Abstract
Introduction
As the number of Alzheimer's disease (AD) prevention studies grows, many individuals will need to learn their genetic and/or biomarker risk for the disease to determine trial eligibility. An alternative to traditional models of genetic counseling and disclosure is needed to provide comprehensive standardized counseling and disclosure of apolipoprotein E (APOE) results efficiently, safely, and effectively in the context of AD prevention trials.
Methods
A multidisciplinary Genetic Testing, Counseling, and Disclosure Committee was established and charged with operationalizing the Alzheimer's Prevention Initiative (API) Genetic Counseling and Disclosure Process for use in the API Generation Program trials. The objective was to provide consistent information to research participants before and during the APOE counseling and disclosure session using standardized educational and session materials.
Results
The Genetic Testing, Counseling, and Disclosure Committee created a process consisting of eight components: requirements of APOE testing and reports, psychological readiness assessment, determination of AD risk estimates, guidance for identifying providers of disclosure, predisclosure education, APOE counseling and disclosure session materials, APOE counseling and disclosure session flow, and assessing APOE disclosure impact.
Discussion
The API Genetic Counseling and Disclosure Process provides a framework for large-scale disclosure of APOE genotype results to study participants and serves as a model for disclosure of biomarker results. The process provides education to participants about the meaning and implication(s) of their APOE results while also incorporating a comprehensive assessment of disclosure impact. Data assessing participant safety and psychological well-being before and after APOE disclosure are still being collected and will be presented in a future publication.
Keywords: Alzheimer's disease, APOE, Genetic counseling, Genetic disclosure, Alzheimer's Prevention Initiative, Generation Program, Clinical trials, Clinical research, Prevention
Highlights
-
•
Participants may need to learn their risk for Alzheimer's disease to enroll in studies.
-
•
Alternatives to traditional models of apolipoprotein E counseling and disclosure are needed.
-
•
An alternative process was developed by the Alzheimer's Prevention Initiative.
-
•
This process has been implemented by the Alzheimer's Prevention Initiative Generation Program.
1. Introduction
Alzheimer's disease (AD) poses a tremendous medical, economic, and societal burden [1]. In response, the National Plan to Address Alzheimer's Disease has set an ambitious goal of developing treatments to delay onset of AD and related dementias by 2025 [2]. Given this heightened urgency, at least eight trials of preclinical AD (i.e., prevention) treatments have been attempted, are ongoing, or are in various stages of planning [3]. Unlike trials conducted in individuals in the symptomatic stages of AD, preclinical AD trials enroll cognitively healthy participants who are at increased risk for developing clinical symptoms of AD because of their age, genetics, or the presence of AD-related biomarkers. Trials that use genes or biomarkers as eligibility criteria therefore typically require participants to learn sensitive information along with the associated implications for their individual risk [[4], [5], [6], [7]].
The Alzheimer's Prevention Initiative (API), established in 2010, is a collaborative research program led by Banner Alzheimer's Institute in partnership with other key organizations working to accelerate the evaluation of interventions to prevent AD dementia [4,8]. In 2012, API received a National Institutes of Health (NIH) grant to help support the Colombia Autosomal Dominant AD Trial (NCT01998841), the first funded prevention trial of an investigational disease-modifying treatment in cognitively unimpaired mutation carriers and noncarriers from the world's largest autosomal dominant AD kindred. Because many kindred members do not want to know their genetic status and there was no established paradigm for genetic disclosure in Colombia, this ongoing study includes both mutation carriers who are randomized to active treatment or placebo and noncarriers who receive placebo, and it does not include genetic disclosure [4].
In 2013, API received an NIH grant to help support Generation Study 1 (NCT02565511), the first of two complementary trials collectively referred to as the Generation Program and the first funded prevention trial of an investigational disease-modifying treatment in persons at genetic risk for late-onset AD [9,10]. Because this ongoing trial is studying cognitively unimpaired 60–75-year-old persons with two copies of the apolipoprotein E (APOE4) allele, the major genetic risk factor or AD, a mechanism was needed to provide APOE4 homozygotes information about their genetic risk before their enrollment. Generation Study 2 (NCT03131453) was subsequently initiated in cognitively unimpaired APOE4 carriers, including APOE ε4 heterozygotes with a positive amyloid positron emission tomography (PET) scan and additional APOE4 homozygotes irrespective of their amyloid PET results [9,10]. Because it is one of the enrollment criteria, participants in Generation Study 2 must also receive information about their APOE test results, alone or in combination with their amyloid PET results. The design, rationale, and methodology of the API Generation Program has been published previously [10].
The joint practice guidelines for genetic counseling and testing for AD from the American College of Medical Genetics and Genomics as well as the National Society of Genetic Counselors recommend against APOE genetic susceptibility testing in clinical practice. The rationale for this recommendation is the limited clinical utility and prognostic value of testing in ordinary clinical use in individual patients and the potential for unintended psychological and social harms (e.g., distress, genetic discrimination) [11]. However, in a research context, APOE testing is a useful tool to identify potential participants for AD prevention studies and is the only context under which the National Institute on Aging recommends APOE testing [12]. In cases where APOE testing of cognitively unimpaired individuals is necessary, the American College of Medical Genetics and Genomics as well as National Society of Genetic Counselors joint practice guidelines recommend counseling and disclosure be completed with a qualified genetics professional with a protocol modeled after the genetic testing protocol for Huntington's disease (HD) [13], which is based on the International Huntington Association and World Federation of Neurology Research Group on Huntington's Chorea Guidelines, as it is considered by many to be the gold standard for genetic testing for adult onset conditions [14]. The HD protocol incorporates multiple in-person visits that include one or two pretesting genetic counseling sessions incorporating mental health and neurological assessments, followed by genetic testing, a return visit for disclosure of results and counseling support, and finally a follow-up visit or a phone call [13,14].
Available data suggest that APOE disclosure using traditional genetic counseling and disclosure protocols, such as those modeled after the HD protocol guidelines, can be conducted safely and effectively [[15], [16], [17], [18], [19]]. Although traditional models of APOE genetic counseling and disclosure have been shown to be effective, they are not scalable. To identify the number of participants with the appropriate APOE results required by preclinical AD studies, a substantial number of persons will need to learn their APOE results. For example, to enroll approximately 3000 individuals into the Generation Program trials, we estimate that many more thousands of individuals will need to undergo genetic testing and learn their APOE results. Previous research studies developed condensed standardized APOE genetic counseling and disclosure protocols, providing written educational precounseling materials in lieu of the traditional in-person educational pretest counseling session to reduce trial site and participant burden. Compared with the traditional model, this condensed protocol was found to be equivalent in terms of participants' understanding and test-related distress and required significantly less face-to-face clinician time [20,21]. Although most studies using either traditional or condensed protocols report no significant increases in depression or anxiety symptoms among participants learning their APOE results, it is important to note that these outcomes have been studied in restricted settings and populations, with relatively few individuals at the highest risk level (i.e., APOE4 homozygotes) or those close to the estimated age of symptom onset. These studies typically involved disclosure provided by expert genetic counselors to generally well-educated at-risk middle-aged individuals (based on family history of AD) who volunteered to learn such information and were screened to confirm psychological well-being before counseling and disclosure [[15], [16], [17], [18],21].
