Abstract
Tin oxide (SnO2), as electron transport material to substitute titanium oxide (TiO2) in perovskite solar cells (PSCs), has aroused wide interests. However, the performance of the PSCs based on SnO2 is still hard to compete with the TiO2-based devices. Herein, a novel strategy is designed to enhance the photovoltaic performance and long-term stability of PSCs by integrating rare-earth ions Ln3+ (Sc3+, Y3+, La3+) with SnO2 nanospheres as mesoporous scaffold. The doping of Ln promotes the formation of dense and large-sized perovskite crystals, which facilitate interfacial contact of electron transport layer/perovskite layer and improve charge transport dynamics. Ln dopant optimizes the energy level of perovskite layer, reduces the charge transport resistance, and mitigates the trap state density. As a result, the optimized mesoporous PSC achieves a champion power conversion efficiency (PCE) of 20.63% without hysteresis, while the undoped PSC obtains an efficiency of 19.01%. The investigation demonstrates that the rare-earth doping is low-cost and effective method to improve the photovoltaic performance of SnO2-based PSCs.
1. Introduction
As a new generation of thin film photovoltaic technology, organometallic halide perovskite solar cells (PSCs) have been attracting considerable interest owing to their high efficiencies, minor environmental impact, and facile solution processability [1–4]. Since the birth of first prototype in 2009 [5], the power conversion efficiency (PCE) of PSCs has undergone rapid increment from 3.8% to 23.48% (certified) during the past several years [6]. This outstanding progress is attributed to the unremitting efforts of researchers on optimizing chemical composition of perovskite and deposition processes [7–10], as well as perovskite prominent optoelectronic properties, such as bandgap adjustability [11, 12] and long carrier lifetime [13–15]. Interestingly, the emergence of perovskite solar cells originated from dye-sensitized solar cells (DSSCs). In turn, the development of perovskite solar cells promoted the research of DSSCs, especially in polymer electrolytes and flexible devices [16–22].
Titanium dioxide (TiO2) is a frequently used electron transport material for perovskite solar cells. However, TiO2 shows a lower electron mobility of (0.1~1.0 cm2 V–1 s–1), compared with conventional perovskite material (20~30 cm2 V–1 s–1), which causes insufficient charge carrier separation at the TiO2/perovskite interface [23–25]. In addition, the UV instability of TiO2 upon UV exposure triggers a rapid decrease in performance of PSCs via the degradation of the organic components in the PSC (especially for mesoporous TiO2-based PSCs) [26, 27]. Furthermore, high temperature processing (HTP) for TiO2 electron transport layer (ETL) is also unfavorable for the fabrication of low-cost PSCs. To overcome these issues, various metal oxides (i.e., SnO2, ZnO, WO3, In2O3, and SrTiO3) and fullerene are investigated as the substitute electron transport materials for TiO2 [28–32]. Among them, SnO2 has emerged as an especially promising candidate, owing to its low temperature processability, high optical transmittance in visible range, high electron mobility (100~200 cm2 V–1 s–1), less sensitive to UV radiation, and favorable energy level alignment to perovskite absorbers [33]. However, solution-processed planar SnO2 films suffer from temperature-dependent nonideal electron mobility at low annealing temperature or crack defect morphology brought by high annealing temperature (i.e., 500°C), which both lead to inferior contact and electron transport at the ETL/perovskite interfaces and then manifested in J-V hysteresis [34–36]. Fortunately, a thin mesoporous ETL can be designed to ameliorate these issues by improving perovskite coverage condition to get large perovskite grains [37–39]. On this basis, mesoporous SnO2 (m-SnO2) ETL has been highlighted for the industrial production of stable and efficient SnO2-based PSCs [40]. The PSCs embedded with full SnO2 blocking layer (bl)/mesoporous (mp) layer have obtained inspiring advances [41]. Higher PCEs of 13.1% and 17% were obtained employing HTP m-SnO2 and gallium-doped m-SnO2 by Roose et al. [40, 42]. Afterwards, Liu et al. and Yang et al. reported the PSCs with LTP 2D SnO2 nanosheet arrays and yttrium-doped SnO2 nanosheet arrays; the PCEs increased to 16.17% and 17.29% [34, 43]. Recently, Xiong et al. reported a high-stabilized PCE of 19.12% on the planar Mg-SnO2 PSC by using HTP m-SnO2 scaffold [36]. More recently, low-temperature processed (LTP) planar SnO2 PSCs have been achieved a PCE of 21.4% by using tin oxide precursors based on acetylacetonate [44].
In this work, monodisperse mesoporous SnO2 nanospheres with large surface area are synthesized under 300°C posttreatment and are used as mesoporous scaffold in PSCs to improve photovoltaic performance of devices by modifying perovskite coverage condition with large perovskite grains without damaging the surface morphology of SnO2 blocking layer. Moreover, rare-earth cations located in the third subgroup (Sc3+, Y3+, La3+) are introduced into the SnO2 mesoporous scaffold to reduce trap state density and transport resistance, enhance charge carrier concentration, and regulate energy level of SnO2, resulting in enhancement of photovoltaic parameters of the device. By optimizing the amount of Ln3+ dopants, the 3%-SNOY device exhibits a hysteresis-free and high-stabilized power conversion efficiency of 20.63%, superior to those reported previously for full SnO2 mesoporous structure PSCs. Furthermore, the UV stability is also investigated to illustrate the excellent long-term stability of these full SnO2-based PSCs.
2. Results and Discussion
2.1. Structure and Morphologies
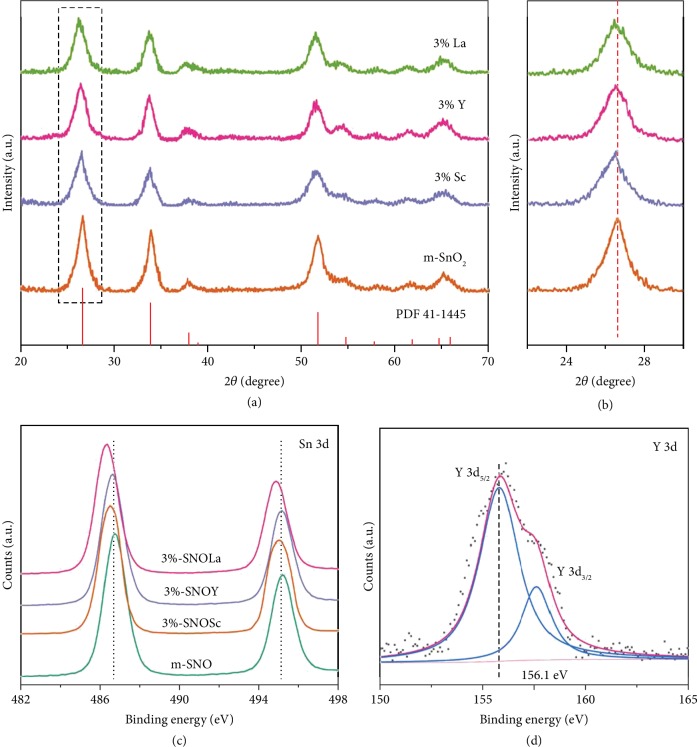
For research, SnO2 were synthesized with planar (p-SNO), mesoporous (m-SNO), and doped Ln in Ln3+/Sn4+ molar ratio (x%-SNOLn). Figure 1(a) shows the X-ray diffraction pattern (XRD) of m-SnO2 and 3% Ln3+ (Sc3+, Y3+, La3+)-doped m-SnO2. All peaks are readily indexed to the tetragonal rutile phase of SnO2 (JCPDS card No. 41-1445); no additional peaks are observed, indicating that Ln3+ (Sc3+, Y3+, La3+) dopants do not change the phase structure of SnO2. The range (2θ = 24~28°) is magnified in Figure 1(b). The peaks for doped samples are slightly broader and shift to lower angels compared with the peak for undoped sample. This is due to the partially substitutional or interstitial incorporation of Ln3+ and the radius difference between Sc3+ (0.087 nm), Y3+ (0.1019 nm), La3+ (0.116 nm), and Sn4+ (0.069 nm), suggesting there is no distortion in the bulk SnO2 lattice due to the small amount of doping.
