months post-MWA, with no evidence of oncologic disease in one patient (negative whole body 18F-FDG PET/CT 2 months before death). One out of sixteen (6%) of patients was lost to follow-up 13 months before death.
Management of LTP and Assisted LTPFS
Repeat MWA was offered in 2/9 (22%) LTPs and one residual tumor. Assisted LTPFS was not reached. Repeat MWA for residual tumor increased local tumor control with MWA from 1.5 to 23.7 months in one patient (after one repeat MWA; however, patient had subsequent LTP which was aggressively retreated with RFA using 3 electrodes and 4 overlapping ablations and no further LTP); from 8.83 to 75.5 months in second patient (two additional MWAs, no further LTP); and from 3.9 to 19.37 months in third patient (single additional MWA, no further LTP). Resulting secondary technique efficacy rate, accounting for all repeat MWAs, was 83/90 (92%). LTPs in the rest of patients were managed with: systemic chemotherapy in 4/9 (44%), lung resection in 1/9 (11%) and lung radiotherapy in 1/9 (11%).
Post-ablation Patient Management, Lung and Extrapulmonary Disease Progression
Six out f fifty (12%) of patients had no lung progression (inside and/or outside the ablation zone). Lung progression outside ablation zone was ablated in 14/41 (34%) of patients. Median overall lung progression-free survival was 8.8 months (Table 7, Fig. 7).
Fig. 7.
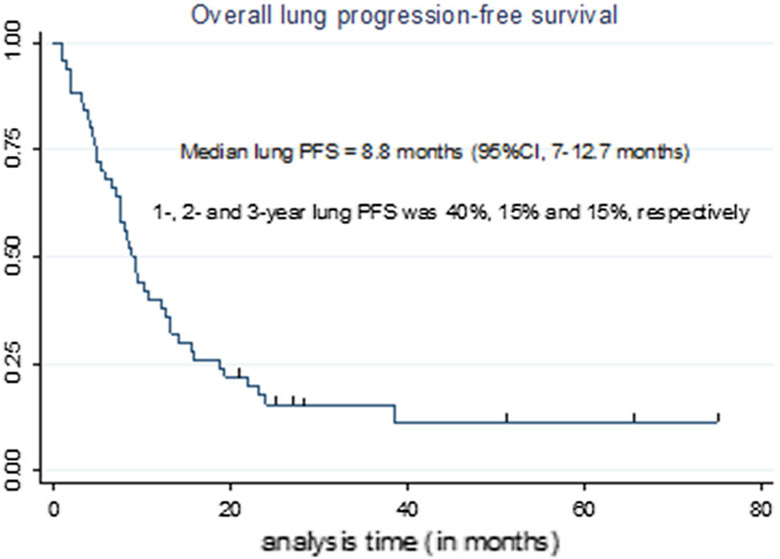
Overall lung progression-free survival (PFS, inside and/or outside the ablation zone)
Thirty-two out of fifty (64%) patients had extrapulmonary disease progression post-MWA (with pulmonary progression in 29/32 (91%), and without in 3/32 (9%) patients).
Three out of fifty (6%) of patients were disease-free post-lung ablation and were off-treatment for 25, 65.4 and 74.9 months. Post-MWA therapies are presented in Table 8.
Table 8.
