Abstract
Rationale: Increasing intensive care unit (ICU) beds and the critical care workforce are often advocated to address an aging and increasingly medically complex population. However, reducing potentially preventable ICU stays may be an alternative to ensure adequate capacity.
Objectives: To determine the proportions of ICU admissions meeting two definitions of being potentially preventable using nationally representative U.S. claims databases.
Methods: We analyzed claims from 2006 to 2015 from all Medicare Fee-for-Service (FFS) beneficiaries and from a large national payer offering a private insurance (PI) plan and a Medicare Advantage (MA) plan. Potentially preventable hospitalizations were identified using existing definitions for ambulatory care sensitive conditions (ACSCs) and life-limiting malignancies (LLMs).
Results: We analyzed 420,369,434 person-years of insurance coverage, during which there were 99,793,416 acute inpatient hospitalizations, of which 16,646,977 (16.7%) were associated with an ICU admission. Of these, the proportions with an ACSC were 12.9%, 12.7%, and 15.8%, and with an LLM were 5.2%, 5.4%, and 6.4%, among those with PI, MA, and FFS, respectively. Over 10 years, the absolute percentages of ACSC-associated ICU stays declined (PI = −1.1%, MA −6.4%, FFS −6.4%; all P < 0.001 for all trends). Smaller changes were noted among LLM-associated ICU stays, declining in the MA cohort (−0.8%) and increasing in the FFS (+0.3%) and PI (+0.2%) populations (P < 0.001 for all trends).
Conclusions: An appreciable proportion of U.S. ICU admissions may be preventable with community-based interventions. Investment in the outpatient infrastructure required to prevent these ICU admissions should be considered as a complementary, if not alternative, strategy to expanding ICU capacity to meet future demand.
Keywords: critical care, risk factors, community medicine
Admissions to the intensive care unit (ICU) are costly and strain health system resources (1, 2). In the United States, the number of critical care beds and their associated costs are increasing (2). Although the total number of ICU admissions appears to be declining over time, there is significant heterogeneity among states (3), and the United States still admits more ICU patients than most Western European countries (4). Because ICU admission rates are particularly high among patients 65 years of age and older (3, 5), and those nearing the end of life (6), the aging and increasingly complex U.S. population may portend a need for increased capacity of critical care delivery services (7, 8).
One potential response to increased demand for critical care services is improved ICU triage and increased interprofessional ICU staffing (9). By contrast, recognizing that some ICU patients may not need, want, or benefit from ICU admission (10–12), another way to meet the growing demand for critical care without increasing costs may be to prevent progression of illnesses that lead to ICU admission (13). However, it is unknown how many ICU admissions are potentially preventable annually in the United States. Despite recent attention on preventable hospitalizations (14, 15), little work has explored preventable hospitalizations associated with ICU services (16).
Therefore, we sought to determine the proportion of hospitalizations associated with an ICU admission in the United States that fall into one of two types of potentially preventable admissions. Because there is no standard definition of a potentially preventable ICU condition, we chose to focus on hospitalizations with ICU admissions that: 1) are associated with an ambulatory care–sensitive condition (ACSC), defined as a hospitalization that may have been avoided with timely and appropriate outpatient care (14, 17, 18); and 2) occur among patients with a life-limiting malignancy (LLM) nearing the end of life, for whom better advance care planning (ACP) or earlier palliative care services may have helped avoid ICU admission (19–24).
Methods
Using the 100% Medicare Provider Analysis and Review files, we counted all acute care hospitalizations and identified those associated with ICU care from 2006 through 2015 among Medicare fee-for-service (FFS) beneficiaries 65 years of age and older. We performed the same analysis with claims from patients enrolled in a large, nationally representative Medicare Advantage (MA) plan and those in a private insurance (PI) plan using the Optum Insight Clinformatics Data Mart. Person-years of coverage were tabulated using each dataset’s beneficiary file. The primary reported outcome is the proportion of ICU admissions meeting the criteria described below for an ACSC or LLM. The Institutional Review Board of the University of Pennsylvania approved this study. Preliminary results were previously reported in an abstract (25).
Administrative Definitions
ICU care was considered present if at least one revenue center code for intensive care or cardiac care unit (CCU), but not for intermediate care, was present in the claims for a given hospitalization (26). We identified hospitalizations as potentially preventable if they included administrative codes for an ACSC (18) or an LLM (27). The latest definition of ACSCs encompasses 13 clinical categories of acute and chronic conditions, including hypertension, urinary tract infection, and uncontrolled diabetes, for which timely outpatient care may have prevented a hospital admission. Details of the development process and construct validity of each ACSC have been previously reported (18). This construct does not identify the direct reason for an ICU admission conditional on an already hospitalized patient. Rather, it identifies hospitalizations that could have been prevented with early outpatient care, regardless of whether or not that hospitalization ended up requiring ICU care. The definition of LLMs is based on a clinician-developed palliative care screening program at a large academic cancer center, with administrative codes restricted to those with associated high 1-year mortality based on Medicare claims data (27). Further details of all administrative definitions are provided in the online supplement.
Temporal Trends and Group Comparisons
We evaluated changes over time for counts and proportions using simple Poisson regression and the chi-square test for trend, respectively. Confidence intervals and comparisons between observations around observed estimates were reported using Poisson and binomial distributions and tests with a two-sided α of 0.05 for counts and proportions, respectively. Comparisons between groups were analyzed with the t test and chi-square test for continuous and categorical variables, respectively. In addition, exploratory state-level comparisons are further described in the online supplement. Analytic files were generated from the original databases using SAS (SAS Institute Inc.), and subsequent analyses were performed using the R language for statistical computing version 3.5.0 (28).
