Abstract
Rationale: Obstructive sleep apnea (OSA) is highly prevalent in children and is usually treated by adenotonsillectomy. Nonsurgical therapies for OSA consist primarily of antiinflammatory approaches and have gained popularity, but their efficacy remains to be critically examined.
Objectives: To determine the effect of montelukast on pediatric OSA.
Methods: A prospective randomized double-blind controlled trial of polysomnographically diagnosed OSA in children ages 2–10 years who were treated with either oral montelukast (4 or 5 mg daily) or placebo for 16 weeks. Adherence to the medication was ascertained using automated timed pill dispensers along with weekly telephonic reminders.
Measurements and Main Results: Ninety-two children diagnosed with OSA were approached, and 64 (69.6%) agreed to participate. Of these, 57 (89.0%) completed the 16-week trial, 28 in the montelukast group and 29 in the placebo group. Age, sex, and percentage of obesity were similar in the two groups, as were initial apnea–hypopnea index (AHI) scores. Overall, intention-to-treat analyses revealed that beneficial effects occurred in 20 children receiving montelukast (71.4%), whereas only 2 (6.9%) of the children receiving placebo showed reductions in AHI score (P < 0.001). Indeed, AHI decreased from 9.2 ± 4.1/hour total sleep time (TST) to 4.2 ± 2.8/hour TST (P < 0.0001) in montelukast-treated children, whereas in children receiving placebo, the AHI did not change (from 8.2 ± 5.0/h TST before to 8.7 ± 4.9/h TST at completion of the trial).
Conclusions: When compared with placebo, montelukast for 16 weeks effectively reduced the severity of obstructive sleep apnea in children 2–10 years of age. These results support a therapeutic role for leukotriene modifiers in pediatric OSA provided that long-term trials confirm current findings.
Clinical trial registered with www.clinicaltrials.gov (NCT 00599534).
Keywords: tonsils, obstructive sleep apnea, adenotonsillar hypertrophy, inflammation, leukotrienes
Obstructive sleep apnea (OSA) is a highly prevalent condition in children that imposes a vast array of morbidities including neurocognitive, behavioral, cardiovascular, and metabolic (1). Adenotonsillar hypertrophy has been recognized as the major pathophysiological contributor to OSA in children, and is traditionally treated by surgical removal of enlarged adenoids and tonsils with favorable, albeit variably efficacy being reported (1–11). The realization that a substantial proportion of children with OSA undergoing adenotonsillectomy may develop postoperative complications (12) and have persistent disease after surgery (1–11) has instigated exploration of nonsurgical therapeutic alternatives (13–15) or even prompted consideration of watchful waiting in selected cases (3, 16, 17).
Among the two most commonly employed pharmacological approaches, oral montelukast has been shown to improve respiratory disturbance during sleep in mild pediatric OSA (13, 18–24). However, despite the fact that the biological plausibility on the potential beneficial effects of montelukast-based therapy has been substantiated in in vitro models (13, 25), only one randomized controlled trial has been reported to date involving 23 children with mild OSA who were treated and responded favorably while receiving the active medication (20). We therefore conducted the present randomized, double-blind controlled study to determine whether a 16-week course of montelukast treatment in children with OSA would result in improvements in the severity of sleep-disordered breathing.
Methods
Patients
This prospective, randomized controlled trial study was approved by the institutional human study review committees of the University of Louisville (Louisville, KY) (protocol 474.99) and the University of Chicago (Chicago, IL) (protocol IRB#09-008-A), and registered at clinicaltrials.gov as protocol NCT 00599534.
Subjects for the study were identified from the sleep medicine clinics at Kosair Children’s Hospital (Louisville, KY) and Comer Children’s Hospital at the University of Chicago from January 2008 until December 2012. Children aged 2–10 years, who were referred by their primary care pediatricians or by pediatric otolaryngologists and who underwent overnight sleep studies for suspected OSA, were identified. Symptomatic snoring children with an apnea–hypopnea index (AHI) greater than 2/hour total sleep time (TST) on overnight polysomnography and for whom adenotonsillectomy was contemplated, with or without a history of allergic rhinitis, were deemed eligible for inclusion.
Patients with severe OSA who in the opinion of their treating physicians required early surgical intervention for their OSA were excluded from eligibility for the study. Such criteria generally included an AHI greater than 30/hour TST, nadir SpO2 (arterial oxygen saturation as determined by pulse oximetry) less than 80%, or scheduled surgical adenotonsillectomy within less than 16 weeks. Additional exclusion criteria were as follows: past adenotonsillectomy, genetic disorders, neuromuscular diseases, craniofacial abnormalities, or current treatment with medications such as corticosteroids (oral, inhaled, or intranasal) or oral montelukast. In addition, known hypersensitivity to montelukast, immunodeficiency or receiving immunosuppressant therapy, and the presence of an acute upper respiratory tract infection were also exclusionary criteria.
