Abstract
Advancements in biotechnology and protein engineering expand the availability of various therapeutic proteins including vaccines, antibodies, hormones, and growth factors. In addition, protein drugs hold many therapeutic advantages over small synthetic drugs in terms of high specificity and activity. This has led to further R&D investment in protein-based drug products and an increased number of drug approvals for therapeutic proteins. However, there are many biological and biopharmaceutical obstacles inherent to protein drugs including physicochemical and enzymatic destabilization, which limit their development and clinical application. Therefore, effective formulations of therapeutic proteins are needed to overcome the various physicochemical and biological barriers. In current medical practice, protein drugs are predominantly available in injectable formulations, which have disadvantages including pain, the possibility of infection, high cost, and low patient compliance. Consequently, non-invasive drug delivery systems for therapeutic proteins have gained great attention in the research and development of biomedicines. Therefore, this review covers the various formulation approaches to optimizing the delivery properties of protein drugs with an emphasis on improving bioavailability and patient compliance. It provides a comprehensive update on recent advancements in nanotechnologies with regard to non-invasive protein drug delivery systems, which is also categorized by the route of administrations including oral, nasal, transdermal, pulmonary, ocular, and rectal delivery systems.
Keywords: Protein, Non-parenteral, Drug delivery, Non-invasive, Nanotechnology
Graphical Abstract
1. Introduction
Since endogenous proteins are actively involved in various physiological and pathophysiological processes, the modulation of their functions and activities are relevant to disease control and prevention, providing big opportunities for discovering new therapeutic agents. When compared with conventional low molecular weight synthetic drugs, protein drugs have many advantages including high specificity and potency, low toxicity, and high tolerance by the human body [1]. In addition, advancement in biotechnology, protein engineering, molecular modeling and computational methodologies in formulation development facilitate investment in the research and development of protein drugs, which leads to an increased number of drug approvals for therapeutic proteins [2,3].
Since approving the first protein drug Humulin® (human recombinant insulin) in 1982, the US Food and Drug Administration (FDA) has approved more than 100 therapeutic proteins and many more are currently undergoing clinical and pre-clinical evaluations [4]. Particularly in recent years, the number of biologics approved by the FDA has rapidly grown. In 2018, the Center for Drug Evaluation and Research (CDER) approved 59 novel drugs, of which approximately 25% are biologics (Table 1) [5]. In parallel, the protein drug global market has been continuously growing and is expected to increase from $172.5 billion in 2016 to $228.4 billion in 2021 [6]. Currently, protein drugs are being pursued for the treatment of diverse diseases including metabolic disorders, autoimmune diseases, and cancers. There is also a particular emphasis on intractable diseases.
Table 1.
Biologics approved by FDA in 2018 [5].
| Active ingredient | Product | Route | Class | Targeted disease |
|---|---|---|---|---|
| Burosumab | Crysvita™ | Parenteral (i.v.) | Monoclonal antibody | X-linked dominant hypophosphatemic rickets |
| Calaspargase pegol | Asparlas™ | Parenteral (i.v.) | Pegylated enzyme | Acute lymphoblastic leukemia |
| Cemiplimab | Libtayo™ | Parenteral (i.v.) | Monoclonal antibody | Cutaneous squamous cell carcinoma |
| Cenegermin | Oxervate™ | Eye drop | Recombinant human nerve growth factor | Neurotrophic keratitis |
| Elapegademase | Revcovi™ | Parenteral (i.m.) | Pegylated enzyme | Adenosine deaminase severe combined immunodeficiency |
| Emapalumab-lzsg | Gamifant™ | Parenteral (i.v.) | Monoclonal antibody | Haemophagocytic lymphohistiocytosis |
| Erenumab | Aimovig™ | Parenteral (s.c.) | Monoclonal antibody | Migraine prevention |
| Fremanezumab-vfrm | Ajovy™ | Parenteral (s.c.) | Monoclonal antibody | Migraine prevention |
| Galcanezumab-gnlm | Emgality™ | Parenteral (s.c.) | Monoclonal antibody | Migraine prevention |
| Ibalizumab-uiyk | Trogarzo™ | Parenteral (i.v.) | Monoclonal antibody | Multidrug-resistant HIV-1 |
| Lanadelumab | Takhzyro™ | Parenteral (s.c.) | Monoclonal antibody | Hereditary angioedema attacks |
| Mogamulizumab-kpkc | Poteligeo™ | Parenteral (i.v.) | Monoclonal antibody | Mycosis Fungoides and Sézary Syndrome |
| Moxetumomab pasudotox | Lumoxiti™ | Parenteral (i.v.) | Monoclonal antibody | Relapsed or refractory hairy cell leukemia |
| Pegvaliase-pqpz | Palynziq™ | Parenteral (s.c.) | Pegylated enzyme | Phenylketonuria |
| Ravulizumab | Ultomiris™ | Parenteral (i.v.) | Monoclonal antibody | Paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome |
| Tagraxofusp-erzs | Elzonris™ | Parenteral (i.v.) | Fusion protein | Blastic plasmacytoid dendritic cell neoplasm |
| Tildrakizumab | IIumya™ | Parenteral (s.c.) | Monoclonal antibody | Moderate-to-severe plaque psoriasis |
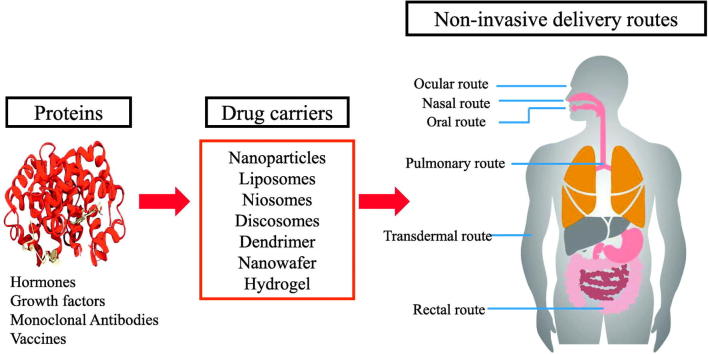
Although many therapeutic proteins are now in the pharmaceutical market, the most common route of administration for therapeutic proteins is parenteral injection (Table 1). In general, parenteral injection is not the preferred route of administration due to pain, risk of infection, high cost, and low patient compliance. In addition, the safety issues related to the disposal of needles discourages parenteral administration [7]. On the other hand, non-invasive drug delivery systems via oral, nasal, pulmonary, ophthalmic, rectal, or transdermal routes offer alternative platforms for the delivery of macromolecules with substantial benefits including self-medication, free of needle stick injury, low risk of infection, cost-effectiveness, and better patient compliance [2]. However, the inherent assets of proteins such as large molecular size, hydrophilicity, low permeability, and chemical/enzymatic instability create a big hurdle in developing non-invasive drug delivery systems [8]. In order to overcome those issues, various formulation approaches have been adopted, which target alternative non-invasive routes of administrations. Particularly, advancements in nanotechnologies promote novel nanoformulations with controlled particle size and surface modification, which improves the target selectivity, systemic half-life, and bioavailability of protein drugs. Cutting-edge nanotechnology has a great impact on the development of non-invasive drug delivery carriers that lead to better clinical outcomes for protein drugs [9]. Selected examples of commercially available non-invasive formulations of biopharmaceuticals are summarized in Table 2.
Table 2.
Selected examples of commercially available drugs for non-invasive routes of administration [[181], [182], [183], [184]].
| Product | Drug | Route | Indications |
|---|---|---|---|
| Minirin® | Desmopressin | Oral, Nasal | Cranial diabetes insipidus or nocturia associated with multiple sclerosis |
| Sandimmune® | Cyclosporine A | Oral | Immunosuppressants |
| Colomycin® | Colistin | Oral | Intestinal infection (caused by sensitive Gram-negative organisms) |
| Cytorest® | Cytochrome C | Oral | Leucopenia |
| Cachexon® | Glutathione | Oral | AIDS- related cachexia |
| Ceredist®OD | Taltirelin | Oral | Spinocerebellar ataxia |
| Anginovag® | Tyrothricin | Oral | Pharyngitis |
| Vancocin® | Vancomycin | Oral | Infection, clostridium difficile-associated diarrhea |
| Oral-Lyn™ | Insulin | Buccal | Diabetis mellitus |
| Suprifact™ | Buserelin | Nasal | Prostate cancer, endometriosis |
| Suprecur® | Buserelin | Nasal | Prostate cancer, endometriosis |
| Synarel® | Nafarelin | Nasal | Endometriosis |
| Kryptocur® | LHRH | Nasal | Cryptorchism |
| Miacalcin® | Salmon calcitonin | Nasal | Hypercalcemia or osteoporosis |
| Fortical® | Salmon calcitonin | Nasal | Hypercalcemia or osteoporosis |
| Desmospray® | Desmopressin | Nasal | Cranial diabetes insipidus or nocturia associated with multiple sclerosis |
| Syntocinin™ | Oxytocin | Nasal | Indicated for the initiation or improvement of uterine contractions |
| Antepan® | Protirelin | Nasal | Hypothyroidism and acromegaly |
| FluMist® Quadrivalent | Vaccine | Nasal | Influenza |
| Eylea® | Aflibercept | Ocular | Wet age-related macular degeneration (WAMD), Diabetic macular edema (DME) or Diabetic retinopathy (DR) in DME, Macular edema following retinal vein occlusion (MEtRVO) |
| Lucentis® | Ranibizumab | Ocular | WAMD, DME or DR in DME, MEtRVO, Myopic choroidal neovascularization (mCNV) |
This review details various formulation approaches used to optimize the delivery properties of protein drugs. A particular emphasis is placed on recent advancements in nanotechnologies and their implications on non-invasive delivery systems of protein drugs via various routes of administration.
2. Barriers to Non-Invasive Formulation Development for Proteins
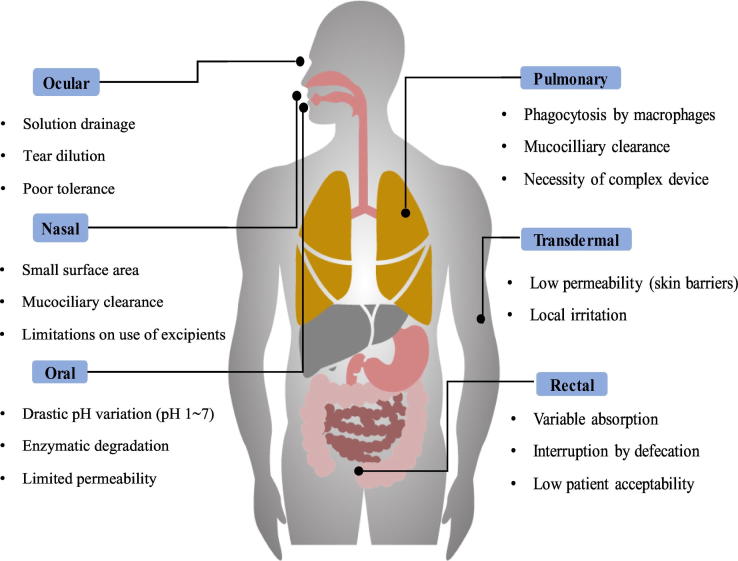
Over the past decades, various non-invasive routes of administration including oral, transdermal, nasal, and pulmonary routes draw the substantial attention for the development of more patient-friendly dosage forms of protein drugs. Among them, oral administration is the most preferred route of administration, which has the advantages of high patient compliance, self- and comfortable medication, free from pain and infection risk, and low cost. Presently, the oral formulations of IFN-α and human growth hormone are under clinical studies [4]. In the last few decades, the nasal route has also been widely explored as an alternative route of administration for bypassing the extensive hepatic first-pass effect. The nasal route offers some advantages including enhanced bioavailability and seamless administration, but more importantly, it offers the opportunity of targeting drugs directly to the brain from the nose [10]. Similarly, the lungs are exceptionally permeable to macromolecules; thus, pulmonary delivery can be an effective non-invasive route for protein delivery [11]. It has been reported that inhaled insulin was more rapidly absorbed than subcutaneously injected insulin and provides an enhanced physiological response to a meal [11]. Likewise, the transdermal delivery system provides a painless alternative to parenteral injections. The pain, needle phobia, and risk of infection associated with the parenteral injection can be overcome by these alternate routes. However, there are crucial physicochemical and biological barriers in formulating protein drugs [12]. The drug absorption resulting from alternate routes depends on numerous factors such as the size, concentration, solubility, degree of ionization of the drug, drug carrier, venous drainage of the mucosal tissue, mucosal contact time, the pH of the absorption site, and so on [12]. Therefore, a better understanding of the potential barriers against protein delivery is essential in designing optimal formulations. The main barriers against protein delivery are summarized in Fig. 1, according to the non-invasive routes of administration and more details on each barrier are described in the following sections.
Fig. 1.
Main barriers for non-invasive routes of administration.
2.1. Physicochemical Drug Properties
Intrinsic properties of protein-based drugs such as their large size, poor membrane permeation, physicochemical instability, and susceptibility to enzymatic degradation create a formidable challenge with respect to the delivery of protein drugs, particularly for non-invasive drug delivery. Therefore, physicochemical drug properties are one of the key considerations for selecting the route of administration and designing formulations.
First, the high molecular mass and the large size of drug molecules often result in poor membrane permeability, which causes low absorption. In general, drugs with a relatively small molecular mass of <500 Da readily penetrate membranes in the GI tract and skin through passive diffusion [13]. With respect to ocular delivery, the human retina restricts the diffusion of macromolecules >76 kDa due to inner and outer plexiform layers and macromolecules above 150 kDa are unable to reach the inner retina [14]. The nasal mucosa also exhibit low membrane permeability for molecules larger than 1 kDa [12]. Given that proteins above 3–5 kDa are generally regarded as peptidyl molecules, the molecular mass and size of proteins should be the most critical characteristics affecting the absorption of protein drugs [15].
The hydrophilicity of therapeutic proteins also has a great impact on their cellular transport. Most protein drugs are highly hydrophilic with a log P value of less than zero [16], which hampers drug permeation across biological membranes and creates challenges with respect to delivery of protein drugs to intracellular targets. Due to the lipophilic nature of biological membranes and the paracellular space which is 3–10 Å, the large size and hydrophilic nature of proteins limit their diffusion and passage through paracellular pathways. Therefore, the cellular uptake of hydrophilic proteins is primarily controlled by active transport or endocytosis rather than passive diffusion [17]. For proteins, one of the major disadvantages of the endocytic pathway is entrapment by endosomes, which eventually leads to degradation by lysosomal enzymes.
Another physicochemical drug property influencing absorption is the surface charge of a therapeutic protein, which is derived by the amino acid sequence of the protein and the pH of its surroundings. This physicochemical property is complex and heterogeneous. It is typically caused by deamination, isomerization, or post-translational modification, leading to a change in the net charge of a protein and the formation of acidic and basic variants [15]. The surface charge can cause protein drugs to interact with molecules on the cell surfaces or tissue components, thereby affecting absorption, distribution, and elimination of proteins in the body.
Furthermore, proteins are susceptible to enzymatic and physicochemical destabilization during various stages of their life cycle, which spans from formulation development to systemic exposure after intake. This can lead to low bioavailability and short biological half-life [7]. Particularly, the complex secondary, tertiary, and quaternary structures of proteins are vulnerable in various physicochemical environments, resulting in the loss of bioactivity [16]. Some of the amino acid components of proteins are very sensitive to destabilization reactions including denaturation, adsorption, aggregation/precipitation, oxidation, and hydrolysis during manufacturing or storage. Factors such as pH, temperature, agitation, ionic strength, and the presence of the metal ions or surfactants can cause the destabilization of proteins [18].
2.2. Biological Barriers
2.2.1. pHs of Biological Environments
The pH conditions in various biological environments can affect the ionization, chemical instability, and absorption of protein-based drugs and their delivery systems. For example, protein drugs are often unstable at physiological pHs. Particularly, the strongly acidic gastric environment (pH 1 – 3) causes destabilization of protein drugs in the stomach, but chemical degradation reduces significantly in the ileum and colon due to higher pHs [12,16,19]. In an ocular delivery system, the buffering agent plays an important role since hyperosmotic solutions cause transient dehydration of the anterior chamber tissues while hypotonic solutions may cause edema [14].
2.2.2. Enzymatic Barriers
Enzymatic degradation in the gastrointestinal (GI) tract is a formidable barrier against the oral delivery of proteins and leads to low bioavailability. While the enzymatic activity of proteases is high in the small intestine, the proteolytic activity in the colon is comparatively lower; therefore, colon-targeted delivery systems have gained a great deal of attention as an effective delivery system for protein drugs [12]. Moreover, colon-targeted drug delivery can also achieve prolonged drug absorption with a longer residence time. In addition to the systemic delivery of protein drugs, colon-targeted drug delivery systems are suitable for the treatment of local bowel diseases such as colon cancer, ulcerative colitis, Crohn’s disease, and amoebiasis [12]. Even though the non-oral routes of administration can avoid the hepatic first-pass effect, enzymatic barriers may create a “pseudo-first-pass effect”. For example, even low metabolic enzyme activity may act as a barrier against nasal and pulmonary delivery of protein drugs [12,20].
2.2.3. Mucosal Barriers
Mucus and epithelial cell layers act as the major absorption barrier against non-injectable drug administration. All mucosal epithelia are covered in a layer of mucus, which serves as the first line of defense at the surfaces of the eye, respiratory tract, and gastrointestinal tract against mechanical damage or the entrance of harmful substances [12]. In addition, the mucus layer forms a physical diffusion barrier for large molecules. Its hydrophilic nature and negative charge cause the drug to interact with mucus components, retarding drug diffusion and thereby limiting intestinal drug absorption [20].
The major components of mucus are secreted mucin-type glycoproteins and the thickness of the mucus layer varies greatly throughout the body. For example, airway mucus may range in thickness from 5 to 55 μm but the nasal tract has a mucus layer of limited thickness. Thus it is more permeable compared to other mucosal surfaces [21]. In the eye, the secreted precorneal mucin gel covering the conjunctiva may be as thick as 30–40 μm [21]. In the GI tract, mucus layer thickness significantly varies depending on the site and digestive activity [12]. While the mucus layer is thickest in the stomach and the colon, it ranges between 10 and more than 170 μm in ileum and stomach, respectively [12]. As a result, while the colon offers a favorable absorption site for proteins due to the lack of proteolytic activity, drugs must pass across a thicker mucus layer.