Relationship with ICU Beds
We measured the correlation between each type of potentially preventable ICU admission and the total number of ICU beds in each state with the FFS population. State indicators were not available for the MA and private insurance populations. Total ICU beds were defined as the number of medical, surgical, and cardiac intensive care beds in each state in a given year based on the American Hospital Association Annual Survey Database (29). We also measured correlations using Spearman’s ρ with 95% confidence intervals (CIs) constructed using 10,000 bootstrap replicates. Differences in correlations were determined by estimating a bootstrapped difference over 10,000 replicates with a two-sided α level of 0.05.
Sensitivity Analysis
Medical billing procedures in the United States used International Classification of Diseases Ninth Revision, Clinical Modification 9th revision diagnostic and procedure codes through the third quarter of 2015, after which 10th revision codes were used. Because this transition in coding occurred during our study period, and could confound results based on differences in coding practices and administrative definitions (30), we compared the proportions of each type of potentially preventable ICU admission between the fourth quarters of 2014 and 2015. We then projected the 2015 fourth quarter rates forward based on the 2014 fourth quarter rates and repeated the analyses of temporal trends using the projected rates.
Results
We analyzed 420,369,434 person-years of insurance coverage during which there were 99,793,416 acute inpatient hospitalizations, of which 16,646,977 (16.7%) were associated with an ICU (including CCU) admission (Table 1). The unadjusted incidence of ICU admission and overall hospitalization varied across payer groups (P < 0.001 for all comparisons). ICU admission rates were highest in the FFS population (5,110 per 100,000 person-years) and lowest among the privately ensured (1,052 per 100,000 person-years).
Table 1.
Summary characteristics of claims data from three nationally representative insurance plans from 2006 to 2015
| Measure | All | Fee for Service | Medicare Advantage | Private Insurance |
|---|---|---|---|---|
| Total hospitalizations | 99,793,416 | 88,402,008 | 4,112,550 | 7,278,858 |
| Person-years of coverage | 420,369,434 | 289,391,447 | 19,619,445 | 111,358,542 |
| Total ICU admissions | 13,206,444 | 11,503,628 | 545,095 | 1,157,721 |
| Total CCU admission | 3,885,634 | 3,705,677 | 163,555 | 16,402 |
| Any ICU or CCU admission | 16,646,977 | 14,787,690 | 687,318 | 1,171,969 |
| Age, mean weighted by 5-yr bucket | ||||
| Population (range) | 66 (0–120) | 78 (65–120) | 77 (65–120) | 35 (0–120) |
| Hospitalizations without ICU admission | 79 | 82 | 79 | 38 |
| Hospitalizations with ICU admission | 78 | 81 | 78 | 50 |
| Total hospitalizations per 100,000 person-years | 23,739 | 30,548 | 20,962 | 6,536 |
| Total ICU or CCU Admissions per 100,000 person-years | 3,960 | 5,110 | 3,503 | 1,052 |
Definition of abbreviations: CCU = cardiac care unit; ICU = intensive care unit.
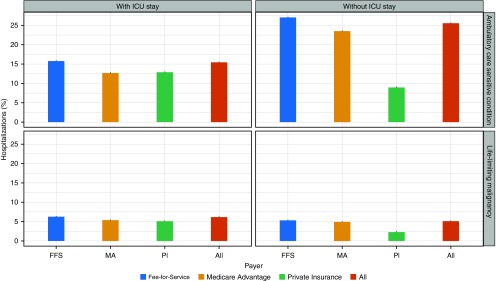
Among hospitalizations with ICU care, more than 2,580,378 (15.5%) were associated with a potentially preventable diagnosis (Figure 1). Over the course of the 10-year study period, this is equivalent to an average of 258,037 potentially preventable ICU admissions per year.
Figure 1.
Percentage of hospitalizations, stratified by the presence of an intensive care unit (ICU) stay and payer, for each potentially preventable condition. Each bar displays the 95% binomial confidence interval. FFS = Fee-for-Service; MA = Medicare Advantage; PI = private insurance.
Relationship with ICU Beds
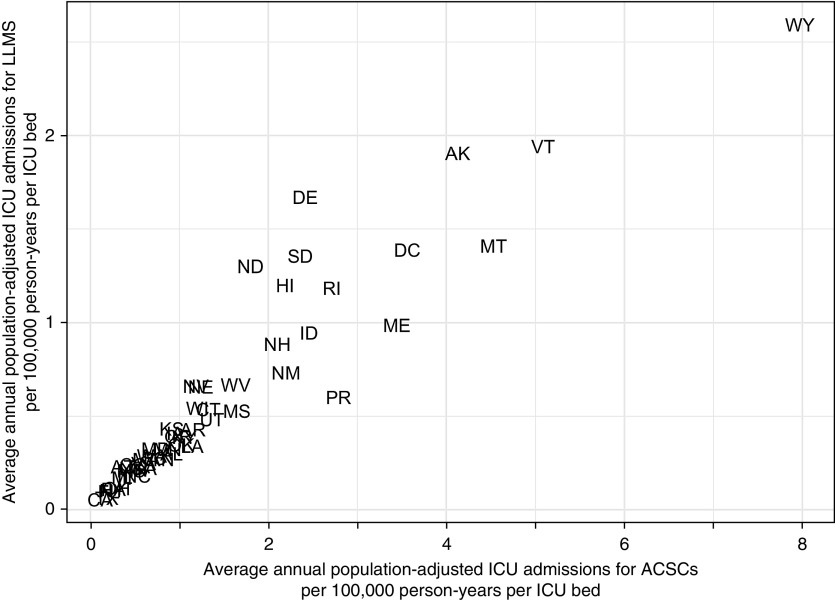
After adjustment for state demographics and available ICU beds, there is an almost eightfold difference among states in the rates of ICU admissions (Figure 2). We found significant correlations between each type of ICU admission and the total number of ICU beds in each state (see Figure E1 in the online supplement). For example, in 2015, the correlations between ACSCs (ρ = 0.54, 95% CI = 0.26–0.73) and LLMs (ρ = 0.61, 95% CI = 0.38–0.76) with ICU beds were similar to that for all ICU admissions (ρ = 0.58, 95% CI = 0.30–0.78). This pattern was consistent across the study period for both ACSCs (Figure E2) and LLMs (Figure E3).