Children receiving maintenance oral antihistamine preparations or nasal decongestants were required to continue using these medications throughout the duration of the study. Patients receiving immunotherapy for allergic conditions were also expected to continue the same regimen without escalation of dose and frequency throughout the duration of the study.
A clinical research coordinator randomly assigned each child to either one of the two treatment groups by block randomization (n = 4/block), using a table of random digits to generate the allocation sequence. Each child received 16 weeks of treatment with montelukast (Singulair; Merck Co., Whitehouse Station, NJ) or placebo tablets. Montelukast was given at 4 and 5 mg/day to children less than 6 and at least 6 years of age, respectively. The placebo tablets were provided by Merck Co. and were identical in appearance to the active medication, and the clinical pharmacy at the medical center handled delivery of medication to subjects per protocol randomization.
Adherence to the treatment was monitored by providing the assigned treatment in 28-day electronic pill dispensers equipped with electronic locks and alarms (Med-O-Wheel SECURE; e-pill, Wellesley, MA). In addition, weekly telephone calls were made to all participants to promote adherence and to address any potential problems. Failure to use the assigned medication at a frequency corresponding to more than once per week was considered as nonadherence to the prescribed therapy. At the end of the 16-week protocol, all subjects who did not withdraw, including nonadherent participants, underwent a repeat overnight polysomnographic study.
Demographic information including age, sex, ethnicity, height, and weight were obtained for all subjects. Tonsil size derived from a score of 0 (no tonsils present) to 4 (kissing tonsils) (19), Mallampati score (Likert scale range, 1 to 4) (26), and adenoid size as estimated from lateral neck X-ray film based on the degree of choanal obstruction on a Likert scale range 1 to 4 (4, 75–100%; 3, 50–75%; 2, 25–50%; 1, 0–25%), were tabulated when available, as previously described (27, 28).
BMI z-score calculation
Height and weight were recorded for each child on arrival for night-time polysomnography. Body mass index z-score was calculated with an online BMI z-score calculator provided by the Centers for Disease Control and Prevention (Atlanta, GA; http://www.cdc.gov/epiinfo/). Children with BMI z-score values greater than 1.65 were considered obese (29).
Overnight Sleep Studies
An overnight polysomnographic study was performed in the laboratory in the presence of a trained polysomnographic technologist at each of the sleep centers, using the computerized clinical data acquisition system in use at that site. Briefly, the bilateral electro-oculogram, eight channels of electroencephalogram, chin and anterior tibial electromyograms, tracheal sounds, and analog output from a body position sensor were monitored, along with chest and abdominal wall movement, ECG, and airflow using nasal pressure catheter, end-tidal capnography, and an oronasal thermistor. SpO2 was assessed by pulse oximetry with simultaneous recording of the pulse waveform. In addition, a digital time-synchronized video recording was performed.
After removal of movement and technical artifacts, the studies were scored according to standard criteria as defined by the American Academy of Sleep Medicine, with all scoring technologists being supervised by one of the authors to ensure consistency across centers (30). The proportion of time spent in each sleep stage was expressed as the percentage of total sleep time (%TST). Central, obstructive, mixed apneic events were counted, and hypopneas were assessed. Obstructive apnea was defined as the absence of airflow with continued chest wall and abdominal movement for a duration of at least two breaths.
Hypopneas were defined as a decrease in oronasal flow greater than 50% on either the thermistor or nasal pressure transducer signal with a corresponding decrease in SpO2 greater than 3% or arousal. The obstructive AHI was defined as the number of apneas and hypopneas per hour of TST, and an AHI greater than 2/hour TST made the patient eligible for inclusion in the study (31). In addition, the number of 3% reductions in SpO2 per hour of sleep was calculated (oxygen desaturation index 3% [ODI3%]).
Data Analysis
Data are presented as means ± SD unless stated otherwise. We considered a significant improvement to be present when a reduction in AHI by at least 3 events/hour TST occurred. Data were assessed for kurtosis and confirmed as being normally distributed. Statistical analyses were conducted with SPSS 21.0 (SPSS, Chicago, IL) (32). A P value less than 0.05 was considered to have achieved statistical significance.
