Abstract
Background
Psoriasis is an incurable, chronic skin disorder with considerable impact on the quality of life. No drugs are available for treating the disease. Clarifying the progression, exploring the risk factors affecting progression, and finding effective treatments with few side-effects and low recurrence rates is critical. This protocol describes a future study that will analyze psoriasis vulgaris progression risk factors and trends, establish a multicenter clinical registration platform, evaluate clinical evidence for Chinese Medicine (CM) intervention for psoriasis, and evaluate therapeutic effectiveness and recurrence rate advantages of CM.
Methods
The study is a prospective cohort clinical trial planned for October 2019 to September 2021 involving 20 clinics. The trial will enroll 1,500 participants in a psoriasis vulgaris group, and 500 healthy participants in a control group (no intervention). The psoriasis vulgaris group will be divided into three equal-sized subgroups: blood heat syndrome group (BHS), blood stasis syndrome group (BSS), and non-blood heat nor blood stasis syndrome group (NHS) group. Participants will be grouped according to CM syndrome classification and receive oral CM herbal medication (according to the CM syndrome classification, and tailored to the participant’s disease progression). Medication will be administered twice every day during the intervention phase (eight weeks of intervention, and eight weeks of follow-up). Exposure measures include demographic variables, risk factors, and intervention factors.
Discussion
The primary outcome measures include improvement in both the psoriasis and severity index scores after eight weeks of intervention. Secondary outcome measures include body surface area affected, Physician Global Assessment scores, Dermatology Life Quality Index, pain-relat ed quality of life, pain on visual analog scale, CM syndromes, and recurrence. Other outcome measures include CM physical scale, personal history, medical expenses, and patient satisfaction. The number, nature, and severity of adverse events will be carefully recorded.
Trial registration
The trial has been registered at ClinicalTrials.gov (ID: NCT03942185).
Keywords: Psoriasis, characteristics, cohort study, Chinese Medicine (CM), protocol
Introduction
Psoriasis is a common, chronic, relapsing, inflammatory, and systemic skin disease. Clinical expression involves well-defined erythema covered with silvery-white flakes, mostly at limb extensor surfaces, the scalp, the lumbosacral region, and the navel. Psoriasis vulgaris is the most common disease type. Psoriasis impacts quality of life, including depression, anxiety, and suicide (1). Estimates of the prevalence of psoriasis in adults and children range from 0.51% to 11.43%, and 0% to 1.37%, respectively, and the incidence is increasing globally (2). In China, the prevalence of the disease’s year-over-year increase has accelerated from 0.12% in 1984 to 0.47% in recent years (3).
Psoriasis is a complex disease caused by multiple factors. Genetic factors have an impact on the disease, and other inducing factors, such as external environmental factors, also play important roles in the pathogenesis. Cells involved in the complex interactions include keratinocytes, dendritic cells (DC), T-lymphocytes (T cells), neutrophils, and mast cells (4). However, the specific etiology, risk factors associated with disease progression, and the overall progression of the disease are not fully understood (5). At the same time, psoriasis still has no disease-specific treatment, and social, medical, and economic burdens are increasing. Therefore, to alleviate the disease progression and social burden, it is necessary to analyze the risk factors associated with the progression of psoriasis vulgaris and identify the disease progression process, to make specific and targeted efforts in prevention and control.
The systematic treatment of psoriasis is currently dominated by acitretin, immunosuppressants, and biological agents, which have different degrees of adverse reactions and high economic costs, limiting widespread clinical use (6). Chinese Medicine (CM) provide unique advantages for the treatment of psoriasis. Herbal CM has been shown to effectively regulate the activation of T-cells, inhibiting the proliferation of keratinocytes and angiogenesis, and is used to treat psoriasis (7). Over the years, CM gradually identified the CM syndrome category "Blood" as the entry point etiology, and the pathogenesis of psoriasis indicated that the disease location is in the blood (8). The classic CM syndromes of psoriasis include “Blood Heat”, “Blood Stasis”, and “Blood Deficiency”. Among them, “Blood Heat” and “Blood Stasis” syndromes are the most common. Treatments are based on the principles of clearing heat and cooling blood, promoting blood circulation, and removing blood stasis, among others (according to the different CM syndromes) (9). Previous studies (10,11) also confirmed that Th cell differentiation correlates with different psoriasis syndromes in different immune states, and the proportion of Treg/Th17 cells in the peripheral blood. The expression of major cytokines and transcription factors are different in patients with different psoriasis CM syndromes. Drugs are more targeted, and maximize therapeutic effect when considering the CM syndrome types for appropriate treatments.