Recognizing the significance and sensitive nature of disclosing this type of information, as well as the need to address the scale of APOE genetic counseling and disclosure required by the Generation Program trials, the API team established a multidisciplinary Genetic Testing, Counseling, and Disclosure (GTCD) Committee to develop an APOE genetic counseling and disclosure process to responsibly provide participants with clear and consistent communication of genetic information in the Generation Program and to serve as a model for dissemination into clinical practice, complementing processes developed by other large-scale AD prevention programs for communication of biomarker results [22,23]. This article describes the rationale for, and methods used to develop the API Genetic Counseling and Disclosure Process, as well as challenges and future directions.
2. Methods
2.1. Process development
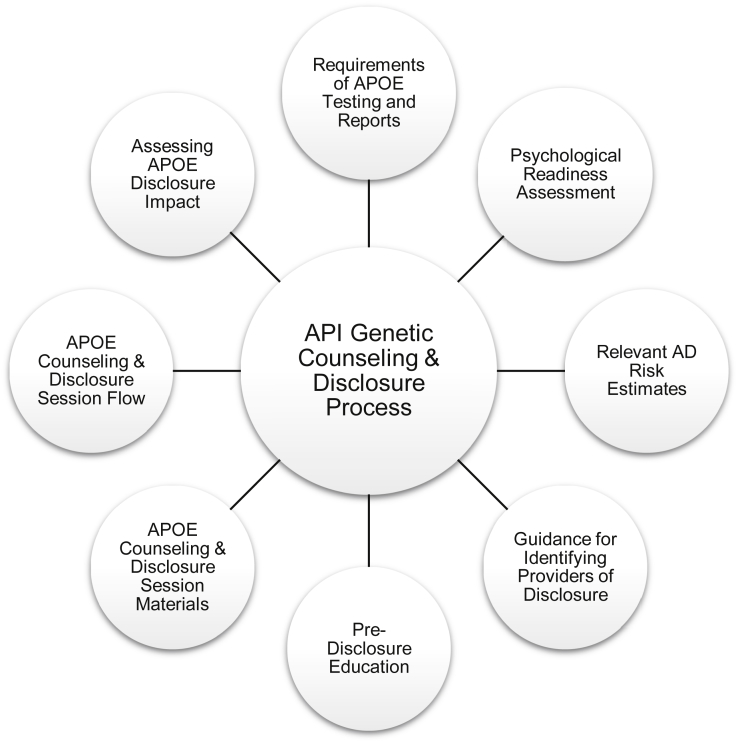
The API team recognized early in the planning stages for the Generation Program the need for special attention and consultation from qualified experts in the development of a program-specific APOE genetic counseling and disclosure process. The GTCD Committee was established to inform and guide the design of the API Genetic Counseling and Disclosure Process for use in the Generation Program. It comprises physicians, social science researchers, health educators, legal experts, and genetic counselors, with expertise in AD, medical ethics, and disclosure of genetic risk factors. At the start of protocol development, the GTCD Committee met every 2 weeks by telephone and approximately quarterly in person. The committee continues to meet monthly by telephone during study implementation. The committee operationalized eight components comprising the API Genetic Counseling and Disclosure Process whose core features include guidelines for APOE testing and reports and providers of disclosure, standardized education and structured content of counseling and disclosure sessions, in-clinic assessment of well-being, assessment of psychological readiness for disclosure and evaluation of APOE disclosure impact (Fig. 1).
Fig. 1.
Alzheimer's Prevention Initiative Genetic Counseling and Disclosure Process components. Abbreviations: APOE, apolipoprotein E; API, Alzheimer's Prevention Initiative; AD, Alzheimer's disease.
2.2. APOE genetic testing
Some individuals enroll in the Generation Program already knowing their APOE genotype from prior genetic testing (e.g., via direct-to-consumer testing); nevertheless, for quality control and standardization purposes, the API Genetic Counseling and Disclosure Process requires all participants to complete an APOE counseling and disclosure session. This requirement also ensures all participants have access to the same information about APOE and the associated risk for AD, as well as providing all participants an opportunity to discuss their results with a provider. All participants complete APOE testing via the study's central laboratory as part of screening regardless of prior testing, but in cases where a report for previous APOE genotyping can be obtained and confirmed as completed by an appropriately certified laboratory (per local regulations, e.g., Clinical Laboratory Improvement Amendments and College of American Pathologists accreditation), these initial results may be used for the APOE counseling and disclosure session.
2.3. Assessing psychological readiness for APOE disclosure
To reduce the risk of negative psychological reactions to disclosure of APOE results, measures to assess psychological readiness to receive results are administered by trained site personnel before the genetic counseling and disclosure session can proceed. The 15-item version of the Geriatric Depression Scale (GDS) [24] and the Six-Item Subset of the State-Trait Anxiety Inventory for Adults (STAI-AD) [25,26] are used as assessments of psychological readiness in both Generation Program studies as they are well-known measures of mood and anxiety with established clinically meaningful defined score ranges. Generation Study 2 adds the electronic version of the Columbia Suicide Severity Rating Scale (C-SSRS) [27] to assess current and prior lifetime suicidal ideation and behaviors, whereas in Generation Study 1, this was assessed by clinical judgment before genetic disclosure. However, the C-SSRS is administered to APOE4 homozygotes in part 2 of Generation Study 1 as described previously [10].
In Generation Study 1, psychological readiness assessment scores above a specified threshold (GDS >6 and STAI-AD >19) results in automatic exclusion, whereas scores within flagged ranges require additional investigator review for inclusion, with special attention to responses on specific questions (Table 1). Generation Study 2 also uses GDS and STAI-AD as assessments of psychological readiness, with specified scores requiring additional investigator review for inclusion (Table 1), but no exclusionary cutoffs are included for GDS or STAI-AD to allow for increased flexibility for investigators to use their clinical judgment when evaluating participants' psychological readiness. However, Generation Study 2 adds the electronic version of the C-SSRS as an additional measure of psychological readiness, resulting in exclusion of participants who report active suicidal ideation (with at least some intent to act) in the past 6 months and/or those reporting suicidal behavior (not including nonsuicidal self-injurious behavior) in the past 2 years. If participants are excluded from study participation based on suicidality, participant safety and well-being continue to be monitored per trial protocol. The process for referral and follow-up with mental health resources varies by country as does site access to mental health professionals (e.g., some site investigators are psychiatrists).
