Abstract
Alzheimer’s disease (AD) is a multifactorial disease which involves both the periphery and central nervous system (CNS). It has been recently recognized that gut microbiota interacts with the gut and brain (microbiota-gut-brain axis), contributing to the pathogenesis of neurodegenerative diseases, such as AD. Dysbiosis of gut microbiota can induce increased intestinal permeability and systemic inflammation, which may lead to the development of AD pathologies and cognitive impairment via the neural, immune, endocrine, and metabolic pathways. Toll-like receptors (TLRs) play an important role in the innate immune system via recognizing microbes-derived pathogens and initiating the inflammatory process. TLRs have also been found in the brain, especially in the microglia, and have been indicated in the development of AD. In this review, we summarized the relationship between microbiota-gut-brain axis and AD, as well as the complex role of TLRs in AD. Intervention of the gut microbiota or modulation of TLRs properly might emerge as promising preventive and therapeutic strategies for AD.
Keywords: Alzheimer’s disease, Toll-like receptors, Aging, Microbiota-gut-brain axis, Inflammation, Dysbiosis
1. Introduction
Modern medicine has achieved great victory in life span expanding. As the incidence of numerous geriatric diseases increases with age, the new challenges include alleviating symptoms, reducing the complications of diseases, and delaying the onset with the intervention of risk factors. Dementia is one of these diseases, which exerts heavy burden on both the family and society. The prevalence of dementia increases exponentially with age. The global prevalence of dementia is about 0.7–1.8% in population aged 60–64 years, while in people aged over 90 years the figure is between 28.7% and 63.9% [1]. Alzheimer’s disease (AD) is the most common type of dementia accounting for 50–60% of all cases. Extracellular senile plaques and intracellular neurofibrillary tangles are the main pathological hallmarks of the disease. The biomarker framework has been established for clinical diagnosis, including amyloid β-42 (Aβ42) level, total tau and phosphorylated tau (p-Tau) level in cerebrospinal fluid (CSF), positron emission tomography (PET) amyloid imaging, Fluorodeoxyglucose (FDG) uptake on PET and structural magnetic resonance imaging (MRI) [2], [3]
Although great efforts have been made targeting the two pathological hallmarks, there still remains no disease modifying treatment for AD. To date, therapeutic strategies targeting against Aβ, including active vaccines, passive immunization, as well as β- and γ-secretases inhibitors, were fraught with failure and confusing results [4], [5], [6], [7], [8], [9], [10]. Clinical trials focused on tau protein immunotherapy are still pending [11]. Several hypotheses other than amyloid cascade hypothesis have been raised [12], [13], [14], [15], [16]. It is considered that the imbalance between Aβ production and clearance leads to Aβ accumulation and subsequently neuronal dysfunction. Accumulating studies have implicated that neuroinflammation might participate in the clearance of Aβ and even promote the pathological process of AD [17], [18], [19]. Epidemiological and observational studies indicate that non-steroidal anti-inflammatory drugs (NSAIDs) users had a lower risk of developing dementia [20], [21], [22]. Genome-wide association studies (GWAS) revealed that some genes involved in immune response were associated with AD risk [23]. Acute systemic inflammation events including various infections, surgical interventions, myocardial infarction and so on, could boost neuroinflammation and exaggerate cognitive decline [24]. In animal experiments, it has also been shown that systemic inflammation could accelerate AD-like pathological changes [25]. All these evidences indicate a close relationship between AD and inflammation. It is assumed that infection event could exaggerate neuroinflammation, promote Aβ production, and then resulting in exacerbation of cognitive impairment.
In recent years, a large number of studies revealed that dysbiosis or the localized intestinal infection may trigger systemic immune response, resulting in exacerbated inflammatory response in AD brain [26]. Dysbiosis refers to microbial imbalance on or inside the body. Intestinal microbiota can bidirectionally interplay with the central nervous system (CNS) through neural, immune, endocrine, and metabolic signals, which is regarded as the microbiota-gut-brain axis [27]. There were over 150,000 microbial genomes reconstructed from global, body-wide metagenomes [28]. The human gastrointestinal (GI) tract is the largest reservoir and harbors approximately 1014 microorganisms. Gut microbiota not only has metabolic and trophic functions, but also promotes host defense and immune homeostasis [29], [30]. As numerous lymphoid tissues locate in the intestinal mucosa, GI tract incessantly monitors the pathogens and the dynamic microenvironment of the gut. The alteration of gut microbiota may lead to intestinal infection or inflammatory bowel disease, and prime the immune response [31], [32].
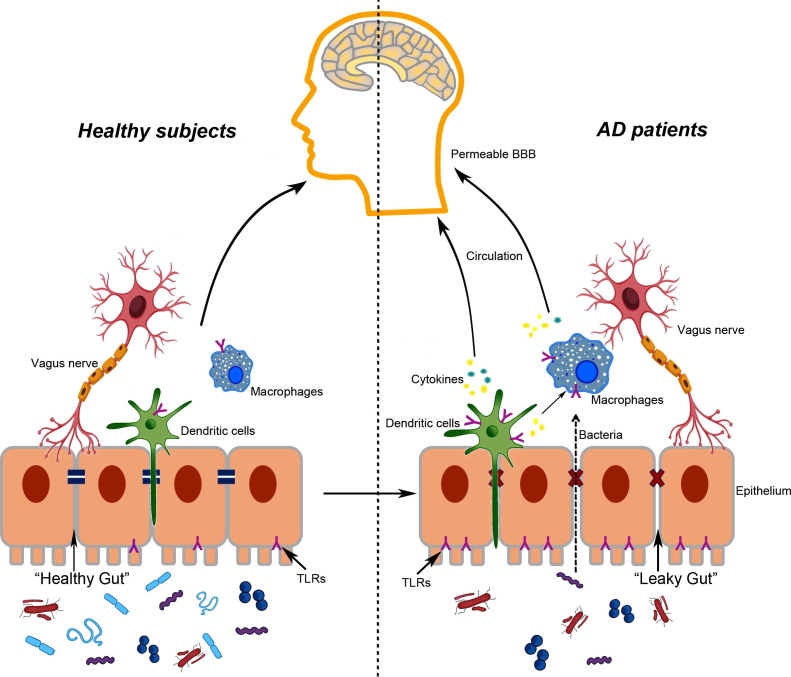
Toll-like receptors (TLRs), the crucial sentinels, are the first line of defenders, participating in recognizing molecules broadly shared by pathogens and the activated immune system. TLRs are involved in commensal colonization, maintenance of the homeostasis, and integrity of the intestinal barrier [33]. Apart from an assortment of gut bacteria and their excreta, Aβ is also a ligand of TLRs, which, under certain conditions, can initiate the inflammatory process in the gut and the brain, leading to the development of neurodegenerative diseases, including AD. Here, we review the current knowledge concerning the relationship between TLRs and microbiota-gut-brain axis in AD, and discuss the potential mechanisms underlying the role of TLRs in AD (Fig. 1).
Fig. 1.
Potential implications of TLRs and gut-brain-axis for AD. In healthy subjects, the gut epithelium is guaranteed by tight junctions between the cells. TLRs are expressed on macrophages, dendritic cells (DCs), and intestinal epithelial cells, serving as sentinels to monitor the pathogens in gut. Vagus nerve appears to modulate communication between the gut and the brain. The whole microenvironment maintains in homeostasis. During aging, the tight junction of intestinal and BBB become permeable. In AD patients, the diversity of gut microbiota decreased, while the population of pro-inflammation bacteria increased. Bacteria and their excretions could cross the leaky gut and then activate the TLRs in epithelium, IECs and macrophages, leading to production of pro-inflammation cytokines. These cytokines make their way through circulation or vague nerves to the brain, enlarge the neuroinflammatory responses, and promote neurodegeneration in CNS.
2. Toll-like receptors signaling
TLRs are a family of transmembrane pattern recognition receptors. TLRs initiate the downstream signaling transduction upon recognition of damage- and pathogen-associated molecular patterns (DAMPs and PAMPs). To date, 11 human and 13 mouse TLRs have been identified. The TLRs can roughly be classified into two groups referring to their space distribution. TLR1, TLR2, TLR4, TLR5, TLR6, and TLR11 are expressed on the plasma membrane, which recognize microbial products such as lipids, lipoproteins, and proteins, whereas TLR3, TLR7, TLR8, and TLR9 are localized in cytoplasmic compartments, which can be activated by nucleic acid species [34].