Although various clinical studies of oral CM herbal therapy for psoriasis have been reported, high-quality randomized controlled trials (RCT) are still lacking, and no systematic, prospective cohort studies with large data sets have been implemented (12).
In conclusion, this study is designed to develop a cohort of patients with psoriasis vulgaris for the collection of baseline and psoriasis vulgaris progression data, analysis of progression risk factors and disease change trend, and intervention with CM herbal therapy. The ultimate goal is to evaluate the therapeutic advantages of CM in clinical effectiveness and recurrence rate of psoriasis vulgaris.
Methods
Participants
Study setting
The proposed cohort study is multicentered and prospective. The trial has been registered at ClinicalTrials.gov (ID: NCT03942185). At the time of writing this protocol report (version 2.0, April 2, 2019), the trial has not yet begun enrollment. Participant recruitment will take place from October 1, 2019 to December 31, 2021.
During the period before the trial begins, all investigators are conducting unified training to ensure that the staff involved in the study fully understands all trail aspects. The study will be conducted in the following 20 clinical centers in China: Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Zhejiang Provincial Hospital of Traditional Chinese Medicine, Shanghai Dermatology Hospital, The First Affiliated Hospital of Henan University of Traditional Chinese Medicine, Changhai Hospital, Shanghai 10th People’s Hospital, Beijing Hospital of Traditional Chinese Medicine, Wuhan No.1 Hospital, Jiangsu Province Hospital of Traditional Chinese Medicine, Chinese Medicine Hospital Affiliated to Southwest Medical University, First Affiliated Hospital of Heilongjiang Chinese Medicine University, Affiliated Hospital of Jiangxi University of Traditional Chinese Medicine, The Second Affiliated Hospital of Jiangxi University of Traditional Chinese Medicine, The First Affiliated Hospital of Anhui University of Traditional Chinese Medicine, The Guangxi Zhuang Autonomous Region Institute of Dermatology and Control, The First Affiliated Hospital of Guiyang College of Traditional Chinese Medicine, The Second People’s Hospital Affiliated to Fujian University of Traditional Chinese Medicine, Affiliated Hospital of Gansu University of Traditional Chinese Medicine, Shanxi Provincial Hospital of Traditional Chinese Medicine, and Shijiazhuang Hospital of Traditional Chinese Medicine.
Sample size
According to the China chest cancer research collaborative group established by the Jieping Wu Medical Foundation (13) and Real World Research (2018 edition) guidelines, and considering the release cases, we determined that the sample size should be 500 cases in each group, with a total of 2,000 participants in four groups [blood heat syndrome (BHS) group, blood stasis syndrome (BSS) group, non-blood heat nor blood stasis syndrome (NHS) group, and a healthy (control) group].
Study population
A baseline cohort of 2,000 participants will be recruited between October 2019 and September 2021. Participants will be recruited from 20 research centers, with each center targeting 25 BHS patients, 25 BSS patients, 25 NHS, and 25 healthy volunteers. In this study, each patient and healthy volunteer will participate only once. Participant follow-up will depend on the date of recruitment.
Eligibility criteria
Inclusion criteria of patients with psoriasis: (I) condition must conform with both Western and CM diagnosis standards for psoriasis vulgaris and CM syndrome diagnosis standards for psoriasis, (II) be 18 to 65 years old (male or female), (III) the selected subjects cannot be omitted until the pre-designed number of cases is completed, and (IV) informed consent must be obtained. Eligible participants must fulfill all inclusion criteria.
Inclusion criteria of healthy volunteers: (I) are not diagnosed with psoriasis vulgaris under Western and CM standards for psoriasis vulgaris and CM syndrome diagnosis standards for psoriasis, (II) are 18 to 65 year old, male or female, (III) the selected subjects cannot be omitted until the pre-designed number of cases is completed, and (IV) informed consent must be obtained. Eligible participants must fulfill all inclusion criteria.
Exclusion criteria of patients with psoriasis: (I) patients with a history (or family history) of severe mental illness, and (II) any other reasons that the investigator considered inappropriate to participate in the study. Candidates satisfying one or more exclusion criteria will be excluded.
