Abstract
Purpose of Review
The improvement in prostate cancer survival over time, even in those with advanced disease, has led to an increasing recognition of the impact of prostate cancer and its treatment on bone health. Cancer treatment–induced bone loss (CTIBL) is a well-recognized entity but greater awareness of the risks associated with CTIBL and its treatment is required.
Recent Findings
The principal culprit in causing CTIBL is hormonal ablation induced by prostate cancer treatment, including several new agents which have been developed in recent years which significantly improve survival, but may cause CTIBL. This review discusses the impact of prostate cancer and its treatment on bone health, including published evidence on the underlying pathophysiology, assessment of bone health, and strategies for prevention and treatment.
Summary
It is important to recognize the potential cumulative impact of systemic prostate cancer treatments on bone health.
Keywords: Prostate cancer, Bone health, Osteoporosis, Androgen deprivation therapy
Introduction
Prostate cancer is the second most commonly diagnosed cancer in men with an estimated 1.3 million cases diagnosed in 2018 according to the most recent International Agency for Research on Cancer (IARC) report [1]. Men diagnosed with prostate cancer are now living longer. Prostate cancer survival has tripled in the last 40 years in the UK, with about 84% of men surviving their disease for ten years or more (2010–2011) [2]. In the USA, prostate cancer mortality has declined by 51% from 1993 to 2016 [3]. This improved survival is mainly attributed to advances in treatment, with some dispute about the impact of screening and earlier detection on mortality [4]. As patients are now living with prostate cancer for longer, the long-term impact of prostate cancer and its treatment on bone health in men is increasingly recognized.
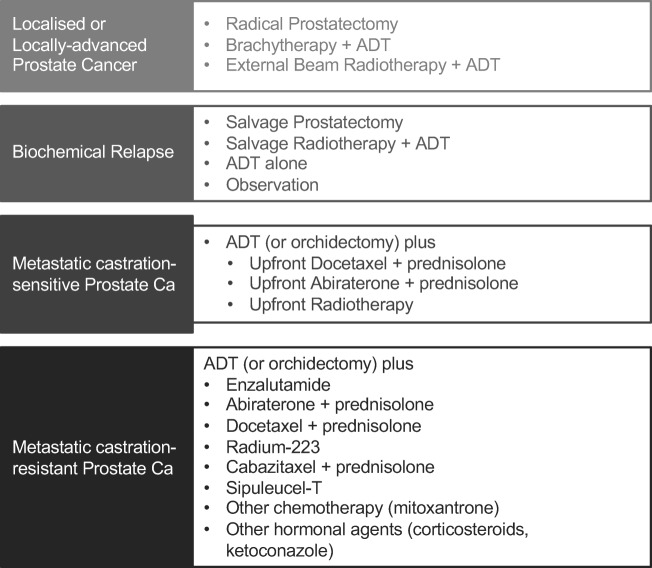
Androgens and the Androgen Receptor (AR) signalling pathway play a key role in prostate cancer pathophysiology. Androgen deprivation therapy (ADT), which can be achieved surgically (by orchidectomy) or chemically using luteinizing hormone-releasing hormone (LHRH) agonists and LHRH antagonists is therefore a cornerstone in the treatment of prostate cancer (Fig. 1). ADT is used in prostate cancer treatment at various stages: in men who present with or progress to metastatic disease; men who receive radical radiotherapy for localized or locally advanced disease and men who progress on a period of watchful waiting and are not fit for radical treatment [5–8]. Novel means for hormonal manipulation such as androgen synthesis inhibitors or AR signalling inhibitors are utilised in addition to ADT in the advanced disease setting.
Fig. 1.
Treatment of prostate cancer at different stages
Long-term ADT has well-recognized negative impact on bone mineral density and increases fracture risk in men [9–12]. A large observational study conducted by Shahinian et al. looked at the outcomes in 50,613 patients with prostate cancer. Of those who survived for at least 5 years after diagnosis, data showed that 19.4% of those who received ADT sustained a fracture, compared to 12.6% of those who did not receive ADT (p < 0.001) [13]. This negative impact on bone health also applies to other prostate cancer treatments including chemotherapy, glucocorticoids and novel hormone manipulation agents. These are used, in addition to background ADT, for the treatment of advanced prostate cancer, which involves bone in an estimated 90% of cases [14] with significant potential for morbidity and skeletal-related events (SREs) such as pathological fractures, pain, spinal cord compression and need for radiotherapy.
There is also evidence to suggest that even before initiating ADT, men with advanced prostate cancer have a higher incidence of osteoporosis and osteopenia compared to age-matched controls [15]. A separate population-based cohort study showed that men who have a high baseline risk of skeletal complications developed more fractures after initiating ADT [16]. Osteoporotic fractures in men with prostate cancer have been shown to correlate with poor survival outcomes [16, 17]. In addition, osteoporotic fractures also have a significant socio-economic impact. A report published by Hernlund et al. on osteoporosis in the European Union (EU) revealed that there were 3.5 million new fragility fractures in the EU in 2010 with an economic burden estimated at 37 billion euros and that this is expected to rise by 25% in 2025 [18].
Clinical guidelines from the National Institute for Health and Care Excellence (NICE) in the UK recommend that fracture risk is considered for all men receiving ADT and that treatment is offered to all those found to have osteoporosis [19]. Similarly, the European Association of Urology (EAU), European Society for Radiotherapy and Oncology (ESTRO) and International Society for Geriatric Oncology (SIOG) guidelines suggest that BMD assessment should be performed prior to the initiation of long-term ADT [20].
In this review, we will discuss current molecular and clinical understanding of the impact of metastatic disease and cancer treatment–induced bone loss (CTIBL) on bone health in prostate cancer patients and its management.
Pathophysiology
Prostate Cancer Bone Metastases
Bone is the most common site of metastasis from prostate cancer as shown in an autopsy study of 1589 patients with prostate cancer in which 90% were found to have bone involvement [14]. Bone metastases are associated with an increased morbidity and a negative impact on quality of life mainly through SREs [21]. Treatment strategies are therefore directed at delaying the onset of SREs and hence preserving the quality of life and functional status in this patient group [22].
The exact mechanisms for development of bone metastases in prostate cancer patients remain unclear and studies are ongoing in this field. The bone microenvironment, however, is recognized as a significant mediator of prostate cancer bone tropism and this is mediated by the CXCL16/CXCR6 axis. Circulating tumour cells migrate towards the bone based on a gradient of chemokines and ligands released by the bone marrow. These tumour cells then parasitize the bone microenvironment for haematopoietic stem cells (HSC) and become dormant in the bone marrow. It is therefore suggested that a specific component of the bone marrow microenvironment can serve as a potential therapeutic target in prostate cancer patients with bone metastases [23].
RANKL is a major mediator of normal bone remodelling and binds to its receptor RANK on the surface of osteoclast progenitors, resulting in osteoclast differentiation and bone resorption. Disseminated prostate cancer cells enhance RANKL expression on osteoblasts by secreting parathyroid hormone-related protein leading to osteoclastogenesis and increased bone resorption, which in turn creates space for tumour cells to grow within the bone marrow [24].
Further studies looking at the specific molecular mechanisms controlling the formation and progression of bone metastases in prostate cancer patients are important as they can set new targets for the development of novel therapies in this patient group.