TLRs are composed of three major domains, a leucine-rich repeat (LRR) ligand-binding domain, a single membrane spanning helix, and a signaling Toll-interleukin-1 receptor (TIR) domain. Upon recognizing PAMPs or DAMPs, TLRs undergo conformational changes following dimerization to recruit the downstream signaling adaptors, which triggers the activation of specific transcription factors and the subsequent innate immune responses. A total of four adaptor proteins have been identified, including myeloid differentiation primary response protein 88 (MyD88), TIR domain–containing adaptor molecule (TIRAP, also known as MyD88-adaptor-like protein, MAL), TRIF-related adaptor molecule (TRAM, also known as TIR-domain-containing molecule 2, TICAM2), TIR domain–containing adaptor protein inducing interferon-β (TRIF, also known as TIR-domain-containing molecule 1, TICAM1) [35]. MyD88 is a universal adaptor protein for all TLR-mediated signaling pathways except for TLR3 [36]. MyD88-dependent pathway can induce the activation of nuclear factor kappa B (NF-κB) and activator protein 1 (AP-1), leading to the expression of pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin (IL)-1, and IL-6. Both TLR3 and TLR4 are capable of recruiting TRIF, resulting in the production of type-I interferon (IFN). TRAM is specifically necessary for TRIF-dependent signaling pathway through TLR4, but not TLR3 [37]. Besides, TLR7, TLR8, and TLR9 can also induce the production of type-I IFN through the MyD88-dependent pathway [36].
The activation of TLRs can be regulated by sialic acid-binding immunoglobulin superfamily lectin receptors (Siglecs), which are known to inhibit the immune response. Extensive Siglec-TLR interactions negatively regulate the activation of TLRs [38]. Disruption of their interactions can result in the activation of TLRs and the immune responses [38]. Siglec-3 (CD33) has been shown to regulate the presentation of LPS to TLR4, leading to down-regulation of TLR4-mediated signaling [39]. Besides, triggering receptors expressed on myeloid cells-1 (TREM-1) also acts synergistically with receptors for PAMPs, including TLRs. TREM-1 amplifies the TLR-mediated immune response to microbial products, resulting in a dramatic upregulation of pro-inflammatory cytokines secretion [40].
3. TLRs in Alzheimer’s disease
Broad expression of TLRs have been found in human CNS. TLR1-9 encoding mRNA were detected in primary cultures of microglia from postmortem human brain. Astrocytes and oligodendrocytes were also found to express TLR2 and TLR3, and to some extent, TLR1 and TLR4 [41]. Besides, certain TLRs were also found in neurons [42], [43].
Microglia are the resident macrophages and primary immune cells in the CNS, responsible for the elimination of invading pathogens and injured neurons. As early as in the 1990s, microglia had been revealed can be activated by Aβ [44], [45]. TLRs are also the endogenous binding sites for Aβ. It has been revealed that CD14, TLR2, and TLR4 are required for the activation of microglia by Aβ [46]. Overexpression of TLR2, TLR4, and CD14 (the lipopolysaccharide (LPS) receptor) have been found in the brains of both AD patients and AD mouse models [47], [48]. AD mouse models, mostly generated by over-expression of mutated human PS1, APP and/or tau, are valuable tools to investigate the mechanisms of AD. These transgenic mouse models generally develop amyloid plaques and/or neurofibrillary tangles in the brain, resembling the hallmarks of AD. Triple-transgenic AD (3xTg-AD) mouse, APP/presenilin 1 (APP/PS1) mouse, and tau transgenetic mouse are the commonly used AD mouse models to investigate the role of TLRs in AD [49].
The role of TLR2 in AD is controversial. TLR2 can recognize Aβ42, triggering the release of pro-inflammatory cytokines, including TNF-α, interleukin-6 (IL-6), and interleukin 1-β (IL-1β), which are detrimental to the CNS, promoting the pathogenesis of AD [50], [51]. Inhibiting TLR2 by anti-TLR2 antibody could attenuate Aβ-induced pro-inflammatory cytokines release and amyloid accumulation, leading to improved performance in spatial learning in AD mouse models [52], [53]. Additionally, TLR2 deficiency enhanced phagocytosis and clearance of Aβ in cultured microglia [54]. All these evidences indicate that inhibition of TLR2, which participates in Aβ deposition and Aβ-induced neuroinflammation, might be beneficial for AD. However, Richard et al. reported that TLR2 knockout APP/PS1 mice had lower amyloid burden, but higher toxic Aβ1-42 species and more heavily cognitive damage [55]. This was supported by another study, which demonstrated a markedly increased uptake of Aβ42 by microglia via the activation of TLR2 [56]. These discrepancies might be due to different animal models used, as well as their representing disease stages. Thus, the role of TLR2 in AD still needs further investigation.
Similar to TLR2, TLR4 seems to play a dual role in the pathogenesis and progression of AD as well. On one side, microglial TLR4 mediates Aβ-induced neurotoxicity [48]. Cytokines, including IL-1β, IL-10, IL-17, and TNF-α, were upregulated in a TLR4-dependent way in AD mice [57]. On the other side, TLR4-mutant AD mice had less microglial activation, and as a result, more Aβ accumulation and severer cognitive deficits than TLR4 wild type AD mice, suggesting that activation of microglia via TLR4 signaling could enhance the clearance of Aβ and preserve cognitive function from Aβ-induced neurotoxicity [58]. Another study found that neuroinflammation could promote neuronal autophagy, and that chronic mild stimulation of TLR4 was associated with a reduction in cerebral p-Tau levels and improved cognitive function of tau-transgenic AD mice [59]. However, activation of microglia by LPS, a TLR4 ligand, was markedly blunted in 12-month-old APP/PS1 mice compared to their 2-month-old counterparts, indicating TLR4 signaling dysfunction due to chronic exposure of microglia to Aβ deposits [60]. Collectively, these studies suggest that TLR4 signaling is essential for the clearance of Aβ by microglia. However, persistent chronic activation of microglia by Aβ exposure would dampen the TLR4 signaling, leading to further Aβ accumulation and neurodegeneration. Thus, modulating the activation of TLR4 towards facilitating Aβ clearance without activating neuroinflammation should be a promising treatment target for AD.
In addition to TLR2 and TLR4, TLR9 can also be detected in both microglia and neurons. TLR9 polymorphism has been reported to be associated with a decreased risk of AD [61]. Activation of TLR9 signaling could protect neurons from stress [62]. TLR9 knockout mice showed impaired synaptic function [63]. Targeting CpG motifs, which function as TLR9 agonists, can reduce both Aβ and tau pathologies in various AD transgenic mouse models, and rescue their cognitive deficits [64], [65], [66]. These studies provide valuable evidence in support of immunomodulation via TLR9 as a potential therapeutic approach for AD.
4. Gut microbiota and aging
Human aging is an intrinsic physiological process with a gradually function decline in the organs, including intestine, brain, and gut microbiota. The normal intestinal barrier is comprised of tight junctions between epithelial cells, mucus, bicarbonate, and anti-microbial peptides secreted from Paneth cells [67].
An integrated and healthy gut wall is essential to protect the host from the attack of pathogenic bacteria. Disorders of the GI tract are prevalent amongst the elderly population. For example, chronic constipation is common in the elderly and reaches an incidence rate of 30–40% among those over 65 years of age [68]. The underlying mechanism is poorly understood, but impaired intestine mobility, intrinsic aging of the cells in the gut, and some extrinsic factors like gut microbiota, may influence the physiological function of the GI tract [69]. Age related loss of enteric neurons by about 38% was found in old man [70]. Animal studies also demonstrated neuronal loss and degenerative changes with age in the enteric nervous system (ENS), which might be associated with a age-related phenotypic shift of macrophages and altered neural response to inflammatory signals, resulting in increased apoptosis and loss of enteric neurons and neural stem cells [71]. Besides, intestinal epithelial stem cells (IESC), which are responsible for the renewal of the intestinal epithelium, have also been shown to experience age-related dysfunction in mice, such as hyper-proliferation and expansion, and increased expression of genes associated with cellular stress, DNA damage and apoptosis [72].