Exclusion criteria of healthy volunteers: (I) presence of other active skin diseases which may affect the condition assessment, (II) those with severe, uncontrollable local or systemic acute or chronic infections, (III) a history (or family history) of severe mental illness, and (IV) any other reasons that the investigator considered inappropriate for participation in the study. Candidates satisfying one or more exclusion criteria will be excluded.
Study design
Study timeline
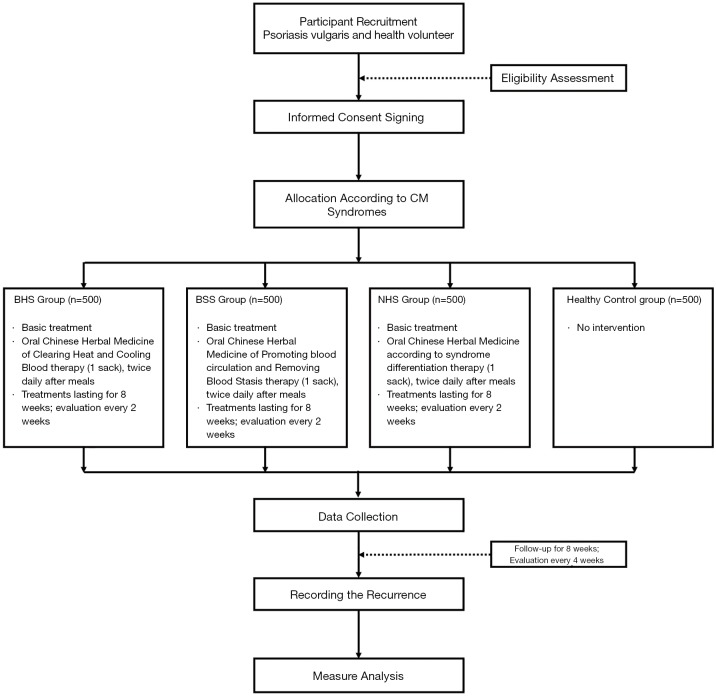
The study protocol consists of four main phases over sixteen weeks: Screening-Enrollment-Allocation, Treatment-Intervention, End-of-Intervention, and Follow-up (Figure 1).
Figure 1.
Flow diagram of the study progress.
The eight-week treatment period is planned in four cycles of two weeks, with a return visit between cycles to check for adverse events (AE), and monitor compliance. All interventions will be stopped after eight weeks. Follow-up will be conducted during the twelfth and sixteenth weeks.
Different demographic characteristics, medical and medication history, skin lesion photographs, relative disease scores, recurrence, CM physical scale, personal life history, medical expenses, patient satisfaction, vital signs, drug combinations, physical examinations, and adverse effects, (among other data) will be recorded (Table 1).
Table 1. Schedule for enrollment, intervention, and assessment.
| Activity | Phase | Screening/enrollment allocation | Treatment/Intervention | End of intervention | Follow-up | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Time point | Week 0 | Weeks 1–2 | Weeks 3–4 | Weeks 5–6 | Weeks 7–8 | Week 12 | Week 16 | ||||
| Enrollment of participants | Eligibility screening | ● | |||||||||
| Acquisition of informed consent | ● | ||||||||||
| Demographic characteristic | ● | ||||||||||
| Medical history taking | ● | ||||||||||
| Enrollment | ● | ||||||||||
| Skin lesion photograph | ● | ● | ● | ● | ● | ● | ● | ● | |||
| Treatment/intervention | Basic treatment + CHCB | ★ | ----------------------------------------------------------------------- | ★ | |||||||
| Basic treatment + PBCRBS | ☆ | ---------------------------------------------------------------------- | ☆ | ||||||||
| Basic treatment + SDT | ▲ | ----------------------------------------------------------------------- | ▲ | ||||||||
| Outcome assessment | PASI score | ● | ● | ● | ● | ● | ● | ● | ● | ||
| Affected BSA | ● | ● | ● | ● | ● | ● | ● | ● | |||
| PGA score | ● | ● | ● | ● | ● | ● | ● | ● | |||
| DLQI score | ● | ● | ● | ● | ● | ||||||
| PRQoL score | ● | ● | ● | ● | ● | ||||||
| VAS score | ● | ● | ● | ● | ● | ● | ● | ● | |||
| CM syndrome | ● | ● | ● | ● | ● | ● | ● | ||||
| recurrence | ● | ● | |||||||||
| Other outcomes | CM physical scale | ● | ● | ● | ● | ||||||
| Personal life history | ● | ● | ● | ● | ● | ● | ● | ● | |||
| Medical expenses | ● | ● | ● | ● | ● | ● | |||||
| Patient satisfaction | ● | ● | ● | ● | ● | ● | ● | ● | |||
| Safety assessment | Vital sign | ● | ● | ● | |||||||
| Drug combination | ● | ● | ● | ● | ● | ● | ● | ● | |||
| Physical examination | ● | ● | ● | ||||||||
| Adverse effect | ● | ● | ● | ● | ● | ● | ● | ● | |||
★Intervention in the blood heat syndrome (BHS) group. ✩Intervention in the blood stasis syndrome (BSS) group. ▲Non-blood heat or blood stasis syndrome (NHS) group. ●Corresponding point. Lines show that there are interventions going on over the period of time. CHCB, Oral Chinese herbal medicine of Clearing Heat and Cooling Blood therapy; PBCRBS, Oral Chinese herbal medicine of Promoting Blood Circulation and Removing Blood Stasis therapy; SDT, Oral Chinese Herbal Medicine according to Syndrome Differentiation therapy; CM, Chinese Medicine.