Results
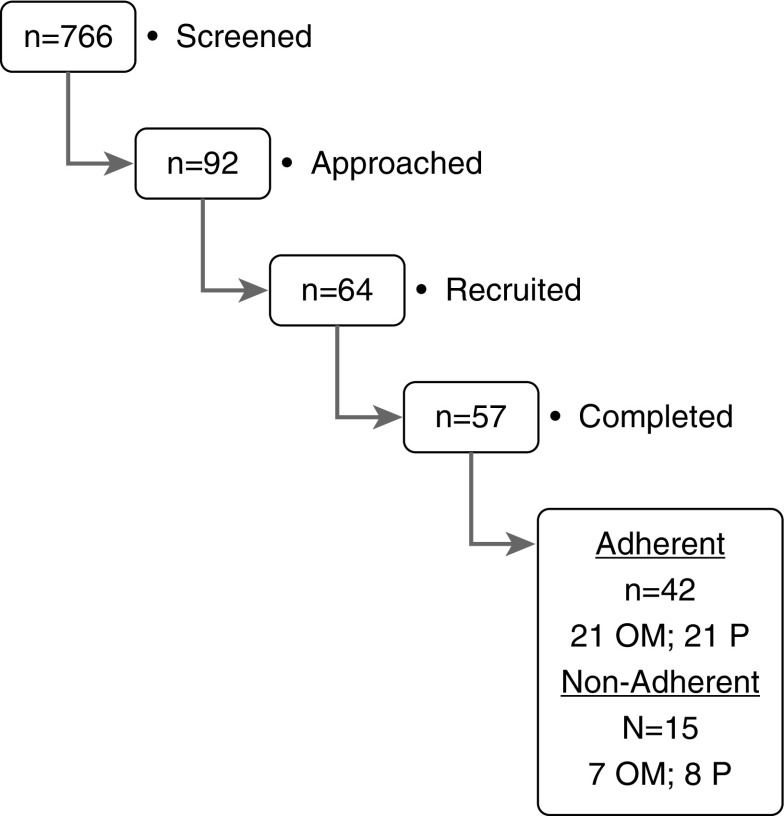
During the period of recruitment, 92 children diagnosed with OSA were approached from a total of 766 screened children, and 64 (69.6%) agreed to participate (Figure 1). The major reasons for a priori exclusion included ongoing treatment with montelukast (n = 128), intranasal corticosteroids (n = 372), previous adenotonsillectomy or adenoidectomy (n = 154), and other chronic or congenital diseases (n = 20). Of the 64 participants, 57 (89.0%) completed the 16-week trial and of these 42 were adherent to the assigned treatment (21 in the montelukast group and 21 in the placebo group). Among the 15 children who were initially enrolled, but were not adherent to the protocol, 8 subjects were in the placebo group and 7 were in the montelukast group (P value, not significant). These 15 children were included in the intention-to-treat analysis, as reported below. Similarly, of the seven children who withdrew from the study after enrollment, four were in the montelukast group and three children were in the placebo group (P value, not significant).
Figure 1.
Schematic flow diagram illustrating the trial recruitment process. OM = oral montelukast; P = placebo.
For the two treatment groups that completed the study, age, sex, ethnicity, percentage of obesity, and adenotonsillar size were similar (Table 1), as were their AHI and several other polysomnographically derived measures (Table 1). Adverse events reported were minor, and included the following: headache in two children (one montelukast, one placebo) and nausea in three subjects (two placebo and one montelukast).
Table 1.
