Abstract
Objective
The objective of this article is to discuss the technical details of perineoscopic radical prostatectomy (perineoscopic-RP), which we performed for the first time as a surgical treatment for prostate cancer (PCa), and to present the outcomes of three patients who underwent this procedure.
Material and methods
Using a robotic scope as an optical system, we performed perineoscopic-RP in 3 patients in March 2018. Technical details of the procedure have been explained step-by-step in this article. Preoperative, perioperative, and postoperative data of all the patients was analyzed.
Results
Perineoscopic-RP was completed successfully without the need to convert to other approaches and/or techniques in all three patients. The patients were in a low-risk group for PCa. The mean time that elapsed to reach the prostate apex was 50±3.6 minutes, including the time required to install the optic and retractor system. The mean total operative time was 144.3±8.4 minutes. No intraoperative or postoperative complications were observed. Surgical margins were negative in all patients. Incontinence was observed in 2 patients after the removal of the Foley catheter. All patients achieved complete continence in the 3rd month during the follow-up.
Conclusion
This technique, of which we presented the initial results in this article, can be successfully performed as a surgical intervention method for PCa. Prospective and comparative studies with larger patient series are required to place this method in routine practice.
Keywords: Novel surgical technique, optical magnification, perineoscopic approach, radical prostatectomy
Introduction
Radical prostatectomy (RP) remains the major treatment modality in prostate cancer (PCa). More than one technique is available, which enables surgeons to perform RP, including open retropubic RP (RRP), open perineal RP (PRP), laparoscopic RP (LRP), and robotic RP (RALRP).[1] Along with the technological improvements introduced in surgical practice, robotic surgery has gained momentum around the world in the last decade. Data reported from the United States of America reveal that the most common procedure performed during PCa surgery is RALRP.[2]
The use of robotic surgery in the perineal approach has emerged as an exciting development and was followed by more studies demonstrating the reliability and feasibility of robotic PRP (r-PRP).[3–5] In our surgical practice, we have already performed a modified r-PRP technique without it without the use of gas and GelPort.[6] Following this improvement, the idea of the hybridization of PRP with the robotic optic system emerged in order to benefit also from the advantages provided by the latter. This led to the development of perineoscopic-RP as a novel surgical technique. The technical details of this new procedure are presented in this article.
Material and methods
The study was approved by the Ethics Committee of Bakirkoy Dr. Sadi Konuk Training and Research Hospital. Detailed information was given to all eligible patients about all optional RP techniques. Informed consent was obtained from each patient who volunteered to participate in the study. Perineoscopic-RP was performed in 3 patients within the scope of this study at our clinic in March 2018. All procedures were performed by two surgeons experienced in perineal surgery and RP.
Preoperative demographic data, patient characteristics, technical details of the surgery, and intraoperative and postoperative data were analyzed.
Case 1
A 62-year-old male patient had a history of hypertension and laparoscopic cholecystectomy. The prostate-specific antigen (PSA) was found to be 4.4 ng/mL and a transrectal ultrasound-guided prostate biopsy (TRUS Bx) was performed. A Gleason score of 6 (3 + 3) adenocarcinoma was reported in 4 of the 12 cores. In the multiparametric magnetic resonance imaging (mpMRI), a Prostate Imaging Reporting and Data System (PI-RADS) IV lesion was detected on the left base of the peripheral zone. No extraprostatic extension was observed. The prostate volume (PV) was 45 cc and the International Index of Erectile Function-5 (IIEF-5) score was 17.
Case 2
A 68-year-old male patient had coronary artery disease and no previous surgeries. The PSA was 7.5 ng/mL. TRUS Bx revealed a Gleason score of 6 (3 + 3) adenocarcinoma in 3 of the 12 cores. In mpMRI, PI-RADS III lesions were detected in the left apex in the peripheral zone and no extraprostatic extension was observed. The PV was 30 cc and the IIEF-5 score was 18.