The screening-enrollment-allocation phase
In the screening-enrollment-allocation phase, patients with psoriasis and healthy volunteers will be recruited through a dermatological outpatient service, physical examination, and an inclusion evaluation will be performed. Eligible participants will be invited to sign the written informed consent regarding participation in the trial (including the procedures, risks, and options for dropping out of the study). A study investigator or medical staff member with adequate training will explain the study clearly to the participant. After providing consent, the participant must sign and date an informed consent form. Subsequently, a participant identification (PID) number will be assigned to facilitate identification throughout the study. All participants will be grouped according to CM syndromes. Patients with psoriasis will be divided into the BHS group, the BSS group, and the NHS group according to the diagnostic criteria (14) of CM syndromes for psoriasis. Healthy volunteers will enter a corresponding control group.
Healthy volunteers must only record medical and surgical history, physical characteristics, and demographic data, as well as submit to a physical examination, and recording of vital signs (blood pressure, heart rate, height, weight, waist circumference, and hip circumference). No follow-up is required.
The treatment-intervention phase
Patients with psoriasis will be treated with different internal treatment methods in each group, and external treatment methods are not limited. The systematic treatment will last for eight weeks, and the patients will receive follow-up as required for eight weeks after the intervention.
Interventions
The therapeutic interventions for patients with psoriasis will be oral CM herbal internal treatments according to the corresponding therapeutic principle of CM syndromes. The treating physician may adjust CM herbal-medicine dosage at any time based on disease changes.
BHS group: participants in the BHS group will receive oral CM herbal Clearing Heat therapy and Cooling Blood therapy via warm water decoction; one daily dose will be administered in two parts during the day, 180–200 mL total for each part over eight weeks.
BSS group: participants in the BSS group will receive oral CM herbal therapy promoting Blood Circulation, and removing Blood Stasis therapy via warm water decoction; one daily dose will be administered in two parts during the day, 180–200 mL total for each part over eight weeks.
NHS group: participants in the NHS group will receive oral CM herbal therapy according to the CM principle of syndrome differentiation via warm water decoction; one daily dose will be administered in two parts during the day, 180–200 mL total for each part over eight weeks.
Control group: no intervention.
Basic treatment: all psoriasis groups will receive basic treatment with moisturizer and a urea cream (Shanghai Yun Jia Huangpu Pharmaceutical Co. Ltd., No. H20063666) selected for this study. Participants will be told to clean their bodies correctly and to apply moisturizer within two minutes of bathing to avoid dehydration. Participants will be instructed to continue their regular daily routine, and maintain communication with treating physician as part of the study.
Intervention drugs
All psoriasis groups will be treated with oral CM herbal medicine for eight weeks. Research drugs will be labeled per Chinese laws and regulations and only used after passing quality inspection. Distribution of research drugs must be recorded correctly in the form provided by the sponsor.
Drug combination
All drugs (except research medicine used for the study) are considered as combination drugs and must be recorded by type (trade name), dosage, indication, and time taken. The study investigator or researchers will judge whether the participant should withdraw from the study due to administration of the combined drugs. The use of steroid hormones during the study period shall not be allowed under any circumstances.
The end-of-intervention phase
Patients stop intervention in this phase and are monitored for relative indicators.