Figure 1.
(a) XRD patterns of undoped m-SnO2 and 3% Ln3+ (Sc3+, Y3+, La3+)-doped m-SnO2. (b) Magnified XRD diffraction peaks for the selected region. (c), (d) XPS spectra of Sn3d and Y3d, respectively.
The X-ray photoelectron spectroscopy (XPS) scans for all samples, as shown in –, highlight that no impurities are found in the spectra, suggesting that the Ln3+ ions are incorporated successfully into m-SnO2. From the high-resolution XPS spectra of Sn 3d (Figure 1(c)), undoped m-SnO2 shows two signal peaks at 486.7 and 495.2 eV, respectively, corresponding to Sn 3d 5/2 and Sn 3d 3/2 states of Sn4+. After doping, all Sn 3d peaks shift to lower binding energies, arising from the variations of the chemical environment for Sn4+. In detail, the different binding interactions between Ln-O and Sn-O lead to the charge transfer effect around Sn4+ species [45]. Besides, compared with the binding energy of Sc2O3 (401.9 eV), Y2O3 (156.6 eV), and Ln2O3 (835.2 eV) [46], the shift to lower binding energy with different degrees indicates that Ln3+ exists in a substitutional or interstitial bonding mode of Sn-O-Ln [47]. The narrow spectra of Sc 2p, Y 3d, and La 3d are manifested in Figure 1(d) and , ; all characteristic peaks can be assigned to +3 oxidation state of Ln [48, 49].
Figure 2(a) shows TEM image of 3% Y-doped m-SnO2, which mainly consists of monodisperse nanospheres with the average diameter about 54.19 ± 4.44 nm (Figure 2(c)). The enlarged TEM image shown in Figure 2(b) reveals that the 3% Y-doped m-SnO2 nanospheres have granular structure and coarse surface that consist of small SnO2 nanoparticles with a size of 6~8 nm, which endows the 3% Y-doped m-SnO2 nanospheres with high surface area. From Figure 2(b) and , the lattice spacing for m-SnO2 and 3% Ln3+-doped m-SnO2 is calculated as 0.334 nm, corresponding to the rutile SnO2 phase of (110) lattice planes, indicating that the Ln3+ dopants have no influence on the crystalline phase of m-SnO2, which is accorded with the XRD patterns (Figure 1(b)).
Figure 2.
(a) TEM image of 3% Y-doped m-SnO2. (b) HRTEM image of 3% Y-doped m-SnO2. (c) Histogram of particle diameters from (b). (d) Nitrogen adsorption-desorption isotherms of the as-synthesized samples.
The pore width and the specific surface area of undoped m-SnO2 and 3% Y3+-doped m-SnO2, derived from nitrogen adsorption-desorption measurements, are shown in Figure 2(d) and . All samples exhibit a type H3 hysteresis loop according to the Brunauer-Deming-Deming-Teller (BDDT) classification, indicating the presence of mesopores (2~50 nm) [50, 51]. For 3% Y3+-doped m-SnO2, the Barrett-Joyner-Halenda (BJH) desorption cumulative pore width is 13.8 nm, which is in agreement with the above BDDT classification. Compared with the pore width of undoped m-SnO2 sample (9.80 nm), an increment of 40.8% is achieved by 3% Y3+ dopant [34]. According to the BET method, the specific surface areas of undoped m-SnO2 and 3% Y3+-doped m-SnO2 are 120.2 and 130.0 m2 g−1, respectively. Based on the BET results, the pore feature (especially for pore width) of the doped sample is improved due to the addition of rare-earth ions, which is profitable for the penetration of the perovskite into the mesoporous scaffold and then the formation of well-aligned perovskite morphology.
Figures – show the FE-SEM images of m-SnO2 nanospheres with similar particle diameter for undoped and doped samples, indicating the doping hardly changes the morphology of particles; thus, the influence of morphology by doping on device performance can be excluded. However, the granular structure of m-SnO2 nanospheres collapses when the Ln3+ concentration is increased to 4% (, ) [34, 37]. Thus, a suitable Ln doping amount is crucial. In our experiment condition, the Ln optimal concentration is 3%.
Figure 3 shows the FE-SEM images of SnO2 films (annealed at 300°C), perovskite films, and the devices. shows the corresponding FE-SEM images annealed at 180°C. In Figure 3(a) and , the morphologies of the p-SNO annealed at 300°C and 180°C are similar, indicating that the p-SNO films are thermal stable. From Figure 3(b), 3%-SNOY thin film consists of many monodisperse and well-aligned nanospheres, which increases contact area between m-SnO2 scaffold/perovskite layer and results in the improvement of JSC and FF. From Figures 3(d)–3(f), 3%-SNOY perovskite film presents uniform, smooth-surface, and larger grains compared with that of the p-SNO perovskite film, while heavily Ln3+-doped m-SnO2 (4%-SNOY) (Figure 3(c)) leads to a rough and pin hole perovskite surface, which may have a detrimental effect on device performance. Compared with the cross-sectional SEM image of Figures 3(g) and 3(h), the SNOY-based perovskite film shows dense, large perovskite particles and thickness about 400 nm, proving that the perovskite is well crystallized in the 3%-SNOY scaffold.
Figure 3.
Top view FE-SEM images of (a) p-SNO, (b) 3%-SNOY, and (c) 4%-SNOY thin films deposited on FTO substrates. Top view FE-SEM images of perovskite films on (d) p-SNO, (e) 3%-SNOY, and (f) 4%-SNOY. Cross view FE-SEM image of the PSC based on (g) p-SNO and (h) 3%-SNOY mesoporous scaffold (annealed at 300°C).
2.2. Energy Band Structure
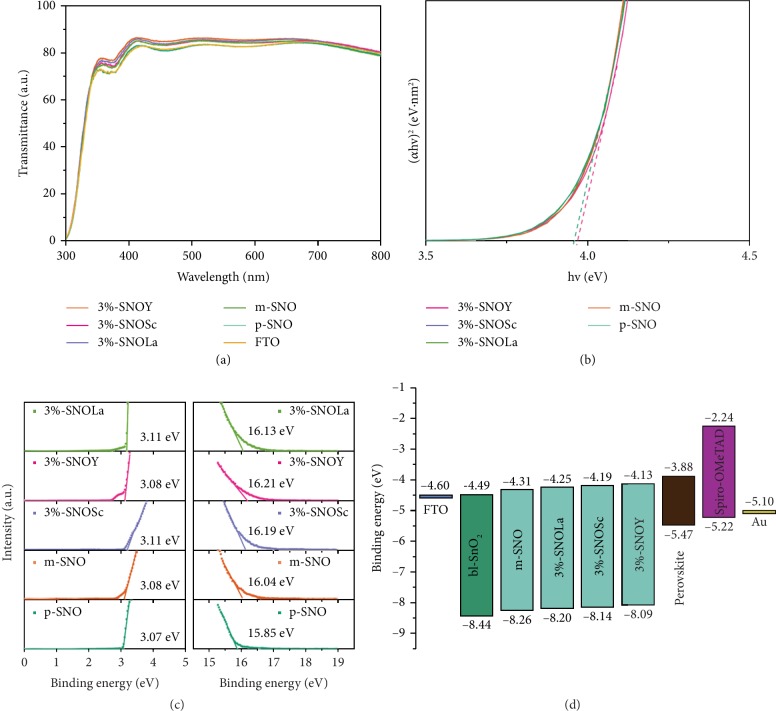
High optical transmission facilitates efficient utilization of sunlight and leads to an improved JSC and PCE for PSCs. Figure 4(a) shows the optical transmission of the films in the order: 3% − SNOY > 3% − SNOSc > 3% − SNOLa > m − SNO > p − SNO, which agrees with the photovoltaic performance of the devices. From Figure 4(b), the optical band edges (Eg) are calculated according to the formula: (αhν)n = A(hν − Eg) (n = 1/2), and results are listed . Various samples have almost the same Eg values (3.95 eV), indicating that the low amount of Ln doping does not affect Eg of the film.