Table 1.
Psychological readiness criteria
Generation Study 1
|
Generation Study 2
|
Abbreviations: APOE, apolipoprotein E; GDS, Geriatric Depression Scale; STAI-AD, State-Trait Anxiety Inventory for Adults; C-SSRS, Columbia Suicide Severity Rating Scale.
2.4. Risk estimates
To provide estimates of risk for development of mild cognitive impairment (MCI) or dementia due to AD tailored to the Generation Program study population, data from four large prospective longitudinal studies were analyzed for the incidence of MCI or dementia among initially cognitively unimpaired individuals aged 60–75 stratified by APOE genotype. The results from these analyses were published previously [28]. In brief, 5-year risk estimates by age group (e.g., 60–64, 65–69) were found to be highly variable in comparison to lifetime risk estimates (defined as through age 85); therefore, the API Genetic Counseling and Disclosure Process provides lifetime risk estimates in the corresponding APOE genetic counseling and disclosure materials [28]. In addition, the AD risk estimates developed for the Generation Program are lower than those published previously and used for APOE disclosure in the REVEAL studies and by direct-to-consumer genetic testing companies [29,30]. These lower risk estimates supported the decision to require all Generation Program participants to complete a genetic counseling and disclosure session, regardless of prior knowledge of APOE status, to ensure all participants are provided with these updated population-specific AD risk estimates.
2.5. Genetic counseling and disclosure providers
Multiple strategies are used to address limited site access to genetic counselors or other genetics-trained professionals. In the United States and Canada, sites can not only use local providers of genetic counseling and disclosure but also have access to centralized remote genetics services, where an off-site genetic counselor completes the APOE counseling and disclosure session via telephone or videoconference. Outside the United States and Canada, local providers are used primarily. Local providers of genetic counseling and disclosure must be qualified per local laws and regulations governing genetic counseling and associated licensure requirements of providers. In areas where no regulations exist, the Sponsors recommend that providers of genetic counseling and disclosure be trained clinical professionals with an advanced understanding of genetics and experience with providing potentially sensitive medical results. In cases of uncertainty, qualifications of the proposed provider are reviewed and approved by the Sponsors.
2.6. Predisclosure genetic education materials
To reduce participant burden and address the considerable number of APOE genetic counseling and disclosure sessions expected to meet enrollment goals of the Generation Program, the API Genetic Counseling and Disclosure Process uses a condensed model that can be completed in a single visit to a Generation Program study site. To accommodate a condensed model, we developed self-directed learning materials, including an Educational Predisclosure Video and Disclosure Informational Brochure (both accessible to participants before the genetic counseling and disclosure visit) covering content typically addressed in a pretesting genetic counseling session. The Video and Brochure provide a summary of the APOE gene and its relationship to AD, as well as considerations for learning one's APOE genotype for both the participant and their family (e.g., potential emotional responses, implications of AD risk for blood relatives). The Video incorporates several multiple-choice questions with feedback explaining the correct response to help confirm and reinforce participant understanding. Participants are strongly encouraged to review these materials in advance of the APOE counseling and disclosure session to orient and familiarize themselves with the information addressed during the session. These key points are also included in the study of informed consent form to ensure participants are presented with this information before enrolling in the trial.
2.7. Genetic counseling and disclosure session materials
Several supportive materials are used in the API Genetic Counseling and Disclosure Process to help standardize the APOE counseling and disclosure session. A Genetic Counseling and Disclosure Session Handout and a set of Genetic Counseling and Disclosure Session Talking Points (Table 2) help standardize the content of the session. The provider uses the Handout as a visual aid during the counseling and disclosure session (participants are provided with a copy for note taking and to keep) and uses the Talking Points as a reference guide for the session. The Talking Points are intended to help address the variability of experience and expertise providers may have in educating participants in genetics and with disclosing APOE results. The Talking Points highlight key topics to be addressed during the session but are not intended to be exhaustive or restrictive.
Table 2.
Genetic counseling and disclosure session talking points for participants undergoing first time disclosure of their APOE results
API Genetic Counseling and Disclosure Process: genetic counseling and disclosure session talking points with individuals receiving first-time∗ disclosure
|
Abbreviations: APOE, apolipoprotein E; API, Alzheimer's Prevention Initiative; AD, Alzheimer's disease; MCI, mild cognitive impairment.
Talking points for participants already aware of their APOE status is available in the supplemental online information.
Participants who enroll in the Generation Program already knowing their APOE results are required to complete an APOE counseling and disclosure session as part of the API Genetic Counseling and Disclosure Process, but a slightly modified set of talking points are used with these participants (See Supplementary Table 1). Talking points for participants already aware of their APOE genotype do not include review of disclosure implications or confirmation of their desire to learn results (as they are already known) but add a discussion of the previous disclosure experience and any impact it had on the participant and their family.
APOE Risk Estimate Summary Sheets are available for each possible genotype and provided to participants after the session to serve as a record of their results and the associated risk information reviewed during the session. For purposes of the Generation Program, a Genetic Counseling and Disclosure Session Manual outlines the entire API Genetic Counseling and Disclosure Process. The materials used in the Process were written in American English but were designed to be generally applicable worldwide, often only requiring translation and/or subtitles. The materials were designed to be edited easily (e.g., made with commonly available software packages in editable formats) in case modifications are needed for cultural adaptation. The API Genetic Counseling and Disclosure Process materials are available on request at the discretion of the authors.
2.8. Genetic counseling and disclosure sessions
The APOE counseling and disclosure session begins with a brief review of the educational content from the Educational Predisclosure Video and Disclosure Informational Brochure, as well as a discussion of the participant's family history, feelings toward APOE disclosure, and considerations for learning one's APOE results. Before disclosure of APOE results and associated risk estimates, the provider is required to confirm the participants' continued desire to learn their APOE results (for people who do not already know their APOE genotype). After disclosure, with consideration given to the emotional and informational needs of the participant, the provider also discusses inheritance implications, degree of risk comparisons, potential risk modifiers, and offers resources for more information. The Risk Estimate Summary Sheet corresponding with the participant's APOE result is given to the participant at the end of the session. All genetic counseling and disclosure sessions are completed with the participant at a study site, even if the site is using remote genetic services, to ensure study staff are immediately available if needed. Post-disclosure follow-up with the participant can be completed by telephone or in person. The materials used in the API Genetic Counseling and Disclosure Process were designed to be easily adaptable to local standards and regulations. Fig. 2 presents a flowchart summarizing the API Genetic Counseling and Disclosure Process.
Fig. 2.
Alzheimer's Prevention Initiative Genetic Counseling and Disclosure Process flow. Abbreviation: APOE, apolipoprotein E.
