Abstract
Background
It is well known that the dysregulation of microRNAs (miRNAs) has been identified in papillary thyroid carcinoma (PTC), but their roles in the progression and metastasis of PTC remain unclear. MicroRNA-3619-3p (miR-3619-3p) is associated with cancer progression as an oncogene which is predicted to target at the Wnt/β-catenin signaling pathway. Our study aimed to investigate the role of miR-3619-3p on PTC cell migration and invasion, as well as the underlying mechanisms.
Methods
The expression of miR-3619-3p in 36 PTC tissues and corresponding tumor-adjacent tissues, as well as 3 PTC cell lines (BCPAP, K1, TPC-1) and the normal thyroid epithelial cell line (N-thy-ori 3-1) were detected by quantitative real-time polymerase chain reaction (qRT-PCR). The relationship between miR-3619-3p expression and clinicopathologic status of PTC patients was analyzed. Migration, invasion, and wound healing, were used to evaluate the role of miR-3619-3p in PTC. The activation of β-catenin and the possible molecular pathway were detected by western blotting.
Results
The expression of miR-3619-3p in PTC tissues was significantly higher than the corresponding tumor-adjacent tissues (P<0.01), and its high expression positively correlated with extrathyroidal invasion, multicentricity, and cervical lymph node metastasis. Moreover, the miR-3619-3p was also up-regulated in PTC cell lines when compared to N-thy-ori 3-1. MiR-3619-3p enhanced the capabilities of migration and invasion in PTC cell lines. Furthermore, miR-3619-3p activated Wnt/β-catenin pathway via maintaining the mRNA stability of β-catenin.
Conclusions
miR-3619-3p promoted PTC cell migration and invasion as an oncogene via activating the Wnt/β-catenin pathway through increasing the stability of β-catenin.
Keywords: Papillary thyroid carcinoma (PTC), miR-3619-3p, migration, invasion, β-catenin
Introduction
As the most common malignancy in the endocrine system, thyroid carcinoma accounts for 90% of all endocrine-related cancer cases. In the past 30 years, the incidence of thyroid carcinoma has increased more than three-fold worldwide (1), with papillary thyroid carcinoma (PTC) accounting for more than 90% of the new cases (2). PTC has a propensity to invade the lymphatic region, leading to multimodal lesions in the thyroid gland as well as a high incidence of lymph node metastases (3), which highly affects the prognosis and the therapeutic regimen (4,5), especially in the elderly patients (6). Several recent publications suggest that the increasing number, size, and ratio of positive lymph node and the presence of an extranodal spread may all impact prognosis (7-9). It has been confirmed that genetic alteration such as BRAF mutation, plays a crucial role in the tumorigenesis of PTC (10), but the metastasis and invasion mechanism of PTC is still unclear. Therefore, exploring the underlying molecular mechanism of the migration in PTC cells is of great significance for providing new therapeutic targets and improving the prognosis of PTC patients.
MicroRNAs (miRNAs), are a highly conserved class of small noncoding RNAs, consisting of 21–25 nucleotides (11). MiRNAs regulate the expression of genes by influencing post-transcription or translation, which play a major role in cellular processes including cell growth, reproduction, and apoptosis. A vast of publications have indicated that the expression of miRNAs is dysregulated in various human tumors (12), including thyroid carcinoma. A series of miRNAs, such as miR-222, miR-146b, and miR-20b (13-15), have been proven to play an important role in the development and metastasis of thyroid carcinoma. For example, miR-146b-5p positively regulates migration and invasion of PTC cells by targeting at SMAD4 and influencing the TGF-β signaling pathway (16), and miR-222 promotes invasion and metastasis of PTC through targeting protein phosphatase 2 regulatory subunit B alpha expression (PPP2R2A) (13). Recently, some miRNAs have been proven to upregulate protein or mRNA levels in unconventional ways (17). MiR-122 and miR-1254 were shown capable of enhancing the stability of target mRNA by reducing their degradation (18,19).
A recent study has showed that microRNA-3619-3p (miR-3619-3p) is significantly correlated with postoperative tumor relapse, and it is up-regulated in recurrent esophageal carcinoma tissue, compared to nonrecurrent esophageal carcinoma (20). Using bioinformatic software to predict target genes and pathway analysis, it was found that miR-3619-3p is mainly involved in the Wnt/β-catenin pathway. Previous studies have concluded that this pathway plays a primary role in the invasion and metastasis of thyroid carcinoma (21), and alteration of the Wnt/β-catenin pathway was a late event in thyroid cell transformation (22). However, the role of miR-3619-3p in PTC has not been confirmed, and the expression and molecular mechanism of miR-3619-3p in PTC remain unclear.
In this study, we aimed to elucidate the expression level and biological functions of miR-3619-3p in PTC, and its role in regulating the Wnt/β-catenin pathway.
Methods
Clinical tissue samples
Thirty-six pairs of PTC tissues and corresponding adjacent noncancerous thyroid tissues were collected at the First Affiliated Hospital of Sun Yat-sen University and Sun Yat-sen University Cancer Center (Guangzhou, China) between September 2016 and September 2017. All patients underwent surgical treatment and the postoperative pathology was diagnosed as PTC. The tissue samples were immediately frozen in liquid nitrogen and stored at −80 °C refrigerator for further investigation. The samples were obtained with informed consent from all patients, and the study was approved by the Institutional Research Ethics Committee of Sun Yat-sen University.
