Abstract
The research into vascular contributions to cognitive impairment and dementia (VCID) aims to understand the importance of cerebrovascular biology in cognitive decline. Prevention and treatment of VCID is poised to have major impact on dementia-related disease burden and is thus a critical emerging objective in dementia research. This article presents VCID consortia focused on multidisciplinary approaches to identify key pathologic targets and develop diagnostic tools with the goal of bridging the divide between basic research and clinical trials. Members of these multi-institute, multidisciplinary consortia provide a prospective on the history and emerging science of VCID and how VCID consortia can address some of the more complex questions in VCID and drive the field forward. These consortia, and others like them, are uniquely suited to tackle some of the most difficult obstacles in translating research to the clinic.
1. Introduction
The science of vascular contributions to cognitive impairment and dementia (VCID) seeks to understand the aging neurovascular system when it fails to cope with biological insults due to vascular disease, proteinopathy, metabolic disease, and immune affront, resulting in cognitive decline. VCID takes many forms and occurs in the context of numerous clinical diagnoses and comorbidities [[1], [2], [3]]. In some of these conditions, the role of vascular disease is evident, such as in dementia after stroke, whereas in others, it is more covert as in insidious vascular damage co-occurring with pathological Alzheimer's disease (AD). Importantly, it has become clear that vascular disease is present in the majority of patients with dementia. It contributes to the dementia phenotype to a large degree in some individuals and to a smaller but still significant degree in others [4]. The impact of VCID is substantial. Because of the frequent co-occurrence of other etiologies, it is not straightforward to assess the exact population impact of VCID with existing data. Nonetheless, it likely affects many millions of people worldwide who are diagnosed with one of the vascular dementias, the clinical syndrome of AD, or another diagnosis. Furthermore, based on our understanding of cardiovascular and cerebrovascular risk and observations of dementia incidence, it is likely that there is disproportionately high VCID burden among certain health disparity populations [5,6]. Prevention and treatment of VCID is poised to have a major impact and is thus a critical emerging objective in dementia research [[7], [8], [9]].
VCID research integrates diverse fields of biology and spans basic neuroscience, translational, and clinical research. The extensive scope and overlapping diagnostic and disciplinary boundaries of VCID has historically resulted in relevant research being fragmented and siloed, for example, among different forms of VCID and from other dementia etiologies, and as a result, VCID has not until recently been widely recognized as a potentially critical contributor to the public burden of cognitive impairment and dementia. Traditionally used concepts of vascular or multi-infarct dementia have only captured a relatively narrow subset of VCID with multiple clinical strokes. Another consideration that traditionally diminished the VCID research footprint, relative to the actual disease burden, is that VCID is infrequently called out diagnostically even though it is present in the majority of cases, in particular when the affected person is older than 80 years, which accounts for the vast majority of dementia.
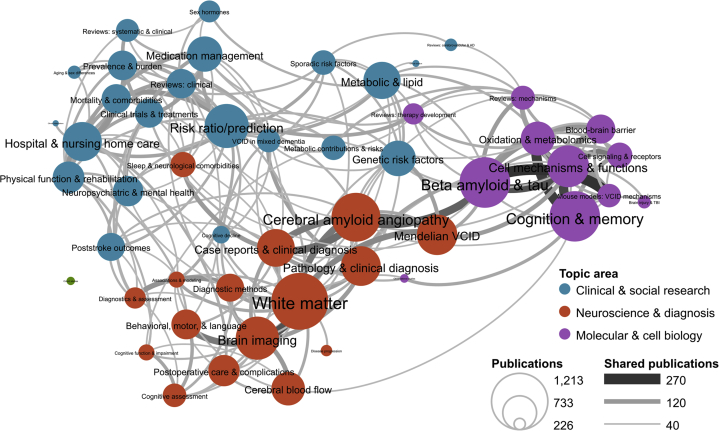
A bibliometric landscape analysis of VCID was completed to identify trends in the production and research topics of these publications using data from PubMed articles from 2002 to 2018 (Fig. 1). Bibliometric methods are quantitative methods of studying scientific research using publications as a proxy for research. Although bibliometric analysis has limitations, such as inability to interpret impact or assess scientific rigor, this technique represents one method to quantitatively evaluate the VCID field. This analysis highlights the multidisciplinary scientific nature of the field, but also, underscores the vast and, at times, separated nature of the research. However, there have been efforts to enhance the field of VCID research by harmonizing terminology, methodology and coming together to form multidisciplinary research teams and consortia [10,11]. These groups are well-positioned to address some of the more complex questions in VCID.