Figure 4.
(a) Transmittance spectra of films. (b) Tauc plots corresponding to the transmission spectra. (c) Ultraviolet photoelectron spectroscopy (UPS) spectra of films in the on-set (left) and the cutoff (right) region. (d) Experimentally determined energy level diagrams (relative to the vacuum level) of different component layers in the PSC devices.
Based on the equation of (Fermi level) EF = Ecutoff(cutoff binding energy) − 21.2 eV (emission energy from He irradiation) and Figure 4(c), EF of samples are calculated and the results are listed in . The gradual upward shift of the EF confirms the improvement in carrier concentration crosschecked by conductivity and Mott-Schottky measurements mentioned after [43, 52]. According to EVB = EF − Eon−set (on-set binding energy) and ECB = EVB + Eg, the valence band positions (EVB) and the conduction band (ECB) are calculated and expressed in Figure 4(d) and . SnO2 mesoporous scaffold plays a functional bridging role between SnO2 bl and perovskite layer; the increase of ECB values of p-SNO, m-SNO to SNOLn films is beneficial for the charge carrier extraction, which the results are good coincidence with the J-V measurements. Meanwhile, the enhancement of VOC can be attributed to conduction band upward shift, influenced by the ECB and EVB of ETL and hole transport layer (HTL). According to the above discussions, the enhancement in photovoltaic parameters is ascribed to the optimization of morphology of perovskite film and charge transport dynamics.
2.3. Photoelectrochemical Properties
The current-voltage (I-V) curves of the FTO/ETL/Au devices were measured in dark and are shown in . The conductivities of samples are calculated as 1.15 × 10−5 (m-SNO) to 2.76 × 10−5 S cm−1 (3%-SNOY), according to σ = d/AR, where d is the film thickness, A is the film area, and R is the film resistance. The enhanced conductivity indicates the passivation of charge trap states by Ln3+ doping and facilitates the charge extraction for improving JSC and FF values.
shows Mott-Schottky (M-S) analysis of samples; all films show positive slope for n-type. The flat band potential Vfb, calculated from the intersection of the linear region with the X-axis, is 0.49 V (m-SNO), 0.35 V (3%-SNOSc), 0.29 V (3%-SNOY), and 0.41 V (3%-SNOLa) vs. Ag/AgCl (equivalent to 0.69, 0.55, 0.49, and 0.61 V vs. NHE). Fermi level (EF) is calculated according to the empirical formula EF = −(Vfb + 4.5) eV by assuming the energy level of normal hydrogen electrode as −4.5 eV [53]. Thus, the EF values of the films are −5.19 eV (m-SNO), −5.05 eV (3%-SNOSc), −4.99 eV (3%-SNOY), and −5.11 eV (3%-SNOLa), which are consistent with the UPS results. The M-S curves can be used to analyze the number of free electrons (Ne), which is inversely proportional to the straight line slope of the M-S plot using the equation: slope = 2/εε0A2qNe, where ε is the relative dielectric constant for SnO2, ε0 is the vacuum permittivity, A is the sample area, and q is the elementary charge [43, 54, 55]. The slope of the 3%-SNOY film (2.10 × 1016) is much smaller than that of m-SNO film (10.22 × 1016), suggesting a considerable increment of Ne. Simultaneously, it also confirms that Ln3+ ions are incorporated interstitially into SnO2 as p-type dopants, which make the increment of Ne [43].
To further elucidate the effect of Ln3+ doping on hysteretic behavior of the device, we carried out steady-state photoluminescence (PL) quenching experiment and time-resolved photoluminescence (TRPL) intensity decay measurement. As presented in , the samples with mesoporous scaffold show a faster electron quenching efficiency than the p-SNO film. According to the result, 3%-SNOY shows the fastest quenching rate, indicating an efficient electron transport and charge extraction at the interface of perovskite/SnO2, which can prevent the accumulation of redundant capacitive charge that leads to hysteresis. and show the TRPL decays of samples; the curves were fitted with a two-component exponential decay function I = A1e−(t − t0)/τ1 + A2e−(t − t0)/τ2, where τ1 refers to the faster component of trap-mediated nonradiative recombination and τ2 is the slower component correlated to radiative recombination. The average PL decay times (τave) can be calculated with the formula τave = (A1τ12 + A2τ22)/(A1τ1 + A2τ2), in which A1 and A2 represent the decay amplitudes. The fitted result for the 3%-SNOY film delivered a much faster PL decay rate (58.89 ns) than that of the p-SNO film (171.35 ns), validating the trap-assisted recombination is partly eliminated, which is beneficial for improving PCE output.
illustrates the Nyquist plots of PSCs devices; the intercept point on the real axis in the high-frequency range is the series resistance (Rs), the semicircle in the high-frequency range is the charge-transfer resistance (Rct) between at HTL/Au interface, and the value in low-frequency range is the recombination resistance (Rrec) at mesoporous/perovskite layer interface. The measured resistances are listed in , the Rrec trend is in good coincidence with the J-V measurements, and the improvement of FF can be attributed to the optimization of charge-transfer resistance of devices owing to Ln3+ doping.
2.4. Photovoltaic Performance
Figure 5(a) shows the structure diagram of fabricated PSCs. Figure 5(b) shows the characteristic photocurrent density-voltage curves (J-V) curves of the optimized p-SNO, m-SNO, and 3%-SNOLn (Sc3+, Y3+, La3+) devices; the relative photovoltaic data are given in . Impressively, compared to the p-SNO device, m-SNO cell shows an improved PCE of 19.01%, indicating a 10.46% enhancement in PCE (19.01% vs. 17.21%), which attributes to efficient electron transport brought by dense, large size, and vertical distribution of perovskite (Figure 3(h)) and improved interfacial connection between SnO2 scaffold and perovskite. We note that PCE reach a highest value of 20.63% for 3%-SNOY device, owing to the morphology optimization of perovskite layer, the adjustment of energy level alignment with perovskite layer, and the improvement of charge transport dynamics by Y3+ doping. The performance of PSCs is further improved owing to Ln ion doping, obeying an order of 3% − SNOY > 3% − SNOSc > 3% − SNOLa > m − SNO > p − SNO, yielding a champion PCE as high as 20.63% for 3%-SNOY tailored PSC. The photovoltaic performance of the 3%-SNOY device is higher than that of state-of-the-art PSCs based on mesoporous SnO2 scaffold ().
Figure 5.
(a) Schematic device structure. (b) J-V curves of the PSCs with different SnO2. (c) Steady-state output of JSC and PCE for m-SNO and 3%-SNOY devices. (d) IPCE spectra of the PSCs with different SnO2. (e) J-V curves of the PSCs with different SnO2 under backward and forward scanning.