Case 3
A 62-year-old male patient had diabetes mellitus and no history of previous surgeries. The PSA was 3.2 ng/mL. TRUS Bx revealed a Gleason score of 6 (3 + 3) adenocarcinoma in 5 of the 12 cores. No significant features were detected in the mpMRI. The PV was 40 cc and the IIEF-5 score was 5.
Patient selection and preoperative preparation
Patients who had been diagnosed with localized PCa and were found to have a risk of <5% for lymph node metastasis according to Briganti nomogram were found eligible for this study.[7] After TRUS-Bx, at least 1 month was required to elapse as the rest period before the perineoscopic-RP procedure could be performed. For the bowel preparation, an enema was used on the day before the surgery and not on the morning of surgery to prevent contamination of the surgical site. Patients were required to wear antithrombotic and pneumatic compression stockings.
Positioning the patient and preparation for surgery
Under general anesthesia, the patient was placed in the exaggerated lithotomy position, i.e., the 15° Trendelenburg position (Figure 1). A roll was located under the patient at the level of the root of the penis. The lithotomy position can be exaggerated further at this point to achieve a more adequate vision. A urethral catheter was inserted into the bladder to empty it. A sterile glove was placed in the rectum and its edges circumferentially were sutured to the anus. This step enabled a digital rectal examination, which made the dissection procedure much safer. Then, the retractor system was mounted to the operating table so that it could be used immediately when required (Figure 2).
Figure 1.
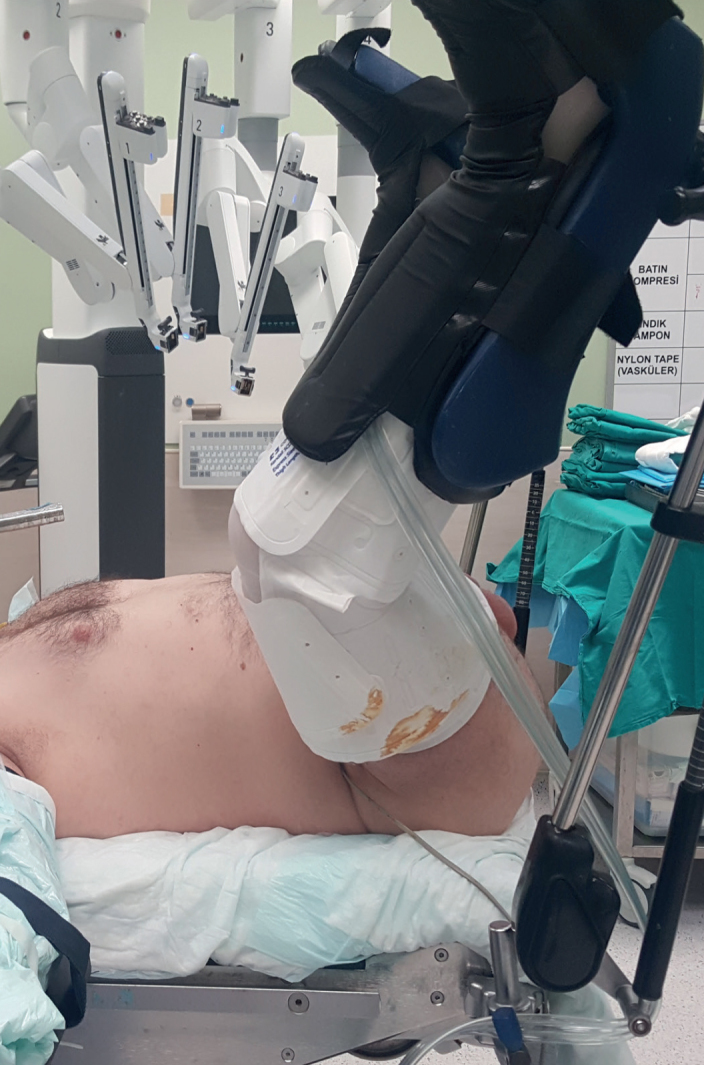
Exaggerated lithotomy performed in the 15° Trendelenburg position
Figure 2. a–b.