Gut microbiota transmission occurs during the peri-partum period from mothers to their infants, which could be affected by several perturbations, including birth by Cesarean section and the use of antibiotics during pregnancy [73], [74]. The composition and diversity of the infant microbiota is highly dynamic during the first year of life, and gets to resemble those of adult microbiota by around 3 years old [75]. Thereafter, the gut microbiota generally maintains stable. However, the increasing disappearances of microbiota and its diversity due to decreased vertical transmission from mother to child, decreased horizontal acquisition of commensal microbiota from other humans, and disrupted maintenance of key microbiota taxa in the early life by multiple insults like antibiotics exposure have exerted cumulative effects over generations, particularly the development of immunity in the gut [76], [77], [78], [79]. Gut microbiota has been suggested to be associated with the development and organization of ENS, and the formation of gut immune system, although little is known about how the balance between immune response and host health is maintained [76], [80]. In terms of aging, age-dependent, microbiome-modulated immunosenescence have been identified. The diversity and configuration of microbiota in the elderly can be affected by factors including residence location, diet, and health status, leading to the incidence of a wide variety of aging-related diseases [81], [82].
5. The blood brain barrier during aging
The blood brain barrier (BBB) is a highly selective barrier that acts to separate the circulating blood from the brain in the CNS. It is composed of the continuous capillary endothelium connected by the tight junctions, astrocytic end feet, and basal membrane. The physical function of BBB is selectively impermeable to the microscopic objects (e.g., bacteria), large or hydrophilic elements diffusing into the CSF [83]. However, BBB dysfunction and leakage, associated with tight junction impairment and pericytes loss, are common during aging [84], [85]. BBB needs much more mitochondrial volumes than tissues from non-BBB area to maintain its unique structure and the corresponding function [86]. However, this high mitochondrial content makes it vulnerable to accumulated oxidative stress and damage during aging, such as reactive oxygen species (ROS). The compromised BBB allows pathogens to get into the brain, leading to neuronal damages.
Cross-talks exist between the gut and brain, though the exact mechanisms have not been fully elucidated. The impact of gut microbiota on gut-brain axis is proposed to involve neural, immune, neuroendocrine, and metabolic systems [87]. The vagus nerve is the longest cranial nerve in the body and has afferent (sensory) and motor (efferent) nerves. Neurochemical and behavioral changes induced by bacteria exposure to the gut were not found in vagotomized mice, suggesting the vagus as a modulatory communication pathway between the gut and brain [88]. The spread of certain pathologies between the gut and brain have also been identified in animal studies via the vagus nerve [89]. Certain live bacteria may be beneficial to the establishment of BBB defense [90]. Germ-free mice showed significantly increased permeability of BBB, as well as lowered levels of endothelial tight junction proteins [91]. On the other hand, overresponse of the immune system due to gut dysbiosis can result in increased intestinal permeability, gut-vascular barrier (GVB) disruption, and systemic inflammation, which may further lead to the impairment of BBB integrity and neuroinflammation [92]. The existence of GVB was firstly identified by Spadoni et al, who demonstrated the disruption of GVB by pathogenic bacteria, leading to a systemic immune response [67]. Additionally, metabolic products produced by microbiota, such as short-chain fatty acids (SCFAs), can be sensed by vagus nerve. They can modulate the function of cholinergic neurons of the gut and neuronal activity in the brain after crossing the BBB, resulting in behavioral and cognitive changes.
Environmental and dietary influences, including chronic bacterial or viral infections can progressively alter BBB permeability and thereby facilitate cerebral colonization by opportunistic pathogens as we age. Given the existence of the gut wall, gut microbiota, immune system, and BBB dysfunction during aging, the microbiota-gut-brain axis may play an important role in age related neurodegenerative diseases such as AD.
6. The gut microbiota in Alzheimer’s disease
Early in 1989, Aβ protein deposits were detected in the intestine [93]. Amyloid-β protein precursor (AβPP) from which Aβ is derived, and total tau, are also expressed in the enteric neurons, making it plausible that AD pathophysiology could involve the ENS [94], [95]. However, this concept still needs further verification due to controversial reports which showed similar amount of Aβ and tau pathologies between AD patients and elderly controls [96], [97].
Animal studies have shown a direct effect of gut microbiota on AD pathologies. Intestinal inflammation induced by gut microbiota perturbation has been identified contributing to the pathogenesis and progression of AD. The local gut inflammation induced by infection significantly enhanced microglia activation and neuroinflammatory response in 3xTg-AD mice [26]. A lower level of pro-inflammatory cytokine IL-17 was found in gut-associated lymphoid tissue (GALT) cells of aged AD mice compared to their controls, suggesting that the surveillance to gut microbiota and immune barrier were impaired in AD [98]. Altered gut microbiota composition in the fecal samples from AD patients and AD mouse models have been reported [99], [100], [101]. The altered microbial composition could influence the levels of Aβ42, amyloid deposition, and pro-inflammatory cytokines in the brain [99]. A recent report revealed different genera abundance of fecal microbiota between AD patients and cognitively normal controls (increased in AD: Dorea, Lactobacillus, Streptococcus, Bifidobacterium, Blautia, and Escherichia; decreased in AD: Alistipes, Bacteroides, Parabacteroides, Sutterella, and Paraprevotella) [102]. A significantly negative relationship between amyloid burden and relative abundance of Lactobacillus in AD feces was observed [102]. In another study, cognitively impaired patients with brain amyloidosis showed lower abundance of the anti-inflammatory E. rectale and higher abundance of pro-inflammatory Escherichia/Shigella in their fecal samples compared to healthy controls or amyloid negative controls. Besides, amyloidosis-positive patients had increased serum levels of the pro-inflammatory cytokines, including IL-6, CXCL2, NLRP3 and IL-1β, and lower serum levels of anti-inflammatory cytokine IL-10 [103]. These findings support that there is an association between gut-microbiota-related inflammation and brain amyloidosis in AD.
Although great efforts have been made focusing on the role of gut-brain-axis in AD, the relationship between antibiotic treatment and the development of AD in humans has not been identified. Animal studies have demonstrated that antibiotic-induced perturbations in gut microbiota could influence neuroinflammation and amyloidosis in the brain. Antibiotic treatment over 6 months induced distinct alterations in microbial diversity in APP/PS1 mice, alongside alterations in peripheral inflammatory cytokines and chemokines, which coincided with attenuated Aβ plaque deposition and neuroinflammatory responses [104]. The same group also found that 1 week postnatal antibiotic treatment of APP/PS1 mice resulted in altered gut microbial diversity and reduced Aβ deposition at 6.5 months of age [105]. The underlying mechanism has not been elucidated. However, these findings indicate the close relationship between altered host innate immunity and amyloidosis in AD.
7. Microbiota-gut-brain axis and TLRs: potential implications for AD
It is well known that TLRs are expressed on numerous cell types in gut, including macrophages, dendritic cells (DCs), T lymphocytes, and intestinal epithelial cells (IECs). Intestinal epithelial cells are located on the front line of a microbial-rich environment, therefore, TLRs act as the essential mediators between microbiota and the host.
A broad spectrum of compounds are excreted by GI microbiota, including bacterial amyloids and LPS. The alterations of gut microbiota composition might induce perturbation of bacterial amyloids and LPS. Both of them can directly activate TLRs. Bacterial amyloids have been detected in both gram-negative and gram-positive bacteria, like Proteobacteria, Bacteriodetes, Chloroflexi, Actinobacteria, and Firmicutes [106], [107]. There are a variety of bacterial amyloids which contribute to numerously different functions [108]. It has been known that bacterial amyloids are involved in biofilms formation and host defense [109]. However, bacterial amyloids also function as toxins, triggering apoptosis in some human cell lines [110]. The existence of vast quantities of amyloids imply that human physiology may be potentially exposed to a tremendous systemic amyloid burden. It is remarkable that amyloids produced by human microorganisms are biologically similar to CNS amyloids, such as CsgA, Aβ42 [111]. When bacteria invade the intestinal mucosa, following interaction with a receptor complex of TLR1/TLR2, bacterial amyloids can initiate a robust release of inflammation cytokines, including IL-17 and IL-22 [112].
Higher bacterial LPS level was found in AD brains than that of the controls [113]. The mean LPS levels varied from 2 to 26 folds increases in brain samples from AD over age-matched controls, depending on the brain area and the severity of the disease [114]. Infusion of bacterial LPS into the fourth ventricle of rat brains reproduced AD-like pathological alterations and cognitive impairment, which did not recover with time [115]. Administration of LPS peripherally led to prolonged elevation of Aβ and cognitive deficits [116]. Besides, AD mice exhibited enhanced expression of microglial LPS receptor, CD14, the blockade of which reduced excessive microglial activation and toxicity [117]. Additionally, an in vitro study demonstrated that LPS could potentiate Aβ fibrillogenesis [118]. These results suggest that bacterial infection events are potential catalyst to promote the progression of AD. On the other hand, Aβ has also been reported to be an innate immune protein, which protects the brain from invading pathogens by entrapping and neuralizing them within the β-amyloid [119]. Aβ has been shown to exert antimicrobial activity in vitro [119]. The antimicrobial activity was significantly higher in brain homogenates from AD than in samples from age matched controls, which can be ablated with the treatment of anti-Aβ antibodies [119]. However, chronic sustained activation of this protective antimicrobial pathway leads to excessive Aβ deposition and tangle formation, and subsequently neurodegeneration and dementia [120].
