Abstract
Background
Blumgart anastomosis (BA) during pancreaticoduodenectomy (PD) had reduced postoperative pancreatic fistula (POPF) after PD in literatures. The aim of this study is to report surgical results from a consecutive series of operation using the modified BA method.
Methods
Data from 50 consecutive patients who underwent PD using modified BA between September 2011 and August 2018 were prospectively collected and retrospectively analyzed, regarding POPF, other morbidities, and mortality.
Results
Overall incidence of POPF was 10.0%, the rate of grade B POPF was 8.0% (4/50) and grade C was 2.0% (1/50). Among 50 patients, 5 post pancreatectomy hemorrhages (PPHs) (10.0%) including 2 POPF related bleeding, and 4 abscesses including 2 related to POPF occurred. Fistula risk grades were as follows: 0 negligible, 6 low, 36 intermediate, and 8 high. Except for one patient, fistulas improved over the clinical course after radiologic intervention drainage and angiography. The mortality occurred due to POPF followed by jejunal detachment from the remnant pancreas stump. In this case, jejunum was too thin, compared to the pancreatic thickness.
Conclusions
This retrospective single-center result demonstrated that the modified BA had an acceptable rate of POPF. Modified BA may be risky and potentially provoke fatal POPF, when joining a thin jejunum and thick pancreas.
Keywords: Blumgart anastomosis (BA), postoperative pancreatic fistula (POPF), vulnerable, pancreaticoduodenectomy
Introduction
There is no standard method of pancreaticojejunostomy (PJ) that reduces postoperative pancreatic fistula (POPF), which is the most challenging complications closely related to perioperative death in pancreaticoduodenectomy (PD).
In recent years, blumgart anastomosis (BA) has been introduced and increasingly more accepted by more medical centers (1-3). Retrospective studies regarding its modification reported a low POPF rate, compared to other types of reconstruction such as the Kakita (4) method of PJ and pancreaticogastrostomy (PG) (5-7). The authors suggested that BA was superior to other PJ methods regarding POPF reduction, and was a reproducible even for young surgeons and therefore can be recommended as a standard method for PJ (6). Previously, our group reported lower rate of POPF after applying the modified BA (8). However, a recent prospective study suggested that the modified BA technique did not reduce clinically relevant (CR)-POPF compared to the interrupted suture technique (9).
In this study, we reported the surgical results of a consecutive series of PJ using the modified BA method, based on initial experience and point out its vulnerability.
Methods
A retrospective review of institutional pancreatic resection database was performed on prospectively and retrospectively collected data, including clinical, operative, and pathologic information on all patients undergoing pancreatic head resection. Permission for this study was granted from the Institutional Review Board.
The database was analyzed to evaluate patients who underwent pancreatoduodenectomy (PD) or pylorus-preserving PD (PPPD) between September 2011 and August 2018 in a single institution by a single surgeon. Patients undergoing total pancreatectomy were excluded. Operative details including operative (OP) time (from time of incision to closure of the wound), and estimated blood loss (EBL) were obtained from the anesthesia record. Final pathologic findings were retrieved from the medical chart.
PD was performed using conventional PD or PPPD. Anastomosis to the remnant pancreas was performed between the pancreas and jejunum by the transpancreatic U-suture technique PJ, as described in 2009 (2). The outer layer, consisting of the remnant pancreatic parenchyma and seromuscular layer of the jejunum, was sutured using a 4-0 polypropylene suture (Prolene; Ethicon, Somerville, NJ, USA) and the inner layer, consisting of the pancreatic duct and mucosa of the jejunum, was sutured using an interrupted 5-0 polydioxanone suture (PDS IITM Ethicon, Sommerville, NJ, USA).
Trans-anastomotic stenting was used variably through the study period. When the pancreatic duct was smaller than 2 mm, a 5–8 Fr silastic catheter with multiple side holes was inserted approximately 2 to 3 cm into the pancreatic duct. The largest stents that could pass into the pancreatic duct were used. Catheter migration was prevented by placing an anchoring suture, using one of the inner posterior layer sutures. The catheter exited via a small enterotomy near the jejunal stump. The enterostomy site used for the catheter exit was closed with a purse-string suture and anchored using absorbable suture material. Stents were externalized by making a stab incision in the anterior abdominal wall, and totally externalized pancreatic stents were connected to a Jackson-Pratt (JP) drain to generate negative pressure. Internal stent was used in pancreatic duct with the diameters over 3 mm.