Demographic and polysomnographic characteristics of 57 children randomly assigned to either receive montelukast or placebo, and who completed the study
| Oral Montelukast (n = 28) | Placebo (n = 29) | P Value | |
|---|---|---|---|
| Age, yr | 5.5 ± 2.5 | 5.6 ± 2.4 | NS |
| Sex, male:female | 14:14 | 14:15 | NS |
| Ethnicity, % | |||
| White | 57.1 | 58.6 | NS |
| African American | 42.9 | 41.4 | NS |
| BMI z-score | 1.40 ± 0.46 | 1.44 ± 0.51 | NS |
| % obese (BMI z-score > 1.65) | 42.8 | 48.3 | NS |
| Parentally reported: | |||
| Asthma, % | 25.0 | 24.1 | NS |
| Allergic rhinitis, % | 32.1 | 34.5 | NS |
| Tonsillar size | 2.7 ± 0.4 | 2.6 ± 0.5 | NS |
| Adenoid size | 2.4 ± 0.8 (n = 24) | 2.5 ± 0.7 (n = 26) | NS |
| Mallampati score, n | 2.1 ± 0.5 | 2.1 ± 0.5 | NS |
| Total sleep time, min | 483.5 ± 49.1 | 477.4 ± 50.7 | NS |
| Stage N1, % | 4.5 ± 3.0 | 4.6 ± 3.2 | NS |
| Stage N2, % | 39.5 ± 8.9 | 39.1 ± 8.6 | NS |
| Stage N3, % | 34.8 ± 16.6 | 35.7 ± 15.2 | NS |
| REM sleep, % | 18.8 ± 7.3 | 19.1 ± 7.8 | NS |
| Sleep latency, min | 23.9 ± 15.3 | 23.4 ± 14.2 | NS |
| REM latency, min | 106.4 ± 55.2 | 108.1 ± 57.5 | NS |
| Total arousal index, events/h TST | 17.0 ± 7.3 | 17.3 ± 8.2 | NS |
| Respiratory arousal index, events/h TST | 6.1 ± 4.2 | 5.8 ± 4.9 | NS |
| Obstructive AHI, events/h TST | 9.2 ± 4.1 | 8.2 ± 5.0 | NS |
| SpO2 nadir, % | 85.2 ± 7.4 | 84.8 ± 7.5 | NS |
| ODI3%, /h TST | 7.2 ± 3.1 | 7.0 ± 3.3 | NS |
| PetCO2 > 50 mm Hg, %TST | 5.5 ± 4.6 | 5.4 ± 5.2 | NS |
Definition of abbreviations: AHI = apnea–hypopnea index; BMI = body mass index; NS = not significant; ODI3% = oxygen desaturation index 3% (number of 3% reductions in SpO2 per hour of sleep); PetCO2 = end-tidal (partial) carbon dioxide pressure; SpO2 = arterial oxygen saturation as measured by pulse oximetry; TST (total sleep time) = time spent in the sleep state during the overnight polysomnographic study.
All data are expressed as means ± SD.
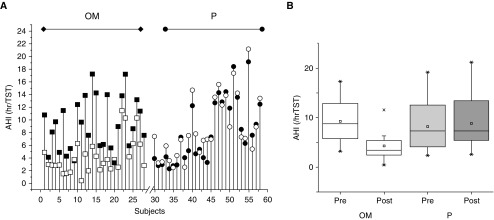
Overall, beneficial effects occurred in 20 children receiving montelukast (71.4%), whereas only 2 (6.9%) of the children receiving placebo showed reductions in AHI (P < 0.001; Figures 1 and 2). Indeed, AHI decreased from 9.2 ± 4.1/hour TST to 4.2 ± 2.8/hour TST (P < 0.0001) in montelukast-treated children, whereas in children receiving placebo, AHI did not change (from 8.2 ± 5.0/h TST before to 8.7 ± 4.9/h TST on completion of the trial) (Table 2 and Figure 2). Similarly, the ODI3% and arousal indices were significantly improved in montelukast-treated children, whereas no significant changes occurred in placebo-treated children (Table 2).
Figure 2.
Effects of a 16-week treatment with oral montelukast or placebo on the severity of sleep-disordered breathing. (A) Individual apnea–hypopnea indices before (solid symbols) and after (open symbols) treatment with oral montelukast (squares) or placebo (circles). (B) Boxplots illustrating median and 95% confidence intervals of apnea–hypopnea indices in response to montelukast or placebo, based on intention-to-treat analyses (P < 0.0001).
Table 2.
Changes in polysomnographic findings after 16-week adherent treatment with either oral montelukast or placebo, based on intention-to-treat analysis
| Montelukast |
Placebo |
|||||
|---|---|---|---|---|---|---|
| Premontelukast (n = 28) | Postmontelukast (n = 28) | P Value (Pre vs. Post) | Preplacebo (n = 29) | Postplacebo (n = 29) | P Value (Pre vs. Post) | |
| BMI z-score | 1.40 ± 0.46 | 1.41 ± 0.49 | NS | 1.44 ± 0.51 | 1.43 ± 0.50 | NS |
| Tonsillar size | 2.7 ± 0.4 | 2.3 ± 0.4 | <0.01 | 2.6 ± 0.5 | 2.5 ± 0.6 | NS |
| Adenoid size | 2.4 ± 0.8 (n = 24) | 2.0 ± 0.5 (n = 20) | <0.001 | 2.5 ± 0.7 (n = 26) | 2.4 ± 0.8 (n = 23) | NS |
| Mallampati score | 2.1 ± 0.5 | 2.1 ± 0. 4 | NS | 2.1 ± 0.5 | 2.2 ± 0.5 | NS |
| Total sleep duration, min | 483.5 ± 49.1 | 478.2 ± 58.1 | NS | 477.4 ± 50.7 | 485.7 ± 54.3 | NS |
| Stage 1, % | 4.5 ± 3.0 | 4.0 ± 3.3 | NS | 4.6 ± 3.2 | 4.7 ± 3.9 | NS |