Figure 2.
State-level differences in rates of intensive care unit (ICU) admissions for a potentially preventable cause among Medicare Fee-for-Service beneficiaries at least 65 years old adjusted for the age, sex, distribution, and ICU bed capacity in each state, per 100,000 person-years per ICU bed. ACSCs = ambulatory care sensitive conditions; LLMS = life-limiting malignancies.
We observed wide variation in unadjusted ICU admitting patterns across states for ACSCs (Figure E4) and LLMs (Figure E5). Among states, admission rates per capita for ACSCs and LLMs were positively correlated, whereas these rates were negatively correlated when expressed as percentages of all ICU admissions (Figure E6).
Temporal Trends
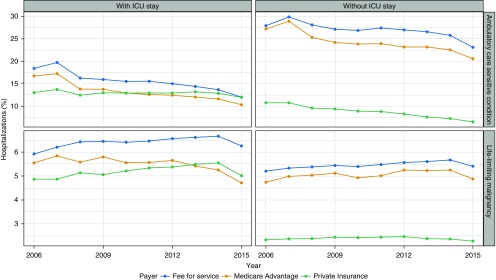
The percentages of ACSC hospitalizations both with and without ICU care declined between 2006 and 2015 across all payer groups (P < 0.001 for all tests; Figure 3). In contrast, the percentages of hospitalizations with LLMs showed more heterogeneous trends over time. Over the 10-year period, the absolute percentages of ACSC-associated ICU stays declined across all payer groups (PI = −1.1%, MA = −6.4%, FFS = −6.4%; P < 0.001 for all trends). Smaller changes were noted among LLM-associated ICU stays, declining in the MA cohort (−0.8%) and increasing in the FFS (+0.3%) and PI (+0.2%) populations (P < 0.001 for all trends).
Figure 3.
Unadjusted temporal trends in potentially preventable hospitalizations with and without an intensive care unit (ICU) admission in the United States. The plot shows the percentage of admissions by insurance coverage for hospitalizations associated with diagnostic codes for ambulatory care–sensitive conditions (top panels) and for life-limiting malignancies (bottom panels).
Age and Payer Comparisons
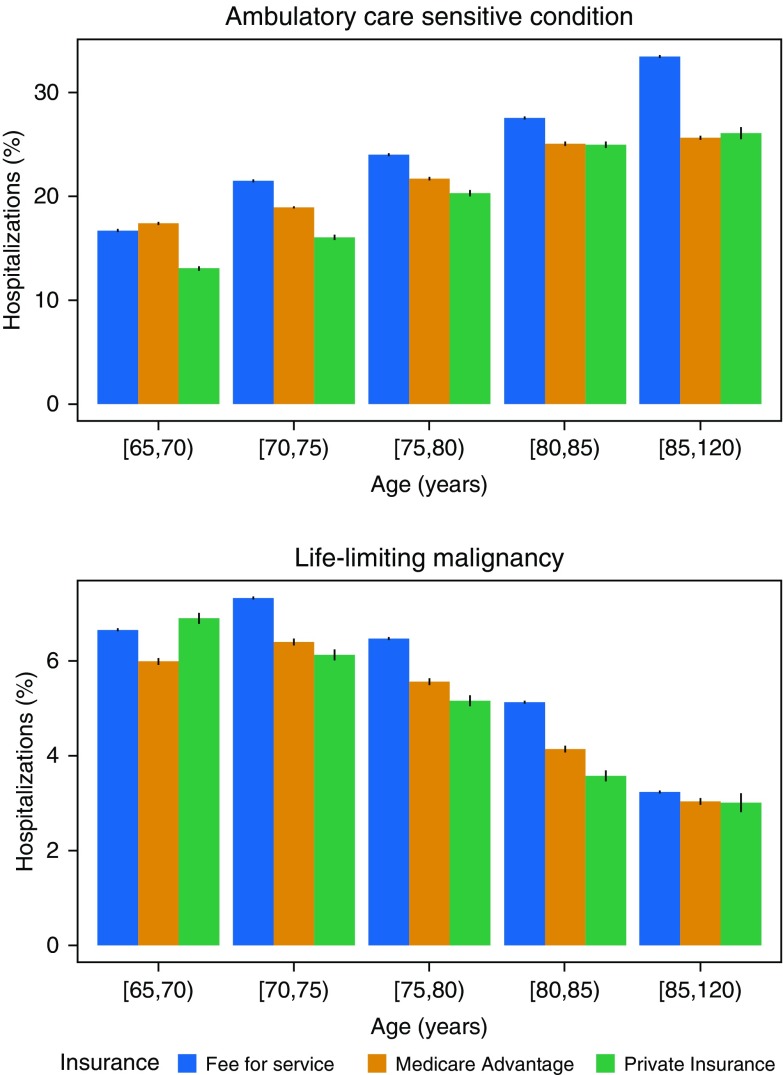
The proportion of hospital admissions with ICU care that were potentially preventable varied by age (Figure 4). Among those at least 65 years old, the proportion of hospitalizations with ICU care associated with an ACSC increased with age, whereas those for an LLM declined with age across all payer groups (P < 0.001). Among the privately ensured, the proportion of potentially preventable hospitalizations with an ICU admission was higher than for hospitalizations without an ICU admission. This difference from the other payer groups was primarily driven by admission patterns among patients younger than 65 years of age (Figure E8).