2.3. Others
Each non-invasive route of administration has its own limitations depending on the anatomical size and position, microclimate, specific physiological conditions, and formulations. For example, the ocular delivery of proteins is hindered by the blood-retinal barrier and efflux transporters (MRP1, 4 and 5) expressed in the posterior segment [22]. In addition, the viscosity of formulations can affect ocular drug delivery. High viscosity increases the corneal contact time but leads to reflex tearing and blinking of the eye, which alters the viscosity of the formulations. The volume and viscosity of the fluid in the rectum may also affect drug absorption.
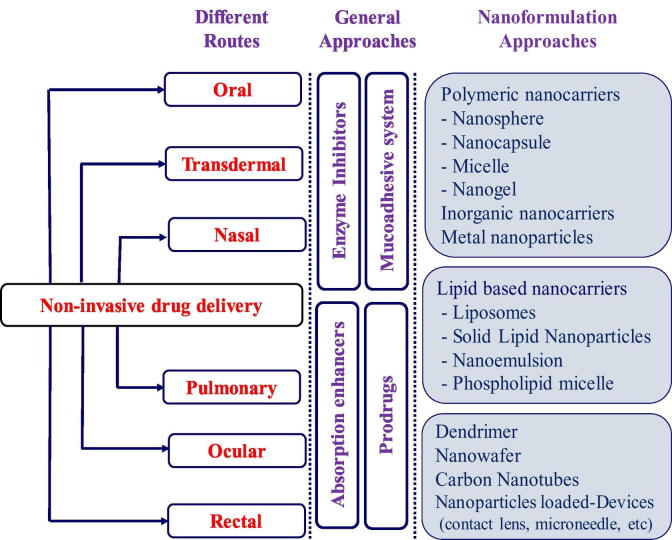
Overall, there are multiple barriers against protein delivery via a non-invasive route of administration, resulting in a loss of biological activity of the proteins and low bioavailability. Therefore, various formulation strategies have been attempted to overcome those barriers as exemplified in Fig. 2. More details on non-invasive protein delivery systems are discussed in the next section according to the route of administration.
Fig. 2.
Approaches for protein drug delivery via non-invasive routes of administration.
3. Formulation Strategies for Non-Invasive Protein Drug Delivery
3.1. Oral Delivery
Oral ingestion is typically the most preferred route of administration due to good patient compliance. Furthermore, oral dosage forms are cost-effective compared to parental dosage forms [23]. However, the development of oral formulations for protein drugs presents a difficult challenge due to many barriers including the large molecular mass, size, physicochemical instability, enzymatic degradation, and low membrane permeability, as detailed in previous sections. Accordingly, most protein drugs in the pharmaceutical market are available in parenteral formulations, and only a few in oral formulations [23].
Despite the hurdles, great efforts and many different approaches have been taken to develop oral dosage forms of protein drugs. This has led to significant progress in recent years. Particularly, advancements in nanotechnology have fueled progress in oral protein delivery systems and some of them are highlighted in the following sections.
3.1.1. Polymer-Based Formulations
3.1.1.1. Hydrogels
Hydrogels are three-dimensional polymeric networks composed of cross-linked hydrophilic and biocompatible polymers, which also exhibit a thermodynamic compatibility with water allowing them to swell in aqueous media [24,25]. Hydrogels are widely used for various clinical applications such as contact lenses, biosensors, materials for tissue engineering, and drug delivery carriers [25,26]. Significantly, hydrogels can also offer an effective and convenient way to administer protein drugs. Among various polymers, 2-hydroxyethyl methacrylate, ethylene glycol dimethylacrylate, N-isopropyl acrylamide, acrylic acid, methacrylic acid (MAA), poly (ethylene glycol) (PEG) and poly (vinyl alcohol) (PVA) are commonly used in hydrogels for protein delivery [26].
Given that hydrogels must maintain their integrity in order to protect proteins until they are released at target sites, the mechanical properties of hydrogels are also important for pharmaceutical applications. The degree of hydrogel crosslinking must be adjusted to obtain the desired mechanical properties since a higher degree of crosslinking results in a stronger but more brittle structure [26]. Copolymerization can also be utilized to obtain relatively strong but elastic hydrogels.
Hydrogels are often designed to respond to certain physiological stimuli such as pH, ionic strength and temperature. In response to environmental stimuli, such hydrogels can dramatically change their swelling behavior, network structure, permeability, and mechanical strength [25]. Particularly, for more effective oral delivery of protein drugs, pH-triggered drug release systems are widely adopted in order to protect the proteins from the harsh gastric environments. The pH-responsive hydrogels are ionic hydrogels containing pendant groups ionized in response to environmental pH changes; this causes the hydrogel network to swell. There are many previous reports investigating pH-responsive hydrogels loaded with protein drugs. Recently, Carrillo-Conde at al. [27] have prepared P(MAA-g-EG) and poly[methacrylic acid-grafted-(N-vinylpyrrolidone)] [P(MAA-co-NVP)] hydrogel systems for anti-tumor necrosis factor (TNF)-α monoclonal antibody (mAb). These hydrogelsystems protected TNF-α mAb from the acidic environment of the stomach and released the mAb in the small intestine. Consequently, these hydrogel systems preserved antibody bioactivity, leading to the systemic circulation of an intact antibody retaining its biological function [27]. Other research groups have also demonstrated that P(MAA-g-EG) based hydrogels were applicable to the oral delivery of vaccines and cholera toxin [28]. Overall, P(MAA-g-EG) hydrogels appear to be a promising oral delivery carrier for protein drugs, therapeutic antibodies, and vaccines.
Recently, Lima etal. [29] fabricated an alginate-based hydrogel loaded with BSA as a model therapeutic. Their hydrogel system exhibited pH-dependent swelling performance with higher value at pH 7.4 and enhanced pharmacological activity [29]. Similarly, a xanthan gum/poly (N-vinyl imidazole) hydrogel system loaded with BSA also exhibited high loading efficiency and encapsulation efficiency [30]. The release profile of BSA was dependent on the concentration of the polymers. Overall, alginate and xanthan gum-based hydrogel systems seem to be suitable for protein delivery via the oral route of administration.
3.1.1.2. Nanoparticles
In recent years, various nanoparticles have been explored as potential carriers for oral protein delivery. Nanoparticles are able to improve the physicochemical stability of proteins in the GI tract by imprisoning proteins in the polymeric matrix with a size range of 10–1000 nm. As an oral protein carrier, nanoparticles should be non-toxic and non-immunogenic [31]. In addition, the size of nanoparticles often plays a key role in intestinal absorption, distribution, elimination, and in-vivo activity. For instance, nanoparticles <100 nm can be well absorbed across the intestinal mucosa, but intestinal absorption is dramatically reduced for nanoparticles >500 nm [31]. The surface of nanoparticles can be decorated with specific ligands for targeting the receptor-mediated transport pathways [32].
Various natural and synthetic polymers are used in the preparation of nanoparticles. Among them, poly (lactic acid) (PLA), poly (lactic-co-glycolic acid) (PLGA), chitosan, gelatin, polymethylmethacrylates, and poly-alkyl-cyanoacrylate are the most widely used for the preparation of nanoparticles [33,34]. Chitosan, which is derived from the deacetylation of chitin, is a copolymer consisting of glucosamine and N-acetyl-glucosamine [34,35]. Many properties of chitosan, including biocompatibility,mucoadhesion, and low toxicity, make it a suitable candidate as protein delivery carriers [33,35]. Moreover, chitosan enhances cellular uptake by opening the tight junction [33,34]. Therefore, many researchers have attempted to use chitosan as a protein delivery carrier. For example, Mukhopadhyay etal. [33] developed the self-assembled chitosan/insulin nanoparticles for successful oral insulin delivery. They prepared nanoparticles with spherical or subspherical shapes, an average particle size of 200–550 nm, and a high encapsulation efficiency of about 85%. The oral administration of chitosan/insulin nanoparticles effectively decreased the blood glucose level in alloxan-induced diabetic mice, suggesting that chitosan nanoparticles have great potential as oral insulin carriers [33].
Many chitosan derivatives were proposed to improve the solubility of natural chitosan in a broader pH range. Among them, N-trimethyl chitosan chloride (TMC), a partially quaternized derivative of chitosan, overcomes issues of solubility at a neutral pH while retaining the advantages of chitosan including mucoadhesive properties and tight junction opening. Sandri etal. [36] compared the cellular uptake of insulin-loadedTMC nanoparticles with chitosan nanoparticles. In their studies, both chitosan and TMC nanoparticles significantly enhanced the permeation of insulin into Caco-2 cells; however, TMC NPs were more efficient in jejunum tissue (pH 6–6.5) due to their high mucoadhesive potential. This indicates that TMC nanoparticles are a suitable carrier for the oral administration of insulin [36]. In addition, Jin etal. [37] prepared insulin-loadedTMC nanoparticles modified to target goblet cells using a C-Src tyrosine kinase (CSK) targeting peptide. Compared with unmodified nanoparticles, the CSK peptide modification facilitated the uptake of nanoparticles in villi. In addition, the orally administrated CSKpeptide-modifiedTMC nanoparticles showed increased bioavailability and a greater hypoglycemic effect of oral insulin when compared to unmodified ones. Accordingly, CSKpeptide-modifiedTMC nanoparticlesappeared to be effective as goblet cell-targeting nanocarriers for oral delivery of insulin [37]. Carboxymethyl chitosan (CMC) may be also applicable as a drug carrier for hydrophilic macromolecules since some of previous studies confirmed the superior stability, low toxicity, and controlled drug release properties of CMC-basedpH-sensitive nanoparticles [38,39]. Besides these examples, there have been many attempts to overcome the disadvantages of chitosan by using derivatives such as diethyl methyl chitosan, triethyl chitosan, and lauryl succinyl chitosan, which also have high potential as effective oral delivery carriers for protein drugs.
Alginate is a natural anionic polymer that is widely used as a drug carrier [40,41]. Due to its anionic surface charge, it can readily undergo gel formation via electrostatic interaction with cationic materials. However, alginate beads have a large porosity, which leads to drug leakage. To overcome this issue, chitosan or dextran sulfate are commonly used in combination with alginate. For example, Mukhopadhyay etal. [42] suggested that chitosan-alginate nanoparticles could be a promising oral delivery carrier for insulin via pH-dependent drug release in the GI tract. They demonstrated that the insulin release from chitosan-alginate nanoparticles were effectively suppressed at acidic pHs, followed by a sustained release at intestinal pHs [42]. Accordingly, chitosan-alginate nanoparticles protected insulin from the harsh gastric environment upon oral administration. Furthermore, chitosan-alginate nanoparticles significantly improved the hypoglycemic effects and bioavailability of oral insulin when compared to free insulin solution in diabetic mice [42].
In addition to natural polymer-based nanoparticles, various synthetic polymers are commonly used as oral delivery carriers for protein drugs. Among them, PLGA is a representative polymer widely used for oral protein delivery. PLGA is a copolymer of lactic acid and glycolic acid created via ring-opening polymerization. The biodegradability and biocompatibility of PLGA facilitate its application as a drug delivery carrier [43,44]. Yang etal. [45] prepared insulin-loadedPLGA nanoparticles by double-emulsion solvent evaporation methods and evaluated invivo efficacy after oral administration in diabetic rats [45]. Their results indicated that blood glucose in the diabetic rats decreased to normal levels within 8 h after oral administration of insulin-loadedPLGA nanoparticles [45].
In many ways, PLGA nanoparticles are promising vehicles for oral protein delivery; however, they also present difficulties such as initial burst drug release in acidic pHs and poor mucosal penetration [45]. To overcome these issues, PLGA nanoparticles can undergo the surface modification. Zhang etal. [46] reported the enhanced bioavailability of insulin by using PLGA nanoparticle coated with chitosan. PLGA nanoparticles are applicable to targeted drug delivery of orally administered proteins. Hurkat etal. [47] prepared PLGA nanoparticles coated with targeting ligands specific for M cells located in Peyer's patches, the main gateway for macromolecules such as bacteria and virus. As a targeting ligand, they used Con A lectins which can specifically bind to alpha-fucosyl groups presented by M cells. Con A-coatedPLGA nanoparticles suppressed drug release in acidic environments and gradually released insulinup to 70% with retaining the structural stability of insulin. Furthermore, invivo experiments suggested that Con A-coatedPLGA nanoparticles were effective at improving the oral bioavailability of insulin [47]. Another approach for targeted oral protein delivery utilizes the neonatal Fc receptor (FcRn) present in the apical region of the small intestine and colon epithelial cells. The Fc-conjugated nanoparticles bind to FcRn and diffuse through lamina propria via receptor-mediated endocytosis. Recently, Shi etal. [32] developed exenatide-loadedPEG-PLGA nanoparticles modified with Fc, which lead to the enhanced absorption and better hypoglycemic effect of exenatide. This study suggests that nanoparticles targeted to FcRn overcome the epithelial barrier and improve the effectiveness of oral protein delivery systems [32].
Some studies reported the application of polylactic acid (PLA) as a novel oral protein delivery carrier. PLA is a biodegradable aliphatic polymer that can be hydrolyzed into monomeric units inside the body. In a recent study, Xiong etal. [48] prepared insulin-loadedblock-copolymer containing PLA and Pluronic P85 (PLA-P85-PLA), which resulted in a prolonged and enhanced hypoglycemic effect. Similarly, insulin-loadedPLA-PEG nanoparticles conjugated with modified Fc exhibited >11-fold higher absorption than non-targeted nanoparticles [49].
Overall, nanoparticles decorated with specific targeting ligands may have a significant impact on the treatment of many diseases by enabling protein drugs, which are currently limited by low bioavailability, to be efficiently delivered through oral administration. FcRn targeting may also be applicable for other routes of administration since FcRn is expressed in the vascular endothelium, blood-brain barrier, renal system, hepatic system, lungs, and throughout the hematopoietic system [49].
Redox-activated nanocarriers are also suggested as effective delivery systems for drugs and genes, which are often sensitive to glutathione (GSH) as a regulator of cellular redox potential [[50], [51], [52]]. For example, Cai etal. [52] demonstrated the effective gene delivery using stimulus-responsive catiomer designed with redox-sensitive disulfide and acid-labile imine linkers. The experimental results confirmed the high potential of redox-responsive systems for nonviral gene delivery applications with high DNA binding ability, low cytotoxicity, and high transfection efficiency.
3.1.2. Lipid Based Formulations
3.1.2.1. Emulsion
An emulsion is a well-blended mixture of two immiscible liquids such as oil and water in the presence of emulsifying or surface-active agents [53]. Multiple emulsions such as oil-in-water-in-oil (O/W/O) and water-in-oil-in-water (W/O/W) emulsions are often used for delayed or controlled drug release [54]. In addition, W/O/W emulsion has been widely studied for protein encapsulation since it can effectively entrap hydrophilic drugs in the internal aqueous chamber. However, the application of W/O/W emulsion as a protein drug delivery system faces issues including reduced protein activity, difficulties in size control, and instability against pH, heat, and storage [55].
Wang etal. [56] suggested a simple preparation method for anhydrous reversed micelles (ARMs) by lyophilization of a water-in-oil emulsion. Lyophilized ARMs contained insulin and phosphatidylcholine in an aqueous phase and an oil phase, respectively. After oral administration, ARMs released the drug slowly and significantly reduced plasma glucose levels in diabetic rats [56]. Recently, protein-loaded, pH-sensitive nanofibers were also developed by using an emulsion electrospinning method, which exhibited pH-dependent drug release and significantly enhanced protein stability during long term storage [57].
On the other hand, self-nanoemulsifying drug delivery systems (SNEDDS) have received considerable attention as a promising alternative to orally administered emulsions due to their high physical stability and ease of manufacture [58,59]. SNEDDS consist of oils, surfactants, co-solvents/co-surfactants, and drugs. These can be orally administered in gelatin capsules and spontaneously form a fine O/W emulsion with nano-sized droplets upon aqueous dilution by the gentle agitation of the gastrointestinal fluids [60,61]. In addition to the enhanced dissolution of drugs by SNEDDS, bypassing the hepatic first-pass effect via lymphatic transport is another factor that contributes to the increased bioavailability of drugs by SNEDDS [60,61]. Compared to conventional W/O/W emulsions, SNEDDS have some advantages as an oral delivery system for protein drugs in terms of better stability, better oral bioavailability, and easier particle size control. Sakloetsakon etal. [62] fabricated the thiolated chitosan based SNEDDS for oral delivery of insulin, which displayed a significant increase in oral exposure of insulin.
In addition, Qi etal. [61] proposed a self-double-emulsifying drug delivery system (SDEDDS) by formulating mixtures of hydrophilic surfactants and water-in-oil (W/O) emulsions to resolve the instability issue of W/O/W emulsions. SDEDDS spontaneously emulsify into W/O/W double emulsions in the aqueous gastrointestinal environment, resulting in drug encapsulation within the internal water phase of the double emulsions. When they encapsulated pidotimod as a model protein in SDEDDS, the protein retained its stability for 6 months at 25°C. Moreover, SDEDDS increased the oral absorption of pidotimod by 2.56-fold when compared to aqueous protein solution [61].
3.1.2.2. Liposome
Liposomes are widely used to improve the membrane permeability of protein drugs by encapsulating proteins inside the aqueous core [16]. The structural similarity of liposomes with the cellular membrane is also beneficial in facilitating intestinal absorption. However, liposomes have some weaknesses as an oral protein carrier such as chemical and enzymatic instability in the GI tract [63]. Since surface coating should be advantageous for oral drug delivery in liposomes to overcome instability issues, various approaches have been attempted to modify the surface of liposomes by using ligands that interact with specific receptors on the cellular membrane. For example, lectins, a type of glycoprotein from plants, are promising ligands for specific binding to their carbohydrate receptors on the mucosal surface [64]. Makhlof etal. [65] prepared liposomes coated with wheat germ agglutinin (WGA) lectin, which strongly bound to N-acetyl-D-glucosamine and sialic acid on the intestinal mucosal membrane. In their studies, three types of calcitonin encapsulated liposomes were prepared: uncoated liposomes (LIP), carbopol coated liposomes (CP-Lip), and WGA-carbopol coated liposomes (WGA-CP-Lip). Among the tested liposomes, WGA-CP-Lip significantly improved the cellular uptake of calcitonin without any toxicity [65]. Furthermore, after oral administration to rats, relatively high plasma calcium levels suggested that WGA enhances the oral absorption of calcitonin by specifically binding to a receptor on the intestinal membrane.