The follow-up phase
Patients will receive follow-ups during the eight weeks after the treatment-intervention, on the twelfth and sixteenth week (from the beginning of the study), and will be monitored for relative indicators.
Measures
Exposure measures
The exposures of interest include demographic variables, risk factors, and intervention factors. Demographic variables include age, sex, height, weight, and ethnicity, among others. Risk factors include occupational exposure, long-term residence, smoking and alcohol history, family history of psoriasis, and inducing or aggravating factors, among others. Intervention factors include external medicine, physiotherapy, and internal medicine.
Outcome measures
Primary outcome measure
The proposed primary outcome of the study is the improvement in the Psoriasis Area Severity Index (PASI) scores after eight weeks of treatment. PASI scores will be assessed every two weeks during treatment and every four weeks during follow-up. All participants completing the eight-week treatment period will receive follow-up during the eight weeks following the treatment-intervention phase.
Secondary outcome measures
Secondary outcomes include affected body surface area (BSA), scores for Physician Global Assessment (PGA), Dermatology Life Quality Index (DLQI), pain-related quality of life (PRQoL), pain on the visual analog scale (VAS) and CM syndromes, and disease relapse. Relapse is defined as a PASI score decrease of more than 50% by the twelfth or sixteenth week 16, versus the maximum value recorded from enrollment. The timeline for efficacy evaluation is shown in Table 1.
Other outcome measures
Other outcome measures include CM physical scale, medical expenses, and patient satisfaction. The timeline for efficacy evaluation is shown in Table 1.
Safety evaluation
All the CM herbal medicines used in this study are included in the pharmacopeia of the People’s Republic of China, and all administered doses are within the prescribed dose range of the pharmacopeia of the People’s Republic of China. Additionally, several measures will be taken for safety, including observation of vital signs, drug combination, physical examination, AE, and severe AE outcomes. The timeline for the safety evaluation is shown in Table 1.
If an AE occurs, the investigator may decide whether to stop the observation or not, depending on the condition. In case of a serious AE, the study investigators must take immediate action to ensure the safety of the participants. Also, any serious AE will be reported to the Yueyang Hospital of Integrated Traditional Chinese and Western Medicine and the Ethics Committee within 24 hours. The Yueyang Hospital of Integrated Traditional Chinese and Western Medicine shall inform other participating hospitals promptly and ensure compliance with all legal and regulatory reporting procedures.
Data management and monitoring
All study data including informed consent forms, will be collected and maintained in a dedicated database for medical research records and must be submitted by investigators at the end of the follow-up period. The principal investigators at the participating hospitals are responsible for collecting the medical research records from the study investigators, as well as for reviewing and storing these records.
The data report adopts the electronic Case Report Form (eCRF), and a specially-assigned person is in charge of data entry at each center. To ensure the quality and consistency of the source data and the database input, two researchers will work independently to verify the source data with the eCRF data. In case of any discrepancy, a query list will be created, and the researchers must respond to the query list to resolve the discrepancy. All documentation related to quality control will be maintained to allow future objective evaluation of the safety and critical efficacy outcomes.
Statistical analysis
Professional statisticians developed the statistical analysis plan upon consultation with the primary trial investigators. The data will be stored in the Data Management Center of Jiangsu Famous Medical Technology Co. Ltd., and processed by the in-house statisticians blinded to group allocation. The analyses will be conducted using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA), and will cover the number of participants enrolled in each group, the number of patients who dropped out of the study (and the reason for the drop-out), demographic and other baseline characteristics, compliance, efficacy analysis, and safety analysis.
Analytical methods will be combined with descriptive analysis and comparative analysis. Qualitative data will be described by frequency table, percentage, or composition ratio, and comparative analyses will be conducted by a chi-square test, Fisher’s exact test, Wilcoxon rank-sum test, Cochran-Mantel-Haenszel test, and weighted least squares covariance. Quantitative data will be described as mean, standard deviation, median, quartile, minimum, and maximum. The t-test will be used for comparative analysis of data with normal distributions, and with Satterthwaite correction if the variance is uneven. Quantitative data exhibiting a non-normal distribution on the Wilcoxon rank-sum test will be analyzed using the Wilcoxon signed-rank sum test and covariance generalized linear models. The hypothesis test will be two-sided, and the test statistics and their corresponding p-values will be reported. A value of P<0.05 will be considered statistically significant, while P<0.01 will be considered highly statistically significant.
