Abstract
BACKGROUND
The global epidemiology of type 1 diabetes (T1D) is not yet well known, as no precise data are available from many countries. T1D is, however, characterized by an important variation in incidences among countries and a dramatic increase of these incidences during the last decades, predominantly in younger children. In the United States and Europe, the increase has been associated with the gross domestic product (GDP) per capita. In our previous systematic review, geographical variation of incidence was correlated with socio-economic factors.
AIM
To investigate variation in the incidence of T1D in age categories and search to what extent these variations correlated with the GDP per capita.
METHODS
A systematic review was performed to retrieve information about the global incidence of T1D among those younger than 14 years of age. The study was carried out according to the PRISMA recommendations. For the analysis, the incidence was organized in the periods: 1975-1999 and 2000-2017. We searched the incidence of T1D in the age-groups 0-4, 5-9 and 10-14. We compared the incidences in countries for which information was available for the two periods. We obtained the GDP from the World Bank. We analysed the relationship between the incidence of T1D with the GDP in countries reporting data at the national level.
RESULTS
We retrieved information for 84 out of 194 countries around the world. We found a wide geographic variation in the incidence of T1D and a worldwide increase during the two periods. The largest contribution to this increase was observed in the youngest group of children with T1D, with a relative increase of almost double when comparing the two periods (P value = 2.5 × e-5). Twenty-six countries had information on the incidence of T1D at the national level for the two periods. There was a positive correlation between GDP and the incidence of T1D in both periods (Spearman correlation = 0.52 from 1975-1999 and Spearman correlation = 0.53 from 2000-2017).
CONCLUSION
The incidence increase was higher in the youngest group (0-4 years of age), and the highest incidences of T1D were found in wealthier countries.
Keywords: Type 1 diabetes, Incidence, Children, Age categories, Gross domestic product per capita
Core tip: Currently, there is information on the incidence of T1D of 43.3% of the 194 countries of the world, of which only 44 countries have national coverage information. We found a wide geographic variation in the incidence of T1D and a worldwide increase in the two periods (1975-1999 and 2000-2017). Comparing the two periods, the relative increase in the incidence occurred in the 0-4 group (1.9 times), followed by the 5-9 group (1.8 times) and 10-14 group (1.4 times). There was a positive correlation between GDP per capita, and the incidence of T1D, where wealthier countries have higher values of incidence.
INTRODUCTION
Type 1 diabetes (T1D) is one of the most common endocrine diseases in childhood and adolescence. Additionally, the diagnosis of T1D has increased considerably in adults[1]. According to the International Diabetes Federation (IDF), it was estimated that more than 86000 children were living with T1D in 2015 around the world[2]. There is a wide geographic variation in the incidence of T1D, both among countries and within the different regions in these countries. In North America and Europe, the incidence varies between 4 and 41 per 100000. The countries that report the highest rates are Switzerland, Finland, Norway, the United Kingdom and Sardinia, with values > 20 per 100000 per year. In contrast, T1D is rare in Asian countries, such as China, where the incidence is approximately 0.1 case per 100000 people each year[3-5]. In Latin America, according to IDF, it is estimated that 45100 children younger than 15 years have T1D[2].(Table 1)
Table 1.
Incidence of type 1 diabetes in individuals aged 0-14 years
| Country | Area | Study period | Inc | Asce % | Info Source | Data collection | Ref. |
| Algeria | |||||||
| Oran | 1990-1999 | 8.60 | NA | PBDR | P or H | DIAMOND et al[6] | |
| Argentina | |||||||
| Cordoba | 1991-1992 | 7.00 | 90.0 | PBDR | P or H | DIAMOND et al[6] | |
| Avellaneda | 1990-1996 | 6.30 | 94.0 | PBDR | P or H | DIAMOND et al[6] | |
| Tierra del Fuego | 1993-1996 | 10.30 | 100.0 | PBDR | P or H | DIAMOND et al[6] | |
| Corrientes | 1992-1999 | 6.60 | 95.0 | PBDR | P or H | DIAMOND et al[6] | |
| Australia | |||||||
| NWa | 2000-2011 | 23.6 | 97 | PBDR | H | Hayne et al[18] | |
| Austria | |||||||
| NW | 2004-2008 | 17.50 | 97.2 | PBDR | P | Patterson et al[19] | |
| Bahamas | |||||||
| NW | 2001-2002 | 10.10 | NA | MBR | P | Peter et al[20] | |
| Barbados | |||||||
| NW | 1990-1993 | 2.00 | NA | PBDR | P or H | DIAMOND et al[6] | |
| Belarus | |||||||
| Gomel, Minsk | 1997-2002 | 5.60 | 100.0 | PBDR | NA | Zalutskaya et al[21] | |
| Belgium | |||||||
| Antwerp | 2004-2008 | 15.90 | 94.9 | PBDR | P | Patterson et al[19] | |
| Bosnia and Herzegovina | |||||||
| Tuzla Canton | 1995-2004 | 6.93 | 100.0 | PBDR | P | Tahirović et al[22] | |
| Republic of Srpska | 1998-2010 | 8.13 | 100.0 | PBDR | P | Radosevic et al[23] | |
| Brazil | |||||||
| São Pauloa (Bauru) | 1986-2015 | 12.8 | 97.7 | PBDR | P | Negrato et al[24] | |
| Rio Grande do Sul (Passo Fundo) | 1996-1999 | 7.00 | 82.5 | PBDR | P or H | DIAMOND et al[6] | |
| Bulgaria | |||||||
| Eastern | 1989-1994 | 6.80 | 99.9 | PBDR | P | Patterson et al[7] | |
| Varna | 1990-1999 | 8.10 | 100.0 | PBDR | P or H | DIAMOND et al[6] | |
| Western | 1990-1999 | 10.70 | 99.5 | PBDR | P or H | DIAMOND et al[6] | |
| Canada | |||||||
| Toronto | 1976-1978 | 9.00 | 97.2 | PBDR | P, H | Ehrlich et al[25] | |
| Manitoba | 1985-1993 | 20.65 | 95.0 | PBDR | P | Blanchard et al[26] | |
| Prince Edward Island | 1990-1993 | 24.50 | 100.0 | PBDR | P or H | DIAMOND et al[6] | |
| Alberta (Edmonton) | 1990-1996 | 23.30 | 85.5 | PBDR | P or H | DIAMOND et al[6] | |
| Calgary | 1990-1999 | 20.60 | 100.0 | PBDR | P or H | DIAMOND et al[6] | |
| Québec | 1989-2000 | 15.34 | NA | PBDR | P | Legault et al[27] | |
| Newfoundland and Labrador | 1987-2010 | 38.68 | NA | PBDR | P, H | Newhook et al[28] | |
| Chile | |||||||
| IX Region | 1980-1993 | 1.37 | 97.0 | PBDR | P | Larenas et al[29] | |
| Santiago of Chile (Communes of Metropolitan region) | 2000-2005 | 6.30 | 100.0 | PBDR | P | Torres-Avilés et al[30] | |
| China | |||||||
| NW | 1988-1994 | 0.47 | 93.0 | PBDR | P, H | Yang et al[31] | |
| Zhejianga | 2007-2013 | 2.02 | 94,6 | PBDR | H | Wu et al[32] | |
| Beijinga | 1995-2010 | 1.7 | NA | PBDR | H | Gong et al[33] | |
| Shanghaia | 1997-2011 | 3.1 | 90 | PBDR | H | Zhao et al[34] | |
| Colombia | |||||||
| Bogotá | 1990-1990 | 3.80 | 97.0 | PBDR | P or H | DIAMOND et al[6] | |
| Cali | 1995-1999 | 0.50 | NA | PBDR | P or H | DIAMOND et al[6] | |
| Croatia | |||||||
| NWa | 2004-2012 | 17.2 | 96.69 | PBDR | H | Rojnic Putarek et al[35] | |
| Cuba | |||||||
| NW | 1990-1999 | 2.30 | 62.5 | PBDR | P or H | DIAMOND et al[6] | |
| Cyprus | |||||||
| NW | 1990-2009 | 12.34 | 50.0 | PBDR | NA | Skordis et al[36] | |
| Czech Republic | |||||||
| NW | 2004-2008 | 19.30 | 97.4 | PBDR | P | Patterson et al[19] | |
| Dem. People's Republic of Korea | |||||||
| NWa | 2012-2014 | 3.1 | NA | PBDR | P or H | Kim et al[37] | |
| Denmark | |||||||
| NW | 2004-2008 | 25.10 | 99.2 | PBDR | P | Patterson et al[19] | |
| Dominican Republic | |||||||
| NW | 1995-1999 | 0.50 | 53.0 | PBDR | P or H | DIAMOND et al[6] | |
| Egypt | |||||||
| Alexandria, Damanhour | 1992-1992 | 8.00 | NA | OPD | NA | Arab et al[38] | |
| Northerna | 1996-2011 | 1.93 | NA | MBR | H | El-Ziny et al[39] | |
| Estonia | |||||||
| NW | 1983-2006 | 13.09 | 98.0 | PBDR | P, H | Teeäär et al[40] | |
| Ethiopia | |||||||
| Gondar | 1995-2008 | 0.33 | NA | MBR | P | Alemu S et al[41] | |
| Jimma | 2002-2008 | 0.33 | NA | MBR | P | Alemu S et al[41] | |
| Fiji | |||||||
| NWa | 2001-2012 | 0.93 | NA | PBDR | H | Ogle et al[42] | |
| Finland | |||||||
| NW | 2006-2011 | 62.42 | NS | OPD | NA | Harjutsalo et al[43] | |
| France | |||||||
| Franche-Comté | 1980-1998 | 7.01 | 80.6 | PBDR | H | Mauny et al[44] | |
| Aquitanie, Lorraine, Normandia Basse, Normandia Haut | 1990-1994 | 8.50 | 97.0 | PBDR | P or H | DIAMOND et al[6] | |
| Aquitaine | 1998-2004 | 12.20 | NA | OPD | NA | Barat et al[45] | |
| Languedoc-Roussillona | 2000-2010 | 16.2 | NA | PBDR | NA | Trellu et al[46] | |
| Georgia | |||||||
| NW | 1998-1999 | 4.60 | NA | OPD | NA | Arab et al[38] | |
| Germany | |||||||
| NWa | 2004-2008 | 22.9 | 97 | PBDR | H | Bendas et al[47] | |
| Greece | |||||||
| NW | 1992-1992 | 6.03 | NA | PBDR | P | Dacou-Voutetakis et al[48] | |
| Hungary | |||||||
| 18 of 19 countries (All, less Budapest) | 2004-2008 | 18.30 | 98.7 | PBDR | P | Patterson et al[19] | |
| Iceland | |||||||
| NW | 1989-1994 | 13.50 | 100.0 | PBDR | P | Patterson et al[7] | |
| India | |||||||
| Madras | 1991-1994 | 11.00 | 90.0 | PBDR | H | Ramachandran et al[49] | |
| Iran (Islamic Republic of) | |||||||
| Fars | 1991-1996 | 3.68 | 100.0 | PBDR | P | Pishdad[50] | |
| Ireland | |||||||
| NWa | 2008-2013 | 28.3 | 96,8 | PBDR | P | Roche et al[51] | |
| Israel | |||||||
| NWa: Population: Arabs | 2004-2010 | 9.14 | NA | PBDR | P | Blumenfeld et al[52] | |
| NWa: Population: Jews | 2004-2010 | 13 | NA | PBDR | P | Blumenfeld et al[52] | |
| Italy | |||||||