Cancer Treatment–Induced Bone Loss
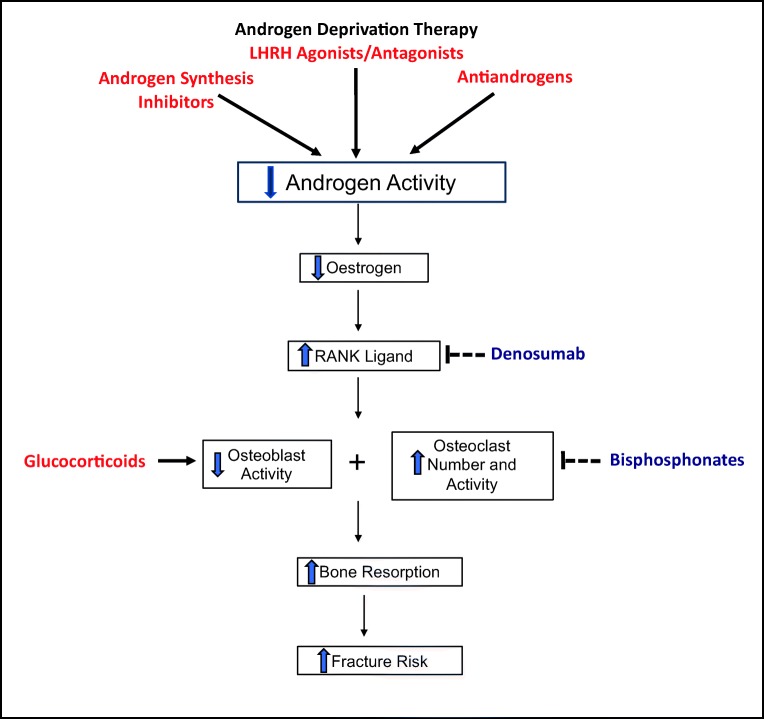
The role of sex steroids on bone homeostasis has been extensively studied and, in recent years, the development of mouse models with global and cell-specific deletions in Oestrogen and Androgen Receptors (ERα, ERβ, AR) has evolved our understanding of this role [25–28]. Androgen receptor (AR) signalling in osteoblasts is responsible for the protective effects of androgens on trabecular bone mass, leading to a decrease in osteoclast numbers and bone resorption. Oestrogens, produced via aromatization of androgens in males, protect against endocortical resorption, at least in part, via ERα signalling in mesenchymal/stromal cells [26]. Oestrogens play an important role in regulating the RANKL/RANK/OPG pathway, which influences osteoclast activity and has important therapeutic implications [26–29]. Collective evidence from several interventional and observational human studies supports the theory that oestrogen plays a much more significant role in regulating bone metabolism in men than testosterone [30–33].
Following initiation of ADT, the levels of both testosterone and oestradiol fall rapidly and significantly, leading to disruption of bone integrity. A prospective study conducted at an academic medical centre in the USA investigated the rate of bone loss following initiation of ADT in men with prostate cancer. This showed the reduction in bone mineral density to be most significant in the first 12 months after initiation of ADT; hence, the importance of early initiation of preventative measures [34]. It also showed that the rate of bone loss in prostate cancer patients initiating ADT was 5- to 10-fold higher than in either healthy age-matched controls or men with prostate cancer with normal hormone levels [34]. This deleterious effect on bone health is directly related to the duration of androgen deprivation. A progressive decline in bone mineral density was observed, even up to 10 years, with prolonged use of ADT in a separate cross-sectional study, and this was more pronounced with continuous ADT and surgical castration compared to intermittent ADT [35].
Glucocorticoid-induced bone loss is well-recognized and mediated through increased apoptosis of osteoblasts and osteocytes, impaired differentiation of osteoblasts and increased life span of osteoclasts [36]. Glucocorticoids are an important component of the treatment of metastatic prostate cancer where they are often used in conjunction with chemotherapy; with adrenal synthesis inhibitors (such as abiraterone); or sometimes at a low dose as monotherapy. The resultant increase in fracture risk should therefore be taken into consideration when used in these settings [37, 38]. Studies are needed to investigate the potential cumulative effect on bone health when combining ADT with chemotherapy and glucocorticoids. Enzalutamide, a novel oral androgen receptor signalling inhibitor, now approved for use in patients with metastatic castration-resistant prostate cancer, has been shown to be associated with a smaller change in bone mineral density when compared to the effect caused by leuprolide [39••, 40]. Apalutamide is a next-generation non-steroidal androgen receptor antagonist being studied in patients with metastatic castration-resistant prostate cancer and further results and analysis would be needed to investigate its impact on bone health [41••] (Fig. 2).
Fig. 2.
Mechanistic role of androgen deprivation therapy
Assessment of Bone Health in Prostate Cancer Patients
There is an inverse relationship between fracture risk and BMD with an approximately two-fold increase in fracture risk with each standard deviation reduction in BMD [42, 43]. Dual energy X-ray absorptiometry (DXA) is the most common method of assessing bone mineral density (BMD). Specific skeletal locations are typically measured, including the proximal femur (total hip or femoral neck) and lumbar spine [44, 45]. Measurements are usually reported as a T-score and osteoporosis is defined as a T-score ≥ 2.5 standard deviation below the mean value for young healthy adults [46].
While highly specific, DXA assessment of BMD has a low sensitivity for prediction of fractures with many fragility fractures occurring in individuals who have a non-osteoporotic BMD. Many individuals who sustain a fracture are subsequently found to have non-osteoporotic BMD [47]. Several other factors therefore contribute to fracture risk including advanced age, sex, falls risk, history of previous fractures, family history of fractures, and other lifestyle factors. Nevertheless, DXA remains the gold standard for assessing bone mineral density in this population.
FRAX® (http://www.shef.ac.uk/FRAX) is a risk assessment tool that has been developed to incorporate BMD measurements in addition to these variables for more accurate prediction of fracture risk. It calculates the 10-year probability of a major osteoporotic fracture and of hip fracture [48, 49]. Launched in 2008, it is now the most widely used risk assessment tool in clinical practice having been approved by both the FDA and NICE [49]. Other tools such as QFracture (http://www.qfracture.org), which is based on a UK prospective open cohort of over 2 million patients have also been developed and validated for clinical use [50, 51].
Prevention and Treatment of CTIBL
Systemic anticancer therapies as well as bone-targeted agents such as zoledronic acid, denosumab and radium-223 have proven effective for the prevention of skeletal-related events from prostate cancer bone metastases and this has been the subject of previous extensive review [52] and is beyond the remit of this article. With improved survival of patients living with bone-metastatic prostate cancer however, the impact of CTIBL continues to grow and is gaining recognition. Several strategies have therefore been evaluated to prevent and treat CTIBL.
Awareness and Education
Studies have consistently shown that patients often lack knowledge about the risk of CTIBL and means for prevention and treatment [53–55]. There also appears to be a discrepancy between what physicians assume that patients know and what the patients’ perception is about their bone health [56]. It is also interesting that despite physicians’ good knowledge about bone health, there appears to be inadequate adherence to guidelines for screening, monitoring and treatment of CTIBL [57, 58].