Archaeosomes are another type of lipid-based oral delivery system fabricated from the polar lipids of various Archaeobacteria. They have unique structural features that help them retain stability at high temperatures, low or high pHs, and in the presence of phospholipases and bile salts, which may lead to superior stability in the GI tract [66,67]. Therefore, they have gained increasing attention as a carrier for proteins, genes, and vaccines. Li etal. [66,67] explored the potential of archaeosomes constructed with the polar lipid fraction E from S. acidocaldarius as an oral drug delivery carrier. In their studies, archaeosomes exhibited superior stability in simulated GI fluids, and invivo experiments indicated that archaeosomes containing insulin-induced lower levels of blood glucose when compared to conventional liposomal formulations [66]. They also examined the immunogenic potentials of archaeosomes as oral vaccine delivery vehicles. After oral administration, archaeosomes greatly enhanced the immune response against the model antigen ovalbumin (OVA), eliciting a substantial IgG response systemically as well as an IgA response mucosally [67]. Archaeosomes also facilitated antigen-specific CD8+T cell proliferation. The above indicates that archaeosomes may be a potential vaccine carrier and adjuvant for effective oral immunization [67].
3.1.3. Others
In order to facilitate the effective oral delivery of biologics, various novel and cutting-edge approaches have been pursued. One of them includes drug delivery with microneedle-based technology where the capsule is filled with micro-needles for oral delivery of biologics. Here, shielded microneedles with insulin pass through the hostile environment of the stomach and once exposed to intestinal pH, the shield coating dissolves and then the bare microneedles pierce the intestinal epithelium to enhance the insulin absorption. The researchers performed proof-of-concept studies, demonstrating the superior hypoglycemic effect of shielded microneedles with insulin over subcutaneous injection and transdermal microneedles in pigs [68]. Furthermore, it was found that microneedle-containing devices were safe with no evidence of tissue damage in the GI tract. This study strongly supports the applicability of microneedle technology for use in the GI tract. Similarly, Banerjee et al. [69] developed a mucoadhesive device loaded into a pH-responsive enteric-coated capsule for oral delivery of insulin. The enteric coating aids in escaping the acidic environment of the stomach and its mucoadhesive properties enhance the intestinal resident time.
3.2. Nasal Delivery
The blood-brain barrier (BBB) controls the passage of most therapeutics, including proteins, into the central nervous system (CNS) and also represents a significant hurdle in drug delivery to the CNS for the treatment of many neuronal degenerative disorders [70]. In this context, the nasal route can be the most reliable alternative when compared to oral and parenteral routes [71,72]. Although the nasal route is typically used for infection of many pathogens, it is a convenient mucosal site for drug delivery. Primarily, the nasal mucosa is accountable for airways homeostasis, humidification, mucous secretion, mucociliary clearance and antimicrobial protection [73]. The nasal delivery route is advantageous to drug delivery because it is non-invasive, readily accessible due to a thin and porous epithelial barrier, and highly vascularized. Additionally, it can bypass the hepatic first-pass metabolism, has a relatively low activity of proteolytic enzymes, and provides a rapid onset of action [[73], [74], [75]]. Furthermore, it is effective and patient-friendly with respect to self-medication. Lastly, it allows drugs to be delivered directly to the brain tissue or cerebrospinal fluid through olfactory neurons, bypassing the BBB [75]. All those advantages make the nasal route suitable for local and systemic drug delivery. Particularly, the nasal route exhibited high potential for an effective protein drug delivery.
Various formulation strategies are available to improve the bioavailability of protein drugs via nasal administration. They include the use of enzyme inhibitors to circumvent nasal metabolism by proteolytic enzymes, the incorporation of absorption enhancers to promote permeation through the membrane, mucoadhesive formulations to improve nasal residential time, and prodrug approaches for optimizing favorable physiochemical properties. Among them, the use of absorption enhancers such as bile salts, surfactants, fluidic acid derivatives, phosphatidylcholines, fatty acids (taurodihydrofusidate), cyclodextrins(CDs), cationized polymers, chelators, and cell penetration peptides facilitate drug permeation across the nasal membrane [76]. Since the bioavailability of proteins can be limited by short residence time in the nasal cavity, mucoadhesive systems are used to prolong nasal retention time. Accordingly, the improved nasal bioavailability of calcitonin and insulin using Carbopol 941 and carboxymethyl cellulose was reported in previous studies [2]. Mucoadhesive polymers also act as permeation enhancers by opening the tight junctions of the nasal epithelium. Thus, mucoadhesive micro-/nano-particles can be effective carriers for the nasal delivery of protein drugs as they offer longer residence time with enhanced permeation across the nasal membrane. These formulation approaches are discussed in detail below.
3.2.1. Polymer-Based Formulations
3.2.1.1. Nano-/Micro-Particles
The nasal administration of protein drugs using nanoparticles has been actively pursued in recent years [71,73]. Particularly, mucoadhesive nanoparticles have a longer residence time in the nasal cavity. Chitosan nanoparticles have been widely explored for the nasal delivery of insulin [73]. The positive charge of chitosan nanoparticles prolonged the contact time between insulin and the nasal mucosal membrane, thereby enhancing the bioavailability of insulin. Likewise, the intranasal administration of chitosan-N-acetyl-L-cysteine nanoparticles and PEG-g-chitosan nanoparticles enhanced the bioavailability of insulin [75,77]. The nasal-associated lymphoid tissue (NALT) is the main target in intranasal vaccination, and polymeric nanoparticles of chitosan, PLGA, and polystyrene were effective with respect to antigen uptake by NALT [73]. Trimethyl chitosan nanoparticles increased the residence time of the antigen and enhanced IgA and IgG production [73,78]. Likewise, mucoadhesive chitosan nanoparticles dramatically increased the brain uptake of nerve growth factor (NGF) via nasal administration [73,79].
3.2.1.2. Nanogel
Nanogels are water-swollen and crosslinked polymer nanoparticles with hydrodynamic sizes in the 10–100 nm range and may be dispersed in an aqueous medium while maintaining their fixed conformation [80,81]. They can be prepared using natural and/or synthetic polymers [82]. The main advantage of nanogels is that their size, surface charge, network density, and chemical functional groups can be controlled and tailored to obtain the desired structural and functional properties.
Insulin molecules have been covalently attached to soft, highly hydrophilic, and multifunctional nanogels for nasal delivery. After intranasal administration, poly(N-vinyl pyrrolidone)-based nanogels covalently attached to insulin were able to cross the BBB and exhibit greater neuroprotection against amyloid β-induced dysfunction when compared to free-insulin [83]. These results suggest intranasal nanogels are a promising tool for developing new therapies for neurodegenerative diseases.
Nochi etal. [84] developed an intranasal vaccine-delivery system with a nanometer-sized hydrogel consisting of a cationic cholesteryl-group-bearing pullulan (cCHP). Anon-toxic subunit fragment of Clostridium botulinumtype-A neurotoxin BoHc/A was administered intranasally with cCHP nanogel (cCHP-BoHc/A), which was effectively taken up by mucosal dendritic cells after its release from the cCHP nanogel [84]. Moreover, tetanus toxoid intranasally administered with cCHP nanogels induced strong tetanus-toxoid-specific systemic and mucosal immune responses, which suggest that cCHP nanogels can be used as a protein-basedantigen-delivery vehicle for adjuvant-free intranasal vaccination [84].
3.2.2. Lipid-Based Formulations
The nasal administration of drug-loaded liposomes showed effectiveness in the treatment of CNS disorders because it allows direct nose-to-brain drug delivery by means of lipid nanoparticles. Liposomes are applicable for the intranasal delivery of therapeutic proteins and peptides. Kakhi et al. [85] developed liposomal vaccines for intranasal administration. The liposomal vaccine was fabricated with the ErbB2 T-cytotoxic epitope, the influenza-derived HA T-helper epitope, and the lipopeptide adjuvant, Pam2CAG. Nasal administration of the liposomal vaccine resulted in efficient anti-lung tumor activity, where the size, structure, and flexibility of liposomes did not affect vaccine immunity and antitumoral efficiency. These results suggest the feasibility of liposomal carriers for antitumor vaccine delivery by nasal route [86].
Spray-dried polymer-coated liposomes could improve the penetration of proteins across the nasal mucosa [86]. Among the polymer-coated liposomes, TMC-coated liposomes have shown the high entrapment efficiency of BSA, a model protein, while retaining the structural integrity of the entrapped protein [86]. Recently, Kaplan et al. [87] developed liposomes with sizes ranging between 200 and 250 nm and entrapment efficiency of 73%. The liposomes were further dispersed in a chitosan gel (lipogel) to enhance the mucosal retention time [87].
3.3. Transdermal Delivery
Drug delivery via the skin offers some advantages including reduced dosing frequency with sustained drug release, bypassing the hepatic first-pass metabolism, cost-effectiveness, and high patient compliance [2,88,89]. Particularly, when compared to oral delivery, transdermal administration can prevent the chemical and enzymatic destabilization of protein drugs in the harsh gastrointestinal environment. Furthermore, transdermal administration is also applicable for topical drug delivery, which minimizes the adverse systemic effects. As a result, in the past few decades, there have been many attempts at delivering macromolecules via the transdermal route; however, inherent low absorption of macromolecules by impermeable stratum corneum is a major challenge [90].
The skin consists of three stratiform tissues; epidermis, dermis and subcutaneous tissue. The epidermis comprises the stratum corneum and viable epidermis. While the viable epidermis and dermis are hydrophilic, the hydrophobic and fibrous structure of the stratum corneum impedes the passage of exogenous materials and serves as a major barrier against the perfusion of large hydrophilic proteins [88]. In addition, adhesive membrane proteins form tight junctions in the viable epidermis, which forms an additional physical barrier. To overcome these barriers, various approaches have been developed including the addition of biochemical enhancers such as terpenes and glycols, and the use of physical penetration enhancement techniques such as iontophoresis, sonophoresis, electroporation, and microneedles [90]. Although these approaches have been successful, to a certain extent, in enhancing the skin penetration of macromolecules, chemical enhancers can cause local irritation and permanent skin damage. Physical enhancement approaches also have disadvantages including high cost and patient discomfort [90]. Recent advancements in nanotechnologies have promoted improvements in the transdermal delivery of hydrophilic macromolecules such as proteins and peptides. Some representative nano-formulations for transdermal delivery of protein drugs are described in the following sections.
3.3.1. Polymer-Based Formulations
Nanoparticles have lipid-fluidizing functions which alter the extracellular lipids of the stratum corneum and thus play an important role in altering skin permeability. Sadhasivam et al. [91] developed a nanoparticle system with chitosan for transdermal delivery of insulin. These insulin loaded-chitosan nanoparticles exhibited a quasi-circular structure with sizes ranging between 465 and 661 nm. Transdermal patches of insulin–chitosan nanoparticles were prepared using HPMC, PVP K30, and PEG 400 with Tween 80 as plasticizer [91]. The insulin release rate from patches increased as the concentration of hydrophilic polymer was increased [91]. Groot et al. [92] developed ovalbumin (OVA)-loaded poly(lactic-co-glycolic acid) (PLGA) nanoparticles with and without the TLR3 agonist, poly(I:C). Intradermal injection of the OVA-loaded PLGA nanoparticles by hollow microneedles primed both adoptively transferred antigen-specific naïve transgenic CD8+ and CD4+ T cells with markedly high efficiency. The immune response following immunization with anionic PLGA nanoparticles co-encapsulated with OVA and poly(I:C) provided protection against a recombinant strain of intracellular bacterium Listeria monocytogenes [92]. This study demonstrates that PLGA nanoparticles are an effective delivery vehicle for protein antigen into the skin. Monkare et al. [93] also developed hyaluronan-based dissolving microneedles loaded with PLGA nanoparticles (NPs) co-encapsulating OVA and poly(I:C) for intradermal immunization. The immunogenicity of PLGA NPs delivered by dissolving microneedles was compared with that of hollow microneedle-delivered NPs in mice. While NP-loaded dissolving microneedles achieved equally strong immune responses compared to hollow microneedles, humoral and cellular immune responses produced by NP-loaded dissolving microneedles were lower than those produced by NPs delivered through hollow microneedles [93]. Recently, Tu et al. [94] evaluated the transdermal delivery of protein drugs by N-trimethyl chitosan nanoparticles (TMC NPs) in the presence of electret. Electret prepared by a corona charging system with polypropylene film is a dielectric material that can maintain a quasi-permanent electrical charge for a long duration. In this study, superoxide dismutase (SOD)-loaded TMC NPs, when combined with electret, exhibited a strong inhibitory effect on edemas in the ears of mice, which indicates that the transdermal delivery of protein drugs in TMC NPs was significantly enhanced in the presence of electret [94]. This study suggests that TMC NPs combined with electret is a novel platform for the transdermal delivery of protein drugs. In addition, Chen et al. [95] developed a cardiac patch by using electrospinning cellulose nanofibers modified with chitosan/silk fibroin (CS/SF) multilayers, to improve the retention of the engrafted adipose tissue-derived mesenchymal stem cells (AD-MSCs). They demonstrated that CS/SF-modified nanofibrous patches promoted the retention and functional survival of engrafted AD-MSCs [95].
3.3.2. Lipid-Based Formulations
3.3.2.1. Emulsion
Nanocarriers dispersed in lipophilic vehicles should penetrate the stratum corneum efficiently since the stratum is hydrophobic. Among nanocarriers, nanoemulsion is a low viscosity isotropic dispersed system containing two immiscible liquid phases. In general, nanoemulsions can be prepared by high-pressure homogenization, phase-inversion temperature, and micro-fluidization [96]. Since nanoemulsion is a thermodynamically unstable system, its major drawback is physical instability such as creaming and flocculation during long-term storage [97]. However, optimizing the particle size and the composition of surfactants can produce a metastable state in the system. Although nanoemulsions for transdermal delivery are less popular due to their thermodynamic instability, transcutaneous immunization of nanodispersions has shown some potential as a transdermal antigen delivery vehicle [98,99]. Transcutaneous immunization is safe, non-invasive, economic, patient-friendly, and avoids first-pass metabolism [96]. Ledet etal. [98] prepared a novel nanoemulsion with FITC-BSA, squalane, Tween 80, and Span 80 by using high-pressure homogenization. These nanoemulsions exhibited optimized physical characteristics and superior skin permeation, indicating their flexibility for incorporating diverse active ingredients and the potential of their nanoemulsion system as a transcutaneous vaccine delivery vehicle [98]. Lopez etal. [99] also developed a novel imiquimod solid nanoemulsion (IMI-Sol) for transcutaneous immunization, which induced high-qualityT cell responses for enhanced protection against infections. Their study demonstrates that IMI-Sol can overcome the limitations of previous imiquimod based transcutaneous immunization approaches and promoted the prevention and treatment of infectious diseases and cancers [99]. Malakar etal. [100] developed insulin-loaded microemulsions for transdermal delivery using isopropyl myristate or oleic acid as the oil phase, Tween 80 as the surfactant, and isopropyl alcohol as the cosurfactant. They demonstrated that a microemulsion containing 10% oleic acid, 38% aqueous phase, 50% surfactant phase, and 2% dimethyl sulfoxide as a permeation enhancer was highly effective at improving the skin permeation of insulin.
To overcome the instability issues associated with nanoemulsions, S/O nanodispersion systems have been proposed, which are oil-based dispersions of hydrophilic molecules in solid powder form. S/O dispersions are prepared by removing water and cyclohexane from W/O emulsions via lyophilization followed by redispersion of the surfactant–drug complex in another oil vehicle [101]. Therefore, W/O nanoemulsions are precursors to S/O nanodispersions. For example, Araki etal. [102] developed a transcutaneous immunization vaccine based on S/O nanodispersion using OVA as a model antigen. OVA was coated with a surfactant to form nano-sizedsurfactant-OVA complexes and dispersed in the oil phase, which penetrated the skin without any adjuvant. Incorporation of ionic lipids in an S/O system caused higher amounts of OVA-specific serum IgG than S/O nanodispersions without ionic lipids [102]. Consequently, this study demonstrates that ionic lipid-mediated S/O nanodispersions were effective in delivering macromolecules via transcutaneous administration [102]. Likewise, S/O nanodispersion of BSA appeared to be an effective transdermal delivery system with enhanced skin permeation of model proteins [103]. Tahara etal. [104] also demonstrated that the collaborative effect of S/O nanodispersion and arginine-rich peptides as a permeation enhancer could enhance the skin permeation of insulin.
3.3.2.2. Liposome
Liposomes are efficient carriers for the delivery of therapeutics into the skin [89,105]. Due to the similarity between liposomal components and skin lipids, liposomes are easily absorbed by the epidermis and reach the deepest layers of the skin. In addition to delivering higher concentrations of a drug into the layers of the skin, liposomes enhance skin hydration via molecular mixing of the liposome bilayer with intracellular lipids in the stratum corneum [106]. Particularly, the topical application of liposomes offers a wide range of advantages such as increased moisturization, enhanced local effects, minimized unwanted systemic side effects, biodegradability, and biocompatibility.