Cell lines and cell culture
The human normal thyroid epithelial cell line Nthy-ori 3-1 and human PTC cell lines, TPC-1 and BCPAP, were given generously by Professor Haixia Guan (The First Affiliated Hospital of China Medical University). Another PTC cell line, K1, was purchased from the European Collection of Authenticated Cell Cultures. Cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS) (Gibco, Grand Island, NY, USA) at 37 °C in a 5% CO2 incubator.
RNA transfection and treatment
For this purpose, BCPAP and TPC-1 cells were seeded in 6-well plates for 24 h before transfection in order to achieve 50–60% confluence. Cells were transfected using Lipo3000 (Invitrogen) with RNA oligonucleotides according to the manufacturer’s protocols. After 48 h of miRNA transfection, the cells were harvested for further assays. The miR-3619-3p mimics, inhibitor and negative control (NC) were purchased from GenePharma (Shanghai, China). The sequence of the miR-3619-3p mimics was, 5’-GGGACCAUCCUGCCUGCUGUGG-3' and 5'-ACAGCAGGCAGGAUGGUCCCUU-3', while the miR-3619-3p inhibitor was 5'-CCACAGCAGGCAGGAUGGUCCC-3'. ICG-001 (Selleck, Houston, TX, USA) was dissolved in dimethyl sulfoxide (Sigma, St. Louis, MO, USA) and its final concentration in medium was 10 µM. The LiCl (Sigma) was dissolved in DMEM, and its final concentration in medium was 20 mM.
RNA isolation and quantitative real-time polymerase chain reaction (qRT-PCR) analysis
Total RNA from cell lines and patient tissue samples were isolated with Trizol reagent (Invitrogen) according to manufacturer’s instructions. RNA samples were reverse transcribed into cDNA by PrimeScriptTM RT-PCR Kit (TaKaRa, Dalian, China) and PrimeScriptTM miRNA RT-PCR Kit (TaKaRa), respectively. qRT-PCR was performed using a Light Cycler 480II system (Roche Diagnostics, Basel, Switzerland) with SYBR Premix Ex TaqTM (TaKaRa). U6 served as an internal control. The sequences of the primers used for amplification were as follows: miR-3619-3p forward, 5'-GACCATCCTGCCTGCTGTG-3'; reverse, Uni-miR qRCP primer (TaKaRa); U6 forward, 5'-ACGCAAATTCGTGAAGCGTT-3'; reverse, Uni-miR qRCP primer (TaKaRa); CTNNB1 forward, 5'-CTGACAAAACTGCTAAATGACGAGG-3'; reverse, 5'-TTATGCAAGGTCCCAGCGGTAC-3'; GAPDH forward, 5'- GCACCGTCAAGGCTGAGAAC-3'; and reverse, 5'- TGGTGAAGACGCCAGTGGA-3'. Data processing used the 2-ΔΔCT method.
Wound healing assay
Wound healing assays were performed to evaluate the cell motility. After 48 h of transfection, cells were about 90–95% confluence in a 6-well plate. The cells were carefully scratched with sterile pipette tips to form streaks, and then cells were cultured in medium without serum for 24 h. The cultures were observed and photographed using an inverted microscope (Leica DMI4000B, Wetzlar, Germany).
Cell migration and invasion assays
Cell migration and invasion assays were detected in a 24-well plate with 8 µm pore size chamber inserts (Corning, NY, USA). Eighty thousand cells resuspended in 100 µL serum-free medium were seeded into the upper chamber per well with the non-coated membrane for migration assay and with the Matrigel-coated membrane (BD Bioscience, San Jose, CA, USA) for the invasion assay. In the lower chamber, 600 µL of DMEM supplemented with 10% FBS was added. After several hours of incubation at 37 °C, the upper chamber was wiped to remove the cells on the upper surface with a cotton swab, and then the cells, having migrated to the bottom surface of the membrane, were fixed for 15 min with 4% polyformaldehyde, and stained with 0.05% crystal violet for 15 min. Then, the cells were observed and photographed using an inverted microscope (Leica DMI4000B).
Western blot
The cells were lysed on ice with RIPA lysis buffer supplemented with protease inhibitor cocktail (Roche), and the lysates were cleared by centrifugation for 20 min at 14,000 rpm. The protein concentration of each sample was quantified with BCA Kit (Pierce, Rockford, IL, USA). Total protein (30–50 µg) was separated by 10% SDS-PAGE, and transferred to PVDF membranes (Merck Millipore, Billerica, MA, USA). The membranes were blocked in 5% non-fat milk for 1h and then probed with specific primary antibodies overnight at 4 °C. The membranes were incubated with corresponding secondary antibodies and specific bands were detected using the SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher, Waltham, MA, USA). GAPDH antibody was used as control. The primary antibodies were as follow: rabbit anti-human β-catenin (Abcam, #32572, Cambridge, MA, USA), anti-human GAPDH (Santa Cruz, #sc-25778, CA, USA).
Statistical analysis
All experiments were performed independently at least three times. All data are presented as the mean ± standard deviation (SD). The significance of differences between groups were assessed by a paired, two-tailed Student’s t-test. The χ2 tests were used to analyze miR-3619-3p levels between human PTC samples and the matched non-tumorous thyroid tissues. All statistical analyses were performed using SPSS 22.0 software. P values of less than 0.05 were considered statistically significant.