Post-microwave ablation (MWA) therapies received
| Post-MWA therapy | Incidence |
|---|---|
| Systemic chemotherapy | |
| Yes. Number of regimens received: | 37 (74%) |
| 1 Regimen | 12/37 (32%) |
| 2 Regimens | 12/37 (32%) |
| ≥ 3 Regimens | 13/37 (35%) |
| No | 13 (26%) |
| Target therapy (EGFR inhibitors/antibody, anti-VEGF, tyrosine kinase inhibitors) | |
| Yes | 29 (58%) |
| No | 21 (42%) |
| Lung ablation | |
| Yes | 19 (38%) |
| No | 31 (62%) |
| Lung resection | |
| Yes | 5 (10%) |
| No | 45 (90%) |
| Other therapies (radiotherapy, resection or ablation of the other tumor sites, Y-90 RAE, HAIP, immunotherapy) | |
| Yes | 28 (56%) |
| No | 22 (44%) |
| Patients off-treatment after MWA due to no evidence of disease, low volume of disease or due side effects/comorbidities | |
| Yes | 5 (10%) |
| No | 45 (90%) |
RAE radioembolization, HAIP hepatic artery infusion pump
Cancer-Specific Survival Prognosticators
Pre-MWA CEA level > 10 ng/ml (p = 0.046, Table 9, Fig. 8) and ≥ 3 pre-MWA chemotherapy ± target therapy lines were associated with cancer-specific survival on univariate analysis (p = 0.02, Table 9, Fig. 9).
Table 9.
Overall survival, cancer-specific survival and prognosticators of them
| Overall survival (OS) | |
| Median OS | 58.6 months (95% CI 28.2-NR) |
| 1-Year OS | 94% (95% CI 83–98%) |
| 2-Year OS | 82% (95% CI 67–91%) |
| 3-Year OS | 61% (95% CI 42–75%) |
| Cancer-specific survival (CSS) | |
| Median CSS | Not reached |
| 1-Year CSS | 98% (95% CI 86–100%) |
| 2-Year CSS | 90% (95% CI 74–96%) |
| 3-Year CSS | 70% (95% CI 49–83%) |
| Prognosticators of shorter CSS | |
| Pre-MWA CEA level > 10 ng/ml | p = 0.046*, HR = 3.53, 95% CI 1.02–12.2 |
| ≥ 3 Chemotherapy ± target therapy regimens | p = 0.019*, HR = 3.92, 95% CI 1.3–12.3 |
| Prior lung resection | p = 0.1, HR = 0.27 |
| Prior liver resection | p = 0.43, HR = 0.62 |
| Lung as a first site of metastatic disease | p = 1, HR = 1 |
CEA carcinoembryonic antigen level, HR hazard ratio, CI confidence interval
Statistically significant values
Fig. 8.
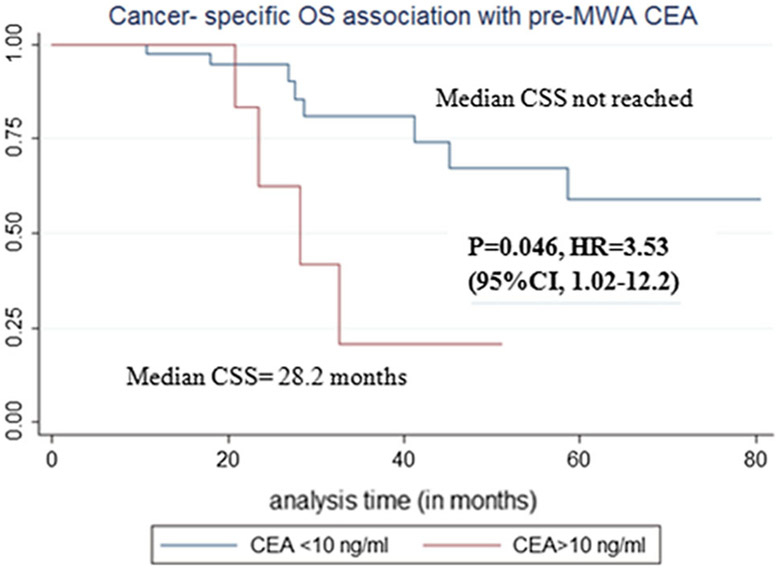
Cancer-specific survival (CSS) association with pre-MWA carcinoembryonic antigen (CEA) level
Fig. 9.