The encapsulation of proteins within liposomes has been used to develop a non-invasive transdermal delivery system for macromolecules. To overcome the limited success of conventional liposomes [107], many advanced forms of liposomes have been used to improve skin permeation of macromolecules because the stratum corneum often limits the efficiency of drug delivery through the skin. Among them, the incorporation of drugs within flexible liposomal vesicles such as Transfersomes®, ethosome, niosome, and invasomes is a promising approach for delivering drugs into deeper layers of the skin or systemic circulation [108]. Transfersomes®, also called deformable liposomes, ultra-flexible liposomes, or elastic liposomes, have the special feature of elasticity induced by an incorporated edge activator (a single chain surfactant) [108,109]. While the first and second generation transfersomes® were mainly composed of phospholipids and surfactants, third-generation transferosomes are composed of amphiphilic surfactant combinations with or without phospholipids and exhibit greater elasticity and skin permeability than conventional liposomes [109]. Previous studies have demonstrated the effectiveness of Transfersomes® as a transdermal delivery system of proteins. A transferosomal gel containing insulin was also successful in enhancing the skin permeation of insulin and achieving a prolonged hypoglycemic effect in alloxan-induced diabetic rats over 24 h after transdermal administration [110]. Similarly, interleukin-2 and interferon-alpha also showed an enhanced immunologic effect when formulated into transfersomes, which indicates the potential of transfersomes as a transdermal delivery system of macromolecules. In addition, improved immunogenicity was observed when the carboxyl-terminal19 kDa fragment of merozoite surface protein-1 (PfMSP-119) from Plasmodium falciparum was administered subcutaneously through elastic liposomes as an improved vaccine delivery system [111].
In addition to Transfersomes®, niosomes and ethosomes are also applicable for transdermal delivery of macromolecules. Niosomes are self-assembling elastic nanovesicles composed of one or more non-ionic surfactants and/or lipids [108]. They are second-generation elastic vesicles in which molecular clusters are formed by self-aggregation of non-ionic surfactants in an aqueous phase or in combination with cholesterol [112]. They are chemically stable because they lack phospholipids, which thereby avoids oxidative degradation [112]. They also offer enhanced penetration and a localized depot for sustained drug release through the skin. Ethosomes are a multi-phase dispersion system, composed of phospholipids, water, and highly concentrated alcohol. The head of the lipid bilayer contains alcohol, which enhances the flexibility and fluidity of ethosomes [112]. Meng etal. [113] evaluated the transdermal potential of novel testosterone propionate (TP) ethosomes and liposomes prepared by surfactant modification. The generated TP-loaded ethosomes were nanometric in size, displayed high entrapment efficiency, and exhibiting greater skin permeation and stability than conventional liposomes.
For more effective delivery of macromolecules into the skin, liposomal formulations are incorporated into a dissolving microneedle array. For example, Zhao etal. [114] developed ovalbumin (OVA)/platycodin (PD)-loaded liposomes (OVA-PD-Lipos) and incorporated them into a dissolving microneedle array (OVA-PD-Lipos-MNs) to enhance transdermal immunization. These liposomes exhibited high stability of OVA, low toxicity of PD, and enhanced cellular uptake. Subsequently, OVA-PD-Lipos-MNs showed a significantly enhanced immune response, suggesting this combined system of liposome and dissolving microneedle array is a promising delivery vehicle for subunit vaccines and their adjuvants into the skin [114]. Guo etal. [115] also developed a transcutaneous vaccine device consisting of a dissolving polyvinylpyrrolidone (PVP) microneedle array where the tips were loaded with antigenand adjuvants encapsulated in liposomes. They selected ovalbumin (OVA) as the model antigen, CpG OND as the adjuvant, and cationic liposomes (Lip) for co-delivering antigens and adjuvant. An enhanced immune response was observed with this system, and it also achieved a shift of immune type from a predominately Th2 type to a balance of Th1/Th2 types [115].
3.3.3. Others
3.3.3.1. Microneedle
Microneedles are tiny needles with lengths between 50 and 900 μm, and they can pass through stratum corneum to create microchannels [116,117]. As a pain-free device, microneedles improve the patient’s compliance and provide a versatile platform to overcome the skin barrier for hydrophilic and high molecular weight drugs, including protein drugs [118]. They can be fabricated using silicon, metals, biodegradable polymers, or carbohydrates. First-generation microneedles were solid microneedles that perforated the skin membrane and enhanced the permeability of drugs. Although solid microneedles appeared to be effective in delivering insulin [119], the application of solid microneedles is limited by low delivery efficiency, complex administration, a lack of precise dosing, and risk of infection [120]. Recently, solid microneedles were modified by coating the drug payload directly onto the surface of microneedles. Dip-coating, casting, and deposition techniques are widely used for coating the microneedles. Many studies on protein delivery using coated microneedles have been successfully carried out. These studies examined the delivery of recombinant human erythropoietin alfa, desmopressin, insulin, and various antigens [13,121]. Kusamori at al. [122] also developed interferon-alpha (IFNα)-coated polyvinyl alcohol-based microneedles. They reported efficient delivery of INFα from microneedles with minimal invasion. Moreover, the pharmacokinetic parameters and anti-tumor activity of IFNα-microneedles were comparable to those of IFNα subcutaneous administration [122].
Hollow microneedles contain a fluid drug formulation, which pierces the skin and transports the drug through the openings [13]. An important benefit of hollow microneedles over solid microneedles is their ability to facilitate force-driven fluid flow, which allows faster rates of drug delivery [13]. Furthermore, the dose of the desired drug in the solution can be more easily controlled according to the need of the patient.
Additional strategies such as the use of dissolvable or degradable polymers and the incorporation of bio-responsive materials have also been used to improve the efficacy of microneedles [121]. Dissolvable microneedles are completely dissolved upon insertion into the skin, and this triggers the release of the drug. Duration of action ranges from an hour to days depending on the dissolution rate of the polymer [123]. Hydrophilic polymers swell rapidly and dissociate the chain network, which drives drug release upon insertion of a microneedle into the skin. Mönkäre etal. [124] reported the preparation of monoclonal IgG-loaded hyaluronic acid-based dissolving microneedles. After the dissolution of the microneedles, more than 80% of the incorporated IgG was recovered and no conformational changes were observed. In an ex-vivo study, the majority of the original tip length was dissolved within 10 min, and both IgG and hyaluronan were deposited to a depth of 150–200 μm in the skin. This study indicated that hyaluronan-based dissolving microneedles could be useful for rapid non-invasive intradermal protein delivery.
Incomplete insertion is a common problem associated with polymer microneedles, leading to inefficient drug delivery and wastage of valuable medication. To resolve this issue, Chen etal. [125] developed a fully insertable insulin-loaded microneedle system using poly-γ-glutamic acid (γ-PGA) microneedles and polyvinyl alcohol (PVA)/polyvinyl pyrrolidone (PVP) for supporting structures. This microneedle system dissolved within 4 min after insertion into the skin and quickly released its insulin payload. Furthermore, the hypoglycemic effect of insulin-loaded microneedles was comparable to that of subcutaneous insulin injections in diabetic rats [125]. This study indicated that γ-PGA microneedles containing a supporting structure design have great potential for the relatively rapid and convenient transdermal delivery of protein drugs [125].
Biodegradable microneedles provide sustained drug release upon the hydrolysis of the biodegradable polymer matrix [13]. Polymers with high molecular weight and high crosslinking density are preferred when fabricating this type of microneedles. Recently, Seong etal. [126] fabricated bullet-shapeddouble-layered microneedle arrays using water-swellable tips made of polystyrene-block-poly(acrylic acid) and insulin as a model protein drug. The bullet-shape provides an optimal geometry for mechanical interlocking with soft tissues via selective distal swelling after skin insertion. In an in-vivo pilot study, this microneedle system showed a prolonged release of insulin and a gradual decrease in blood glucose levels [126].
Bioresponsive microneedles are triggered to release protein drugs in response to physiological signals such as variations in pH, glucose, reactive oxygen species and enzymes, which offer opportunities for on-demand release of drugs in a relatively precise manner [127]. Wang etal. [128] developed a bio-inspiredglucose-responsive insulin delivery system, which was a painless core-shell microneedle array patch consisting of a degradable cross-linked gel. This gel-based device partially dissociated and subsequently released its insulin payload when triggered by hydrogen peroxide (H2O2) generated during the oxidation of glucose by a glucose-specific enzyme covalently attached inside the gel. This resulted in the effective regulation of blood glucose levels in a diabetic mouse model [128].
Collectively, advancements in materials and nanotechnology have facilitated the fabrication of more sophisticated microneedle devices that improve efficacy, biocompatibility, and safety. Furthermore, advancements in the formulation technologies necessary to load drugs into microneedles signal a bright future for microneedles as an effective transdermal delivery system for hydrophilic macromolecules.
3.3.3.2. Iontophoresis
Iontophoresis is one of the technigues to improve the transdermal drug delivery by using mild electric current (usually <0.5 mA/cm2 of skin) to the skin [129]. The charged drug is placed under an electrode with the same polarity; therefore, when electirc current is applied, the drug is moved away from the electrode into the skin by electro-repulsion [13]. Electromigration and electroosmosis are two main drug transport mechanisms for iontophoretic delivery [129]. As the repulsion of the charged drug by an electrode with the same polarity, electromigration is the principal transport mechanism for charged drugs [129]. Electroosmosis is the flow of solvent from anode to cathode and it is the transport mechanism for neutral hydrophilic drugs into the skin [129]. Iontophoretic drug delivery is affected by multiple factors including the physicochemical drug properties (e.g. size, overall charge, structure, and lipophilicity), current density, duration of current application, and electrodes. Therefore, these factors should be adjusted to optimize the iontophoresis-mediated drug delivery. In addition, the main limitations of iontophoretic drug delivery should be also considered, which include the size limit of drug molecules up to ~10–15 kDa, potential malfunction of the device itself, and the possibility of dose dumping [129].
Badkar etal. [130] demonstrated the transdermal delivery of interferon alpha-2b (IFNalpha2b) in hairless rats using iontophoresis and microporation, where iontophoresis enhanced the penetration of IFNalpha2b through microporated skin in hairless rats. In addition, many studies were carried out with small molecules and demonstrated the synergistic effect of iontophoresis in the transdermal drug delivery [107].
3.3.3.3. Sonophoresis
Sonophoresis uses ultrasound waves to increase the skin permeability of drugs. Ultrasound waves induce expansion and oscillation of the air pockets in the stratum corneum, consequently disrupting the lipid bilayer and forming cavities which enhance the drug permeability through the skin [131]. In addition to the net ultrasound exposure time and the pulse "on" duration, the degree of enhancementin drug delivery by sonophoresis depends on the physicochemical drug properties of the drug. Even though sonophoresis can enhance the transdermal delivery of therapeutic proteins, potential instability issues of proteins by ultrasound should be carefully evaluated.
Combination of sonophoresis with other enhancement methods such as chemical enhancers, electroporation, and iontophoresis can be more effective in enhancing the drug delivery through the skin as compared with sonophoresis alone. Liao etal. [132] demonstraed that combined treatment with optimal ultrasound and microbubbles could enhance the transdermal delivery of α-arbutin to inhibit melanogenesis without damaging the skin in mice [132]. The combined system improved the penetration depth, concentration, and efficiency of transdermal α-arbutin delivery when compared to either the drug alone or ultrasound alone.
3.3.3.4. Electroporation
Electroporation is an electrical technique applying high voltage electronic pulses for short durations of time to increase the skin permeability by opening the aqueous pores in a reversible manner [13,129,133]. Both electroporation and iontophoresis rely on the application of an electric field: however, electroporation acts mainly on the skin to alter the membrane permeability to enhance drug penetration while iontophoresis directly acts on the drug to propel it into the skin [13,129,133].
Many studies reported the effectiveness of electroporation to enhance the transdermal delivery of proteins. For example, Mohammad etal. [134] investigated the effects of electroporation parameters on the transdermal delivery of insulin. Results showed that the increase in the number of pulses (from 15 up to 60 successive pulses) and the decrease in applied field strength (from 200 to 100 V/cm) significantly decreased the blood sugar level in rabbits. Furthermore, combination of electroporation with other enhancement techniques such as iontophoresis offers a synergistic effect on the transdermal delivery of macromolecules. For example, Medi etal. [135] demonstrated the synergistic effect of electroporation in combination with iontophoresis on skin permeability of human parathyroid hormone.
3.4. Pulmonary Delivery
The lung allows rapid and high drug absorption due to its large surface area (approximately 80–140 m2), very thin alveolar epithelium thickness (0.1–0.5 mm), and large blood supply [2,136]. Pulmonary drug delivery is advantageous because it avoids the hepatic first-pass effect and it is also non-invasive, efficacious at lower doses, and applicable for local or systemic delivery [2,136]. Lung tissue has relatively low enzymatic activity when compared to the GI tract and pulmonary epithelium has many immunological properties [137]. However, pulmonary delivery does have some disadvantages including short duration of activity due to the rapid removal of the drug. Inhaled drugs can be swept from the airways toward the mouth and can also be removed via phagocytosis by alveolar macrophages after their deposition in the lungs [137]. Therefore, an effective slow-drug release requires a means of avoiding or suspending the lungs’ natural clearance mechanisms until encapsulated drugs have been effectively delivered. In general, proteins with molecular weights between 6000 and 50,000 D have good bioavailability following inhalation [11]. Therefore, pulmonary administration has gained a great deal of attention as an attractive delivery route for protein drugs.
The delivery device plays a critical role in the effectiveness of pulmonary drug administration and thus the selection of a delivery device is one of the key factors in the formulation design for pulmonary drug delivery. The most commonly used devices used to deliver therapeutics as aerosols are nebulizers (e.g. jet nebulizers, ultrasonic nebulizers, and vibrating mesh nebulizers), metered-dose inhalers, and dry powder inhalers. Selection of a delivery device depends on the nature of the drug, its formulation including aerosol properties, the site of action, and the pathophysiology of the lungs [138]. Protein nebulizer formulations are mainly aqueous solutions and are relatively easy to develop. Mechanically generated vibration mesh technologies are currently being used in clinical trials for the delivery of proteins and peptides.
Various nanotechnology-based formulation approaches have been extensively examined for effective protein delivery via the pulmonary route. In general, nanoparticles appear to be promising as a pulmonary delivery carrier of proteins due to their targeting capability and controlled drug release. In addition, nanoparticles <200 nm in size might escape the recognition by alveolar macrophages, resulting in more effective uptake and drug action [139]. Besides polymeric nanoparticles, other types of nanocarriers including liposomes and solid lipid nanoparticles have been used for pulmonary delivery of protein drugs [140]. More detailed discussion on these nanocarriers can be found in the following sections.
3.4.1. Polymer-Based Formulations
Polymeric nanoparticles (NPs) are widely used as a pulmonary drug delivery carrier due to biocompatibility and the ease of surface modification and copolymerization [139]. The most commonly used natural polymeric carriers include chitosan, alginate, and gelatin. Similarly, poloxamer, poly(lactic-co-glycolic) acid (PLGA), and polyethylene glycol (PEG) are the most commonly used synthetic nanocarriers for pulmonary drug delivery [136,139,141]. Recently, Menon et al. [139] examined the capabilities of six polymeric NPs (gelatin, chitosan, alginate, PLGA, PLGA-chitosan, and PLGA- PEG) with regard to inhalational protein/DNA delivery. Among them, gelatin and PLGA NPs exhibited the smallest particle sizes (<200 nm), which was within the optimal range for deposition in the alveolar region by nebulization while avoiding phagocytosis by alveolar macrophages [139]. Based on these profiles, gelatin and PLGA NPs were used to encapsulate plasmid DNA encoding yellow fluorescent protein (YFP) or rhodamine-conjugated erythropoietin (EPO) for pulmonary delivery to rats. Following a single inhalation, widespread pulmonary EPO distribution persisted for up to 10 days while increasing YFP expression was observed for at least 7 days for both NPs [139]. These results support both PLGA and gelatin NPs as promising carriers for pulmonary protein/DNA delivery.
Alfagih et al. [137] also developed bovine serum albumin (BSA)-loaded NPs using a biodegradable polymer poly(glycerol adipate-co-ω-pentadecalactone) (PGA-co-PDL), for pulmonary delivery. NP size was mainly affected by the polymer mass used and the structural stability of proteins was well maintained. The effective uptake of NPs was observed in target dendritic cells with >85% cell viability [137]. These results indicate the optimal process parameters for the preparation of protein-loaded PGA-co-PDL NCMPs suitable for inhalation.
3.4.2. Lipid-Based Formulations
Liposomes are the most effective pulmonary carrier for proteins drugs, offering enhanced and sustained drug release, biocompatibility, biodegradation, and non-immunogenicity [2]. Liposomes can enhance drug permeation through alveolar epithelium by altering the physicochemical properties of the drug (e.g. rendering the drug hydrophobic) and by decreasing the mucociliary clearance due to their surface viscosity [2]. Therefore, liposomal formulations have been developed for the pulmonary delivery of protein drugs. In addition to liposomes, solid lipid nanoparticles (SLN) have also been studied as carriers for the delivery of therapeutic proteins through the lung epithelium. Particularly, the production of spray-dried powders containing SLNs has overcome the problem of low inertia which can prevent nanoparticles from depositing in the lung. Gaspar et al. [142] developed a new hybrid microencapsulated SLN for pulmonary administration. Papain, a model protein, was adsorbed onto glyceryl dibehenate and glyceryl tristearate SLN and then, incorporated into mannitol and trehalose microspheres by a spray-drying process, leading to spherical microparticles with suitable aerodynamic characteristics for lung deposition [142]. Protein stability was maintained throughout microsphere production, and the protein released from the dry powder formulations retained its enzymatic activity. These results suggest the utility of microencapsulated SLN as protein carriers for pulmonary delivery.
3.5. Ocular Delivery
Due to the urgent need for advanced therapeutic approaches for the treatment of chronic ocular diseases, the ophthalmology market has grown tremendously over the last 20 years [14]. Particularly, the emergence of the anti-vascular endothelial growth factor (anti-VEGF) aptamer (Macugen; Pegaptanib sodium; OSI Pharmaceuticals, New York) and monoclonal antibody (Lucentis; Ranibizumab; Genentech, California) in the early 2000s dramatically accelerated the expansion of ophthalmic protein and peptide-based drug market. Global sales of biological drugs for ophthalmic indications are expected to increase to $35.7 billion by 2025 [143]. Compared to small molecule drugs, proteins-based drugs have shown great promise as novel therapeutics for the treatment of ocular diseases, with some advantages including high potency, less toxicity, low drug-drug interaction, and increased biological and chemical diversity [14,17]. However, the ocular delivery of these macromolecules faces several challenges which include physicochemical degradation, low permeability, short in-vivohalf-life, and risk of immunogenic effect [14,17]. Consequently, there is a strong need to develop novel ophthalmic delivery systems for macromolecules and to target these macromolecules to biologically relevant ocular tissues.