Under physiological conditions, despite constant exposure to microbial-derived TLR ligands, IEC is in a state of hypo-responsiveness with low expression of TLRs. If the intestine is infected by pathogenic bacteria or when inflammatory bowel disease occurs, TLRs are upregulated in an inflammation-dependent way in IECs and macrophages [121], [122]. As a result, tremendous pro-inflammation cytokines and chemokines are released into the blood. Altered gut microbiota profile has been found associated with elevated levels of plasma LPS, inflammatory cytokines (IL-6, IL-8, IL-12, and TNF), and activated T-cells [123]. Gut infection could also enhance systemic pro-inflammatory response, characterized by the production of pro-inflammatory cytokines and chemokines such as TNF-α, IL-6, CCL5, and CXCL-1, which was associated with increased activation of microglia in 3xTg-AD mouse brain [124]. Once the pro-inflammatory cytokines are released by TLRs, they make their way to the brain by crossing the BBB via both diffusion and cytokine transporters, especially during aging when the GI epithelial barrier and BBB become significantly more restructured and permeable. In the brain, these cytokines act on receptors expressed by neurons and glial cells, particularly microglia, altering their activation status and physiology [125]. A recently published work by Wang et al. observed accumulation of Aβ in the brains of 5xFAD mice, which is accompanied by shifts in gut microbial population [126]. Besides, as activated M1 microglia increased in the brain, so did the number and pattern of peripheral pro-inflammatory T helper 1 (Th1) cells, indicating that gut dysbiosis alters peripheral inflammation, promoting the activation of microglia and amyloidosis, and eventually cognitive impairment. A drug named GV-971, remodeled the gut microbiota, could reduce Th1 cell proliferation in the blood and harness neuroinflammation and cognitive impairment, which has also been demonstrated effective in a phase 3 clinical trial [126].
8. The potential therapeutic targets
To date, there is no disease modifying therapy available for AD. Therefore, novel insights into AD pathologies are imperative to discover new therapeutic strategies. The existing interactions between TLRs and the gut-microbiota-brain axis in AD might provide opportunity for intervention. Emerging studies are focusing on regulating inflammatory response in the gut and brain to delay the progression of AD.
Administration of probiotics appears to be a novel and safe method to preserve a healthy intestinal microbiota and intestinal barrier, reducing the initiation of pro-inflammatory responses and propagation of neuroinflammation in neurodegeneration diseases [125]. Cell surface macromolecules in probiotics, such as peptidoglycan, cell wall teichoic, and lipoteichoic acid (LTA), exopolysaccharides, surface layer associated proteins (SLAPS), and fibronectin binding proteins are able to interact directly with the intestinal epithelium, mucus, and TLRs of the GI mucosa [127], [128], [129], [130], [131], [132]. It has been found that LPS-induced neuroinflammation and memory impairment could be attenuated by consumption of probiotics [133]. In animal experiments, treatment of probiotics, including Bifidobacterium and Lactobacillus, could ameliorate cognitive impairment, decrease the size and number of amyloid plaques, and reduce the immune response and neuroinflammation [134], [135]. Clinical trials also demonstrated that probiotics administration could significantly increase the mini-mental state examination score of the AD patients [136], [137]. Antibiotic treatment and fecal microbiota transplantation are potential options, but still need further investigation. As mentioned above, TLRs might be the possible therapeutic targets for AD. Although the role of TLR2 in AD brain is still controversial, studies have demonstrated the association of TLR2 signaling with the activation of microglia and the clearance of Aβ. Further investigations are needed to better characterize the TLR2 signaling, which would shed light on how to target TLR2 as a therapy for AD. Activation of TLR4 signaling have been found to promote microglia-mediated Aβ clearance. Besides, TLR4 activation could also be probably beneficial due to its autophagy effect. However, LPS-induced TLR4 signaling activation was dampened in AD mice during aging, suggesting TLR4 signaling might become tolerant to persist Aβ exposure in the brain [60]. Chronic and systemic administration of Monophosphoryl lipid A (MPL, a non-pyrogenic TLR4 agonist), through enhancing phagocytic capacity without inducing immune tolerance of innate immune cells, can attenuate the cerebral Aβ load [138]. What’s more, TLR9 could be another possible therapeutic target. Intraperitoneal injection of TLR9 agonist significantly reduced Aβ and tau pathologies, as well as levels of toxic oligomers in AD mouse models [64], [66], [139]. TLR9 stimulation also effectively ameliorated the cognitive deficits of these mice. These beneficial effects might result from increased phagocytic activity and upregulation of anti-inflammatory cytokines [66]. Given the complexity of the roles of TLRs in AD, a more profound understanding of the TLR signaling pathway and their association with AD pathologies are essential for the development of effective treatments.
9. Summary and outlook
In conclusion, we summarized the role of microbiota-gut-brain axis and TLRs in the pathogenesis of AD. It can be assumed that when gut dysbiosis occurs, microbial amyloids, LPS and other small compounds segregated can disrupt the gut wall and increase its permeability, which further undermine the BBB via blood or ENS pathway. TLRs can be activated by microbial amyloids or LPS in the gut, leading to the release of pro-inflammatory and/or anti-inflammatory cytokines, which results in the imbalance of the immune system, contributing to the progression of AD pathologies and cognitive decline. Attempts to restore the gut microbiota to a composition that found in healthy adults may slow down the progression of AD. However, interventions directly targeting TLRs still have a long way to go before more extensive studies carried out to elucidate the TLR signaling pathway and its impact on the immune system.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (81870826), Zhejiang Provincial Natural Science Foundation of China (LY18H090004), and Department of Health of Zhejiang Province (No. 2013KYB134).