| Apuliaa | 2001-2013 | 17.99 | NA | PBDR | P | Di Ciaula[53] | |
| Friuli-Venezia Giuliaa | 2010-2013 | 17.55 | NA | MBR | H | Valent et al[54] | |
| Abruzzoa | 1999-2008 | 14.30 | 95 | PBDR | H | Altobelli et al[55] | |
| Venetoa | 2006-2013 | 17.00 | NA | PBDR | H | Marigliano et al[56] | |
| NW-39.7% population | 1990-2003 | 12.55 | NA | PBDR | P | Bruno et al[57] | |
| Japan | |||||||
| NWa | 2005-2012 | 2.14 | NA | PBDR | H | Onda et al[58] | |
| Jordan | |||||||
| NW | 1992-1996 | 3.33 | 95.0 | NS | P, H | Ajlouni et al[59] | |
| Kuwait | |||||||
| NWa | 2011-2013 | 41.7 | 96.7 | PBDR | H | Shaltout et al[60] | |
| Latvia | |||||||
| NW | 1990-1999 | 7.40 | NA | PBDR | P or H | DIAMOND et al[6] | |
| Libyan Arab Jamahiriya | |||||||
| Benghazi | 1991-1999 | 9.00 | NA | PBDR | P or H | DIAMOND et al[6] | |
| Lithuania | |||||||
| NW | 2004-2008 | 14.20 | NA | PBDR | P | Patterson et al[19] | |
| Luxembourg | |||||||
| NW | 2004-2008 | 19.00 | 100.0 | PBDR | P | Patterson et al[19] | |
| Malta | |||||||
| NW | 2006-2010 | 23.87 | 100.0 | PBDR | P | Formosa et al[61] | |
| Mauritius | |||||||
| NW | 1990-1994 | 1.30 | 67.5 | PBDR | P or H | DIAMOND et al[6] | |
| Mexico | |||||||
| NW | 2000-2010 | 5.93 | NA | PBDR | H | Gómez-Díaz et al[62] | |
| Montenegro | |||||||
| NWa | 1997-2011 | 18.6 | 100 | PBDR | H | Samardžić et al[63] | |
| Netherlands | |||||||
| NWa | 1999-2011 | 25.2 | NA | PBDR | H | Fazeli et al[64] | |
| New Zealand | |||||||
| NW | 1999-2000 | 18.00 | 95.0 | PBDR | NA | Campbell-Stokes et al[65] | |
| Norway | |||||||
| NWa | 2004-2012 | 32.7 | NA | PBDR | H | Skrivarhaug et al[66] | |
| Oman | |||||||
| NW | 1993-1995 | 2.59 | 96.0 | PBDR | P | Soliman et al[67] | |
| Pakistan | |||||||
| Karachi | 1990-1999 | 0.50 | 51.0 | PBDR | P or H | DIAMOND et al[6] | |
| Papua New Guinea | |||||||
| NW | 1996-2000 | 0.08 | NA | MBR | P | Ogle et al[68] | |
| Paraguay | |||||||
| NW | 1990-1999 | 0.90 | NA | PBDR | P or H | DIAMOND et al[6] | |
| Peru | |||||||
| Lima | 1990-1994 | 0.50 | 67.5 | PBDR | P or H | DIAMOND et al[6] | |
| Poland | |||||||
| NW | 1989-2004 | 11.23 | NA | PBDR | P | Jarosz-Chobot et al[69] | |
| Krakow and the Lesser Polanda | 2004-2011 | 15.87 | NA | PBDR | H | Wojcik et al[70] | |
| Podlasie, Silesia, Łódzkie, Pomorskie, Bydgoszcza | 2005-2012 | 20.22 | NA | PBDR | H | Chobot et al[71] | |
| Portugal | |||||||
| Algarve | 1990-1994 | 14.60 | 87.0 | PBDR | P or H | DIAMOND et al[6] | |
| Portoalegre | 1990-1994 | 21.30 | 93.0 | PBDR | P or H | DIAMOND et al[6] | |
| Coimbra | 1990-1999 | 9.60 | 100.0 | PBDR | P or H | DIAMOND et al[6] | |
| Madeira Island | 1990-1999 | 6.90 | 100.0 | PBDR | P or H | DIAMOND et al[6] | |
| Qatar | |||||||
| NW | 1992-1996 | 11.40 | NA | OPD | NA | Al-Zyoud et al[72] | |
| Republic of China (Taiwan) | |||||||
| NWa | 2003-2010 | 5.45 | NA | PBDR | H | Lin et al[73] | |
| Romania | |||||||
| NWa | 2002-2011 | 9.6 | 96,2 | PBDR | H | Serban et al[74] | |
| Russian Federation | |||||||
| Novosibirsk | 1990-1999 | 6.90 | 93.5 | PBDR | P or H | DIAMOND et al[6] | |
| Moscow | 1996-2005 | 12.07 | 94.0 | PBDR | P | Pronina et al[75] | |
| Rwanda | |||||||
| capital and 6 regionsa | 2004-2011 | 2.7 | NA | MBR | H | Marshal et al[76] | |
| Saudi Arabia | |||||||
| Eastern Province | 1986-1997 | 12.30 | 100.0 | PBDR | NA | Kulaylat et al[77] | |
| Al-Madinah (North West) | 2004-2009 | 30.88 | NA | PBDR | P | Habeb et al[78] | |
| Serbia | |||||||
| Belgrade | 2000-2004 | 12.90 | NA | OPD | NA | Vlajinac et al[79] | |
| Singapore | |||||||
| NW | 1992-1994 | 2.42 | 92.2 | PBDR | P | Lee et al[80] | |
| Slovakia | |||||||
| NW | 1999-2003 | 13.60 | 100.0 | PBDR | P | Patterson et al[19] | |
| Slovenia | |||||||
| NW | 1998-2010 | 13.83 | 100.0 | PBDR | P | Radosevic et al[23] | |
| Spain | |||||||
| Madrid | 1985-1988 | 10.60 | 90.0 | PBDR | H | Serrano Ríos et al[81] | |
| Cáceres | 1988-1999 | 16.67 | 99.2 | PBDR | H | Lora-Gómez et al[82] | |
| Badajoz | 1992-1996 | 17.23 | 95.0 | PBDR | P | Morales-Pérez et al[83] | |
| Navarrea | 1975-2011 | 13.2 | NA | PBDR | H | Forga et al[84] | |
| Catalonia | 2004-2008 | 12.10 | 97.6 | PBDR | P | Patterson et al[19] | |
| Castilla y Leóna | 2000-2013 | 10.8 | NA | PBDR | H | Vega et al[85] | |
| Biscaya | 1990-2013 | 10.7 | 99,1 | PBDR | H or P | Fernández-Ramos et al[86] | |
| Sudan | |||||||
| Gezira | 1990-1990 | 5.00 | 100.0 | PBDR | P or H | DIAMOND et al[6] | |
| Khartoum | 1991-1995 | 10.10 | 97.0 | PBDR | NA | Elamin et al[87] | |
| Sweden | |||||||
| NWa | 2007-2011 | 42 | 99 | PBDR | N | Rawshani et al[88] | |
| Switzerland | |||||||
| NW | 2004-2008 | 13.10 | 91.3 | PBDR | P | Patterson et al[19] | |
| TFYR Macedonia | |||||||
| NW | 2004-2008 | 5.80 | 100.0 | PBDR | P | Patterson et al[19] | |
| Thailand | |||||||
| North-eastern | 1996-2005 | 0.58 | NA | MBR | H | Panamonta et al[89] | |
| Tunisia | |||||||
| Beja, Monastir, Gafsa | 1990-1994 | 6.69 | 96.0 | PBDR | P | Ben Khalifa et al[90] | |
| Kairoan | 1991-1993 | 7.60 | NA | PBDR | P or H | DIAMOND et al[6] | |
| Beja | 1990-1999 | 7.70 | NA | PBDR | P or H | DIAMOND et al[6] | |
| Gafsa | 1990-1999 | 8.50 | NA | PBDR | P or H | DIAMOND et al[6] | |
| Monastir | 1990-1999 | 5.80 | NA | PBDR | P or H | DIAMOND et al[6] | |
| Turkey | |||||||
| NWa | 2011-2013 | 10.8 | 99 | PBDR | H | Yeşilkaya et al[91] | |
| Ukraine | |||||||
| NW | 1985-1992 | 8.10 | NA | OPD | NA | Timchenko et al[92] | |
| United Kingdom | |||||||
| NW | 1991-2008 | 19.32 | NA | PBDR | P | Imkampe et al[93] | |
| United Republic of Tanzania | |||||||
| Dar es Salaam | 1982-1991 | 0.92 | NA | MBR | P | Swai et al[94] | |
| United States of America | |||||||
| Olmsted, Minnesotaa | 1994-2010 | 19.9 | NA | MBR | H | Cartee et al[95] | |
| Five areasa | 2002-2013 | 19.5 | 98,9 | PBDR | H | Mayer-Davis et al[96] | |
| Uruguay | |||||||
| Montevideo | 1992-1992 | 8.30 | 97.0 | PBDR | P or H | DIAMOND et al[6] | |
| Uzbekistan | |||||||
| NWa | 1998-2014 | 2.48 | 100 | PBDR | H | Rakhimova et al[97] | |
| Venezuela (Bolivarian Republic of) | |||||||
| Caracas | 1990-1994 | 0.10 | NA | PBDR | P or H | DIAMOND et al[6] | |
Update of the publications that report the incidence of type 1 diabetes from population-based studies.
Updated studies. Area and NW: Study at the national level. ASCE%: Percentage of completeness between primary and secondary sources of registers. PBDR: Registration of population-based data; MBR: Medical-based record; OPD: Other population denominators; NS: Non-specified; P: Prospective -incident cases collected prospectively-; H: Historical -incident cases collected retrospectively-; NA: Information not available.
There are differences in the incidence rates among age categories (0-4, 5-9, 10-14) in almost all countries. According to DIAMOND[6], for the period between 1991-1996, it was noted that the incidence increased with age; children between 5 and 9 years had 1.62 times the risk of children 0-4 years, i.e., a 62% excess risk, and the 10-14 age group had 1.93 times the risk of the 0-4 age group. Recently, there have been signs suggesting that this trend is changing. Records of the Patterson et al[7] between 1999 and 2008, showed that the incidence was highest in the youngest age group (0 to 4 years), with an increase of 5.4% compared to 4.3% in the 5-9 age group and 2.9% in the 10-14 age group.
In addition, the IDF has suggested the existence of a relationship between income level and the incidence of T1D[2]. In the United States, where the incidence of T1D in different socio-economic groups was studied, it was found that there was a higher incidence of T1D in the highest income groups[8]. The same pattern occurs in Europe, where it was shown that the incidence of T1D correlates strongly with the gross domestic product (GDP). GDP is most commonly used to measure the size of a country’s economy. However, there have been conflicting results. For example, an ecological study carried out in North Rhine-Westphalia, Germany showed that the risk of T1D was higher in children living in socially disadvantaged areas[9].
In a previous systematic review, we identified T1D incidence in 80 out of 194 countries and found significant associations between the geographical variation of incidence and a series of economic, climatic and environmental, and health conditions factors[10]. Among these factors, GPD per capita was highly correlated with the 0-14-year incidence of T1D (Spearman Correlation = 0.72, P value = 9.05 × e-14).
Here, we focus on three age categories (0-4, 5-9, 10-14) and two periods (1975-1999 and 2000-2017). We searched, through a systematic review of the literature, the global variation in the incidence of T1D in these age categories and periods. We then searched to what extent these variations correlated with the GDP per capita in these countries.
MATERIALS AND METHODS
In this study, we updated the review on the global T1D incidence published by Diaz-Valencia et al[10] with new papers. Once the incidence data were obtained through the systematic review, we conducted an exploratory ecological analysis. Following the procedures mentioned by Morgenstern[11] for ecological studies, we analysed the relations of population rates of T1D incidence and the average GDP of these countries, retrieved from the World Bank database. This analysis was divided into two periods (1975-1999 and 2000-2017).