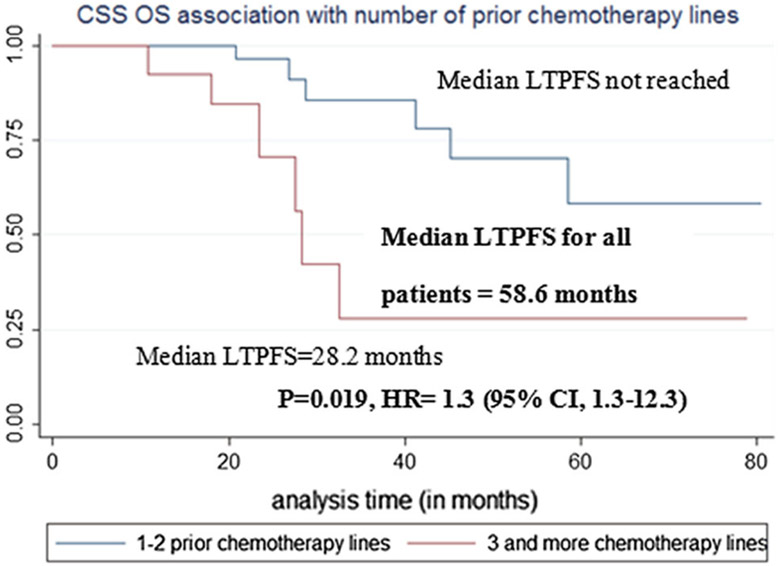
Cancer-specific survival (CSS) association with number of pre-MWA chemotherapy ± target therapy lines
Prior lung or liver resection and lung as first site of metastatic disease were not associated with cancer-specific survival (Table 9).
Small number of deaths and intermediate follow-up time precluded multivariate analysis of any predictors.
Side Effects and Complications
Incidence of side effects was 12/60 (20%). Minor complications rate was 23/60 (38%) and included pneumothorax, requiring thoracostomy. Major complications rate was 8/60 (13%); 75% of major complications included pneumothoraces requiring prolonged hospitalization (Table 10).
Table 10.
Side effects, minor and major complications following lung MW ablation
| 1. Side effects (asymptomatic pneumothoraces, no treatment) | 12/60 (20%) |
| 2. Minor complications (pneumothoraces, requiring thoracostomy) | 23/60 (38%) |
| 1. Moderate pneumothoraces | 12/23 (52%) |
| 2. Enlarging (hemo)pneumothoraces | 4/23 (17%) |
| 3. Large pneumothoraces | 1/23 (4%) |
| 4. Small recurrent/circumferential/symptomatic pneumothorax or small pneumothorax with persistent air leak/small pleural effusion/pneumomediastinum | 5/23 (22%) |
| 5. Small asymptomatic non-circumferential pneumothorax, not requiring admission | 1/23 (4%) |
| Median length of stay of patients with pneumothorax | 1 day (range 0–7) |
| 3. Major complications | 8/60 (13%) |
| Thoracostomy, requiring prolonged hospitalization (3–7 days) for persistent air leak, desaturation, severe pain or intraparenchymal bleeding with large hemopneumothorax | 6/60 (10%) |
| Aspergilloma formation in the ablation zone cavity 2 months after MWA. This became chronic pulmonary aspergillosis requiring hospitalization for 2 days with prolonged treatment with voriconazole (6 weeks 200 mg p/os every 12 h and subsequent 6 weeks of 100–400 mg IVPB every 12 h) | 1/60 (2%) |
| Pleuritic pain 12 days after MWA with underlying pneumonia, empyema, bronchopleural fistula and dehydration requiring hospitalization for 10 days with intravenous antibiotics (combination of piperacillin and tazobactam), infusion therapy and analgesics (fentanyl and morphine). After discussion with thoracic surgery service, the underlying bronchopleural fistula was successfully managed with prolonged thoracostomy for 5 months in the outpatient setting without further sequelae. | 1/60 (2%) |
| 4. Deaths within 90 days post-MWA | 0/60 (0%) |
MWA microwave ablation, IVPB piggy bag (short-term or secondary infusion)
Discussion
The LTP rate of 10% in this cohort compared favorably to reported 12–26% LTP rates in prior pulmonary tumor MWA series [33-35, 37]. This may be attributed to the relatively small tumor size and the creation of minimal margin ≥ 5 mm in 56% of patients. We found a strong association between tumor size (p = 0.001), minimal ablation margin (p < 0.001), lesion location (p = 0.002) and LTP. The small LTP number precluded multivariate analysis.