Results
miR-3619-3p is upregulated in PTC and correlates with patients’ clinicopathologic characteristics
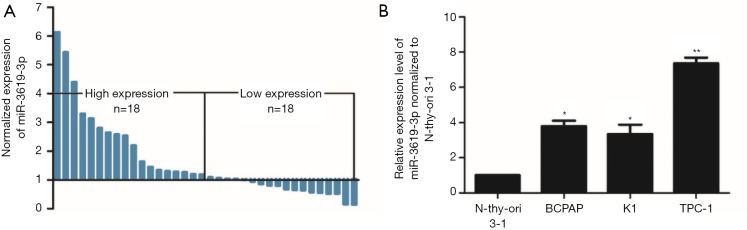
To investigate whether miR-3619-3p regulated the progression of PTC, we performed qRT-PCR to determine the expression level of miR-3619-3p in 36 pairs of PTC tissues and corresponding adjacent thyroid tissues. Compared with the matched normal thyroid tissues, the expression of miR-3619-3p was significantly upregulated in PTC tissues (P<0.01, Figure 1A). In addition, the expression of miR-3619-3p in PTC cell lines, including BCPAP, K1, and TPC-1, was similarly increased as expected compared with normal thyroid cell line Nthy-ori 3-1 (P<0.05, Figure 1B).
Figure 1.
miR-3619-3p is upregulated in human PTC tissues and cell lines. (A) The relative expression level of miR-3619-3p was significantly up-regulated in PTC tissues compared with corresponding adjacent non-tumor tissues by qRT-PCR (n=36); (B) the expression of miR-3619-3p in PTC cell lines was higher compared to the normal N-thy-ori 3-1 cells. The data represent the mean ± SD from three independent experiments. *, P<0.05, **, P<0.01. PTC, papillary thyroid carcinoma; qRT-PCR, quantitative real-time polymerase chain reaction.
To explore the correlation between the expression of miR-3619-3p and clinicopathologic characteristics of PTC patients, 36 patients were divided into two groups (n=18 > median; n=18 ≤ median), according to the median expression level of miR-3619-3p (23). As shown in Table 1, miR-3619-3p expression positively correlated with multicentricity (P=0.044), extrathyroidal invasion (P=0.011), and lymph node metastasis (P=0.026), although there was no significant correlation with age, gender, tumor size, and TNM stage.
Table 1. Correlation between miR-3619-3p expression and clinicopathologic characteristics of PTC patients.
| Characteristics | miR-3619-3p | P value | |
|---|---|---|---|
| Low (%) | High (%) | ||
| Age (years) | 0.289 | ||
| <55 | 17 (94.4) | 15 (83.3) | |
| ≥55 | 1 (5.6) | 3 (16.7) | |
| Gender | 0.07 | ||
| Male | 3 (16.7) | 8 (44.4) | |
| Female | 15 (83.3) | 10 (55.6) | |
| Tumor size (cm) | 0.083 | ||
| <2 | 14 (77.8) | 9 (50.0) | |
| ≥2 | 4 (22.2) | 9 (50.0) | |
| Multicentricity | 0.044* | ||
| Negative | 13 (72.2) | 7 (38.9) | |
| Positive | 5 (27.8) | 11 (61.1) | |
| Extrathyroidal invasion | 0.011* | ||
| Negative | 9 (50.0) | 2 (11.1) | |
| Positive | 9 (50.0) | 16 (88.9) | |
| Lymphatic metastasis | 0.026* | ||
| Negative | 8 (44.4) | 2 (11.1) | |
| Positive | 10 (55.6) | 16 (88.9) | |
| TNM stage | 0.289 | ||
| I | 17 (94.4) | 15 (83.3) | |
| II | 1 (5.6) | 3 (16.7) | |
The TNM stage is based on the Eighth Edition of the AJCC TNM Staging System of differentiated thyroid cancer. *, P values <0.05 indicate statistical significance. PTC, papillary thyroid carcinoma; AJCC, American Joint Committee on Cancer.
Collectively, these results showed that miR-3619-3p was upregulated in human PTC tissues and cell lines, and its high expression correlated with patients’ clinicopathologic characteristics, indicating that it may be a potential oncogenic microRNA in PTC.