| Stage 2, % | 39.5 ± 8.9 | 34.3 ± 9.8 | <0.05 | 39.1 ± 8.6 | 40.6 ± 9.5 | NS |
| Stage 3, % | 34.8 ± 16.6 | 41.9 ± 13.8 | <0.02 | 35.7 ± 15.2 | 36.4 ± 17.0 | NS |
| REM sleep, % | 18.8 ± 7.3 | 23.4 ± 8.4 | <0.01 | 19.1 ± 7.8 | 18.6 ± 7.8 | NS |
| Sleep latency, min | 23.9 ± 15.3 | 29.2 ± 15.2 | NS | 23.4 ± 14.2 | 25.9 ± 16.0 | NS |
| REM latency, min | 106.4 ± 55.2 | 112.9 ± 74.7 | NS | 108.1 ± 57.5 | 107.2 ± 58.8 | NS |
| Total arousal index, events/h TST | 17.0 ± 7.3 | 11.7 ± 7.5 | <0.01 | 17.3 ± 8.2 | 18.3 ± 8.4 | NS |
| Respiratory arousal index, events/h TST | 6.1 ± 4.2 | 2,5 ± 2.6 | <0.001 | 5.8 ± 4.9 | 6.6 ± 5.4 | NS |
| Obstructive AHI, events/h TST | 9.2 ± 4.1 | 4.2 ± 2.8 | <0.0001 | 8.2 ± 5.0 | 8.7 ± 4.9 | NS |
| SpO2 nadir, % | 85.2 ± 7.4 | 91.0 ± 2.5 | <0.0001 | 84.8 ± 7.5 | 86.1 ± 7.3 | NS |
| ODI3%, /h TST | 7.2 ± 3.1 | 2.8 ± 1.8 | <0.001 | 7.0 ± 3.3 | 6.8 ± 3.1 | NS |
| PetCO2 > 50 mm Hg, %TST | 5.5 ± 4.6 | 5.9 ± 5.6 | NS | 5.4 ± 5.2 | 5.3 ± 6.1 | NS |
Definition of abbreviations: AHI = apnea–hypopnea index; BMI = body mass index; NS = not significant; ODI3% = oxygen desaturation index 3% (number of 3% reductions in SpO2 per hour of sleep); PetCO2 = end-tidal (partial) carbon dioxide pressure; SpO2 = arterial oxygen saturation as measured by pulse oximetry; TST (total sleep time) = time spent in the sleep state during the overnight polysomnographic study.
All data are expressed as means ± SD.
Discussion
This study shows that when compared with placebo, a 16-week treatment course with montelukast significantly reduces the severity of OSA in children.
Before addressing the potential implications of our study, several methodological issues require comment. As part of our study design, we enrolled patients whose polysomnographic findings of OSA were not so severe as to justify urgent clinical intervention. These issues were described in the protocol considerations that preceded the implementation of the randomized trial for adenotonsillectomy (Childhood Adenotonsillectomy Trial [CHAT]) (33). Although capping the severity of OSA could have hampered the effect size, significant improvements emerged from the intervention with montelukast, whereas treatment with placebo did not yield any significant beneficial changes, and even led to a slight and significant trend toward worsening of AHI during the 16-week treatment period.
Of note, the treatment period was predicated on the average wait times between diagnosis of OSA and surgical treatment, such that we cannot infer whether additional improvements would occur with lengthier interventions. In addition, both the ineligibility rates and the enrollment refusal rates were relatively high, reflecting the large numbers of children currently being treated with antiinflammatory agents before referral for polysomnographic or otolaryngologist evaluation among the former, whereas the latter essentially indicate the preconceived parental decision to adopt adenotonsillectomy as the treatment. Similarly, trial dropout rates reflect the usual attrition associated with a drug-related trial, particularly considering the strict adherence criteria and the implementation of adherence electronic monitoring procedures.
Finally, although the sample size was sufficient to illustrate a significant beneficial effect of montelukast, thereby corroborating the findings in the previous randomized controlled trial by Goldbart and colleagues (20), it is clear that adoption of this therapeutic option into the routine management of pediatric OSA will require further confirmation from expanded multicenter studies, and improved identification of those pediatric patients with OSA who are most likely to benefit from montelukast therapy. In this context, it will be important to implement assessments of quality of life and behavioral patterns because such assessments are not linearly correlated with the severity of OSA as measured by AHI (3, 34).
