Abstract
The layered transition metal dichalcogenides (TMDs) and transition metal phosphides are low-cost, earth-abundant, and robust electrocatalysts for hydrogen evolution reaction (HER). Integrating them into hybrid nanostructures is potentially promising to further boost the catalytic activity toward HER based on their synergistic effects. Herein, we report a general method for the synthesis of a series of MoSe2-based hybrid nanostructures, including MoSe2-Ni2P, MoSe2-Co2P, MoSe2-Ni, MoSe2-Co, and MoSe2-NiS, by postgrowth of Ni2P, Co2P, Ni, Co, and NiS nanostructures on the presynthesized MoSe2 nanosheet-assembled nanospheres, respectively, via a colloidal synthesis method. As a proof-of-concept application, the as-synthesized hybrid nanostructures are used as electrocatalysts for HER, exhibiting high activity and stability in acidic media. Among them, the MoSe2-Co2P composite shows the highest HER activity with an overpotential of 167 mV at 10 mA cm−2.
1. Introduction
With increasing concerns on the global environmental contamination and energy shortage caused by the excessive consumption of fossil fuels, hydrogen as a promising chemical fuel has been considered as a clean and sustainable alternative [1–4]. However, the massive and sustainable hydrogen production from the electrocatalytic water splitting requires highly efficient and robust catalysts [5–9]. Although platinum (Pt) and other precious metals have shown superior catalytic performance in the hydrogen evolution reaction (HER) at low overpotentials in acidic media, their scarcity and high cost limit their practical applications [10–12]. Therefore, it is still a great challenge to find cost-effective and earth-abundant electrocatalysts with high HER activity, low overpotential, and excellent stability to replace the rare and expensive noble metal electrocatalysts.
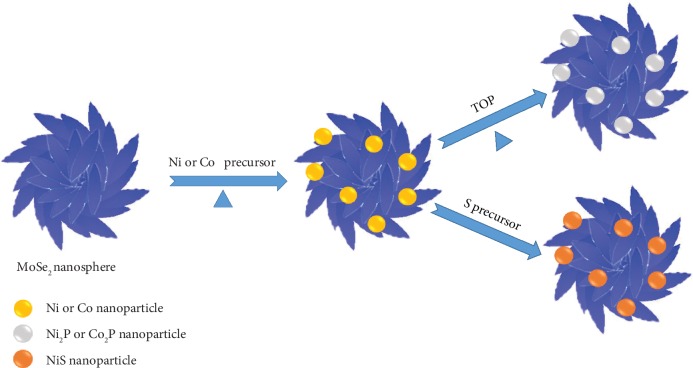
Among various non-noble-metal HER catalysts, transition metal semiconductor nanomaterials, e.g., dichalcogenides and phosphides, have been extensively studied [13–21], because of their low cost, high abundancy, and high HER catalytic activity [22–28]. Recently, theoretical calculations and experimental studies on MoS2 nanosheets suggested that the exposed edge is one of the catalytically active sites for hydrogen evolution [29, 30], which inspired researchers to prepare edge-rich MoS2 nanostructures to enhance the HER performance [31–34]. Furthermore, based on the density functional theory, Tsai et al. found that the HER performance of MoSe2 can be comparable to or even better than that of MoS2 [35]. However, there are only a few reports on designing the catalytically active MoSe2 nanostructures for HER [36–41]. Besides transition metal dichalcogenides (TMDs), the emerging transition metal phosphides have also drawn extensive attention as effective HER catalysts due to their good durability, corrosion resistance, and high current density at low overpotential [42–50]. Among them, the Co- and Ni-based phosphide nanostructures have exhibited their great potential as the electrocatalysts for HER [26, 51–54]. Therefore, it is very important to design and synthesize MoSe2-based hybrid HER electrocatalysts by combining the advantages of both MoSe2 and transition metal phosphides. Herein, we report a general colloidal method for the synthesis of a series of hybrid nanostructures using the MoSe2 nanosheet-assembled nanospheres as templates, including MoSe2-Ni2P, MoSe2-Co2P, MoSe2-Ni, MoSe2-Co, and MoSe2-NiS (Scheme 1). First, MoSe2 nanospheres were prepared by a hot-injection method. Then, the obtained MoSe2 nanospheres mixed with transition metal cations, oleylamine, and trioctylphosphine in a three-necked flask. Because the surface potential of the freshly prepared MoSe2 nanospheres is negative, the transition metal cations in the solution can be easily adsorbed on the surface of MoSe2 nanosheets via the electrostatic interaction. As shown in Scheme 1, when the reaction temperature was increased to about 220°C, Ni or Co nanoparticles were formed. If the reaction temperature was further increased to 320°C, the trioctylphosphine would react with transition metals to form the transition metal phosphides on MoSe2 nanospheres. However, if the reaction temperature was 220°C, after addition of the S precursor, the transition metal sulfide, such as NiS, nanoparticles were formed on MoSe2 nanospheres. As a proof-of-concept application, the as-prepared hybrid nanostructures exhibit high electrocatalytic HER activity and stability in acidic media.
Scheme 1.
Schematic illustration of synthesis of the MoSe2 nanosphere-based hybrid nanostructures.
2. Results
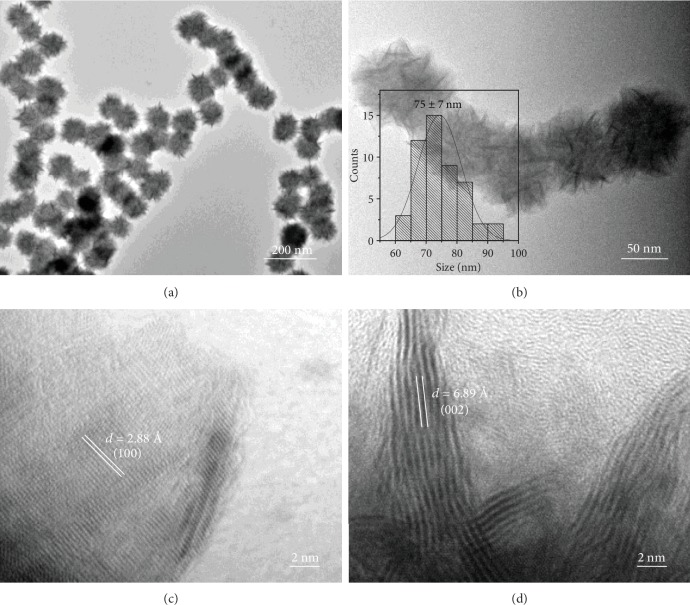
Briefly, the colloidal MoSe2 nanosheet-assembled nanospheres were prepared by injection of the selenium-octadecene precursor into a mixture of octadecene and stearic acid containing MoCl5 at 300°C, which was kept for 30 min (see Materials and Methods for details). The morphologies of the obtained colloidal MoSe2 nanospheres were characterized by a transmission electron microscope (TEM). As shown in Figures 1(a) and 1(b), the prepared MoSe2 nanospheres with size of 75 ± 7 nm (inset in Figure 1(b)) were formed by the assembly of MoSe2 nanosheets. High-resolution TEM (HRTEM) image of a typical edge of MoSe2 nanosheet confirms its single-crystalline nature (Figure 1(c)). The lattice distances of 2.88 Å and 6.89 Å can be assigned to the (100) and (002) planes of 2H phase MoSe2 [37], respectively (Figures 1(c) and 1(d)). The powder X-ray diffraction (XRD) pattern () further confirms that the nanosphere consists of crystalline 2H phase MoSe2 (JCPDS No. 15-0029, hexagonal, a = 3.288 Å, c = 12.89 Å). As shown in , the peaks located at 31.69°, 37.38°, and 56.10° correspond to the (100), (103), and (110) planes of the hexagonal MoSe2, respectively.