Figure 4.
Differences by age in percentage of intensive care unit (ICU) admissions for a potentially preventable cause. The proportion of ICU admissions associated with an ambulatory care–sensitive condition increases with age, whereas those with a life-limiting malignancy decreases. These trends are consistent across payer groups. Each bar displays the 95% binomial confidence interval. Brackets indicate a value included in the age interval, whereas parentheses indicate a value excluded from it.
Sensitivity Analysis
The sensitivity analysis examining the effect of new administrative definitions in the fourth quarter of 2015 revealed substantial differences between the observed and projected ICU admission rates (Figure E7). In nearly all cases, the observed rates were much lower than the projected rates. However, regardless of the approach used to quantify 2015 fourth-quarter rates, the 10-year trends over the study period were unchanged.
Discussion
Understanding which types of ICU admissions may be preventable is important, due to increasing strains on ICU capacity and changes in healthcare financing that prioritize outpatient management. Because a gold standard definition of the “preventability” of hospital or ICU admissions remains elusive (31–33), a focus on admissions with specific, potentially preventable mechanisms may elucidate targets for policy and care management interventions that improve the cost effectiveness of care delivery. In addition, describing the burden of potentially preventable hospitalizations associated with ICU care would help to inform decisions about modifying the critical care workforce capacity. These results are based on datasets that represent about 13% of the entire U.S. population and about 64% of the U.S. population of at least 65 years of age.
Although the proportion of ICU-associated hospitalizations with an ACSC has been slowly decreasing over time, the proportion of those with an LLM has been increasing. These divergent trends may reflect known changes in coding practices, such as increased billing for sepsis that may mask otherwise equivalent admissions for ACSCs (34, 35). Such patterns may also be explained by changes in population health management and care strategies, attributed to federal reimbursement programs or due to other secular trends (36, 37). By contrast, there have been fewer changes in federal policy to incentivize ACP among patients with LLM, and even recent federal reimbursements for ACP are rarely used (38, 39).
In this context, we found that the proportion of ICU admissions that may be potentially preventable in the United States is substantial, ranging from 16% to 20%, with variation across payer groups. This figure underestimates the total proportion of potentially preventable ICU admissions, because we did not address other mechanisms of potentially preventable admissions, such as those after an opioid overdose (40), firearm-related injuries (41, 42), and motor vehicle collisions due to distracted driving (43), or intoxication (44). The possibility that an appreciable proportion of ICU admissions may be preventable with early, community-based interventions highlights several opportunities for policy change and further study.
First, our results suggest that expansion of ICU bed supply in the United States contributes to the total number of potentially preventable ICU admissions, though perhaps not to the proportion of ICU admissions that are potentially preventable. Put another way, ICU bed elasticity of demand (45) does not differ between admissions for ACSCs, LLMs, and general ICU admissions. Over the last decade, ICU beds in the United States increased by 15–18% (2, 46). Thus, reversals of this trend, if not outright reductions in ICU bed supply, might similarly reduce preventable and nonpreventable ICU admissions alike. However, because ICU clinicians may become more efficient when faced with bed scarcity (47), triage could change differentially between preventable and nonpreventable admissions. Even after adjustment for baseline demographics and available ICU beds, there remains wide variation in the state-level admission rates for potentially preventable ICU admissions. Although no optimal ICU admission rate for these diagnoses has been determined, the wide variation likely suggests room to improve appropriate triage and care delivery. Subsequent cost savings could support investment in outpatient services designed to prevent such hospitalizations in the first place.
Second, although ACSCs were generally more common in hospitalizations without an ICU stay among Medicare beneficiaries, rates of ACSCs were still high in hospitalizations with an ICU stay. By contrast, among privately ensured patients, ACSC and LLM rates were higher in hospitalizations with an ICU stay, mostly among those 15–50 years of age. We could not determine if the differences in this younger cohort were driven by variation in administrative coding practices in a younger, less-complex population, by triage differences within hospitals, or other reasons. Even so, our national estimates align with the one prior study on this topic from a single center (16). This is an important finding, because historical efforts to reduce admissions for ACSCs have ignored ICU status, which is associated with significantly higher costs (48, 49). Estimated costs for hospitalizations with ICU care far exceed those for corresponding outpatient preventive services (50). Targeting prevention of hospitalizations with an ICU stay are more likely to be cost effective or even cost saving overall compared with targeting hospitalizations without an ICU stay. However, evaluation of cost sharing under different payment plans is warranted to evaluate the potential impact on patients and families who may incur out-of-pocket or informal costs associated with more outpatient or home services.