Figures – and show J-V curves and photovoltaic parameters of the PSCs based on m-SNO layer doped with different Ln ion concentrations. To ensure the reliability and repeatability of data, average photovoltaic parameters of the each PSC device were obtained from 20 devices. As mentioned above, excessive dopants are detrimental to the device performance either by the hoist of conduction band leading to an inefficient electron injection, in which case VOC keeps increasing, but JSC decreases, or the dopants induce trap states, leading to a decrease of all device parameters [43]. In our case, both the trap state density increase induced by heavily Ln3+-doped m-SnO2 (4%-Sc3+, Y3+, La3+) and the dreadful recombination occurring at the interface of mesoporous scaffold/perovskite induced by the scaffold breakdown drag the parameters of PSC devices. The reproducibility statistics of 20 devices shown in Figures – and further confirm the better photovoltaic data for the 3%-SNOLn devices than the p-SNO devices. Apparently, the 3%-SNOY device exhibits the highest average PCE value (20.26 ± 0.116%), and average JSC, VOC, and FF values of 3%-SNOY device are the highest among all devices. The reliability and repeatability tests distinctly demonstrate the champion performance of the 3%-SNOY devices.
Figure 5(c) shows the steady-state output of JSC and PCE for m-SNO and 3%-SNOY devices by tracking maximum power point (MPP) at a bias voltage 0.87 V and 0.94 V as shown in Figure 5(b). The p-SNO and 3%-SNOY devices yield stabilized PCE of 16.79% and 20.28%, respectively, which are comparable to the PCE obtained from the fresh J-V curves. Figure 5(d) demonstrates incident photon-to-current conversion efficiency (IPCE) spectra of p-SNO, m-SNO, and 3%-SNOY champion devices to verify the validity of the power output. The 3%-SNOY device exhibits the highest integrated JSC value of 22.75 mA cm−2 compared to m-SNO (22.18 mA cm−2) and p-SNO (21.15 mA cm−2), concluding an efficient charge injection and well energy level alignment for 3%-SNOY film. In our case, the JSC values derived from IPCE spectra are undervalued compared with that obtained from the corresponding J-V curves (Figure 5(b)), which is acceptable due to the spectral mismatch of the solar simulator and the theoretical AM 1.5G spectrum as reported before [56].
Figure 5(e) shows the J-V curves of the devices under backward (B 2 V ⟶ −0.2 V) and forward (F −0.2 V ⟶ 2 V) scanning, and the related data are summarized in . Apparently, p-SNO device shows a mild hysteresis while 3%-SNOY device embodies a character of hysteresis-free. It denotes that the introduction of rare-earth ions improves charge carrier transport dynamics and suppresses the charge accumulation at the interfaces of SnO2 bl/m-SnO2 and m-SnO2/perovskite layers.
Figures and show the dependence of JSC and VOC on light intensity of the PSCs. The power law dependence of JSC on the illumination intensity is generally expressed as JSC ∝ Iα, where α is the exponential factor related to bimolecular recombination [56]. The α value of 3%-SNOY (0.983) device is closer to 1 than that of m-SNO (0.973) and p-SNO (0.953) devices, suggesting a reduction of the bimolecular radiative recombination in the Ln3+ ion-doped devices due to more effective carrier transportation through the interfaces of SnO2 bl/m-SnO2/perovskite layers.
As shown in , the trap-assisted Shockley-Read-Hall recombination associated with trap state density is significantly suppressed due to the Ln3+ dopants determined as VOC = nkTln(I)/q + constant [54], where n is an ideal factor related to monomolecular recombination, k represents the Boltzmann constant, and T is the absolute temperature. The slope of the plot (n) for the 3%-SNOY device is lower (1.32 kT/q) than that of the m-SNO (1.48 kT/q) and p-SNO devices (1.86 kT/q), indicating that the fewer monomolecular recombination in the 3%-SNOY device by Ln3+ doping, which is consistent with the .
shows the dark J-V curves of PSCs, which is correlated to the leakage current from carrier recombination in the devices. According to the equation, VOC = nkTln(JSC/J0)/q, where JSC and J0 are the photogenerated and dark saturation current densities, respectively [57]. The value of J0 for the 3%-SNOY device is calculated as 8.41 × 10−11 mA cm−2, which is five orders of magnitude smaller than that of the p-SNO device (1.32 × 10−6 mA cm−2). While all three devices exhibit approximate output current, implying a higher rectification ratio and a greatly restrained leakage current induced by charge recombination for the device Ln3+ doped.
illustrates the dark J-V curves of the electron-only devices with a structure of FTO/SnO2/perovskite/PCBM/Au. Apparently, the trap-filled limit voltages (VTFL) for 3%-SNOY device show a decreased trend in comparison with the other two devices, which is proportional to trap state density (ntrap) according to VTFL = qntrapL2/2εε0, where L is the thickness of the electron-only device, ε is the relative dielectric constant for SnO2, and ε0 is the vacuum permittivity [58]. These results provide strong evidences that the trap defects are healed significantly owing to Ln3+ ion doping, crosschecking the aforementioned conclusion.
2.5. Stability
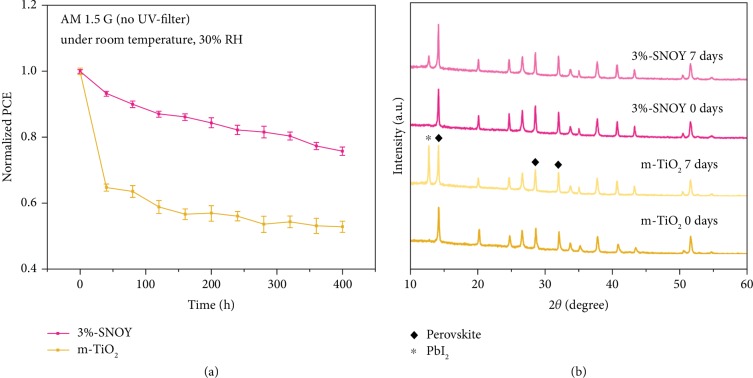
The long-term stability of perovskite solar cells should consider both ultraviolet light and humidity sensitivities [39, 59]. Figure 6(a) shows the normalized PCE vs. time of the devices without encapsulation under simulated solar light illumination for 400 h in 30% RH ambient condition. The m-TiO2 PSCs manifest a dramatic degradation to 64.8% of the highest PCE in the first 40 h arising from the photocatalytic properties of TiO2 degrading perovskite and then followed by a plain to 53.2% of the highest PCE originating from that the defects in TiO2 can be passivated by the adsorption of atmospheric oxygen [39]. While the 3%-SNOY devices decrease steadily at nearly 75.8% of the highest PCE under the same testing conditions, exhibiting remarkablely improved UV stability. shows the change of average PCE values of 3%-SNOY and m-TiO2 devices with time, presenting similar results as Figure 6(a).
Figure 6.
(a) Normalized PCE change with time for m-TiO2 and 3%-SNOY devices without encapsulation under simulated solar light illumination for 400 h. (b) XRD patterns of m-TiO2 and 3%-SNOY perovskite films in ambient condition (RH ~30%) before and after simulated solar light illumination for 7 days.
After exposure of the perovskite based on m-TiO2 to AM1.5 illumination under 30% RH ambient condition for one week shown in Figure 6(b), a new sharp diffraction peak at 12.7° corresponding to the PbI2 (001) lattice plane is observed in the XRD pattern, arising from the decomposition of the aged perovskite film caused by UV light and ambient humidity. The PbI2 would block the charge transport, resulting in a decrease of PCE. Notably, the intensity of PbI2 diffraction peak for the 3%-SNOY-based perovskite film aged in the same situations is very less, implying a much lower degree of decomposition, which is due to the passivation effect on defects and UV light stability by rare-earth ion doping.
3. Conclusions
In conclusion, rare-earth Ln-doped monodisperse SnO2 nanospheres with specific surface area of 130.0 m2 g−1 are successfully synthesized by a solution-phase route. The doped SnO2 nanospheres are used as scaffold to fabricate mesoporous perovskite solar cells. The observation of morphology, microstructure characterization, energy band analysis, photoelectric property investigation, and photovoltaic performance measurement indicate that the doping of rare-earth Ln ions promotes the formation of dense, even and large perovskite crystals, which facilitate better interfacial contacts of electron transport layer/perovskite layer. On the other hand, Ln dopants optimize the energy level of electron transport layer and reduce the resistance and charge trap states, resulting in an efficient electron transport and charge extraction. As a result, the Y3+ (3%)-doped mesoporous SnO2-based PSC achieved a champion efficiency of 20.63% with hysteresis-free, while the planar and mesoporous SnO2-based PSCs obtain efficiency of 17.21% and 19.01%, respectively. This investigation demonstrates a novel strategy for developing efficient and low-cost full SnO2-based PSCs.