Figure 1.
TEM and HRTEM measurements of the as-prepared MoSe2 nanospheres. (a, b) TEM images and (c, d) HRTEM images. Inset in (b): statistical analysis of the size of 50 MoSe2 nanospheres measured from TEM images.
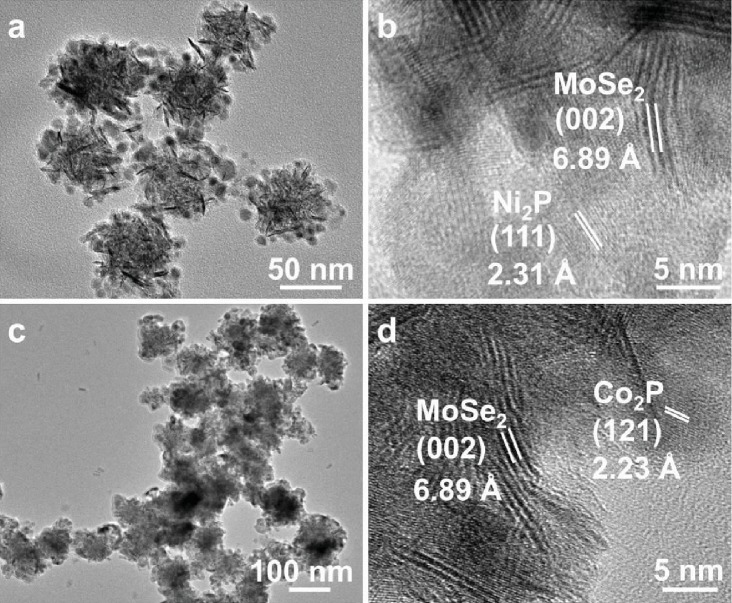
The as-prepared MoSe2 nanosheet-assembled nanospheres were then used as templates for the growth of hybrid nanostructures. For example, Ni2P nanoparticles have been grown on the surface of MoSe2 nanospheres to form the MoSe2-Ni2P hybrid nanostructures (see Materials and Methods for details). From the TEM image (Figure 2(a)), it can be seen that the Ni2P nanoparticles are coated on the surface of MoSe2 nanospheres. Based on the XRD analysis (), the as-obtained hybrid nanostructure is composed of crystalline 2H phase of MoSe2 (JCPDS No. 15-0029) and hexagonal phase of Ni2P (JCPDS No. 65-1989, a = 5.859 Å, c = 3.382 Å). The HRTEM image (Figure 2(b)) further confirms that the Ni2P nanoparticles are highly crystalline with a lattice spacing of 2.31 Å attributing to the (111) planes of the hexagonal phase Ni2P. The measured lattice distance of 6.89 Å corresponds to the (002) plane of 2H phase MoSe2. The size of Ni2P nanoparticles in the hybrid nanostructures is 8.7 ± 1.3 nm (). The energy-dispersive X-ray spectroscopy (EDS) spectrum () shows that the estimated molar ratios of Mo/Se and Ni/P are 1/2.2 and 1.6/1, respectively, close to the calculated stoichiometric ratios. The corresponding EDS elemental mapping () confirms the homogeneous distribution of Mo, Se, Ni, and P, further revealing the successful growth of Ni2P on the surface of MoSe2 nanospheres.
Figure 2.
TEM and HRTEM measurements of the MoSe2-Ni2P and MoSe2-Co2P hybrid nanostructures. (a) TEM and (b) HRTEM images of MoSe2-Ni2P hybrid nanostructures. (c) TEM and (d) HRTEM images of MoSe2-Co2P hybrid nanostructures.
Importantly, our method is general, which can be used to grow other nanostructures on MoSe2 nanospheres. For example, Co2P nanoparticles can also be grown on the surface of MoSe2 nanospheres to form MoSe2-Co2P hybrid nanostructures (Figure 2(c)). The measured lattice fringes of 6.89 Å and 2.23 Å match well with the (002) planes of MoSe2 and (121) planes of Co2P, respectively. The size of Co2P nanoparticles in the hybrid nanostructures is 7.5 ± 1.4 nm (). The XRD analysis () demonstrates the coexistence of MoSe2 (JCPDS No. 15-0029) and Co2P (JCPDS No. 32-0306, orthorhombic, a = 5.6465 Å, b = 6.6099 Å, c = 3.513 Å). The molar ratios of Mo/Se and Co/P are 1/1.5 and 2.2/1, respectively, as characterized by EDS (). The presence of Mo, Se, Co, and P and their homogeneous distributions can be clearly observed in the EDS elemental mapping of MoSe2-Co2P hybrid nanostructures ().
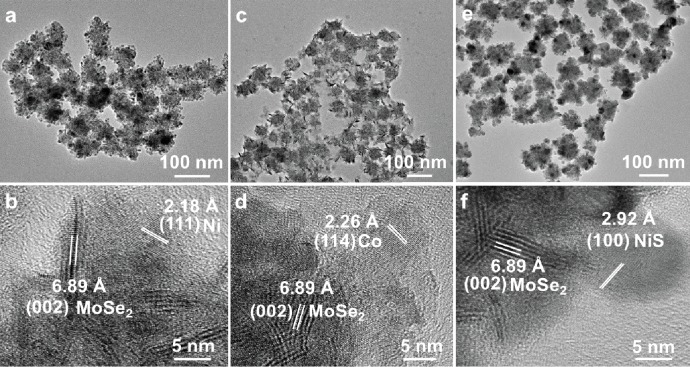
Besides Ni2P and Co2P nanoparticles, metallic Ni and Co nanoparticles have also been successfully grown on the surface of MoSe2 nanospheres (see Materials and Methods for details). Figure 3(a) shows that the Ni nanoparticles have been grown on the surface of MoSe2 nanospheres to form the MoSe2-Ni hybrid nanostructures. The size of Ni nanoparticles in the hybrid nanostructures is 7.4 ± 1.5 nm (). In the HRTEM image (Figure 3(b)), the measured interplanar distances between lattice fringes are estimated to be 6.89 Å and 2.18 Å, which match well with the (002) planes of MoSe2 and (111) planes of Ni, respectively. The cubic Ni (JCPDS No. 01-1258, a = 3.54 Å) was confirmed by the XRD pattern (). confirms the magnetic property of Ni nanoparticles in the prepared MoSe2-Ni hybrid nanostructures. The EDS () and corresponding elemental mapping () demonstrate the presence of Mo, Se, and Ni and their homogeneous distribution. Using the similar method, the MoSe2-Co hybrid nanostructure can also be prepared (Figure 3(c)). The corresponding HRTEM image reveals that the observed lattice spacing for MoSe2 (002) planes and Co (114) planes are 6.89 Å and 2.26 Å, respectively. The size of Co nanoparticles in the hybrid nanostructures is 6.4 ± 1.4 nm (). The XRD pattern identifies that the MoSe2 (JCPDS No. 15-0029) and hexagonal phase Co (JCPDS No. 65-9722, a = 8.288 Å, c = 10.542 Å) coexist (). confirms the magnetic property of Co nanoparticles in the prepared MoSe2-Co hybrid nanostructures. Furthermore, the presence of Mo, Se, and Co and their homogeneous distributions have been shown in the EDS spectrum () and corresponding elemental mapping ().