All LTPs occurred in tumors ≥ 1 cm, ablated with minimal margin < 5 mm, with 41% LTP rate for tumors with both these factors (p = 0.0004). This observation supports the value of minimal margin as LTP predictor [7, 22, 31, 38, 39]. Tumor size remains a limiting factor for the widespread use of ablation regardless of energy used [23, 40-42]. Pleural-based tumors had more than seven times higher LTP risk. Minimal ablation margin remained significant predictor of LTP for non-pleural-based metastases.
One-, 2- and 3-year survival rates of 94, 84 and 60% compared favorably to reported ranges of 84–95, 56–72 and 35–65%, respectively [43].
LTP did not affect overall survival (p = 0.54). The majority of patients received systemic chemotherapy and/or targeted therapy post-MWA; more than a third of patients had subsequent lung ablation or lung resection for progression. Therefore, overall survival could not be solely attributed to lung ablation, but rather to the overall therapeutic management of oligo-metastatic disease. Local therapy for pulmonary metastases significantly prolonged 3-year survival in patients receiving adjuvant radiotherapy or ablation, compared to those treated with chemotherapy alone (88 vs. 33%, respectively) for pulmonary metastases [44].
Pre-MWA CEA level and number of prior chemotherapy and target therapy regimens predicted cancer-specific survival. CEA association with survival can be explained by CEA circulating cancer cell death inhibition and activation of cell adhesion-related molecules [45] that has been reported after metastasectomy of CRC lung metastases [46, 47].
Thoracostomy rate of 38% rate is higher than what is reported after RFA (13–33%) [43]. This may be related to our clinical protocol of thoracostomy for any circumferential pneumothorax even in asymptomatic patients. The rate of moderate/large or enlarging pneumothorax was 28%, requiring prolonged hospitalization after 10% of procedures. The overall major complication rate of 13% is comparable to the 20% rate in prior lung MWA series [48, 49].
Approximately a third of patients underwent MWA for progression of disease after lung metastatectomy and only 11% of were biopsy-proven, since radiological diagnosis of lung metastases in the setting of CRC is generally reliable [50].
There currently is an ongoing debate on the impact of local therapy and pulmonary metastasectomy on survival in this population. Most of the evidence supporting complete removal of pulmonary metastases originated from surgical series of well-selected patients with favorable characteristics (a few or small size metastases) without any comparison to patients that were not resected [47, 51]. The lack of randomized data and comparative studies make conclusions about the impact of resection and locoregional therapies difficult [51]. Lung metastatectomy can improve survival; however, recurrence rates are high with repeat thoracotomy being associated with higher morbidity, whereas lobectomy offers no survival benefit when compared to limited sub-lobar or wedge resections [51]. Therefore, ablation could be advocated to be the preferable local therapy for small metastases that can be ablated with sufficient margins.
The study had several limitations including its retrospective nature, the small number of patients and the intermediate length of follow-up, precluding long-term estimations. Another limitation is the lack of pathologic complete ablation confirmation, a common limitation of interventional oncology therapies. The impact of the MWA device choice on local tumor control was limited since 91% of tumors were ablated using the Neuwave device. Known genetic signatures and predictive biomarkers were not available for the entire cohort of patients and could not be assessed as potential factors impacting outcomes [39, 52-55].
To summarize, this work indicated the efficacy of MWA that compared favorably to prior results of thermal ablation in terms of local tumor control, local progression-free and overall survival. Factors associated with LTPFS and cancer-specific survival were identified and described. Further validation is certainly needed in larger studies with longer follow-up to better define the role of MWA in the management of CRC metastatic disease.
Acknowledgements
The research funded by the Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748).
Footnotes
Ethical Approval IRB waiver of approval was obtained for this retrospective cohort study. The database was HIPAA registered and compliant.