Two drains were routinely placed anterior and posterior to the PJ and exteriorized through the lateral abdominal wall. Although the use of a surgical drain varied throughout the course of the series, the most common practice was placing a single drain posterior to the choledocho-jejunostomy and PJ. Occasionally, a second drain was placed anterior to the PJ for high-risk anastomoses, most often for a soft or fatty infiltrated pancreatic remnant. Recently a risk model for POPF was established by Callery and colleagues (10) with emphasis on CR-POPF. Ten-point clinical risk score for pancreatic fistula (CRS-PF) accurately predicts CR-POPF after PD. This model, the CRS-PF, has been verified in its predictive performance by external validations (11). We used this score for risk grades.
Results
In perioperative factors, (Table 1) mean age was 67.2, mean body mass index (BMI) was 23.4 and American Society of Anesthiologists (ASA) grade 3 was 18% (n=9). The majority of patients underwent PPPD (62.0%) and 2 laparoscopic PPPD (LAPPPD) were performed. The most common pathology was distal bile duct cancer (DBDc) (38.0%, n=19) followed by ampulla of vater cancer (AOVc) (24.0%), pancreatic ductal adenocarcinoma (PDAC) (20.0%), cystic neoplasm (14.0%) and neuroendocrine tumor (NET) (4.0%). Preoperative bile drainage (PBD) was performed in 29 patients (58.0%).
Table 1. Perioperative factors of consecutive 50 modified BA in PD.
| Clinical factors | N=50 |
|---|---|
| Age (year, mean ± SD) | 67.2±3.6 |
| Gender (M:F) | 26:24 |
| Pathology (%) | |
| PDAC | 10 (20.0) |
| AOVc | 12 (24.0) |
| DBDc | 19 (38.0) |
| Cystic tumor | 7 (14.0) |
| NET | 2 (4.0) |
| DM (%) | 11 (22.0) |
| BMI (kg/m2, mean ± SD) | 23.4±6.5 |
| OP method (%) | |
| PD | 17 (34.0) |
| PPPD | 31 (62.0) |
| LAPPPD | 2 (4.0) |
| ASA (%) | |
| 1 | 17 (34.0) |
| 2 | 24 (48.0) |
| 3 | 9 (18.0) |
| PBD (%) | 29 (58.0) |
| OP time (min, mean ± SD) | 431.5±85.2 |
| Blood loss (cc, mean ± SD) | 593.5±120.0 |
| Internal stent (%) | 24 (48.0) |
| External stent (%) | 26 (52.0) |
| Pancreas texture (%) | |
| Soft | 12 (24.0) |
| Firm | 26 (52.0) |
| Hard | 12 (24.0) |
| Main P-duct size (mm, mean ± SD) | 3.36±0.2 |
| POD#1 drain amylase (mean ± SD) | 2,429.1±150.5 |
| POD#1 drain amylase >5,000 (%) | 5 (10.0) |
BA, blumgart anastomosis; PD, pancreaticoduodenectomy; SD, standard deviation; PDAC, pancreatic ductal adenocarcinoma; AOVc, ampulla of vater cancer; DBDc, distal bile duct cancer; NET, neuroendocrine tumor; DM, diabetes mellitus; BMI, body mass index; OP, operative; PPPD, pylorus-preserving PD; LAPPPD, laparoscopic PPPD; ASA, American Society of Anesthiologists; PBD, preoperative bile drainage; POD, postoperative day.
Operative and postoperative factors were analyzed. Mean OP time was 403 minutes and mean EBL was 593 mL. Twenty-four patients underwent insertion of an internal stent on PJ site and the remaining 26 patients underwent placement of external stent.
Mean pancreatic duct size was 3.6 (1.5–5.0) mm. Most pancreas texture was mostly firm or hard (76.0%), and 12 patients had soft pancreases (24.0%).
Postoperative results summurized in Table 2. Incidence of grade B POPF was 8.0% (4/50) and grade C was 2.0% (1/50). Among 50 patients, five post pancreatectomy hemorrhages (PPHs) (10.0%) including two POPF related hemorrhages, and four abscesses including two related to POPF occurred. Fistula risk grades were as follows: 0 negligible, 6 low, 36 intermediate, and 8 high. Except for one patient, fistulas improved over the clinical course after radiologic intervention drainage and angiography.