Figure 3.
TEM and HRTEM measurements of the MoSe2-Ni, MoSe2-Co, and MoSe2-NiS hybrid nanostructures. (a) TEM and (b) HRTEM images of the MoSe2-Ni hybrid nanostructures. (c) TEM and (d) HRTEM images of the MoSe2-Co hybrid nanostructures. (e) TEM and (f) HRTEM images of the MoSe2-NiS hybrid nanostructures.
Moreover, after the sulfurization of the MoSe2-Ni hybrid nanostructure, the MoSe2-NiS hybrid nanostructure can also be synthesized by adding sulfur precursor to the reaction solution (see Materials and Methods for details). The as-synthesized nanostructure was characterized by XRD (). The XRD analysis of the final product reveals a mixture of hexagonal MoSe2 (JCPDS No. 15-0029) and hexagonal NiS (JCPDS No. 02-1273, a = 3.440 Å, c = 5.350 Å). The TEM image (Figure 3(e)) shows that the NiS nanoparticles are uniformly coated on the surface of the MoSe2 nanospheres. The size of NiS nanoparticles in the hybrid nanostructures is 11.7 ± 1.4 nm (). As shown in the corresponding HRTEM image, the lattice spacing of 6.89 Å corresponds to the (002) planes of MoSe2, while the other one of 2.92 Å is attributed to the (100) planes of NiS (Figure 3(f)). The EDS spectrum in shows that the Mo/Se and Ni/S molar ratios are estimated to 1/2.4 and 1/1, respectively, close to the corresponding stoichiometric ratios. The EDS elemental mapping () further demonstrates the homogeneous distributions of Mo, Se, Ni, and S in the as-prepared nanostructure.
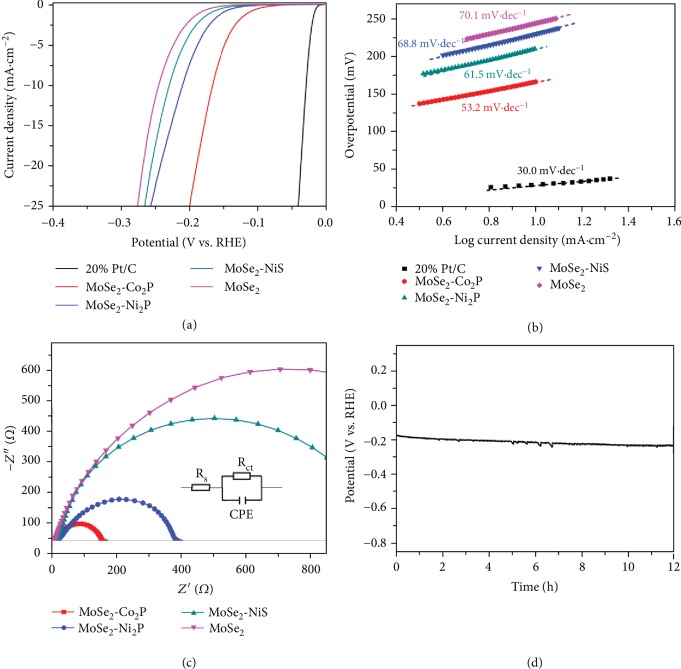
As a proof-of-concept application, the aforementioned synthesized hybrid nanostructures, including MoSe2-Ni2P, MoSe2-Co2P, and MoSe2-NiS, were used as catalysts for the electrochemical HER. The electrochemical HER activities were tested in 0.5 M H2SO4 aqueous solution using a standard three-electrode system. Figure 4(a) shows typical polarization curves of these as-prepared hybrid nanostructures and the 20 wt% Pt/C. The MoSe2 nanosphere shows the lowest HER activity with overpotential of 245 mV at 10 mA cm−2. After growth of NiS, Ni2P, and Co2P on the MoSe2 nanospheres, the catalytic activities of hybrid nanostructures were significantly enhanced. The overpotentials at 10 mA cm−2 are 231, 211, and 167 mV for the MoSe2-NiS, MoSe2-Ni2P, and MoSe2-Co2P hybrid nanostructures, respectively. Furthermore, the Tafel slopes were used to evaluate the HER kinetics. As shown in Figure 4(b), the measured Tafel slope of Pt/C is 30.0 mV dec−1, close to the reported value [10–12]. The Tafel slope of MoSe2 nanospheres is 70.1 mV/dec, which decreases to 68.8, 61.5, and 53.2 mV/dec for MoSe2-NiS, MoSe2-Ni2P, and MoSe2-Co2P hybrid nanostructures, respectively. In addition, as shown in Figure 4(c), the electrochemical impedance spectroscopy (EIS) reveals that these hybrid nanostructures exhibit a faster electron/charge transfer rate than does the MoSe2 nanosphere (), suggesting that the growth of NiS, Ni2P, and Co2P on MoSe2 nanospheres can significantly enhance the electrical conductivity. The good electron-transfer kinetics is important for electrocatalysts to exhibit high activity [55–57]. Note that the performance of MoSe2-Co2P is comparable to or even better than those previously reported similar materials for the acid HER ().
Figure 4.
HER electrochemical performances of the hybrid nanostructures. (a) Polarization curves of the 20 wt% Pt/C, MoSe2-Co2P, MoSe2-Ni2P, and MoSe2-NiS hybrid nanostructures, and MoSe2 nanosphere with iR correction. (b) The corresponding Tafel plots. (c) Nyquist plots of MoSe2-Co2P, MoSe2-Ni2P, and MoSe2-NiS hybrid nanostructures and MoSe2 nanosphere collected at a bias voltage of -150 mV. Inset: the equivalent circuit used for fitting the Nyquist plots. (d) Chronopotentiometry response for MoSe2-Co2P hybrid nanostructures at 10 mA cm−2 for 12 h.