2.9. Assessing impact of APOE disclosure
Given the limited available data on the outcomes of APOE disclosure, specifically in the context of clinical trials, and the desire to demonstrate the utility of the process, the API Genetic Counseling and Disclosure Process incorporates follow-up measures to assess the impact of participants learning their APOE results. Generation Study 1 includes a robust battery assessing impact of APOE disclosure administered 2–7 days, 6 weeks, 6 months, and 12 months after disclosure (Table 3). Assessments included in Generation Study 1 genetic disclosure follow-up period include measures of participant psychological well-being, understanding and retention of information presented throughout the API Genetic Counseling and Disclosure Process, perceptions of perceived risk and threat of AD, and assessments of the general impact of APOE testing and motivation for learning these results, as well as participant satisfaction with the genetic services provided, including satisfaction with remote counseling, when applicable.
Table 3.
Generation Program genetic disclosure follow-up schedule of assessments
| Visit timepoint |
Day 1 (disclosure visit) |
2–7 Days |
6 Weeks |
6 Months |
12 Months |
|---|---|---|---|---|---|
| Visit number | 1 | 101 | 102 | 103 | 104 |
| Generation Sudy 1 | |||||
| Genetic counseling | X | ||||
| Disclosure of genotype | X | ||||
| Six-Item Subset of the STAI-AD [25,26] | X | X | X | X | X |
| GDS [24] | X | X | X | X | X |
| Knowledge of genetic disease [[31], [32], [33]] | X | X | X | X | X |
| Disease-specific distress (adapted Impact of Events Scale) [[34], [35], [36]] | X | X | X | X | X |
| Perceived risk of AD [37,38] | X | X | X | X | X |
| Perceived threat about AD [39] | X | X | X | X | X |
| Motivation for learning APOE results | X | ||||
| REVEAL IGT-AD [40] | X | X | X | X | |
| Satisfaction with remote counseling [[41], [42], [43]] | X | ||||
| Satisfaction with genetic services [44] | X | ||||
| Generation Study 2 | |||||
| Genetic counseling | X | ||||
| Disclosure of genotype | X | ||||
| Six-Item Subset of the STAI-AD [25,26] | X | X | |||
| GDS [24] | X | X | |||
Abbreviations: STAI-AD, State-Trait Anxiety Inventory for Adults; GDS, Geriatric Depression Scale; AD, Alzheimer's disease; APOE, apolipoprotein E; IGT-AD, Impact of Genetic Testing for Alzheimer's disease.
In contrast, Generation Study 2, which began recruitment approximately 18 months after Generation Study 1, includes an abbreviated battery, with only a 2–7-day follow-up using the GDS and STAI-AD, because thorough APOE disclosure follow-up is being completed in an adequately large sample as part of Generation Study 1. To reduce participant burden, all postdisclosure follow-up assessments may be administered over the phone or in person for both studies. The schedule of assessments for genetic disclosure follow-up in both Generation Study 1 and Generation Study 2 is provided in Table 3. It is important to note that key safety assessments (e.g., assessment of adverse events and serious adverse events, assessments of suicidality) are completed in all participants throughout both studies, independent of APOE disclosure follow-up.
3. Discussion
At this time, APOE counseling and disclosure are usually not recommended in the clinical setting because of lack of clinical utility [11]; however, when disclosure is necessary, the recommended traditional models of genetic counseling and disclosure require multiple visits and a substantial amount of time with a genetics-trained professional [13,14]. The Generation Program (and potentially other future preclinical AD trials) requires identification of individuals at high risk for developing dementia due to AD based in part on APOE genotype [3,9,10]. Thousands of individuals will need to undergo APOE genetic testing, counseling, and disclosure as part of the sizable recruitment and screening funnel required to meet prevention study program enrollment goals [45]. As such, an alternative to traditional models of genetic counseling and disclosure is needed to provide comprehensive standardized counseling and disclosure of APOE results efficiently, safely, and effectively in the context of AD prevention trials. The API Genetic Counseling and Disclosure Process was designed to meet these needs for the Generation Program and to serve as a much-needed framework for future endeavors requiring large-scale APOE genetic counseling and disclosure with cognitively healthy older adults.
Enlisting multidisciplinary experts was critical in the design of the API Genetic Counseling and Disclosure Process. Including committee members with wide-ranging areas of expertise and backgrounds led to the creation of an ethically sound, practicable, and comprehensive process strengthened by the wealth of knowledge and insight offered by the GTCD committee. Numerous factors were taken into consideration, including how best to provide comprehensive standardized genetic counseling and disclosure-related information to participants. In the Generation Program, APOE counseling and disclosure is completed at sites across the world by providers with varying levels of relevant training and experience. To ensure consistency of communication of information to participants before and during the APOE counseling and disclosure session, the GTCD committee developed educational and session materials (e.g., Education Predisclosure Video, Genetic Counseling and Disclosure Session Handout, Genetic Counseling and Disclosure Session Talking Points) to direct the API Genetic Counseling and Disclosure Process and support providers of genetic counseling and disclosure, especially those with limited experience in delivering APOE results. To provide applicable estimates of APOE-associated risk of developing MCI or dementia because of AD for use in the API Genetic Counseling and Disclosure Process materials, updated lifetime risk estimates tailored to the Generation Program study population were calculated based on four large longitudinal studies [28].
It was also necessary to address the logistical considerations of completing a substantial number of APOE counseling and disclosure sessions. To reduce participant burden and amount of time needed with a provider, the API Genetic Counseling and Disclosure Process is completed during one visit to the study site. To facilitate a condensed model, participants complete precounseling education independently and/or remotely via a web-accessible Education Predisclosure Video and written Disclosure Informational Brochure instead of the pretest in-person genetic counseling visit typically included in traditional models of genetic counseling and disclosure [14]. Also, all postdisclosure participant follow-up may be completed over the phone, further reducing the number of visits to the study site.
Preclinical AD studies such as the Generation Program are conducted globally at numerous sites with varying levels of access to genetics-trained professionals with varying experience disclosing APOE results. The API Genetic Counseling and Disclosure Process was designed to provide a consistent participant experience regardless of the genetic counseling and disclosure provider's experience level by incorporating standardized materials, including a Genetic Counseling and Disclosure Session Handout and Genetic Counseling and Disclosure Session Talking Points to guide the session. In addition, APOE Risk Estimate Summary Sheets are available for each possible genotype result and are provided to participants after the session to serve as a record of their results and the associated risk information reviewed during the session.