Fig. 1.
VCID bibliometric topic map. Research topics are based on the results of the Latent Dirichlet Analysis (LDA) algorithm. LDA works by first creating a set of term vocabularies for each of a prespecified number of topics based on the terms’ co-occurrence in publication abstracts. The algorithm then uses those vocabularies to assign individual publications to one or more of these topics based on the frequency with which terms from that topic appear in the publication's abstract. Publications were pulled from PubMed using the following search string: (((dementia OR (cognitive impairment) OR (cognitive dysfunction)) AND (vascular OR cardiovascular OR cerebrovascular OR lacunar OR stroke)) OR CADASIL OR (cerebral autosomal dominant arteriopathy) OR (Binswanger[tiab] OR Binswanger's[tiab]) OR (cerebral amyloid angiopathy)) AND 2002:2016[dp]. For this analysis, the number of topics was set to 50, the algorithm was run on the publication abstracts in this data set. A topic similarity network was generated in which topics are connected if more than 45 articles in the data set were assigned to both of the connected topics. Descriptions were assigned to each topic based on the abstract and MeSH terms that most frequently appeared in each topic and on manual inspection of the papers assigned to each topic.
1.1. Perspective on the history and emerging science of VCID
The development of highly specific and sensitive diagnostic criteria is a rigorous process that requires both strong scientific evidence and adoption by the scientific and medical communities. At the same time, applied VCID research depends on appropriate diagnostic criteria and biologically valid, robust, sensitive, and specific markers of disease processes. This must be balanced with the priority of ensuring a diagnosis that will facilitate the best care of patients possible while maintaining scientific interoperability that ensures utility for clinicians and researchers. Achieving this has been a challenge.
Initial diagnostic constructs strove to come up with definitions that would capture vascular disease as the sole cause of cognitive impairment [12]. In accordance with the present insights, this approach has important drawbacks, the most prominent of which is that in the majority of patients with cognitive impairment, vascular disease co-occurs with other etiologies [4,13]. Excluding the other etiologies narrows the field and ignores combined and synergistic effects. Moreover, from a clinical perspective, it is questionable if co-occurring etiologies can be reliably identified based on clinical phenotypes, and if co-occurring etiologies can be identified, is it scientifically or clinically appropriate to exclude them? Over the past decades, we have seen evolution of diagnostic constructs for VCID from vascular as the sole cause (VaD criteria) to encompassing shared etiologies [11,14]. Studies now show that a substantial proportion of patients who are assumed to have Alzheimer's pathology, including early onset inherited forms, additionally have evidence of vascular pathology.
Like for dementia and cancer, a second challenge in diagnostic constructs for VCID is the fact that “vascular” is an umbrella term and as such cannot be considered as a specific etiology that guides treatment. For example, mechanisms, diagnosis, and treatment of VCID in a patient with lasting cognitive deficits after an acute subarachnoid hemorrhage are evidently completely different from those in a patient with progressive cognitive deficits due to accumulating white matter hyperintensities due to cerebral small vessel disease, although both clinical phenotypes are due to VCID and may have shared mechanistic pathways. To date, the challenge has been to come up with diagnostic constructs that are broad enough to capture the full spectrum of VCID, yet also specific enough to classify targetable disease processes within the VCID spectrum.
To address this challenge, biomarker frameworks of disease classification are being developed in a number of fields, including pathological AD and VCID [[15], [16], [17]]. There is strong evidence that AD pathology often coexists with cerebrovascular disease with many risk factors being shared, resulting in additive or synergistic effects on cognitive decline [4]. Thus, biomarkers that inform VCID pathology and etiology during life can have a major impact across a number of diseases. Possibly, the ways forward are approaches similar to those currently seen in the AD field where the “clinical syndrome” and the pathology, although clearly interrelated, are defined separately [15].