The rationale for implementing a clinical management paradigm consisting of a nonsurgical treatment such as montelukast for pediatric OSA was predicated on the overall disappointing success rate of adenotonsillectomy when normalization of respiratory abnormalities is set as the success criterion (1–11, 35). When the relatively high persistent OSA rates after adenotonsillectomy are paired with the potential risks of the surgery (12, 36), the availability of efficacious nonsurgical options becomes highly desirable. Of note, there is evidence indicating that watchful waiting may result in improvements in the severity of OSA (3, 16, 17), and a portion of the current temporal changes in AHI could be assigned to such temporal factors. However, this is unlikely considering the absence of any measurable improvements among placebo-treated children.
Evidence from in vitro experiments in our laboratory employing primary dissociated tissue cultures of tonsillar and adenoid tissues revealed marked reductions in proliferation with montelukast (25), a finding that was also supported by the original open study in children with OSA (18). Furthermore, findings from a retrospective analysis of a large cohort receiving a combination of montelukast and intranasal corticosteroids (15) further reinforce the concept that inclusion of montelukast is potentially beneficial in the management of pediatric OSA. Indeed, current findings provide initial confirmation in a clinical setting that the combination of inhaled corticosteroid and montelukast is a potentially effective intervention for the treatment of mild OSA in children, and that such results need to be confirmed by prospective multicenter, randomized, controlled trial approaches, particularly when considering the favorable safety profile associated with the use of montelukast (37).
Summary
In this randomized, double-blind, placebo-controlled trial, montelukast emerges as favorably reducing the severity of OSA short-term in children 2–10 years of age. These findings add to the existing evidence supporting a therapeutic role for antiinflammatory approaches in the management of this highly prevalent condition in children, and clearly justify future studies targeting the long-term benefits of these approaches in children with OSA.
Acknowledgments
Acknowledgment
The authors are grateful to Richa Kulkarni for expert and dedicated assistance in the recruitment of subjects. The authors thank the parents and children for participation in the study.
Footnotes
Supported by a Merck Sharp and Dohme Co. Investigator-Initiated Grant. L.K.-G. is also supported by National Institutes of Health grant 1HL130984-01. D.G. is supported by the Herbert T. Abelson Chair in Pediatrics.
Author Contributions: L.K.-G. provided the conceptual framework for the study, acquired data, analyzed and interpreted data, drafted the initial manuscript, is responsible for the financial support of the project and the manuscript content, and approved the final manuscript as submitted. H.P.R.B. acquired data, participated in data interpretation, and approved the final manuscript as submitted. D.G. provided conceptual framework for the study, analyzed and interpreted data, provided critical editing of the initial manuscript, and approved the final manuscript as submitted.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Marcus CL, Brooks LJ, Draper KA, Gozal D, Halbower AC, Jones J, Schechter MS, Ward SD, Sheldon SH, Shiffman RN, et al. American Academy of Pediatrics. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130:e714–e755. doi: 10.1542/peds.2012-1672. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharjee R, Kheirandish-Gozal L, Spruyt K, Mitchell RB, Promchiarak J, Simakajornboon N, Kaditis AG, Splaingard D, Splaingard M, Brooks LJ, et al. Adenotonsillectomy outcomes in treatment of obstructive sleep apnea in children: a multicenter retrospective study. Am J Respir Crit Care Med. 2010;182:676–683. doi: 10.1164/rccm.200912-1930OC. [DOI] [PubMed] [Google Scholar]