References
- 1.Prince M., Bryce R., Albanese E., Wimo A., Ribeiro W. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimer's & Dementia. 2013;9(63–75) doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Jack C.R., Vemuri P., Wiste H.J., Weigand S.D., Aisen P.S. Evidence for ordering of Alzheimer disease biomarkers. Arch Neurol. 2011;68:1526–1535. doi: 10.1001/archneurol.2011.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jack C.R., Bennett D.A., Blennow K., Carrillo M.C., Feldman H.H. A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016;87:539–547. doi: 10.1212/WNL.0000000000002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilman S., Koller M., Black R.S., Jenkins L., Griffith S.G. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64:1553–1562. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- 5.Salloway S., Sperling R., Gilman S., Fox N.C., Blennow K. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology. 2009;73:2061–2070. doi: 10.1212/WNL.0b013e3181c67808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doody R.S., Thomas R.G., Farlow M., Iwatsubo T., Vellas B. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer's disease. N Engl J Med. 2014;370:311–321. doi: 10.1056/NEJMoa1312889. [DOI] [PubMed] [Google Scholar]
- 7.Ostrowitzki S., Lasser R.A., Dorflinger E., Scheltens P., Barkhof F. A phase III randomized trial of gantenerumab in prodromal Alzheimer's disease. Alzheimer's Res Therapy. 2017;9:95. doi: 10.1186/s13195-017-0318-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landen J.W., Andreasen N., Cronenberger C.L., Schwartz P.F., Börjesson-Hanson A. Ponezumab in mild-to-moderate Alzheimer's disease: randomized phase II PET-PIB study. Alzheimer's & Dementia (New York, N. Y.) 2017;3:393–401. doi: 10.1016/j.trci.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salloway S., Honigberg L.A., Cho W., Ward M., Friesenhahn M. Amyloid positron emission tomography and cerebrospinal fluid results from a crenezumab anti-amyloid-beta antibody double-blind, placebo-controlled, randomized phase II study in mild-to-moderate Alzheimer's disease (BLAZE) Alzheimer's Res Therapy. 2018;10:96. doi: 10.1186/s13195-018-0424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doody R.S., Raman R., Farlow M., Iwatsubo T., Vellas B. A phase 3 trial of semagacestat for treatment of Alzheimer's disease. N Engl J Med. 2013;369:341–350. doi: 10.1056/NEJMoa1210951. [DOI] [PubMed] [Google Scholar]
- 11.Hoskin J.L., Sabbagh M.N., Al-Hasan Y., Decourt B. Tau immunotherapies for Alzheimer’s disease. Expert Opin Invest Drugs. 2019;28:545–554. doi: 10.1080/13543784.2019.1619694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ballard C., Gauthier S., Corbett A., Brayne C., Aarsland D. Alzheimer's disease. The Lancet. 2011;377:1019–1031. doi: 10.1016/S0140-6736(10)61349-9. [DOI] [PubMed] [Google Scholar]
- 13.Barage S.H., Sonawane K.D. Amyloid cascade hypothesis: pathogenesis and therapeutic strategies in Alzheimer's disease. Neuropeptides. 2015;52:1–18. doi: 10.1016/j.npep.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Area-Gomez E., Schon E.A. On the pathogenesis of Alzheimer's disease: the MAM hypothesis. FASEB J. 2017;31:864–867. doi: 10.1096/fj.201601309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swerdlow R.H., Burns J.M., Khan S.M. The Alzheimer's disease mitochondrial cascade hypothesis: progress and perspectives. Biochim Biophys Acta. 2014;1842:1219–1231. doi: 10.1016/j.bbadis.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alzheimer's Association Calcium Hypothesis Workgroup Calcium Hypothesis of Alzheimer's disease and brain aging: a framework for integrating new evidence into a comprehensive theory of pathogenesis. Alzheimers Dement. 2017;13(178–182) doi: 10.1016/j.jalz.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Regen F., Hellmann-Regen J., Costantini E., Reale M. Neuroinflammation and Alzheimer's disease: implications for microglial activation. Curr Alzheimer Res. 2017;14:1140–1148. doi: 10.2174/1567205014666170203141717. [DOI] [PubMed] [Google Scholar]
- 18.Heneka M.T., Carson M.J., El Khoury J., Landreth G.E., Brosseron F. Neuroinflammation in Alzheimer's disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamer A.R. Systemic inflammation and disease progression in Alzheimer disease. Neurology. 2010;74:1157. doi: 10.1212/WNL.0b013e3181d5df7f. author reply 1157–1158. [DOI] [PubMed] [Google Scholar]
- 20.Vlad S.C., Miller D.R., Kowall N.W., Felson D.T. Protective effects of NSAIDs on the development of Alzheimer disease. Neurology. 2008;70:1672–1677. doi: 10.1212/01.wnl.0000311269.57716.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stewart W.F., Kawas C., Corrada M., Metter E.J. Risk of Alzheimer's disease and duration of NSAID use. Neurology. 1997;48:626–632. doi: 10.1212/wnl.48.3.626. [DOI] [PubMed] [Google Scholar]
- 22.Leoutsakos J.M., Muthen B.O., Breitner J.C., Lyketsos C.G. Effects of non-steroidal anti-inflammatory drug treatments on cognitive decline vary by phase of pre-clinical Alzheimer disease: findings from the randomized controlled Alzheimer's disease anti-inflammatory prevention trial. Int J Geriatr Psychiatry. 2012;27:364–374. doi: 10.1002/gps.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lambert J.C., Grenier-Boley B., Chouraki V., Heath S., Zelenika D. Implication of the immune system in Alzheimer's disease: evidence from genome-wide pathway analysis. J Alzheimers Dis. 2010;20:1107–1118. doi: 10.3233/JAD-2010-100018. [DOI] [PubMed] [Google Scholar]
- 24.Holmes C., Cunningham C., Zotova E., Woolford J., Dean C. Systemic inflammation and disease progression in Alzheimer disease. Neurology. 2009;73:768–774. doi: 10.1212/WNL.0b013e3181b6bb95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krstic D., Madhusudan A., Doehner J., Vogel P., Notter T. Systemic immune challenges trigger and drive Alzheimer-like neuropathology in mice. J Neuroinflammation. 2012;9:151. doi: 10.1186/1742-2094-9-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montacute R., Foley K., Forman R., Else K.J., Cruickshank S.M. Enhanced susceptibility of triple transgenic Alzheimer's disease (3xTg-AD) mice to acute infection. J Neuroinflammation. 2017;14:50. doi: 10.1186/s12974-017-0826-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collins S.M., Surette M., Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol. 2012;10:735. doi: 10.1038/nrmicro2876. [DOI] [PubMed] [Google Scholar]
- 28.Pasolli E., Asnicar F., Manara S., Zolfo M., Karcher N. Extensive unexplored human microbiome diversity revealed by Over 150,000 genomes from metagenomes spanning age, geography, and lifestyle. Cell. 2019;176 doi: 10.1016/j.cell.2019.01.001. 649–662.e620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pickard J.M., Zeng M.Y., Caruso R., Núñez G. Gut microbiota: role in pathogen colonization, immune responses, and inflammatory disease. Immunol Rev. 2017;279:70–89. doi: 10.1111/imr.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takiishi T., Fenero C.I.M., Câmara N.O.S. Intestinal barrier and gut microbiota: shaping our immune responses throughout life. Tissue Barriers. 2017;5 doi: 10.1080/21688370.2017.1373208. e1373208-e1373208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McIlroy J., Ianiro G., Mukhopadhya I., Hansen R., Hold G.L. Review article: the gut microbiome in inflammatory bowel disease—avenues for microbial management. Aliment Pharmacol Ther. 2018;47:26–42. doi: 10.1111/apt.14384. [DOI] [PubMed] [Google Scholar]
- 32.Bibbò S., Lopetuso L.R., Ianiro G., Di Rienzo T. Role of microbiota and innate immunity in recurrent Clostridium difficile infection. J Immunol Res. 2014;2014 doi: 10.1155/2014/462740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rakoff-Nahoum S., Paglino J., Eslami-Varzaneh F., Edberg S., Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 34.Moresco E.M., LaVine D., Beutler B. Toll-like receptors. Curr Biol. 2011;21:R488–493. doi: 10.1016/j.cub.2011.05.039. [DOI] [PubMed] [Google Scholar]