Results from a phase 2 study evaluating two education-based models, incorporating patient pamphlets, involvement of family physicians and Bone Health Care Coordinators, to improve bone health care in men receiving ADT have shown these to be feasible and they were associated with improved requesting of baseline Bone Mineral Density (BMD) scans [59••]. In many cases, it would be possible for the family physicians to play a key role in the treatment and monitoring of these patients given their experience in non-cancer-related osteoporosis. In addition, given that the internet is the most widely used source of information for patients, the development of approved online educational tools/websites is thought to be beneficial in raising awareness and adherence with healthy bone behaviour [60].
Lifestyle Modification
Osteoporosis and osteopenia are common in men with prostate cancer even before initiating ADT, particularly in the elderly population. Several studies have identified factors that affect bone loss in this patient group, including maintenance of high BMI, weight-bearing exercise, avoidance of alcohol and smoking, and possibly high dietary calcium intake could help reduce bone loss [61–64]. However, further studies are required to objectively quantify the impact of these interventions on BMD.
On the basis of potential roles in prostate cancer pathogenesis, calcium and vitamin D supplementation in men with prostate cancer has been a subject of several studies [65–68]. However, no trials to date have evaluated the risk-benefit ratio of calcium and vitamin D supplementation in men receiving ADT. Notably, a systematic review of 12 clinical trials in men with prostate cancer undergoing ADT showed that the currently recommended doses of calcium and vitamin D supplementation for prevention of osteoporosis are inadequate in preventing BMD loss in this group [68]. Further studies are therefore needed to determine the safety and efficacy of higher doses in this population.
Another important consequence of exposure to ADT is sarcopenia, a degenerative loss of muscle mass that is in turn associated with frailty and increased falls risk [69]. When combined with the effects of ADT on BMD, these patients are at an even higher fracture risk with potentially life-threatening complications [70]. Measures such as muscle-strengthening exercise and maintaining healthy nutrition with adequate protein intake have been evaluated and shown to potentially help ameliorate the risk of sarcopenia and its attendant consequences in patients on ADT [71].
Bone-Targeted Agents
Bisphosphonates
The role of bisphosphonates in reducing BMD loss in men with prostate cancer receiving ADT has been extensively studied in several randomized controlled trials (RCTs) (summarised in Table 1). Among the largest of these was the RADAR study conducted by Denham et al. who enrolled 1071 men receiving radical radiotherapy for locally advanced prostate cancer and had in addition, 6 or 18 months of ADT with or without zoledronic acid (4 mg q3-monthly for 18 months) [73]. They found that compared with baseline DEXA measurements, BMD at the hip measured at 4 years was reduced with both 6 and 18 months of ADT (1.7% and 3.7%, respectively; p < 0.01) and this BMD reduction was prevented by concomitant administration of zoledronate. There was however, no significant difference in the primary endpoint of incidence of vertebral fractures seen although this was attributed to insufficient sample size and duration of follow-up [86].
Table 1.
Summary of RCTs of bisphosphonates in men receiving ADT
| Study | Study population | N | Study groups | Follow-up | Key findings |
|---|---|---|---|---|---|
| Smith et al. 2001 [72] | advanced or recurrent PCa and no bone metastases | 47 | ADT only vs ADT + Pam | 48 weeks |
-ADT only arm: decrease in BMD of LS (− 3.3%), trochanter (− 2.1%), and total hip (− 1.8%), p < 0.001 for all. -No significant change in mean BMD at any skeletal site in ADT + Pam arm. |
| Michaelson et al. 2007 [73] | Non-metastatic PCa | 40 | ADT + placebo vs ADT + Zol | 12 months | -Increase in BMD in Zol arm compared to placebo in both LS (4% vs − 3.1%, p < 0.001) and total hip (0.7% vs − 1.9%, p = 0.004), with suppression of serum NTP levels. |
| Bhoopalam et al. 2009 [74] | Non-metastatic PCa on ADT for ≤ 1 year or > 1 year | 93 | ADT + placebo vs ADT + Zol | 12 months | -Increase in LS BMD in Zol arm seen in both groups (ADT ≤ 1 year: 5.95% in Zol arm vs − 3.23% in placebo arm [p = 0.0044], ADT > 1 year: 6.08% in Zol arm vs 1.57% in placebo arm [p = 0.0005]), including patients with multiple risk factors for osteoporosis. |
| Smith et al. 2003 [75] | Non-metastatic PCa | 106 | ADT + placebo vs ADT + Zol | 12 months | -Increase in LS BMD in Zol arm compared to placebo arm (5.6% vs − 2.2%, p < 0.001) |
| Magno et al. 2005 [76] | Locally advanced PCa with osteoporosis at baseline | 60 | MAB vs MAB + Ner vs Bicalutamide vs Bicalutamide + Ner | 12 months |
-MAB only arm: significant loss in BMD of LS (− 4.9%, p = 0.002) and total hip (− 1.9%, p = 0.004). -MAB + Ner arm: no significant BMD change. -Bicalutamide arm: no significant BMD change. -Bicalutamide + Ner arm: increase in LS (+ 2.5%) and total hip (+ 1.6%) BMD—both p < 0.05. |
| Ryan et al. 2006 [77] | PCa without bone metastases, on ADT for ≤ 12 months | 120 | ADT + placebo vs ADT + Zol | 12 months | -Zol arm: increase in femoral neck, total hip, and LS BMD by 3.6% (p = 0.0004), 3.8% (p < 0.0001), and 6.7% (p < 0.0001) respectively. |
| Ryan et al. 2007 [78] | PCa with or without bone metastases, on ADT for ≤ 12 months | 42 | ADT + placebo vs ADT + Zol | 12 months | -After excluding BMD data from sites of known metastases, patients in the Zol arm had a relative increase in BMD compared to placebo, at the femoral neck (4.2%, p = 0.001) and LS (7.1%, p < 0.001). |
| Klotz et al. 2013 [79] | Non-metastatic PCa | 186 | ADT + placebo vs ADT + Alen | 12 months | -Increase in LS BMD in Alen arm compared to placebo (1.7% vs − 1.9%, p < 0.0001). |
| Choo et al. 2013 [80] | Non-metastatic PCa, undergoing RT + 2–3 years of ADT | 104 | ADT + placebo vs ADT + Ris | 24 months | -Non-significant decrease in BMD loss in Ris arm at 2 years compared to placebo. |
| Greenspan et al. 2007/2008 [81, 82] | Non-metastatic PCa | 112 | ADT + placebo vs ADT + Alen, crossover at 12 months | 24 months |
-ADT + Alen arm: increase in BMD of LS by 3.7% (p ≤ 0.001) and femoral neck by 1.6% (p = 0.008) at 1 year. -At crossover, those continuing Alen had additional BMD gains at both LS and hip, both p < 0.01; those who switched to placebo maintained BMD at LS and hip but had BMD loss at radius - p < 0.01. |
| Rodrigues et al. 2007 [83] | PCa patients who had prostatectomy and rising PSA | 94 | Placebo vs Clo vs Zol | 36 months |
Placebo arm: mean BMD loss of − 1.82. Clo arm: mean BMD loss − 0.72. Zol arm: mean BMD loss − 0.82. |
| Israeli et al. 2007 [84] | Locally advanced PCa during first year of ADT | 213 | ADT + placebo vs ADT + Zol | 12 months | -Mean BMD percentage differences were 6.7% for LS and 3.7% for total hip (p < 0.0001 for both). |
| Kachnic et al. 2013 [85] | high grade and/or locally advanced, non-metastatic PCa receiving ADT + RT | 96 | Zol vs observation | 36 months | Increase in BMD in LS (6% vs − 5%, p < 0.0001), left total hip (1% vs − 8%, p = 0.0002), and left femoral neck (3% vs − 8%, p = 0.0007) in Zol arm compared to observation arm. |
