Abstract
Introduction
Currently, there is no cure for Alzheimer's disease (AD), and it is widely accepted that AD is a complex disease with multiple approaches necessary to prevent and treat the disease.
Methods
Using amyloid biomarkers in human cerebrospinal fluid, pharmacokinetic, safety, and metabolism studies, we investigate the properties of NGP 555, γ-secretase modulator, for the first time in human clinical trials.
Results
Our preclinical and clinical studies combined show beneficial effects with NGP 555 on synaptic response and amyloid cerebrospinal fluid biomarkers while avoiding negative side effects. Importantly, pharmacokinetic and pharmacodynamic parameters combined with safety outcomes indicate that NGP 555 penetrates the blood-brain barrier and increases the ratio of amyloid-β peptide Aβ37 and Aβ38 compared with that of Aβ42, establishing a proof of target engagement in humans in a 14 day, once-daily oral dosing trial.
Discussion
In humans, NGP 555 has demonstrated a beneficial shift in the production of Aβ37 and Aβ38 versus Aβ42 biomarker levels in the cerebrospinal fluid while maintaining an adequate safety profile. The overall clinical goal is to achieve an optimal balance of efficacy for altering amyloid-β peptide (Aβ) biomarkers while maintaining a safe profile so that NGP 555 can be given early in AD to prevent production of Aβ42 and accumulation of amyloid plaques, in an effort to prevent aggregation of tau and destruction of neurons and synapses resulting in cognitive decline.
Keywords: Alzheimer's, Amyloid, Biomarkers, γ-secretase modulator, Pathology, Prevention, Pharmacokinetic, Pharmacodynamics, Cognition, Cerebrospinal fluid, Dementia, Synapses
1. Introduction
Alzheimer's disease (AD) is characterized by amyloid plaques and neurofibrillary tangles in the brain [1], [2], [3], [4]. These pathological changes precede the onset of dementia by many years and are thought to cause the destruction of neuronal cells and synapses, culminating in the eventual loss of cognitive function [5], [6], [7]. While no single approach is likely to cure or prevent AD, targeting amyloid and tau while treating neuroinflammatory and synaptic dysfunction may all be required to effectively mitigate the disease [8], [9], [10], [11], [12], [13], [14]. Treatment of AD has focused on the amyloid cascade hypothesis [13], [14] and led to studies focused on prevention of the damaging effects of Aβ42, the hallmark peptide in amyloid plaques [4], [15], [16]. Multiple small molecule and biological approaches targeting the Aβ42 peptide resulted in many failures because most of these agents had limited efficacy and significant side effects, particularly when inhibiting the enzymes that cleave the amyloid precursor protein resulting in amyloid-β (Aβ) peptide liberation [16], [17], [18], [19], [20], [21]. An alternative approach to the use of molecules that fully inhibit enzyme activity is to use modulators of the gamma secretase complex (GSMs) which selectively lower Aβ42 and raise the levels of shorter Aβ peptides [22], [23], [24], [25], [26], [27]. In contrast to the mechanism of γ-secretase inhibitors, this approach is more selective because it does not disrupt the Notch pathway or result in accumulation of amyloid precursor protein carboxyl-terminal fragments [28], [29], [30], [31]. Although GSMs represented a minority of the molecules tested in the clinic [16], to date most of these candidates have not succeeded because of compound toxicity based on structure or limited effectiveness to lower cerebrospinal fluid (CSF) amyloid biomarkers [32], [33], [34]. However, several GSMs are currently on the horizon and new discoveries of optimal chemical structures carry hope for the future of GSMs as therapeutics for AD [35], [36], [37], [38], [39], [40], [41], [42], [43], [44]. One such molecule is NGP 555, with previous studies demonstrating the ability to favorably alter amyloid biomarkers and pathology in the brain while preventing cognitive decline preclinically. Combined with the preclinical findings, this compound represents a new possibility for therapy to further advance a clinically safe molecule for AD [42], [43], [44]. To date, no GSM has gone the distance to confirm the translation of preclinical findings on Aβ biomarker reductions (Aβ42 CSF levels and brain amyloid plaques) to prevention of cognitive decline (with an adequate safety margin) in humans. We expand our knowledge of NGP 555's function and describe our phase 1 clinical results for the small molecule modulator of Aβ production, NGP 555.