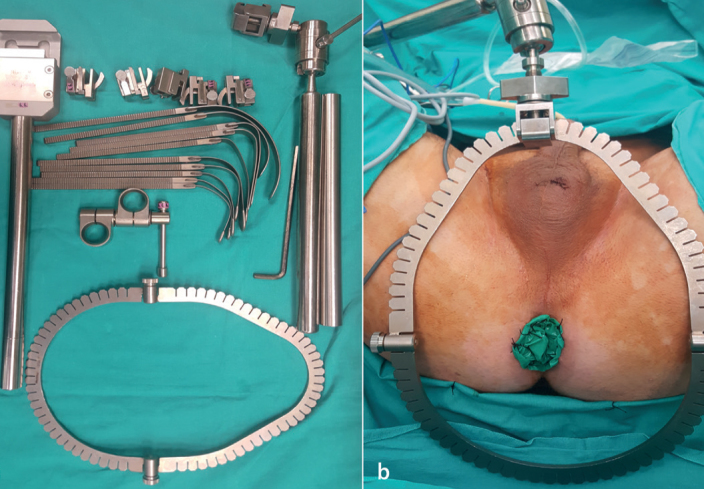
(a) The retractor system, (b) Placement of the glove in the rectum and mounting of the retractor system
Surgeon position and operating room setup
The surgeon took a seat facing the patient’s perineum. A monitor was placed on the right side of the patient over his head. The da Vinci Xi Robotic system (Intuitive Surgical Inc., Sunnyvale, CA, USA) was placed on the left side of the patient. The first robotic arm was placed on the median line (Figure 3). All stages of the operation were generally performed using a 30° up scope. The surgeon performed the surgery while simultaneously checking out the image on the screen. The reverse, right, left, up, or down manipulations of the optic system were performed by the resident surgeon or the co-surgeon on the console.
Figure 3.
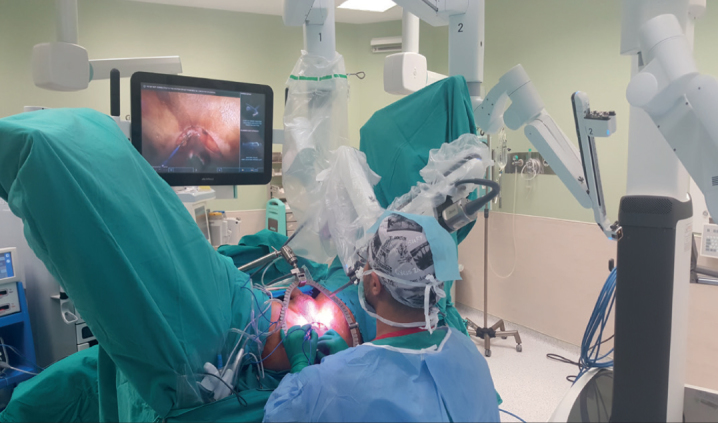
The view of the operating room
Perineoscopic radical prostatectomy
A semilunar skin incision was made between the two ischial tuberosities at an approximate distance of 6 cm from each other. The subcutaneous tissue was dissected and the bilateral ischiorectal fossa were opened using an index finger. The central tendon of the perineal body was dissected. Based on the surgeon’s choice, supra-, infra-, or trans-sphincteric access was established. The membranous urethra and the prostate apex were reached after separating the rectourethral muscles. The surrounding perineal tissues were hung with sutures on the retractor ring to provide better exposure at this stage (Figure 4).
Figure 4.