Another way to improve access to professionals trained in genetics is the use of remote genetics services. Several studies in cancer have shown telephone-based delivery of genetic results to be safe [[46], [47], [48], [49], [50], [51]], and one study has shown delivery of APOE results by telephone to be equivalent to in-person sessions on scores of anxiety, depression, and test-related distress [18]. Currently, only Generation Program trial sites in the United States and Canada have access to centralized remote genetics services; however, the comparison of telephone-versus videoconferencing-based remote disclosure is being assessed at some sites in the United States as an investigator-initiated ancillary study (NCT02978729) to better understand implications of remote delivery modality [52]. Data from this ancillary study are being collected and will be shared in a future article.
The API Genetic Counseling and Disclosure Process was also designed to protect and monitor participant safety and well-being. As in previous research investigating impact of APOE disclosure, measures verifying psychological well-being are administered before the genetic counseling and disclosure session can proceed. Several assessments intended to evaluate psychological readiness to receive APOE results are administered, and in conjunction with investigator judgment, are used to determine whether it is appropriate for the participant to continue with APOE counseling and disclosure. Given the limitations of available research assessing the impact of APOE disclosure, particularly in the context of clinical trials, the API Genetic Counseling and Disclosure Process includes measures assessing psychological well-being as well as assessments evaluating how learning this information affects participants' understanding of, feelings toward, and beliefs about AD. These outcomes of APOE disclosure are being evaluated across all sites to inform real-world variation outcomes in a global trial. These data are being collected, and we look forward to reporting the results in the future.
The work done to develop the API Genetic Counseling and Disclosure Process for return of APOE results to cognitively normal AD prevention study participants complements processes developed by the Anti-Amyloid Treatment in Asymptomatic Alzheimer's Disease (A4) Study to disclose amyloid imaging results in the context of a preclinical AD trial [22] as well efforts from the European Prevention Alzheimer's Disease project assessing perspectives related to communication of biomarker-based risk of AD [23]. These programs, along with our work, will likely inform future strategies for communicating biomarker-based AD risk results to optimize outcomes in both the research and clinical settings.
Although the API Genetic Counseling and Disclosure Process offers a framework to complete large-scale APOE genetic counseling and disclosure in the context of an AD prevention clinical trial, we acknowledge several limitations. First, the API Genetic Counseling and Disclosure Process has only been implemented in two companion AD prevention studies of similar design but was designed to serve as a framework informed by standard genetic counseling and education principles to provide standardized APOE counseling and disclosure that accounts for anticipated real-world practice variations. For example, the API Genetic Counseling and Disclosure Process has already served as the foundation for the amyloid disclosure process in Generation Study 2. Second, applicability of the API Genetic Counseling and Disclosure Process outside a clinical trial setting is unclear as it has only been implemented as part of the Generation Program and has yet to be tested in a nonresearch-based clinical setting and population. In addition, to implement the API Genetic Counseling and Disclosure Process for another purpose, several of the materials may need to be updated and modified as those used in the API Genetic Counseling and Disclosure Process are tailored to the Generation Program. Certain materials may also need to be updated as the field's understanding of APOE and its associated risk of AD evolves over time. Third, measures of APOE disclosure impact included in the Generation Program focus on participant safety, well-being, understanding of and thoughts about AD, but other areas of impact warrant investigation. As such, additional areas of impact are being assessed as ancillary studies, including whether disclosure of APOE results is associated with worsening of subjective and objective cognitive functioning and stigma, as well as assessing potential differences based on delivery modality of remote genetic services (i.e., telephone vs. videoconference) [52].
3.1. Future directions
The API Genetic Counseling and Disclosure Process provides a novel framework for completing APOE counseling and disclosure in the context of AD prevention trials, such as those in our API studies. The APOE disclosure impact data we are collecting will help us understand the risks and benefits of modifying traditional genetic counseling and disclosure models and set the stage for future adaptations of the current disclosure model in the research and clinical settings. Although comprehensive disclosure outcome data are being collected as part of the Generation Program and several ancillary studies are underway, additional areas of APOE disclosure impact warrant further examination, including evaluation of potential modifications to health and lifestyle behaviors, changes in financial behaviors, employment, and other economic factors, as well as potential impact of stigma and discrimination in response to APOE disclosure. It is imperative that we better understand the impact of APOE disclosure as an increasing number of individuals are learning this information as part of research and other activities, notably direct to consumer genetic testing [53].
Incorporating additional risk factors and modifiers to create a more comprehensive and individualized assessment of AD risk is another area warranting further study. For example, because PET, cerebrospinal fluid, and promising blood-based biomarker measurements may be used to screen or select participants in AD prevention studies, it will help to further optimize our approach to estimate a person's AD risk based on a combination of biomarker and APOE genotype results [3,54]. It will also be beneficial to understand better the impact of other potential modifiers (e.g., sex, health conditions) on personalized risk estimates for developing MCI or dementia because of AD. In addition, future research may not just rely on the APOE gene to identify participants at risk for AD but may use polygenic risk scores [55]. Better understanding the various risk factors, how they interact, and implications of various modifiers could eventually lead to the creation of a personalized AD risk calculator similar to the one developed by the American College of Cardiology and American Heart Association, which provides a personalized numerical risk value of developing atherosclerotic cardiovascular disease based on variety factors [56].
4. Conclusion
As the number of AD prevention studies continues to increase, an alternative to traditional genetic counseling and disclosure models is needed because of the substantial number of visits and face-to-face clinician time required, which is not always feasible in the context of a clinical trial. To address this need, the API Genetic Counseling and Disclosure Process was developed to provide a comprehensive and standardized alternative process for APOE counseling and disclosure in the Generation Program and to serve as a framework for large-scale APOE genetic counseling and disclosure programs in the future. The APOE disclosure impact data being collected will be vital in helping to better understand the risks and benefits of modifying traditional genetic counseling and disclosure models and will likely inform future adaptations to optimize patient outcomes for both the research and clinical settings.
Research in context.
-
1.
Systematic review: We used sources such as PubMed to review the literature on genetic counseling and disclosure, with a focus on disclosure of apolipoprotein E (APOE) for risk of Alzheimer's disease.
-
2.
Interpretation: The Alzheimer's Prevention Initiative's Genetic Counseling and Disclosure Process consists of eight components whose core features include guidelines for APOE testing requirements and providers of disclosure, standardized education and structured content of disclosure sessions, assessment of well-being and psychological readiness for disclosure, and evaluation of disclosure impact. The process provides a framework for large-scale disclosure of APOE genotype results to study participants and serves as a model for disclosure of biomarker results.
-
3.
Future directions: Results from these studies will help us understand the impact of APOE disclosure as well as the implications of modifying traditional counseling and disclosure models and set the stage for future adaptations of the current model in research and clinical settings.