It is important to understand the mechanism for the improved HER performance of MoSe2-Co2P hybrid nanostructures. First, the more postgrowth nanostructures mean the more exposure of active sites, enabling a high utilization ratio of catalysts [58–61]. The effect of the surface area on HER was evaluated through the electrochemical double-layer capacitance (Cdl, ). The fitted Cdl of MoSe2-Co2P is close to that of MoSe2, revealing that the promoted HER performance should be derived from the enhanced intrinsic activity rather than the increased electrochemical active specific area or active sites. On the other hand, the Rct value of the MoSe2-Co2P hybrid nanostructures is smaller than the pure MoSe2 nanospheres (), indicating the fastest charge transfer process. These results clearly show the crucial role of the synergetic effect between MoSe2 and Co2P in MoSe2-Co2P hybrid nanostructures, which is responsible for the enhanced electrochemical hydrogen evolution.
The stability of the MoSe2-Co2P hybrid nanostructures for HER catalysis was tested using the chronopotentiometry method. As shown in Figure 4(d), only a slight voltage drop is observed even after 12 h electrolysis of water at current density of 10 mA cm−2, indicating the excellent stability of the MoSe2-Co2P hybrid nanostructures. Moreover, after the stability test, the polarization curve only shows a slight negative shift () as compared with the initial one. These results imply the long-term stability of MoSe2-Co2P catalyst for HER.
3. Discussion
In summary, by using the synthesized MoSe2 nanospheres as templates, nickel and cobalt-based nanomaterials can be synthesized on them to form a series of the MoSe2 nanosphere-based hybrid nanostructures, including MoSe2-Ni2P, MoSe2-Co2P, MoSe2-Ni, MoSe2-Co, and MoSe2-NiS. Importantly, as a proof-of-concept application, when used as electrocatalysts, the MoSe2-Ni2P, MoSe2-Co2P, and MoSe2-NiS hybrid nanostructures exhibited high HER activities in acidic environment. Among them, the MoSe2-Co2P hybrid nanostructures exhibit excellent stability and highest HER activity with an overpotential of 167 mV at 10 mA cm−2. We believe that as a general method for hybridizing layered TMD nanostructures with transition metal chalcogenide/phosphide nanocrystals, this strategy is applicable for growth of many other nanomaterials to form hybrid nanostructures with enhanced electrochemical activity and stability.
4. Materials and Methods
4.1. Chemicals
Molybdenum chloride (MoCl5) was purchased from Alfa Aesar. Sulfur, selenium, nickel acetylacetonate (Ni(acac)2), cobalt acetylacetonate (Co(acac)2), trioctylphosphine (TOP), octadecene (ODE), stearic acid (SA), oleylamine (OLA), and toluene were purchased from Aldrich. All the chemicals were used as received without further purification.
4.2. Synthesis of MoSe2 Nanosheet-Assembled Nanospheres
In a typical procedure, 0.5 mmol of MoCl5, 1 g of SA, and 9 mL of ODE were added into a 100 mL three-necked flask. The aforementioned mixture, denoted as solution A, was degassed under a vacuum at 120°C for 10 min. Then, the temperature was heated up to 300°C under nitrogen. At the same time, 1 mmol of selenium powder was dissolved into 2 mL of ODE at 250°C, which was denoted as solution B. Solution B was cooled to 130°C and subsequently injected into solution A when the temperature of mixed solution finally reached 300°C, which was then kept at 300°C for 30 min. After it was cooled down to room temperature, the product, i.e., MoSe2 nanospheres, was collected by centrifugation (10000 rpm, 5 min) and washed several times with toluene and acetone (technical grade) before further characterization. All products in this work were collected and washed by the same method.
4.3. Synthesis of MoSe2-Ni2P, MoSe2-Co2P, MoSe2-Ni, MoSe2-Co, and MoSe2-NiS Hybrid Nanostructures
In order to synthesize the MoSe2-Ni2P hybrid nanostructures, the freshly prepared MoSe2 nanospheres were redispersed into 10 mL of OLA containing 0.5 mmol Ni(acac)2 and 2 mL TOP. The mixture was degassed under a vacuum at 110°C for 10 min. Then, the temperature was increased to 320°C under nitrogen atmosphere and kept for 1 h before cooling down to room temperature. The MoSe2-Co2P hybrid nanostructures were synthesized by the same method, except that Co(acac)2 was used instead of Ni(acac)2. The preparation procedure of MoSe2-Ni and MoSe2-Co hybrid nanostructures was the same as the aforementioned procedure except that the reaction temperature was changed to 220°C from 320°C. For the synthesis of MoSe2-NiS hybrid nanostructures, the procedure was the same as that used for synthesis of MoSe2-Ni hybrid nanostructures, except that after the reaction was proceeded at 220°C for 1 h, 2 mL of sulfur powder (0.5 mmol) in oleylamine (OLA) was injected. The resulting mixed solution was kept at 220°C for another 1 h.
4.4. Characterization
The XRD were recorded on a Bruker D8 diffractometer (German) with a slit of 1/2° at a scanning rate of 2° min−1, using Cu Kα radiation (λ = 1.5406 Å). TEM, HRTEM, and EDS mapping characterizations were performed on JEOL 2010F (Japan) and JEOL 2100F (Japan) with an acceleration voltage of 200 kV.
4.5. Electrode Preparation
Experimentally, 4 mg of acetylene black was first mixed with 5 mL of hexane and then sonicated to form a uniform suspension. Then, 8 mg of MoSe2, MoSe2-Ni2P, MoSe2-Co2P, or MoSe2-NiS in hexane was added into acetylene black suspension under sonication for 1 h. The mixed catalysts were separated by centrifugation, then washed with ethanol. The prepared catalysts were annealed at 400°C for 30 min under a flow 5% H2/Ar to remove surfactants. 4 mg of the respective catalyst powders was dispersed in 1 mL of 1 : 1 (v/v) water/ethanol mixed solvents under ultrasonication for 1 h. 5 μL of the resulting solution was dropped onto the surface of a cleaned glassy carbon electrode by a microliter syringe and dried at room temperature. After drying at room temperature, the surface of the catalyst-based electrode was covered by 5 μL of 1% Nafion solution.
4.6. Electrochemical Measurements
All the electrochemical experiments were performed on an electrochemical workstation (CHI 760C, CH Instruments Inc., USA), using a conventional three-electrode system, i.e., a Hg/Hg2Cl2 (saturated KCl, SCE) reference electrode, a carbon rod counter electrode, and the prepared working electrode. 0.5 M H2SO4 aqueous solution was used as the electrolyte throughout the experiments. Before the electrochemical measurement, the electrolyte was degassed by bubbling N2 for at least 30 min. The polarization curves were obtained by sweeping the potential from 0 to -0.6 V (vs. SCE) at a sweep rate of 2 mV s−1. Electrochemical impedance spectroscopy (EIS) was recorded in 0.5 M H2SO4 aqueous solution using an alternating current (AC) voltage of 5 mV and direct current (DC) voltage of -0.15 V (vs. RHE) within the frequency range from 100 kHz to 0.1 Hz. Voltage-time responses were monitored by chronopotentiometry measurement at 10 mA cm−2 for 12 h.