Table 2. Surgical outcomes after consecutive 50 modified BA in PD.
| Surgical outcomes | N=50 |
|---|---|
| CR-POPF | 5 (10.0) |
| Biochemical leak (%) | 7 (14.0) |
| Grade B (%) | 4 (8.0) |
| Grade C (%) | 1 (2.0) |
| POPF related PPH (%) | 2 (4.0) |
| POPF related abscess (%) | 2 (4.0) |
| Bile leakage (%) | 0 |
| DGE (%) | 2 (4.0) |
| Clavien-Dindo >III (%) | 5 (10.0) |
| Re-operation (%) | 2 (4.0) |
| Mortality (%) | 1 (2.0) |
| Length of stay (mean ± SD) | 19.5±2.6 |
BA, blumgart anastomosis; PD, pancreaticoduodenectomy; CR-POPF, clinically relevant-postoperative pancreatic fistula; PPH, post pancreatectomy hemorrhage; DGE, delayed gastric emptying; SD, standard deviation.
The mortality occurred owing to POPF followed by jejunal detachment from the pancreas stump, because the jejunum was too thin compared to the thickness of pancreas. The jejunum was not likely to cover the pancreas tightly enough (Figure 1). Considering the mortality case, a 77-year-old man with a history of chronic lung disease presented to the emergency department with a jaundice. Serum level of cancer antigen (CA) 19-9 was 85 U/mL. Radiologic evaluation revealed the mid bile duct cancer. He underwent PPPD and had soft pancreas with 2 mm pancreatic duct. The jejunum couldn’t cover enough the pancreas stump on Figure 2. His jejunum was very thin just like a pediatric jejunum and its length (diameter) was less than surgeon’s little finger (15 mm). Pancreas stump height at the right side of portal vein on computed tomography (CT) was 13 mm. On 7 days after the surgery, CT revealed fluid collection around PJ (Figure 3) and JP amylase level was around 15,500 U/L. On postoperative 10 days, JP bleeding was detected and underwent angiography revealed the pseudoaneurysm of gastroduodenal artery. The coil embolization of it was done successfully. However, the patient showed hematemesis and hemoperitoneum 2 days after embolization. We decided the exploration and did completion pancreatectomy. On surgical findings, the detachment of the jejunum from the pancreas stump. Five days after reoperation, he died due to hypovolemic shock induced by re-bleeding on intra abdomen.
Figure 1.
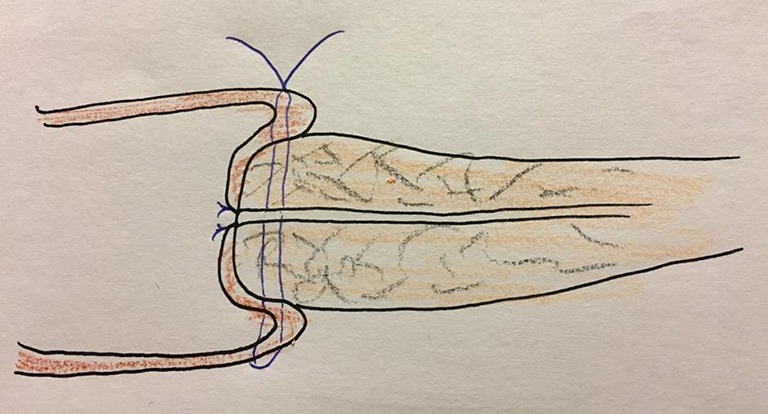
Vulnerable joining slender jejunum and fatty pancreas.
Figure 2.
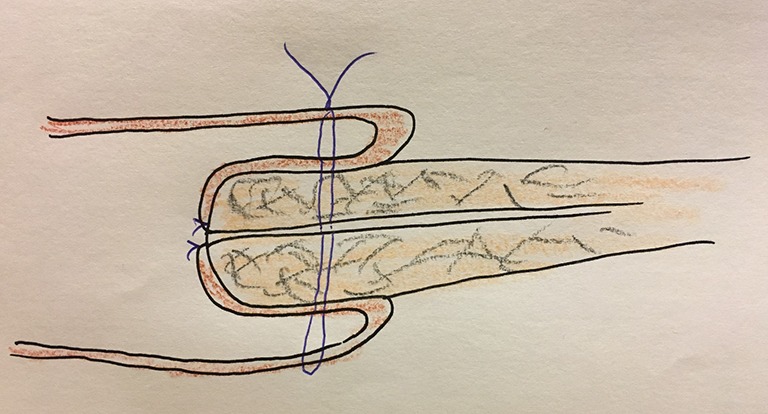
Safe BA requires wide jejunum and slender pancreas. BA, blumgart anastomosis.