Acknowledgments
This Generation Program is funded by Novartis Pharma AG, Basel, Switzerland and Amgen, Thousand Oaks, CA, USA, in collaboration with the Banner Alzheimer's Institute located in Phoenix, AZ, USA. Generation Study 1 is supported by funding from the National Institute on Aging (1UF1AG046150), part of the National Institutes of Health, as well as the Alzheimer's Association, Fidelity Biosciences Research Initiative, GHR Foundation, and Banner Alzheimer's Foundation. The authors acknowledge Drs Doris Zallen, Linda Patrick-Miller, Richard Caselli, Gary Marchant, Ana Graf, Cristina Lopez Lopez, Kristin Harkins, and Trisha Walsh. The opinions expressed by Scott Y.H. Kim are his own and do not represent the views of the NIH, US Department of Health and Human Services, or any other part of the US government.
Authors' contributions: All authors participated in the study design, implementation, and/or conduct of the API Genetic Counseling and Disclosure Process. All authors contributed to the review of the protocol and approved the final article.
Footnotes
Conflicts of interest: Carolyn Langlois: no financial conflicts; Angela Bradbury: research support from the National Institute on Aging (1UF1AG046150); Beth McCarty Wood: research support from the National Institute on Aging (1UF1AG046150); J. Scott Roberts: research support from the National Institute on Aging (1UF1AG046150 and P30 AG053760); Scott Kim: no financial conflicts; supported by NIH Intramural Research Program; Marie-Emmanuelle Riviere: full or former employees of, and shareholders in Novartis Pharma AG/Novartis Pharmaceuticals Corporation; Fonda Liu: full or former employees of, and shareholders in Novartis Pharma AG/Novartis Pharmaceuticals Corporation; Eric Reiman: research support from the National Institute on Aging (P30 AG19610, R01 AG055444, UF1 AG046150, R01 AG031581), NIH Office of the Director (UG3OD023171), NINDS (U01NS093334), grants from Novartis, Amgen, Banner Alzheimer's Foundation, and Genentech/Roche; research support from Alzheimer's Association, Banner Alzheimer's Foundation, Fidelity Biosciences Research Initiative (FBRI), GHR, NOMIS Foundation, and the Flinn Foundation; Compensated consultation services to Aural Analytics, Alkahest, Alzheon, Denali, Green Valley, Roche (expenses only), United Neuroscience, and Takeda/Zinfandel Pharma; research contracts from Avid, Eli Lilly, Genentech/Roche, Novartis, and Amgen; and a patent for the accelerated evaluation of AD prevention therapies in persons at genetic or biomarker risk; Pierre Tariot: research support from the National Institute on Aging (UF1 AG046150, R01AG055444), grants from Novartis, Amgen, Banner Alzheimer's Foundation, and Genentech/Roche; research support from Arizona Department of Health Services, Alzheimer's Association, Banner Alzheimer's Foundation, FBRI, GHR, NOMIS FOUNDATION, and the Flinn Foundation; consultant fees from Acadia, Abbott Laboratories, AbbVie, AC Immune, Auspex, Boehringer-Ingelheim, Brain Test Inc, California Pacific Medical Center, Chase Pharmaceuticals, CME Inc, GliaCure, Insys Therapeutics, Pfizer, and T3D; consulting fees and research support from AstraZeneca, Avanir, Lilly, Lundbeck, Merck & Co, Roche, and Takeda; research support only from Amgen, Avid, Biogen, Elan, Functional Neuromodulation [f(nm)], GE, Genentech, Novartis, and Targacept; and stock options in Adamas Pharmaceuticals; Jason Karlawish: research support from the National Institute on Aging (1UF1AG046150); site investigator for API Generation Program (Novartis) and A4 Trial (Lilly) and a consultant for Squintmetrics; Jessica Langbaum: research support from National Institute on Aging (UF1 AG046150, R01AG055444), Genentech/Roche, Novartis, Amgen, Alzheimer's Association (API-16-388841), Arizona Alzheimer's Consortium (state of Arizona), Banner Alzheimer's Foundation, FBRI, Flinn Foundation, GHR, and NOMIS Foundation.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.trci.2019.09.013.
Supplementary Data
References
- 1.Wimo A., Guerchet M., Ali G.C., Wu Y.T., Prina A.M., Winblad B. The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimers Dement. 2017;13:1–7. doi: 10.1016/j.jalz.2016.07.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Plan to Address Alzheimer's Disease: 2018 Update. US Department of Health and Human Services, Office of the Assistant Secretary for Planning and Evaluation; 2018. Available at: https://aspe.hhs.gov/system/files/pdf/259581/NatPlan2018.pdf. Accessed October 23, 2019. [Google Scholar]
- 3.Cummings J., Lee G., Ritter A., Zhong K. Alzheimer's disease drug development pipeline: 2018. Alzheimers Dement (N Y) 2018;4:195–214. doi: 10.1016/j.trci.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tariot P.N., Lopera F., Langbaum J.B., Thomas R.G., Hendrix S., Schneider L.S. The Alzheimer's Prevention Initiative Autosomal-Dominant Alzheimer's Disease Trial: a study of crenezumab versus placebo in preclinical PSEN1 E280A mutation carriers to evaluate efficacy and safety in the treatment of autosomal-dominant Alzheimer's disease, including a placebo-treated noncarrier cohort. Alzheimers Dement (N Y) 2018;4:150–160. doi: 10.1016/j.trci.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim S.Y., Karlawish J., Berkman B.E. Ethics of genetic and biomarker test disclosures in neurodegenerative disease prevention trials. Neurology. 2015;84:1488–1494. doi: 10.1212/WNL.0000000000001451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andrieu S., Coley N., Lovestone S., Aisen P.S., Vellas B. Prevention of sporadic Alzheimer's disease: lessons learned from clinical trials and future directions. Lancet Neurol. 2015;14:926–944. doi: 10.1016/S1474-4422(15)00153-2. [DOI] [PubMed] [Google Scholar]
- 7.Wang J., Tan L., Yu J.T. Prevention trials in Alzheimer's disease: current status and future perspectives. J Alzheimers Dis. 2016;50:927–945. doi: 10.3233/JAD-150826. [DOI] [PubMed] [Google Scholar]
- 8.Reiman E.M., Langbaum J.B., Fleisher A.S., Caselli R.J., Chen K., Ayutyanont N. Alzheimer's prevention initiative: a plan to accelerate the evaluation of presymptomatic treatments. J Alzheimers Dis. 2011;26:321–329. doi: 10.3233/JAD-2011-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopez Lopez C., Caputo A., Liu F., Riviere M.E., Rouzade-Dominguez M.L., Thomas R.G. The Alzheimer's Prevention Initiative Generation Program: evaluating CNP520 efficacy in the prevention of Alzheimer's disease. J Prev Alzheimers Dis. 2017;4:242–246. doi: 10.14283/jpad.2017.37. [DOI] [PubMed] [Google Scholar]