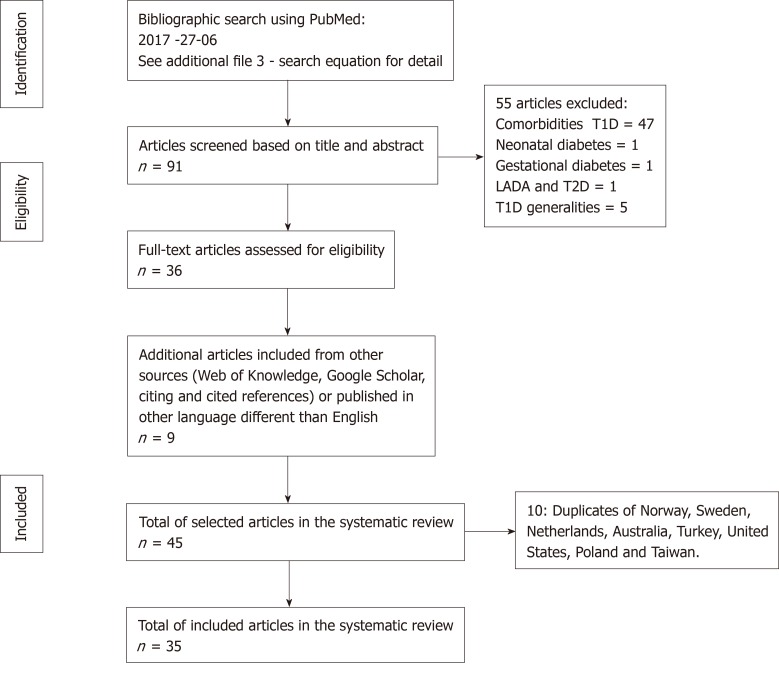
We extracted information on the incidence of T1D in children under 14 years from population-based studies conducted in clearly defined geographical areas at the local, regional or national level, published in original articles in English, Spanish or French, following the PRISMA recommendations. The databases used for the literature search were Medline and Thomson Reuters (Web of Knowledge). Additionally, we explored Google Scholar. This study followed the protocol search deposited in the International Prospective of Systematic Reviews with the Registration Number: CRD42012002369. Figure 1 presents the flow diagram of the bibliographic search.
Figure 1.
Flow diagram of the bibliographic search strategy. T1D: Type 1 diabetes; T2D: Type 2 diabetes; LADA: Latent autoimmune diabetes in adults.
During this systematic review, several procedures were standardized to minimize the possibility of incurring biases in the identification of literature, selection and the interpretation of evidence. To reduce potential biases during the design and execution of the systematic review, a team was created; initially, this team was formed by a senior expert researcher (AJV) and a researcher (PAD). During the update of this systematic review, the team consisted of two researchers (NGL and PAD). The initial search was undertaken between November 2011 and January 2014 and the update between January and June 2017. For this systematic review a query equation was used, which implemented the same strategy as that validated by Diaz-Valencia et al[10] (Supplementary material).To build the original query equation, we performed an exploratory search, from which 92 references were selected that reported on the incidence of T1D. From these 92 references, we analysed the MeSH terms and incorporated them into a preliminary search equation. Using this equation, we excluded the MeSH terms of references that did not report the incidence of T1D in the search equation. We repeated this process until the search equation included the 92 references used initially.
For all query equations, studies were excluded if (1) The main objective was not to study the incidence of T1D (e.g., genetic factors, complications, treatments); (2) The study was not population-based and instead it was performed in selected groups, such as studies based on volunteer subjects or people belonging to a specific health insurance organization; (3) The study did not report using the World Health Organization (1985 or 1999) or American Diabetes Association (1997 or 2011) diagnosis criteria; (4) The study described the incidence of T1D as a general topic, with no description by year and age at diagnosis; (5) We could not translate the article; or (6) The full text of the article was unavailable.
Quality assessment
The quality of the included studies was evaluated independently by 2 reviewers (NGL-PAD) using the evaluation criteria proposed by Loney et al[12] as an external validation. We also implemented an internal quality assessment. The external validation consisted of eight criteria, (1) Was the target population clearly described? (2) Were cases ascertained either by survey of the entire population or by probability sampling? (3) Was the response rate > 70%? (4) Were the non-responders clearly described? (5) Was the sample representative of the population? (6) Were data collection methods standardized? (7) Were validated diagnostic criteria or approaches used to assess the presence/absence of disease? and (8) Were the estimates of incidence given with confidence intervals? An article’s score was obtained by adding up the number of criteria it satisfied. Every satisfied criterion was given 1 point. There was no cut-off score for rating low-quality studies; we arbitrarily considered 0-4, 5-6 and 7-8 points as high, medium and low risk of bias, respectively.
The internal validation was based on 5 criteria. (1) The percentage of completeness between primary and secondary sources of registers. A percentage greater than 90 scored a 1, less than 90 scored a 0.5 and unavailable information scored a 0; (2) Information source: If the data came from the registration of population-based data, it was assigned a 1; if the data came from medical-based records or population denominators it was assigned a 0.5; and if the source was non-specified, it scored a 0; (3) Data collection: If the cases were collected prospectively (P) or retrospectively (H) was assigned a 1; and if the information was not available, it scored a 0; (4) Clear criterion for diagnosis scored a 1; And (5) if the study was population-based, it scored a 1. We arbitrarily considered 0-3, 3.5 and 4-5 as high, medium and low risk of bias, respectively.
Data collection
Two reviewers (Diaz-Valencia PA and Gomez-Lopera N) extracted and reached agreement on the data from included articles using a standard data collection form. We included in this systematic review the most updated and comprehensive data. In each of the articles analysed, we extracted the following information: (1) Identification of the study: Authors, title, journal, year of publication; (2) Period and country of study: Countries were categorized by region according to the United Nations[13]; (3) Geographical coverage of the study: Nationwide (when the study was conducted across the whole nation) or local (when it was restricted to a region, city or geographically defined population); (4) Incidence rates expressed as new cases per 100000 people (both sexes) per year in the age categories 0-4, 5-9, 10-14, and 0-14. The rates were retrieved from either tables or graphs. If we found incidence values in the graphics, we extracted them using GraphClick[14]. This program allows the user to automatically retrieve the original data from the x and y coordinates of images. Efforts were made to obtain the value of incidence of T1D for each country at the national level. When no information was retrieved at the national level, local studies were considered. In the database, we identified the level of coverage as national or local; (5) The incidence information from two periods was searched: The first was between 1975-1999, and the second was between 2000-2017. We based this separation on a bimodal trend observed in the years of the publications identified in the previous systematic review[10]; And (6) We collected the percentage of completeness/ ascertainment when available.
GPD per capita
The GDP per capita was used to carry out an exploratory ecologic analysis of the relationship between the change in the incidence of T1D and the differences in socio-economic levels during two periods (1975-1999 and 2000-2017). The World Bank database[15] was used to extract the information for GDP per capita that indicated the relationship between the total value of all the goods and services generated during a year by the economy of a nation or state and the number of its inhabitants in that year. For each study period, we calculated the average of the values of the T1D incidence. In addition, for homogeneity in our analysis, we only chose countries with data at the national level.
Statistical analysis
We presented all the collected data graphically on maps that contain the information obtained from countries at the national level, in two timeframes (1975-1999 and 2000-2017) using the software Tableau[16]. We compared the incidence of T1D for countries that have information from 1975-1999 and 2000-2017 at the national level in the categories of ages 0-4, 5-9 and 10-14, comparing the means of paired samples.
We performed a correlation to analyse the relationship between the change in incidence of T1D and the change in GDP per capita using the Spearman test and linear regression models to predict the change in the incidence of T1D according to change in the GDP per capita by countries at the national level. Model assumptions for linear models were checked by visual inspection of the residuals. We used the program R version 3.3.3[17] to perform the statistical analysis and create graphics related to the study. In all cases, we considered that a P value less than 0.05 was statistically significant.
RESULTS
This systematic review of the literature presented information available at the global level on the incidence of T1D and retrieved data for 84 countries, representing 43.3% of the 194 countries of the world. We included 35 additional studies from the previous systematic review[10]. Among these 35 new papers, we retrieved information for 25 countries; some of them reported data at the national and others at the local level (Figure 1). Updated studies were identified by superscript letters (a) in Table 1. It was possible to update the information published by Diaz-Valencia et al[10] for 21 countries and obtain data for 4 additional countries: Fiji, Turkey, Rwanda and Republic of China (Taiwan). Of the 84 countries, data were collected at the national level for 44 and the local level for 40.
The median study quality score for studies reporting on the incidence of T1D was high in both cases, with a mean quality score of 7.18 of 8 possible [standard deviation (SD): 0.80] using the external validation, and with a mean quality score of 4.37 of 5 possible [standard deviation (SD): 0.71] using the internal validation. All studies described the target population in detail and used validated diagnostic criteria to assess the presence of disease. Most studies used standardized data collection methods and reported estimates with their accompanying confidence intervals. We found 93.94% concordance between the internal and external validation.
We observed a wide geographical variation in the incidence of T1D at the global level (Table 1). In general, the incidence of T1D was highest in Europe (> 15 per 100000 per year), followed by North America, Australia, Asia, Central and South America. In children from 0-14 years-old, the lowest incidence at the national level (< 1 per 100000 per year) occurred in Thailand; Papua, New Guinea; Fiji; the Dominican Republic; and Paraguay. In contrast, the highest incidence at the national level occurred in Finland, Sweden, Norway and Kuwait, with 62.42, 42, 32.7 and 41.7 per 100000 inhabitants per year, respectively.
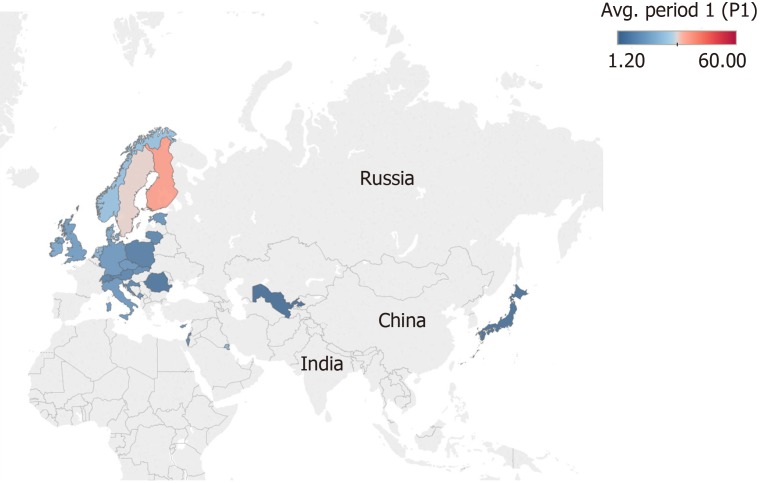
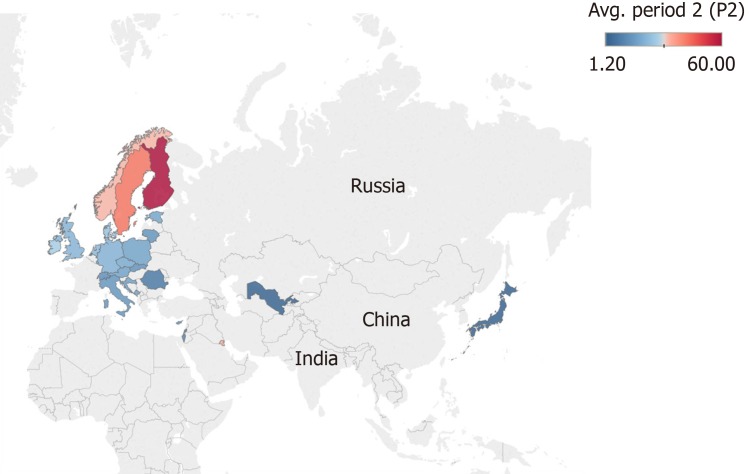
We retrieved and compared 26 countries that had information at the national level regarding the incidence of T1D for the periods 1975-1999 and 2000-2017 in individuals from 0-14 years (Figures 2 and 3). In general, an increase in the incidence of T1D is noted at the global level. In the 26 countries, these values were almost double. For example, in Kuwait, the incidence value was 22.3 for the period of 1992-1997[98] and 41.7 for the period of 2011-2013[60] (equivalent to a ratio of 1.86).