Since topically-applied conventional dosage forms, such eye drops have the main drawbacks of low bioavailability and subsequent low therapeutic efficiency, various new strategies have been developed to overcome the ocular delivery barriers and enhance the bioavailability of proteins via the ocular route of administration. For example, the utilization of chemical chaperones and co-administration of recombinant human hyaluronidase have been attempted to facilitate protein delivery through the ocular route [144,145]. Since protein aggregation is one of the primary concerns in the formulation of proteins for ocular diseases, a novel strategy to utilize chemical chaperones (protein aggregation inhibitors) was developed to prevent protein misfolding and/or inhibit the self-assembly of aggregation-prone sequences in native protein structures [144]. The co-administration of recombinant hyaluronidases has also been used for decades to increase the penetration of biological drugs across ocular tissue barriers because hyaluronidases catalyze the degradation of hyaluronic acid, a key structural component of tissues [145]. Furthermore, various nanocarriers including polymeric micelles, liposomes, nanospheres, nanowafers, and dendrimers are being extensively evaluated for their controlled and targeted delivery of proteins via the ocular route. Some formulation approaches that enhance the ocular delivery of protein drugs are described in the following section.
3.5.1. Polymer-Based Formulations
3.5.1.1. Nanoparticles
Nanoparticles can be administered through various routes including topical, periocular, suprachoroidal and intravitreal. However, intravitreal injection of nanoparticles often leads to clouding of the vitreous due to the light scattering properties of polymeric particles. The possible loss of bioactivity, low stability of proteins due to interactions with nanoparticle matrix and extensive nanoencapsulation methods may complicate the delivery of nanoformulations of proteins [14]. So far, only a few nanoparticle-mediated ocular delivery systems are reported for protein drugs, and they are in the early stage of development.
Many studies have proven that polymeric nanoparticles, including a polymer-basedthermo-gelling system, are successful in ocular drug delivery. Cho etal. [146] developed a thermosensitive hexanoyl glycol chitosan (HGC) as a new drug carrier for topical drug delivery to the eye, by modulating the degree of N-hexanoylation to control thermogelling. Since HGC was retained on the preocular surface for a comparatively longer period of time due to its enhanced viscosity at body temperature, brimonidine-loadedHGC achieved better bioavailability and prolonged duration of action compared to the marketed formulation (Alphagan P)[146]. This study indicates that an HGC-based formulation can overcome the limited bioavailability of conventional eye drops due to the rapid drug removal from the surface of the eye. Agrahari etal. [147] synthesized a pentablock (PB) copolymer (PB-1: PCL-PLA-PEG-PLA-PCL)-based nanoformulation suspended in a thermosensitive gelling copolymer (PB-2: mPEG-PCL-PLA-PCL-PEGm) as a novel ocular delivery system for proteins. Subsequently, IgG-Fab encapsulated nanoparticles suspended in thermosensitive gelling copolymer demonstrated a continuous zero-order release, avoiding the potential toxicity associated with peaks and valleys of the protein concentration in the target tissues [147]. This study suggests that PBcopolymer-based composite nanoformulations can serve as a platform for ocular delivery of therapeutic proteins, which may reduce the side effects from frequent intravitreal injections.
3.5.1.2. Polymeric Micelles
Polymeric micelles are nanocarriers with sizes ranging between 10 and 100 nm and are composed of amphiphilic block copolymers with hydrophilic chains forming a shell and hydrophobic chains forming a core [14,148]. They can be self-assembled in aqueous media to form an organized supramolecular structure at concentrations exceeding their critical micellar concentrations [14,148]. Polymeric micelles for ocular delivery are prepared by using various polymers including poloxamer 407, poloxamer 188, methoxy poly (ethylene glycol)-poly(ɛ-caprolactone), poly (butylene oxide)-poly (ethylene oxide)-poly (butylene oxide), polyhydroxyethylaspartamide, and, N-isopropylacrylamide (NIPAAM) [148]. They can also be fabricated by using the various methods examined in a recent review by Mandal et. al. [148].
Polymeric micelles have improved stability, high drug loading capacity, a low risk for toxicity, highly modifiable surfaces, and controlled drug release [14,148,149]. Particularly, mucoadhesive polymeric micelles can enhance the residence time at the ocular surface, and the nano-range size of polymeric micelles offer better penetration into ocular tissues. In addition, they have high patient compliance because they are easy to apply and produce no visual interference [148,149]. Previous studies have demonstrated significant improvements in the in-vivo performance of macromolecules via the ocular administration of polymeric micelles. For example, anti-Flt1 peptide (an antagonistic peptide for vascular endothelial growth factor receptor 1 (VEGFR1 or Flt1)) was chemically conjugated to tetra-n-butyl ammonium modified hyaluronate (HA-TBA) via amide bond formation for the treatment of retinal neovascularization and diabetic retinopathy. The resulting conjugate self-assembled to form micelle-like nanoparticles in aqueous solution [150]. Anti-Flt1peptide-HA conjugates in the form of micelles effectivelyinhibited retinal choroidal neovascularization (CNV) in laser-inducedCNV model rats. They also significantly reduced the retinal vascular permeability and the deformation of retinal vascular structure in a diabetic retinopathy rat model [150]. These conjugate micelles also increased the mean residence time of anti-Flt1 peptide to longer than 2 weeks.
In addition to conventional polymeric micelles, new ocular delivery technologies have emerged for facilitating controlled drug release, including micelles as nano-scale microbubble precursors and stimuli-responsive micelles [151]. Particularly, innovative carriers such as stimuli-responsive intelligent polymeric vesicles biomimicking the biological activities of lipid bilayers can lead to effective ocular delivery of protein drugs to both anterior and posterior segments of the eye [151]. Although only a few stimuli have been investigated in this field to date, the stimuli applicable for ocular delivery may include temperature, pH, light, magnetic fields, or disease-specific biological components [151]. Based on more extensive feasibility studies with various proteins and peptides in future, the stimuli-responsive intelligent polymeric vesicles may provide a new platform for the effective ocular delivery of macromolecules, although the limitation of biomaterials and effective stimuli responses are still a hurdle that needs to be overcome.
3.5.1.3. Dendrimers
Dendrimers are nano-sized polymeric carriers that can entrap and/or conjugate high molecular weight molecules [152]. They are radially symmetric molecules with well-defined, homogeneous, and monodisperse structures consisting of tree-like branches. Some commonly used dendrimers are based on polyamidoamines, polyamines, polyamides (polypeptides), poly (aryl ethers), polyesters, and carbohydrates, of which polyamidoamines (PAMAM) are the most common and commercially available [152]. Their molecular weight and surface charge play a crucial role in determining tissue accumulation profiles and drug release rates from the polymer. The absorption is maximal in the cationic dendrimers due to their ability to interact with lipid bilayers, and it is decreased with the uncharged or anionic dendrimers [153]. Additionally, the multiple functional groups on their surface make it possible to target any location in the body, which can reinforce ligand-receptor binding, ameliorate the targeting of attached components, and accelerate dendrimer stimuli-responsive functions [14]. Holden etal. [154] fabricated a PAMAM dendrimer hydrogel by crosslinking responsive acrylate groups from PEG-acrylate chains, which were activated by UV light. They found that the dendrimer hydrogel was highly mucoadhesive and nontoxic to human corneal epithelial cells. In addition, high cellular uptake and enhanced “bovine corneal transport” of drugs were reported [154]. They also fabricated a novel hybrid PAMAM dendrimer hydrogel/poly (lactic-co-glycolic acid) (PLGA) nanoparticle platform for co-delivery of drugs. The formulation was found to be effective, non-toxic, highly absorbed, and exhibited a sustained drug release profile on the ocular surface of rabbits [155]. Studies have also confirmed the delivery of therapeutic peptides and proteins using different conjugation techniques with dendrimers. As proteins can form an adsorbed layer called a “protein corona”, the mechanism of formation of a protein corona allows for a better understanding of dendrimer conjugates in a biological environment, which thereby helps in effective formulation design as well [156]. Even though dendrimers are not yet approved for clinical use in the eye, their promising preclinical results may lead to significant advancements in clinical development as some of the drawbacks associated with dendrimers (multistep syntheses, high preparation costs, inadequate quality control assays, etc.) are improved.
3.5.1.4. Others
Nanowafers are tiny transparent circular or rectangular membranes that contain arrays of drug-loadednano-reservoirs that release drugs in a highly controlled manner and for a longer duration than eye drops (a few hours to several days) [14,157]. They are composed of various polymers such as poly (vinyl alcohol) (PVA), polyvinyl pyrrolidone (PVP), hydroxypropyl methylcellulose (HPMC) and carboxymethyl cellulose (CMC) [157]. Nanowafers are applied with the patient’s fingertip, can withstand constant blinking without removal, and release the drug slowly. This enhances the drug resident time and absorption into ocular tissues and improves therapeutic efficacy [157]. Furthermore, during drug release, the nanowafer slowly dissolves rendering ocular surfaces free of polymers. The feasibility of nanowafers as an ocular delivery system has been examined in previous studies [158]. Yuan etal. [158] developed PVA-based nanowafers loaded with axitinib for the treatment of corneal neovascularization (CNV). These nanowafers were nontoxic, and in a murine ocular burn model, once-a-day dosing with an axitinib nanowafer was two-fold more effective, when compared to twice-a-day dosing with a topical eye drop formulation [158]. Consequently, these results confirmed that the nanowafer formulation was more effective than topical eye drops even at a lower dosing frequency. These promising results provide a rationale for the preclinical and clinical evaluation of nanowafer for the ocular delivery of macromolecules including proteins and peptides.
Drug-loaded contact lenses are also candidates for effective ocular drug delivery and therapy. Contact lenses provide longer drug residence time on the eye, which enhances drug permeation through the cornea [159]. The slow diffusion of drug molecules from the lens matrix offers sustained drug release. The residence time and drug release rate can be further improved by entrapping the drug in nanocarriers and dispersing the drug-loaded nanocarriers in the lens matrix [159]. The application of drug-loaded contact lenses as an ocular delivery system for macromolecules needs to be further explored to achieve the desired therapeutic objectives. However, limitations including drug leaching during storage and distribution and safety issues related to surface roughness must be resolved.
3.5.2. Lipid-Based Formulations
Lipid-based nanocarriers such as liposomes, solid lipid nanoparticles, and nanostructured lipid carriers have been examined for ocular protein delivery. A number of repeated intravitreal injections can increase the risk of ocular complications including vitreous hemorrhage, endophthalmitis, retinal detachment, and cataracts; therefore, sustained drug release should be beneficial in reducing the risk of these ocular complications. Li etal. [160] developed novel pigment epithelium-derived factor (PEDF)-loaded immuno-nanoliposomes (INLs) for the treatment of choroidal neovascularization (CNV). After injection into CNV models of BN rats, PEDF-loaded INLs exclusively bound to CNV but not to normal choroidal vessels, which led to a significant reduction in the CNV area of PEDF treatment groups [160]. Moreover, PEDF-loaded INLs exposed to ultrasound were more efficient in reducing the CNV area. This suggests that INLs, in combination with ultrasonic exposure, can transmit PEDF into the cytoplasm with high specificity and efficiency, thereby enhancing the inhibitory effects of PEDF on CNV and reducing its side effects [160]. Consequently, PEDF-loaded INLs could represent a new treatment paradigm for patients with ocular neovascularization.
Solid-lipid nanoparticles (SLNs) also appear to be a promising ocular delivery system for improving therapeutic efficiency without compromising drug safety and patient compliance. As a drug carrier, SLNs offers many advantages including physical stability, targetability, controlled release, easy scale-up, and non-toxicity since they are composed of physiological lipids [161]. In addition, SLNs can enhance the corneal absorption of drugs and improve the ocular bioavailability of both hydrophilic and lipophilic drugs. SLNs are also amenable to autoclave sterilization, a required step for ocular formulations, and they are dispersible in aqueous media, enabling them to be formulated as modified eye drops [161]. Based on these assets, SLNs are especially useful as an ocular drug delivery carrier. However, drawbacks of SLNs include low drug loading efficiency, initial burst release of drugs, and rapid elimination by the mononuclear phagocytic system [161]. To overcome these drawbacks, a nanostructured lipid carrier has been developed, which is composed of highly disordered solid and liquid lipids and improves the entrapment efficiency compared to SLNs [162]. Although SLNs and nanostructured lipid carriers have shown high potential in delivering small molecules to ocular tissues, their efficiency in delivering macromolecules has not been fully exploited, yet. Vicente-Pascual etal. [163] developed SLN systems for the delivery of p-IL10, which could allow for the sustained de novo synthesis of the cytokine in corneal cells and provide long-termanti-inflammatory effects. This SLN system was able to enter and transfect human corneal epithelial cells, which suggests that SLN-basedvectors are promising gene delivery systems for corneal diseases [163]. Although SLNs meet many of the requirements for ocular formulations, as described above, more extensive evaluations are needed to expand the application of SLNs for the ocular delivery of macromolecules such as protein drugs.
Niosomes, self-assembling nanovesicles composed of non-ionic surfactants that behave like liposomes, are also preferred for topical ocular drug delivery due to their chemical stability, biodegradability, biocompatibility, lack of immunogenicity, and low toxicity [164]. They can also encapsulate both lipophilic and hydrophilic drugs, offering structural flexibility. Discomes are a modified form of niosomes with large structures (12–16 mm) created by the addition of Solulan C24 (non-ionic surfactant). They are well-suited for the ocular route of administration since their large size may prevent their drainage into the systemic pool [164]. In addition, discomes are disc-shaped, which helps them fit better into the cul-de-sac of the eyes. Niosomes and discomes are still in early-stage development, and their potential as an ocular delivery system of proteins should be further explored.
3.6. Rectal Delivery
The rectal administration of drugs has been a long-lasting medical practice for the management of local and systemic diseases due to the fairly stable physicochemical and enzymatic environment of the colorectal mucosa and the possibility of avoiding the hepatic first-pass effect [133,165]. In addition, rectal administration is useful when patients are susceptible to nausea, vomiting, convulsion, or are unconscious. The lower part of the rectal venous drainage is directly connected to the general circulation, avoiding the hepatic first-pass effect. Furthermore, extensive rectal vasculature, the presence of lymphatic vessels, and lower protease activity can enhance drug absorption via the rectal route of administration [133]. Rectal drug delivery may improve the bioavailability of protein drugs which are very vulnerable to physicochemical and enzymatic- destabilization [133]. Therefore, various approaches to improve the bioavailability of drugs via the rectal administration have been attempted, including the utilization of absorption enhancers, protease inhibitors, prodrugs, and nanoformulations. In general, absorption enhancers are required to increase rectal absorption of macromolecules such as insulin, heparin, calcitonin, recombinant human granulocyte colony-stimulating factor (rhG-CSF), and human chorionic gonadotrophin [133,166]. Although a number of absorption enhancers can be used in rectal drug delivery, some of them cause membrane damage and irritation; thus, non-toxic and effective enhancers are in high demand. Similarly, the use of protease inhibitors is also effective at improving rectal bioavailability by reducing the degradation of proteins and enhancing the enzymatic stability of protein drugs. Prodrug approaches are being pursued to enhance the absorption of proteins and peptides by protecting them against degradation from peptidases and other mucosal enzymes. In addition to those approaches, nanotechnology-based formulation approaches are available for improving protein drug delivery via rectal administration. Although rectal formulations of macromolecules have been explored less extensively when compared to other routes of administration, continuous research and development may reveal the hidden potential of rectal drug delivery systems for macromolecules such as proteins and peptides. Some of the formulation approaches for rectal protein delivery are introduced in the following sections.
3.6.1. Polymer-Based Formulations
3.6.1.1. Hydrogel
Previous studies demonstrated the effectiveness of hydrogel formulations at improving the bioavailability of protein drugs via rectal administration. Shi etal. [167] developed a novel hydroxypropyl methyl cellulose-co-polyacrylamide-co-methacrylic acid (HPMC-co-PAM-co-PMAA) hydrogel as a rectal suppository. The HPMC-co-PAM-co-PMAA hydrogel was fabricated via free-radical polymerization and loaded with insulin to regulate blood glucose levels in diabetic cases. This insulin-loaded hydrogel was non-cytotoxic and could release insulinin a continuous manner in pH 7.4 buffer (rectal conditions), exhibiting an obvious invivo hypoglycemic effect [167]. Xue etal. [168] also reported a novel binary hydrogel containing insulin as rectal suppository via solution polymerization. Many of the micro-pores inside binary hydrogels can accommodate a large amount of drug molecules. And an insulin-loaded hydrogel was able to diffuse drugs in a sustained manner, which resulted in reduced blood glucose levels in diabetic rats [168]. These promising preclinical observations strongly support hydrogel formulations as effective rectal delivery systems for protein drugs.
3.6.1.2. Nanoparticles
While the valuable features of polymeric nanoparticles have already been proven for most delivery routes, the development of polymeric nanoparticles for rectal administration is still limited [169]. Chitosan and its derivatives, PLGA, PLA, and methacrylic acid copolymers are commonly used to fabricate polymeric nanoparticles for rectal drug delivery [169]. Furthermore, these polymeric nanoparticles often undergo surface modification to offer additional assets such as site-specificity or longer circulation time. Zuo etal. [170] developed a novel nano-complex by using galactosylated low molecular weight chitosan (gal-LMWC) and an antisense oligonucleotide (ASO) against TNFα for the treatment of Crohn's disease. They successfully demonstrated targeted delivery of gal-LMWC/ASO complexes into activated colonic macrophages, which significantly reduced colonic TNFα in mice with colitis via intracolonic administration [170]. Recently, Nunes etal. [171] reported that dense surface modification of PLGA nanoparticles with short-chain polyethylene glycol (PEG) in a non-covalent fashion could enhance drug distribution and retention after rectal delivery when compared to non-modified nanoparticles. This indicates the usefulness of PEG-modified nanoparticles as mucus-penetrating carriers. Furthermore, the intestinal organoids-based delivery system may be a potential approach for delivering protein drugs to inflamed areas, since intestinal organoids can improve the functionality of the drug-loaded nanoparticles due to their ability to adsorb small nanoparticle inside the lumen and attach to the damaged area [172].