Discussion
The proposed research will establish a study cohort and track the treatment progress of psoriasis vulgaris, and analyze the related changes of the disease and risk factors in order to make a specific and targeted effort towards prevention and control of psoriasis. So far, research on psoriasis vulgaris has explained related influencing factors. However, the importance of each influencing factor is still unclear. For example, we still cannot assess the risk of disease progression based on a patient’s lifestyle and living environment. Therefore, through the construction of a big-data cohort study, we will thoroughly evaluate the influence of each factor on the disease, and track the disease progression process.
Psoriasis is currently an incurable, chronic skin disorder, not only affecting the skin but also causing chronic multisystem inflammation. These effects have a considerable impact on a person’s quality of life, appearance and mental health (15-17). Over the years, oral CM herbal medicine has been used for the prevention and treatment of psoriasis, and there have been many reports of clinical trials, but a lack of systematic big-data collection and analysis. CM literature suggests that the pathogenesis of psoriasis is closely related to blood. In recent years, there have been many reports of blood treatment as a fundamental CM treatment for psoriasis with favorable outcomes. Reportedly, Chinese scholars have applied the oral CM herbal Clearing Heat and Cooling Blood therapy to treat psoriasis patients with BHS, and the results showed that the PASI scores decreased significantly after treatment (18,19), which may be related to the regulation of peripheral blood IL-23/Th17 (18). In the slow-phase of psoriasis, blood stasis, skin lost in scratching, and treatment are well suited for therapies promoting blood circulation and removing blood stasis. The oral CM herbal Promoting Blood Circulation and Removing Blood Stasis therapies are shown to significantly decrease the PASI score and serum levels of TNF-α, IL-8 (8).
Our study is a prospective and multicenter cohort study of psoriasis vulgaris. The multicenter model considers the impact of environmental factors and an individual participant’s characteristics on the trial results, providing more reliable clinical evidence. At the same time, the objective PASI score was adopted as the primary outcome measure, and BSA, PGA, DLQI, PRQoL, VAS, and CM syndrome scores as the secondary outcome measures, which should improve the reliability of the results. Additionally, the CM physical scale, personal life history, medical expenses, patient satisfaction, and other outcome measures have been added. Keeping participants closer to the clinics helps better track the disease progression in a clinical setting. However, the study design has the following limitations: The duration of this study is limited to 16 weeks, and the sample size is only 2,000 participants, therefore it might be challenging to observe the full progression of psoriasis vulgaris within the limited period and sample size. We will use unique statistical and outcome measures to improve the accuracy and sensitivity of the study, mitigating these limitations. Additionally, we are planning to conduct further RCT studies involving psoriasis vulgaris with more participants to identify the process of disease progression.
In conclusion, this prospective, multicenter cohort study of psoriasis vulgaris will analyze progression risk factors and disease change trends, establish a multicenter clinical registration platform, and establish clinical evidence by evaluating the therapeutic advantages (clinical effectiveness and recurrence reduction), of CM therapy. The results will contribute to decision-making in the management and treatment of psoriasis and provide essential information that may be incorporated into future guidelines.
Acknowledgments
Funding: This study was supported by the National Key Research and Development Program of China (No. 2018YFC1705301), the NSFC of China (No. 81874470, 81973860), the Shanghai Development Office of TCM [Nos. ZY(2018-2020)-FWTX-1008, ZY(2018-2020)-CCCX-2004-08, and ZY(2018-2020)-FWTX-4010], the Shanghai Science and Technology Committee (No. 18401932300), the Young Talent Supporting Program of China Association of Traditional Chinese Medicine [No. CACM-2017-QNRC2-(B05)], and the Development Fund for Shanghai Talents (No. 2017047).