| Denham et al. 2014 [86] | Locally advanced PCa | 1071 | ADT for 6 months before RT ± additional 12 months ADT ± 18 months Zol | 3 years |
-Incidence of vertebral fractures was not increased by 18 months compared to 6 months ADT and was not affected by addition of Zol. -Incidence of non-vertebral fractures was significantly related to ADT duration (p = 0.013) but not to the addition of Zol. |
| Taxel et al. 2010 [87] | Locally advanced PCa | 40 | Placebo vs weekly risedronate | 6 months |
-The Ris group had no change in femoral neck or total hip BMD, while the placebo group decreased by 2% (p = 0.004) and 2.2% (p = 0.001), respectively. -The Ris group had an increase in LS BMD of 1.7% from baseline (p = 0.04), with no change in the placebo group. |
| Casey et al. 2010 [88] | Non-metastatic PCa | 200 | ADT + Zol for 24 months vs ADT alone for 24 months vs ADT alone for 12 months crossing over to ADT + Zol for 12 months. | 24 months |
-Significant BMD differences between patients receiving ADT alone and ADT + Zol were observed at the 12 months (p < or = 0.01 for each site) and 24 months (p < 0.05 for each site). -Initiating Zol after 12 months of ADT alone provided BMD benefits but was insufficient to completely restore BMD. |
| Kapoor et al. 2011 [89] | Non-metastatic PCa with osteoporosis or osteopenia | 41 | ADT + placebo vs ADT + Zol | 12 months | -The change in vertebral BMD in the Zol group (+ 7.93%) was significantly greater (p < 0.05) than the change in the placebo group (+ 0.82%). |
RCT randomized controlled trial, PCa prostate cancer, Pam pamidronate, Zol zoledronate, BMD bone mineral density, LS lumbar spine, PF proximal femur, NTP N-telopeptide (a bone turnover marker), MAB maximum androgen blockade, Ner neridronate, Alen alendronate, RT radiotherapy, Ris risedronate, Clo clodronate
Their results however confirmed those from several prior smaller studies [72–74, 77] that all demonstrated a benefit with bisphosphonates in reducing BMD loss among prostate cancer patients on ADT. It is important to note however, that variations in trial design, agents investigated and the heterogeneous populations included in the studies makes direct comparison of their results difficult. In addition, the primary endpoint in most of these studies was the BMD change rather than the incidence of fractures, which is of greater clinical relevance.
Serpa Neto et al. carried out a 2012 meta-analysis of 15 randomized controlled trials on the effects of bisphosphonates in men with prostate cancer treated with ADT [90]. The authors concluded that the use of bisphosphonates had a substantial effect in prevention of fractures (Risk Ratio (RR) 0.80; p = 0.005) and osteoporosis (RR 0.39; p < 0.00001), without causing any major side effects [90]. This analysis however included two large RCTs of patients with metastatic CRPC, which makes assessing the impact of bisphosphonates specifically on benign fractures difficult. No bisphosphonate is currently licensed for prevention of BMD loss or fractures in prostate cancer patients on ADT.
Denosumab
There are relatively few trials evaluating the effect of denosumab on BMD and fracture risk in men with non-metastatic prostate cancer receiving ADT. The most important of these is a large double-blind RCT by Smith et al. that randomized 1468 patients to receiving 6-monthly denosumab injections (60 mg subcutaneously for 3 years) or placebo. The results demonstrated a significant increase in BMD of the lumbar spine at 24 months in the denosumab group compared to placebo (+ 5.6% vs − 1.0%, p < 0.001), with a decrease in the incidence of new vertebral fractures at 36 months (1.5% vs 3.9% with placebo, HR 0.38; 95% confidence interval, 0.19 to 0.78; p = 0.006). These beneficial effects were observed as early as 1 month and were sustained at 36 months [91].
A comparison of the effects of denosumab and alendronate on BMD and fracture risk was performed in a randomized study of 234 prostate cancer patients on ADT. The authors found that denosumab was associated with a significant increase in BMD (measured at the lumbar spine) at 24 months compared to alendronate (5.6% vs 1.1%, p < 0.001) with a concomitant lower incidence of vertebral fractures, which was not statistically significant [92]. Denosumab is currently the only agent that has regulatory approval for the treatment of bone loss associated with hormone ablation in men with prostate cancer at increased risk of fractures.
Endocrine Agents
The effect of selective oestrogen receptor modulators such as toremifene and raloxifene in prevention of BMD loss in men receiving ADT has also been investigated given the growing recognition of the role of oestrogen in bone metabolism in men. A randomized controlled trial of 48 men with non-metastatic prostate cancer on ADT showed that the addition of raloxifene significantly increased the BMD at the hip (p < 0.001) and tended to increase the BMD at the spine (p = 0.07) [93]. A larger study of 646 men with prostate cancer on ADT showed that toremifene was associated with a significant relative risk reduction in the incidence of new vertebral fractures of 50% (p = 0.05), with a significant increase in BMD at the lumbar spine, hip and femoral neck compared to placebo (p < 0.0001). However, more venous thromboembolic events occurred in the toremifene arm [94]. Neither agent is currently licensed for this indication.
Conclusion and Future Directions
Prostate cancer patients are now living longer, and many patients receive several lines of therapy, which can have a cumulative impact on bone health over a period of years. Early recognition and optimization of bone health is therefore important in this patient group. A number of new agents have been approved and licensed for treatment of prostate cancer in recent years, and more agents are under development, like apalutamide, which will further extend the treatment options available once licensed, and may impact on bone health.
There is a need to raise awareness among patients about the risks of CTIBL as well as developing models to assist physicians to adhere to guidelines for screening and treatment. Several lifestyle modifications have been investigated but in order to objectively quantify their impact on BMD, further studies are required in this patient group. Bisphosphonates have been shown to reduce BMD loss in prostate cancer patients receiving ADT, however, few studies have investigated reduction in fractures and further larger studies are needed in this area.. Denosumab is the only agent that has shown a significant impact on fracture incidence in this patient population and is currently recommended for treatment of CTIBL associated with androgen deprivation therapy.
Compliance with Ethical Standards
Conflict of Interest
Salma El Badri and Abdulazeez Salawu declare no conflict of interest. Janet Brown reports grants and personal fees from Amgen, Novartis, and Bayer; and personal fees from BMS, Daiichi-Sankyo, Ipsen, Sandoz, and Merck, Sharp, Dome, outside the submitted work.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Cancer-induced Musculoskeletal Diseases
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Cancer Research UK. https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/prostate-cancer#heading-Two. 25 June 2019.