Third, the appreciable rates of ICU admissions among patients with LLMs also highlight opportunities to improve preference-concordant care. We found that 6.3% of all ICU admissions are among patients with malignancies with limited expected survival, but many other seriously ill patients with similar prognoses, such as those with chronic lung disease (51), heart failure (52), and neurodegenerative disorders (20), also spend significant time in the ICU near the end of life. Prior work has shown that national ICU bed supply is associated with the proportion of patients dying with cancer who are admitted to the ICU near the end of life (53), and similar associations are likely for these other groups of seriously ill patients. Early palliative care in patients with serious illness may reduce ICU admissions and costs, whereas simultaneously improving patient- and family-centered outcomes (19, 21–24). Thus, greater investment in early, outpatient palliative care may be both cost saving and quality enhancing compared with investment in ICU bed and workforce expansion.
Fourth, although based on an exploratory analysis, regional differences in ICU utilization patterns for ACSCs and LLMs highlight potentially important heterogeneity in demographic patterns, severity of illness, and ICU triage decisions. Puerto Rico, for example, had the highest proportion of ICU admissions for ACSCs, but the lowest for LLMs, indicating that the ICU may play a different role in health systems with fewer trained intensivists and a more limited primary care infrastructure (54, 55). In a previous study of seven U.S. states, Medicaid expansion was associated with decreased rates of mechanical ventilation in select populations (56). This preliminary finding suggests that, for some diseases and in some regions, expanded primary care access may reduce the incidence of severe respiratory failure. Decisions to expand ICU capacity based on admission rates should account for both the potential preventability of ICU admissions and also the needs of the local health system outside of the ICU.
This study should be interpreted in light of its limitations. First, the assessment of “preventability” (33) or “goal concordance” (57, 58) is difficult even with manual chart review, and is further confounded when using only administrative claims. At the individual patient level, preventability for ACSCs and LLMs is probably best determined through a mixed-methods analysis that accounts for both patient preferences for care and local availability of relevant care delivery services—neither of which were examined in this study (17, 31). In addition, although ACSCs have previously been evaluated in hospitalizations with an ICU stay, it has only been in aggregate with those hospitalizations without an ICU stay. The fact that ACSCs (including those requiring the presence of a primary diagnosis) were still found in some hospitalizations even in the presence of an ICU admission may increase confidence in the prominent role of that diagnosis. This approach also does not distinguish between a hospitalization that could have been prevented entirely and one that would only have required ward-level, rather than ICU-level, care with an earlier community-based intervention. The difference between these two cases highlights the need for defining and evaluating both the constructs of preventable ICU admissions and preventable hospitalizations associated with an ICU admission. Thus, we avoided making claims of the preventability of any individual hospitalization, but rather focused on population-level groups of potentially preventable hospitalizations. Although the rates we report may therefore be imprecise, the consistently high proportions of potentially preventable admissions across time and payer suggest ample opportunities for improvement.
Second, there is significant heterogeneity in ICU triage decisions across hospitals (59) and hospital referral regions (60) that cannot be accounted for in this study. Thus, it will be important to ascertain how strategies to reduce potentially preventable ICU admissions may work differentially well across settings. Third, other than age and sex, we did not adjust for demographic or other patient-level characteristics across states when calculating hospital or ICU admission rates. Fourth, we did not report trends broken down by individual category of ACSCs. Future work that is able to analyze these more granular trends could inform targeting of particular outpatient services. Such an analysis would also disaggregate the contributions of rising rates of chronic obstructive pulmonary disease admissions with simultaneously falling rates of pneumonia (61). Fifth, the databases used in this study did not contain unique patient identifiers that would allow for identification of the same patient across multiple insurance plans. This limitation prevents attributing temporal trends in ICU admission rates to potential factors such as plan switching. Finally, due to the datasets available for this analysis, our estimates did not account for people who were uninsured, covered by Medicaid, or had private insurance through a different payer, thus precluding extrapolation of our findings to the entire U.S. population.
With these caveats in mind, this study nonetheless supports a general conclusion that a substantial portion of ICU admissions in the United States may be prevented. This finding is important, because it points to population-health approaches that may improve care quality, while also alleviating strain on the national critical care delivery system. Investing in outpatient, preventive, and palliative services should therefore be viewed as important complementary, if not alternative, strategies to increasing the critical care workforce in seeking to best care for the nation’s sickest patients.
Supplementary Material
Acknowledgments
Acknowledgment
The authors are grateful to the technical support service at the Research Data Assistance Center (ResDAC) for their assistance with understanding claims codes in the Medicare Provider Analysis and Review dataset.
Footnotes
Supported by National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute grants T32-HL098054 and K23-HL141639 (G.E.W.), and, in part, by NIH/National Institute on Aging grant K24-AG047908 (Y.Y. and R.M.W.).