4. Materials and Methods
4.1. Materials
Formamidinium iodide (FAI, 99.5%), methylammonium iodide (MAI, 99.5%), and methylammonium bromide (MABr, 99.5%) were obtained from Xi'an Polymer Light Technology Corp, China. Lead iodide (PbI2, 99.99%), lead bromide (PbBr2, 99%), and cesium iodide (CsI, 99%) were purchased from TCI. Bis(trifluoromethane) sulfonimide lithium salt (Li-TFSI, 99.95%), anhydrous dimethyl sulfoxide (DMSO, 99.9%), anhydrous N,N-dimethylformamide (DMF, 99.8%), 4-tert-butylpyridine (98%), anhydrous chlorobenzene (CB, 99.8%), anhydrous acetonitrile (99.8%), anhydrous 1-butanol (99.8%), and SnCl2·2H2O (99.995%) were received from Sigma-Aldrich. K2SnO3·3H2O (99.5%), urea (99.995%), YCl3·6H2O (99.99%), LaCl3·6H2O (99.99%), ScCl3·6H2O (99.9%), and ethylene glycol (EG, 99%) were obtained from Aladdin. All the chemicals were used as received without further purification. Deionized water (resistivity > 18 MΩ) was obtained through a Millipore water purification system. Prepatterned fluorine-doped tin oxide-coated (FTO) substrates with a sheet resistance of 14 Ω sq−1 were purchased from Pilkington.
4.2. Preparation of SnO2 and Ln-Doped Solutions
SnO2 solution was prepared by dissolving SnCl2·2H2O in 1-butanol in the concentration of 0.1 M. Monodisperse SnO2 nanospheres were synthesized by a solution-phase route [50]. In a typical procedure, 8 mL of aqueous solution containing LnCl3 (Sc3+, Y3+, La3+) and K2SnO3 with different Ln3+/Sn4+ molar ratios (0~4%) mixed with 15 mL of EG was added into a 50 mL Teflon-lined autoclave and maintained at 170°C for 13 h. The air-cooled precipitation was washed thoroughly with deionized water for removal of K+ and organic residue followed by a centrifugation and then diluted with deionized water in the concentration of 0.1 g mL−1 prior to use.
4.3. Fabrication of Perovskite Solar Cells
Laser-patterned FTO glass with size of 1.5 × 1.5 cm2 was cleaned by detergent and sonicating in isopropanol, acetone, deionized water, and ethanol and finally treated with UV ozone for 30 min. SnO2 block layer (bl) was deposited on FTO by a spin-coating step (3000 rpm, 30 s) and then annealed at 150°C for 1 h, named as p-SNO. SnO2 scaffold layer contained different Ln3+/Sn4+ molar ratios (0~4%) with thickness about 100~200 nm was covered by spin-coating the SnO2 solution at 2000 rpm for 30 s and then annealed at 300°C for 1 h to remove the organic residue, named, respectively, as m-SNO and x%-SNOLn (x = 1, 2, 3, 4). The cesium-containing triple cation perovskite was deposited by an antisolvent method according to the literatures [27, 60]. The perovskite precursor solution was spin-coated at 1000 rpm for 10 s and then at 6000 rpm for 20 s on the p-SNO and full SnO2 as bl/mp layers substrates. During the second step, 130 μL of chlorobenzene was poured on the spinning substrate 5 s prior to the end of the program to rinse out residual DMSO and DMF in the precursor films. Afterwards, the substrates were heated immediately at 100 °C for 1 h and then were cooled down to room temperature naturally. Subsequently, the spiro-OMeTAD layers were subsequently deposited on top of the as-prepared perovskite layers by spin-coating 20 μL of chlorobenzene solution containing chlorobenzene (1 mL), spiro-OMeTAD (80 mg), 4-tert-butylpyridine (28.8 μL), and Li-TFSI (17.5 μL, 520 mg Li-TFSI in 1 mL acetonitrile) at 4000 rpm for 30 s. Finally, about 100 nm thick Au electrodes were thermally evaporated on the spiro-OMeTAD layers under high vacuum via a shadow mask. Thus, the PSCs with the active area of 0.1 cm2 (0.25 × 0.4 cm2) were prepared. For clarity, PSC devices based on planar structure SnO2 and mesoporous structure SnO2 with different Ln3+/Sn4+ molar ratios (0~4%) are denoted as p-SNO device, m-SNO device, and x%-SNOLn devices (x = 1, 2, 3, 4). The PSCs with full TiO2 as bl/mp layers named as m-TiO2 were also fabricated as previously reported for comparative study [27].
4.4. Characterization
The crystal structures of samples were determined by powder X-ray diffraction (XRD, Smart Lab, Rigaku) using graphite monochromatic copper radiation (λ = 1.5418 Å). The morphology characterizations were performed on the field emission scanning electron microscopy (FE-SEM, SU8000, Hitachi) and a JEOL JEM-2100 transmission electron microscopy (TEM). Surface electronic states and UV photoelectron spectroscopy (UPS) were carried out using a XPS/UPS system (Thermo Scientific, ESCLAB 250XI, USA). UPS was performed using He I radiation at 21.22 eV with bias (−5 V) on the samples to separate the sample and analyzer low kinetic energy cutoffs. For XPS, all binding energies were referenced to the C1s peak (284.8 eV) of the surface adventitious. The Brunauer-Emmett-Teller (BET) specific surface area was determined using N2 adsorption apparatus (ASAP 2020 HD88, micromeritics) at 77 K after a pretreatment at 453 K for 3 h. The flat band potential was performed by using a CHI760E (Chenhua Co. Ltd, Shanghai) electrochemical workstation with a standard three-electrode configuration, which employed a Pt plate as the counter electrode and Ag/AgCl (saturated Na2SO4) as the reference electrode. Ultraviolet-visible (UV-vis) absorption spectra of samples were recorded on a PerkinElmer Lambda 950UV/VIS/NIR spectrometer in the wavelength range of 300~800 nm. The steady-state photoluminescence (PL) spectra were acquired using a fluorescence spectrophotometer (Lumina, Thermo Fisher) equipped with a Xenon lamp at an excitation wavelength of 507 nm. The time-resolved photoluminescence (TRPL) spectrum was recorded on an Omni-λ monochromator excited with a 760 nm laser.
4.5. Measurement
The photocurrent density-voltage (J-V) curves of PSCs were recorded with a Keithley 2420 source-measure unit under 100 mW cm−2 (AM 1.5G) with presweep delay of 0.04 s, max reverse bias of 0.2 V, max forward bias of 2.0 V, and dwell time of 30 ms in ambient environment. The illumination source was a solar light simulator (Newport Oriel Sol 3A class, USA, calibrated by a Newport reference cell). Average photovoltaic parameters of the PSC devices were obtained from 20 devices to ensure the reliability and repeatability of data. Dark J-V curves were measured on a Keithley 2420 source meter in the dark. The stabilized power output was recorded close to the maximum power point, which was extracted from the J-V curves on an electrochemical work station (CHI660E, Chenhua Co. Ltd, Shanghai) under simulated sunlight irradiation with intensity of 100 mW cm−2 at AM 1.5G. The incident photo-to-current conversion efficiency (IPCE) curves were measured as a function of wavelength from 300 nm to 800 nm using a QE-R quantum efficiency measurement system (Enli Technology Co. Ltd). The electrochemical impedance spectroscopy (EIS) measurements were conducted on a Zennium electrochemical workstation (IM6) under AM 1.5G with the frequencies from 100 mHz to 1 MHz, the bias of 0 V, and the amplitude of 20 mV. Long-term stability under persistent moisture (30% RH) was tested by XRD measurement for 7 days and record of time-dependent photovoltaic performances for 400 h under ambient condition with 30% RH. All the average values for long-term stability were obtained from 8 devices for each sample.