As nanotechnology has advanced, carbon nanotubes (CNTs) have appeared as ground breaking nanocarriers for the delivery of diverse compounds that suffer from short half-lives and poor solubility [173,174]. CNTs belong to the fullerene family of carbon allotropes with cylindrical shapes and exhibit unique physiochemical properties with easy surface modification [174,175]. They also possess superior mechanical properties, high thermal conductivities, and, more importantly, the ability to penetrate cell membranes, which make them suitable as nanocarriers for targeted or controlled drug delivery, biosensing, and bioimaging [173,174]. Hassanzadeh etal. [173] fabricated multi-walled carbon nanotube (MWCNTs) complexes loaded with 2-arachidonoylglycerol (2-AG) to improve the pharmacological effect of 2-AG. Once-daily intrarectal administration of this MWCNTs-2-AG complex enhanced the therapeutic effects of 2-AG with longer duration in a rat colitis model [173]. In addition to small molecules, CNTs can also deliver proteins [174]. Although there are few examples of CNTs being used for the rectal delivery of proteins, some invitro studies suggest that carbon nanotubes combined with functional proteins may open new pathways for rectal delivery of protein drugs.
3.6.2. Lipid-Based Formulations
Nano-sized liposomes are also applicable for rectal administration, and a few studies examining liposomal formulations have been conducted regarding the rectal delivery of macromolecules. Recently, modified nanoliposomes containing hepatitis B surface antigen were also proposed for mucosal immunization, which composed of 1,2-dipalmitoyl-sn-glycero-3-phosphocholine bilayer engulfing a solid fat core (mainly glyceryl tripalmitate) and use monophosphoryl lipid A as an adjuvant [176]. These hybrid liposomes showed greater stability and significant humoral and cellular immune responses in rats after intracolonic administration [176]. As an additional nanocarrier, solid lipid nanoparticles are applicable for rectal drug delivery. Many examples of their rectal application have been reported so far, however, they have failed to demonstrate an advantage over conventional formulations [169].
4. Computer-Aided Formulation Development
Computational methods are widely used to support the various formulation design including tablets, nanoparticles, and topical formulations [177]. Computational methods can aid in selecting optimal excipients and predicting potential toxicity, stability, and biological performance of drug delivery systems [177]. In addition, computational methods help to identify the factors influencing the formulation design in a hypothetical manner [178]. Various computational tools are available, which include molecular modeling, molecular mechanics, discrete element modeling, finite element method, computational fluid dynamics, and physiologically based pharmacokinetics modeling. These tools facilitate the formulation development with reducing time and cost, by predicting in vitro/in vivo properties of formulations including stability, solubility, dissolution, and in vivo performance [178,179]. The computational approach is also applicable for the formulation development of protein drugs during early preclinical development. For example, Barata et al. [180] identified protein–excipient interaction hotspots using computational approaches. For the selection of excipients preventing protein aggregation, they used a molecular docking approach for the identification of protein–excipient hotspots. Potential interactions between Fab A33 and several excipients predicted by molecular docking were also confirmed by Tm values from a thermal study [180]. This approach provided information on intermolecular interactions responsible for the protective effects of excipients in protein formulations.
Given that the computational approach can enhance our understanding of the mechanism of action of drug carriers and their underlying interactions with drugs, cellular membranes, and other biological components, computational modeling along with laboratory experiments should be an integral part of formulation development.
5. Summary and Outlook
Protein drugs are indispensable in current medical practice due to their potent therapeutic effects and clinical benefit; however, their industrial manufacturing and clinical applications still face to a big challenge due to inherent physical and/or chemical instability, enzymatic degradation and rapid elimination from systemic circulation. Also, their drug delivery viability, large size, and hydrophilicity make it difficult for them to overcome absorption barriers. Although the parenteral administration of protein drugs is often useful in bypassing absorption barriers, it suffers from issues associated with repeated dosing, high costs, and pain, raising the need for non-invasive delivery systems via alternative routes of administration. Each route of administration faces certain biological barriers originating from anatomical and physiological characteristics that stretch from the site of administration to systemic circulation or target organs. These barriers significantly affect the bioavailability and therapeutic outcomes of protein drugs. Therefore, various formulation approaches have been developed to overcome these barriers associated with each route of administration and this review provided an overview of the recent advancement in non-invasive delivery systems for therapeutic macromolecules. Particularly, advancements in nanotechnology offer a great opportunity to improve protein drug delivery via non-invasive routes of administration. Furthermore, the combined use of nano-formulations with the sophisticated medical devices, electric or magnetic forces, or sonic waves can maximize the effectiveness of non-invasive drug delivery systems. Computational approach is also helpful in more successful formulation design with reduced time and cost.
Taken together, the non-invasive drug delivery systems of therapeutic macromolecules are evolving innovatively via the technical convergence of IT, BT, and NT (information technology, biotechnology, and nanotechnology). Looking forward, the effective combination of nano-formulation techniques with novel bio-devices or wearable digital devices will open a new era of sophisticated digital medicines and provide new platforms for non-invasive and more precise protein drug delivery in the near future.
Declaration of Competing Interest
None.
Acknowledgment
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea Government (MSIT) (No. 2019R1A2C2004873 and No. 2018R1A5A2023127).
References
- 1.Herrero E.P., Alonso M.J., Csaba N. Polymer-based oral peptide nanomedicines. Ther Deliv. 2012;3:657–668. doi: 10.4155/tde.12.40. [DOI] [PubMed] [Google Scholar]
- 2.Jitendra Sharma P.K., Bansal S., Banik A. Noninvasive routes of proteins and peptides drug delivery. Indian J Pharm Sci. 2011;73:367–375. doi: 10.4103/0250-474X.95608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tiwari M.K., Singh R., Singh R.K., Kim I.-W., Lee J.-K. Computational approaches for rational design of proteins with novel functionalities. Comput Struct Biotechnol. 2012;2 doi: 10.5936/csbj.201209002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sachdeva S., Lobo S., Goswami T. What is the future of noninvasive routes for protein- and peptide-based drugs? Ther Deliv. 2016;7:355–357. doi: 10.4155/tde-2016-0031. [DOI] [PubMed] [Google Scholar]
- 5.U.S. Food & Drug Administration's Center for drug evaluation and research . New drug therapy approvals. 2019. Advancing health through innovation 2018.https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2018 Available: Accessed 2019 Feb 03. [Google Scholar]
- 6.Dewan S.S. Global markets for bioengineered protein drugs. 2017. https://www.bccresearch.com Available. Accessed 2018 Dec 18.
- 7.Lewis A.L., Richard J. Challenges in the delivery of peptide drugs: an industry perspective. Ther Deliv. 2015;6:149–163. doi: 10.4155/tde.14.111. [DOI] [PubMed] [Google Scholar]
- 8.Pisal D.S., Kosloski M.P., Balu-Iyer S.V. Delivery of therapeutic proteins. J Pharm Sci. 2010;99:2557–2575. doi: 10.1002/jps.22054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kesharwani P., Gorain B., Low S.Y., Tan S.A., Ling E.C.S. Nanotechnology based approaches for anti-diabetic drugs delivery. Diabetes Res Clin Pract. 2018;136:52–77. doi: 10.1016/j.diabres.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 10.Touitou E., Illum L. Nasal drug delivery. Drug Deliv Transl Res. 2013;3:1–3. doi: 10.1007/s13346-012-0111-1. [DOI] [PubMed] [Google Scholar]
- 11.Patton J.S., Fishburn C.S., Weers J.G. The lungs as a portal of entry for systemic drug delivery. Proc Am Thorac Soc. 2004;1:338–344. doi: 10.1513/pats.200409-049TA. [DOI] [PubMed] [Google Scholar]
- 12.Thwala L.N., Santander-Ortega M.J., Lozano M.V., Csaba N.S. Functionalized polymeric nanostructures for mucosal drug delivery. In: Sarmento B., das Neves J., editors. Biomedical applications of functionalized nanomaterials. Elsevier; Amsterdam: 2018. pp. 449–487. [Google Scholar]
- 13.Zhang Y., Yu J., Kahkoska A.R., Wang J., Buse J.B. Advances in transdermal insulin delivery. Adv Drug Deliv Rev. 2019;139:51–70. doi: 10.1016/j.addr.2018.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mandal A., Pal D., Agrahari V., Trinh H.M., Joseph M. Ocular delivery of proteins and peptides: challenges and novel formulation approaches. Adv Drug Deliv Rev. 2018;126:67–95. doi: 10.1016/j.addr.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tibbitts J., Canter D., Graff R., Smith A., Khawli L.A. Key factors influencing ADME properties of therapeutic proteins: a need for ADME characterization in drug discovery and development. MAbs. 2016;8:229–245. doi: 10.1080/19420862.2015.1115937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muheem A., Shakeel F., Jahangir M.A., Anwar M., Mallick N. A review on the strategies for oral delivery of proteins and peptides and their clinical perspectives. Saudi Pharm J. 2016;24:413–428. doi: 10.1016/j.jsps.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vyas S.P., Paliwal R., Paliwal S.R. Ocular delivery of peptides and proteins. In: Van Der Walle C., editor. Peptide and protein delivery. Academic Press; Boston: 2011. pp. 87–103. [Google Scholar]
- 18.Andrade F., Videira M., Ferreira D., Sarmento B. Nanocarriers for pulmonary administration of peptides and therapeutic proteins. Nanomedicine. 2011;6:123–141. doi: 10.2217/nnm.10.143. [DOI] [PubMed] [Google Scholar]
- 19.Renukuntla J., Vadlapudi A.D., Patel A., Boddu S.H., Mitra A.K. Approaches for enhancing oral bioavailability of peptides and proteins. Int J Pharm. 2013;447:75–93. doi: 10.1016/j.ijpharm.2013.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boegh M., Foged C., Müllertz A., Mørck Nielsen H. Mucosal drug delivery: barriers, invitro models and formulation strategies. J Drug Deliv Sci Technol. 2013;23:383–391. [Google Scholar]
- 21.Lai S.K., Wang Y.-Y., Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv Drug Deliv Rev. 2009;61:158–171. doi: 10.1016/j.addr.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim J., Schlesinger E.B., Desai T.A. Nanostructured materials for ocular delivery: nanodesign for enhanced bioadhesion, transepithelial permeability and sustained delivery. Ther Deliv. 2015;6:1365–1376. doi: 10.4155/tde.15.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aguirre T.A.S., Teijeiro-Osorio D., Rosa M., Coulter I.S., Alonso M.J. Current status of selected oral peptide technologies in advanced preclinical development and in clinical trials. Adv Drug Deliv Rev. 2016;106:223–241. doi: 10.1016/j.addr.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Khodaverdi E., Maftouhian S., Aliabadi A., Hassanzadeh-Khayyat M., Mohammadpour F. Casein-based hydrogel carrying insulin: preparation, invitro evaluation and invivo assessment. J Pharm Investig. 2018;48:1–7. [Google Scholar]
- 25.Peppas N.A., Wood K.M., Blanchette J.O. Hydrogels for oral delivery of therapeutic proteins. Expert Opin Biol Ther. 2004;4:881–887. doi: 10.1517/14712598.4.6.881. [DOI] [PubMed] [Google Scholar]
- 26.Sri B., Ashok V., Arkendu C. As a review on hydrogels as drug delivery in the pharmaceutical field. Int J Pharm Chem Sci. 2012;1:642–661. [Google Scholar]
- 27.Carrillo-Conde B.R., Brewer E., Lowman A., Peppas N.A. Complexation hydrogels as oral delivery vehicles of therapeutic antibodies: an invitro and exvivo evaluation of antibody stability and bioactivity. Ind Eng Chem Res. 2015;54:10197–10205. doi: 10.1021/acs.iecr.5b01193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshida M., Kamei N., Muto K., Kunisawa J., Takayama K. Complexation hydrogels as potential carriers in oral vaccine delivery systems. Eur J Pharm Biopharm. 2017;112:138–142. doi: 10.1016/j.ejpb.2016.11.029. [DOI] [PubMed] [Google Scholar]
- 29.Lima D.S., Tenório-Neto E.T., Lima-Tenório M.K., Guilherme M.R., Scariot D.B. pH-responsivealginate-based hydrogels for protein delivery. J Mol Liq. 2018;262:29–36. [Google Scholar]
- 30.Sabaa M.W., Hanna D.H., Abu Elella M.H., Mohamed R.R. Encapsulation of bovine serum albumin within novel xanthan gum based hydrogel for protein delivery. Mater Sci Eng C. 2019;94:1044–1055. doi: 10.1016/j.msec.2018.10.040. [DOI] [PubMed] [Google Scholar]
- 31.Kumari A., Yadav S.K., Yadav S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B Biointerfaces. 2010;75:1–18. doi: 10.1016/j.colsurfb.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 32.Shi Y., Sun X., Zhang L., Sun K., Li K. Fc-modifiedexenatide-loaded nanoparticles for oral delivery to improve hypoglycemic effects in mice. Sci Rep. 2018;8:726. doi: 10.1038/s41598-018-19170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mukhopadhyay P., Sarkar K., Chakraborty M., Bhattacharya S., Mishra R. Oral insulin delivery by self-assembled chitosan nanoparticles: invitro and invivo studies in diabetic animal model. Mater Sci Eng C. 2013;33:376–382. doi: 10.1016/j.msec.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 34.Ma X., Williams R.O. Polymeric nanomedicines for poorly soluble drugs in oral delivery systems: an update. J Pharm Investig. 2018;48:61–75. [Google Scholar]
- 35.Luangtana-anan M., Nunthanid J., Limmatvapirat S. Potential of different salt forming agents on the formation of chitosan nanoparticles as carriers for protein drug delivery systems. J Pharm Investig. 2019;49:37–44. [Google Scholar]
- 36.Sandri G., Bonferoni M.C., Rossi S., Ferrari F., Boselli C. Insulin-loaded nanoparticles based on N-trimethyl chitosan: invitro (Caco-2 model) and exvivo (excised rat jejunum, duodenum, and ileum) evaluation of penetration enhancement properties. AAPS Pharm Sci Tech. 2010;11:362–371. doi: 10.1208/s12249-010-9390-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin Y., Song Y., Zhu X., Zhou D., Chen C. Goblet cell-targeting nanoparticles for oral insulin delivery and the influence of mucus on insulin transport. Biomaterials. 2012;33:1573–1582. doi: 10.1016/j.biomaterials.2011.10.075. [DOI] [PubMed] [Google Scholar]
- 38.Hu R., Zheng H., Cao J., Davoudi Z., Wang Q. Synthesis and invitro characterization of carboxymethyl chitosan-CBA-doxorubicin conjugate nanoparticles as pH-sensitive drug delivery systems. J Biomed Nanotechnol. 2017;13:1097–1105. doi: 10.1166/jbn.2017.2407. [DOI] [PubMed] [Google Scholar]
- 39.Zhang X., Zhang H., Yin L., Hu R., Qiu T. A pH-sensitive nanosystem based on carboxymethyl chitosan for tumor-targeted delivery of daunorubicin. J Biomed Nanotechnol. 2016;12:1688–1698. doi: 10.1166/jbn.2016.2278. [DOI] [PubMed] [Google Scholar]
- 40.Chan A.W., Neufeld R.J. Tuneable semi-synthetic network alginate for absorptive encapsulation and controlled release of protein therapeutics. Biomaterials. 2010;31:9040–9047. doi: 10.1016/j.biomaterials.2010.07.111. [DOI] [PubMed] [Google Scholar]
- 41.Patole V.C., Pandit A.P. Mesalamine-loaded alginate microspheres filled in enteric coated HPMC capsules for local treatment of ulcerative colitis: invitro and invivo characterization. J Pharm Investig. 2018;48:257–267. [Google Scholar]
- 42.Mukhopadhyay P., Chakraborty S., Bhattacharya S., Mishra R., Kundu P. pH-sensitive chitosan/alginate core-shell nanoparticles for efficient and safe oral insulin delivery. Int J Biol Macromol. 2015;72:640–648. doi: 10.1016/j.ijbiomac.2014.08.040. [DOI] [PubMed] [Google Scholar]
- 43.Luo Y.Y., Xiong X.Y., Tian Y., Li Z.L., Gong Y.C. A review of biodegradable polymeric systems for oral insulin delivery. Drug Deliv. 2016;23:1882–1891. doi: 10.3109/10717544.2015.1052863. [DOI] [PubMed] [Google Scholar]
- 44.Perinelli D.R., Cespi M., Bonacucina G., Palmieri G.F. PEGylated polylactide (PLA) and poly (lactic-co-glycolic acid) (PLGA) copolymers for the design of drug delivery systems. J Pharm Investig. 2019;49:1–16. [Google Scholar]