In this light, biomarker development has been a key area of interest in VCID research. The use of biomarkers in basic and clinical research as well as in clinical practice has become a commonplace occurrence. Biomarkers are needed in all areas of research, including clinical research, clinical trials, and care settings, and a biomarker validated for one setting in one population many not be universally applicable. In many cases, however, the validity of biomarkers still needs to be evaluated and, at times, reevaluated as our understanding of the diseases and the uses of the biomarker are considered [18]. In VCID, biomarkers are often focused on imaging (e.g., brain or other tissue such as the retina) and fluid-based (e.g., blood, plasma, serum, urine, and cerebral spinal fluid). The characteristics they capture and measure, such as downstream tissue injury or actual disease processes, determine their clinical and scientific utility. After biomarkers have been well validated, they can be appropriately used by the research and medical communities. The advancement of the science of VCID toward therapeutic treatment is also enhanced by consortia that are more focused on foundational discoveries on the mechanistic underpinnings of VCID and on working to find ways to correct the biological imbalance.
New medicines are identified through a process that involves a wide range of scientific disciplines, including several basic science disciplines (e.g., but not limited to: vascular biology, metabolism, immunology, biochemistry, chemistry, and pharmacology), as well as a diverse spectrum of clinical sciences ranging from clinical stroke through clinical cognitive impairment and dementia, and related relevant disciplines. VCID consortia around the world are working to identify targets and are moving them out of the preclinical discovery phase and through the drug discovery process.
Research consortia offer the unique ability to move a basic mechanism and clinical finding forward by addressing barriers, such as the traditional silos between basic researcher and clinical researcher, and providing a diverse set of scientific perspectives to a single problem. A single consortium can work with molecular information gained from the basic science and use clinical samples to study diagnosis, expression of disease biomarkers, differences between normal and disease states, and response to therapy. The multidisciplinary nature of the group is more likely to allow for easy application of what is learned to move a basic mechanistic observation to a clinical discovery.
1.2. Consortia
VCID consortia discussed here are focused on multidisciplinary approaches to identify key pathologic targets and develop diagnostic or prognostic tools with the goal of bridging the divide between basic research and clinical trials. The criteria used to identify these consortia are as follows: (1) pursuing the science of VCID; (2) multi-institute, multidisciplinary teams; (3) identifying treatable etiological targets; and/or developing biomarkers for use in clinical trials (e.g., diagnosis, patient stratification, disease progression, target engagement, and prognosis). The primary purpose of this review is to bring together information about these consortia, summarized in Table 1, as a resource that will help both inform and further facilitate the science of VCID, with a particular focus on etiology and treatable targets with the potential to decrease disease burden.
Table 1.
Detailed Information about VCID consortia
| Name | Date founded | Location(s) | Current leadership | Funding | Website |
|---|---|---|---|---|---|
| Understanding the role of the perivascular space in cerebral small-vessel disease | 2017 | United Kingdom, Denmark, France, Germany, North America: United States of America, Canada | European Coordinator: Joanna M Wardlaw, North American Coordinator: Berislav Zlokovic | Fondation Leducq Transatlantic Network of Excellence | https://www.fondationleducq.org/network/understanding-the-role-of-the-perivascular-space-in-cerebral-small-vessel-disease/ |
| Stroke and Cognition Consortium (STROKOG) | 2015 | Europe: United Kingdom, Poland, the Netherlands, Scotland, Ireland, France, Germany, Finland, Sweden, Bulgaria, Asia: Korea, Singapore, China, Hong Kong, North America: United States of America, Africa: South Africa, Nigeria, Australia: Australia | Consortium Leader: Professor Perminder Sachdev | National Health & Medical Research Council (NHMRC) (Australia), The Dementia Momentum fund from the Center of Healthy Brain Aging (CHeBA), UNSW Medicine, University of New South Wales, and the Vincent Fairfax Family Foundation. | https://cheba.unsw.edu.au/group/strokog |