Figure 2.
Map showing the mean incidence of type 1 diabetes in 26 countries from 1975-1999. The colour scale represents the level of incidence ranging from 1.20 in blue to 60 per 100000 individuals in red aged 0-14 years.
Figure 3.
Map showing the mean incidence of type 1 diabetes in 26 countries from 2000-2017. The colour scale represents the level of incidence ranging from 1.20 in blue to 60 per 100000 individuals in red aged 0-14 years.
Additionally, we analysed three distinct categories for age in 15 countries that had information at the national level in the two periods considered in this study (Table 2). We observed an increased in the incidence. In absolute numbers, the period 1975-1999 showed that the incidence increased with age, where the lowest incidence was found in children under the age of 5 years and the highest in children older than 10 years. In the period 2000-2017, there was a higher incidence in the category of 5-9 years, followed by 10-14, and the lowest was found in 0-4. However, comparing the two periods, the relative increase in the incidence of T1D occurred in the 0-4 group (1.9 times), followed by the 5-9 group (1.8 times) and 10-14 group (1.4 times). We performed an extra analysis of all countries reporting incidence values for each age category without taking into account whether they reported the incidence at the local or national level, finding equivalent results (data not shown).
Table 2.
Summary values for the comparison of the incidence of type 1 diabetes for countries with nationwide data by age category in the periods analysed in the study
| 0-4 yr | 5-9 yr | 10-14 yr | ||||
| Period | 1975-1999 | 2000-2017 | 1975-1999 | 2000-2017 | 1975-1999 | 2000-2017 |
| Mean | 6.68 | 12.59 | 11.92 | 21.99 | 14.04 | 19.54 |
| 95%CI | (4.49, 8.87) | (9.23, 15.96) | (7.95, 15.96) | (14.80, 29.18) | (9.72, 18.38) | (15.09, 23.99) |
| T student | -6.31 | -4.58 | -3.22 | |||
| P value | 0.00002 | 0.00043 | 0.006 | |||
| Ratio periods | 1.9 | 1.8 | 1.4 | |||
CI: Confidence Interval.
GDP per capita
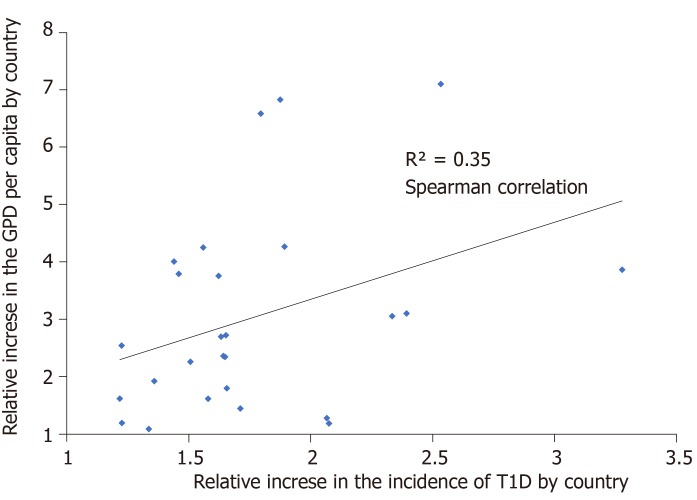
In general, there was a positive correlation between GDP per capita and the incidence of T1D. A positive correlation was found for the relation between the relative change in T1D incidence and the relative change in GDP for the countries reporting data at national level (Spearman correlation 0.35) (Figure 4).
Figure 4.
Observed relation between the ratios of the increase in incidence and gross domestic product in 26 countries.
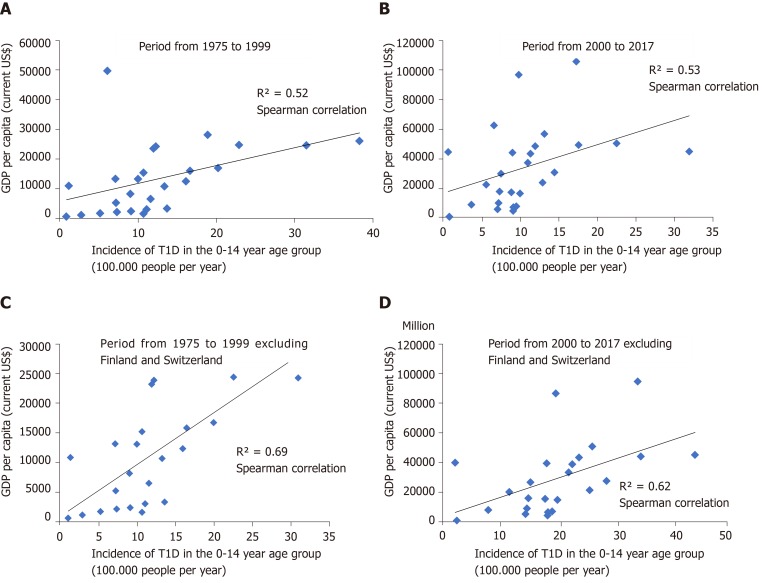
Analysing the two periods, we found a positive correlation between incidence of T1D and GPD per capita among 26 countries (Spearman correlation = 0.52 between 1975-1999 and Spearman correlation = 0.53 between 2000-2017). Excluding Finland and Switzerland because of their extreme values in T1D incidence and GDP per capita, respectively, we retrieved a Spearman correlation = 0.69 between 1975-1999 and Spearman correlation = 0.62 between 2000-2017 (Figure 5). From the linear regression model, including the 26 countries, it was suggested that for 1991 the country-to-country variation in GDP explained 9% of the country-to-country variation in incidence (adjusted R2 of the model 0.09), while, for the year 2006, it was 17% (adjusted R2 of 0.17) (Table 3). We performed the same analysis excluding Finland and Switzerland. We found for 1991 that the change in the GDP explained 44% the change in incidence of T1D (adjusted R2 of the model 0.44), while, for 2006, it was 22% (adjusted R2 of 0.22) (Table 4).
Figure 5.
Correlation between incidence of type 1 diabetes and gross domestic product per capita for countries with information at the national level in two periods. A: Analysis for 26 countries period from 1975 to 1999; B: Analysis for 26 countries, period from 2000 to 2017; C: Analysis excluding Finland and Switzerland, period from 1975 to 1999; D: Analysis excluding Finland and Switzerland, period from 2000 to 2017.
Table 3.
Models of change in the incidence of type 1 diabetes for 26 countries with nationwide data according to the change in gross domestic product
| Model GDP per capita | Coefficient | Est | CI 2.5% | CI 97.5% | SE | t value | P value |
| Year 1991: Residual standard error: 6.63 Adjusted R2: 0.09509; F-statistic: 3.312 on 1 and 21 DF; P value: 0.08307 | Intercept | 8.99 | 4.63 | 13.36 | 2.1 | 4.28 | 0.0003 |
| GDP per capita | 0.0002 | 0.0000 | 0.0005 | 0.0001 | 1.8000 | 0.0830 | |
| Year 2006: Residual standard error: 8.6; Adjusted R2: 0.176; F-statistic: 5.698 on 1 and 21 DF. P value: 0.02647 | Intercept | 13.5 | 7.34 | 19.66 | 2.96 | 4.56 | 0.0002 |
| GDP per capita | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 2.39 | 0.0260 |
Est: Estimator; CI: Confidence Interval; SE: Standard Error; Df: Degree freedom; GDP: Gross domestic product.
Table 4.
Models of change in the incidence of type 1 diabetes for countries with nationwide data according to the change in gross domestic product, excluding Finland and Switzerland
| Model GDP per capita | Coefficient | Est. | CI 2.5% | CI 97.5% | SE | t value | P value |
| Year 1991: standard residual error: 5.08; adjusted R2: 0.44. F statistic: 19.37. 22 DF. P value: 0.0002 | Intercept | 5.82 | 2.27 | 9.36 | 1.71 | 3.40 | 0.002 |
| GDP Per capita | 0.0005 | 0.0003 | 0.0008 | 0.0001 | 4.1220 | 0.0002 | |
| Year 2006: Standard residual error: 8.08; adjusted R2: 0.22; F statistic: 7.62. 22 DF. P value: 0.01 | Intercept | 13.57 | 8.16 | 18.98 | 2.61 | 5.21 | 0.00003 |
| GDP Per capita | 0.0002 | 0.00004 | 0.0003 | 0.0001 | 2.76 | 0.01 |
Est: Estimator; CI: Confidence Interval; SE: Standard Error; Df: Degree freedom; GDP: Gross domestic product.
DISCUSSION
In this study, we updated our previous results on the global incidence of T1D in individuals aged 0-14 years and its variation over time. We analysed the trend in two periods for age categories and GDP per capita. In general, there was a wide geographic variation in the 84 countries for which the incidence of T1D was reported. This variability could be explained to some extent by ethnic differences in allele and haplotype frequencies of risk alleles between populations, for example, in the HLA region, which explains almost 50% of the genetic component of the disease[99]. There has been a strong association between a high frequency of pre-disposition for HLA haplotypes and a high incidence of T1D. For example, research in the United States, based on the presence of two high-risk haplotypes of HLA-DR3/DR4, revealed that Caucasians have a higher risk of developing T1D than other ethnic groups[100]. It has been demonstrated that unlike Europeans, DR susceptibility alleles in Asian populations (whose incidence is lower) are in strong linkage disequilibrium with DQ neutral alleles or even protectors, and it is believed that these effects contribute to the low incidence of T1D in these populations[101,102].
Although there is a consensus on the effect of the genetic susceptibility to T1D between different ethnic groups, these differences cannot fully explain the global variability and the increase in incidence. In this study, we observed an increase in the incidence of T1D worldwide when comparing the periods 1975-1999 and 2000-2017 (Figures 2 and 3). The mechanisms behind the enigma in the increase in the incidence are unknown. However, the mechanism have been attributed to external factors, such as those related to the environment and lifestyle, which may be involved in the epidemiology of the disease[103].
We also observed an increase in the incidence of T1D in all age categories (0-4, 5-9 and 10-14). In the period 1975-1999, the incidence increased with age, with a peak in children aged 10-14 years. This pattern could be attributed to the onset of puberty with resistance to insulin; therefore, the demand for insulin secretion increases[104]. In contrast, for the period 2000-2017, there appeared to be an increasing number of patients in the 5-9 age group and a greater relative rise in the 0-4 age group. The mechanisms underlying the increased incidence of T1D in the youngest children are unknown but have largely been attributed to environmental influences[55].
The environment may act in diverse ways, either by enhancing autoimmunity or modulating normal mechanisms of protection against the development of the disease[105]. It can be speculated that the plausible causes of temporal changes in the incidence of T1D are attributed to environmental factors, such as social, economic, dietary and health-related factors, which have changed rapidly over the last century.
An example of these changes is socio-economic factors. This study analysed the relationship between one socio-economic indicator (GDP per capita) and the incidence of T1D and found that the highest incidences of the disease were reported in wealthier countries. This same pattern was found by Patterson et al[106] in a study conducted throughout Europe. Similarly, studies conducted at the country level in Sweden[107], United Kingdom[108], and Italy[109] described similar associations between T1D and the socio-economic variables. The influence of changes in GDP per capita on the incidence of the disease was higher for the average year 1991 than that after 2006. We wonder if this behaviour could be explained because 2006 was before the financial crisis of 2008 that provoked a fall in the economies of all regions[15].