Ethical Statement: Ethical approval has been obtained from the Ethics Committee of the Yueyang Hospital of Integrated Traditional Chinese and Western Medicine (approval no. 2019-028). All participants will be enrolled only after providing written informed consent. No clinical data or bio-samples will be collected without the participants’ consent. The trial is conducted per national laws, Good Clinical Practice guidelines, and the Declaration of Helsinki as revised in 2013. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Tampa M, Sarbu MI, Mitran MI, et al. The Pathophysiological Mechanisms and the Quest for Biomarkers in Psoriasis, a Stress-Related Skin Disease. Dis Markers 2018;2018:5823684. 10.1155/2018/5823684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol 2017;31:205-12. 10.1111/jdv.13854 [DOI] [PubMed] [Google Scholar]
- 3.Zhang JZ. Epidemiology and risk factors of psoriasis. Pract J Clin Med 2013;01:4-6. [Google Scholar]
- 4.Georgescu SR, Tampa M, Caruntu C, et al. Advances in Understanding the Immunological Pathways in Psoriasis. Int J Mol Sci 2019;20. doi: . 10.3390/ijms20030739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tampa M, Nicolae IL, Ene CD, et al. Vitamin C and thiobarbituric acid reactive substances (TBARS) in psoriasis vulgaris related to psoriasis area severity index (PASI). Rev Chim 2017;68:43-7. [Google Scholar]
- 6.Zheng Q, Jiang WC, Sun XY, et al. Total glucosides of paeony for the treatment of psoriasis: A systematic review and meta-analysis of randomized controlled trials. Phytomedicine 2019;62:152940. 10.1016/j.phymed.2019.152940 [DOI] [PubMed] [Google Scholar]
- 7.Li FL, Xu R, Zeng QC, et al. Tanshinone IIA Inhibits Growth of Keratinocytes through Cell Cycle Arrest and Apoptosis: Underlying Treatment Mechanism of Psoriasis. Evid Based Complement Alternat Med 2012;2012:927658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dai YJ, Li YY, Zeng HM, et al. Effect of Yinxieling decoction on PASI, TNF-α and IL-8 in patients with psoriasis vulgaris. Asian Pac J Trop Med 2014;7:668-70. 10.1016/S1995-7645(14)60113-9 [DOI] [PubMed] [Google Scholar]
- 9.Li X, Xiao QQ, Li FL, et al. Immune Signatures in Patients with Psoriasis Vulgaris of Blood-Heat Syndrome: A Systematic Review and Meta-Analysis. Evid Based Complement Alternat Med 2016;2016:9503652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J, Cao XX, Xu R, et al. Research on different expressions of peripheral blood Th1/Th2 cells in psoriasis patients of blood heat syndrome and of blood stasis syndrome. Zhongguo Zhong Xi Yi Jie He Za Zhi 2014;34:46-50. [PubMed] [Google Scholar]
- 11.Fan B, Li X, Ze K, et al. Expression of T-helper 17 cells and signal transducers in patients with psoriasis vulgaris of blood-heat syndrome and blood-stasis syndrome. Chin J Integr Med 2015;21:10-6. 10.1007/s11655-014-1769-7 [DOI] [PubMed] [Google Scholar]
- 12.Parker S, Zhang CS, Yu JJ, et al. Oral Chinese herbal medicine versus placebo for psoriasis vulgaris: A systematic review. J Dermatolog Treat 2017;28:21-31. 10.1080/09546634.2016.1178377 [DOI] [PubMed] [Google Scholar]
- 13.Wu Jieping Medical Foundation, China breast cancer research collaboration group. Real-World Research guidelines (2018 edition).
- 14.Dermatologist branch of China association of Chinese medicine. Traditional Chinese medicine (Baibi) with psoriasis vulgaris of evidence-based clinical practice guidelines (2013 edition). J Tradit Chin Med 2014;1:76-82. [Google Scholar]
- 15.Li X, Miao X, Wang H, et al. Association of Serum Uric Acid Levels in Psoriasis: A Systematic Review and Meta-Analysis. Medicine (Baltimore) 2016;95:e3676. 10.1097/MD.0000000000003676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X, Kong LJ, Li FL, et al. Association between Psoriasis and Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-analysis. PLoS One 2015;10:e0145221. 10.1371/journal.pone.0145221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parisi R, Webb RT, Kleyn CE, et al. Psychiatric morbidity and suicidal behaviour in psoriasis: a primary care cohort study. Br J Dermatol 2019;180:108-15. 10.1111/bjd.17004 [DOI] [PubMed] [Google Scholar]
- 18.Wang L, Fang YP, Zhou GX, et al. Study on clinical efficacy and mechanism of Qingying Tang for treating psoriatic blood-heat syndrome based on IL-23/Th17. Zhongguo Zhong Yao Za Zhi 2019;44:175-80. [DOI] [PubMed] [Google Scholar]
- 19.Zhang LX, Bai YP, Song PH, et al. Effect of Chinese herbal medicine combined with acitretin capsule in treating psoriasis of blood-heat syndrome type. Chin J Integr Med 2009;15:141-4. 10.1007/s11655-009-0145-5 [DOI] [PubMed] [Google Scholar]