| Biomarkers for Vascular Contributions to Cognitive Impairment and Dementia Consortium (MarkVCID) | 2016 | North America: United States of America | Coordinating Center: Dr. Steven M. Greenberg Sites: Dr. Hanzhang Lu, Dr. Danny J. J. Wang, Dr. Sudha Seshadri, Dr. Gary A. Rosenberg, Dr. Julie A. Schneider, Dr. Joel H. Kramer, Dr. Donna M. Wilcock |
NINDS/NIA/NIH | https://markvcid.partners.org/ |
| SVDs@target | 2016 | Europe: Germany, the United Kingdom, the Netherlands | Leader consortium: Prof. Martin Dichgans, Leader clinical study Zoom@SVDs: Prof. Geert Jan Biessels, Leader clinical study INVESTIGATE-SVDs: Prof. Joanna Wardlaw, Leader clinical trial TREAT-SVDs: Prof. Martin Dichgans | European Union's Horizon 2020 research and innovation programme under grant agreement No. 666881 | https://www.svds-at-target.eu/ |
| The Heart-Brain Connection: the missing link in the pathophysiology of vascular cognitive impairment (HBC) | 2013 | Europe: Netherlands | Current program leaders: Prof. Dr. M. J. Daemen, Prof. Dr. G. J. Biessels, Past program leader: Prof. Dr. M. A. van Buchem | Dutch Heart Foundation | http://www.heart-brain.nl/ |
| International Stroke Genetics Consortium (ISGC) | 2007 | Members come from all continents and more than 30 countries | Chair: Dr. Stephanie Debette, Vice Chair: Dr. Jin-Moo Lee, Immediate Past Chair: Dr. Daniel Woo Founded: Dr. Jonathan Rosand | Registration fees for workshops, private donations (for travel scholarships, prizes, workshop proceedings, website etc.), and project-specific grants | http://www.strokegenetics.org/ |
Stroke affects people differently, with recovery from stroke involving changes that can be debilitating and vary from person to person in the physical, social, cognitive, and emotional aspects of life. For this reason, poststroke VCID is an active area of study. The Stroke and Cognition Consortium (STROKOG), developed under the auspices of Society for the Study of Vascular Cognitive and Behavioral Disorders (VASCOG), brings together international longitudinal cohort studies of cognitive decline and dementia after stroke or TIA. The goals of the consortium are to facilitate a better understanding of the determinants and manifestations of vascular contributions to cognitive disorders and to improve the diagnosis and treatment of VCID [19]. An additional key objective is to harmonize shared, nonidentifiable data from member studies and perform individual participant data meta-analyses. Studies in the consortium have examined poststroke or other high vascular risk cohorts longitudinally, with cognitive decline, including dementia, as primary outcome variables. As of April 2019, the consortium has 32 member studies, and in total, this includes approximately 18,000 enrolled individuals from 18 countries.
Another consortium focused on stroke and cognition, the International Stroke Genetics Consortium (ISGC), was launched in April 2007 at the Annual Meeting of the American Academy of Neurology. The initial aim was to bring together resources and expertise to advance research on stroke genomics. This has evolved toward broader goals of advancing research on the biological mechanisms underlying stroke risk, stroke severity, and consequences of stroke, such as cognitive and functional outcomes. The consortium has diversified its activities, broadening the scope to encompass not only genomics but also other ‘omics’ approaches, as well as translational studies. Extensive research is also conducted to better understand the genetic underpinnings of imaging-based stroke endophenotypes, especially MRI markers of cerebral small vessel disease, and the impact of these on VCID. Participating studies are both clinic-based and population-based (in collaboration with the Cohorts for Heart and Aging Research in Genomic Epidemiology [CHARGE] consortium), including large biobanks [[20], [21], [22]].
The MarkVCID consortium has a translational focus by aiming to deliver discrete high-quality biomarker kits ready for use in clinical trials by developing and validating biomarkers for small vessel VCID [23]. Diseases of the brain's network of small blood vessels, most prominently arteriolosclerosis and cerebral amyloid angiopathy, have also been shown to be major contributors to cognitive impairment and dementia. These small-vessel disease pathologies often overlap with other conditions such as clinical and pathological AD. The mission of the consortium is to analyze and optimize candidate VCID biomarkers and participate in a consortium-wide program of biomarker scaling-up, multisite protocol implementation, and multisite validation with discrete biomarker kits emerging at the end of the process that can be moved to large scale validation and handed off to clinical trials in the future [24].