Conflict of interest C.T. Sofocleous has received research support from BTG, Ethicon (Neuwave); HS Medical, Angiodynamics; Sota Medical; and is a consultant for Ethicon and GE. S.B. Solomon, shareholder of Johnson & Johnson, has received personal fees from Medtronics, Astra Zeneca, Johnson & Johnson and GE Heathcare. Other authors have no conflict of interest.
References
- 1.Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67(3):177–93. [DOI] [PubMed] [Google Scholar]
- 2.Cook AD, Single R, McCahill LE. Surgical resection of primary tumors in patients who present with stage IV colorectal cancer: an analysis of surveillance, epidemiology, and end results data, 1988–2000. Ann Surg Oncol. 2005;12(8):637–45. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi H, Mochizuki H, Sugihara K, Morita T, Kotake K, Teramoto T, et al. Characteristics of recurrence and surveillance tools after curative resection for colorectal cancer: a multicenter study. Surgery. 2007;141(1):67–75. [DOI] [PubMed] [Google Scholar]
- 4.Mitry E, Guiu B, Cosconea S, Jooste V, Faivre J, Bouvier AM. Epidemiology, management and prognosis of colorectal cancer with lung metastases: a 30-year population-based study. Gut. 2010;59(10):1383–8. [DOI] [PubMed] [Google Scholar]
- 5.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):104–17. [DOI] [PubMed] [Google Scholar]
- 6.Fiorentino F, Hunt I, Teoh K, Treasure T, Utley M. Pulmonary metastasectomy in colorectal cancer: a systematic review and quantitative synthesis. J R Soc Med. 2010;103(2):60–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shady W, Petre EN, Gonen M, Erinjeri JP, Brown KT, Covey AM, et al. Percutaneous radiofrequency ablation of colorectal cancer liver metastases: factors affecting outcomes—a 10-year experience at a single center. Radiology. 2016;278(2):601–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruers T, Van Coevorden F, Punt CJ, Pierie JE, Borel-Rinkes I, Ledermann JA, et al. Local treatment of unresectable colorectal liver metastases: results of a randomized phase II trial. J Natl Cancer Inst. 2017;109(9):djx015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solbiati L, Ahmed M, Cova L, Ierace T, Brioschi M, Goldberg SN. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology. 2012;265(3):958–68. [DOI] [PubMed] [Google Scholar]
- 10.Embun R, Fiorentino F, Treasure T, Rivas JJ, Molins L. Pulmonary metastasectomy in colorectal cancer: a prospective study of demography and clinical characteristics of 543 patients in the Spanish colorectal metastasectomy registry (GECMP-CCR). BMJ Open. 2013;3(5):e002787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez M, Poncet A, Combescure C, Robert J, Ris HB, Gervaz P. Risk factors for survival after lung metastasectomy in colorectal cancer patients: a systematic review and meta-analysis. Ann Surg Oncol. 2013;20(2):572–9. [DOI] [PubMed] [Google Scholar]
- 12.Rios A, Galindo PJ, Torres J, Roca MJ, Robles R, Lujan JA, et al. Factors causing early relapse after lung metastasis surgery. Eur J Cancer Care (Engl). 2007;16(1):26–32. [DOI] [PubMed] [Google Scholar]
- 13.Pfannschmidt J, Hoffmann H, Dienemann H. Reported outcome factors for pulmonary resection in metastatic colorectal cancer. J Thorac Oncol. 2010;5(6 Suppl 2):S172–8. [DOI] [PubMed] [Google Scholar]