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 4.Taitt HE. Global Trends and Prostate Cancer: A Review of Incidence, Detection, and Mortality as Influenced by Race, Ethnicity, and Geographic Location. Am J Mens Health. 2018;12(6):1807–1823. doi: 10.1177/1557988318798279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denham JW, Steigler A, Lamb DS, Joseph D, Turner S, Matthews J, Atkinson C, North J, Christie D, Spry NA, Tai KH, Wynne C, D'Este C. Short-term neoadjuvant androgen deprivation and radiotherapy for locally advanced prostate cancer: 10-year data from the TROG 96.01 randomised trial. Lancet Oncol. 2011;12(5):451–459. doi: 10.1016/S1470-2045(11)70063-8. [DOI] [PubMed] [Google Scholar]
- 6.Bolla M, de Reijke TM, Van Tienhoven G, Van den Bergh AC, Oddens J, Poortmans PM, et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med. 2009;360(24):2516–2527. doi: 10.1056/NEJMoa0810095. [DOI] [PubMed] [Google Scholar]
- 7.Hanks GE, Pajak TF, Porter A, Grignon D, Brereton H, Venkatesan V, Horwitz EM, Lawton C, Rosenthal SA, Sandler HM, Shipley WU, Radiation Therapy Oncology Group Phase III trial of long-term adjuvant androgen deprivation after neoadjuvant hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the prostate: the Radiation Therapy Oncology Group Protocol 92-02. J Clin Oncol. 2003;21(21):3972–3978. doi: 10.1200/JCO.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 8.Hussain M, Tangen CM, Berry DL, Higano CS, Crawford ED, Liu G, Wilding G, Prescott S, Kanaga Sundaram S, Small EJ, Dawson NA, Donnelly BJ, Venner PM, Vaishampayan UN, Schellhammer PF, Quinn DI, Raghavan D, Ely B, Moinpour CM, Vogelzang NJ, Thompson IM Jr Intermittent versus continuous androgen deprivation in prostate cancer. N Engl J Med. 2013;368(14):1314–1325. doi: 10.1056/NEJMoa1212299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu CT, Yang YH, Chen PC, Chen MF, Chen WC. Androgen deprivation increases the risk of fracture in prostate cancer patients: a population-based study in Chinese patients. Osteoporos Int. 2015;26(9):2281–2290. doi: 10.1007/s00198-015-3135-9. [DOI] [PubMed] [Google Scholar]
- 10.Wang A, Obertova Z, Brown C, Karunasinghe N, Bishop K, Ferguson L, et al. Risk of fracture in men with prostate cancer on androgen deprivation therapy: a population-based cohort study in New Zealand. BMC Cancer. 2015;15:837. doi: 10.1186/s12885-015-1843-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen C, Lairson DR, Swartz MD, Du XL. Risks of Major Long-Term Side Effects Associated with Androgen-Deprivation Therapy in Men with Prostate Cancer. Pharmacotherapy. 2018;38(10):999–1009. doi: 10.1002/phar.2168. [DOI] [PubMed] [Google Scholar]
- 12.Smith MR, Boyce SP, Moyneur E, Duh MS, Raut MK, Brandman J. Risk of clinical fractures after gonadotropin-releasing hormone agonist therapy for prostate cancer. J Urol. 2006;175(1):136–139. doi: 10.1016/S0022-5347(05)00033-9. [DOI] [PubMed] [Google Scholar]
- 13.Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352(2):154–164. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 14.Bubendorf L, Schopfer A, Wagner U, Sauter G, Moch H, Willi N, et al. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31(5):578–583. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- 15.Hussain SA, Weston R, Stephenson RN, George E, Parr NJ. Immediate dual energy X-ray absorptiometry reveals a high incidence of osteoporosis in patients with advanced prostate cancer before hormonal manipulation. BJU Int. 2003;92(7):690–694. doi: 10.1046/j.1464-410X.2003.04471.x. [DOI] [PubMed] [Google Scholar]
- 16.Shao YH, Moore DF, Shih W, Lin Y, Jang TL, Lu-Yao GL. Fracture after androgen deprivation therapy among men with a high baseline risk of skeletal complications. BJU Int. 2013;111(5):745–752. doi: 10.1111/j.1464-410X.2012.11758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oefelein MG, Ricchiuti V, Conrad W, Resnick MI. Skeletal fractures negatively correlate with overall survival in men with prostate cancer. J Urol. 2002;168(3):1005–1007. doi: 10.1097/01.ju.0000024395.86788.cc. [DOI] [PubMed] [Google Scholar]
- 18.Hernlund E, Svedbom A, Ivergard M, Compston J, Cooper C, Stenmark J, et al. Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA) Arch Osteoporos. 2013;8:136. doi: 10.1007/s11657-013-0136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Institute for Health and Care Excellence (NICE). Prostate cancer: diagnosis and management NICE guideline [NG131]. https://www.nice.org.uk/guidance/ng131. 14 August 2019. [PubMed]
- 20.Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol. 2017;71(4):618–629. doi: 10.1016/j.eururo.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Saad F, Ivanescu C, Phung D, Loriot Y, Abhyankar S, Beer TM, Tombal B, Holmstrom S. Skeletal-related events significantly impact health-related quality of life in metastatic castration-resistant prostate cancer: data from PREVAIL and AFFIRM trials. Prostate Cancer Prostatic Dis. 2017;20(1):110–116. doi: 10.1038/pcan.2016.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vignani F, Bertaglia V, Buttigliero C, Tucci M, Scagliotti GV, Di Maio M. Skeletal metastases and impact of anticancer and bone-targeted agents in patients with castration-resistant prostate cancer. Cancer Treat Rev. 2016;44:61–73. doi: 10.1016/j.ctrv.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Park SH, Keller ET, Shiozawa Y. Bone Marrow Microenvironment as a Regulator and Therapeutic Target for Prostate Cancer Bone Metastasis. Calcif Tissue Int. 2018;102(2):152–162. doi: 10.1007/s00223-017-0350-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sottnik JL, Keller ET. Understanding and targeting osteoclastic activity in prostate cancer bone metastases. Curr Mol Med. 2013;13(4):626–639. doi: 10.2174/1566524011313040012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohamad NV, Soelaiman IN, Chin KY. A concise review of testosterone and bone health. Clin Interv Aging. 2016;11:1317–1324. doi: 10.2147/CIA.S115472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Almeida M, Laurent MR, Dubois V, Claessens F, O'Brien CA, Bouillon R, Vanderschueren D, Manolagas SC. Estrogens and Androgens in Skeletal Physiology and Pathophysiology. Physiol Rev. 2017;97(1):135–187. doi: 10.1152/physrev.00033.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vanderschueren D, Laurent MR, Claessens F, Gielen E, Lagerquist MK, Vandenput L, Börjesson AE, Ohlsson C. Sex steroid actions in male bone. Endocr Rev. 2014;35(6):906–960. doi: 10.1210/er.2014-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu J, Henning P, Sjogren K, Koskela A, Tuukkanen J, Moverare-Skrtic S, et al. The androgen receptor is required for maintenance of bone mass in adult male mice. Mol Cell Endocrinol. 