A novel trans-Atlantic VCID consortium funded by the Fondation Leducq is called Understanding the Role of the Perivascular Space in Cerebral Small Vessel Disease. This consortium is using parallel studies in humans who had stroke stroke or cognitive presentations of small vessel diseases, and a corresponding range of representative laboratory models, with harmonized protocols for human and rodent MRI to pursue the hypothesis that small-vessel VCID–associated brain injury is related to structural changes in and dysfunction of the perivascular space and that a better understanding of the perivascular space and small vessel VCID interactions, including pathology, will result in the identification of novel therapeutic targets [25].
SVDs@target brings together basic scientists and clinicians to make use of novel animal models, technologies, and expertly phenotyped patient cohorts. The consortium's aim is to identify key mechanisms common to multiple small-vessel diseases that are relevant to VCID and to validate these novel mechanisms through manipulations in model systems and interventions in human subjects, with the ultimate goal of reducing the burden of VCID. A key feature of this program is that it combines and leverages both preclinical and clinical research toward its applied goals in VCID.
Understanding the relationship among diseases of the heart, blood vessels, and brain is an emerging scientific area that is highly relevant to VCID. The Heart-Brain Connection is a Dutch interdisciplinary network with origins that lie in studies of hemodynamic changes in VCID that is in part motivated by the AHA/ASA and the National Plan to Address Alzheimer's Disease research priorities. With the goal of identifying new strategies for prevention and treatment of VCID, this consortium pursues comprehensive animal research and diagnostic hospital- and population-based studies to increase the understanding of the mechanisms involved in linking cardiovascular and cognitive dysfunction. This consortium will also develop new tools that, combined with new pathophysiological insights, will lead to innovative and personalized therapeutic options to improve or stabilize declining cognition.
2. Conclusion
The NIH officially recognized the science of VCID by starting to track spending on VCID research in NIH Reporter, aligning the acronym with “vascular cognitive impairment/dementia.” Investment in VCID research has risen from $45M when tracking began in 2014 to $259M in 2018 [26]. Funding of VCID research has also increased internationally because investigators, the public, and funding agencies increasingly recognize its public health impact and synergy at the intersection of stroke and dementia, two of the most pressing health issues around the world [27].
Although the multidisciplinary research of VCID science continues to expand our understanding of cellular and molecular mechanisms, the scope and complexity of VCID pathologies and clinical syndromes continue to present large challenges to translating basic science findings into the clinic. Here, we have described how six consortia are working to address this bottleneck and to improve our ability to diagnose and treat VCID. Many of these efforts include large cohorts of human subjects that also act as resources for other researchers who want to address the science of VCID. The NIH, led by the NINDS, NHLBI, and NIA, has identified such VCID cohorts (https://www.ninds.nih.gov/Current-Research/Focus-Disorders/VCID-Cohorts-Tool), although this represents only a partial list of all of the human-based VCID efforts around the world. The multinational Joint Programme for Neurodegenerative Diseases have funded efforts to catalog (https://www.neurodegenerationresearch.eu/jpnd-global-cohort-portal/) completed and ongoing population and cohort observational studies and clinical trial data sets with data relevant to vascular cognitive impairment that are available for secondary analyses and meta-analyses and also created a searchable portal hosting studies relevant to neurodegenerative disease research including VCID [28]. In addition to the consortia and cohort efforts mentioned in this study, there are many other international collaborative activities aimed at understanding, diagnosing, or ameliorating the effects of VCID, including the Joint Programme for Neurodegenerative Diseases–funded HARNESS initiative (https://harness-neuroimaging.org/) to disseminate standardized image acquisition and analysis tools to advance research in the field [10]. Furthermore, there is ample scientific space and need for new VCID consortia and cohorts to move the community forward. By increasing multidisciplinary collaboration, including researchers with diverse scientific backgrounds and perspectives, the biomedical community can begin to address the significant public-health burden associated with this heterogeneous group of conditions.