- 45.Yang J., Sun H., Song C. Preparation, characterization and invivo evaluation of pH-sensitive oral insulin-loaded poly (lactic-co-glycolicacid) nanoparticles. Diabetes Obes Metab. 2012;14:358–364. doi: 10.1111/j.1463-1326.2011.01546.x. [DOI] [PubMed] [Google Scholar]
- 46.Zhang X., Sun M., Zheng A., Cao D., Bi Y. Preparation and characterization of insulin-loaded bioadhesive PLGA nanoparticles for oral administration. Eur J Pharm Sci. 2012;45:632–638. doi: 10.1016/j.ejps.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 47.Hurkat P., Jain A., Jain A., Shilpi S., Gulbake A. Concanavalin A conjugated biodegradable nanoparticles for oral insulin delivery. J Nanopart Res. 2012;14:1219. [Google Scholar]
- 48.Xiong X.Y., Li Q.H., Li Y.P., Guo L., Li Z.L. Pluronic P85/poly (lactic acid) vesicles as novel carrier for oral insulin delivery. Colloids Surf B Biointerfaces. 2013;111:282–288. doi: 10.1016/j.colsurfb.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 49.Pridgen E.M., Alexis F., Kuo T.T., Levy-Nissenbaum E., Karnik R. Transepithelial transport of Fc-targeted nanoparticles by the neonatal Fc receptor for oral delivery. Sci Transl Med. 2013;5:213ra167. doi: 10.1126/scitranslmed.3007049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng H., Yin L., Zhang X., Zhang H., Hu R. Redox sensitive shell and core crosslinked hyaluronic acid nanocarriers for tumor-targeted drug delivery. J Biomed Nanotechnol. 2016;12:1641–1653. doi: 10.1166/jbn.2016.2279. [DOI] [PubMed] [Google Scholar]
- 51.Karimi M., M Moosavi Basri S., Vossoughi M., S Pakchin P., Mirshekari H. Redox-sensitive smart nanosystems for drug and gene delivery. Curr Org Chem. 2016;20:2949–2959. [Google Scholar]
- 52.Cai X., Dong C., Dong H., Wang G., Pauletti G.M. Effective gene delivery using stimulus-responsive catiomer designed with redox-sensitive disulfide and acid-labile imine linkers. Biomacromolecules. 2012;13:1024–1034. doi: 10.1021/bm2017355. [DOI] [PubMed] [Google Scholar]
- 53.Feas D.A., Igartúa D.E., Calienni M.N., Martinez C.S., Pifano M. Nutraceutical emulsion containing valproic acid (NE-VPA): a drug delivery system for reversion of seizures in zebrafish larvae epilepsy model. J Pharm Investig. 2017;47:429–437. [Google Scholar]
- 54.Khalil N.M., do Nascimento T.C.F., Casa D.M., Dalmolin L.F., de Mattos A.C. Pharmacokinetics of curcumin-loadedPLGA and PLGA–PEG blend nanoparticles after oral administration in rats. Colloids Surf B Biointerfaces. 2013;101:353–360. doi: 10.1016/j.colsurfb.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 55.Koga K., Takarada N., Takada K. Nano-sizedwater-in-oil-in-water emulsion enhances intestinal absorption of calcein, a high solubility and low permeability compound. Eur J Pharm Biopharm. 2010;74:223–232. doi: 10.1016/j.ejpb.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 56.Wang T., Wang N., Hao A., He X., Li T. Lyophilization of water-in-oil emulsions to prepare phospholipid-based anhydrous reverse micelles for oral peptide delivery. Eur J Pharm Sci. 2010;39:373–379. doi: 10.1016/j.ejps.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 57.Frizzell H., Ohlsen T.J., Woodrow K.A. Protein-loaded emulsion electrospun fibers optimized for bioactivity retention and pH-controlled release for peroral delivery of biologic therapeutics. Int J Pharm. 2017;533:99–110. doi: 10.1016/j.ijpharm.2017.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ahsan M.N., Verma P.R.P. Enhancement of invitro dissolution and pharmacodynamic potential of olanzapine using solid SNEDDS. J Pharm Investig. 2018;48:269–278. [Google Scholar]
- 59.Madhav K.V., Kishan V. Self microemulsifying particles of loratadine for improved oral bioavailability: preparation, characterization and invivo evaluation. J Pharm Investig. 2018;48:497–508. [Google Scholar]
- 60.Kohli K., Chopra S., Dhar D., Arora S., Khar R.K. Self-emulsifying drug delivery systems: an approach to enhance oral bioavailability. Drug Discov Today. 2010;15:958–965. doi: 10.1016/j.drudis.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 61.Qi X., Wang L., Zhu J., Hu Z., Zhang J. Self-double-emulsifying drug delivery system (SDEDDS): a new way for oral delivery of drugs with high solubility and low permeability. Int J Pharm. 2011;409:245–251. doi: 10.1016/j.ijpharm.2011.02.047. [DOI] [PubMed] [Google Scholar]
- 62.Sakloetsakun D., Dünnhaupt S., Barthelmes J., Perera G., Bernkop-Schnürch A. Combining two technologies: Multifunctional polymers and self-nanoemulsifying drug delivery system (SNEDDS) for oral insulin administration. Int J Biol Macromol. 2013;61:363–372. doi: 10.1016/j.ijbiomac.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 63.Park K., Kwon I.C., Park K. Oral protein delivery: current status and future prospect. React Funct Polym. 2011;71:280–287. [Google Scholar]
- 64.Minko T. Drug targeting to the colon with lectins and neoglycoconjugates. Adv Drug Deliv Rev. 2004;56:491–509. doi: 10.1016/j.addr.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 65.Makhlof A., Fujimoto S., Tozuka Y., Takeuchi H. In vitro and invivo evaluation of WGA–carbopol modified liposomes as carriers for oral peptide delivery. Eur J Pharm Biopharm. 2011;77:216–224. doi: 10.1016/j.ejpb.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 66.Li Z., Chen J., Sun W., Xu Y. Investigation of archaeosomes as carriers for oral delivery of peptides. Biochem Biophys Res Commun. 2010;394:412–417. doi: 10.1016/j.bbrc.2010.03.041. [DOI] [PubMed] [Google Scholar]
- 67.Li Z., Zhang L., Sun W., Ding Q., Hou Y. Archaeosomes with encapsulated antigens for oral vaccine delivery. Vaccine. 2011;29:5260–5266. doi: 10.1016/j.vaccine.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 68.Traverso G., Schoellhammer C.M., Schroeder A., Maa R., Lauwers G.Y. Microneedles for drug delivery via the gastrointestinal tract. J Pharm Sci. 2015;104:362–367. doi: 10.1002/jps.24182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Banerjee A., Lee J., Mitragotri S. Intestinal mucoadhesive devices for oral delivery of insulin. Bioeng Transl Med. 2016;1:338–346. doi: 10.1002/btm2.10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Poovaiah N., Davoudi Z., Peng H., Schlichtmann B., Mallapragada S. Treatment of neurodegenerative disorders through the blood–brain barrier using nanocarriers. Nanoscale. 2018;10:16962–16983. doi: 10.1039/c8nr04073g. [DOI] [PubMed] [Google Scholar]
- 71.Meredith M.E., Salameh T.S., Banks W.A. Intranasal delivery of proteins and peptides in the treatment of neurodegenerative diseases. AAPS J. 2015;17:780–787. doi: 10.1208/s12248-015-9719-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Patel M.R., Hirani S.N., Patel R.B. Microemulsion for nasal delivery of Asenapine maleate in treatment of schizophrenia: formulation considerations. J Pharm Investig. 2018;48:301–312. [Google Scholar]
- 73.Dombu C.Y., Betbeder D. Airway delivery of peptides and proteins using nanoparticles. Biomaterials. 2013;34:516–525. doi: 10.1016/j.biomaterials.2012.08.070. [DOI] [PubMed] [Google Scholar]
- 74.Nikolić V., Ilić-Stojanović S., Petrović S., Tačić A., Nikolić L. Administration routes for nano drugs and characterization of nano drug loading. In: Mohapatra S.S., Ranjan S., Dasgupta N., Mishra R.K., Thomas S., editors. Characterization and biology of nanomaterials for drug delivery. Elsevier; 2019. pp. 587–625. [Google Scholar]
- 75.Duan X., Mao S. New strategies to improve the intranasal absorption of insulin. Drug Discov Today. 2010;15:416–427. doi: 10.1016/j.drudis.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 76.Yuba E., Kono K. Nasal delivery of biopharmaceuticals. In: das Neves J., Sarmento B., editors. Mucosal delivery of biopharmaceuticals: biology, challenges and strategies. Springer US; Boston, MA: 2014. pp. 197–220. [Google Scholar]
- 77.Wang X., Zheng C., Wu Z., Teng D., Zhang X. Chitosan-NAC nanoparticles as a vehicle for nasal absorption enhancement of insulin. J Biomed Mater Res B Appl Biomater. 2009;88:150–161. doi: 10.1002/jbm.b.31161. [DOI] [PubMed] [Google Scholar]
- 78.Slütter B., Bal S.M., Que I., Kaijzel E., Löwik C. Antigen−adjuvant nanoconjugates for nasal vaccination: an improvement over the use of nanoparticles? Mol Pharm. 2010;7:2207–2215. doi: 10.1021/mp100210g. [DOI] [PubMed] [Google Scholar]
- 79.Vaka S.R.K., Sammeta S.M., Day L.B., Murthy S.N. Delivery of nerve growth factor to brain via intranasal administration and enhancement of brain uptake. J Pharm Sci. 2009;98:3640–3646. doi: 10.1002/jps.21674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mohtashamian S., Boddohi S. Nanostructured polysaccharide-based carriers for antimicrobial peptide delivery. J Pharm Investig. 2017;47:85–94. [Google Scholar]
- 81.Soni K.S., Desale S.S., Bronich T.K. Nanogels: an overview of properties, biomedical applications and obstacles to clinical translation. J Control Release. 2016;240:109–126. doi: 10.1016/j.jconrel.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aderibigbe B., Naki T. Design and efficacy of nanogels formulations for intranasal administration. Molecules. 2018;23:1241. doi: 10.3390/molecules23061241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Picone P., Ditta L.A., Sabatino M.A., Militello V., San Biagio P.L. Ionizing radiation-engineered nanogels as insulin nanocarriers for the development of a new strategy for the treatment of Alzheimer's disease. Biomaterials. 2016;80:179–194. doi: 10.1016/j.biomaterials.2015.11.057. [DOI] [PubMed] [Google Scholar]
- 84.Nochi T., Yuki Y., Takahashi H., Sawada S.-i., Mejima M. Nanogel antigenic protein-delivery system for adjuvant-free intranasal vaccines. Nat Mater. 2010;9:685. doi: 10.1038/nmat2784. [DOI] [PubMed] [Google Scholar]
- 85.Kakhi Z., Frisch B., Heurtault B., Pons F. Liposomal constructs for antitumoral vaccination by the nasal route. Biochimie. 2016;130:14–22. doi: 10.1016/j.biochi.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 86.Chen K.H., Di Sabatino M., Albertini B., Passerini N., Kett V.L. The effect of polymer coatings on physicochemical properties of spray-dried liposomes for nasal delivery of BSA. Eur J Pharm Sci. 2013;50:312–322. doi: 10.1016/j.ejps.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 87.Kaplan M., Tuğcu-Demiröz F., Vural İ., Çelebi N. Development and characterization of gels and liposomes containing ovalbumin for nasal delivery. J Drug Deliv Sci Technol. 2018;44:108–117. [Google Scholar]
- 88.Zeb A., Arif S.T., Malik M., Shah F.A., Din F.U. Potential of nanoparticulate carriers for improved drug delivery via skin. J Pharm Investig. 2018;48:1–33. [Google Scholar]
- 89.Pham C.V., Cho C.-W. Application of d-α-tocopheryl polyethylene glycol 1000 succinate (TPGS) in transdermal and topical drug delivery systems (TDDS) J Pharm Investig. 2017;47:111–121. [Google Scholar]
- 90.Jain S., Patel N., Shah M.K., Khatri P., Vora N. Recent advances in lipid-based vesicles and particulate carriers for topical and transdermal application. J Pharm Sci. 2017;106:423–445. doi: 10.1016/j.xphs.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 91.Sadhasivam L., Dey N., Francis A.P., Devasena T. Transdermal patches of chitosan nanoparticles for insulin delivery. Int J Pharm Pharm Sci. 2015;7:84–88. [Google Scholar]
- 92.de Groot A.M., Du G., Monkare J., Platteel A.C.M., Broere F. Hollow microneedle-mediated intradermal delivery of model vaccine antigen-loadedPLGA nanoparticles elicits protective Tcell-mediated immunity to an intracellular bacterium. J Control Release. 2017;266:27–35. doi: 10.1016/j.jconrel.2017.09.017. [DOI] [PubMed] [Google Scholar]
- 93.Monkare J., Pontier M., van Kampen E.E.M., Du G., Leone M. Development of PLGA nanoparticle loaded dissolving microneedles and comparison with hollow microneedles in intradermal vaccine delivery. Eur J Pharm Biopharm. 2018;129:111–121. doi: 10.1016/j.ejpb.2018.05.031. [DOI] [PubMed] [Google Scholar]
- 94.Tu Y., Wang X., Lu Y., Zhang H., Yu Y. Promotion of the transdermal delivery of protein drugs by N-trimethyl chitosan nanoparticles combined with polypropylene electret. Int J Nanomedicine. 2016;11:5549. doi: 10.2147/IJN.S109552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen J., Zhan Y., Wang Y., Han D., Tao B. Chitosan/silk fibroin modified nanofibrous patches with mesenchymal stem cells prevent heart remodeling post-myocardial infarction in rats. Acta Biomater. 2018;80:154–168. doi: 10.1016/j.actbio.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 96.Escobar-Chávez J.J., Rodríguez-Cruz I.M., Domínguez-Delgado C.L., Díaz-Torres R., Revilla-Vázquez A.L. Recent advances in novel drug carrier systems. InTech; 2012. Nanocarrier systems for transdermal drug delivery; pp. 201–240. [Google Scholar]
- 97.Rai V.K., Mishra N., Yadav K.S., Yadav N.P. Nanoemulsion as pharmaceutical carrier for dermal and transdermal drug delivery: formulation development, stability issues, basic considerations and applications. J Control Release. 2018;270:203–225. doi: 10.1016/j.jconrel.2017.11.049. [DOI] [PubMed] [Google Scholar]
- 98.Ledet G., Pamujula S., Walker V., Simon S., Graves R. Development and invitro evaluation of a nanoemulsion for transcutaneous delivery. Drug Dev Ind Pharm. 2014;40:370–379. doi: 10.3109/03639045.2012.763137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lopez P.A., Denny M., Hartmann A.-K., Alflen A., Probst H.C. Transcutaneous immunization with a novel imiquimod nanoemulsion induces superior T cell responses and virus protection. J Dermatol Sci. 2017;87:252–259. doi: 10.1016/j.jdermsci.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 100.Malakar J., Sen S.O., Nayak A.K., Sen K.K. Development and evaluation of microemulsions for transdermal delivery of insulin. ISRN Pharmaceutics. 2011;2011 doi: 10.5402/2011/780150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kitaoka M., Wakabayashi R., Kamiya N., Goto M. Solid-in-oil nanodispersions for transdermal drug delivery systems. Biotechnol J. 2016;11:1375–1385. doi: 10.1002/biot.201600081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Araki S., Wakabayashi R., Moniruzzaman M., Kamiya N., Goto M. Ionic liquid-mediated transcutaneous protein delivery with solid-in-oil nanodispersions. Med Chem Comm. 2015;6:2124–2128. [Google Scholar]
- 103.Martins M., Azoia N.G., Ribeiro A., Shimanovich U., Silva C. In vitro and computational studies of transdermal perfusion of nanoformulations containing a large molecular weight protein. Colloids Surf B Biointerfaces. 2013;108:271–278. doi: 10.1016/j.colsurfb.2013.02.032. [DOI] [PubMed] [Google Scholar]
- 104.Tahara Y., Honda S., Kamiya N., Goto M. Transdermal delivery of insulin using a solid-in-oil nanodispersion enhanced by arginine-rich peptides. Med Chem Comm. 2012;3:1496–1499. [Google Scholar]
- 105.Seo J., Kim M.J., Jeon S.O., Oh D.H., Yoon K.H. Enhanced topical delivery of fish scale collagen employing negatively surface-modified nanoliposome. J Pharm Investig. 2018;48:243–250. [Google Scholar]
- 106.Rahimpour Y., Hamishehkar H. Liposomes in cosmeceutics. Expert Opin Drug Deliv. 2012;9:443–455. doi: 10.1517/17425247.2012.666968. [DOI] [PubMed] [Google Scholar]
- 107.Carter P., Narasimhan B., Wang Q. Biocompatible nanoparticles and vesicular systems in transdermal drug delivery for various skin diseases. Int J Pharm. 2019;555:49–62. doi: 10.1016/j.ijpharm.2018.11.032. [DOI] [PubMed] [Google Scholar]
- 108.Ashtikar M., Nagarsekar K., Fahr A. Transdermal delivery from liposomal formulations – evolution of the technology over the last three decades. J Control Release. 2016;242:126–140. doi: 10.1016/j.jconrel.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 109.Kumar A. Transferosome: a recent approach for transdermal drug delivery. J Drug Deliv Ther. 2018;8:100–104. [Google Scholar]
- 110.Malakar J., Sen S.O., Nayak A.K., Sen K.K. Formulation, optimization and evaluation of transferosomal gel for transdermal insulin delivery. Saudi Pharm J. 2012;20:355–363. doi: 10.1016/j.jsps.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tyagi R.K., Garg N.K., Jadon R., Sahu T., Katare O.P. Elastic liposome-mediated transdermal immunization enhanced the immunogenicity of P.falciparum surface antigen, MSP-119. Vaccine. 2015;33:4630–4638. doi: 10.1016/j.vaccine.2015.06.054. [DOI] [PubMed] [Google Scholar]
- 112.Zhou X., Hao Y., Yuan L., Pradhan S., Shrestha K. Nano-formulations for transdermal drug delivery: a review. Chin Chem Lett. 2018;29:1713–1724. [Google Scholar]