2019;479:159–169. doi: 10.1016/j.mce.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 29.Todenhofer T, Stenzl A, Hofbauer LC, Rachner TD. Targeting bone metabolism in patients with advanced prostate cancer: current options and controversies. Int J Endocrinol. 2015;2015:838202. doi: 10.1155/2015/838202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khosla S. New Insights Into Androgen and Estrogen Receptor Regulation of the Male Skeleton. J Bone Miner Res. 2015;30(7):1134–1137. doi: 10.1002/jbmr.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drake MT, Khosla S. Male osteoporosis. Endocrinol Metab Clin N Am. 2012;41(3):629–641. doi: 10.1016/j.ecl.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Argoud T, Boutroy S, Claustrat B, Chapurlat R, Szulc P. Association between sex steroid levels and bone microarchitecture in men: the STRAMBO study. J Clin Endocrinol Metab. 2014;99(4):1400–1410. doi: 10.1210/jc.2013-3233. [DOI] [PubMed] [Google Scholar]
- 33.Piot Anne, Chapurlat Roland D, Claustrat Bruno, Szulc Pawel. Relationship Between Sex Steroids and Deterioration of Bone Microarchitecture in Older Men: The Prospective STRAMBO Study. Journal of Bone and Mineral Research. 2019;34(9):1562–1573. doi: 10.1002/jbmr.3746. [DOI] [PubMed] [Google Scholar]
- 34.Greenspan SL, Coates P, Sereika SM, Nelson JB, Trump DL, Resnick NM. Bone loss after initiation of androgen deprivation therapy in patients with prostate cancer. J Clin Endocrinol Metab. 2005;90(12):6410–6417. doi: 10.1210/jc.2005-0183. [DOI] [PubMed] [Google Scholar]
- 35.Kiratli BJ, Srinivas S, Perkash I, Terris MK. Progressive decrease in bone density over 10 years of androgen deprivation therapy in patients with prostate cancer. Urology. 2001;57(1):127–132. doi: 10.1016/S0090-4295(00)00895-5. [DOI] [PubMed] [Google Scholar]
- 36.den Uyl D, Bultink IE, Lems WF. Advances in glucocorticoid-induced osteoporosis. Curr Rheumatol Rep. 2011;13(3):233–240. doi: 10.1007/s11926-011-0173-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dorff TB, Crawford ED. Management and challenges of corticosteroid therapy in men with metastatic castrate-resistant prostate cancer. Ann Oncol. 2013;24(1):31–38. doi: 10.1093/annonc/mds216. [DOI] [PubMed] [Google Scholar]
- 38.Auchus RJ, Yu MK, Nguyen S, Mundle SD. Use of prednisone with abiraterone acetate in metastatic castration-resistant prostate cancer. Oncologist. 2014;19(12):1231–1240. doi: 10.1634/theoncologist.2014-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoffman-Censits J, Kelly WK. Enzalutamide: a novel antiandrogen for patients with castrate-resistant prostate cancer. Clin Cancer Res. 2013;19(6):1335–1339. doi: 10.1158/1078-0432.CCR-12-2910. [DOI] [PubMed] [Google Scholar]
- 40.Merseburger AS, Haas GP, von Klot CA. An update on enzalutamide in the treatment of prostate cancer. Ther Adv Urol. 2015;7(1):9–21. doi: 10.1177/1756287214555336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rathkopf DE, Antonarakis ES, Shore ND, Tutrone RF, Alumkal JJ, Ryan CJ, et al. Safety and Antitumor Activity of Apalutamide (ARN-509) in Metastatic Castration-Resistant Prostate Cancer with and without Prior Abiraterone Acetate and Prednisone. Clin Cancer Res. 2017;23(14):3544–3551. doi: 10.1158/1078-0432.CCR-16-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnell O, Kanis JA, Oden A, Johansson H, De Laet C, Delmas P, et al. Predictive value of BMD for hip and other fractures. J Bone Miner Res. 2005;20(7):1185–1194. doi: 10.1359/JBMR.050304. [DOI] [PubMed] [Google Scholar]
- 43.Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312(7041):1254–1259. doi: 10.1136/bmj.312.7041.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kanis JA, Burlet N, Cooper C, Delmas PD, Reginster JY, Borgstrom F, Rizzoli R, European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2008;19(4):399–428. doi: 10.1007/s00198-008-0560-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.International Society for Clinical Densitometry (ISCD). 2019 ISCD Official Positions – Adult. 2019. https://www.iscd.org/official-positions/2019-iscd-official-positions-adult/. Accessed 14 August 2019.
- 46.Kanis JA, Melton LJ, 3rd, Christiansen C, Johnston CC, Khaltaev N. The diagnosis of osteoporosis. J Bone Miner Res. 1994;9(8):1137–1141. doi: 10.1002/jbmr.5650090802. [DOI] [PubMed] [Google Scholar]
- 47.Siris ES, Miller PD, Barrett-Connor E, Faulkner KG, Wehren LE, Abbott TA, Berger ML, Santora AC, Sherwood LM. Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: results from the National Osteoporosis Risk Assessment. JAMA. 2001;286(22):2815–2822. doi: 10.1001/jama.286.22.2815. [DOI] [PubMed] [Google Scholar]
- 48.Kanis JA, Hans D, Cooper C, Baim S, Bilezikian JP, Binkley N, Cauley JA, Compston JE, Dawson-Hughes B, el-Hajj Fuleihan G, Johansson H, Leslie WD, Lewiecki EM, Luckey M, Oden A, Papapoulos SE, Poiana C, Rizzoli R, Wahl DA, McCloskey E, Task Force of the FRAX Initiative Interpretation and use of FRAX in clinical practice. Osteoporos Int. 2011;22(9):2395–2411. doi: 10.1007/s00198-011-1713-z. [DOI] [PubMed] [Google Scholar]
- 49.Kanis JA, Johansson H, Harvey NC, McCloskey EV. A brief history of FRAX. Arch Osteoporos. 2018;13(1):118. doi: 10.1007/s11657-018-0510-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Collins GS, Mallett S, Altman DG. Predicting risk of osteoporotic and hip fracture in the United Kingdom: prospective independent and external validation of QFractureScores. BMJ. 2011;342:d3651. doi: 10.1136/bmj.d3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hippisley-Cox J, Coupland C, Brindle P. The performance of seven QPrediction risk scores in an independent external sample of patients from general practice: a validation study. BMJ Open. 2014;4(8):e005809. doi: 10.1136/bmjopen-2014-005809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salawu A, Handforth C, Brown J. Bone-Targeted Therapies in Prostate Cancer. In: Bolla M, Van Poppel H, editors. Management of Prostate Cancer: Springer International Publishing; 2017; 343–356