Research in context.
-
1.
Systematic review: The Criteria used to identify these consortia are as follows: (1) pursuing the science of VCID; (2) multi-institute, multidisciplinary teams; (3) identifying treatable etiological targets; and/or developing biomarkers for use in clinical trials. Once identified the consortia leads were invited to provide a description of their efforts.
-
2.
Interpretation: The review brings together information about multiple consortia as a resource that will help both inform and further facilitate the science of VCID, with a particular focus on etiology and treatable targets with the potential to decrease disease burden.
-
3.
Future directions: The consortia discussed in this article, and others like them, are uniquely suited to tackle some of the most difficult obstacles in translating research to the clinic. Over the next years, as the research efforts of these consortia continue, we will see advances in identifying key pathologic targets and developing diagnostic or prognostic tools.
Acknowledgments
The authors thank all the consortium members and staff who contributed to the efforts mentioned in this manuscript and assisted in providing background material.
References
- 1.Snyder H.M., Corriveau R.A., Craft S., Faber J.E., Greenberg S.M., Knopman D. Vascular contributions to cognitive impairment and dementia including Alzheimer's disease. Alzheimer's Dement. 2015;11:710–717. doi: 10.1016/j.jalz.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corriveau R.A., Bosetti F., Emr M., Gladman J.T., Koenig J.I., Moy C.S. The Science of Vascular Contributions to Cognitive Impairment and Dementia (VCID): a framework for advancing research priorities in the cerebrovascular biology of cognitive decline. Cell Mol Neurobiol. 2016;36:281–288. doi: 10.1007/s10571-016-0334-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Flier W.M., Skoog I., Schneider J.A., Pantoni L., Mok V., Chen C.L.H. Vascular cognitive impairment. Nat Rev Dis Primers. 2018;4:18003. doi: 10.1038/nrdp.2018.3. [DOI] [PubMed] [Google Scholar]
- 4.Kapasi A., DeCarli C., Schneider J.A. Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol. 2017;134:171–186. doi: 10.1007/s00401-017-1717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corriveau R.A., Koroshetz W.J., Gladman J.T., Jeon S., Babcock D., Bennett D.A. Alzheimer's disease-related dementias summit 2016: National Research Priorities. Neurology. 2017;89:2381–2391. doi: 10.1212/WNL.0000000000004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayeda E.R., Glymour M.M., Quesenberry C.P., Whitmer R.A. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimer's Dement. 2016;12:216–224. doi: 10.1016/j.jalz.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.SPRINT MIND Investigators for the SPRINT Research Group. Williamson J.D., Pajewski N.M., Auchus A.P., Bryan R.N., Chelune G., Cheung A.K. Effect of intensive vs standard blood pressure control on probable dementia: a randomized clinical trial. JAMA. 2019;321:553–561. doi: 10.1001/jama.2018.21442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ngandu T., Lehtisalo J., Solomon A., Levalahti E., Ahtiluoto S., Antikainen R. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385:2255–2263. doi: 10.1016/S0140-6736(15)60461-5. [DOI] [PubMed] [Google Scholar]
- 9.Marengoni A., Rizzuto D., Fratiglioni L., Antikainen R., Laatikainen T., Lehtisalo J. The effect of a 2-year intervention consisting of diet, physical exercise, cognitive training, and monitoring of vascular risk on chronic morbidity-the FINGER Randomized Controlled Trial. J Am Med Dir Assoc. 2018;19:355–360.e1. doi: 10.1016/j.jamda.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 10.Smith E.E., Biessels G.J., De Guio F., de Leeuw F.E., Duchesne S., During M. Harmonizing brain magnetic resonance imaging methods for vascular contributions to neurodegeneration. Alzheimer's Dement (Amst) 2019;11:191–204. doi: 10.1016/j.dadm.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hachinski V., Iadecola C., Petersen R.C., Breteler M.M., Nyenhuis D.L., Black S.E. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006;37:2220–2241. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- 12.Roman G.C., Tatemichi T.K., Erkinjuntti T., Cummings J.L., Masdeu J.C., Garcia J.H. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 13.Viswanathan A., Rocca W.A., Tzourio C. Vascular risk factors and dementia: how to move forward? Neurology. 2009;72:368–374. doi: 10.1212/01.wnl.0000341271.90478.8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorelick P.B., Scuteri A., Black S.E., Decarli C., Greenberg S.M., Iadecola C. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jack C.R., Jr., Bennett D.A., Blennow K., Carrillo M.C., Dunn B., Haeberlein S.B. NIA-AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimer's Dement. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Connor J.P., Aboagye E.O., Adams J.E., Aerts H.J., Barrington S.F., Beer A.J. Imaging biomarker roadmap for cancer studies. Nat Rev Clin Oncol. 2017;14:169–186. doi: 10.1038/nrclinonc.2016.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shlipak M.G., Day E.C. Biomarkers for incident CKD: a new framework for interpreting the literature. Nat Rev Nephrol. 2013;9:478–483. doi: 10.1038/nrneph.2013.108. [DOI] [PubMed] [Google Scholar]
- 18.Sweeney M.D., Montagne A., Sagare A.P., Nation D.A., Schneider L.S., Chui H.C. Vascular dysfunction-The disregarded partner of Alzheimer's disease. Alzheimer's Dement. 2019;15:158–167. doi: 10.1016/j.jalz.2018.07.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sachdev P.S., Lo J.W., Crawford J.D., Mellon L., Hickey A., Williams D. STROKOG (stroke and cognition consortium): an international consortium to examine the epidemiology, diagnosis, and treatment of neurocognitive disorders in relation to cerebrovascular disease. Alzheimer's Dement (Amst) 2017;7:11–23. doi: 10.1016/j.dadm.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Traylor M., Adib-Samii P., Harold D., Alzheimer's Disease Neuroimaging Initiative. International Stroke Genetics Consortium (ISGC) UK Young Lacunar Stroke DNA resource. Dichgans M. Shared genetic contribution to ischaemic stroke and Alzheimer's Disease. Ann Neurol. 2016;79:739–747. doi: 10.1002/ana.24621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neurology Working Group of the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium. the Stroke Genetics Network (SiGN) and the International Stroke Genetics Consortium (ISGC) Identification of additional risk loci for stroke and small vessel disease: a meta-analysis of genome-wide association studies. Lancet Neurol. 2016;15:695–707. doi: 10.1016/S1474-4422(16)00102-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crawford K.M., Gallego-Fabrega C., Kourkoulis C., Miyares L., Marini S., Flannick J. Cerebrovascular disease knowledge portal: an open-access data resource to accelerate genomic discoveries in stroke. Stroke. 2018;49:470–475. doi: 10.1161/STROKEAHA.117.018922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.NINDS . NINDS; National Institutes of Health (NIH), Bethesda, Maryland: 2017. NIH Consortium Takes Aim at Vascular Disease-Linked Cognitive Impairment and Dementia. [Google Scholar]
- 24.Greenberg S.M., William M. Feinberg award for excellence in clinical stroke: big pictures and small vessels. Stroke. 2017;48:2628–2631. doi: 10.1161/STROKEAHA.117.017246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown R., Benveniste H., Black S.E., Charpak S., Dichgans M., Joutel A. Understanding the role of the perivascular space in cerebral small vessel disease. Cardiovasc Res. 2018;114:1462–1473. doi: 10.1093/cvr/cvy113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.NIH . NIH; 2019. Estimates of Funding for Various Research, Condition, and Disease Categories (RCDC) [Google Scholar]
- 27.Liggins C., Snyder H.M., Silverberg N., Petanceska S., Refolo L.M., Ryan L. International Alzheimer's Disease Research Portfolio (IADRP) aims to capture global Alzheimer's disease research funding. Alzheimer's Dement. 2014;10:405–408. doi: 10.1016/j.jalz.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 28.METACOHORTS Consortium for the study of vascular disease and its contribution to cognitive decline and neurodegeneration: an initiative of the Joint Programme for Neurodegenerative Disease Research. Alzheimer's Dement. 2016;12:1235–1249. doi: 10.1016/j.jalz.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]