Figure 3.
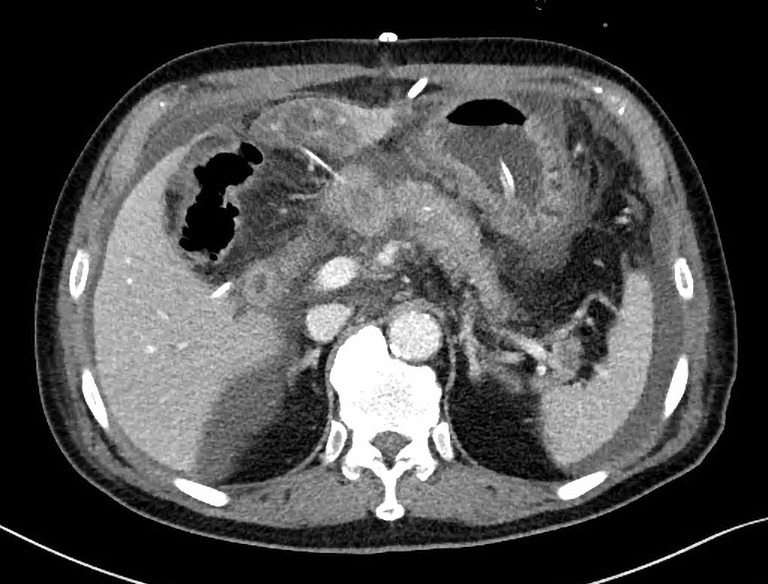
On 7 days after surgery, CT finding revealed fluid collection around PJ. CT, computed tomography; PJ, pancreaticojejunostomy.
Discussion
This is the first report pointing out the drawback of BA, which might have incomplete and unstable covering of pancreas stump vulnerable to evoke POPF, and result in mortality.
The original BA sutures through the remnant pancreas and is free from tangential tension and shear forces, and firmly anchors the gland to the jejunum, preventing full disruption of the gland from the pancreas, allowing for the accurate and careful placement of the duct-to-mucosal sutures and may result in fewer leaks from accessory pancreatic ducts (2,3).
Considering the drawback of original BA, Interrupted suture of the pancreatic parenchyma and the jejunal seromuscular layer might develop tangential shear forces during the tightening of the knots, and the suture material could easily lacerate pancreatic parenchyma. So, the modified technique had been introduced to tie knots on the ventral wall of the jejunum to make the anastomosis more feasible and safe (5,6).
Irrespective of modification, it is obligatory to cover the remnant pancreas and any circumstance unable to keep this condition should be avoided (Figure 2). It may be risky to do BA safely when joining a bulky pancreas and/or slender jejunum. During the modified BA, I make the distance 10 mm between suture on stump and an edge of stump. So, I think minimal 20 mm over the stump may be needed when we do anastomosis to make a safe BA.
In a review of trans-pancreatic sutures similar to the BA, Kakita (4) suggested that the presence of many sutures and their tightness might reduce blood flow in the pancreatic stump, causing ischemia and necrosis of the pancreatic stump by restricting of tissue blood flow. So, he applied as few sutures as possible, taking care to not tie the suture too tightly, thus maintaining blood flow in the pancreatic stump. Retrospective studies regarding modified BA reported a lower POPF rate, compared to other types of reconstruction such as Kakita and PG (5-7). Moreover, when the cases were limited to the risky soft pancreatic textures, the modified BA group showed lower POPF rates in comparison to the Kakita group (6).
The accumulated evidence proves the safety and superiority of modified BA rather than other methods of PJ. The authors suggested that BA was superior to other PJ methods regarding POPF reduction and it is reproducible even for young surgeons and therefore can be recommended as a standard method for PJ (6).