miR-3619-3p promotes PTC cell migration and invasion
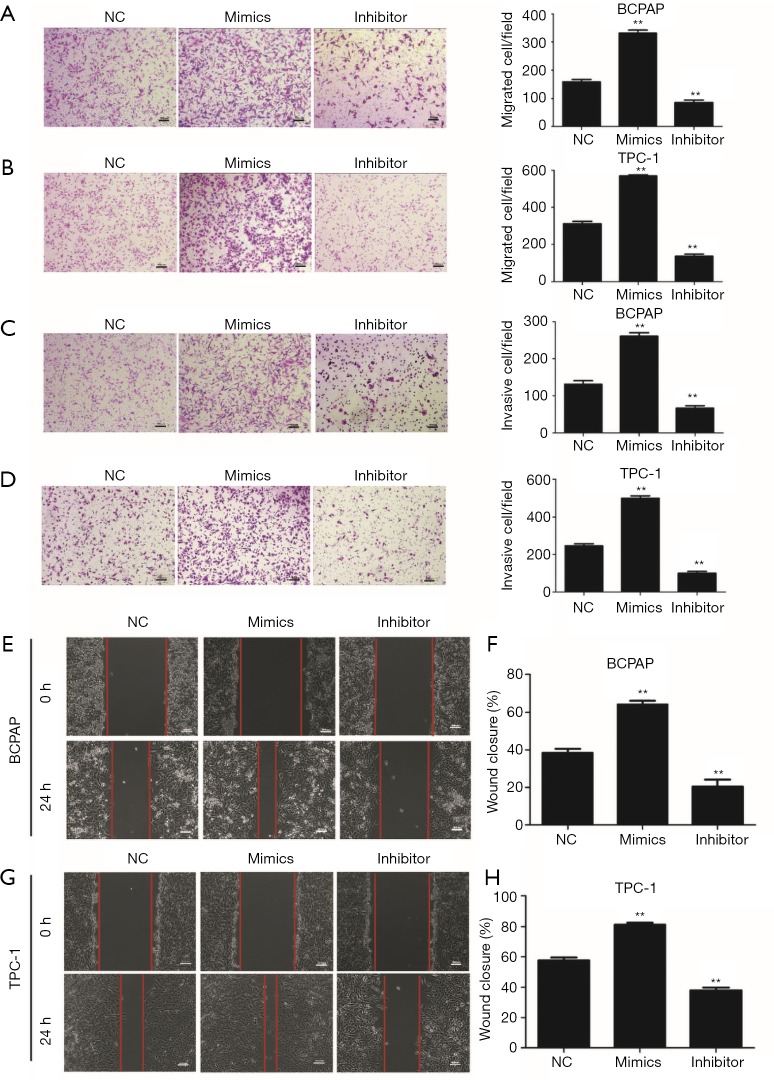
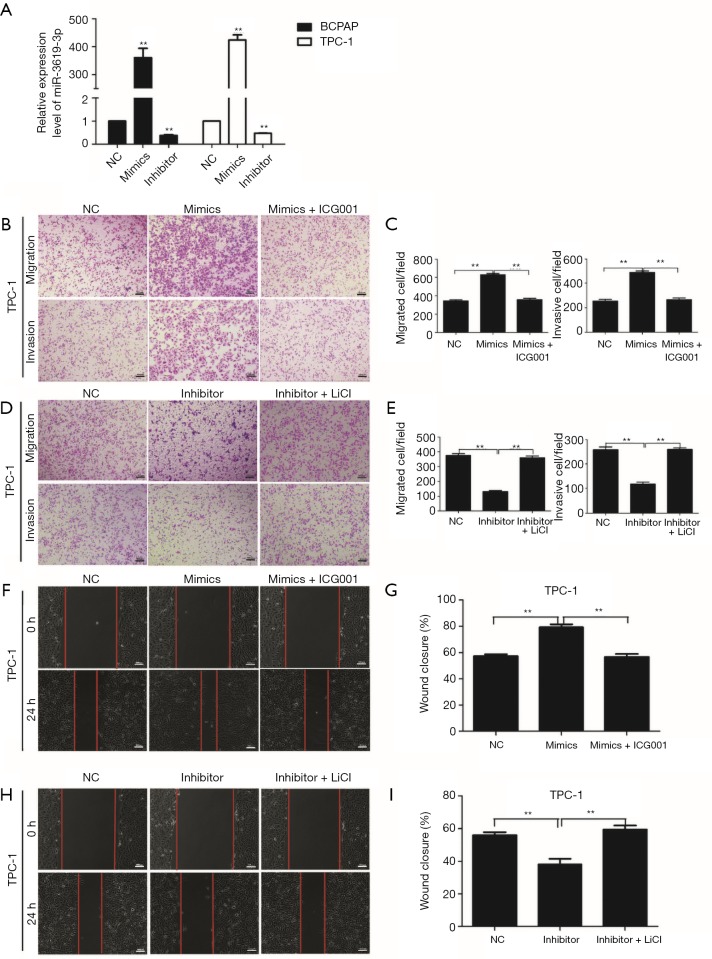
To evaluate the oncogenic role of miR-3619-3p in PTC, we transfected its mimics, inhibitor, or the NC into BCPAP or TPC-1, and detected the expression of miR-3619-3p by qRT-PCR to confirm the efficiencies of mimics and inhibitor (Figure S1A). After that, transwell assays with or without Matrigel were employed to observe the capabilities of migration and invasion in BCPAP and TPC-1 cells. The result showed that ectopic expression of miR-3619-3p promoted cell migration and invasion, when compared to the NC group. Inversely, knocking down endogenous miR-3619-3p in cells via inhibitor substantially suppressed cell migration and invasion (Figure 2A,B,C,D).
Figure 2.
miR-3619-3p promotes PTC cell migration and invasion in vitro. (A,B) Transwell migration assays were performed to analyze the migration of BCPAP and TPC-1 cells that were transiently transfected with NC, miR-3619-3p mimics, or inhibitor. (C,D) Transwell invasion assays were performed to analyze the invasion of BCPAP and TPC-1 cells that were transiently transfected with NC, miR-3619-3p mimics, or inhibitor. (A,B,C,D) The cells were stained with 0.05% crystal violet. Representative images of invasion and migration are shown; all images were obtained at 100× magnification under an inverted microscope. The cells that had migrated were counted from ten randomly chosen fields. Error bars correspond to the mean ± SD of triplicate experiments. (E,F) Wound healing assays of BCPAP and TPC-1 after treatment, respectively. (G,H) Percentages of wound closures in the wound healing assays. **, P<0.01. PTC, papillary thyroid carcinoma.
Similarly, the wound healing assay demonstrated that high miR-3619-3p significantly improved the migration; to the contrary, the reduction of miR-3619-3p impaired the wound closure (Figure 2E,F,G,H).