- 10.Lopez Lopez C., Tariot P.N., Caputo A., Langbaum J.B., Liu F., Riviere M.E. The Alzheimer's Prevention Initiative Generation Program: study design of two randomized controlled trials for individuals at risk for clinical onset of Alzheimer's disease. Alzheimers Dement (N Y) 2019;5:216–227. doi: 10.1016/j.trci.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldman J.S., Hahn S.E., Catania J.W., LaRusse-Eckert S., Butson M.B., Rumbaugh M. Genetic counseling and testing for Alzheimer disease: joint practice guidelines of the American College of Medical Genetics and the National Society of Genetic Counselors. Genet Med. 2011;13:597–605. doi: 10.1097/GIM.0b013e31821d69b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Institutes of Health. National Institute on Aging . National Institute on Aging: US Department of Health and Human Services; 2015. Alzheimer's Disease Genetics Fact Sheet. Available at: https://www.nia.nih.gov/health/alzheimers-disease-genetics-fact-sheet. Accessed October 23, 2019. [Google Scholar]
- 13.Genetic Testing Protocol for Huntington's Disease. Huntington's Disease Society of America; 2016. Available at: http://hdsa.org/wp-content/uploads/2015/02/HDSA-Gen-Testing-Protocol-for-HD.pdf. Accessed October 23, 2019. [Google Scholar]
- 14.Guidelines for the molecular genetics predictive test in Huntington's disease. International Huntington Association (IHA) and the World Federation of Neurology (WFN) Research Group on Huntington's Chorea. Neurology. 1994;44:1533–1536. [PubMed] [Google Scholar]
- 15.Romero L.J., Garry P.J., Schuyler M., Bennahum D.A., Qualls C., Ballinger L. Emotional responses to APO E genotype disclosure for Alzheimer disease. J Genet Couns. 2005;14:141–150. doi: 10.1007/s10897-005-4063-1. [DOI] [PubMed] [Google Scholar]
- 16.Green R.C., Roberts J.S., Cupples L.A., Relkin N.R., Whitehouse P.J., Brown T. Disclosure of APOE genotype for risk of Alzheimer's disease. N Engl J Med. 2009;361:245–254. doi: 10.1056/NEJMoa0809578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts J.S., Christensen K.D., Green R.C. Using Alzheimer's disease as a model for genetic risk disclosure: implications for personal genomics. Clin Genet. 2011;80:407–414. doi: 10.1111/j.1399-0004.2011.01739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christensen K.D., Uhlmann W.R., Roberts J.S., Linnenbringer E., Whitehouse P.J., Royal C.D.M. A randomized controlled trial of disclosing genetic risk information for Alzheimer disease via telephone. Genet Med. 2018;20:132–141. doi: 10.1038/gim.2017.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ashida S., Koehly L.M., Roberts J.S., Chen C.A., Hiraki S., Green R.C. The role of disease perceptions and results sharing in psychological adaptation after genetic susceptibility testing: the REVEAL Study. Eur J Hum Genet. 2010;18:1296–1301. doi: 10.1038/ejhg.2010.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts J.S., Chen C.A., Uhlmann W.R., Green R.C. Effectiveness of a condensed protocol for disclosing APOE genotype and providing risk education for Alzheimer disease. Genet Med. 2012;14:742–748. doi: 10.1038/gim.2012.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Green R.C., Christensen K.D., Cupples L.A., Relkin N.R., Whitehouse P.J., Royal C.D. A randomized noninferiority trial of condensed protocols for genetic risk disclosure of Alzheimer's disease. Alzheimers Dement. 2015;11:1222–1230. doi: 10.1016/j.jalz.2014.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harkins K., Sankar P., Sperling R., Grill J.D., Green R.C., Johnson K.A. Development of a process to disclose amyloid imaging results to cognitively normal older adult research participants. Alzheimers Res Ther. 2015;7:26. doi: 10.1186/s13195-015-0112-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milne R., Bunnik E., Diaz A., Richard E., Badger S., Gove D. Perspectives on communicating biomarker-based assessments of Alzheimer's disease to cognitively healthy individuals. J Alzheimers Dis. 2018;62:487–498. doi: 10.3233/JAD-170813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheikh J.I., Yesavage J.A. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontologist. 1986;5:165–173. [Google Scholar]
- 25.Marteau T.M., Bekker H. The development of a six-item short-form of the state scale of the Spielberger State-Trait Anxiety Inventory (STAI) Br J Clin Psychol. 1992;31:301–306. doi: 10.1111/j.2044-8260.1992.tb00997.x. [DOI] [PubMed] [Google Scholar]
- 26.Tluczek A., Henriques J.B., Brown R.L. Support for the reliability and validity of a six-item state anxiety scale derived from the State-Trait Anxiety Inventory. J Nurs Meas. 2009;17:19–28. doi: 10.1891/1061-3749.17.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Posner K., Brown G., Stanley B., Yershova K., Oquendo M., Currier G. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168:1266–1277. doi: 10.1176/appi.ajp.2011.10111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qian J., Wolters F.J., Beiser A., Haan M., Ikram M.A., Karlawish J. APOE-related risk of mild cognitive impairment and dementia for prevention trials: an analysis of four cohorts. PLoS Med. 2017;14:e1002254. doi: 10.1371/journal.pmed.1002254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cupples L.A., Farrer L.A., Sadovnick A.D., Relkin N., Whitehouse P., Green R.C. Estimating risk curves for first-degree relatives of patients with Alzheimer's disease: the REVEAL study. Genet Med. 2004;6:192–196. doi: 10.1097/01.gim.0000132679.92238.58. [DOI] [PubMed] [Google Scholar]
- 30.23andMe . 23andMe; Mountain View, CA: 2019. Alzheimer's Disease (APOE variants): Established Research Report on 2 Reported Markers. [Google Scholar]
- 31.Kaphingst K.A., McBride C.M., Wade C., Alford S.H., Reid R., Larson E. Patients' understanding of and responses to multiplex genetic susceptibility test results. Genet Med. 2012;14:681. doi: 10.1038/gim.2012.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lerman C., Narod S., Schulman K., Hughes C., Gomez-Caminero A., Bonney G. BRCA1 testing in families with hereditary breast-ovarian cancer. A prospective study of patient decision making and outcomes. JAMA. 1996;275:1885–1892. [PubMed] [Google Scholar]
- 33.Kelly K., Leventhal H., Marvin M., Toppmeyer D., Baran J., Schwalb M. Cancer genetics knowledge and beliefs and receipt of results in Ashkenazi Jewish individuals receiving counseling for BRCA1/2 mutations. Cancer Control. 2004;11:236–244. doi: 10.1177/107327480401100405. [DOI] [PubMed] [Google Scholar]