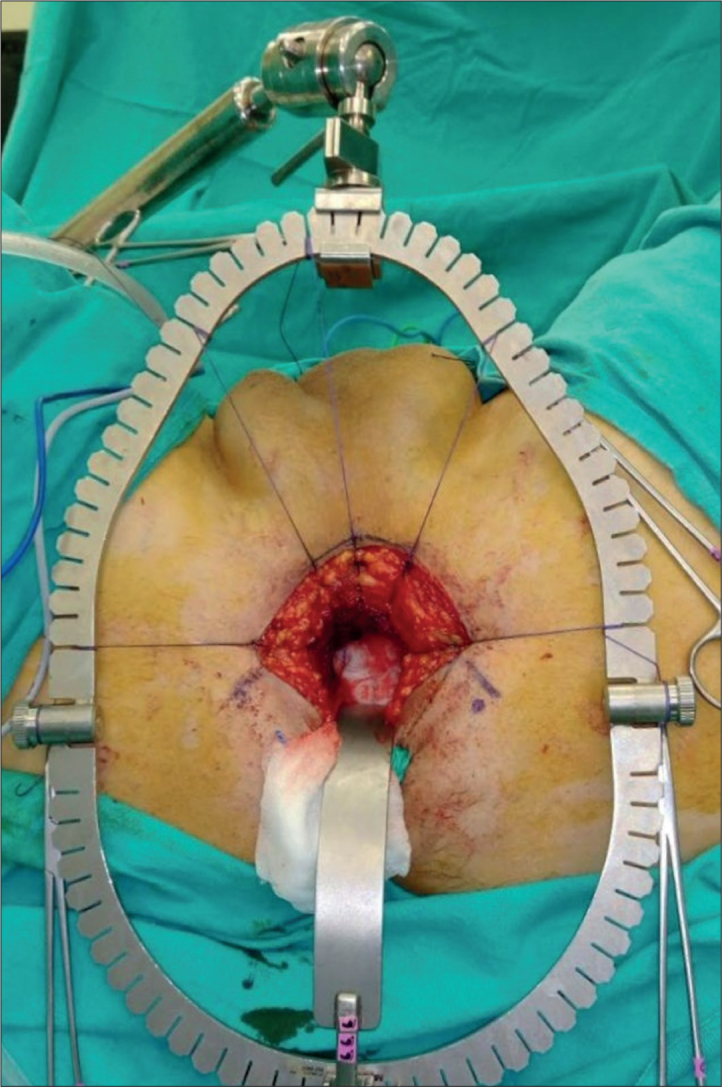
Placement of the inferior retractor and hanging of the perineal tissues on the retractor ring using sutures
After both of the lateral sides of the prostate were dissected, Denonvilliers’ fascia was opened. Then, the seminal vesicles and vas deferens were identified bilaterally. Posterolateral pedicles were dissected by preserving the neurovascular bundle. The membranous urethra was cut. A Foley catheter was clamped for the purpose of containing the fluid in the catheter balloon, after which it was cut. The anterior surface of the prostate was dissected by preserving the deep dorsal vein complex and the endopelvic fascia. Then, the bladder neck was identified and the prostatectomy was completed. A vesicourethral anastomosis was performed with two 3/0 V-Loc™ sutures (Covidien, Mansfield, MA, USA) (Figure 5). The adequacy of the anastomosis was checked by filling the bladder with 200 mL isotonic saline.
Figure 5.