Collectively, miR-3619-3p functioned as an oncogene in regulating PTC cell migration and invasion.
miR-3619-3p targets CTNNB1 in PTC
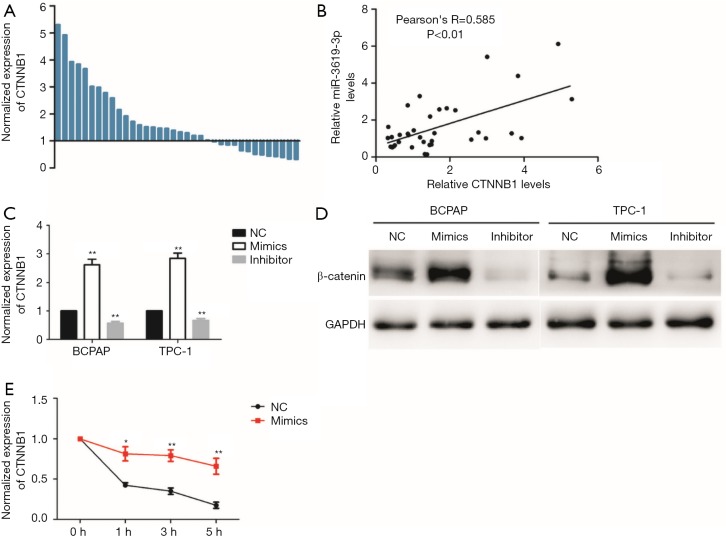
To understand how miR-3619-3p promoted PTC cell migration and invasion, we searched for potential targets by using the public miRNA prediction databases (DIANA Tools: http://www.microrna.gr/webServer and Targetscan: http://www.targetscan.org/vert_71/). Both databases showed the target genes of miR-3619-3p could be annotated to the Wnt/β-catenin pathway, and CTNNB1 (β-catenin), which is the core gene coding the protein of β-catenin, contained a highly conserved sequence paired with miR-3619-3p which is located in the 3′-UTR of CTNNB1 mRNA. Therefore, we detected the expression of CTNNB1 in PTC tissues and found that the expression of CTNNB1 was significantly upregulated in PTC tissues compared with the corresponding adjacent thyroid tissues (P<0.01, Figure 3A), and there was a positive correlation between miR-3619-3p and CTNNB1 expression in 36 pairs of PTC tissues (Figure 3B). Strikingly, this result contradicts the most common way of miRNA by binding to the 3' untranslated region (3'-UTR) of target genes. To confirm this result, we detected the mRNA and protein level of CTNNB1 in both BCPAP and TPC-1 cells after they were transfected with the miR-3619-3p mimics or inhibitor. The results showed that overexpression of miR-3619-3p dramatically increased both mRNA and protein levels of CTNNB1 in BCPAP and TPC-1 cells. However, the expression of CTNNB1 (β-catenin) was decreased after knock down of the miR-3619-3p (Figure 3C,D). According to recent research, microRNAs are involved not only in post-transcription regulation, but also in the decay of target mRNAs (18,19). Interestingly, we found that the half-life time of CTNNB1 mRNA was prolonged when enforced by the expression of miR-3619-3p after the treatment of transcription inhibitor actinomycin D (10 ng/mL), which indicates that miR-3619-3p could enhance the CTNNB1 mRNA stability (Figure 3E). Overall, these results suggest that miR-3619-3p may promote migration and invasion via targeting at CTNNB1.
Figure 3.
miR-3619-3p activates Wnt/β-catenin pathway in PTC. (A) The relative expression level of CTNNB1(β-catenin) was significantly up-regulated in PTC tissues compared with corresponding adjacent non-tumor tissues by qRT-PCR (n=36); (B) the association analysis of the relationship between miR-3619-3p and CTNNB1 expression levels in 36 paired PTC tissues; (C) the relative mRNA expression level of CTNNB1 in BCPAP and TPC-1 after treatment by qRT-PCR; (D) immunoblotting detected protein levels of β-catenin in BCPAP and TPC-1 that were transiently transfected with NC, miR-3619-3p mimics or inhibitor; (E) the CTNNB1 RNA stability assay in TPC cells with treatment of 10 ng/mL actinomycin D after miR-3619-3p mimics or NC transfection. The data represent the mean ± SD from three independent experiments. *, P<0.05; **, P<0.01. PTC, papillary thyroid carcinoma.