Acknowledgments
This work was supported by MOE under AcRF Tier 2 (ARC 19/15; Nos. MOE2014-T2-2-093, MOE2015-T2-2-057, MOE2016-T2-2-103, and MOE2017-T2-1-162) and AcRF Tier 1 (2016-T1-001-147, 2016-T1-002-051, 2017-T1-001-150, and 2017-T1-002-119) and Nanyang Technological University under the Start-Up Grant (M4081296.070.500000) in Singapore. S.H. thanks the support from the Fundamental Research Funds for the Central Universities (No. PA2018GDQT0013) in China. We would like to acknowledge the Facility for Analysis, Characterization, Testing and Simulation, Nanyang Technological University, Singapore, for use of their electron microscopy (and/or X-ray) facilities. H.Z. thanks the support from the Innovation and Technology Commission via the Hong Kong Branch of National Precious Metals Material Engineering Research Center and the Start-Up Grant from the City University of Hong Kong.
Conflicts of Interest
The authors declare no competing financial interests.
Authors' Contributions
H. Zhang proposed the research direction and guided the project. S. Han synthesized and characterized the materials. K. Zhou measured the electrochemical performance of the materials. S. Han, K. Zhou, Y. Yu, C. Tan, J. Chen, Y. Huang, Q. Ma, Y. Chen, H. Cheng, W. Zhou, and H. Zhang analyzed and discussed the experimental results and drafted the manuscript. Shikui Han and Kai Zhou contributed equally to this work.
Supplementary Materials
Figure S1: XRD pattern of the as-prepared MoSe2 nanosheet-assembled nanospheres. Figure S2: XRD pattern of the as-prepared MoSe2-Ni2P nanostructures. Figure S3: statistical analysis of the size of 50 Ni2P nanoparticles measured from HRTEM images. Figure S4: EDS spectrum of the as-prepared MoSe2-Ni2P nanostructures. Figure S5: EDS mapping of the as-prepared MoSe2-Ni2P hybrid nanostructures. Figure S6: statistical analysis of the size of 50 Co2P nanoparticles measured from HRTEM images. Figure S7: XRD pattern of the as-prepared MoSe2-Co2P hybrid nanostructures. Figure S8: EDS spectrum of the as-prepared MoSe2-Co2P hybrid nanostructures. Figure S9: EDS elemental mapping of the as-prepared MoSe2-Co2P hybrid nanostructures. Figure S10: statistical analysis of the size of 50 Ni nanoparticles measured from HRTEM images. Figure S11: (a) XRD pattern of the as-prepared MoSe2-Ni hybrid nanostructures. (b) Top: photograph of MoSe2-Ni in toluene. Bottom: photograph showing the magnetic property of MoSe2-Ni in toluene in the presence of a magnet. Figure S12: EDS spectrum of the as-prepared MoSe2-Ni hybrid nanostructures. Figure S13: EDS elemental mapping of the as-prepared MoSe2-Ni hybrid nanostructures. Figure S14: statistical analysis of the size of 50 Co nanoparticles measured from HRTEM images. Figure S15: (a) XRD pattern of the as-prepared MoSe2-Co hybrid nanostructures. (b) Top: photograph of MoSe2-Co in toluene. Bottom: photograph showing the magnetic property of MoSe2-Co in toluene in the presence of a magnet. Figure S16: EDS spectrum of the as-prepared MoSe2-Co hybrid nanostructures. Figure S17: EDS elemental mapping of the as-prepared MoSe2-Co hybrid nanostructures. Figure S18: XRD pattern of the as-prepared MoSe2-NiS hybrid nanostructures. Figure S19: statistical analysis of the size of 50 NiS nanoparticles measured from HRTEM images. Figure S20: EDS spectrum of the as-prepared MoSe2-NiS hybrid nanostructures. Figure S21: EDS elemental mapping of the as-prepared MoSe2-NiS hybrid nanostructures. Figure S22: cyclic voltammetry curves of (a) MoSe2-Co2P hybrid nanostructures and (b) MoSe2 nanosphere in the region of 0.15-0.25 V vs. RHE. (c) The differences in current density at 0.20 V vs. RHE plotted against scan rate fits to a linear regression. Figure S23: polarization curves of MoSe2-Co2P hybrid nanostructures before and after 12 h-HER tests at 10 mA cm−2. Table S1: the charge transfer resistances (Rct) and constant phase elements (CPEs) of the prepared electrocatalysts. Table S2: HER activities of the MoSe2-Co2P hybrid nanostructures and the reported electrocatalysts.
References
- 1.Caban-Acevedo M., Stone M. L., Schmidt J. R., et al. Efficient hydrogen evolution catalysis using ternary pyrite-type cobalt phosphosulphide. Nature Materials. 2015;14(12):1245–1251. doi: 10.1038/nmat4410. [DOI] [PubMed] [Google Scholar]
- 2.Xie J. F., Zhang J. J., Li S., et al. Controllable disorder engineering in oxygen-incorporated MoS2 ultrathin nanosheets for efficient hydrogen evolution. Journal of the American Chemical Society. 2013;135(47):17881–17888. doi: 10.1021/ja408329q. [DOI] [PubMed] [Google Scholar]
- 3.Glenk G., Reichelstein S. Economics of converting renewable power to hydrogen. Nature Energy. 2019;4(3):216–222. doi: 10.1038/s41560-019-0326-1. [DOI] [Google Scholar]
- 4.Upham D. C., Agarwal V., Khechfe A., et al. Catalytic molten metals for the direct conversion of methane to hydrogen and separable carbon. Science. 2017;358(6365):917–921. doi: 10.1126/science.aao5023. [DOI] [PubMed] [Google Scholar]
- 5.Greeley J., Jaramillo T. F., Bonde J., Chorkendorff I. B., Norskov J. K. Computational high-throughput screening of electrocatalytic materials for hydrogen evolution. Nature Materials. 2006;5(11):909–913. doi: 10.1038/nmat1752. [DOI] [PubMed] [Google Scholar]
- 6.Chhowalla M., Shin H. S., Eda G., Li L. J., Loh K. P., Zhang H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nature Chemistry. 2013;5(4):263–275. doi: 10.1038/nchem.1589. [DOI] [PubMed] [Google Scholar]
- 7.Zhang H. Ultrathin two-dimensional nanomaterials. ACS Nano. 2015;9(10):9451–9469. doi: 10.1021/acsnano.5b05040. [DOI] [PubMed] [Google Scholar]
- 8.Lu Q., Wang A.-L., Gong Y., et al. Crystal phase - based epitaxial growth of hybrid noble metal nanostructures on 4 h/Fcc Au nanowires. Nature Chemistry. 2018;10(4):456–461. doi: 10.1038/s41557-018-0012-0. [DOI] [PubMed] [Google Scholar]