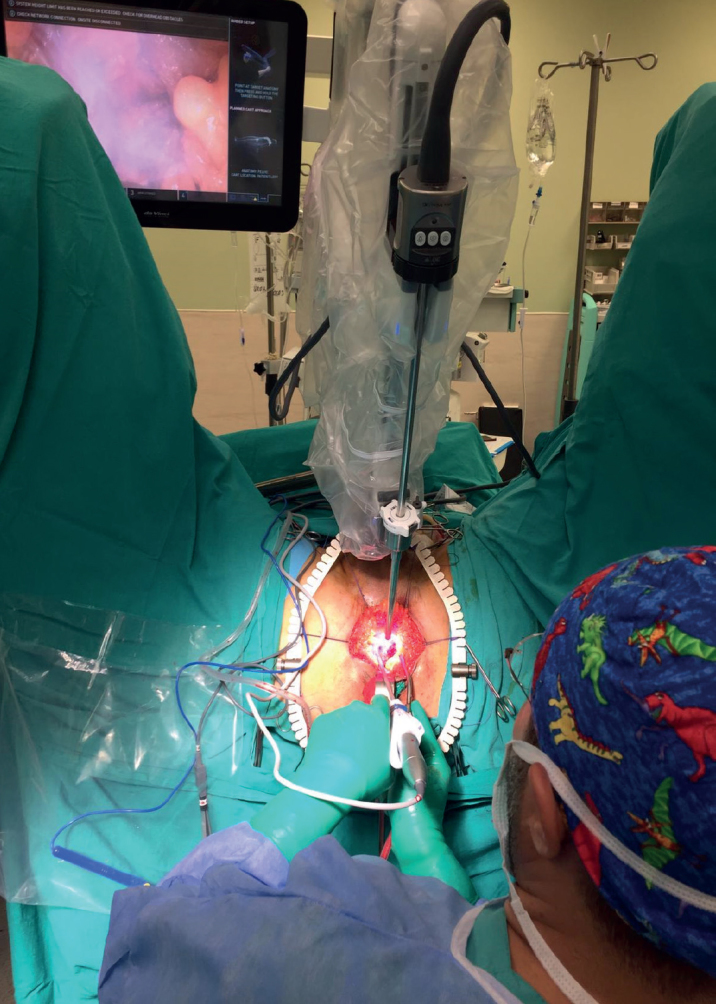
Perineoscopic radical prostatectomy being performed
Results
Perineoscopic-RP was completed successfully without the need to convert to other approaches and/or techniques in all the 3 patients. The mean age of the patients was 64±3.5 years. The mean body mass index was 28.4±2.9 kg/m2, mean PSA was 5±2.2 ng/mL, and mean PV was 38.3±7.6 cc. The mean time elapsed to reach the prostate apex was 50±3.6 minutes, including the time required to install the optic and retractor system. The mean total operative time was 144.3±8.4 minutes. The mean estimated blood loss was 111.7±22.5 cc. No intraoperative or postoperative complications were observed. The mean length of the hospital stay was 2.3±0.6 days and the mean catheter removal time was 9±1 days. The postoperative pathology reports of all 3 patients confirmed that the disease was organ-confined with negative surgical margins. The Gleason score was upgraded from 6 (3+3) to 7 (3+4) in the 3rd patient. All patients had undetectable levels of PSA in the 6th month during the follow-up period. The 2nd patient achieved complete continence immediately after the removal of the catheter. Complete continence was observed in all patients in the 3rd month during the follow-up period. The 1st and 2nd patients who experienced satisfactory penile erections in the preoperative period also achieved adequate erections in the postoperative 6th month by using a daily dose of 5 mg of tadalafil and 20 mg of the same drug on demand.
Discussion
Innovation in surgical techniques parallel to technological developments is an inevitable and positive phenomenon. PRP, which is the first and the oldest method of PCa surgery, presents similar oncologic outcomes as compared to the retropubic approach. Furthermore, it is associated with lower amounts of blood loss, a reduced need for blood transfusion, earlier recovery of intestinal functions, less postoperative pain, and shorter lengths of hospital stay.[8,9] However, in spite of all these advantages, PRP is performed at a limited number of centers because RRP is preferred more commonly by urologists. The reasons for this can be either the fact that many urologists are not familiar with the anatomy of the perineal region or that they face exposure-related problems and ergonomic challenges because of the deep and narrow features of the respective surgical area.[10] The use of robotic surgery in PRP is an exciting innovation for overcoming these difficulties. Moreover, PRP is a more cost-effective procedure as compared to RRP and RALRP and it is also associated with a lower cost among all RP techniques.[11] Our main goal for developing this technique was that patients and surgeons should be able to benefit from the advantages of optical magnification at a lower cost.
We believe that a superior anatomic perspective can be achieved with optical magnification. In particular, the neurovascular bundle can be preserved more effectively and bladder neck dissection and vesicourethral anastomosis can be performed more safely. It can also simplify the process of separating the deep dorsal vein complex and provide better preservation of small vessels.