miR-3619-3p promotes cell migration and invasion by regulating Wnt/β-catenin pathway in PTC
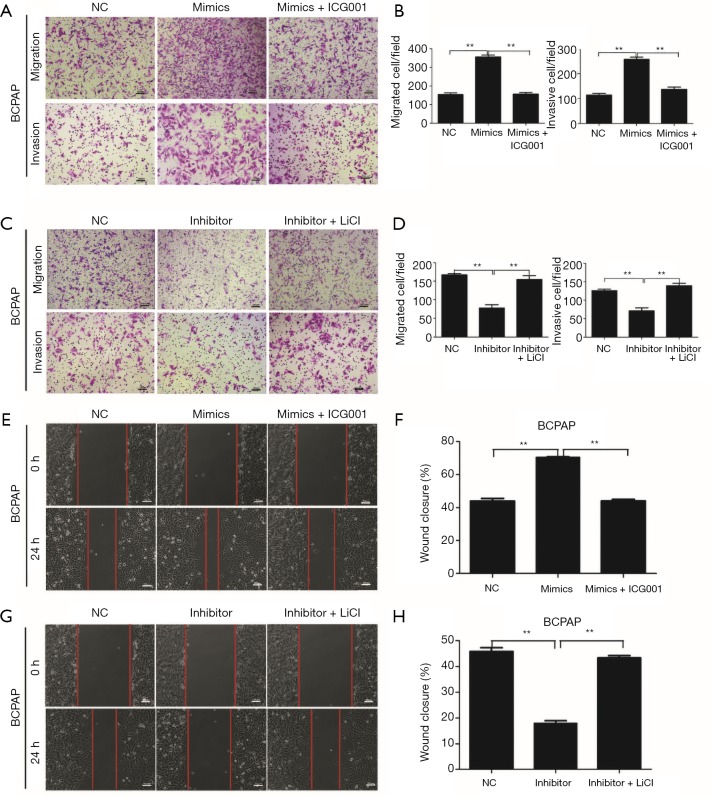
Since miR-3619-3p is targeted at CTNNB1 (β-catenin), the coding gene of β-catenin, we hypothesized that miR-3619-3p promoted cell migration and invasion by regulating the Wnt/β-catenin pathway. To confirm this hypothesis, a β-catenin transcription inhibitor, ICG-001, was introduced to inhibit the activation of the Wnt/β-catenin pathway in both BCPAP and TPC-1 cells, and miR-3619-3p mimics or NC were transfected into these two cells at the same time. The migration and invasion assays showed that ICG-001 prominently abolished miR-3619-3p-induced cell migration and invasion (Figure 4A,B; Figure S1B,C). Furthermore, the activator of the Wnt/β-catenin pathway, LiCl, could abrogate the functions of silencing miR-3619-3p on cell migration and invasion (Figure 4C,D; Figure S1D,E). Similar findings were also observed in wound healing assays (Figure 4E,F,G,H; Figure S1F,G,H,I). In short, miR-3619-3p promotes cell migration and invasion by regulating the Wnt/β-catenin pathway in PTC cells.
Figure 4.
miR-3619-3p enhances cell migration and invasion via activating Wnt/β-catenin pathway in PTC. (A,B) Transwell migration and invasion assays for BCPAP that were transfected with miR-3619-3p mimics or NC followed by treatment with ICG-001 (10 µm) for 24 h. (C,D) Transwell migration and invasion assays for BCPAP that were transfected with miR-3619-3p inhibitor or NC after treatment with LiCL (20 mM) for 24 h. (A,C) The cells were stained with 0.05% crystal violet. Representative images of migration and invasion are shown; all images were obtained at 100× magnification under an inverted microscope. The cells that had migrated were counted from ten randomly chosen fields. (E,F,G,H) Wound healing assays of BCPAP after treatment, respectively. **, P<0.01. Error bars correspond to the mean ± SD of triplicate experiments. PTC, papillary thyroid carcinoma.
Discussion
The expression of miR-3619-3p in small cell carcinoma of the esophagus was found to be more abundant in a postoperative relapse group than in a non-relapse group, which suggests miR-3619-3p is associated with esophageal cancer relapse and predicts poor clinical prognosis (20). In our study, miR-3619-3p was highly expressed in PTC tissues and its expression was related to multicentricity, extrathyroidal invasion, and lymph node metastasis in patients. In addition, enforced expression of miR-3619-3p enhanced the migration and invasion of PTC cells in vitro. It revealed that miR-3619-3p may function as an oncogene to promote aggression in PTC.
It is known to all that miRNAs negatively regulate target gene expression post-transcriptionally, leading to the instability and degradation of target mRNAs (84%) or translation depression (16%) through argonaute-catalyzed mRNA cleavage (24). Nevertheless, Dragomir’ and Knutsen’s groups summarized seven unconventional ways that miRNAs function outside the paradigm mentioned above (17), posting that miRNAs could upregulate protein expression by activating translation (25) and directly activating transcription by binding the promoter RNA of genes (26). Moreover, Shimakami and Yamane found that miR-122 bound to the 5'-UTR of HCV RNA with Ago2 and slowed its decay by protecting it from the 5’ exonuclease of the host mRNA decay machinery, resulting in translation stimulation (18). Meanwhile, another study indicates that miR-1254 interacts with cell cycle and apoptosis regulator 1 (CCAR1) 5'-UTR and enhances the stability of both molecules (19). In our study, we found over-expression of miR-3619-3p could prolong the mRNA half-life of β-catenin and enhance its stability unconventionally, leading to an accumulation of β-catenin mRNA and the activation of the Wnt/β-catenin pathway. Because both 3'-UTR and 5'-UTR are involved in the mRNA decay (27), we hypothesized that one mechanism of how miR-3619-3p enhanced β-catenin mRNA stability was that miR-3619-3p bound to the 3'-UTR or 5'-UTR of β-catenin mRNA to slow its decay. Since Rbm46 (28) and Dishevelled-KSRP complex (29) are demonstrated to regulate the Wnt/β-catenin pathway through post-transcriptional stabilization of β-catenin mRNA, miR-3619-3p may regulate the stability of β-catenin mRNA in coordination with these proteins.