2. Methods
2.1. Synaptic recordings
Primary mouse neuronal cultures were prepared from embryos day 18 and plated on coverslips at 10 K per well. Cells days in vitro (11-13) harvested from the same pups were treated on different coverslips 24 or 72 hours before recording with either dimethyl sulfoxide (control, n = 4), gamma-secretase inhibitor, difluorphenacetyl-alanyl phenylglycine t-butyl ester (n = 5), or NGP 555 (GSM, n = 4) and recorded on the same day. Extracellular and intracellular solutions were prepared as previously described (Chen, Durakoglugil et al. 2010). The membrane-resistances were 400 ± standard error of the mean (SEM) MΩ (control), 390 ± SEM MΩ (inhibitor), and 450 ± SEM MΩ (modulator), whereas access-resistance varied between 2 MΩ and 14 MΩ.
2.2. Metabolism
Liquid chromatography (LC), ultraviolet, and mass spectrometry (MS) methods were used to identify metabolites in rat, dog, and human plasma samples. Pooled plasma samples were extracted with 3 volumes acetonitrile. Metabolite profiling and identification were performed using a LC/MS system consisting of a Surveyor high performance liquid chromatography system equipped with a diode array ultraviolet detector interfaced to an ion trap mass spectrometer (Thermo Scientific, San Jose, CA). Mass spectra were acquired in full scan (MS) (m/z 150-1000).
2.3. NGP 555 formulation for clinical studies
NGP 555 was formulated at 25 mg and 100 mg as a solid dispersion of amorphous drug substance with polymer blended dispersants put into capsules for oral administration. The final product contained approximately 25% NGP 555, 22% hydroxypropyl methylcellulose-AS, 5% croscarmellose sodium, 1% magnesium stearate, and 47% Avicel PH101. Placebo contained 100% Avicel PH102. NGP 555 or placebo capsules were given at 9 a.m. each morning, approximately 30 min after consuming at least 50% of a balanced breakfast.
2.4. Clinical trial design
The Phase 1a (NGP 555-001) was a randomized, double-blind, placebo-controlled, parallel group, single ascending dose study conducted on 40 subjects (18–55 year old, inclusive) that included an equal representation of male and female volunteers across cohorts. The mean age of subjects was 35.9 years, ranging from 21 to 55 years across treatment groups. An equal number of male and female subjects (20 of each gender) were enrolled in the study with sex balance approximately equal across all treatment groups. Subjects enrolled by ethnicity include Hispanic or Latino ethnicity (37.5%), black or African American (32.5%), white (25%), Asian (2.5%), and Native Hawaiian or other Pacific Islander (2.5%). Five cohorts (cohort 1 = 25 mg, cohort 2 = 50 mg, cohort 3 = 100 mg, cohort 4 = 200 mg, and cohort 5 = 300 mg) of 8 volunteers were randomized to receive NGP 555 or placebo in a 3:1 ratio (6 to NGP 555 and 2 to placebo).
Phase 1b (NGP 555-002) was a randomized, placebo-controlled, double-blind, multiple-ascending dose study on healthy volunteers (40–65 year old, inclusive) with both male and female volunteers enrolled. The dose escalation was as follows: cohort 1 = 100 mg, cohort 2 = 200 mg, and cohort 3 = 400 mg); 8 volunteers were randomized to receive NGP 555, given for 14 days by oral capsule, or placebo, administered as matched capsule in a 3:1 ratio (6 to NGP 555 and 2 to placebo). For a subset of the 200 mg and 400 mg cohorts, subjects had CSF samples analyzed for Aβ alloforms. The mean age of subjects was 48.9 years, ranging from 40 to 61 years across treatment groups. Male (15) and female (9) subjects were of the following ethnicities: white (41.7%), Hispanic or Latino ethnicity (29.2%), black or African American (20.8%), and Asian (12.5%). Overall, the demographic profiles of subjects receiving NGP 555 and placebo were similar and do not suggest any imbalances between treatment groups that may affect the interpretation of study results.
General inclusion/exclusion criteria include age (18–55 year old, phase 1a or 40–65 year old, phase 1b) with a body mass index ranging from 18.0 to 32.0 kg/m2 with overall good health and no known genetic, liver, kidney, central nervous system, or cardiovascular disease.
The following safety endpoints were routinely evaluated: adverse event (AE) assessments, vital signs (blood pressure [BP] and heart rate [HR], oral body temperature, respiratory rate), 12-lead electrocardiogram (PR, RR, QRS, QT, QTcB, and QTcF intervals), clinical laboratory testing (hematology, clinical chemistry, coagulation, and urinalysis), concomitant medications, Columbia Suicide Severity Rating Scale.