In addition to being more convenient for the surgeon, this technique also affords convenience to the assistant surgeon and nurse. It is difficult for the assistant surgeon and nurse to clearly visualize the narrow and deep surgical site in PRP; only the primary surgeon is able to have a complete vision of the site. By means of this new perineoscopic-RP technique, the assistant surgeon and nurse may follow the surgical procedures clearly via the monitor and understand the process better. Therefore, the manipulations are made faster and easier, including retraction, aspiration, cutting, and clamping. Another advantage is that this system allows for recording the surgery, which is useful in creating an efficient training material for the interested surgeons and for providing feedback to the operating surgeon. Except for the optical magnification, perineoscopic-RP is exactly the same procedure as PRP and it contributes positively to the learning curve of PRP.
The use of an optic system to facilitate PRP has been reported previously.[12–15] Heaton performed a video-assisted perineal and open surgery in 1994. Over 50 perineal and open surgery techniques reportedly performed with a laparoscope. The use of the laparoscope similar to our technique was described, however, in this case, the assistant surgeon manually manipulated a non-stabilized optic system.[12] In our technique, the optic system was stabilized using the arm and camera of the robot and the manipulations were performed during the operation. At the same time, a co-assistant surgeon made optic manipulations from a console. When required, the system can be used as a 30° down scope or can be changed to a 0° scope. Endoscope-assisted techniques have also been defined, to easily perform dissections of the bladder neck and seminal vesicles at the most distal end of a perineal dissection.[13,14] Further, Saito and Murakami used a laparoscope for lymph node dissection after PRP.[15] We are of the opinion that lymph node dissection is a big handicap of PRP, but it can be overcome by including the optical magnification.
Based on our experience of these three cases, the main disadvantage of perineoscopic-RP, in our opinion, was that there was significant difficulty in achieving eye-hand coordination for the operator. However, after we experienced this setback in our first case, this error gradually decreased in the following cases. It is obvious that this limitation can be solved by gaining more experience with a corresponding increase in the patient number. We argue that successful results can be accomplished with better laparoscopic optic systems and 4K or 3D-imaging systems in the absence of robotic surgical systems in surgical centers. Furthermore, we believe that the perineoscopic approach can be performed successfully in other perineal reconstructive surgeries as well.
In conclusion, perineoscopic-RP is an appropriate and applicable method that combines the optical magnification advantage of r-PRP and the low-cost advantage of PRP. At the same time, we believe that perineoscopic-RP would contribute to improving the learning curve for surgery and help with advanced resident training. To be able to obtain robust and reliable information, larger prospective and comparative series are required, which are able to present long-term results of perineoscopic-RP.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Bakirkoy Dr. Sadi Konuk Training and Research Hospital.