- 35.Akira S., Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 36.Kawai T., Akira S. TLR signaling. Semin Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 37.Fitzgerald K.A., Rowe D.C., Barnes B.J., Caffrey D.R., Visintin A. LPS-TLR4 signaling to IRF-3/7 and NF-kappaB involves the toll adapters TRAM and TRIF. J Exp Med. 2003;198:1043–1055. doi: 10.1084/jem.20031023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen G.Y., Brown N.K., Wu W., Khedri Z., Yu H. Broad and direct interaction between TLR and Siglec families of pattern recognition receptors and its regulation by Neu1. eLife. 2014;3 doi: 10.7554/eLife.04066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ishida A., Akita K., Mori Y., Tanida S., Toda M. Negative regulation of Toll-like receptor-4 signaling through the binding of glycosylphosphatidylinositol-anchored glycoprotein, CD14, with the sialic acid-binding lectin, CD33. J Biol Chem. 2014;289:25341–25350. doi: 10.1074/jbc.M113.523480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klesney-Tait J., Turnbull I.R., Colonna M. The TREM receptor family and signal integration. Nat Immunol. 2006;7:1266–1273. doi: 10.1038/ni1411. [DOI] [PubMed] [Google Scholar]
- 41.Bsibsi M., Ravid R., Gveric D., van Noort J.M. Broad expression of Toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol. 2002;61:1013–1021. doi: 10.1093/jnen/61.11.1013. [DOI] [PubMed] [Google Scholar]
- 42.Prehaud C., Megret F., Lafage M., Lafon M. Virus infection switches TLR-3-positive human neurons to become strong producers of beta interferon. J Virol. 2005;79:12893–12904. doi: 10.1128/JVI.79.20.12893-12904.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wadachi R., Hargreaves K.M. Trigeminal nociceptors express TLR-4 and CD14: a mechanism for pain due to infection. J Dent Res. 2006;85:49–53. doi: 10.1177/154405910608500108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McGeer P.L., Itagaki S., Tago H., McGeer E.G. Reactive microglia in patients with senile dementia of the Alzheimer type are positive for the histocompatibility glycoprotein HLA-DR. Neurosci Lett. 1987;79:195–200. doi: 10.1016/0304-3940(87)90696-3. [DOI] [PubMed] [Google Scholar]
- 45.Styren S.D., Civin W.H., Rogers J. Molecular, cellular, and pathologic characterization of HLA-DR immunoreactivity in normal elderly and Alzheimer's disease brain. Exp Neurol. 1990;110:93–104. doi: 10.1016/0014-4886(90)90054-v. [DOI] [PubMed] [Google Scholar]
- 46.Reed-Geaghan E.G., Savage J.C., Hise A.G., Landreth G.E. CD14 and toll-like receptors 2 and 4 are required for fibrillar A{beta}-stimulated microglial activation. J Neurosci. 2009;29:11982–11992. doi: 10.1523/JNEUROSCI.3158-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Letiembre M., Liu Y., Walter S., Hao W., Pfander T. Screening of innate immune receptors in neurodegenerative diseases: a similar pattern. Neurobiol Aging. 2009;30:759–768. doi: 10.1016/j.neurobiolaging.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 48.Walter S., Letiembre M., Liu Y., Heine H., Penke B. Role of the toll-like receptor 4 in neuroinflammation in Alzheimer’s disease. Cell Physiol Biochem. 2007;20:947–956. doi: 10.1159/000110455. [DOI] [PubMed] [Google Scholar]
- 49.Bilkei-Gorzo A. Genetic mouse models of brain ageing and Alzheimer's disease. Pharmacol Ther. 2014;142:244–257. doi: 10.1016/j.pharmthera.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 50.Jana M., Palencia C.A., Pahan K. Fibrillar amyloid-beta peptides activate microglia via TLR2: implications for Alzheimer's disease. J Immunol (Baltimore, Md.: 1950) 2008;(181):7254–7262. doi: 10.4049/jimmunol.181.10.7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin W., Ding M., Xue J., Leng W. The role of TLR2/JNK/NF-κB pathway in amyloid β peptide-induced inflammatory response in mouse NG108-15 neural cells. Int Immunopharmacol. 2013;17:880–884. doi: 10.1016/j.intimp.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 52.McDonald C.L., Hennessy E., Rubio-Araiz A., Keogh B., McCormack W. Inhibiting TLR2 activation attenuates amyloid accumulation and glial activation in a mouse model of Alzheimer's disease. Brain Behav Immun. 2016;58:191–200. doi: 10.1016/j.bbi.2016.07.143. [DOI] [PubMed] [Google Scholar]
- 53.Costello D.A., Carney D.G., Lynch M.A. alpha-TLR2 antibody attenuates the Abeta-mediated inflammatory response in microglia through enhanced expression of SIGIRR. Brain Behav Immun. 2015;46:70–79. doi: 10.1016/j.bbi.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 54.Liu S., Liu Y., Hao W., Wolf L., Kiliaan A.J. TLR2 is a primary receptor for Alzheimer's amyloid β peptide to trigger neuroinflammatory activation. Journal of immunology (Baltimore, Md.: 1950) 2012;(188):1098–1107. doi: 10.4049/jimmunol.1101121. [DOI] [PubMed] [Google Scholar]
- 55.Richard K.L., Filali M., Prefontaine P., Rivest S. Toll-like receptor 2 acts as a natural innate immune receptor to clear amyloid beta 1–42 and delay the cognitive decline in a mouse model of Alzheimer's disease. J Neurosci. 2008;28:5784–5793. doi: 10.1523/JNEUROSCI.1146-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen K., Iribarren P., Hu J., Chen J., Gong W. Activation of Toll-like receptor 2 on microglia promotes cell uptake of Alzheimer disease-associated amyloid beta peptide. J Biol Chem. 2006;281:3651–3659. doi: 10.1074/jbc.M508125200. [DOI] [PubMed] [Google Scholar]
- 57.Jin J.J., Kim H.D., Maxwell J.A., Li L., Fukuchi K. Toll-like receptor 4-dependent upregulation of cytokines in a transgenic mouse model of Alzheimer's disease. J Neuroinflammation. 2008;5:23. doi: 10.1186/1742-2094-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Song M., Jin J., Lim J.E., Kou J., Pattanayak A. TLR4 mutation reduces microglial activation, increases Abeta deposits and exacerbates cognitive deficits in a mouse model of Alzheimer's disease. J Neuroinflammation. 2011;8:92. doi: 10.1186/1742-2094-8-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qin Y., Liu Y., Hao W., Decker Y., Tomic I. Stimulation of TLR4 attenuates alzheimer's disease-related symptoms and pathology in tau-transgenic mice. J Immunol. 2016;197:3281–3292. doi: 10.4049/jimmunol.1600873. [DOI] [PubMed] [Google Scholar]
- 60.Go M., Kou J., Lim J.E., Yang J., Fukuchi K.I. Microglial response to LPS increases in wild-type mice during aging but diminishes in an Alzheimer's mouse model: implication of TLR4 signaling in disease progression. Biochem Biophys Res Commun. 2016;479:331–337. doi: 10.1016/j.bbrc.2016.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Y.L., Tan M.S., Yu J.T., Zhang W., Hu N. Toll-like receptor 9 promoter polymorphism is associated with decreased risk of Alzheimer's disease in Han Chinese. J Neuroinflammation. 2013;10:101. doi: 10.1186/1742-2094-10-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shintani Y., Drexler H.C.A., Kioka H., Terracciano C.M.N., Coppen S.R. Toll-like receptor 9 protects non-immune cells from stress by modulating mitochondrial ATP synthesis through the inhibition of SERCA2. EMBO Rep. 2014;15:438–445. doi: 10.1002/embr.201337945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Patel V., Patel A.M., McArdle J.J. Synaptic abnormalities of mice lacking toll-like receptor (TLR)-9. Neuroscience. 2016;324:1–10. doi: 10.1016/j.neuroscience.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 64.Scholtzova H., Chianchiano P., Pan J., Sun Y., Goni F. Amyloid beta and Tau Alzheimer's disease related pathology is reduced by Toll-like receptor 9 stimulation. Acta Neuropathol Commun. 2014;2:101. doi: 10.1186/s40478-014-0101-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scholtzova H., Kascsak R.J., Bates K.A., Boutajangout A., Kerr D.J. Induction of toll-like receptor 9 signaling as a method for ameliorating Alzheimer's disease-related pathology. J Neurosci. 2009;29:1846–1854. doi: 10.1523/JNEUROSCI.5715-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Scholtzova H., Do E., Dhakal S., Sun Y., Liu S. Innate immunity stimulation via toll-like receptor 9 ameliorates vascular amyloid pathology in Tg-SwDI mice with associated cognitive benefits. J Neurosci. 2017;37:936–959. doi: 10.1523/JNEUROSCI.1967-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Spadoni I., Zagato E., Bertocchi A., Paolinelli R., Hot E. A gut-vascular barrier controls the systemic dissemination of bacteria. Science. 2015;350:830–834. doi: 10.1126/science.aad0135. [DOI] [PubMed] [Google Scholar]
- 68.Stevens T.K., Soffer E.E., Palmer R.M. Fecal incontinence in elderly patients: common, treatable, yet often undiagnosed. Clevel Clin J Med. 2003;70:441–448. doi: 10.3949/ccjm.70.5.441. [DOI] [PubMed] [Google Scholar]
- 69.Saffrey M.J. Aging of the mammalian gastrointestinal tract: a complex organ system. Age (Dordr) 2014;36:9603. doi: 10.1007/s11357-013-9603-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.de Souza R.R., Moratelli H.B., Borges N., Liberti E.A. Age-induced nerve cell loss in the myenteric plexus of the small intestine in man. Gerontology. 1993;39:183–188. doi: 10.1159/000213532. [DOI] [PubMed] [Google Scholar]