Author Contributions: G.E.W., M.P.K., Y.Y., R.K., G.L.A., P.W.G., R.M.W., and S.D.H. contributed substantively to the conception or design of the work, or the acquisition, analysis, or interpretation of data for the work, drafting the work, or revising it critically for important intellectual content, and final approval of the version submitted for publication.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Halpern SD. ICU capacity strain and the quality and allocation of critical care. Curr Opin Crit Care. 2011;17:648–657. doi: 10.1097/MCC.0b013e32834c7a53. [DOI] [PubMed] [Google Scholar]
- 2.Halpern NA, Goldman DA, Tan KS, Pastores SM. Trends in critical care beds and use among population groups and Medicare and Medicaid beneficiaries in the United States: 2000–2010. Crit Care Med. 2016;44:1490–1499. doi: 10.1097/CCM.0000000000001722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weissman GE, Kerlin MP, Yuan Y, Gabler NB, Groeneveld PW, Werner RM, et al. Population trends in intensive care unit admissions in the United States among Medicare beneficiaries, 2006–2015. Ann Intern Med. 2018;170:213–215. doi: 10.7326/M18-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wunsch H, Angus DC, Harrison DA, Collange O, Fowler R, Hoste EAJ, et al. Variation in critical care services across North America and Western Europe. Crit Care Med. 2008;36:2787–2793. doi: 10.1097/CCM.0b013e318186aec8. e1–9. [DOI] [PubMed] [Google Scholar]
- 5.Sjoding MW, Prescott HC, Wunsch H, Iwashyna TJ, Cooke CR. Longitudinal changes in ICU admissions among elderly patients in the United States. Crit Care Med. 2016;44:1353–1360. doi: 10.1097/CCM.0000000000001664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teno JM, Gozalo P, Trivedi AN, Bunker J, Lima J, Ogarek J, et al. Site of death, place of care, and health care transitions among US Medicare beneficiaries, 2000–2015. JAMA. 2018;320:264–271. doi: 10.1001/jama.2018.8981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halpern NA, Pastores SM, Oropello JM, Kvetan V. Critical care medicine in the United States: addressing the intensivist shortage and image of the specialty. Crit Care Med. 2013;41:2754–2761. doi: 10.1097/CCM.0b013e318298a6fb. [DOI] [PubMed] [Google Scholar]
- 8.Pastores SM, Halpern NA, Oropello JM, Kvetan V. Intensivist workforce in the United States: the crisis is real, not imagined. Am J Respir Crit Care Med. 2015;191:718–719. doi: 10.1164/rccm.201501-0079LE. [DOI] [PubMed] [Google Scholar]
- 9.Kahn JM, Rubenfeld GD. The myth of the workforce crisis: why the United States does not need more intensivist physicians. Am J Respir Crit Care Med. 2015;191:128–134. doi: 10.1164/rccm.201408-1477CP. [DOI] [PubMed] [Google Scholar]
- 10.Ward NS, Teno JM, Curtis JR, Rubenfeld GD, Levy MM. Perceptions of cost constraints, resource limitations, and rationing in United States intensive care units: results of a national survey. Crit Care Med. 2008;36:471–476. doi: 10.1097/CCM.0B013E3181629511. [DOI] [PubMed] [Google Scholar]
- 11.Le Guen J, Boumendil A, Guidet B, Corvol A, Saint-Jean O, Somme D. Are elderly patients’ opinions sought before admission to an intensive care unit? Results of the ICE-CUB study. Age Ageing. 2016;45:303–309. doi: 10.1093/ageing/afv191. [DOI] [PubMed] [Google Scholar]
- 12.Zimmerman JE, Kramer AA. A model for identifying patients who may not need intensive care unit admission. J Crit Care. 2010;25:205–213. doi: 10.1016/j.jcrc.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 13.Weissman GE, Halpern SD. Kairotropy: discovering critical illness trajectories using clinical phenotypes with big data. In: Vincent JL, editor. Annual update in intensive care and emergency medicine 2016. Switzerland: Springer International Publishing; 2016. pp. 483–496. [Google Scholar]
- 14.Billings J, Anderson GM, Newman LS. Recent findings on preventable hospitalizations. Health Aff (Millwood) 1996;15:239–249. doi: 10.1377/hlthaff.15.3.239. [DOI] [PubMed] [Google Scholar]
- 15.Zuckerman RB, Sheingold SH, Orav EJ, Ruhter J, Epstein AM. Readmissions, observation, and the hospital readmissions reduction program. N Engl J Med. 2016;374:1543–1551. doi: 10.1056/NEJMsa1513024. [DOI] [PubMed] [Google Scholar]
- 16.Burr J, Sherman G, Prentice D, Hill C, Fraser V, Kollef MH. Ambulatory care–sensitive conditions: clinical outcomes and impact on intensive care unit resource use. South Med J. 2003;96:172–178. doi: 10.1097/01.SMJ.0000050680.55019.32. [DOI] [PubMed] [Google Scholar]
- 17.Bindman AB, Grumbach K, Osmond D, Komaromy M, Vranizan K, Lurie N, et al. Preventable hospitalizations and access to health care. JAMA. 1995;274:305–311. [PubMed] [Google Scholar]
- 18.AHRQ. AHRQ quality indicators: guide to prevention quality indicators: hospital admission for ambulatory care sensitive conditions. Rockville, MD: Agency for Healthcare Research and Quality; 2014. AHRQ Publication No. 02-R0203.