Acknowledgments
We acknowledge the financial support from the National Natural Science Foundation of China (Nos. U1705256, 51972123, 21771066) and the Cultivation Program for Postgraduate in Scientific Research Innovation Ability of Huaqiao University (No. 17011081001).
Conflicts of Interest
The authors declare no conflict of interest.
Supplementary Materials
Figure S1: (a)–(c) survey XPS spectra of 3% Ln3+(Sc3+, Y3+, La3+)-doped m-SnO2. (d), (e) Narrow XPS spectra of Sc 2p and La 3d. Figure S2: (a) TEM and (b) HRTEM images of undoped m-SnO2. Figure S3: FE-SEM images of (a) m-SnO2, (b) 3% Sc3+, (c) 3% La3+, (d) 4% Sc3+, and (e) 4% La3+-doped m-SnO2. Top view FE-SEM images of (f) bare FTO glass and (g) p-SNO (annealed at 180°C). Figure S4: (a) I-V curves of various films under dark condition. (b) M-S plot of various films. The electrodes were submerged in a 0.5 M KCl solution with a Pt counter electrode and Ag/AgCl reference electrode. (c) PL spectra and (d) TRPL spectra of the perovskite films based on various SnO2 film. (e) Fitting curves from Nyquist plots for various SnO2 devices. The inset is the corresponding equivalent circuit. Figure S5: J-V curves of the PSCs based on m-SNO doped with (a) Sc3+, (b) Y 3+, and (c) La 3+ in different concentrations. (d)–(g) Average photovoltaic data of the devices with Ln-doped SnO2 scaffold. Average photovoltaic parameters of the each PSC device were obtained from 20 devices to ensure the reliability and repeatability of data. Figure S6: (a), (b) Dependence of JSC and VOC on light intensity of PSCs. (c) Dark J-V curves of PSCs. (d) J-V curves under dark conditions for the electron-only devices with the inserted structure. Table S1: pore properties of 3% Y-doped m-SnO2 and undoped m-SnO2. Table S2: band edge (Eg), Fermi level (EF), valence band (EVB), and conduction band (ECB) of samples. Table S3: fitted data from TRPL spectra in Figure S4d. Table S4: photovoltaic and impedance data of the PSCs. Table S5: PCE comparison of the PSCs based on full SnO2 mesoporous structure [S1-S8]. Table S6: photovoltaic data of the PSCs with different Ln3+ (Sc3+, Y3+, La3+) concentrations. Table S7: average photovoltaic data of the PSCs. The average values were obtained from 20 devices. Table S8: photovoltaic parameters of the PSCs scanning in different directions. Table S9: PCE values for 3%-SNOY and m-TiO2 devices. Average values were obtained from 8 devices.
References
- 1.Hodes G. Perovskite-based solar cells. Science. 2013;342(6156):317–318. doi: 10.1126/science.1245473. [DOI] [PubMed] [Google Scholar]
- 2.Service R. F. Perovskite solar cells keep on surging. Science. 2014;344(6183):p. 458. doi: 10.1126/science.344.6183.458. [DOI] [PubMed] [Google Scholar]
- 3.Wu J., Lan Z., Lin J., et al. Counter electrodes in dye-sensitized solar cells. Chemical Society Reviews. 2017;46(19):5975–6023. doi: 10.1039/C6CS00752J. [DOI] [PubMed] [Google Scholar]
- 4.Tu Y., Yang X., Su R., et al. Diboron‐assisted interfacial defect control strategy for highly efficient planar perovskite solar cells. Advanced Materials. 2018;30(49, article 1805085) doi: 10.1002/adma.201805085. [DOI] [PubMed] [Google Scholar]
- 5.Kojima A., Teshima K., Shirai Y., Miyasaka T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. Journal of the American Chemical Society. 2009;131(17):6050–6051. doi: 10.1021/ja809598r. [DOI] [PubMed] [Google Scholar]
- 6.Kim M., Kim G.-H., Lee T. K., et al. Methylammonium chloride induces intermediate phase stabilization for efficient perovskite solar cells. Joule. 2019;3(9):2179–2192. doi: 10.1016/j.joule.2019.06.014. [DOI] [Google Scholar]
- 7.Xiao M., Huang F., Huang W., et al. A fast deposition‐crystallization procedure for highly efficient lead iodide perovskite thin‐film solar cells. Angewandte Chemie International Edition. 2014;53(37):9898–9903. doi: 10.1002/anie.201405334. [DOI] [PubMed] [Google Scholar]
- 8.Jeon N. J., Noh J. H., Kim Y. C., Yang W. S., Ryu S., Seok S. I. Solvent engineering for high-performance inorganic–organic hybrid perovskite solar cells. Nature Materials. 2014;13(9):897–903. doi: 10.1038/nmat4014. [DOI] [PubMed] [Google Scholar]
- 9.Yang W. S., Noh J. H., Jeon N. J., et al. High-performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science. 2015;348(6240):1234–1237. doi: 10.1126/science.aaa9272. [DOI] [PubMed] [Google Scholar]
- 10.Jacobsson T. J., Correa-Baena J.-P., Pazoki M., et al. Exploration of the compositional space for mixed lead halogen perovskites for high efficiency solar cells. Energy & Environmental Science. 2016;9(5):1706–1724. doi: 10.1039/C6EE00030D. [DOI] [Google Scholar]
- 11.Pellet N., Gao P., Gregori G., et al. Mixed‐organic‐cation perovskite photovoltaics for enhanced solar‐light harvesting. Angewandte Chemie International Edition. 2014;53(12):3151–3157. doi: 10.1002/anie.201309361. [DOI] [PubMed] [Google Scholar]
- 12.McMeekin D. P., Sadoughi G., Rehman W., et al. A mixed-cation lead mixed-halide perovskite absorber for tandem solar cells. Science. 2016;351(6269):151–155. doi: 10.1126/science.aad5845. [DOI] [PubMed] [Google Scholar]
- 13.Green M. A., Ho-Baillie A., Snaith H. J. The emergence of perovskite solar cells. Nature Photonics. 2014;8(7):506–514. doi: 10.1038/nphoton.2014.134. [DOI] [Google Scholar]
- 14.Yin W.-J., Shi T., Yan Y. Unique properties of halide perovskites as possible origins of the superior solar cell performance. Advanced Materials. 2014;26(27):4653–4658. doi: 10.1002/adma.201306281. [DOI] [PubMed] [Google Scholar]
- 15.Frost J. M., Butler K. T., Brivio F., Hendon C. H., van Schilfgaarde M., Walsh A. Atomistic origins of high-performance in hybrid halide perovskite solar cells. Nano Letters. 2014;14(5):2584–2590. doi: 10.1021/nl500390f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu J., Lan Z., Lin J., et al. Electrolytes in dye-sensitized solar cells. Chemical Reviews. 2015;115(5):2136–2173. doi: 10.1021/cr400675m. [DOI] [PubMed] [Google Scholar]
- 17.Bella F., Popovic J., Lamberti A., Tresso E., Gerbaldi C., Maier J. Interfacial effects in solid–liquid electrolytes for improved stability and performance of dye-sensitized solar cells. ACS Applied Materials & Interfaces. 2017;9(43):37797–37803. doi: 10.1021/acsami.7b11899. [DOI] [PubMed] [Google Scholar]
- 18.Bella F., Sacco A., Massaglia G., Chiodoni A., Pirri C. F., Quaglio M. Dispelling clichés at the nanoscale: the true effect of polymer electrolytes on the performance of dye-sensitized solar cells. Nanoscale. 2015;7(28):12010–12017. doi: 10.1039/C5NR02286J. [DOI] [PubMed] [Google Scholar]