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – A.İ.T.; Design – A.İ.T., A.Ş.; Supervision – A.İ.T., A.Ş., S.Ş.; Resources – A.Ş., E.Ş., K.G.Ş., F.A.A.; Materials – E.Ş., K.G.Ş., F.A.A.; Data Collection and/or Processing – E.Ş., K.G.Ş., S.Ş.; Analysis and/or Interpretation – A.İ.T., A.Ş., E.Ş., K.G.Ş., F.A.A., S.Ş.; Literature Search – A.Ş., E.Ş., K.G.Ş.; Writing Manuscript – A.Ş., E.Ş., K.G.Ş.; Critical Review – A.İ.T., A.Ş., E.Ş., K.G.Ş., F.A.A., S.Ş.; Other – A.Ş., E.Ş., K.G.Ş.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Song W, Park JH, Jeon HG, Jeong BC, Seo SI, Jeon SS, et al. Comparison of Oncologic Outcomes and Complications According to Surgical Approach to Radical Prostatectomy: Special Focus on the Perineal Approach. Clin Genitourin Cancer. 2017;15:e645–e52. doi: 10.1016/j.clgc.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 2.Lowrance WT, Eastham JA, Savage C, Maschino AC, Laudone VP, Dechet CB, et al. Contemporary open and robotic radical prostatectomy practice patterns among urologists in the United States. J Urol. 2012;187:2087–92. doi: 10.1016/j.juro.2012.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laydner H, Akça O, Autorino R, Eyraud R, Zargar H, Brandao LF, et al. Perineal robot-assisted laparoscopic radical prostatectomy: feasibility study in the cadaver model. J Endourol. 2014;28:1479–86. doi: 10.1089/end.2014.0244. [DOI] [PubMed] [Google Scholar]
- 4.Kaouk JH, Akca O, Zargar H, Caputo P, Ramirez D, Andrade H, et al. Descriptive Technique and Initial Results for Robotic Radical Perineal Prostatectomy. Urology. 2016;94:129–38. doi: 10.1016/j.urology.2016.02.063. [DOI] [PubMed] [Google Scholar]
- 5.Tuğcu V, Akça O, Şimşek A, Yiğitbaşı İ, Şahin S, Taşçı Aİ. Robot-assisted radical perineal prostatectomy: first experience of 15 cases. Turk J Urol. 2017;43:476–83. doi: 10.5152/tud.2017.35488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taşçı Aİ, Şimşek A, Şam E, Şeker KG, Atar FA, Şahin S, et al. Gasless robotic perineal radical prostatectomy: An initial experience. Turk J Urol. 2018;21:1–4. doi: 10.5152/tud.2018.48085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gandaglia G, Fossati N, Zaffuto E, Bandini M, Dell’Oglio P, Bravi CA, et al. Development and Internal Validation of a Novel Model to Identify the Candidates for Extended Pelvic Lymph Node Dissection in Prostate Cancer. Eur Urol. 2017;72:632–40. doi: 10.1016/j.eururo.2017.03.049. [DOI] [PubMed] [Google Scholar]
- 8.Salomon L, Levrel O, de la Taille A, Anastasiadis AG, Saint F, Zaki S, et al. Radical prostatectomy by the retropubic, perineal and laparoscopic approach: 12 years of experience in one center. Eur Urol. 2002;42:104–11. doi: 10.1016/S0302-2838(02)00263-4. [DOI] [PubMed] [Google Scholar]
- 9.Janoff DM, Parra RO. Contemporary appraisal of radical perineal prostatectomy. J Urol. 2005;173:1863–70. doi: 10.1097/01.ju.0000161483.65619.b3. [DOI] [PubMed] [Google Scholar]
- 10.Horuz R, Göktaş C, Çetinel CA, Akça O, Cangüven Ö, Şahin C, et al. Simple preoperative parameters to assess technical difficulty during a radical perineal prostatectomy. Int Urol Nephrol. 2013;45:129–33. doi: 10.1007/s11255-012-0310-1. [DOI] [PubMed] [Google Scholar]
- 11.Burgess SV, Atug F, Castle EP, Davis R, Thomas R. Cost analysis of radical retropubic, perineal, and robotic prostatectomy. J Endourol. 2006;20:827–30. doi: 10.1089/end.2006.20.827. [DOI] [PubMed] [Google Scholar]
- 12.Heaton JP. Video-assisted perineal and open surgery. J Urol. 1994;152:923. doi: 10.1016/S0022-5347(17)32611-3. [DOI] [PubMed] [Google Scholar]
- 13.Ellison LM, Pinto PA, Kavoussi LR. Radical endoscopic assisted perineal prostatectomy. J Urol. 2003;170:170–3. doi: 10.1097/01.ju.0000072245.68149.0d. [DOI] [PubMed] [Google Scholar]
- 14.Albayrak S, Canguven O, Aydemir H, Goktas C, Cetinel C, Akca O. Endoscope-assisted radical perineal prostatectomy. J Endourol. 2010;24:527–30. doi: 10.1089/end.2009.0398. [DOI] [PubMed] [Google Scholar]
- 15.Saito S, Murakami G. Radical perineal prostatectomy: a novel approach for lymphadenectomy from perineal incision. J Urol. 2003;170:1298–300. doi: 10.1097/01.ju.0000084329.75188.e6. [DOI] [PubMed] [Google Scholar]