- 9.Zhang X., Luo Z., Yu P., et al. Lithiation-induced amorphization of Pd3P2S8 for highly efficient hydrogen evolution. Nature Catalysis. 2018;1(6):460–468. doi: 10.1038/s41929-018-0072-y. [DOI] [Google Scholar]
- 10.Gao M. R., Liang J. X., Zheng Y. R., et al. An efficient molybdenum disulfide/cobalt diselenide hybrid catalyst for electrochemical hydrogen generation. Nature Communications. 2015;6(1, article 5982) doi: 10.1038/ncomms6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang J., Wang T., Pohl D., et al. Interface engineering of MoS2/Ni3S2 heterostructures for highly enhanced electrochemical overall-water-splitting activity. Angewandte Chemie International Edition. 2016;55(23):6702–6707. doi: 10.1002/anie.201602237. [DOI] [PubMed] [Google Scholar]
- 12.Ge X. B., Chen L. Y., Zhang L., Wen Y. R., Hirata A., Chen M. W. Nanoporous metal enhanced catalytic activities of amorphous molybdenum sulfide for high-efficiency hydrogen production. Advanced Materials. 2014;26(19):3100–3104. doi: 10.1002/adma.201305678. [DOI] [PubMed] [Google Scholar]
- 13.Tan C., Cao X., Wu X.-J., et al. Recent advances in ultrathin two-dimensional nanomaterials. Chemical Reviews. 2017;117(9):6225–6331. doi: 10.1021/acs.chemrev.6b00558. [DOI] [PubMed] [Google Scholar]
- 14.Chang K., Hai X., Pang H., et al. Targeted synthesis of 2H- and 1T-phase MoS2 monolayers for catalytic hydrogen evolution. Advanced Materials. 2016;28(45):10033–10041. doi: 10.1002/adma.201603765. [DOI] [PubMed] [Google Scholar]
- 15.Voiry D., Yang J., Chhowalla M. Recent strategies for improving the catalytic activity of 2D TMD nanosheets toward the hydrogen evolution reaction. Advanced Materials. 2016;28(29):6197–6206. doi: 10.1002/adma.201505597. [DOI] [PubMed] [Google Scholar]
- 16.Cheng L., Huang W., Gong Q., et al. Ultrathin WS2 nanoflakes as a high ‐ performance electrocatalyst for the hydrogen evolution reaction. Angewandte Chemie International Edition. 2014;53(30):7860–7863. doi: 10.1002/anie.201402315. [DOI] [PubMed] [Google Scholar]
- 17.Han S., Yang X., Zhu Y., et al. Synthesis of WOn-WX2 (n=2.7, 2.9; X=S, Se) heterostructures for highly efficient green quantum dot light-emitting diodes. Angewandte Chemie International Edition. 2017;56(35):10486–10490. doi: 10.1002/anie.201705617. [DOI] [PubMed] [Google Scholar]
- 18.Yu Y., Nam G.-H., He Q., et al. High phase-purity 1T′-MoS2 and 1T′-MoSe2 layered crystals. Nature Chemistry. 2018;10(6):638–643. doi: 10.1038/s41557-018-0035-6. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Z. W., Chen P., Duan X. D., Zang K. T., Luo J., Duan X. F. Robust epitaxial growth of two-dimensional heterostructures, multiheterostructures, and superlattices. Science. 2017;357(6353):788–792. doi: 10.1126/science.aan6814. [DOI] [PubMed] [Google Scholar]
- 20.Chen J., Wu X.-J., Gong Y., et al. Edge epitaxy of two-dimensional MoSe2 and MoS2 nanosheets on one-dimensional nanowires. Journal of the American Chemical Society. 2017;139(25):8653–8660. doi: 10.1021/jacs.7b03752. [DOI] [PubMed] [Google Scholar]
- 21.Lin H. F., Li Y. Y., Li H. Y., Wang X. Multi-node CdS hetero-nanowires grown with defect-rich oxygen-doped MoS2 ultrathin nanosheets for efficient visible-light photocatalytic H2 evolution. Nano Research. 2017;10(4):1377–1392. doi: 10.1007/s12274-017-1497-3. [DOI] [Google Scholar]
- 22.Huang Z., Luo W., Ma L., et al. Dimeric [Mo2S12]2- cluster: a molecular analogue of MoS2 edges for superior hydrogen-evolution electrocatalysis. Angewandte Chemie International Edition. 2015;54(50):15181–15185. doi: 10.1002/anie.201507529. [DOI] [PubMed] [Google Scholar]
- 23.Xu J., Cui J., Guo C., et al. Ultrasmall Cu7S4@MoS2 hetero-nanoframes with abundant active edge sites for ultrahigh-performance hydrogen evolution. Angewandte Chemie International Edition. 2016;55(22):6502–6505. doi: 10.1002/anie.201600686. [DOI] [PubMed] [Google Scholar]
- 24.Voiry D., Yamaguchi H., Li J., et al. Enhanced catalytic activity in strained chemically exfoliated WS2 nanosheets for hydrogen evolution. Nature Materials. 2013;12(9):850–855. doi: 10.1038/nmat3700. [DOI] [PubMed] [Google Scholar]
- 25.Liu W., Hu E., Jiang H., et al. A highly active and stable hydrogen evolution catalyst based on pyrite-structured cobalt phosphosulfide. Nature Communications. 2016;7, article 10771 doi: 10.1038/ncomms10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Popczun E. J., McKone J. R., Read C. G., et al. Nanostructured nickel phosphide as an electrocatalyst for the hydrogen evolution reaction. Journal of the American Chemical Society. 2013;135(25):9267–9270. doi: 10.1021/ja403440e. [DOI] [PubMed] [Google Scholar]
- 27.Liu Q., Tian J. Q., Cui W., et al. Carbon nanotubes decorated with CoP nanocrystals: a highly active non-noble-metal nanohybrid electrocatalyst for hydrogen evolution. Angewandte Chemie International Edition. 2014;53(26):6710–6714. doi: 10.1002/anie.201404161. [DOI] [PubMed] [Google Scholar]
- 28.Wang X. G., Kolen'ko Y. V., Bao X. Q., Kovnir K., Liu L. F. One-step synthesis of self-supported nickel phosphide nanosheet array cathodes for efficient electrocatalytic hydrogen generation. Angewandte Chemie International Edition. 2015;54(28):8188–8192. doi: 10.1002/anie.201502577. [DOI] [PubMed] [Google Scholar]
- 29.Kibsgaard J., Chen Z. B., Reinecke B. N., Jaramillo T. F. Engineering the surface structure of MoS2 to preferentially expose active edge sites for electrocatalysis. Nature Materials. 2012;11(11):963–969. doi: 10.1038/nmat3439. [DOI] [PubMed] [Google Scholar]