- 71.Becker L., Nguyen L., Gill J., Kulkarni S., Pasricha P.J. Age-dependent shift in macrophage polarisation causes inflammation-mediated degeneration of enteric nervous system. Gut. 2018;67:827–836. doi: 10.1136/gutjnl-2016-312940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moorefield E.C., Andres S.F., Blue R.E., Van Landeghem L., Mah A.T. Aging effects on intestinal homeostasis associated with expansion and dysfunction of intestinal epithelial stem cells. Aging (Albany NY) 2017;9:1898–1915. doi: 10.18632/aging.101279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Blaser M.J., Dominguez-Bello M.G. The human microbiome before birth. Cell Host Microbe. 2016;20:558–560. doi: 10.1016/j.chom.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 74.Jakobsson H.E., Abrahamsson T.R., Jenmalm M.C., Harris K., Quince C. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section. Gut. 2014;63:559–566. doi: 10.1136/gutjnl-2012-303249. [DOI] [PubMed] [Google Scholar]
- 75.Yatsunenko T., Rey F.E., Manary M.J., Trehan I., Dominguez-Bello M.G. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Blaser M.J. The theory of disappearing microbiota and the epidemics of chronic diseases. Nat Rev Immunol. 2017;17:461. doi: 10.1038/nri.2017.77. [DOI] [PubMed] [Google Scholar]
- 77.Blaser M.J., Falkow S. What are the consequences of the disappearing human microbiota? Nat Rev Microbiol. 2009;7:887–894. doi: 10.1038/nrmicro2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bokulich N.A., Chung J., Battaglia T., Henderson N., Jay M. Antibiotics birth mode, and diet shape microbiome maturation during early life. Sci Transl Med. 2016;8 doi: 10.1126/scitranslmed.aad7121. 343ra382–343ra382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yassour M., Vatanen T., Siljander H., Hämäläinen A.-M., Härkönen T. Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci Transl Med. 2016;8 doi: 10.1126/scitranslmed.aad0917. 343ra381–343ra381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Obata Y., Pachnis V. The effect of microbiota and the immune system on the development and organization of the enteric nervous system. Gastroenterology. 2016;151:836–844. doi: 10.1053/j.gastro.2016.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Claesson M.J., Jeffery I.B., Conde S., Power S.E., O'Connor E.M. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178–184. doi: 10.1038/nature11319. [DOI] [PubMed] [Google Scholar]
- 82.Jeffery I.B., Lynch D.B., O'Toole P.W. Composition and temporal stability of the gut microbiota in older persons. ISME J. 2016;10:170–182. doi: 10.1038/ismej.2015.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Delaney C., Campbell M. The blood brain barrier: insights from development and ageing. Tissue Barriers. 2017;5 doi: 10.1080/21688370.2017.1373897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Goodall E.F., Wang C., Simpson J.E., Baker D.J., Drew D.R. Age-associated changes in the blood-brain barrier: comparative studies in human and mouse. Neuropathol Appl Neurobiol. 2018;44:328–340. doi: 10.1111/nan.12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bell R.D., Winkler E.A., Sagare A.P., Singh I., LaRue B. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Oldendorf W.H., Cornford M.E., Brown W.J. The large apparent work capability of the blood-brain barrier: a study of the mitochondrial content of capillary endothelial cells in brain and other tissues of the rat. Ann Neurol. 1977;1:409–417. doi: 10.1002/ana.410010502. [DOI] [PubMed] [Google Scholar]
- 87.Larroya-Garcia A., Navas-Carrillo D., Orenes-Pinero E. Impact of gut microbiota on neurological diseases: diet composition and novel treatments. Crit Rev Food Sci Nutr. 2018:1–15. doi: 10.1080/10408398.2018.1484340. [DOI] [PubMed] [Google Scholar]
- 88.Bravo J.A., Forsythe P., Chew M.V., Escaravage E., Savignac H.M. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Holmqvist S., Chutna O., Bousset L., Aldrin-Kirk P., Li W. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 2014;128:805–820. doi: 10.1007/s00401-014-1343-6. [DOI] [PubMed] [Google Scholar]
- 90.Leclercq S., Mian F.M., Stanisz A.M., Bindels L.B., Cambier E. Low-dose penicillin in early life induces long-term changes in murine gut microbiota, brain cytokines and behavior. Nat Commun. 2017;8:15062. doi: 10.1038/ncomms15062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Braniste V., Al-Asmakh M., Kowal C., Anuar F., Abbaspour A. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6:263ra158. doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Braniste V., Al-Asmakh M., Kowal C., Anuar F., Abbaspour A. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6 doi: 10.1126/scitranslmed.3009759. 263ra158–263ra158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Joachim C.L., Mori H., Selkoe D.J. Amyloid beta-protein deposition in tissues other than brain in Alzheimer's disease. Nature. 1989;341:226–230. doi: 10.1038/341226a0. [DOI] [PubMed] [Google Scholar]
- 94.Dugger B.N., Whiteside C.M., Maarouf C.L., Walker D.G., Beach T.G. The presence of select tau species in human peripheral tissues and their relation to Alzheimer's disease. J Alzheimers Dis. 2016;51:345–356. doi: 10.3233/JAD-150859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Arai H., Lee V.M.-Y., Messinger M.L., Greenberg B.D., Lowery D.E. Expression patterns of β-amyloid precursor protein (β-APP) in neural and nonneural human tissues from alzheimer's disease and control subjects. Ann Neurol. 1991;30:686–693. doi: 10.1002/ana.410300509. [DOI] [PubMed] [Google Scholar]
- 96.Shankle W.R., Landing B.H., Ang S.M., Chui H., Villarreal-Engelhardt G. Studies of the enteric nervous system in Alzheimer disease and other dementias of the elderly: enteric neurons in Alzheimer disease. Mod Pathol. 1993;6:10–14. [PubMed] [Google Scholar]
- 97.Puig K.L., Lutz B.M., Urquhart S.A., Rebel A.A., Zhou X. Overexpression of mutant amyloid-beta protein precursor and presenilin 1 modulates enteric nervous system. J Alzheimers Dis. 2015;44:1263–1278. doi: 10.3233/JAD-142259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Saksida T., Koprivica I., Vujičić M., Stošić-Grujičić S., Perović M. Impaired IL-17 production in gut-residing immune cells of 5xFAD mice with Alzheimer's disease pathology. J. Alzheimer's Dis.: JAD. 2018;61:619–630. doi: 10.3233/JAD-170538. [DOI] [PubMed] [Google Scholar]
- 99.Harach T., Marungruang N., Duthilleul N., Cheatham V., Mc Coy K.D. Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci Rep. 2017;7:41802. doi: 10.1038/srep41802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bauerl C., Collado M.C., Diaz Cuevas A., Vina J., Perez Martinez G. Shifts in gut microbiota composition in an APP/PSS1 transgenic mouse model of Alzheimer's disease during lifespan. Lett Appl Microbiol. 2018;66:464–471. doi: 10.1111/lam.12882. [DOI] [PubMed] [Google Scholar]
- 101.Zhuang Z.Q., Shen L.L., Li W.W., Fu X., Zeng F. Gut microbiota is altered in patients with Alzheimer's disease. J Alzheimers Dis. 2018;63:1337–1346. doi: 10.3233/JAD-180176. [DOI] [PubMed] [Google Scholar]
- 102.Li B., He Y., Ma J., Huang P., Du J. Mild cognitive impairment has similar alterations as Alzheimer's disease in gut microbiota. Alzheimer's & Dementia. 2019 doi: 10.1016/j.jalz.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 103.Cattaneo A., Cattane N., Galluzzi S., Provasi S., Lopizzo N. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol Aging. 2017;49:60–68. doi: 10.1016/j.neurobiolaging.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 104.Minter M.R., Zhang C., Leone V., Ringus D.L., Zhang X. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer's disease. Sci Rep. 2016;6:30028. doi: 10.1038/srep30028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Minter M.R., Hinterleitner R., Meisel M., Zhang C., Leone V. Antibiotic-induced perturbations in microbial diversity during post-natal development alters amyloid pathology in an aged APP(SWE)/PS1(ΔE9) murine model of Alzheimer's disease. Sci Rep. 2017;7 doi: 10.1038/s41598-017-11047-w. 10411–10411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chapman M.R., Robinson L.S., Pinkner J.S., Roth R., Heuser J. Role of Escherichia coli curli operons in directing amyloid fiber formation. Science. 2002;295:851–855. doi: 10.1126/science.1067484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jordal P.B., Dueholm M.S., Larsen P., Petersen S.V., Enghild J.J. Widespread abundance of functional bacterial amyloid in mycolata and other gram-positive bacteria. Appl Environ Microbiol. 2009;75:4101–4110. doi: 10.1128/AEM.02107-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schwartz K., Boles B.R. Microbial amyloids–functions and interactions within the host. Curr Opin Microbiol. 2013;16:93–99. doi: 10.1016/j.mib.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Larsen P., Nielsen J.L., Dueholm M.S., Wetzel R., Otzen D. Amyloid adhesins are abundant in natural biofilms. Environ Microbiol. 2007;9:3077–3090. doi: 10.1111/j.1462-2920.2007.01418.x. [DOI] [PubMed] [Google Scholar]