The geographical associations between the socio-economic situation and the incidence of T1D could be attributed to spatial patterns in the composition of the population, which leads to differences in lifestyle and diet. A positive relationship has been demonstrated between the prosperity of the nation, as measured by GDP, with body mass index (BMI)[110]. Recently, the overload and accelerator hypothesis has been proposed for the increase in body size, BMI and insulin resistance, as well as risk factors for developing T1D[111]. This hypothesis suggests that the increase in BMI and a more sedentary lifestyle cause resistance to insulin, which leads to β-cells being overworked. This process results in apoptosis and increased production of antigens, which triggers an autoimmune response. Therefore, individuals with a genetic predisposition to T1D will develop an autoimmune response, further accelerating the loss of β-cells. Although an increase in weight contributes to insulin resistance, another consequence of overweight is the storage of ectopic fat with glucotoxicity associated with inflammation resulting in an inhibition of gene expression of insulin, which is also involved in the process of apoptosis of β-cells[112].
Additionally, the relationship of the socio-economic level with the incidence of T1D could be explained by the improvement of the standards of hygiene, low rates of infection in childhood and low social contact in early childhood, which are possibly experienced in wealthy countries. This theory is known as the hygiene hypothesis, proposed by Gale[113] in 2002. This hypothesis suggests that changes in hygiene and infection patterns in early childhood alter the development of the immune system and the normal mechanisms of protection against autoimmunity. A study in non-obese diabetic mice, showed that there was a 40%-50% increase in the incidence of T1D when the animals were raised in environments free of pathogens[114]. In general, there has been an inverse trend between the incidence of infectious diseases and the incidence of autoimmune and allergic diseases[115].
Other environmental factors potentially related to national economic prosperity must be mentioned. One of these is the nutritional component that has undergone major changes in many developed countries. Early nutrition seems to modulate the development of T1D, for example, the absence or short duration of breastfeeding and early introduction of cow’s milk formulae are thought to be risk factors for this disease[116]. Also, rapid weight gain in infancy, associated with improper feeding, increases the risk of developing T1D[117]. Other possible factors that experiment in wealthier countries are a higher degree of urbanization, which are associated with an increased incidence of T1D, supporting the hygiene hypothesis[10]. In addition, there are differences in caesarean deliveries between low- and high-income countries, where wealthier countries have high levels of caesarean use without medical indication[118]. Delivered by caesarean section are at slightly increased risk of T1D, and it has been postulated that differences in the gut microbiota of these children compared with those born by normal vaginally delivery[103]. Also, the wealth of countries is associated with environmental pollution. An association between air pollution and T1D incidence has been described. Researchers proposed that chemical and air pollutant exposures have multiple effects that may directly affect the risk of T1D[119].
However, a single environmental factor or interaction between factors, has not been identified that could explain these changes in the incidence of T1D. Moreover, there are complex interactions between genetic and environmental factors that remain to be discovered. More epidemiological studies of T1D are needed to develop new hypotheses about the genetic and environmental factors that trigger the disease, which should be further tested.
Currently, there is information on the incidence of 43.3% of the 194 countries of the world, of which only 44 countries have national coverage information; most of them are European. Despite the efforts of the DIAMOND project[6] to describe the incidence of T1D at a global level, there is little information for countries in Africa, Central and South America. Moreover, the data are not entirely representative for some countries. To extrapolate this information for the whole country, there would be a substantial bias, as there may be variability within large nations in both the genetic component and environmental exposures that trigger the disease.
Another aspect to consider is the lack of continuity of the epidemiological studies. Only 21 countries have updated incidence rates in the systematic review conducted between 2014 and 2017. Moreover, this lack of continuity implies a limitation for our study since we retrieved available information to conduct comparisons in two periods, 1975-1999 and 2000-2017; only 26 countries had data at the national level, and age category data are even less available. These 26 countries are mainly from Europe (n = 23), and Asia (n = 3). Regrettably, we do not have information to conduct comparison in the two periods for countries in Africa, Oceania, and America. It is very important to generate more studies on the epidemiology of T1D. This approach will contribute to understanding the dynamic changes in the disease, which, together with studies in basic sciences such as genetics, could identify the factors that modify the risk to the disease and could probably slow down the current increase in the incidence of T1D.
Limitations of this study are worth noting. Although several procedures were standardized during this systematic review to minimize the possibilities of incurring biases in the identification of literature, selection and interpretation of evidence, we cannot rule out having missed relevant studies also due to publication bias. For example, studies that were published in languages other than English, Spanish or French were not included. An important limitation that is shared by all ecological studies is the possibility of making an ecological fallacy. The implication is that the associations we found are only at the group level, and we cannot assume that they are inferred to each individual in these groups. For example, our results do not necessarily imply that all wealthy countries have a higher incidence of T1D, and our findings only reveal potential associations between GPD and population rates of T1D incidence at the group level. Another limitation of our study is the heterogeneity of the different countries included in the statistical analysis. In addition, available secondary sources of GPD data may not have the same accuracy for all countries, leading to imprecise correlation with the incidence of T1D.
We found a wide geographic variation in the incidence of T1D and a worldwide increase in the two periods considered in this study. The greatest increase was observed in the youngest group of children with T1D (0-4 years), with a relative increase of almost double (P value = 2.47 × e-0.5). Finally, there was a positive correlation between the socio-economic level, as measured by GDP per capita, and the incidence of T1D, where wealthier countries have higher values of incidence.
ARTICLE HIGHLIGHTS
Research background
Type 1 Diabetes (T1D) is a complex disease resulting from the interplay of genetic, epigenetic, and environmental factors. There is a dramatic increase in the incidence of T1D, predominantly in younger children (0-4 years old) worldwide. The cause of this increase is still under study.
Research motivation
This work updates the current knowledge on the global incidence of T1D across age categories and its variation over time. The increase of incidence of T1D has been associated with socioeconomic factors, such as gross domestic product (GDP). However, there have been conflicting results about the relationship between income level and the incidence of T1D.
Research objectives
We searched the global variation in the incidence of T1D in the age categories and two periods (1975-1990 and 2000-2017). We then searched to what extent these variations correlated with the GDP per capita in these countries.
Research methods
We updated through a systematic review, our previous results on the global incidence of T1D in individuals aged 0-14 years. We first retrieved the incidence of T1D data in different age categories (0-4, 5-9, 10-14, 0-14) and divided the incidence information into two periods (1975-1999 and 2000-2017). Then, we conducted an exploratory ecological analysis about the relations of population rates of T1D incidence and the average GDP of these countries. Comparisons of means, correlations, linear regression were made.
Research results
We retrieved incidence data for 84 countries, most of them are European. We observed an increase in the incidence of T1D worldwide when comparing the periods 1975-1999 and 2000-2017. We also observed an increase in the incidence of T1D in all age categories (0-4, 5-9 and 10-14). In the period 1975-1999, the incidence increased with age, with a peak in children aged 10-14 years. For the period 2000-2017, there appeared to be an increasing number of patients in the 5-9 age group and a greater relative rise in the 0-4 age group. Also, we found that the highest incidences of the disease were reported in wealthier countries.
Research conclusions
We found a wide geographic variation in the incidence of T1D and a worldwide increase in the two periods considered in this study, especially in younger children (0-4 years old); showing an early age at onset. Also, we confirmed that there was a positive correlation between the socio-economic level and the incidence of T1D. More studies are required to elucidate the interaction between environmental, immunological and genetic factor.
Research perspectives
This study showed the enormous differences in surveillance and epidemiological reports of T1D worldwide. Most of the countries retrieved from the systematic review are European and few studies were carried out in Central and Latin America, Central Asia and Sub-Saharan Africa. It is very important that the scientific community generates more studies on the epidemiology of T1D that contribute to understanding the changes in the dynamics of the disease.
ACKNOWLEDGEMENTS
We are very grateful to Professor Emmanuel Nieto for his advice in economics from Facultad de Salud Publica, Universidad de Antioquia and to Professor Valleron AJ, Membre de l’Académie des Sciences of France, for his critical reading of and helpful suggestions for this manuscript.
Footnotes
Conflict-of-interest statement: The authors declare no conflict of interest.
PRISMA 2009 Checklist statement: The authors have read the PRISMA 2009 Checklist, and the manuscript was prepared and revised according to the PRISMA 2009 checklist.
Manuscript source: Unsolicited manuscript
Peer-review started: March 11, 2019
First decision: March 10, 2019
Article in press: October 27, 2019
Specialty type: Endocrinology and metabolism
Country of origin: Colombia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Koch TR S-Editor: Wang J L-Editor: A E-Editor: Liu MY
Contributor Information
Natalia Gomez-Lopera, Grupo Mapeo Genetico, Departamento de Pediatría, Facultad de Medicina, Universidad de Antioquia, Medellín 050010470, Colombia. natalia.gomezl@udea.edu.co.
Nicolas Pineda-Trujillo, Grupo Mapeo Genetico, Departamento de Pediatría, Facultad de Medicina, Universidad de Antioquia, Medellín 050010470, Colombia.
Paula Andrea Diaz-Valencia, Epidemiology Group. School of Public Health. Universidad de Antioquia, Medellín 050010470, Colombia.