- 30.Li H., Tsai C., Koh A. L., et al. Activating and optimizing MoS2 basal planes for hydrogen evolution through the formation of strained sulphur vacancies. Nature Materials. 2016;15(1):48–53. doi: 10.1038/nmat4465. [DOI] [PubMed] [Google Scholar]
- 31.Li Y. G., Wang H. L., Xie L. M., Liang Y. Y., Hong G. S., Dai H. J. MoS2 nanoparticles grown on graphene: an advanced catalyst for the hydrogen evolution reaction. Journal of the American Chemical Society. 2011;133(19):7296–7299. doi: 10.1021/ja201269b. [DOI] [PubMed] [Google Scholar]
- 32.Benck J. D., Hellstern T. R., Kibsgaard J., Chakthranont P., Jaramillo T. F. Catalyzing the hydrogen evolution reaction (HER) with molybdenum sulfide nanomaterials. ACS Catalysis. 2014;4(11):3957–3971. doi: 10.1021/cs500923c. [DOI] [Google Scholar]
- 33.Yan Y., Xia B. Y., Xu Z. C., Wang X. Recent development of molybdenum sulfides as advanced electrocatalysts for hydrogen evolution reaction. ACS Catalysis. 2014;4(6):1693–1705. doi: 10.1021/cs500070x. [DOI] [Google Scholar]
- 34.Wang H. T., Lu Z. Y., Kong D. S., Sun J., Hymel T. M., Cui Y. Electrochemical tuning of MoS2 nanoparticles on three-dimensional substrate for efficient hydrogen evolution. ACS Nano. 2014;8(5):4940–4947. doi: 10.1021/nn500959v. [DOI] [PubMed] [Google Scholar]
- 35.Tsai C., Chan K. R., Abild-Pedersen F., Nørskov J. K. Active edge sites in MoSe2 and WSe2 catalysts for the hydrogen evolution reaction: a density functional study. Physical Chemistry Chemical Physics. 2014;16(26):13156–13164. doi: 10.1039/c4cp01237b. [DOI] [PubMed] [Google Scholar]
- 36.Zhou X. L., Liu Y., Ju H. X., et al. Design and epitaxial growth of MoSe2-NiSe vertical heteronanostructures with electronic modulation for enhanced hydrogen evolution reaction. Chemistry of Materials. 2016;28(6):1838–1846. doi: 10.1021/acs.chemmater.5b05006. [DOI] [Google Scholar]
- 37.Zhou X. L., Jiang J., Ding T., et al. Fast colloidal synthesis of scalable Mo-rich hierarchical ultrathin MoSe2-x nanosheets for high-performance hydrogen evolution. Nanoscale. 2014;6(19):11046–11051. doi: 10.1039/c4nr02716g. [DOI] [PubMed] [Google Scholar]
- 38.Saadi F. H., Carim A. I., Velazquez J. M., et al. Operando synthesis of macroporous molybdenum diselenide films for electrocatalysis of the hydrogen-evolution reaction. ACS Catalysis. 2014;4(9):2866–2873. doi: 10.1021/cs500412u. [DOI] [Google Scholar]
- 39.Yang Y., Wang S. T., Zhang J. C., Li H. Y., Tang Z. L., Wang X. Nanosheet-assembled MoSe2 and S-doped MoSe2-x nanostructures for superior lithium storage properties and hydrogen evolution reactions. Inorganic Chemistry Frontiers. 2015;2(10):931–937. doi: 10.1039/c5qi00126a. [DOI] [Google Scholar]
- 40.Gholamvand Z., McAteer D., Backes C., et al. Comparison of liquid exfoliated transition metal dichalcogenides reveals MoSe2 to be the most effective hydrogen evolution catalyst. Nanoscale. 2016;8(10):5737–5749. doi: 10.1039/c5nr08553e. [DOI] [PubMed] [Google Scholar]
- 41.Lei Z. Y., Xu S. J., Wu P. Y. Ultra-thin and porous MoSe2 nanosheets: facile preparation and enhanced electrocatalytic activity towards the hydrogen evolution reaction. Physical Chemistry Chemical Physics. 2016;18(1):70–74. doi: 10.1039/c5cp06483j. [DOI] [PubMed] [Google Scholar]
- 42.Kibsgaard J., Jaramillo T. F. Molybdenum phosphosulfide: an active, acid-stable, earth-abundant catalyst for the hydrogen evolution reaction. Angewandte Chemie International Edition. 2014;53(52):14433–14437. doi: 10.1002/anie.201408222. [DOI] [PubMed] [Google Scholar]
- 43.Jiang P., Liu Q., Liang Y. H., Tian J. Q., Asiri A. M., Sun X. P. A cost-effective 3d hydrogen evolution cathode with high catalytic activity: FeP nanowire array as the active phase. Angewandte Chemie-International Edition. 2014;53(47):12855–12859. doi: 10.1002/anie.201406848. [DOI] [PubMed] [Google Scholar]
- 44.McEnaney J. M., Crompton J. C., Callejas J. F., et al. Amorphous molybdenum phosphide nanoparticles for electrocatalytic hydrogen evolution. Chemistry of Materials. 2014;26(16):4826–4831. doi: 10.1021/cm502035s. [DOI] [Google Scholar]
- 45.McEnaney J. M., Crompton J. C., Callejas J. F., et al. Electrocatalytic hydrogen evolution using amorphous tungsten phosphide nanoparticles. Chemical Communications. 2014;50(75):11026–11028. doi: 10.1039/c4cc04709e. [DOI] [PubMed] [Google Scholar]
- 46.Xiao P., Chen W., Wang X. A review of phosphide-based materials for electrocatalytic hydrogen evolution. Advanced Energy Materials. 2015;5(24, article 1500985) doi: 10.1002/aenm.201500985. [DOI] [Google Scholar]
- 47.Ye R., del Angel-Vicente P., Liu Y., et al. High-performance hydrogen evolution from MoS2(1-x)Px solid solution. Advanced Materials. 2016;28(7):1427–1432. doi: 10.1002/adma.201504866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schipper D. E., Zhao Z., Thirumalai H., et al. Effects of catalyst phase on the hydrogen evolution reaction of water splitting: preparation of phase-pure films of FeP, Fe2P, and Fe3P and their relative catalytic activities. Chemistry of Materials. 2018;30(10):3588–3598. doi: 10.1021/acs.chemmater.8b01624. [DOI] [Google Scholar]
- 49.Wu Z. S., Huang L., Liu H., Wang H. L. Element-specific restructuring of anion- and cation-substituted cobalt phosphide nanoparticles under electrochemical water-splitting conditions. ACS Catalysis. 2019;9(4):2956–2961. doi: 10.1021/acscatal.8b03835. [DOI] [Google Scholar]
- 50.Yu F., Zhou H. Q., Huang Y. F., et al. High-performance bifunctional porous non-noble metal phosphide catalyst for overall water splitting. Nature Communications. 2018;9(1, article 2551) doi: 10.1038/s41467-018-04746-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tian J. Q., Liu Q., Asiri A. M., Sun X. P. Self-supported nanoporous cobalt phosphide nanowire arrays: an efficient 3D hydrogen-evolving cathode over the wide range of pH 0-14. Journal of the American Chemical Society. 2014;136(21):7587–7590. doi: 10.1021/ja503372r. [DOI] [PubMed] [Google Scholar]