- 113.Meng S., Chen Z., Yang L., Zhang W., Liu D. Enhanced transdermal bioavailability of testosterone propionate via surfactant-modified ethosomes. Int J Nanomedicine. 2013;8:3051–3060. doi: 10.2147/IJN.S46748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhao J.-H., Zhang Q.-B., Liu B., Piao X.-H., Yan Y.-L. Enhanced immunization via dissolving microneedle array-based delivery system incorporating subunit vaccine and saponin adjuvant. Int J Nanomedicine. 2017;12:4763. doi: 10.2147/IJN.S132456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Guo L., Chen J., Qiu Y., Zhang S., Xu B. Enhanced transcutaneous immunization via dissolving microneedle array loaded with liposome encapsulated antigen and adjuvant. Int J Pharm. 2013;447:22–30. doi: 10.1016/j.ijpharm.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 116.Vora L.K., Donnelly R.F., Larraneta E., Gonzalez-Vazquez P., Thakur R.R.S. Novel bilayer dissolving microneedle arrays with concentrated PLGAnano-microparticles for targeted intradermal delivery: proof of concept. J Control Release. 2017;265:93–101. doi: 10.1016/j.jconrel.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 117.Bruno B.J., Miller G.D., Lim C.S. Basics and recent advances in peptide and protein drug delivery. Ther Deliv. 2013;4:1443–1467. doi: 10.4155/tde.13.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chandrasekhar S., Iyer L.K., Panchal J.P., Topp E.M., Cannon J.B. Microarrays and microneedle arrays for delivery of peptides, proteins, vaccines and other applications. Expert Opin Drug Deliv. 2013;10:1155–1170. doi: 10.1517/17425247.2013.797405. [DOI] [PubMed] [Google Scholar]
- 119.Waghule T., Singhvi G., Dubey S.K., Pandey M.M., Gupta G. Microneedles: a smart approach and increasing potential for transdermal drug delivery system. Biomed Pharmacother. 2019;109:1249–1258. doi: 10.1016/j.biopha.2018.10.078. [DOI] [PubMed] [Google Scholar]
- 120.van der Maaden K., Jiskoot W., Bouwstra J. Microneedle technologies for (trans)dermal drug and vaccine delivery. J Control Release. 2012;161:645–655. doi: 10.1016/j.jconrel.2012.01.042. [DOI] [PubMed] [Google Scholar]
- 121.Ye Y., Yu J., Wen D., Kahkoska A.R., Gu Z. Polymeric microneedles for transdermal protein delivery. Adv Drug Deliv Rev. 2018;127:106–118. doi: 10.1016/j.addr.2018.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kusamori K., Katsumi H., Sakai R., Hayashi R., Hirai Y. Development of a drug-coated microneedle array and its application for transdermal delivery of interferon alpha. Biofabrication. 2016;8 doi: 10.1088/1758-5090/8/1/015006. [DOI] [PubMed] [Google Scholar]
- 123.An M., Liu H. Dissolving microneedle arrays for transdermal delivery of amphiphilic vaccines. Small. 2017;13 doi: 10.1002/smll.201700164. [DOI] [PubMed] [Google Scholar]
- 124.Mönkäre J., Reza Nejadnik M., Baccouche K., Romeijn S., Jiskoot W. IgG-loadedhyaluronan-based dissolving microneedles for intradermal protein delivery. J Control Release. 2015;218:53–62. doi: 10.1016/j.jconrel.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 125.Chen M.-C., Ling M.-H., Kusuma S.J. Poly-γ-glutamic acid microneedles with a supporting structure design as a potential tool for transdermal delivery of insulin. Acta Biomater. 2015;24:106–116. doi: 10.1016/j.actbio.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 126.Seong K.-Y., Seo M.-S., Hwang D.Y., O'Cearbhaill E.D., Sreenan S. A self-adherent, bullet-shaped microneedle patch for controlled transdermal delivery of insulin. J Control Release. 2017;265:48–56. doi: 10.1016/j.jconrel.2017.03.041. [DOI] [PubMed] [Google Scholar]
- 127.Mura S., Nicolas J., Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013;12:991–1003. doi: 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]
- 128.Wang J., Ye Y., Yu J., Kahkoska A.R., Zhang X. Core-shell microneedle gel for self-regulated insulin delivery. ACS Nano. 2018;12:2466–2473. doi: 10.1021/acsnano.7b08152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kalluri H., Banga A.K. Transdermal delivery of proteins. AAPS Pharm Sci Tech. 2011;12:431–441. doi: 10.1208/s12249-011-9601-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Badkar A.V., Smith A.M., Eppstein J.A., Banga A.K. Transdermal delivery of interferon alpha-2B using microporation and iontophoresis in hairless rats. Pharm Res. 2007;24:1389–1395. doi: 10.1007/s11095-007-9308-2. [DOI] [PubMed] [Google Scholar]
- 131.Park D., Park H., Seo J., Lee S. Sonophoresis in transdermal drug deliverys. Ultrasonics. 2014;54:56–65. doi: 10.1016/j.ultras.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 132.Liao A.H., Ma W.C., Wang C.H., Yeh M.K. Penetration depth, concentration and efficiency of transdermal alpha-arbutin delivery after ultrasound treatment with albumin-shelled microbubbles in mice. Drug Deliv. 2016;23:2173–2182. doi: 10.3109/10717544.2014.951102. [DOI] [PubMed] [Google Scholar]
- 133.Shah B., Surti N., Misra A. Challenges in delivery of therapeutic genomics and proteomics. Elsevier; 2011. Other routes of protein and peptide delivery: transdermal, topical, uterine, and rectal; pp. 623–671. [Google Scholar]
- 134.Mohammad E.A., Elshemey W.M., Elsayed A.A., Abd-Elghany A.A. Electroporation parameters for successful transdermal delivery of insulin. Am J Ther. 2016;23:e1560–e1567. doi: 10.1097/MJT.0000000000000198. [DOI] [PubMed] [Google Scholar]
- 135.Medi B.M., Singh J. Electronically facilitated transdermal delivery of human parathyroid hormone (1–34) Int J Pharm. 2003;263:25–33. doi: 10.1016/s0378-5173(03)00337-5. [DOI] [PubMed] [Google Scholar]
- 136.Emami F., Mostafavi Yazdi S.J., Na D.H. Poly(lactic acid)/poly(lactic-co-glycolic acid) particulate carriers for pulmonary drug delivery. J Pharm Investig. 2019;49:1–16. [Google Scholar]
- 137.Alfagih I., Kunda N., Alanazi F., Dennison S.R., Somavarapu S. Pulmonary delivery of proteins using nanocomposite microcarriers. J Pharm Sci. 2015;104:4386–4398. doi: 10.1002/jps.24681. [DOI] [PubMed] [Google Scholar]
- 138.Patil J.S., Sarasija S. Pulmonary drug delivery strategies: a concise, systematic review. Lung India. 2012;29:44–49. doi: 10.4103/0970-2113.92361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Menon J.U., Ravikumar P., Pise A., Gyawali D., Hsia C.C.W. Polymeric nanoparticles for pulmonary protein and DNA delivery. Acta Biomater. 2014;10:2643–2652. doi: 10.1016/j.actbio.2014.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Agu R.U., Ugwoke M.I., Armand M., Kinget R., Verbeke N. The lung as a route for systemic delivery of therapeutic proteins and peptides. Respir Res. 2001;2:198–209. doi: 10.1186/rr58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Elmowafy E.M., Tiboni M., Soliman M.E. Biocompatibility, biodegradation and biomedical applications of poly(lactic acid)/poly(lactic-co-glycolic acid) micro and nanoparticles. J Pharm Investig. 2019;49:1–34. [Google Scholar]
- 142.Gaspar D.P., Serra C., Lino P.R., Gonçalves L., Taboada P. Microencapsulated SLN: an innovative strategy for pulmonary protein delivery. Int J Pharm. 2017;516:231–246. doi: 10.1016/j.ijpharm.2016.11.037. [DOI] [PubMed] [Google Scholar]
- 143.Kim Y.C., Chiang B., Wu X., Prausnitz M.R. Ocular delivery of macromolecules. J Control Release. 2014;190:172–181. doi: 10.1016/j.jconrel.2014.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schymkowitz J., Rousseau F. Protein aggregation: a rescue by chaperones. Nat Chem Biol. 2016;12:58. doi: 10.1038/nchembio.2006. [DOI] [PubMed] [Google Scholar]
- 145.Stern R., Jedrzejas M.J. Hyaluronidases: their genomics, structures, and mechanisms of action. Chem Rev. 2006;106:818–839. doi: 10.1021/cr050247k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cho I.S., Park C.G., Huh B.K., Cho M.O., Khatun Z. Thermosensitive hexanoyl glycol chitosan-based ocular delivery system for glaucoma therapy. Acta Biomater. 2016;39:124–132. doi: 10.1016/j.actbio.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 147.Agrahari V., Agrahari V., Hung W.-T., Christenson L.K., Mitra A.K. Composite nanoformulation therapeutics for long-term ocular delivery of macromolecules. Mol Pharm. 2016;13:2912–2922. doi: 10.1021/acs.molpharmaceut.5b00828. [DOI] [PubMed] [Google Scholar]
- 148.Mandal A., Bisht R., Rupenthal I.D., Mitra A.K. Polymeric micelles for ocular drug delivery: from structural frameworks to recent preclinical studies. J Control Release. 2017;248:96–116. doi: 10.1016/j.jconrel.2017.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nagarwal R.C., Kant S., Singh P.N., Maiti P., Pandit J.K. Polymeric nanoparticulate system: a potential approach for ocular drug delivery. J Control Release. 2009;136:2–13. doi: 10.1016/j.jconrel.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 150.Oh E.J., Choi J.-S., Kim H., Joo C.-K., Hahn S.K. Anti-Flt1peptide–hyaluronate conjugate for the treatment of retinal neovascularization and diabetic retinopathy. Biomaterials. 2011;32:3115–3123. doi: 10.1016/j.biomaterials.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 151.Mahlumba P., Choonara Y.E., Kumar P., Du Toit L.C., Pillay V. Stimuli-responsive polymeric systems for controlled protein and peptide delivery: future implications for ocular delivery. Molecules. 2016;21:1002. doi: 10.3390/molecules21081002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lancina I.I.I., Michael G., Yang H. Dendrimers for ocular drug delivery. Can J Chem. 2017;95:897–902. doi: 10.1139/cjc-2017-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yavuz B., Pehlivan S.B., Unlü N. Dendrimeric systems and their applications in ocular drug delivery. Sci World J. 2013;2013:732340. doi: 10.1155/2013/732340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Holden C.A., Tyagi P., Thakur A., Kadam R., Jadhav G. Polyamidoamine dendrimer hydrogel for enhanced delivery of antiglaucoma drugs. Nanomedicine. 2012;8:776–783. doi: 10.1016/j.nano.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 155.Yang H., Tyagi P., Kadam R.S., Holden C.A., Kompella U.B. Hybrid dendrimer hydrogel/PLGA nanoparticle platform sustains drug delivery for one week and antiglaucoma effects for four days following one-time topical administration. ACS Nano. 2012;6:7595–7606. doi: 10.1021/nn301873v. [DOI] [PubMed] [Google Scholar]
- 156.Chen J., Banaszak Holl M.M. Dendrimer and dendrimer–conjugate protein complexes and protein coronas. Can J Chem. 2017;95:903–906. [Google Scholar]
- 157.Coursey T.G., Henriksson J.T., Marcano D.C., Shin C.S., Isenhart L.C. Dexamethasone nanowafer as an effective therapy for dry eye disease. J Control Release. 2015;213:168–174. doi: 10.1016/j.jconrel.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 158.Yuan X., Marcano D.C., Shin C.S., Hua X., Isenhart L.C. Ocular drug delivery nanowafer with enhanced therapeutic efficacy. ACS Nano. 2015;9:1749–1758. doi: 10.1021/nn506599f. [DOI] [PubMed] [Google Scholar]
- 159.Choi S.W., Kim J. Therapeutic contact lenses with polymeric vehicles for ocular drug delivery: a review. Materials. 2018;11:1125. doi: 10.3390/ma11071125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Li T., Zhang M., Han Y., Zhang H., Xu L. Targeting therapy of choroidal neovascularization by use of polypeptide- and PEDF-loaded immunoliposomes under ultrasound exposure. J Huazhong Univ Sci Technol Med Sci. 2010;30:798–803. doi: 10.1007/s11596-010-0661-8. [DOI] [PubMed] [Google Scholar]
- 161.Alvarez-Trabado J., Diebold Y., Sanchez A. Designing lipid nanoparticles for topical ocular drug delivery. Int J Pharm. 2017;532:204–217. doi: 10.1016/j.ijpharm.2017.09.017. [DOI] [PubMed] [Google Scholar]
- 162.Liu R., Liu Z., Zhang C., Zhang B. Nanostructured lipid carriers as novel ophthalmic delivery system for mangiferin: improving invivo ocular bioavailability. J Pharm Sci. 2012;101:3833–3844. doi: 10.1002/jps.23251. [DOI] [PubMed] [Google Scholar]
- 163.Vicente-Pascual M., Albano A., Solinís M.Á., Serpe L., Rodríguez-Gascón A. Gene delivery in the cornea: invitro & exvivo evaluation of solid lipid nanoparticle-basedvectors. Nanomedicine. 2018;13:1847–1854. doi: 10.2217/nnm-2018-0112. [DOI] [PubMed] [Google Scholar]
- 164.Sahoo S.K., Dilnawaz F., Krishnakumar S. Nanotechnology in ocular drug delivery. Drug Discov Today. 2008;13:144–151. doi: 10.1016/j.drudis.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 165.Ramadan A.A., Elbakry A.M., Esmaeil A.H., Khaleel S.A. Pharmaceutical and pharmacokinetic evaluation of novel rectal mucoadhesive hydrogels containing tolmetin sodium. J Pharm Investig. 2018;48:673–683. doi: 10.1007/s40005-017-0365-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Das Kurmi B., Tekchandani P., Paliwal R., Rai Paliwal S. Nanocarriers in improved heparin delivery: recent updates. Curr Pharm Des. 2015;21:4509–4518. doi: 10.2174/1381612821666150821112808. [DOI] [PubMed] [Google Scholar]
- 167.Shi Y., Xue J., Sang Y., Xu X., Shang Q. Insulin-loaded hydroxypropyl methyl cellulose-co-polyacrylamide-co-methacrylic acid hydrogels used as rectal suppositories to regulate the blood glucose of diabetic rats. Int J Biol Macromol. 2019;121:1346–1353. doi: 10.1016/j.ijbiomac.2018.09.044. [DOI] [PubMed] [Google Scholar]
- 168.Xue J., Shi Y., Li C., Xu X., Xu S. Methylcellulose and polyacrylate binary hydrogels used as rectal suppository to prevent type I diabetes. Colloids Surf B Biointerfaces. 2018;172:37–42. doi: 10.1016/j.colsurfb.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 169.Melo M., Nunes R., Sarmento B., das Neves J. Rectal administration of nanosystems: from drug delivery to diagnostics. Mater Today Chem. 2018;10:128–141. [Google Scholar]
- 170.Zuo L., Huang Z., Dong L., Xu L., Ya Zhu. Targeting delivery of anti-TNFα oligonucleotide into activated colonic macrophages protects against experimental colitis. Gut. 2010;59:470–479. doi: 10.1136/gut.2009.184556. [DOI] [PubMed] [Google Scholar]
- 171.Nunes R., Araújo F., Tavares J., Sarmento B., das Neves J. Surface modification with polyethylene glycol enhances colorectal distribution and retention of nanoparticles. Eur J Pharm Biopharm. 2018;130:200–206. doi: 10.1016/j.ejpb.2018.06.029. [DOI] [PubMed] [Google Scholar]
- 172.Davoudi Z., Peroutka-Bigus N., Bellaire B., Wannemuehler M., Barrett T.A. Intestinal organoids containing poly(lactic-co-glycolic acid) nanoparticles for the treatment of inflammatory bowel diseases. J Biomed Mater Res A. 2018;106:876–886. doi: 10.1002/jbm.a.36305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hassanzadeh P., Arbabi E., Atyabi F., Dinarvand R. Application of carbon nanotubes as the carriers of the cannabinoid, 2-arachidonoylglycerol: towards a novel treatment strategy in colitis. Life Sci. 2017;179:66–72. doi: 10.1016/j.lfs.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 174.Zhang W., Zhang Z., Zhang Y. The application of carbon nanotubes in target drug delivery systems for cancer therapies. Nanoscale Res Lett. 2011;6:555. doi: 10.1186/1556-276X-6-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhang X.-X., Huang H.-Y., Chen L.-Q., Jin H., Lee B.-J. Physicochemical characterization and cytotoxicity of chitosan-modified single walled carbon nanotubes as drug carriers. J Pharm Investig. 2019;49:57–65. [Google Scholar]
- 176.Sahu K.K., Pandey R.S. Immunological evaluation of colonic delivered Hepatitis B surface antigen loaded TLR-4 agonist modified solid fat nanoparticles. Int Immunopharmacol. 2016;39:343–352. doi: 10.1016/j.intimp.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 177.Mehta C.H., Narayan R., Nayak U.Y. Computational modeling for formulation design. Drug Discov Today. 2019;24:781–788. doi: 10.1016/j.drudis.2018.11.018. [DOI] [PubMed] [Google Scholar]
- 178.Huynh L., Neale C., Pomès R., Allen C. Computational approaches to the rational design of nanoemulsions, polymeric micelles, and dendrimers for drug delivery. Nanomedicine. 2012;8:20–36. doi: 10.1016/j.nano.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 179.Ramezanpour M., Leung S.S.W., Delgado-Magnero K.H., Bashe B.Y.M., Thewalt J. Computational and experimental approaches for investigating nanoparticle-based drug delivery systems. Biochim Biophys Acta (BBA) Biomembr. 2016;1858:1688–1709. doi: 10.1016/j.bbamem.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 180.Barata T.S., Zhang C., Dalby P.A., Brocchini S., Zloh M. Identification of protein-excipient interaction hotspots using computational approaches. Int J Mol Sci. 2016;17:853. doi: 10.3390/ijms17060853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Richard J. Challenges in oral peptide delivery: lessons learnt from the clinic and future prospects. Ther Deliv. 2017;8:663–684. doi: 10.4155/tde-2017-0024. [DOI] [PubMed] [Google Scholar]
- 182.Antosova Z., Mackova M., Kral V., Macek T. Therapeutic application of peptides and proteins: parenteral forever? Trends Biotechnol. 2009;27:628–635. doi: 10.1016/j.tibtech.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 183.Rohrer J., Lupo N., Bernkop-Schnürch A. Advanced formulations for intranasal delivery of biologics. Int J Pharm. 2018;553:8–20. doi: 10.1016/j.ijpharm.2018.10.029. [DOI] [PubMed] [Google Scholar]
- 184.Holfinger S., Miller A.G., Rao L.J., Rowland D.Y., Hornik J.H. Effect of regulatory requirement for patient-specific prescriptions for off-label medications on the use of intravitreal bevacizumab. JAMA Ophthalmol. 2016;134:45–48. doi: 10.1001/jamaophthalmol.2015.4331. [DOI] [PubMed] [Google Scholar]