References
- 1.Diaz-Valencia PA, Bougnères P, Valleron AJ. Global epidemiology of type 1 diabetes in young adults and adults: a systematic review. BMC Public Health. 2015;15:255. doi: 10.1186/s12889-015-1591-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.International Diabetes Federation. IDF DIABETES ATLAS. 6th ed; 2013. [Google Scholar]
- 3.Patterson C, Guariguata L, Dahlquist G, Soltész G, Ogle G, Silink M. Diabetes in the young - a global view and worldwide estimates of numbers of children with type 1 diabetes. Diabetes Res Clin Pract. 2014;103:161–175. doi: 10.1016/j.diabres.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Aschner P. Diabetes trends in Latin America. Diabetes Metab Res Rev. 2002;18 Suppl 3:S27–S31. doi: 10.1002/dmrr.280. [DOI] [PubMed] [Google Scholar]
- 5.Gómez-Díaz RA, Garibay-Nieto N, Wacher-Rodarte N, Aguilar-Salinas CA. Epidemiology of type 1 diabetes in Latin America. Curr Diabetes Rev. 2014;10:75–85. doi: 10.2174/1573399810666140223183936. [DOI] [PubMed] [Google Scholar]
- 6.DIAMOND Project Group. Incidence and trends of childhood Type 1 diabetes worldwide 1990-1999. Diabet Med. 2006;23:857–866. doi: 10.1111/j.1464-5491.2006.01925.x. [DOI] [PubMed] [Google Scholar]
- 7.Patterson CC, Dahlquist GG, Gyürüs E, Green A, Soltész G EURODIAB Study Group. Incidence trends for childhood type 1 diabetes in Europe during 1989-2003 and predicted new cases 2005-20: a multicentre prospective registration study. Lancet. 2009;373:2027–2033. doi: 10.1016/S0140-6736(09)60568-7. [DOI] [PubMed] [Google Scholar]
- 8.Grigsby-Toussaint DS, Lipton R, Chavez N, Handler A, Johnson TP, Kubo J. Neighborhood socioeconomic change and diabetes risk: findings from the Chicago childhood diabetes registry. Diabetes Care. 2010;33:1065–1068. doi: 10.2337/dc09-1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.du Prel JB, Icks A, Grabert M, Holl RW, Giani G, Rosenbauer J. Socioeconomic conditions and type 1 diabetes in childhood in North Rhine-Westphalia, Germany. Diabetologia. 2007;50:720–728. doi: 10.1007/s00125-007-0592-5. [DOI] [PubMed] [Google Scholar]
- 10.Diaz-Valencia PA, Bougnères P, Valleron AJ. Covariation of the incidence of type 1 diabetes with country characteristics available in public databases. PLoS One. 2015;10:e0118298. doi: 10.1371/journal.pone.0118298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgenstern H. Uses of ecologic analysis in epidemiologic research. Am J Public Health. 1982;72:1336–1344. doi: 10.2105/ajph.72.12.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loney PL, Chambers LW, Bennett KJ, Roberts JG, Stratford PW. Critical appraisal of the health research literature: prevalence or incidence of a health problem. Chronic Dis Can. 1998;19:170–176. [PubMed] [Google Scholar]
- 13.United Nations. Standard country or area codes for statistical use (M49). Available from: https://unstats.un.org/unsd/methodology/m49/ [Google Scholar]
- 14.GraphClick. Version 3.0. Arizona Software. 2008. Available from: http://www.arizona-software.ch/graphclick/
- 15.The World Bank. World Bank Open Data. Available from: https://data.worldbank.org/
- 16.Reserved-TSAR. Tableau Public. 2015. Available from: https://public.tableau.com/en-us/s/
- 17.R Core Team (2017) R: A language and environment for statistical computing. R Foundation for Statistical Computing. Austria: Vienna. Available from: https://www.R-project.org/
- 18.Haynes A, Bulsara MK, Bower C, Jones TW, Davis EA. Regular peaks and troughs in the Australian incidence of childhood type 1 diabetes mellitus (2000-2011) Diabetologia. 2015;58:2513–2516. doi: 10.1007/s00125-015-3709-2. [DOI] [PubMed] [Google Scholar]
- 19.Patterson CC, Gyürüs E, Rosenbauer J, Cinek O, Neu A, Schober E, Parslow RC, Joner G, Svensson J, Castell C, Bingley PJ, Schoenle E, Jarosz-Chobot P, Urbonaité B, Rothe U, Krzisnik C, Ionescu-Tirgoviste C, Weets I, Kocova M, Stipancic G, Samardzic M, de Beaufort CE, Green A, Dahlquist GG, Soltész G. Trends in childhood type 1 diabetes incidence in Europe during 1989-2008: evidence of non-uniformity over time in rates of increase. Diabetologia. 2012;55:2142–2147. doi: 10.1007/s00125-012-2571-8. [DOI] [PubMed] [Google Scholar]
- 20.Peter SA, Johnson R, Taylor C, Hanna A, Roberts P, McNeil P, Archer B, SinQuee C, Roberts P. The incidence and prevalence of type-1 diabetes mellitus. J Natl Med Assoc. 2005;97:250–252. [PMC free article] [PubMed] [Google Scholar]
- 21.Zalutskaya A, Bornstein SR, Mokhort T, Garmaev D. Did the Chernobyl incident cause an increase in Type 1 diabetes mellitus incidence in children and adolescents? Diabetologia. 2004;47:147–148. doi: 10.1007/s00125-003-1271-9. [DOI] [PubMed] [Google Scholar]
- 22.Tahirović H, Toromanović A. Incidence of type 1 diabetes mellitus in children in Tuzla Canton between 1995 and 2004. Eur J Pediatr. 2007;166:491–492. doi: 10.1007/s00431-006-0257-2. [DOI] [PubMed] [Google Scholar]
- 23.Radosevic B, Bukara-Radujkovic G, Miljkovic V, Pejicic S, Bratina N, Battelino T. The incidence of type 1 diabetes in Republic of Srpska (Bosnia and Herzegovina) and Slovenia in the period 1998-2010. Pediatr Diabetes. 2013;14:273–279. doi: 10.1111/j.1399-5448.2012.00898.x. [DOI] [PubMed] [Google Scholar]
- 24.Negrato CA, Lauris JRP, Saggioro IB, Corradini MCM, Borges PR, Crês MC, Junior AL, Guedes MFS, Gomes MB. Increasing incidence of type 1 diabetes between 1986 and 2015 in Bauru, Brazil. Diabetes Res Clin Pract. 2017;127:198–204. doi: 10.1016/j.diabres.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 25.Ehrlich RM, Walsh LJ, Falk JA, Middleton PJ, Simpson NE. The incidence of type 1 (insulin-dependent) diabetes in Toronto. Diabetologia. 1982;22:289–291. doi: 10.1007/BF00281308. [DOI] [PubMed] [Google Scholar]
- 26.Blanchard JF, Dean H, Anderson K, Wajda A, Ludwig S, Depew N. Incidence and prevalence of diabetes in children aged 0-14 years in Manitoba, Canada, 1985-1993. Diabetes Care. 1997;20:512–515. doi: 10.2337/diacare.20.4.512. [DOI] [PubMed] [Google Scholar]
- 27.Legault L, Polychronakos C. Annual incidence of type 1 diabetes in Québec between 1989-2000 in children. Clin Invest Med. 2006;29:10–13. [PubMed] [Google Scholar]
- 28.Newhook LA, Penney S, Fiander J, Dowden J. Recent incidence of type 1 diabetes mellitus in children 0-14 years in Newfoundland and Labrador, Canada climbs to over 45/100,000: a retrospective time trend study. BMC Res Notes. 2012;5:628. doi: 10.1186/1756-0500-5-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larenas G, Montecinos A, Manosalva M, Barthou M, Vidal T. Incidence of insulin-dependent diabetes mellitus in the IX region of Chile: ethnic differences. Diabetes Res Clin Pract. 1996;34 Suppl:S147–S151. doi: 10.1016/s0168-8227(96)90022-4. [DOI] [PubMed] [Google Scholar]
- 30.Torres-Avilés F, Carrasco E, Icaza G, Pérez-Bravo F. Clustering of cases of type 1 diabetes in high socioeconomic communes in Santiago de Chile: spatio-temporal and geographical analysis. Acta Diabetol. 2010;47:251–257. doi: 10.1007/s00592-010-0189-1. [DOI] [PubMed] [Google Scholar]
- 31.Yang Z, Long X, Shen J, Liu D, Dorman JS, Laporte RE, Chang YF. Epidemics of type 1 diabetes in China. Pediatr Diabetes. 2005;6:122–128. doi: 10.1111/j.1399-543X.2005.00116.x. [DOI] [PubMed] [Google Scholar]
- 32.Wu HB, Zhong JM, Hu RY, Wang H, Gong WW, Pan J, Fei FR, Wang M, Guo LH, Yang L, Yu M. Rapidly rising incidence of Type 1 diabetes in children and adolescents aged 0-19 years in Zhejiang, China, 2007 to 2013. Diabet Med. 2016;33:1339–1346. doi: 10.1111/dme.13010. [DOI] [PubMed] [Google Scholar]
- 33.Gong C, Meng X, Jiang Y, Wang X, Cui H, Chen X. Trends in childhood type 1 diabetes mellitus incidence in Beijing from 1995 to 2010: a retrospective multicenter study based on hospitalization data. Diabetes Technol Ther. 2015;17:159–165. doi: 10.1089/dia.2014.0205. [DOI] [PubMed] [Google Scholar]
- 34.Zhao Z, Sun C, Wang C, Li P, Wang W, Ye J, Gu X, Wang X, Shen S, Zhi D, Lu Z, Ye R, Cheng R, Xi L, Li X, Zheng Z, Zhang M, Luo F. Rapidly rising incidence of childhood type 1 diabetes in Chinese population: epidemiology in Shanghai during 1997-2011. Acta Diabetol. 2014;51:947–953. doi: 10.1007/s00592-014-0590-2. [DOI] [PubMed] [Google Scholar]
- 35.Rojnic Putarek N, Ille J, Spehar Uroic A, Skrabic V, Stipancic G, Krnic N, Radica A, Marjanac I, Severinski S, Svigir A, Bogdanic A, Dumic M. Incidence of type 1 diabetes mellitus in 0 to 14-yr-old children in Croatia--2004 to 2012 study. Pediatr Diabetes. 2015;16:448–453. doi: 10.1111/pedi.12197. [DOI] [PubMed] [Google Scholar]
- 36.Skordis N, Efstathiou E, Kyriakides TC, Savvidou A, Savva SC, Phylactou LA, Shammas C, Neocleous V. Epidemiology of type 1 diabetes mellitus in Cyprus: rising incidence at the dawn of the 21st century. Hormones (Athens) 2012;11:86–93. doi: 10.1007/BF03401541. [DOI] [PubMed] [Google Scholar]
- 37.Kim JH, Lee CG, Lee YA, Yang SW, Shin CH. Increasing incidence of type 1 diabetes among Korean children and adolescents: analysis of data from a nationwide registry in Korea. Pediatr Diabetes. 2016;17:519–524. doi: 10.1111/pedi.12324. [DOI] [PubMed] [Google Scholar]
- 38.Arab M. Diabetes mellitus in Egypt. World Health Stat Q. 1992;45:334–337. [PubMed] [Google Scholar]
- 39.El-Ziny MA, Salem NA, El-Hawary AK, Chalaby NM, Elsharkawy AA. Epidemiology of childhood type 1 diabetes mellitus in Nile Delta, northern Egypt - a retrospective study. J Clin Res Pediatr Endocrinol. 2014;6:9–15. doi: 10.4274/Jcrpe.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teeäär T, Liivak N, Heilman K, Kool P, Sor R, Paal M, Einberg U, Tillmann V. Increasing incidence of childhood-onset type 1 diabetes mellitus among Estonian children in 1999-2006. Time trend analysis 1983-2006. Pediatr Diabetes. 2010;11:107–110. doi: 10.1111/j.1399-5448.2009.00535.x. [DOI] [PubMed] [Google Scholar]
- 41.Alemu S, Dessie A, Seid E, Bard E, Lee PT, Trimble ER, Phillips DI, Parry EH. Insulin-requiring diabetes in rural Ethiopia: should we reopen the case for malnutrition-related diabetes? Diabetologia. 2009;52:1842–1845. doi: 10.1007/s00125-009-1433-5. [DOI] [PubMed] [Google Scholar]
- 42.Ogle GD, Morrison MK, Silink M, Taito RS. Incidence and prevalence of diabetes in children aged <15 yr in Fiji, 2001-2012. Pediatr Diabetes. 2016;17:222–226. doi: 10.1111/pedi.12257. [DOI] [PubMed] [Google Scholar]
- 43.Harjutsalo V, Sund R, Knip M, Groop PH. Incidence of type 1 diabetes in Finland. JAMA. 2013;310:427–428. doi: 10.1001/jama.2013.8399. [DOI] [PubMed] [Google Scholar]