- 19.Khandelwal N, Kross EK, Engelberg RA, Coe NB, Long AC, Curtis JR. Estimating the effect of palliative care interventions and advance care planning on ICU utilization: a systematic review. Crit Care Med. 2015;43:1102–1111. doi: 10.1097/CCM.0000000000000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teno JM, Gozalo P, Khandelwal N, Curtis JR, Meltzer D, Engelberg R, et al. Association of increasing use of mechanical ventilation among nursing home residents with advanced dementia and intensive care unit beds. JAMA Intern Med. 2016;176:1809–1816. doi: 10.1001/jamainternmed.2016.5964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daly B, Hantel A, Wroblewski K, Balachandran JS, Chow S, DeBoer R, et al. No exit: identifying avoidable terminal oncology intensive care unit hospitalizations. J Oncol Pract. 2016;12:e901–e911. doi: 10.1200/JOP.2016.012823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gade G, Venohr I, Conner D, McGrady K, Beane J, Richardson RH, et al. Impact of an inpatient palliative care team: a randomized control trial. J Palliat Med. 2008;11:180–190. doi: 10.1089/jpm.2007.0055. [DOI] [PubMed] [Google Scholar]
- 23.Dionne-Odom JN, Azuero A, Lyons KD, Hull JG, Tosteson T, Li Z, et al. Benefits of early versus delayed palliative care to informal family caregivers of patients with advanced cancer: outcomes from the ENABLE III randomized controlled trial. J Clin Oncol. 2015;33:1446–1452. doi: 10.1200/JCO.2014.58.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kavalieratos D, Corbelli J, Zhang D, Dionne-Odom JN, Ernecoff NC, Hanmer J, et al. association between palliative care and patient and caregiver outcomes: a systematic review and meta-analysis. JAMA. 2016;316:2104–2114. doi: 10.1001/jama.2016.16840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weissman GE, Gabler NB, Kerlin MP, Halpern SD. The incidence of ICU admissions in a privately insured population across the United States [abstract] Am J Respir Crit Care Med. 2016;193:A2955. [Google Scholar]
- 26.Weissman GE, Hubbard RA, Kohn R, Anesi GL, Manaker S, Kerlin MP, et al. Validation of an administrative definition of ICU admission using revenue center codes. Crit Care Med. 2017;45:e758–e762. doi: 10.1097/CCM.0000000000002374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Obermeyer Z, Powers BW, Makar M, Keating NL, Cutler DM. Physician characteristics strongly predict patient enrollment in hospice. Health Aff (Millwood) 2015;34:993–1000. doi: 10.1377/hlthaff.2014.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2016. [Google Scholar]
- 29.American Hospital Association. Washington, DC: AHA; 2006. AHA annual survey database. [Google Scholar]
- 30.Khera R, Dorsey KB, Krumholz HM. Transition to the ICD-10 in the United States: an emerging data chasm. JAMA. 2018;320:133–134. doi: 10.1001/jama.2018.6823. [DOI] [PubMed] [Google Scholar]
- 31.Passey ME, Longman JM, Johnston JJ, Jorm L, Ewald D, Morgan GG, et al. Diagnosing potentially preventable hospitalisations (DaPPHne): protocol for a mixed-methods data-linkage study. BMJ Open. 2015;5:e009879. doi: 10.1136/bmjopen-2015-009879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sentell TL, Seto TB, Young MM, Vawer M, Quensell ML, Braun KL, et al. Pathways to potentially preventable hospitalizations for diabetes and heart failure: a qualitative analysis of patient perspectives. BMC Health Serv Res. 2016;16:300. doi: 10.1186/s12913-016-1511-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Jaghbeer MJ, Tekwani SS, Gunn SR, Kahn JM. Incidence and etiology of potentially preventable ICU readmissions. Crit Care Med. 2016;44:1704–1709. doi: 10.1097/CCM.0000000000001746. [DOI] [PubMed] [Google Scholar]
- 34.Lagu T, Rothberg MB, Shieh M-S, Pekow PS, Steingrub JS, Lindenauer PK. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med. 2012;40:754–761. doi: 10.1097/CCM.0b013e318232db65. [DOI] [PubMed] [Google Scholar]
- 35.Rhee C, Dantes R, Epstein L, Murphy DJ, Seymour CW, Iwashyna TJ, et al. CDC Prevention Epicenter Program. Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009–2014. JAMA. 2017;318:1241–1249. doi: 10.1001/jama.2017.13836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ody C, Msall L, Dafny LS, Grabowski DC, Cutler DM. Decreases in readmissions credited to Medicare’s program to reduce hospital readmissions have been overstated. Health Aff (Millwood) 2019;38:36–43. doi: 10.1377/hlthaff.2018.05178. [DOI] [PubMed] [Google Scholar]
- 37.Wadhera RK, Joynt Maddox KE, Kazi DS, Shen C, Yeh RW. Hospital revisits within 30 days after discharge for medical conditions targeted by the Hospital Readmissions Reduction Program in the United States: national retrospective analysis. BMJ. 2019;366:l4563. doi: 10.1136/bmj.l4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ashana DC, Halpern SD, Umscheid CA, Kerlin MP, Harhay MO.Use of advance care planning billing codes in a retrospective cohort of privately insured patients J Gen Intern Med[online ahead of print] 31 Jul 2019; DOI: 10.1007/s11606-019-05132-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bélanger E, Silver B, Meyers DJ, Rahman M, Kumar A, Kosar C, et al. A retrospective study of administrative data to identify high-need medicare beneficiaries at risk of dying and being hospitalized. J Gen Intern Med. 2019;34:405–411. doi: 10.1007/s11606-018-4781-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stevens JP, Wall MJ, Novack L, Marshall J, Hsu DJ, Howell MD. The critical care crisis of opioid overdoses in the United States. Ann Am Thorac Soc. 2017;14:1803–1809. doi: 10.1513/AnnalsATS.201701-022OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wintemute GJ. The epidemiology of firearm violence in the twenty-first century United States. Annu Rev Public Health. 2015;36:5–19. doi: 10.1146/annurev-publhealth-031914-122535. [DOI] [PubMed] [Google Scholar]