- 52.Laursen A. B., Patraju K. R., Whitaker M. J., et al. Nanocrystalline Ni5P4: a hydrogen evolution electrocatalyst of exceptional efficiency in both alkaline and acidic media. Energy & Environmental Science. 2015;8(3):1027–1034. doi: 10.1039/c4ee02940b. [DOI] [Google Scholar]
- 53.Callejas J. F., Read C. G., Popczun E. J., McEnaney J. M., Schaak R. E. Nanostructured Co2P electrocatalyst for the hydrogen evolution reaction and direct comparison with morphologically equivalent CoP. Chemistry of Materials. 2015;27(10):3769–3774. doi: 10.1021/acs.chemmater.5b01284. [DOI] [Google Scholar]
- 54.Wang C. D., Jiang J., Ding T., Chen G. H., Xu W. J., Yang Q. Monodisperse ternary NiCoP nanostructures as a bifunctional electrocatalyst for both hydrogen and oxygen evolution reactions with excellent performance. Advanced Materials Interfaces. 2016;3(4, article 1500454) doi: 10.1002/admi.201500454. [DOI] [Google Scholar]
- 55.Wang J., Li K., Zhong H., et al. Synergistic effect between metal–nitrogen–carbon sheets and NiO nanoparticles for enhanced electrochemical water-oxidation performance. Angewandte Chemie International Edition. 2015;54(36):10530–10534. doi: 10.1002/anie.201504358. [DOI] [PubMed] [Google Scholar]
- 56.Wang J., Zhong H., Wang Z., Meng F., Zhang X. Integrated three-dimensional carbon paper/carbon tubes/cobalt-sulfide sheets as an efficient electrode for overall water splitting. ACS Nano. 2016;10(2):2342–2348. doi: 10.1021/acsnano.5b07126. [DOI] [PubMed] [Google Scholar]
- 57.Zhong H., Wang J., Meng F., Zhang X. In situ activating ubiquitous rust towards low-cost, efficient, free-standing, and recoverable oxygen evolution electrodes. Angewandte Chemie International Edition. 2016;55(34):9937–9941. doi: 10.1002/anie.201604040. [DOI] [PubMed] [Google Scholar]
- 58.Meng F., Wang Z., Zhong H., Wang J., Yan J., Zhang X. Reactive multifunctional template-induced preparation of Fe-N-doped mesoporous carbon microspheres towards highly efficient electrocatalysts for oxygen reduction. Advanced Materials. 2016;28(36):7948–7955. doi: 10.1002/adma.201602490. [DOI] [PubMed] [Google Scholar]
- 59.Wang Z., Hao X., Jiang Z., et al. C and N hybrid coordination derived Co–C–N complex as a highly efficient electrocatalyst for hydrogen evolution reaction. Journal of the American Chemical Society. 2015;137(48):15070–15073. doi: 10.1021/jacs.5b09021. [DOI] [PubMed] [Google Scholar]
- 60.Wang Z., Xu D., Zhong H., Wang J., Meng F., Zhang X. Gelatin-derived sustainable carbon-based functional materials for energy conversion and storage with controllability of structure and component. Science Advances. 2015;1(1, article e1400035) doi: 10.1126/sciadv.1400035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhong H., Li K., Zhang Q., et al. In situ anchoring of Co9S8 nanoparticles on N and S Co-doped porous carbon tube as bifunctional oxygen electrocatalysts. NPG Asia Materials. 2016;8(9, article e308) doi: 10.1038/am.2016.132. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: XRD pattern of the as-prepared MoSe2 nanosheet-assembled nanospheres. Figure S2: XRD pattern of the as-prepared MoSe2-Ni2P nanostructures. Figure S3: statistical analysis of the size of 50 Ni2P nanoparticles measured from HRTEM images. Figure S4: EDS spectrum of the as-prepared MoSe2-Ni2P nanostructures. Figure S5: EDS mapping of the as-prepared MoSe2-Ni2P hybrid nanostructures. Figure S6: statistical analysis of the size of 50 Co2P nanoparticles measured from HRTEM images. Figure S7: XRD pattern of the as-prepared MoSe2-Co2P hybrid nanostructures. Figure S8: EDS spectrum of the as-prepared MoSe2-Co2P hybrid nanostructures. Figure S9: EDS elemental mapping of the as-prepared MoSe2-Co2P hybrid nanostructures. Figure S10: statistical analysis of the size of 50 Ni nanoparticles measured from HRTEM images. Figure S11: (a) XRD pattern of the as-prepared MoSe2-Ni hybrid nanostructures. (b) Top: photograph of MoSe2-Ni in toluene. Bottom: photograph showing the magnetic property of MoSe2-Ni in toluene in the presence of a magnet. Figure S12: EDS spectrum of the as-prepared MoSe2-Ni hybrid nanostructures. Figure S13: EDS elemental mapping of the as-prepared MoSe2-Ni hybrid nanostructures. Figure S14: statistical analysis of the size of 50 Co nanoparticles measured from HRTEM images. Figure S15: (a) XRD pattern of the as-prepared MoSe2-Co hybrid nanostructures. (b) Top: photograph of MoSe2-Co in toluene. Bottom: photograph showing the magnetic property of MoSe2-Co in toluene in the presence of a magnet. Figure S16: EDS spectrum of the as-prepared MoSe2-Co hybrid nanostructures. Figure S17: EDS elemental mapping of the as-prepared MoSe2-Co hybrid nanostructures. Figure S18: XRD pattern of the as-prepared MoSe2-NiS hybrid nanostructures. Figure S19: statistical analysis of the size of 50 NiS nanoparticles measured from HRTEM images. Figure S20: EDS spectrum of the as-prepared MoSe2-NiS hybrid nanostructures. Figure S21: EDS elemental mapping of the as-prepared MoSe2-NiS hybrid nanostructures. Figure S22: cyclic voltammetry curves of (a) MoSe2-Co2P hybrid nanostructures and (b) MoSe2 nanosphere in the region of 0.15-0.25 V vs. RHE. (c) The differences in current density at 0.20 V vs. RHE plotted against scan rate fits to a linear regression. Figure S23: polarization curves of MoSe2-Co2P hybrid nanostructures before and after 12 h-HER tests at 10 mA cm−2. Table S1: the charge transfer resistances (Rct) and constant phase elements (CPEs) of the prepared electrocatalysts. Table S2: HER activities of the MoSe2-Co2P hybrid nanostructures and the reported electrocatalysts.