- 44.Mauny F, Grandmottet M, Lestradet C, Guitard J, Crenn D, Floret N, Olivier-Koehret M, Viel JF Health Consequences of Chernobyl Fallout in Franche-Comté Study Group. Increasing trend of childhood type 1 diabetes in Franche-Comté (France): analysis of age and period effects from 1980 to 1998. Eur J Epidemiol. 2005;20:325–329. doi: 10.1007/s10654-005-0329-z. [DOI] [PubMed] [Google Scholar]
- 45.Barat P, Valade A, Brosselin P, Alberti C, Maurice-Tison S, Lévy-Marchal C. The growing incidence of type 1 diabetes in children: the 17-year French experience in Aquitaine. Diabetes Metab. 2008;34:601–605. doi: 10.1016/j.diabet.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 46.Trellu M, Lacombe S, Morin D, Dalla-Vale F. [Epidemiology of diabetes in children in Languedoc-Roussillon (France)] Arch Pediatr. 2015;22:241–246. doi: 10.1016/j.arcped.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 47.Bendas A, Rothe U, Kiess W, Kapellen TM, Stange T, Manuwald U, Salzsieder E, Holl RW, Schoffer O, Stahl-Pehe A, Giani G, Ehehalt S, Neu A, Rosenbauer J. Trends in Incidence Rates during 1999-2008 and Prevalence in 2008 of Childhood Type 1 Diabetes Mellitus in Germany--Model-Based National Estimates. PLoS One. 2015;10:e0132716. doi: 10.1371/journal.pone.0132716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dacou-Voutetakis C, Karavanaki K, Tsoka-Gennatas H. National data on the epidemiology of IDDM in Greece. Cases diagnosed in 1992. Hellenic Epidemiology Study Group. Diabetes Care. 1995;18:552–554. doi: 10.2337/diacare.18.4.552. [DOI] [PubMed] [Google Scholar]
- 49.Ramachandran A, Snehalatha C, Krishnaswamy CV. Incidence of IDDM in children in urban population in southern India. Madras IDDM Registry Group Madras, South India. Diabetes Res Clin Pract. 1996;34:79–82. doi: 10.1016/s0168-8227(96)01338-1. [DOI] [PubMed] [Google Scholar]
- 50.Pishdad GR. Low incidence of type 1 diabetes in Iran. Diabetes Care. 2005;28:927–928. doi: 10.2337/diacare.28.4.927. [DOI] [PubMed] [Google Scholar]
- 51.Roche EF, McKenna AM, Ryder KJ, Brennan AA, O'Regan M, Hoey HM. Is the incidence of type 1 diabetes in children and adolescents stabilising? The first 6 years of a National Register. Eur J Pediatr. 2016;175:1913–1919. doi: 10.1007/s00431-016-2787-6. [DOI] [PubMed] [Google Scholar]
- 52.Blumenfeld O, Dichtiar R, Shohat T Israel IDDM Registry Study Group (IIRSG) Trends in the incidence of type 1 diabetes among Jews and Arabs in Israel. Pediatr Diabetes. 2014;15:422–427. doi: 10.1111/pedi.12101. [DOI] [PubMed] [Google Scholar]
- 53.Di Ciaula A. Type I diabetes in paediatric age in Apulia (Italy): Incidence and associations with outdoor air pollutants. Diabetes Res Clin Pract. 2016;111:36–43. doi: 10.1016/j.diabres.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 54.Valent F, Candido R, Faleschini E, Tonutti L, Tortul C, Zanatta M, Zanette G, Zanier L. The incidence rate and prevalence of pediatric type 1 diabetes mellitus (age 0-18) in the Italian region Friuli Venezia Giulia: population-based estimates through the analysis of health administrative databases. Acta Diabetol. 2016;53:629–635. doi: 10.1007/s00592-016-0854-0. [DOI] [PubMed] [Google Scholar]
- 55.Altobelli E, Petrocelli R, Verrotti A, Chiarelli F, Marziliano C. Genetic and environmental factors affect the onset of type 1 diabetes mellitus. Pediatr Diabetes. 2016;17:559–566. doi: 10.1111/pedi.12345. [DOI] [PubMed] [Google Scholar]
- 56.Marigliano M, Tadiotto E, Morandi A, Sabbion A, Contreas G, Avossa F, Fedeli U, Maffeis C. Epidemiology of type 1 diabetes mellitus in the pediatric population in Veneto Region, Italy. Diabetes Res Clin Pract. 2015;107:e19–e21. doi: 10.1016/j.diabres.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 57.Bruno G, Maule M, Merletti F, Novelli G, Falorni A, Iannilli A, Iughetti L, Altobelli E, d'Annunzio G, Piffer S, Pozzilli P, Iafusco D, Songini M, Roncarolo F, Toni S, Carle F, Cherubini V RIDI Study Group. Age-period-cohort analysis of 1990-2003 incidence time trends of childhood diabetes in Italy: the RIDI study. Diabetes. 2010;59:2281–2287. doi: 10.2337/db10-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Onda Y, Sugihara S, Ogata T, Yokoya S, Yokoyama T, Tajima N Type 1 Diabetes (T1D) Study Group. Incidence and prevalence of childhood-onset Type 1 diabetes in Japan: the T1D study. Diabet Med. 2017;34:909–915. doi: 10.1111/dme.13295. [DOI] [PubMed] [Google Scholar]
- 59.Ajlouni K, Qusous Y, Khawaldeh AK, Jaddou H, Batiehah A, Ammari F, Zaheri M, Mashal A. Incidence of insulin-dependent diabetes mellitus in Jordanian children aged 0-14 y during 1992-1996. Acta Paediatr Suppl. 1999;88:11–13. doi: 10.1111/j.1651-2227.1999.tb14334.x. [DOI] [PubMed] [Google Scholar]
- 60.Shaltout AA, Wake D, Thanaraj TA, Omar DM, Al-AbdulRazzaq D, Channanath A, AlKandari H, Abdulrasoul M, Miller S, Conway N, Tuomilehto J, Davidsson L Steering Group for the Study of Childhood Diabetes in Kuwait. Incidence of type 1 diabetes has doubled in Kuwaiti children 0-14 years over the last 20 years. Pediatr Diabetes. 2017;18:761–766. doi: 10.1111/pedi.12480. [DOI] [PubMed] [Google Scholar]
- 61.Formosa N, Calleja N, Torpiano J. Incidence and modes of presentation of childhood type 1 diabetes mellitus in Malta between 2006 and 2010. Pediatr Diabetes. 2012;13:484–488. doi: 10.1111/j.1399-5448.2011.00839.x. [DOI] [PubMed] [Google Scholar]
- 62.Gómez-Díaz RA, Pérez-Pérez G, Hernández-Cuesta IT, Rodríguez-García Jdel C, Guerrero-López R, Aguilar-Salinas CA, Wacher NH. Incidence of type 1 diabetes in Mexico: data from an institutional register 2000-2010. Diabetes Care. 2012;35:e77. doi: 10.2337/dc12-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Samardžić M, Martinović M, Nedović-Vuković M, Popović-Samardžić M. Recent incidence of type 1 diabetes mellitus in montenegro: a shift toward younger age at disease onset. Acta Clin Croat. 2016;55:63–68. doi: 10.20471/acc.2016.55.01.10. [DOI] [PubMed] [Google Scholar]
- 64.Fazeli Farsani S, Souverein PC, van der Vorst MM, Knibbe CA, Herings RM, de Boer A, Mantel-Teeuwisse AK. Increasing trends in the incidence and prevalence rates of type 1 diabetes among children and adolescents in the Netherlands. Pediatr Diabetes. 2016;17:44–52. doi: 10.1111/pedi.12232. [DOI] [PubMed] [Google Scholar]
- 65.Campbell-Stokes PL, Taylor BJ New Zealand Children's Diabetes Working Group. Prospective incidence study of diabetes mellitus in New Zealand children aged 0 to 14 years. Diabetologia. 2005;48:643–648. doi: 10.1007/s00125-005-1697-3. [DOI] [PubMed] [Google Scholar]
- 66.Skrivarhaug T, Stene LC, Drivvoll AK, Strøm H, Joner G Norwegian Childhood Diabetes Study Group. Incidence of type 1 diabetes in Norway among children aged 0-14 years between 1989 and 2012: has the incidence stopped rising? Results from the Norwegian Childhood Diabetes Registry. Diabetologia. 2014;57:57–62. doi: 10.1007/s00125-013-3090-y. [DOI] [PubMed] [Google Scholar]
- 67.Soliman AT, al-Salmi IS, Asfour MG. Epidemiology of childhood insulin-dependent diabetes mellitus in the Sultanate of Oman. Diabet Med. 1996;13:582–586. doi: 10.1002/(SICI)1096-9136(199606)13:6<582::AID-DIA114>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 68.Ogle GD, Lesley J, Sine P, McMaster P. Type 1 diabetes mellitus in children in Papua New Guinea. P N G Med J. 2001;44:96–100. [PubMed] [Google Scholar]
- 69.Jarosz-Chobot P, Polanska J, Szadkowska A, Kretowski A, Bandurska-Stankiewicz E, Ciechanowska M, Deja G, Mysliwiec M, Peczynska J, Rutkowska J, Sobel-Maruniak A, Fichna P, Chobot A, Rewers M. Rapid increase in the incidence of type 1 diabetes in Polish children from 1989 to 2004, and predictions for 2010 to 2025. Diabetologia. 2011;54:508–515. doi: 10.1007/s00125-010-1993-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wojcik M, Sudacka M, Wasyl B, Ciechanowska M, Nazim J, Stelmach M, Starzyk JB. Incidence of type 1 diabetes mellitus during 26 years of observation and prevalence of diabetic ketoacidosis in the later years. Eur J Pediatr. 2015;174:1319–1324. doi: 10.1007/s00431-015-2537-1. [DOI] [PubMed] [Google Scholar]
- 71.Chobot A, Polanska J, Brandt A, Deja G, Glowinska-Olszewska B, Pilecki O, Szadkowska A, Mysliwiec M, Jarosz-Chobot P. Updated 24-year trend of Type 1 diabetes incidence in children in Poland reveals a sinusoidal pattern and sustained increase. Diabet Med. 2017;34:1252–1258. doi: 10.1111/dme.13345. [DOI] [PubMed] [Google Scholar]
- 72.Al-Zyoud M, Al Ali M, Rahim A MI. Insulin dependant diabetes mellitus (IDDM) in children below 13 years of age in Qatar. Diabetes Insights. 1997;4:10. [Google Scholar]
- 73.Lin WH, Wang MC, Wang WM, Yang DC, Lam CF, Roan JN, Li CY. Incidence of and mortality from Type I diabetes in Taiwan from 1999 through 2010: a nationwide cohort study. PLoS One. 2014;9:e86172. doi: 10.1371/journal.pone.0086172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Serban V, Brink S, Timar B, Sima A, Vlad M, Timar R, Vlad A. An increasing incidence of type 1 diabetes mellitus in Romanian children aged 0 to 17 years. J Pediatr Endocrinol Metab. 2015;28:293–298. doi: 10.1515/jpem-2014-0364. [DOI] [PubMed] [Google Scholar]
- 75.Pronina EA, Petraikina EE, Antsiferov MB, Duchareva OV, Petrone A, Buzzetti R, Pozzilli P. A 10-year (1996-2005) prospective study of the incidence of Type 1 diabetes in Moscow in the age group 0-14 years. Diabet Med. 2008;25:956–959. doi: 10.1111/j.1464-5491.2008.02508.x. [DOI] [PubMed] [Google Scholar]
- 76.Marshall SL, Edidin D, Arena VC, Becker DJ, Bunker CH, Gishoma C, Gishoma F, LaPorte RE, Kaberuka V, Ogle G, Sibomana L, Orchard TJ. Prevalence and incidence of clinically recognized cases of Type 1 diabetes in children and adolescents in Rwanda, Africa. Diabet Med. 2015;32:1186–1192. doi: 10.1111/dme.12701. [DOI] [PubMed] [Google Scholar]
- 77.Kulaylat NA, Narchi H. A twelve year study of the incidence of childhood type 1 diabetes mellitus in the Eastern Province of Saudi Arabia. J Pediatr Endocrinol Metab. 2000;13:135–140. doi: 10.1515/jpem.2000.13.2.135. [DOI] [PubMed] [Google Scholar]
- 78.Habeb AM, Al-Magamsi MS, Halabi S, Eid IM, Shalaby S, Bakoush O. High incidence of childhood type 1 diabetes in Al-Madinah, North West Saudi Arabia (2004-2009) Pediatr Diabetes. 2011;12:676–681. doi: 10.1111/j.1399-5448.2011.00765.x. [DOI] [PubMed] [Google Scholar]