- 34.Horowitz M., Wilner N., Alvarez W. Impact of Event Scale: a measure of subjective stress. Psychosom Med. 1979;41:209–218. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Stallard P., Velleman R., Baldwin S. Psychological screening of children for post-traumatic stress disorder. J Child Psychol Psychiatry. 1999;40:1075–1082. [PubMed] [Google Scholar]
- 36.Edwards L., Watson M., St James-Roberts I., Ashley S., Tilney C., Brougham B. Adolescent's stress responses and psychological functioning when a parent has early breast cancer. Psychooncology. 2008;17:1039–1047. doi: 10.1002/pon.1323. [DOI] [PubMed] [Google Scholar]
- 37.Linnenbringer E., Roberts J.S., Hiraki S., Cupples L.A., Green R.C. “I know what you told me, but this is what I think:” perceived risk of Alzheimer disease among individuals who accurately recall their genetics-based risk estimate. Genet Med. 2010;12:219–227. doi: 10.1097/GIM.0b013e3181cef9e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.LaRusse S., Roberts J.S., Marteau T.M., Katzen H., Linnenbringer E.L., Barber M. Genetic susceptibility testing versus family history-based risk assessment: impact on perceived risk of Alzheimer disease. Genet Med. 2005;7:48–53. doi: 10.1097/01.gim.0000151157.13716.6c. [DOI] [PubMed] [Google Scholar]
- 39.Roberts J.S. Anticipating response to predictive genetic testing for Alzheimer's disease: a survey of first-degree relatives. Gerontologist. 2000;40:43–52. doi: 10.1093/geront/40.1.43. [DOI] [PubMed] [Google Scholar]
- 40.Chung W.W., Chen C.A., Cupples L.A., Roberts J.S., Hiraki S.C., Nair A.K. A new scale measuring psychologic impact of genetic susceptibility testing for Alzheimer disease. Alzheimer Dis Assoc Disord. 2009;23:50–56. doi: 10.1097/wad.0b013e318188429e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pieterse A.H., van Dulmen A.M., Beemer F.A., Bensing J.M., Ausems M.G. Cancer genetic counseling: communication and counselees' post-visit satisfaction, cognitions, anxiety, and needs fulfillment. J Genet Couns. 2007;16:85–96. doi: 10.1007/s10897-006-9048-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cella D., Hughes C., Peterman A., Chang C.H., Peshkin B.N., Schwartz M.D. A brief assessment of concerns associated with genetic testing for cancer: the Multidimensional Impact of Cancer Risk Assessment (MICRA) questionnaire. Health Psychol. 2002;21:564–572. [PubMed] [Google Scholar]
- 43.DeMarco T.A., Peshkin B.N., Mars B.D., Tercyak K.P. Patient satisfaction with cancer genetic counseling: a psychometric analysis of the Genetic Counseling Satisfaction Scale. J Genet Couns. 2004;13:293–304. doi: 10.1023/b:jogc.0000035523.96133.bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dick P.T., Filler R., Pavan A. Participant satisfaction and comfort with multidisciplinary pediatric telemedicine consultations. J Pediatr Surg. 1999;34:137–141. doi: 10.1016/s0022-3468(99)90244-0. discussion 41–2. [DOI] [PubMed] [Google Scholar]
- 45.Alber J., Lee A.K.W., Menard W., Monast D., Salloway S.P. Recruitment of at-risk participants for clinical trials: a major paradigm shift for Alzheimer's disease prevention. J Prev Alzheimers Dis. 2017;4:213–214. doi: 10.14283/jpad.2017.32. [DOI] [PubMed] [Google Scholar]
- 46.Schwartz M.D., Valdimarsdottir H.B., Peshkin B.N., Mandelblatt J., Nusbaum R., Huang A.T. Randomized noninferiority trial of telephone versus in-person genetic counseling for hereditary breast and ovarian cancer. J Clin Oncol. 2014;32:618–626. doi: 10.1200/JCO.2013.51.3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jenkins J., Calzone K.A., Dimond E., Liewehr D.J., Steinberg S.M., Jourkiv O. Randomized comparison of phone versus in-person BRCA1/2 predisposition genetic test result disclosure counseling. Genet Med. 2007;9:487–495. doi: 10.1097/gim.0b013e31812e6220. [DOI] [PubMed] [Google Scholar]
- 48.Doughty Rice C., Ruschman J.G., Martin L.J., Manders J.B., Miller E. Retrospective comparison of patient outcomes after in-person and telephone results disclosure counseling for BRCA1/2 genetic testing. Fam Cancer. 2010;9:203–212. doi: 10.1007/s10689-009-9303-3. [DOI] [PubMed] [Google Scholar]
- 49.Klemp J.R., O'Dea A., Chamberlain C., Fabian C.J. Patient satisfaction of BRCA1/2 genetic testing by women at high risk for breast cancer participating in a prevention trial. Fam Cancer. 2005;4:279–284. doi: 10.1007/s10689-005-1474-y. [DOI] [PubMed] [Google Scholar]
- 50.Patrick-Miller L., Egleston B.L., Daly M., Stevens E., Fetzer D., Forman A. Implementation and outcomes of telephone disclosure of clinical BRCA1/2 test results. Patient Educ Couns. 2013;93:413–419. doi: 10.1016/j.pec.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O'Shea R., Meany M., Carroll C., Cody N., Healy D., Green A. Predictive genetic testing and alternatives to face to face results disclosure: a retrospective review of patients preference for alternative modes of BRCA 1 and 2 results disclosure in the Republic of Ireland. J Genet Couns. 2016;25:422–431. doi: 10.1007/s10897-015-9887-8. [DOI] [PubMed] [Google Scholar]
- 52.Bradbury A.R., Egleston B., Patrick-Miller L., MCarty Wood E., Jaeger J., Reddy N. Vol. 12. 2016. p. P324. (Connect 4 APOE: A Randomized Study of Phone versus Videoconference Delivery of APOE Genotype Disclosure in the Generation Study). [Google Scholar]
- 53.Roberts J.S., Gornick M.C., Carere D.A., Uhlmann W.R., Ruffin M.T., Green R.C. Direct-to-consumer genetic testing: user motivations, decision making, and perceived utility of results. Public Health Genomics. 2017;20:36–45. doi: 10.1159/000455006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cummings J., Ritter A., Zhong K. Clinical trials for disease-modifying therapies in Alzheimer's disease: a primer, lessons learned, and a blueprint for the future. J Alzheimers Dis. 2018;64:S3–S22. doi: 10.3233/JAD-179901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chasioti D., Yan J., Nho K., Saykin A.J. Progress in polygenic composite scores in Alzheimer's and other complex diseases. Trends Genet. 2019;35:371–382. doi: 10.1016/j.tig.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goff D.C., Lloyd-Jones D.M., Bennett G., Coady S., D'Agostino R.B., Gibbons R. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. Circulation. 2014;129:S49–S73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.