- 53.Lassemillante AM, Skinner TL, Hooper JD, Prins JB, Wright ORL. Osteoporosis-Related Health Behaviors in Men With Prostate Cancer and Survivors: Exploring Osteoporosis Knowledge, Health Beliefs, and Self-Efficacy. Am J Mens Health. 2017;11(1):13–23. doi: 10.1177/1557988315615956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nadler M, Alibhai S, Catton P, Catton C, To MJ, Jones JM. Osteoporosis knowledge, health beliefs, and healthy bone behaviours in patients on androgen-deprivation therapy (ADT) for prostate cancer. BJU Int. 2013;111(8):1301–1309. doi: 10.1111/j.1464-410X.2012.11777.x. [DOI] [PubMed] [Google Scholar]
- 55.McKean H, Looker S, Hartmann LC, Hayman SR, Kaur JS, McWilliams RR, Peethambaram PP, Stahl JF, Jatoi A. Are cancer survivors/patients knowledgeable about osteoporosis? Results from a survey of 285 chemotherapy-treated cancer patients and their companions. J Nutr Educ Behav. 2008;40(3):144–148. doi: 10.1016/j.jneb.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 56.Tripathy D, Durie BG, Mautner B, Ferenz KS, Moul JW. Awareness, concern, and communication between physicians and patients on bone health in cancer. Support Care Cancer. 2014;22(6):1601–1610. doi: 10.1007/s00520-014-2127-1. [DOI] [PubMed] [Google Scholar]
- 57.Alibhai SM, Rahman S, Warde PR, Jewett MA, Jaffer T, Cheung AM. Prevention and management of osteoporosis in men receiving androgen deprivation therapy: a survey of urologists and radiation oncologists. Urology. 2006;68(1):126–131. doi: 10.1016/j.urology.2006.01.054. [DOI] [PubMed] [Google Scholar]
- 58.Damji AN, Bies K, Alibhai SM, Jones JM. Bone health management in men undergoing ADT: examining enablers and barriers to care. Osteoporos Int. 2015;26(3):951–959. doi: 10.1007/s00198-014-2997-6. [DOI] [PubMed] [Google Scholar]
- 59.Alibhai SMH, Breunis H, Timilshina N, Hamidi MS, Cheung AM, Tomlinson GA, et al. Improving bone health in men with prostate cancer receiving androgen deprivation therapy: Results of a randomized phase 2 trial. Cancer. 2018;124(6):1132–1140. doi: 10.1002/cncr.31171. [DOI] [PubMed] [Google Scholar]
- 60.des Bordes JKA, Suarez-Almazor ME, Volk RJ, Lu H, Edwards B, Lopez-Olivo MA. Online Educational Tool to Promote Bone Health in Cancer Survivors. J Health Commun. 2017;22(10):808–817. doi: 10.1080/10810730.2017.1360415. [DOI] [PubMed] [Google Scholar]
- 61.Agarwal MM, Khandelwal N, Mandal AK, Rana SV, Gupta V, Chandra Mohan V, Kishore GV. Factors affecting bone mineral density in patients with prostate carcinoma before and after orchidectomy. Cancer. 2005;103(10):2042–2052. doi: 10.1002/cncr.21047. [DOI] [PubMed] [Google Scholar]
- 62.Owen PJ, Daly RM, Livingston PM, Fraser SF. Lifestyle guidelines for managing adverse effects on bone health and body composition in men treated with androgen deprivation therapy for prostate cancer: an update. Prostate Cancer Prostatic Dis. 2017;20(2):137–145. doi: 10.1038/pcan.2016.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee CE, Leslie WD, Czaykowski P, Gingerich J, Geirnaert M, Lau YK. A comprehensive bone-health management approach for men with prostate cancer receiving androgen deprivation therapy. Curr Oncol. 2011;18(4):e163–e172. doi: 10.3747/co.v18i4.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grossmann M, Hamilton EJ, Gilfillan C, Bolton D, Joon DL, Zajac JD. Bone and metabolic health in patients with non-metastatic prostate cancer who are receiving androgen deprivation therapy. Med J Aust. 2011;194(6):301–306. doi: 10.5694/j.1326-5377.2011.tb02979.x. [DOI] [PubMed] [Google Scholar]
- 65.Nelson Shakira, Batai Ken, Ahaghotu Chiledum, Agurs-Collins Tanya, Kittles Rick. Association between Serum 25-Hydroxy-Vitamin D and Aggressive Prostate Cancer in African American Men. Nutrients. 2016;9(1):12. doi: 10.3390/nu9010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Batai K, Murphy AB, Ruden M, Newsome J, Shah E, Dixon MA, Jacobs ET, Hollowell CM, Ahaghotu C, Kittles RA. Race and BMI modify associations of calcium and vitamin D intake with prostate cancer. BMC Cancer. 2017;17(1):64. doi: 10.1186/s12885-017-3060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Capiod T, Barry Delongchamps N, Pigat N, Souberbielle JC, Goffin V. Do dietary calcium and vitamin D matter in men with prostate cancer? Nat Rev Urol. 2018;15(7):453–461. doi: 10.1038/s41585-018-0015-z. [DOI] [PubMed] [Google Scholar]
- 68.Datta M, Schwartz GG. Calcium and vitamin D supplementation during androgen deprivation therapy for prostate cancer: a critical review. Oncologist. 2012;17(9):1171–1179. doi: 10.1634/theoncologist.2012-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Owen Patrick J., Daly Robin M., Dalla Via Jack, Mundell Niamh L., Livingston Patricia M., Rantalainen Timo, Fraser Steve F. The clinical relevance of adiposity when assessing muscle health in men treated with androgen deprivation for prostate cancer. Journal of Cachexia, Sarcopenia and Muscle. 2019;10(5):1036–1044. doi: 10.1002/jcsm.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grossmann M, Cheung AS, Zajac JD. Androgens and prostate cancer; pathogenesis and deprivation therapy. Best Pract Res Clin Endocrinol Metab. 2013;27(4):603–616. doi: 10.1016/j.beem.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 71.Martone AM, Marzetti E, Calvani R, Picca A, Tosato M, Santoro L, di Giorgio A, Nesci A, Sisto A, Santoliquido A, Landi F. Exercise and Protein Intake: A Synergistic Approach against Sarcopenia. Biomed Res Int. 2017;2017:2672435. doi: 10.1155/2017/2672435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smith MR, McGovern FJ, Zietman AL, Fallon MA, Hayden DL, Schoenfeld DA, Kantoff PW, Finkelstein JS. Pamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancer. N Engl J Med. 2001;345(13):948–955. doi: 10.1056/NEJMoa010845. [DOI] [PubMed] [Google Scholar]
- 73.Michaelson MD, Kaufman DS, Lee H, McGovern FJ, Kantoff PW, Fallon MA, Finkelstein JS, Smith MR. Randomized controlled trial of annual zoledronic acid to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer. J Clin Oncol. 2007;25(9):1038–1042. doi: 10.1200/JCO.2006.07.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bhoopalam N, Campbell SC, Moritz T, Broderick WR, Iyer P, Arcenas AG, van Veldhuizen P, Friedman N, Reda D, Warren S, Garewal H. Intravenous zoledronic acid to prevent osteoporosis in a veteran population with multiple risk factors for bone loss on androgen deprivation therapy. J Urol. 2009;182(5):2257–2264. doi: 10.1016/j.juro.2009.07.046. [DOI] [PubMed] [Google Scholar]
- 75.Smith MR, Eastham J, Gleason DM, Shasha D, Tchekmedyian S, Zinner N. Randomized controlled trial of zoledronic acid to prevent bone loss in men receiving androgen deprivation therapy for nonmetastatic prostate cancer. J Urol. 2003;169(6):2008–2012. doi: 10.1097/01.ju.0000063820.94994.95. [DOI] [PubMed] [Google Scholar]