The Wnt/β-catenin pathway is a foundational mechanism that regulates cell proliferation, polarity, and cell fate determination during embryonic development and tissue homeostasis (30,31). It plays a significant role in the progression of various malignancies (32,33). Its constitutive activation is commonly found in human cancers; β-catenin accumulates in the cytoplasm and is transferred into the nucleus to function as a transcriptional activator of its target genes. It was widely acknowledged that the activation of the Wnt pathway is the second mutational event involved in the progression from a well-differentiated to a poorly or undifferentiated and more aggressive thyroid carcinoma, which is associated with the poor clinical outcomes (22). The activation of the Wnt/β-catenin pathway in thyroid carcinoma is particularly common (34). Therefore, the Wnt/β-catenin pathway is largely responsible for enhancing the aggressiveness of thyroid carcinoma (21). The complexity of the Wnt/β-catenin pathway offers numerous targets for therapeutic intervention, and β-catenin interacts with multiple partners, which makes small molecular inhibitors an attractive option (35). Quercetin and Aspirin inhibit the Wnt/β-catenin pathway by disrupting the binding of β-catenin and TCF4 or increasing phosphorylation of β-catenin, respectively (36). 2,4-diaminoquinazolines have been demonstrated to be β-catenin antagonists and can inhibit the growth of colorectal cancer (37). Our data suggest that miR-3619-3p promotes cell migration and invasion by regulating the Wnt/β-catenin pathway in PTC, and, is thus a potential target for thyroid carcinoma treatment.
Conclusions
In conclusion, our study suggests that the upregulation of miR-3619-3p in PTC promotes the migration and invasion of PTC cells via the activation of Wnt/β-catenin pathway through increasing the stability of β-catenin mRNA.
Figure S1.
miR-3619-3p promotes papillary thyroid carcinoma progression. (A) qRT-PCR analysis of miR-3619-3p expression in NC, mimics, and inhibitor-treated PTC cells; (B,C) transwell migration and invasion assays for TPC-1 that were transfected with miR-3619-3p mimics or NC followed by treatment with ICG-001 (10 µm) for 24 h; (D,E) transwell migration and invasion assays for TPC-1 that were transfected with miR-3619-3p inhibitor or NC after treatment with LiCL (20 mM) for 24 h. (B,C,D,E) The cells were stained with 0.05% crystal violet. Representative images of migration and invasion are shown; all images were obtained at 100x magnification under an inverted microscope. The cells that had migrated were counted from ten randomly chosen fields. Error bars correspond to the mean ± SD of triplicate experiments. (F,G,H,I) Wound healing assays of TPC-1 after treatment, respectively. The data represented the mean ± SD from three independent experiments. **, P<0.01. PTC, papillary thyroid carcinoma; qRT-PCR, quantitative real-time polymerase chain reaction.
Acknowledgments
We thank Professor Haixia Guan (The First Affiliated Hospital of China Medical University) for his generous gifts of BCPAP, TPC-1 and Nthy-ori 3-1 cells, and thank Professor Hai Hu (Sun Yat-sen Memorial Hospital, Sun Yat-sen University) for his assistance with the experiments.
Funding: This research was supported by grants from the National Natural Science Foundation of China (No. 81772850 and No. 81702648).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The samples were obtained with informed consent from all patients, and the study was approved by the Institutional Research Ethics Committee of Sun Yat-Sen University.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 2.Fagin JA, Wells SJ. Biologic and Clinical Perspectives on Thyroid Cancer. N Engl J Med 2016;375:1054-67. 10.1056/NEJMra1501993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carling T, Udelsman R. Thyroid cancer. Annu Rev Med 2014;65:125-37. 10.1146/annurev-med-061512-105739 [DOI] [PubMed] [Google Scholar]
- 4.Chow SM, Law SC, Chan JK, et al. Papillary microcarcinoma of the thyroid-Prognostic significance of lymph node metastasis and multifocality. Cancer 2003;98:31-40. 10.1002/cncr.11442 [DOI] [PubMed] [Google Scholar]
- 5.Vasko VV, Saji M. Molecular mechanisms involved in differentiated thyroid cancer invasion and metastasis. Curr Opin Oncol 2007;19:11-7. 10.1097/CCO.0b013e328011ab86 [DOI] [PubMed] [Google Scholar]
- 6.Zaydfudim V, Feurer ID, Griffin MR, et al. The impact of lymph node involvement on survival in patients with papillary and follicular thyroid carcinoma. Surgery 2008;144:1070-1077, 1077-8. [DOI] [PubMed]
- 7.Wang LY, Ganly I. Nodal metastases in thyroid cancer: prognostic implications and management. Future Oncol 2016;12:981-94. 10.2217/fon.16.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang LY, Palmer FL, Nixon IJ, et al. Lateral Neck Lymph Node Characteristics Prognostic of Outcome in Patients with Clinically Evident N1b Papillary Thyroid Cancer. Ann Surg Oncol 2015;22:3530-6. 10.1245/s10434-015-4398-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang LY, Palmer FL, Nixon IJ, et al. Central lymph node characteristics predictive of outcome in patients with differentiated thyroid cancer. THYROID 2014; 24:1790-1795. 10.1089/thy.2014.0256 [DOI] [PubMed] [Google Scholar]