- 19.Liu L., Wu Y., Chi F., et al. An efficient quasi-solid-state dye-sensitized solar cell with gradient polyaniline-graphene/PtNi tailored gel electrolyte. Electrochimica Acta. 2019;316:125–132. doi: 10.1016/j.electacta.2019.05.115. [DOI] [Google Scholar]
- 20.Mohamad A. A. Physical properties of quasi-solid-state polymer electrolytes for dye-sensitised solar cells: a characterisation review. Solar Energy. 2019;190:434–452. doi: 10.1016/j.solener.2019.08.016. [DOI] [Google Scholar]
- 21.Sacco A., Bella F., De La Pierre S., et al. Electrodes/electrolyte interfaces in the presence of a surface‐modified photopolymer electrolyte: application in dye‐sensitized solar cells. ChemPhysChem. 2015;16(5):960–969. doi: 10.1002/cphc.201402891. [DOI] [PubMed] [Google Scholar]
- 22.Shanti R., Bella F., Salim Y. S., Chee S. Y., Ramesh S., Ramesh K. Poly(methyl methacrylate- co -butyl acrylate- co -acrylic acid): physico- chemical characterization and targeted dye sensitized solar cell application. Materials & Design. 2016;108:560–569. doi: 10.1016/j.matdes.2016.07.021. [DOI] [Google Scholar]
- 23.Lee Y. H., Luo J., Son M.-K., et al. Enhanced charge collection with passivation layers in perovskite solar cells. Advanced Materials. 2016;28(20):3966–3972. doi: 10.1002/adma.201505140. [DOI] [PubMed] [Google Scholar]
- 24.Rao H.-S., Chen B.-X., Li W.-G., et al. Improving the extraction of photogenerated electrons with SnO2 nanocolloids for efficient planar perovskite solar cells. Advanced Functional Materials. 2015;25(46):7200–7207. doi: 10.1002/adfm.201501264. [DOI] [Google Scholar]
- 25.Yang G., Chen C., Yao F., et al. Effective carrier‐concentration tuning of SnO2 quantum dot electron‐selective layers for high‐performance planar perovskite solar cells. Advanced Materials. 2018;30(14, article 1706023) doi: 10.1002/adma.201706023. [DOI] [PubMed] [Google Scholar]
- 26.Leijtens T., Eperon G. E., Pathak S., Abate A., Lee M. M., Snaith H. J. Overcoming ultraviolet light instability of sensitized TiO2 with meso-superstructured organometal tri-halide perovskite solar cells. Nature Communications. 2013;4(1, article 2885) doi: 10.1038/ncomms3885. [DOI] [PubMed] [Google Scholar]
- 27.Guo Q., Wu J., Yang Y., et al. High performance perovskite solar cells based on β-NaYF4:Yb3+/Er3+/Sc3+@NaYF4 core-shell upconversion nanoparticles. Journal of Power Sources. 2019;426:178–187. doi: 10.1016/j.jpowsour.2019.04.039. [DOI] [Google Scholar]
- 28.Liu D., Kelly T. L. Perovskite solar cells with a planar heterojunction structure prepared using room-temperature solution processing techniques. Nature Photonics. 2014;8(2):133–138. doi: 10.1038/nphoton.2013.342. [DOI] [Google Scholar]
- 29.Wang K., Shi Y., Dong Q., et al. Low-temperature and solution-processed amorphous WOXas electron-selective layer for perovskite solar cells. The Journal of Physical Chemistry Letters. 2015;6(5):755–759. doi: 10.1021/acs.jpclett.5b00010. [DOI] [PubMed] [Google Scholar]
- 30.Qin M., Ma J., Ke W., et al. Perovskite solar cells based on low-temperature processed indium oxide electron selective layers. ACS Applied Materials & Interfaces. 2016;8(13):8460–8466. doi: 10.1021/acsami.5b12849. [DOI] [PubMed] [Google Scholar]
- 31.Bera A., Wu K., Sheikh A., Alarousu E., Mohammed O. F., Wu T. Perovskite oxide SrTiO3 as an efficient electron transporter for hybrid perovskite solar cells. Journal of Physical Chemistry C. 2014;118(49):28494–28501. doi: 10.1021/jp509753p. [DOI] [Google Scholar]
- 32.Xu J., Buin A., Ip A. H., et al. Perovskite–fullerene hybrid materials suppress hysteresis in planar diodes. Nature Communications. 2015;6, article 7081 doi: 10.1038/ncomms8081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang Q., Zhang X., You J. SnO2: a wonderful electron transport layer for perovskite solar cells. Small. 2018;14(31, article 1801154) doi: 10.1002/smll.201801154. [DOI] [PubMed] [Google Scholar]
- 34.Yang G., Lei H., Tao H., et al. Reducing hysteresis and enhancing performance of perovskite solar cells using low‐temperature processed Y‐doped SnO2 nanosheets as electron selective layers. Small. 2017;13(2, article 1601769) doi: 10.1002/smll.201601769. [DOI] [PubMed] [Google Scholar]
- 35.Ke W., Zhao D., Cimaroli A. J., et al. Effects of annealing temperature of tin oxide electron selective layers on the performance of perovskite solar cells. Journal of Materials Chemistry A. 2015;3(47):24163–24168. doi: 10.1039/C5TA06574G. [DOI] [Google Scholar]
- 36.Xiong L., Qin M., Chen C., et al. Fully high‐temperature‐processed SnO2 as blocking layer and scaffold for efficient, stable, and hysteresis‐free mesoporous perovskite solar cells. Advanced Functional Materials. 2018;28(10, article 1706276) doi: 10.1002/adfm.201706276. [DOI] [Google Scholar]
- 37.Yang W. S., Park B.-W., Jung E. H., et al. Iodide management in formamidinium-lead-halide–based perovskite layers for efficient solar cells. Science. 2017;356(6345):1376–1379. doi: 10.1126/science.aan2301. [DOI] [PubMed] [Google Scholar]
- 38.Jung E. H., Jeon N. J., Park E. Y., et al. Efficient, stable and scalable perovskite solar cells using poly(3-hexylthiophene) Nature. 2019;567(7749):511–515. doi: 10.1038/s41586-019-1036-3. [DOI] [PubMed] [Google Scholar]
- 39.Alharbi E. A., Dar M. I., Arora N., et al. Perovskite solar cells yielding reproducible photovoltage of 1.20 V. Research. 2019;2019, article 8474698:9. doi: 10.1155/2019/8474698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roose B., Baena J.-P. C., Gödel K. C., et al. Mesoporous SnO2 electron selective contact enables UV-stable perovskite solar cells. Nano Energy. 2016;30:517–522. doi: 10.1016/j.nanoen.2016.10.055. [DOI] [Google Scholar]
- 41.Li Y., Zhu J., Huang Y., et al. Mesoporous SnO2 nanoparticle films as electron-transporting material in perovskite solar cells. RSC Advances. 2015;5(36):28424–28429. doi: 10.1039/C5RA01540E. [DOI] [Google Scholar]
- 42.Roose B., Johansen C. M., Dupraz K., et al. A Ga-doped SnO2 mesoporous contact for UV stable highly efficient perovskite solar cells. Journal of Materials Chemistry A. 2018;6(4):1850–1857. doi: 10.1039/C7TA07663K. [DOI] [Google Scholar]
- 43.Xiong L., Qin M., Yang G., et al. Performance enhancement of high temperature SnO2-based planar perovskite solar cells: electrical characterization and understanding of the mechanism. Journal of Materials Chemistry A. 2016;4(21):8374–8383. doi: 10.1039/C6TA01839D. [DOI] [Google Scholar]