- 76.Magno C, Anastasi G, Morabito N, Gaudio A, Maisano D, Franchina F, Galì A, Frisina N, Melloni D. Preventing bone loss during androgen deprivation therapy for prostate cancer: early experience with neridronate. Eur Urol. 2005;47(5):575–580. doi: 10.1016/j.eururo.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 77.Ryan CW, Huo D, Demers LM, Beer TM, Lacerna LV. Zoledronic acid initiated during the first year of androgen deprivation therapy increases bone mineral density in patients with prostate cancer. J Urol. 2006;176(3):972–978. doi: 10.1016/j.juro.2006.04.078. [DOI] [PubMed] [Google Scholar]
- 78.Ryan CW, Huo D, Bylow K, Demers LM, Stadler WM, Henderson TO, et al. Suppression of bone density loss and bone turnover in patients with hormone-sensitive prostate cancer and receiving zoledronic acid. BJU Int. 2007;100(1):70–75. doi: 10.1111/j.1464-410X.2007.06853.x. [DOI] [PubMed] [Google Scholar]
- 79.Klotz LH, McNeill IY, Kebabdjian M, Zhang L, Chin JL. Canadian Urology Research C. A phase 3, double-blind, randomised, parallel-group, placebo-controlled study of oral weekly alendronate for the prevention of androgen deprivation bone loss in nonmetastatic prostate cancer: the Cancer and Osteoporosis Research with Alendronate and Leuprolide (CORAL) study. Eur Urol. 2013;63(5):927–935. doi: 10.1016/j.eururo.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 80.Choo R, Lukka H, Cheung P, Corbett T, Briones-Urbina R, Vieth R, et al. Randomized, double-blinded, placebo-controlled, trial of risedronate for the prevention of bone mineral density loss in nonmetastatic prostate cancer patients receiving radiation therapy plus androgen deprivation therapy. Int J Radiat Oncol Biol Phys. 2013;85(5):1239–1245. doi: 10.1016/j.ijrobp.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 81.Greenspan SL, Nelson JB, Trump DL, Resnick NM. Effect of once-weekly oral alendronate on bone loss in men receiving androgen deprivation therapy for prostate cancer: a randomized trial. Ann Intern Med. 2007;146(6):416–424. doi: 10.7326/0003-4819-146-6-200703200-00006. [DOI] [PubMed] [Google Scholar]
- 82.Greenspan SL, Nelson JB, Trump DL, Wagner JM, Miller ME, Perera S, Resnick NM. Skeletal health after continuation, withdrawal, or delay of alendronate in men with prostate cancer undergoing androgen-deprivation therapy. J Clin Oncol. 2008;26(27):4426–4434. doi: 10.1200/JCO.2007.15.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rodrigues P, Hering FO, Bruna P, Meller A, Afonso Y. Comparative study of the protective effect of different intravenous bisphosphonates on the decrease in bone mineral density in patients submitted to radical prostatectomy undergoing androgen deprivation therapy. A prospective open-label controlled study. Int J Urol. 2007;14(4):317–320. doi: 10.1111/j.1442-2042.2006.01721.x. [DOI] [PubMed] [Google Scholar]
- 84.Israeli RS, Rosenberg SJ, Saltzstein DR, Gottesman JE, Goldstein HR, Hull GW, Tran DN, Warsi GM, Lacerna LV. The effect of zoledronic acid on bone mineral density in patients undergoing androgen deprivation therapy. Clin Genitourin Cancer. 2007;5(4):271–277. doi: 10.3816/CGC.2007.n.003. [DOI] [PubMed] [Google Scholar]
- 85.Kachnic LA, Pugh SL, Tai P, Smith M, Gore E, Shah AB, Martin AG, Kim HE, Nabid A, Lawton CA. RTOG 0518: randomized phase III trial to evaluate zoledronic acid for prevention of osteoporosis and associated fractures in prostate cancer patients. Prostate Cancer Prostatic Dis. 2013;16(4):382–386. doi: 10.1038/pcan.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Denham JW, Nowitz M, Joseph D, Duchesne G, Spry NA, Lamb DS, Matthews J, Turner S, Atkinson C, Tai KH, Gogna NK, Kenny L, Diamond T, Smart R, Rowan D, Moscato P, Vimieiro R, Woodfield R, Lynch K, Delahunt B, Murray J, D'Este C, McElduff P, Steigler A, Kautto A, Ball J. Impact of androgen suppression and zoledronic acid on bone mineral density and fractures in the Trans-Tasman Radiation Oncology Group (TROG) 03.04 Randomised Androgen Deprivation and Radiotherapy (RADAR) randomized controlled trial for locally advanced prostate cancer. BJU Int. 2014;114(3):344–353. doi: 10.1111/bju.12497. [DOI] [PubMed] [Google Scholar]
- 87.Taxel P, Dowsett R, Richter L, Fall P, Klepinger A, Albertsen P. Risedronate prevents early bone loss and increased bone turnover in the first 6 months of luteinizing hormone-releasing hormone-agonist therapy for prostate cancer. BJU Int. 2010;106(10):1473–1476. doi: 10.1111/j.1464-410X.2010.09329.x. [DOI] [PubMed] [Google Scholar]
- 88.Casey R, Gesztesi Z, Rochford J. Long term zoledronic acid during androgen blockade for prostate cancer. Can J Urol. 2010;17(3):5170–5177. [PubMed] [Google Scholar]
- 89.Kapoor A, Gupta A, Desai N, Ahn H. Effect of zoledronic Acid on bone mineral density in men with prostate cancer receiving gonadotropin-releasing hormone analog. Prostate Cancer. 2011;2011:176164. doi: 10.1155/2011/176164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Serpa Neto A, Tobias-Machado M, Esteves MA, Senra MD, Wroclawski ML, Fonseca FL, Dos Reis RB, Pompeo AC, Giglio AD. Bisphosphonate therapy in patients under androgen deprivation therapy for prostate cancer: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2012;15(1):36–44. doi: 10.1038/pcan.2011.4. [DOI] [PubMed] [Google Scholar]
- 91.Smith MR, Egerdie B, Hernandez Toriz N, Feldman R, Tammela TL, Saad F, et al. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med. 2009;361(8):745–755. doi: 10.1056/NEJMoa0809003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Doria C, Leali PT, Solla F, Maestretti G, Balsano M, Scarpa RM. Denosumab is really effective in the treatment of osteoporosis secondary to hypogonadism in prostate carcinoma patients? A prospective randomized multicenter international study. Clin Cases Miner Bone Metab. 2016;13(3):195–199. doi: 10.11138/ccmbm/2016.13.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Smith MR, Fallon MA, Lee H, Finkelstein JS. Raloxifene to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer: a randomized controlled trial. J Clin Endocrinol Metab. 2004;89(8):3841–3846. doi: 10.1210/jc.2003-032058. [DOI] [PubMed] [Google Scholar]
- 94.Smith MR, Morton RA, Barnette KG, Sieber PR, Malkowicz SB, Rodriguez D, Hancock ML, Steiner MS. Toremifene to reduce fracture risk in men receiving androgen deprivation therapy for prostate cancer. J Urol. 2013;189(1 Suppl):S45–S50. doi: 10.1016/j.juro.2012.11.016. [DOI] [PubMed] [Google Scholar]