- 10.Xie J, Fan Y, Zhang X. Molecular mechanisms in differentiated thyroid cancer. Front Biosci (Landmark Ed) 2016;21:119-29. 10.2741/4379 [DOI] [PubMed] [Google Scholar]
- 11.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet 2004; 5:522-31. 10.1038/nrg1379 [DOI] [PubMed] [Google Scholar]
- 12.Volinia S, Calin GA, Liu CG, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A 2006;103:2257-61. 10.1073/pnas.0510565103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang Y, Yu S, Cao S, et al. MicroRNA-222 Promotes Invasion and Metastasis of Papillary Thyroid Cancer Through Targeting Protein Phosphatase 2 Regulatory Subunit B Alpha Expression. Thyroid 2018;28:1162-73. 10.1089/thy.2017.0665 [DOI] [PubMed] [Google Scholar]
- 14.Geraldo MV, Yamashita AS, Kimura ET. MicroRNA miR-146b-5p regulates signal transduction of TGF-beta by repressing SMAD4 in thyroid cancer. Oncogene 2012;31:1910-22. 10.1038/onc.2011.381 [DOI] [PubMed] [Google Scholar]
- 15.Hong S, Yu S, Li J, et al. MiR-20b Displays Tumor-Suppressor Functions in Papillary Thyroid Carcinoma by Regulating the MAPK/ERK Signaling Pathway. Thyroid 2016;26:1733-43. 10.1089/thy.2015.0578 [DOI] [PubMed] [Google Scholar]
- 16.Lima CR, Geraldo MV, Fuziwara CS, et al. MiRNA-146b-5p upregulates migration and invasion of different Papillary Thyroid Carcinoma cells. BMC Cancer 2016;16:108. 10.1186/s12885-016-2146-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dragomir MP, Knutsen E, Calin GA. SnapShot: Unconventional miRNA Functions. Cell 2018;174:1038. 10.1016/j.cell.2018.07.040 [DOI] [PubMed] [Google Scholar]
- 18.Shimakami T, Yamane D, Jangra RK, et al. Stabilization of hepatitis C virus RNA by an Ago2-miR-122 complex. Proc Natl Acad Sci U S A 2012;109:941-6. 10.1073/pnas.1112263109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li G, Wu X, Qian W, et al. CCAR1 5' UTR as a natural miRancer of miR-1254 overrides tamoxifen resistance. Cell Res 2016;26:655-73. 10.1038/cr.2016.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okumura T, Shimada Y, Omura T, et al. MicroRNA profiles to predict postoperative prognosis in patients with small cell carcinoma of the esophagus. Anticancer Res 2015; 35:719-27. [PubMed] [Google Scholar]
- 21.Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. NAT REV Cancer 2013;13:184-99. 10.1038/nrc3431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sastre-Perona A, Santisteban P. Role of the wnt pathway in thyroid cancer. Front Endocrinol (Lausanne) 2012;3:31. 10.3389/fendo.2012.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen X, Chen Z, Yu S, et al. Long Noncoding RNA LINC01234 Functions as a Competing Endogenous RNA to Regulate CBFB Expression by Sponging miR-204-5p in Gastric Cancer. Clin Cancer Res 2018;24:2002-14. 10.1158/1078-0432.CCR-17-2376 [DOI] [PubMed] [Google Scholar]
- 24.Guo H, Ingolia NT, Weissman JS, et al. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 2010;466:835-40. 10.1038/nature09267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: microRNAs can up-regulate translation. Science 2007;318:1931-4. 10.1126/science.1149460 [DOI] [PubMed] [Google Scholar]
- 26.Matsui M, Chu Y, Zhang H, et al. Promoter RNA links transcriptional regulation of inflammatory pathway genes. Nucleic Acids Res 2013;41:10086-109. 10.1093/nar/gkt777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu X, Brewer G. The regulation of mRNA stability in mammalian cells: 2.0. Gene 2012;500:10-21. 10.1016/j.gene.2012.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhai L, Wang C, Chen Y, et al. Rbm46 regulates mouse embryonic stem cell differentiation by targeting beta-Catenin mRNA for degradation. PLos One 2017;12:e0172420. 10.1371/journal.pone.0172420 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Bikkavilli RK, Malbon CC. Dishevelled-KSRP complex regulates Wnt signaling through post-transcriptional stabilization of beta-catenin mRNA. J Cell Sci 2010;123:1352-62. 10.1242/jcs.056176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell 2009;17:9-26. 10.1016/j.devcel.2009.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 2004;20:781-810. 10.1146/annurev.cellbio.20.010403.113126 [DOI] [PubMed] [Google Scholar]
- 32.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell 2012;149:1192-205. 10.1016/j.cell.2012.05.012 [DOI] [PubMed] [Google Scholar]
- 33.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell 2006;127:469-80. 10.1016/j.cell.2006.10.018 [DOI] [PubMed] [Google Scholar]
- 34.Garcia-Rostan G, Camp RL, Herrero A, et al. Beta-catenin dysregulation in thyroid neoplasms: down-regulation, aberrant nuclear expression, and CTNNB1 exon 3 mutations are markers for aggressive tumor phenotypes and poor prognosis. Am J Pathol 2001;158:987-96. 10.1016/S0002-9440(10)64045-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jamieson C, Sharma M, Henderson BR. Targeting the beta-catenin nuclear transport pathway in cancer. Semin Cancer Biol 2014;27:20-9. 10.1016/j.semcancer.2014.04.012 [DOI] [PubMed] [Google Scholar]
- 36.Huang K, Zhang JX, Han L, et al. MicroRNA roles in beta-catenin pathway. Mol Cancer 2010;9:252. 10.1186/1476-4598-9-252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sebio A, Kahn M, Lenz HJ. The potential of targeting Wnt/beta-catenin in colon cancer. Expert Opin Ther Targets 2014;18:611-5. 10.1517/14728222.2014.906580 [DOI] [PubMed] [Google Scholar]