Until now, there was no investigation pointed out the drawback of BA even though there was evidence of mortality and morbidity. In our center, after experiencing mortality due to BA, a tailored PE was applied taking into account the extent of jejunum and pancreas volume. When the pancreas stump was too bulky or thickened compared to the anastomotic area of the jejunum, PG was performed. Four PGs were performed in patients with mismatched pancreatic and jejunal thickness without any POPF occurring afterwards.
Recently, Wakayama (9) suggested that simple physical coverage of the pancreatic cut surface might not prevent leakage of pancreatic juice into the abdominal cavity and CR-POPF. They pointed out the reason modified BA could reduce grade B/C POPF incidence has not been met in their investigation.
Even though it is a case series with a small number s of patients regarding operative methods, it may be informative and educational, and may be set up as a standard method of PJ.
Definitely our POPF rate (10%) was relatively high rate of pancreatic fistulae with the results of other data where the fistula rate is less than 2%. This retrospective single-center result demonstrated that the modified BA resulted in an acceptable rate of POPF. When the jejunal diameter is small, compared to the thickness of the pancreas stump, modified BA may be risky and potentially provoke fatal POPF.
Acknowledgments
None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards (No. SC19ZESE0098).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Brennan M. Pancreaticojejunostomy. In: Blumgart LH, Fong Y. Surgery of the liver and biliary tract. 3rd ed. New York: Saunders Co Ltd, 2000. [Google Scholar]
- 2.Kleespies A, Rentsch M, Seeliger H, et al. Blumgart anastomosis for pancreaticojejunostomy minimizes severe complications after pancreatic head resection. Br J Surg 2009;96:741-50. 10.1002/bjs.6634 [DOI] [PubMed] [Google Scholar]
- 3.Grobmyer SR, Kooby D, Blumgart LH, et al. Novel pancreaticojejunostomy with a low rate of anastomotic failure-related complications. J Am Coll Surg 2010;210:54-9. 10.1016/j.jamcollsurg.2009.09.020 [DOI] [PubMed] [Google Scholar]
- 4.Kakita A, Takahashi T, Yoshida M, et al. A simpler and more reliable technique of pancreatojejunal anastomosis. Surg Today 1996;26:532-5. 10.1007/BF00311562 [DOI] [PubMed] [Google Scholar]
- 5.Fujii T, Sugimoto H, Yamada S, et al. Modified blumgart anastomosis for pancreaticojejunostomy: technical improvement in matched historical control study. J Gastrointest Surg 2014;18:1108-15. 10.1007/s11605-014-2523-3 [DOI] [PubMed] [Google Scholar]
- 6.Oda T, Hashimoto S, Miyamoto R, et al. The tight adaptation at pancreatic anastomosis without parenchymal laceration: an institutional experience in introducing and modifying the new procedure. World J Surg 2015;39:2014-22. 10.1007/s00268-015-3075-8 [DOI] [PubMed] [Google Scholar]
- 7.Wang SE, Chen SC, Shyr BU, et al. Comparison of modified blumgart pancreaticojejunostomy and pancreaticogastrostomy after pancreaticoduodenectomy. HPB (Oxford) 2016;18:229-35. 10.1016/j.hpb.2015.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim DJ, Paik KY, Kim W, et al. The effect of modified pancreaticojejunostomy for reducing the pancreatic fistula after pancreaticoduodenectomy. Hepatogastroenterology 2014;61:1421-5. [PubMed] [Google Scholar]
- 9.Hirono S, Kawai M, Okada KI, et al. Modified blumgart mattress suture versus conventional interrupted suture in pancreaticojejunostomy during pancreaticoduodenectomy: randomized controlled trial. Ann Surg 2019;269:243-51. 10.1097/SLA.0000000000002802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Callery MP, Pratt WB, Kent TS, et al. A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg 2013;216:1-14. 10.1016/j.jamcollsurg.2012.09.002 [DOI] [PubMed] [Google Scholar]
- 11.Shubert CR, Wagie AE, Farnell MB, et al. Clinical risk score to predict pancreatic fistula after pancreatoduodenectomy: independent external validation for open and laparoscopic approaches. J Am Coll Surg 2015;221:689-98. 10.1016/j.jamcollsurg.2015.05.011 [DOI] [PubMed] [Google Scholar]