- 44.Abuhelaiqa M., Paek S., Lee Y., et al. Stable perovskite solar cells using tin acetylacetonate based electron transporting layers. Energy & Environmental Science. 2019;12(6):1910–1917. doi: 10.1039/C9EE00453J. [DOI] [Google Scholar]
- 45.Porter H. Q., Turner D. W. Photoelectron spectroscopy. Trends in Biochemical Sciences. 1976;1(11):N254–N255. doi: 10.1016/0968-0004(76)90068-2. [DOI] [Google Scholar]
- 46.Moulder J. F., Stickle W. F., Sobol P. E., Bomben K. D. Handbook of X-ray Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data. Perkin-Elmer Corporation; 1995. [Google Scholar]
- 47.Zhang J., Zhao Z., Wang X., et al. Increasing the oxygen vacancy density on the TiO2 surface by La-doping for dye-sensitized solar cells. The Journal of Physical Chemistry C. 2010;114(43):18396–18400. doi: 10.1021/jp106648c. [DOI] [Google Scholar]
- 48.Arif H. S., Murtaza G., Hanif H., Ali H. S., Yaseen M., Khalid N. R. Effect of La on structural and photocatalytic activity of SnO2 nanoparticles under UV irradiation. Journal of Environmental Chemical Engineering. 2017;5(4):3844–3851. doi: 10.1016/j.jece.2017.07.043. [DOI] [Google Scholar]
- 49.Zhang J., Wang D., Lai L., et al. Probing the reactivity and structure relationship of Ln2Sn2O7 (Ln=La, Pr, Sm and Y) pyrochlore catalysts for CO oxidation. Catalysis Today. 2019;327:168–176. doi: 10.1016/j.cattod.2018.05.009. [DOI] [Google Scholar]
- 50.Wu W., Zhang S., Zhou J., Xiao X., Ren F., Jiang C. Controlled synthesis of monodisperse sub‐100 nm hollow SnO2 nanospheres: a template‐ and surfactant‐free solution‐phase route, the growth mechanism, optical properties, and application as a photocatalyst. Chemistry - A European Journal. 2011;17(35):9708–9719. doi: 10.1002/chem.201100694. [DOI] [PubMed] [Google Scholar]
- 51.Liu J., Wei Y., Meng W., Li P.-Z., Zhao Y., Zou R. Understanding the pathway of gas hydrate formation with porous materials for enhanced gas separation. Research. 2019;2019, article 3206024:10. doi: 10.34133/2019/3206024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bahadur J., Ghahremani A. H., Martin B., Druffel T., Sunkara M. K., Pal K. Solution processed Mo doped SnO2 as an effective ETL in the fabrication of low temperature planer perovskite solar cell under ambient conditions. Organic Electronics. 2019;67:159–167. doi: 10.1016/j.orgel.2019.01.027. [DOI] [Google Scholar]
- 53.Chiu K. Y., Tran T. T. H., Wu C.-G., Chang S. H., Yang T. F., Su Y. O. Electrochemical studies on triarylamines featuring an azobenzene substituent and new application for small-molecule organic photovoltaics. Journal of Electroanalytical Chemistry. 2017;787:118–124. doi: 10.1016/j.jelechem.2017.01.053. [DOI] [Google Scholar]
- 54.Cai F., Cai J., Yang L., et al. Molecular engineering of conjugated polymers for efficient hole transport and defect passivation in perovskite solar cells. Nano Energy. 2018;45:28–36. doi: 10.1016/j.nanoen.2017.12.028. [DOI] [Google Scholar]
- 55.Ang E. H., Dinh K. N., Sun X., et al. Highly efficient and stable hydrogen production in all pH range by two-dimensional structured metal-doped tungsten semicarbides. Research. 2019;2019, article 4029516:14. doi: 10.34133/2019/4029516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tu B., Shao Y., Chen W., et al. Novel molecular doping mechanism for n‐doping of SnO2 via triphenylphosphine oxide and its effect on perovskite solar cells. Advanced Materials. 2019;31(15, article 1805944) doi: 10.1002/adma.201805944. [DOI] [PubMed] [Google Scholar]
- 57.Li C., Song Z., Zhao D., et al. Reducing saturation‐current density to realize high‐efficiency low‐bandgap mixed tin–lead halide perovskite solar cells. Advanced Energy Materials. 2019;9(3, article 1803135) doi: 10.1002/aenm.201803135. [DOI] [Google Scholar]
- 58.Wetzelaer G.-J. A. H., Scheepers M., Sempere A. M., Momblona C., Ávila J., Bolink H. J. Trap‐assisted non‐radiative recombination in organic–inorganic perovskite solar cells. Advanced Materials. 2015;27(11):1837–1841. doi: 10.1002/adma.201405372. [DOI] [PubMed] [Google Scholar]
- 59.Han Y., Meyer S., Dkhissi Y., et al. Degradation observations of encapsulated planar CH3NH3PbI3 perovskite solar cells at high temperatures and humidity. Journal of Materials Chemistry A. 2015;3(15):8139–8147. doi: 10.1039/C5TA00358J. [DOI] [Google Scholar]
- 60.Saliba M., Matsui T., Seo J.-Y., et al. Cesium-containing triple cation perovskite solar cells: improved stability, reproducibility and high efficiency. Energy & Environmental Science. 2016;9(6):1989–1997. doi: 10.1039/C5EE03874J. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: (a)–(c) survey XPS spectra of 3% Ln3+(Sc3+, Y3+, La3+)-doped m-SnO2. (d), (e) Narrow XPS spectra of Sc 2p and La 3d. Figure S2: (a) TEM and (b) HRTEM images of undoped m-SnO2. Figure S3: FE-SEM images of (a) m-SnO2, (b) 3% Sc3+, (c) 3% La3+, (d) 4% Sc3+, and (e) 4% La3+-doped m-SnO2. Top view FE-SEM images of (f) bare FTO glass and (g) p-SNO (annealed at 180°C). Figure S4: (a) I-V curves of various films under dark condition. (b) M-S plot of various films. The electrodes were submerged in a 0.5 M KCl solution with a Pt counter electrode and Ag/AgCl reference electrode. (c) PL spectra and (d) TRPL spectra of the perovskite films based on various SnO2 film. (e) Fitting curves from Nyquist plots for various SnO2 devices. The inset is the corresponding equivalent circuit. Figure S5: J-V curves of the PSCs based on m-SNO doped with (a) Sc3+, (b) Y 3+, and (c) La 3+ in different concentrations. (d)–(g) Average photovoltaic data of the devices with Ln-doped SnO2 scaffold. Average photovoltaic parameters of the each PSC device were obtained from 20 devices to ensure the reliability and repeatability of data. Figure S6: (a), (b) Dependence of JSC and VOC on light intensity of PSCs. (c) Dark J-V curves of PSCs. (d) J-V curves under dark conditions for the electron-only devices with the inserted structure. Table S1: pore properties of 3% Y-doped m-SnO2 and undoped m-SnO2. Table S2: band edge (Eg), Fermi level (EF), valence band (EVB), and conduction band (ECB) of samples. Table S3: fitted data from TRPL spectra in Figure S4d. Table S4: photovoltaic and impedance data of the PSCs. Table S5: PCE comparison of the PSCs based on full SnO2 mesoporous structure [S1-S8]. Table S6: photovoltaic data of the PSCs with different Ln3+ (Sc3+, Y3+, La3+) concentrations. Table S7: average photovoltaic data of the PSCs. The average values were obtained from 20 devices. Table S8: photovoltaic parameters of the PSCs scanning in different directions. Table S9: PCE values for 3%-SNOY and m-TiO2 devices. Average values were obtained from 8 devices.