- 14.Welter S, Jacobs J, Krbek T, Krebs B, Stamatis G. Long-term survival after repeated resection of pulmonary metastases from colorectal cancer. Ann Thorac Surg. 2007;84(1):203–10. [DOI] [PubMed] [Google Scholar]
- 15.Simon CJ, Dupuy DE, DiPetrillo TA, Safran HP, Grieco CA, Ng T, et al. Pulmonary radiofrequency ablation: long-term safety and efficacy in 153 patients. Radiology. 2007;243(1):268–75. [DOI] [PubMed] [Google Scholar]
- 16.Sofocleous CT, May B, Petre EN, Gonen M, Thornton RH, Alago W, et al. Pulmonary thermal ablation in patients with prior pneumonectomy. AJR Am J Roentgenol. 2011;196(5):W606–12. [DOI] [PubMed] [Google Scholar]
- 17.Hess A, Palussiere J, Goyers JF, Guth A, Auperin A, de Baere T. Pulmonary radiofrequency ablation in patients with a single lung: feasibility, efficacy, and tolerance. Radiology. 2011;258(2):635–42. [DOI] [PubMed] [Google Scholar]
- 18.Livraghi T, Solbiati L, Meloni F, Ierace T, Goldberg SN, Gazelle GS. Percutaneous radiofrequency ablation of liver metastases in potential candidates for resection: the “test-of-time approach”. Cancer. 2003;97(12):3027–35. [DOI] [PubMed] [Google Scholar]
- 19.Hiraki T, Gobara H, Iishi T, Sano Y, Iguchi T, Fujiwara H, et al. Percutaneous radiofrequency ablation for pulmonary metastases from colorectal cancer: midterm results in 27 patients. J Vasc Interv Radiol (JVIR). 2007;18(10):1264–9. [DOI] [PubMed] [Google Scholar]
- 20.Yamakado K, Hase S, Matsuoka T, Tanigawa N, Nakatsuka A, Takaki H, et al. Radiofrequency ablation for the treatment of unresectable lung metastases in patients with colorectal cancer: a multicenter study in Japan. J Vasc Interv Radiol (JVIR). 2007;18(3):393–8. [DOI] [PubMed] [Google Scholar]
- 21.de Baere T, Auperin A, Deschamps F, Chevallier P, Gaubert Y, Boige V, et al. Radiofrequency ablation is a valid treatment option for lung metastases: experience in 566 patients with 1037 metastases. Ann Oncol. 2015;26(5):987–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan TD, King J, Sjarif A, Glenn D, Steinke K, Morris DL. Percutaneous radiofrequency ablation of pulmonary metastases from colorectal carcinoma: prognostic determinants for survival. Ann Surg Oncol. 2006;13(11):1529–37. [DOI] [PubMed] [Google Scholar]
- 23.Vogl TJ, Naguib NN, Gruber-Rouh T, Koitka K, Lehnert T, Nour-Eldin NE. Microwave ablation therapy: clinical utility in treatment of pulmonary metastases. Radiology. 2011;261(2):643–51. [DOI] [PubMed] [Google Scholar]
- 24.Egashira Y, Singh S, Bandula S, Illing R. Percutaneous high-energy microwave ablation for the treatment of pulmonary tumors: a retrospective single-center experience. J Vasc Interv Radiol (JVIR). 2016;27(4):474–9. [DOI] [PubMed] [Google Scholar]
- 25.Petre EN, Jia X, Thornton RH, Sofocleous CT, Alago W, Kemeny NE, et al. Treatment of pulmonary colorectal metastases by radiofrequency ablation. Clin Colorectal Cancer. 2013;12(1):37–44. [DOI] [PubMed] [Google Scholar]
- 26.Belfiore G, Moggio G, Tedeschi E, Greco M, Cioffi R, Cincotti F, et al. CT-guided radiofrequency ablation: a potential complementary therapy for patients with unresectable primary lung cancer—a preliminary report of 33 patients. AJR Am J Roentgenol. 2004;183(4):1003–11. [DOI] [PubMed] [Google Scholar]
- 27.Sacks D, McClenny TE, Cardella JF, Lewis CA. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol (JVIR). 2003;14(9 Pt 2):S199–202. [DOI] [PubMed] [Google Scholar]