- 3.Marcus CL, Moore RH, Rosen CL, Giordani B, Garetz SL, Taylor HG, Mitchell RB, Amin R, Katz ES, Arens R, et al. Childhood Adenotonsillectomy Trial (CHAT) A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med. 2013;368:2366–2376. doi: 10.1056/NEJMoa1215881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang YS, Guilleminault C, Lee LA, Lin CH, Hwang FM. Treatment outcomes of adenotonsillectomy for children with obstructive sleep apnea: a prospective longitudinal study. Sleep. 2014;37:71–76. doi: 10.5665/sleep.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang KT, Weng WC, Lee CH, Lee PL, Hsu WC. Discrepancy between objective and subjective outcomes after adenotonsillectomy in children with obstructive sleep apnea syndrome. Otolaryngol Head Neck Surg. 2014;151:150–158. doi: 10.1177/0194599814529534. [DOI] [PubMed] [Google Scholar]
- 6.Alonso-Álvarez ML, Terán-Santos J, Navazo-Egüia AI, Martinez MG, Jurado-Luque MJ, Corral-Peñafiel J, Duran-Cantolla J, Cordero-Guevara JA, Kheirandish-Gozal L, Gozal D Spanish Sleep Network. Treatment outcomes of obstructive sleep apnoea in obese community-dwelling children: the NANOS study. Eur Respir J. 2015;46:717–727. doi: 10.1183/09031936.00013815. [DOI] [PubMed] [Google Scholar]
- 7.Walter LM, Biggs SN, Nisbet LC, Weichard AJ, Hollis SL, Davey MJ, Anderson V, Nixon GM, Horne RS. Long-term improvements in sleep and respiratory parameters in preschool children following treatment of sleep disordered breathing. J Clin Sleep Med. 2015;11:1143–1151. doi: 10.5664/jcsm.5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suri JC, Sen MK, Venkatachalam VP, Bhool S, Sharma R, Elias M, Adhikari T. Outcome of adenotonsillectomy for children with sleep apnea. Sleep Med. 2015;16:1181–1186. doi: 10.1016/j.sleep.2015.02.539. [DOI] [PubMed] [Google Scholar]
- 9.Lee CH, Hsu WC, Chang WH, Lin MT, Kang KT. Polysomnographic findings after adenotonsillectomy for obstructive sleep apnea in obese and non-obese children: a systemic review and meta-analysis. Clin Otolaryngol. In press. [DOI] [PubMed]
- 10.Pomerantz J. Management of persistent obstructive sleep apnea after adenotonsillectomy. Pediatr Ann. 2016;45:e180–e183. doi: 10.3928/00904481-20160329-01. [DOI] [PubMed] [Google Scholar]
- 11.Tan HL, Kheirandish-Gozal L, Gozal D. Obstructive sleep apnea in children: update on the recognition, treatment and management of persistent disease. Expert Rev Respir Med. 2016;21:1–9. doi: 10.1586/17476348.2016.1163224. [DOI] [PubMed] [Google Scholar]
- 12.De Luca Canto G, Pachêco-Pereira C, Aydinoz S, Bhattacharjee R, Tan HL, Kheirandish-Gozal L, Flores-Mir C, Gozal D. Adenotonsillectomy complications: a meta-analysis. Pediatrics. 2015;136:702–718. doi: 10.1542/peds.2015-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kheirandish-Gozal L, Kim J, Goldbart AD, Gozal D. Novel pharmacological approaches for treatment of obstructive sleep apnea in children. Expert Opin Investig Drugs. 2013;22:71–85. doi: 10.1517/13543784.2013.735230. [DOI] [PubMed] [Google Scholar]
- 14.Whitla L, Lennon P. Non-surgical management of obstructive sleep apnoea: a review. Paediatr Int Child Health. 2016 doi: 10.1080/20469047.2016.1162391. Apr 14:1–5. [DOI] [PubMed] [Google Scholar]
- 15.Kheirandish-Gozal L, Bhattacharjee R, Bandla HP, Gozal D. Antiinflammatory therapy outcomes for mild OSA in children. Chest. 2014;146:88–95. doi: 10.1378/chest.13-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chervin RD, Ellenberg SS, Hou X, Marcus CL, Garetz SL, Katz ES, Hodges EK, Mitchell RB, Jones DT, Arens R, et al. Childhood Adenotonsillectomy Trial. Prognosis for spontaneous resolution of OSA in children. Chest. 2015;148:1204–1213. doi: 10.1378/chest.14-2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trosman SJ, Eleff DJ, Krishna J, Anne S. Polysomnography results in pediatric patients with mild obstructive sleep apnea: adenotonsillectomy vs. watchful waiting. Int J Pediatr Otorhinolaryngol. 2016;83:25–30. doi: 10.1016/j.ijporl.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 18.Goldbart AD, Goldman JL, Veling MC, Gozal D. Leukotriene modifier therapy for mild sleep-disordered breathing in children. Am J Respir Crit Care Med. 2005;172:364–370. doi: 10.1164/rccm.200408-1064OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuhle S, Urschitz MS. Anti-inflammatory medications for obstructive sleep apnea in children. Cochrane Database Syst Rev. 2011;(1):CD007074. doi: 10.1002/14651858.CD007074.pub2. [DOI] [PubMed] [Google Scholar]
- 20.Goldbart AD, Greenberg-Dotan S, Tal A. Montelukast for children with obstructive sleep apnea: a double-blind, placebo-controlled study. Pediatrics. 2012;130:e575–e580. doi: 10.1542/peds.2012-0310. [DOI] [PubMed] [Google Scholar]