- 42.Coupet E, Jr, Huang Y, Delgado MK. US emergency department encounters for firearm injuries according to presentation at trauma vs nontrauma centers. JAMA Surg. 2019;154:360–362. doi: 10.1001/jamasurg.2018.4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zatezalo N, Erdogan M, Green RS. Road traffic injuries and fatalities among drivers distracted by mobile devices. J Emerg Trauma Shock. 2018;11:175–182. doi: 10.4103/JETS.JETS_24_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jewett A, Shults RA, Banerjee T, Bergen G. Alcohol-impaired driving among adults—United States, 2012. MMWR Morb Mortal Wkly Rep. 2015;64:814–817. doi: 10.15585/mmwr.mm6430a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gooch RA, Kahn JM. ICU bed supply, utilization, and health care spending: an example of demand elasticity. JAMA. 2014;311:567–568. doi: 10.1001/jama.2013.283800. [DOI] [PubMed] [Google Scholar]
- 46.Wallace DJ, Angus DC, Seymour CW, Barnato AE, Kahn JM. Critical care bed growth in the United States: a comparison of regional and national trends. Am J Respir Crit Care Med. 2015;191:410–416. doi: 10.1164/rccm.201409-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wagner J, Gabler NB, Ratcliffe SJ, Brown SES, Strom BL, Halpern SD. Outcomes among patients discharged from busy intensive care units. Ann Intern Med. 2013;159:447–455. doi: 10.7326/0003-4819-159-7-201310010-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barrett M, Smith M, Elixhauser A, Honigman L, Pines J. Utilization of intensive care services, 2011. Washington, DC: Agency for Healthcare Research and Quality; 2014. [PubMed] [Google Scholar]
- 49. HCUP (Healthcare Cost and Utilization Project). Cost-to-Charge Ratio Files. Rockville, MD: Agency for Healthcare Research and Quality; October 2018 [accessed year/month/day]. Available from: www.hcup-us.ahrq.gov/db/state/costtocharge.jsp.
- 50.Galarraga JE, Mutter R, Pines JM. Costs associated with ambulatory care sensitive conditions across hospital-based settings. Acad Emerg Med. 2015;22:172–181. doi: 10.1111/acem.12579. [DOI] [PubMed] [Google Scholar]
- 51.Au DH, Udris EM, Fihn SD, McDonell MB, Curtis JR. Differences in health care utilization at the end of life among patients with chronic obstructive pulmonary disease and patients with lung cancer. Arch Intern Med. 2006;166:326–331. doi: 10.1001/archinte.166.3.326. [DOI] [PubMed] [Google Scholar]
- 52.Yim CK, Barrón Y, Moore S, Murtaugh C, Lala A, Aldridge M, et al. Hospice enrollment in patients with advanced heart failure decreases acute medical service utilization. Circ Heart Fail. 2017;10:e003335. doi: 10.1161/CIRCHEARTFAILURE.116.003335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bekelman JE, Halpern SD, Blankart CR, Bynum JP, Cohen J, Fowler R, et al. International Consortium for End-of-Life Research (ICELR) Comparison of site of death, health care utilization, and hospital expenditures for patients dying with cancer in 7 developed countries. JAMA. 2016;315:272–283. doi: 10.1001/jama.2015.18603. [DOI] [PubMed] [Google Scholar]
- 54.Egozcue-Dionisi M, Nieves-Nieves J, Torrellas-Ruiz PA, Fernández-González R, Fernández-Medero RL, Adorno-Fontánez J, et al. Challenges in critical care medicine: an overview of Puerto Rico’s intensive care units. P R Health Sci J. 2013;32:165–169. [PubMed] [Google Scholar]
- 55.Perreira K, Peters R, Lallemand N, Zuckerman S. Puerto Rico health care infrastructure assessment. Washington, DC: Urban Institute; 2017. [Google Scholar]
- 56.Admon AJ, Sjoding MW, Lyon SM, Ayanian JZ, Iwashyna TJ, Cooke CR. Medicaid expansion and mechanical ventilation in asthma, chronic obstructive pulmonary disease, and heart failure. Ann Am Thorac Soc. 2019;16:886–893. doi: 10.1513/AnnalsATS.201811-777OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Turnbull AE, Hartog CS. Goal-concordant care in the ICU: a conceptual framework for future research. Intensive Care Med. 2017;43:1847–1849. doi: 10.1007/s00134-017-4873-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Halpern SD. Goal-concordant care and the search for the holy grail. N Engl J Med. 2019;381:1603–1606. doi: 10.1056/NEJMp1908153. [DOI] [PubMed] [Google Scholar]
- 59.Admon AJ, Wunsch H, Iwashyna TJ, Cooke CR. Hospital contributions to variability in the use of ICUs among elderly Medicare recipients. Crit Care Med. 2017;45:75–84. doi: 10.1097/CCM.0000000000002025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cooke CR. Risk of death influences regional variation in intensive care unit admission rates among the elderly in the United States. PLoS One. 2016;11:e0166933. doi: 10.1371/journal.pone.0166933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ford ES. Hospital discharges, readmissions, and ED visits for COPD or bronchiectasis among US adults: findings from the Nationwide Inpatient Sample 2001–2012 and Nationwide Emergency Department Sample 2006–2011. Chest. 2015;147:989–998. doi: 10.1378/chest.14-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.