- 28.King J, Glenn D, Clark W, Zhao J, Steinke K, Clingan P, et al. Percutaneous radiofrequency ablation of pulmonary metastases in patients with colorectal cancer. Br J Surg. 2004;91(2):217–23. [DOI] [PubMed] [Google Scholar]
- 29.Steinke K, King J, Glenn DW, Morris DL. Percutaneous radiofrequency ablation of lung tumors with expandable needle electrodes: tips from preliminary experience. AJR Am J Roentgenol. 2004;183(3):605–11. [DOI] [PubMed] [Google Scholar]
- 30.Sofocleous CT, Sideras P, Petre EN, Solomon SB. Ablation for the management of pulmonary malignancies. AJR Am J Roentgenol. 2011;197(4):W581–9. [DOI] [PubMed] [Google Scholar]
- 31.Mouli SK, Kurilova I, Sofocleous CT, Lewandowski RJ. The role of percutaneous image-guided thermal ablation for the treatment of pulmonary malignancies. AJR Am J Roentgenol. 2017;209(4):740–51. [DOI] [PubMed] [Google Scholar]
- 32.Lubner MG, Brace CL, Hinshaw JL, Lee FT Jr. Microwave tumor ablation: mechanism of action, clinical results, and devices. J Vasc Interv Radiol (JVIR). 2010;21(8 Suppl):S192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vogl TJ, Eckert R, Naguib NN, Beeres M, Gruber-Rouh T, Nour-Eldin NA. Thermal ablation of colorectal lung metastases: retrospective comparison among laser-induced thermotherapy, radiofrequency ablation, and microwave ablation. AJR Am J Roentgenol. 2016;207(6):1340–9. [DOI] [PubMed] [Google Scholar]
- 34.Wolf FJ, Grand DJ, Machan JT, Dipetrillo TA, Mayo-Smith WW, Dupuy DE. Microwave ablation of lung malignancies: effectiveness, CT findings, and safety in 50 patients. Radiology. 2008;247(3):871–9. [DOI] [PubMed] [Google Scholar]
- 35.Lu Q, Cao W, Huang L, Wan Y, Liu T, Cheng Q, et al. CT-guided percutaneous microwave ablation of pulmonary malignancies: results in 69 cases. World J Surg Oncol. 2012;10:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, Charboneau JW, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria—a 10-year update. Radiology. 2014;273(1):241–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolf FJ, Aswad B, Ng T, Dupuy DE. Intraoperative microwave ablation of pulmonary malignancies with tumor permittivity feedback control: ablation and resection study in 10 consecutive patients. Radiology. 2012;262(1):353–60. [DOI] [PubMed] [Google Scholar]
- 38.Giraud P, Antoine M, Larrouy A, Milleron B, Callard P, De Rycke Y, et al. Evaluation of microscopic tumor extension in non-small-cell lung cancer for three-dimensional conformal radiotherapy planning. Int J Radiat Oncol Biol Phys. 2000;48(4):1015–24. [DOI] [PubMed] [Google Scholar]
- 39.Sotirchos VS, Petrovic LM, Gonen M, Klimstra DS, Do RK, Petre EN, et al. Colorectal cancer liver metastases: biopsy of the ablation zone and margins can be used to predict oncologic outcome. Radiology. 2016;280(3):949–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steinke K, Glenn D, King J, Clark W, Zhao J, Clingan P, et al. Percutaneous imaging-guided radiofrequency ablation in patients with colorectal pulmonary metastases: 1-year follow-up. Ann Surg Oncol. 2004;11(2):207–12. [DOI] [PubMed] [Google Scholar]
- 41.Akeboshi M, Yamakado K, Nakatsuka A, Hataji O, Taguchi O, Takao M, et al. Percutaneous radiofrequency ablation of lung neoplasms: initial therapeutic response. J Vasc Interv Radiol (JVIR). 2004;15(5):463–70. [DOI] [PubMed] [Google Scholar]
- 42.Simon TG, Beland MD, Machan JT, Dipetrillo T, Dupuy DE. Charlson Comorbidity Index predicts patient outcome, in cases of inoperable non-small cell lung cancer treated with radiofrequency ablation. Eur J Radiol. 2012;81(12):4167–72. [DOI] [PubMed] [Google Scholar]