- 21.Canadian Agency for Drugs and Technologies in Health. Montelukast for sleep apnea: a review of the clinical effectiveness, cost effectiveness, and guidelines [rapid response report: summary with critical appraisal. 2014 Jan 17 [accessed 2016 Aug 13]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24741731. [PubMed]
- 22.MacLean JE. Montelukast potentially efficacious in children with non-severe obstructive sleep apnoea in the short term. Evid Based Med. 2013;18:173–174. doi: 10.1136/eb-2012-101085. [DOI] [PubMed] [Google Scholar]
- 23.Kar M, Altıntoprak N, Muluk NB, Ulusoy S, Bafaqeeh SA, Cingi C. Antileukotrienes in adenotonsillar hypertrophy: a review of the literature. Eur Arch Otorhinolaryngol. In press doi: 10.1007/s00405-016-3983-8. [DOI] [PubMed] [Google Scholar]
- 24.Shokouhi F, Meymaneh Jahromi A, Majidi MR, Salehi M. Montelukast in adenoid hypertrophy: its effect on size and symptoms. Iran J Otorhinolaryngol. 2015;27:443–448. [PMC free article] [PubMed] [Google Scholar]
- 25.Dayyat E, Serpero LD, Kheirandish-Gozal L, Goldman JL, Snow A, Bhattacharjee R, Gozal D. Leukotriene pathways and in vitro adenotonsillar cell proliferation in children with obstructive sleep apnea. Chest. 2009;135:1142–1149. doi: 10.1378/chest.08-2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brodsky L, Moore L, Stanievich JF. A comparison of tonsillar size and oropharyngeal dimensions in children with obstructive adenotonsillar hypertrophy. Int J Pediatr Otorhinolaryngol. 1987;13:149–156. doi: 10.1016/0165-5876(87)90091-7. [DOI] [PubMed] [Google Scholar]
- 27.Mallampati SR, Gatt SP, Gugino LD, Desai SP, Waraksa B, Freiberger D, Liu PL. A clinical sign to predict difficult tracheal intubation: a prospective study. Can Anaesth Soc J. 1985;32:429–434. doi: 10.1007/BF03011357. [DOI] [PubMed] [Google Scholar]
- 28.Dayyat E, Kheirandish-Gozal L, Sans Capdevila O, Maarafeya MM, Gozal D. Obstructive sleep apnea in children: relative contributions of body mass index and adenotonsillar hypertrophy. Chest. 2009;136:137–144. doi: 10.1378/chest.08-2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL. CDC growth charts: United States. Adv Data. 2000;314:1–27. [PubMed] [Google Scholar]
- 30.Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, Marcus CL, Mehra R, Parthasarathy S, Quan SF, et al. American Academy of Sleep Medicine; Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. J Clin Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montgomery-Downs HE, O’Brien LM, Gulliver TE, Gozal D. Polysomnographic characteristics in normal preschool and early school-aged children. Pediatrics. 2006;117:741–753. doi: 10.1542/peds.2005-1067. [DOI] [PubMed] [Google Scholar]
- 32.Schmider E, Ziegler M, Danay E, Beyer L, Buhner M. Is it really robust? Reinvestigating the robustness of ANOVA against violations of the normal distribution assumption. Methodology. 2010;6:144–151. [Google Scholar]
- 33.Redline S, Amin R, Beebe D, Chervin RD, Garetz SL, Giordani B, Marcus CL, Moore RH, Rosen CL, Arens R, et al. The Childhood Adenotonsillectomy Trial (CHAT): rationale, design, and challenges of a randomized controlled trial evaluating a standard surgical procedure in a pediatric population. Sleep. 2011;34:1509–1517. doi: 10.5665/sleep.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosen CL, Wang R, Taylor HG, Marcus CL, Katz ES, Paruthi S, Arens R, Muzumdar H, Garetz SL, Mitchell RB, et al. Utility of symptoms to predict treatment outcomes in obstructive sleep apnea syndrome Pediatrics 2015135e662–e671.[Published erratum appears in Pediatrics 137(4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tauman R, Gulliver TE, Krishna J, Montgomery-Downs HE, O’Brien LM, Ivanenko A, Gozal D. Persistence of obstructive sleep apnea syndrome in children after adenotonsillectomy. J Pediatr. 2006;149:803–808. doi: 10.1016/j.jpeds.2006.08.067. [DOI] [PubMed] [Google Scholar]
- 36.Subramanyam R, Varughese A, Willging JP, Sadhasivam S. Future of pediatric tonsillectomy and perioperative outcomes. Int J Pediatr Otorhinolaryngol. 2013;77:194–199. doi: 10.1016/j.ijporl.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 37.Bisgaard H, Skoner D, Boza ML, Tozzi CA, Newcomb K, Reiss TF, Knorr B, Noonan G. Safety and tolerability of montelukast in placebo-controlled pediatric studies and their open-label extensions. Pediatr Pulmonol. 2009;44:568–579. doi: 10.1002/ppul.21018. [DOI] [PubMed] [Google Scholar]