- 79.Vlajinac HD, Bojović BM, Sipetić SB, Adanja BJ, Jarebinski MS, Radmanović SZ, Zdravković DS. Insulin dependent diabetes mellitus: incidence in childhood in Belgrade 1982-92. J Epidemiol Community Health. 1995;49:107–108. doi: 10.1136/jech.49.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee WW, Ooi BC, Thai AC, Loke KY, Tan YT, Rajan U, Tan CL. The incidence of IDDM in Singapore children. Singapore Med J. 1998;39:359–362. [PubMed] [Google Scholar]
- 81.Serrano Ríos M, Moy CS, Martín Serrano R, Minuesa Asensio A, de Tomás Labat ME, Zarandieta Romero G, Herrera J. Incidence of type 1 (insulin-dependent) diabetes mellitus in subjects 0-14 years of age in the Comunidad of Madrid, Spain. Diabetologia. 1990;33:422–424. doi: 10.1007/BF00404093. [DOI] [PubMed] [Google Scholar]
- 82.Lora-Gómez RE, Morales-Pérez FM, Arroyo-Díez FJ, Barquero-Romero J. Incidence of Type 1 diabetes in children in Cáceres, Spain, during 1988-1999. Diabetes Res Clin Pract. 2005;69:169–174. doi: 10.1016/j.diabres.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 83.Morales-Pérez FM, Barquero-Romero J, Pérez-Miranda M. Incidence of type I diabetes among children and young adults (0-29 years) in the province of Badajoz, Spain during 1992 to 1996. Acta Paediatr. 2000;89:101–104. doi: 10.1080/080352500750029158. [DOI] [PubMed] [Google Scholar]
- 84.Forga L, Goñi MJ, Ibáñez B, Cambra K, Mozas D, Chueca M. [Incidence of type 1 diabetes in Navarre, 2009-2012] An Sist Sanit Navar. 2014;37:241–247. doi: 10.4321/s1137-66272014000200007. [DOI] [PubMed] [Google Scholar]
- 85.Vega T, Gil M, Lozano J. Age and sex differences in the incidence of diabetes mellitus in a population-based Spanish cohort. J Diabetes. 2015;7:411–417. doi: 10.1111/1753-0407.12183. [DOI] [PubMed] [Google Scholar]
- 86.Fernández-Ramos C, Arana-Arri E, Jiménez-Huertas P, Vela A, Rica I. Incidence of childhood-onset type 1 diabetes in Biscay, Spain, 1990-2013. Pediatr Diabetes. 2017;18:71–76. doi: 10.1111/pedi.12354. [DOI] [PubMed] [Google Scholar]
- 87.Elamin A, Ghalib M, Eltayeb B, Tuvemo T. High incidence of type 1 diabetes mellitus in Sudanese children, 1991-1995. Ann Saudi Med. 1997;17:478–480. doi: 10.5144/0256-4947.1997.478. [DOI] [PubMed] [Google Scholar]
- 88.Rawshani A, Landin-Olsson M, Svensson AM, Nyström L, Arnqvist HJ, Bolinder J, Gudbjörnsdottir S. The incidence of diabetes among 0-34 year olds in Sweden: new data and better methods. Diabetologia. 2014;57:1375–1381. doi: 10.1007/s00125-014-3225-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Panamonta O, Thamjaroen J, Panamonta M, Panamonta N, Suesirisawat C. The rising incidence of type 1 diabetes in the northeastern part of Thailand. J Med Assoc Thai. 2011;94:1447–1450. [PubMed] [Google Scholar]
- 90.Ben Khalifa F, Mekaouar A, Taktak S, Hamhoum M, Jebara H, Kodia A, Zouari B, Chakroun M. A five-year study of the incidence of insulin-dependent diabetes mellitus in young Tunisians (preliminary results) Diabetes Metab. 1997;23:395–401. [PubMed] [Google Scholar]
- 91.Yeşilkaya E, Cinaz P, Andıran N, Bideci A, Hatun Ş, Sarı E, Türker T, Akgül Ö, Saldır M, Kılıçaslan H, Açıkel C, Craig ME. First report on the nationwide incidence and prevalence of Type 1 diabetes among children in Turkey. Diabet Med. 2017;34:405–410. doi: 10.1111/dme.13063. [DOI] [PubMed] [Google Scholar]
- 92.Timchenko OI, Kozachok GS, Turos EI, Omel'chenko EM. [The prevalence of diabetes mellitus in children of different regions of Ukraine] Tsitol Genet. 1996;30:70–73. [PubMed] [Google Scholar]
- 93.Imkampe AK, Gulliford MC. Trends in Type 1 diabetes incidence in the UK in 0- to 14-year-olds and in 15- to 34-year-olds, 1991-2008. Diabet Med. 2011;28:811–814. doi: 10.1111/j.1464-5491.2011.03288.x. [DOI] [PubMed] [Google Scholar]
- 94.Swai AB, Lutale JL, McLarty DG. Prospective study of incidence of juvenile diabetes mellitus over 10 years in Dar es Salaam, Tanzania. BMJ. 1993;306:1570–1572. doi: 10.1136/bmj.306.6892.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cartee AK, Owens LA, Lahr BD, Yawn BP, Murray JA, Kudva YC. Incidence of Type 1 Diabetes Is Not Increasing in a Population-Based Cohort in Olmsted County, Minnesota, USA. Mayo Clin Proc. 2016;91:1066–1073. doi: 10.1016/j.mayocp.2016.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mayer-Davis EJ, Lawrence JM, Dabelea D, Divers J, Isom S, Dolan L, Imperatore G, Linder B, Marcovina S, Pettitt DJ, Pihoker C, Saydah S, Wagenknecht L SEARCH for Diabetes in Youth Study. Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002-2012. N Engl J Med. 2017;376:1419–1429. doi: 10.1056/NEJMoa1610187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rakhimova GN, Alimova NU, Ryaboshtan A, Waldman B, Ogle GD, Ismailov SI. Epidemiological data of type 1 diabetes mellitus in children in Uzbekistan, 1998-2014. Pediatr Diabetes. 2018;19:158–165. doi: 10.1111/pedi.12495. [DOI] [PubMed] [Google Scholar]
- 98.Shaltout AA, Moussa MA, Qabazard M, Abdella N, Karvonen M, Al-Khawari M, Al-Arouj M, Al-Nakhi A, Tuomilehto J, El-Gammal A Kuwait Diabetes Study Group. Further evidence for the rising incidence of childhood Type 1 diabetes in Kuwait. Diabet Med. 2002;19:522–525. doi: 10.1046/j.1464-5491.2002.00703.x. [DOI] [PubMed] [Google Scholar]
- 99.Morran MP, Vonberg A, Khadra A, Pietropaolo M. Immunogenetics of type 1 diabetes mellitus. Mol Aspects Med. 2015;42:42–60. doi: 10.1016/j.mam.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lipton RB, Drum M, Greeley SA, Danielson KK, Bell GI, Hagopian WA. HLA-DQ haplotypes differ by ethnicity in patients with childhood-onset diabetes. Pediatr Diabetes. 2011;12:388–395. doi: 10.1111/j.1399-5448.2010.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rønningen KS, Keiding N, Green A EURODIAB ACE Study Group. Europe and Diabetes. Correlations between the incidence of childhood-onset type I diabetes in Europe and HLA genotypes. Diabetologia. 2001;44 Suppl 3:B51–B59. doi: 10.1007/pl00002955. [DOI] [PubMed] [Google Scholar]
- 102.Park Y, Eisenbarth GS. Genetic susceptibility factors of Type 1 diabetes in Asians. Diabetes Metab Res Rev. 2001;17:2–11. doi: 10.1002/1520-7560(2000)9999:9999<::aid-dmrr164>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 103.Rewers M, Ludvigsson J. Environmental risk factors for type 1 diabetes. Lancet. 2016;387:2340–2348. doi: 10.1016/S0140-6736(16)30507-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Regnell SE, Lernmark Å. Early prediction of autoimmune (type 1) diabetes. Diabetologia. 2017;60:1370–1381. doi: 10.1007/s00125-017-4308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Forlenza GP, Rewers M. The epidemic of type 1 diabetes: what is it telling us? Curr Opin Endocrinol Diabetes Obes. 2011;18:248–251. doi: 10.1097/MED.0b013e32834872ce. [DOI] [PubMed] [Google Scholar]
- 106.Patterson CC, Dahlquist G, Soltész G, Green A EURODIAB ACE Study Group. Europe and Diabetes. Is childhood-onset type I diabetes a wealth-related disease? An ecological analysis of European incidence rates. Diabetologia. 2001;44 Suppl 3:B9–16. doi: 10.1007/pl00002961. [DOI] [PubMed] [Google Scholar]
- 107.Holmqvist BM, Lofman O, Samuelsson U. A low incidence of Type 1 diabetes between 1977 and 2001 in south-eastern Sweden in areas with high population density and which are more deprived. Diabet Med. 2008;25:255–260. doi: 10.1111/j.1464-5491.2007.02342.x. [DOI] [PubMed] [Google Scholar]
- 108.Cardwell CR, Carson DJ, Patterson CC. Secular trends, disease maps and ecological analyses of the incidence of childhood onset Type 1 diabetes in Northern Ireland, 1989-2003. Diabet Med. 2007;24:289–295. doi: 10.1111/j.1464-5491.2007.02080.x. [DOI] [PubMed] [Google Scholar]
- 109.Bruno G, Spadea T, Picariello R, Gruden G, Barutta F, Cerutti F, Cavallo-Perin P, Costa G, Gnavi R Piedmont Study Group for Diabetes Epidemiology. Early life socioeconomic indicators and risk of type 1 diabetes in children and young adults. J Pediatr. 2013;162:600–605.e1. doi: 10.1016/j.jpeds.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 110.Egger G, Swinburn B, Islam FM. Economic growth and obesity: an interesting relationship with world-wide implications. Econ Hum Biol. 2012;10:147–153. doi: 10.1016/j.ehb.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 111.Islam ST, Srinivasan S, Craig ME. Environmental determinants of type 1 diabetes: a role for overweight and insulin resistance. J Paediatr Child Health. 2014;50:874–879. doi: 10.1111/jpc.12616. [DOI] [PubMed] [Google Scholar]
- 112.Wilkin TJ. The accelerator hypothesis: a review of the evidence for insulin resistance as the basis for type I as well as type II diabetes. Int J Obes (Lond) 2009;33:716–726. doi: 10.1038/ijo.2009.97. [DOI] [PubMed] [Google Scholar]
- 113.Gale EA. A missing link in the hygiene hypothesis? Diabetologia. 2002;45:588–594. doi: 10.1007/s00125-002-0801-1. [DOI] [PubMed] [Google Scholar]
- 114.Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347:911–920. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 115.Bach JF, Chatenoud L. The hygiene hypothesis: an explanation for the increased frequency of insulin-dependent diabetes. Cold Spring Harb Perspect Med. 2012;2:a007799. doi: 10.1101/cshperspect.a007799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cardwell CR, Stene LC, Ludvigsson J, Rosenbauer J, Cinek O, Svensson J, Perez-Bravo F, Memon A, Gimeno SG, Wadsworth EJ, Strotmeyer ES, Goldacre MJ, Radon K, Chuang LM, Parslow RC, Chetwynd A, Karavanaki K, Brigis G, Pozzilli P, Urbonaite B, Schober E, Devoti G, Sipetic S, Joner G, Ionescu-Tirgoviste C, de Beaufort CE, Harrild K, Benson V, Savilahti E, Ponsonby AL, Salem M, Rabiei S, Patterson CC. Breast-feeding and childhood-onset type 1 diabetes: a pooled analysis of individual participant data from 43 observational studies. Diabetes Care. 2012;35:2215–2225. doi: 10.2337/dc12-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mejía-León ME, Barca AM. Diet, Microbiota and Immune System in Type 1 Diabetes Development and Evolution. Nutrients. 2015;7:9171–9184. doi: 10.3390/nu7115461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Petrou S, Khan K. An overview of the health economic implications of elective caesarean section. Appl Health Econ Health Policy. 2013;11:561–576. doi: 10.1007/s40258-013-0063-8. [DOI] [PubMed] [Google Scholar]
- 119.Butalia S, Kaplan GG, Khokhar B, Rabi DM. Environmental Risk Factors and Type 1 Diabetes: Past, Present, and Future. Can J Diabetes. 2016;40:586–593. doi: 10.1016/j.jcjd.2016.05.002. [DOI] [PubMed] [Google Scholar]