- 110.Hetz C., Bono M.R., Barros L.F., Lagos R. Microcin E492, a channel-forming bacteriocin from Klebsiella pneumoniae, induces apoptosis in some human cell lines. Proc Natl Acad Sci U S A. 2002;99:2696–2701. doi: 10.1073/pnas.052709699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Alkasir R., Li J., Li X., Jin M., Zhu B. Human gut microbiota: the links with dementia development. Protein Cell. 2017;8:90–102. doi: 10.1007/s13238-016-0338-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nishimori J.H., Newman T.N., Oppong G.O., Rapsinski G.J., Yen J.H. Microbial amyloids induce interleukin 17A (IL-17A) and IL-22 responses via Toll-like receptor 2 activation in the intestinal mucosa. Infect Immun. 2012;80:4398–4408. doi: 10.1128/IAI.00911-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhan X., Stamova B., Jin L.W., DeCarli C., Phinney B. Gram-negative bacterial molecules associate with Alzheimer disease pathology. Neurology. 2016;87:2324–2332. doi: 10.1212/WNL.0000000000003391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhao Y., Jaber V., Lukiw W.J. Secretory products of the human GI tract microbiome and their potential impact on Alzheimer's disease (AD): detection of lipopolysaccharide (LPS) in AD hippocampus. Front Cell Infect Microbiol. 2017;7:318. doi: 10.3389/fcimb.2017.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hauss-Wegrzyniak B., Vraniak P.D., Wenk G.L. LPS-induced neuroinflammatory effects do not recover with time. NeuroReport. 2000;11:1759–1763. doi: 10.1097/00001756-200006050-00032. [DOI] [PubMed] [Google Scholar]
- 116.Kahn M.S., Kranjac D., Alonzo C.A., Haase J.H., Cedillos R.O. Prolonged elevation in hippocampal Abeta and cognitive deficits following repeated endotoxin exposure in the mouse. Behav Brain Res. 2012;229:176–184. doi: 10.1016/j.bbr.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 117.Fassbender K., Walter S., Kuhl S., Landmann R., Ishii K. The LPS receptor (CD14) links innate immunity with Alzheimer's disease. FASEB J. 2004;18:203–205. doi: 10.1096/fj.03-0364fje. [DOI] [PubMed] [Google Scholar]
- 118.Asti A., Gioglio L. Can a bacterial endotoxin be a key factor in the kinetics of amyloid fibril formation? J Alzheimers Dis. 2014;39:169–179. doi: 10.3233/JAD-131394. [DOI] [PubMed] [Google Scholar]
- 119.Eimer W.A., Vijaya Kumar D.K., Navalpur Shanmugam N.K., Rodriguez A.S., Mitchell T. Alzheimer's disease-associated beta-amyloid is rapidly seeded by herpesviridae to protect against brain infection. Neuron. 2018;99(56–63) doi: 10.1016/j.neuron.2018.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Moir R.D., Lathe R., Tanzi R.E. The antimicrobial protection hypothesis of Alzheimer's disease. Alzheimers Dement. 2018;14:1602–1614. doi: 10.1016/j.jalz.2018.06.3040. [DOI] [PubMed] [Google Scholar]
- 121.Hausmann M., Kiessling S., Mestermann S., Webb G., Spöttl T. Toll-like receptors 2 and 4 are up-regulated during intestinal inflammation. Gastroenterology. 2002;122:1987–2000. doi: 10.1053/gast.2002.33662. [DOI] [PubMed] [Google Scholar]
- 122.Cario E., Podolsky D.K. Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect Immun. 2000;68:7010–7017. doi: 10.1128/iai.68.12.7010-7017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jorgensen S.F., Troseid M., Kummen M., Anmarkrud J.A., Michelsen A.E. Altered gut microbiota profile in common variable immunodeficiency associates with levels of lipopolysaccharide and markers of systemic immune activation. Mucosal Immunol. 2016;9:1455–1465. doi: 10.1038/mi.2016.18. [DOI] [PubMed] [Google Scholar]
- 124.Montacute R., Foley K., Forman R., Else K.J., Cruickshank S.M. Enhanced susceptibility of triple transgenic Alzheimer’s disease (3xTg-AD) mice to acute infection. J Neuroinflammation. 2017;14:50. doi: 10.1186/s12974-017-0826-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mancuso C., Santangelo R. Alzheimer's disease and gut microbiota modifications: the long way between preclinical studies and clinical evidence. Pharmacol Res. 2018;129:329–336. doi: 10.1016/j.phrs.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 126.Wang X., Sun G., Feng T., Zhang J., Huang X. Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer’s disease progression. Cell Res. 2019 doi: 10.1038/s41422-019-0216-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kaji R., Kiyoshima-Shibata J., Nagaoka M., Nanno M., Shida K. Bacterial teichoic acids reverse predominant IL-12 production induced by certain <em>Lactobacillus</em> strains into predominant IL-10 production via TLR2-dependent ERK activation in macrophages. J Immunol. 2010;184:3505–3513. doi: 10.4049/jimmunol.0901569. [DOI] [PubMed] [Google Scholar]
- 128.Ryan A., Lynch M., Smith S.M., Amu S., Nel H.J. A role for TLR4 in Clostridium difficile infection and the recognition of surface layer proteins. PLoS Pathog. 2011;7 doi: 10.1371/journal.ppat.1002076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Noh S.Y., Kang S.S., Yun C.H., Han S.H. Lipoteichoic acid from Lactobacillus plantarum inhibits Pam2CSK4-induced IL-8 production in human intestinal epithelial cells. Mol Immunol. 2015;64:183–189. doi: 10.1016/j.molimm.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 130.Wu Z., Pan D., Guo Y., Sun Y., Zeng X. Peptidoglycan diversity and anti-inflammatory capacity in Lactobacillus strains. Carbohydr Polym. 2015;128:130–137. doi: 10.1016/j.carbpol.2015.04.026. [DOI] [PubMed] [Google Scholar]
- 131.Jones S.E., Paynich M.L., Kearns D.B., Knight K.L. Protection from intestinal inflammation by bacterial exopolysaccharides. J Immunol. 2014;192:4813–4820. doi: 10.4049/jimmunol.1303369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bisht S., Singh K.S., Choudhary R., Kumar S., Grover S. Expression of fibronectin-binding protein of L. acidophilus NCFM and in vitro refolding to adhesion capable native-like protein from inclusion bodies. Protein Expr Purif. 2018;145:7–13. doi: 10.1016/j.pep.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 133.Musa N.H., Mani V., Lim S.M., Vidyadaran S., Abdul Majeed A.B. Lactobacilli-fermented cow's milk attenuated lipopolysaccharide-induced neuroinflammation and memory impairment in vitro and in vivo. J Dairy Res. 2017;84:488–495. doi: 10.1017/S0022029917000620. [DOI] [PubMed] [Google Scholar]
- 134.Nimgampalle M., Kuna Y. Anti-Alzheimer properties of probiotic, MTCC 1325 in Alzheimer's disease induced albino rats. J Clin Diagn Res: JCDR. 2017;11:KC01-KC05. doi: 10.7860/JCDR/2017/26106.10428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kobayashi Y., Sugahara H., Shimada K., Mitsuyama EKuhara T. Therapeutic potential of Bifidobacterium breve strain A1 for preventing cognitive impairment in Alzheimer's disease. Sci Rep. 2017;7:13510. doi: 10.1038/s41598-017-13368-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Akbari E., Asemi Z., Daneshvar Kakhaki R., Bahmani F., Kouchaki E. Effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer's disease: a randomized, double-blind and controlled trial. Front Aging Neurosci. 2016;8 doi: 10.3389/fnagi.2016.00256. 256–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tamtaji O.R., Heidari-soureshjani R., Mirhosseini N., Kouchaki E., Bahmani F. Probiotic and selenium co-supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer's disease: a randomized, double-blind, controlled trial. Clin Nutr. 2018 doi: 10.1016/j.clnu.2018.11.034. [DOI] [PubMed] [Google Scholar]
- 138.Michaud J.P., Halle M., Lampron A., Theriault P., Prefontaine P. Toll-like receptor 4 stimulation with the detoxified ligand monophosphoryl lipid A improves Alzheimer's disease-related pathology. Proc Natl Acad Sci U S A. 2013;110:1941–1946. doi: 10.1073/pnas.1215165110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sharma P., Li L., Liu H., Tikiyani V., Hu Z. The claudin-like protein HPO-30 is required to maintain LAChRs at the C. elegans neuromuscular junction. J Neurosci. 2018;38:7072–7087. doi: 10.1523/JNEUROSCI.3487-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]