- 43.Lyons NJ, Pathak S, Daniels IR, Spiers A, Smart NJ. Percutaneous management of pulmonary metastases arising from colorectal cancer; a systematic review. Eur J Surg Oncol. 2015;41(11):1447–55. [DOI] [PubMed] [Google Scholar]
- 44.Inoue Y, Miki C, Hiro J, Ojima E, Yamakado K, Takeda K, et al. Improved survival using multi-modality therapy in patients with lung metastases from colorectal cancer: a preliminary study. Oncol Rep. 2005;14(6):1571–6. [PubMed] [Google Scholar]
- 45.Lee JH, Lee SW. The roles of carcinoembryonic antigen in liver metastasis and therapeutic approaches. Gastroenterol Res Pract. 2017;2017:7521987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McAfee MK, Allen MS, Trastek VF, Ilstrup DM, Deschamps C, Pairolero PC. Colorectal lung metastases: results of surgical excision. Ann Thorac Surg. 1992;53(5):780–5 (discussion 5–6). [DOI] [PubMed] [Google Scholar]
- 47.Higashiyama M, Kodama K, Higaki N, Takami K, Murata K, Kameyama M, et al. Surgery for pulmonary metastases from colorectal cancer: the importance of prethoracotomy serum carcinoembryonic antigen as an indicator of prognosis. Jpn J Thorac Cardiovasc Surg. 2003;51(7):289–96. [DOI] [PubMed] [Google Scholar]
- 48.Splatt AM, Steinke K. Major complications of high-energy microwave ablation for percutaneous CT-guided treatment of lung malignancies: single-centre experience after 4 years. J Med Imaging Radiat Oncol. 2015;59(5):609–16. [DOI] [PubMed] [Google Scholar]
- 49.Zheng A, Wang X, Yang X, Wang W, Huang G, Gai Y, et al. Major complications after lung microwave ablation: a single-center experience on 204 sessions. Ann Thorac Surg. 2014;98(1):243–8. [DOI] [PubMed] [Google Scholar]
- 50.Nordholm-Carstensen A, Krarup PM, Jorgensen LN, Wille-Jorgensen PA, Harling H. Occurrence and survival of synchronous pulmonary metastases in colorectal cancer: a nationwide cohort study. Eur J Cancer. 2014;50(2):447–56. [DOI] [PubMed] [Google Scholar]
- 51.Chua TC, Al-Alem I, Zhao J, Glenn D, Liauw W, Morris DL. Radiofrequency ablation of concomitant and recurrent pulmonary metastases after surgery for colorectal liver metastases. Ann Surg Oncol. 2012;19(1):75–81. [DOI] [PubMed] [Google Scholar]
- 52.Sofocleous CT, Garg SK, Cohen P, Petre EN, Gonen M, Erinjeri JP, et al. Ki 67 is an independent predictive biomarker of cancer specific and local recurrence-free survival after lung tumor ablation. Ann Surg Oncol. 2013;20(Suppl 3):S676–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ziv E, Erinjeri JP, Yarmohammadi H, Boas FE, Petre EN, Gao S, et al. Lung adenocarcinoma: predictive value of KRAS mutation status in assessing local recurrence in patients undergoing image-guided ablation. Radiology. 2017;282(1):251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shady W, Petre EN, Vakiani E, Ziv E, Gonen M, Brown KT, et al. Kras mutation is a marker of worse oncologic outcomes after percutaneous radiofrequency ablation of colorectal liver metastases. Oncotarget. 2017;8(39):66117–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Odisio BC, Yamashita S, Huang SY, Harmoush S, Kopetz SE, Ahrar K, et al. Local tumour progression after percutaneous ablation of colorectal liver metastases according to RAS mutation status. Br J Surg. 2017;104(6):760–8. [DOI] [PMC free article] [PubMed] [Google Scholar]