2.5. Pharmacokinetics
Blood samples for pharmacokinetic (PK) assessments were taken at a time before drug administration and at scheduled times after dosing, specifically at 0.25, 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, 18, 24, 36, 48, 72, 96, and 144 hours after dose for Phase 1a. For Phase 1b, days 1 and 14: 0.25, 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, 16, and 24 hours after dose and at 5 hours after dose for days 2 to 13. NGP 555 concentrations and its metabolite, M436, in the plasma samples were determined by a validated bioanalytical method using LC-MS/MS. NGP 555 and NGP555d8 and M436 and benazepril-d5 were detected by monitoring the precursor and product ions (m/z 407.2→201.0 for NGP 555, m/z 415.2→201.0 for NGP555d8 and m/z 437.2→378.0 for M436, and m/z 430.0→356.0 for benazepril-d5) using an Applied Biosystems API3000 LC-MS/MS. The PK parameters were derived from drug concentrations in plasma using noncompartmental methods with Phoenix WinNonlin Version 6.4. Maximum plasma concentration (Cmax), time to maximum concentration (Tmax), area under the concentration-time curve from zero to infinity (AUC(0-∞)), area under plasma concentration-time curve from zero to time t (AUC(0-t)), slope of the terminal phase (λz), half-life associated with the terminal elimination phase (t 1/2λz), apparent volume of distribution during terminal phase after administration (VZ), apparent total body clearance of drug from plasma (CL).
2.6. Human CSF studies
Lumbar catheter insertion and CSF sampling occurred serially on day 1 prestudy drug administration and day 14. For serial CSF sampling, a 19G Duraflex Plus epidural lumbar catheter was placed at the L3/L4 interspace of each subject on day 1 and day 14. For each day, approximately 2-5 mL CSF was collected per time point. The Aβ peptides from human CSF samples were quantified using the triplex Mesoscale kit (using manufacturer's recommended procedures) or a custom Aβ37 assay on a small spot Streptavidin plate utilizing anti-Aβ37 (kindly provided by Pankaj Mehta, New York State Institute, NY) conjugated to biotin coupled with SULFO-TAG 6E10 (anti-Aβ amino-terminal antibody) as detection.
3. Results
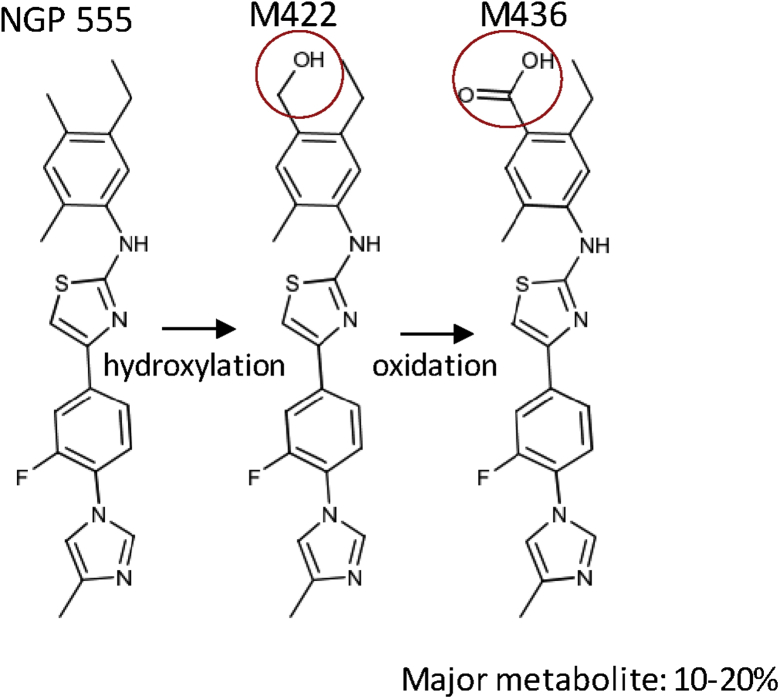
NGP 555 (a small molecule disease-modifying amyloid therapy) demonstrates translation of activity from preclinical to clinical findings. This molecule acts by modulating the γ-secretase complex, and herein, we demonstrate the translation of safety, metabolism, PK, and pharmacodynamic (PD) properties from preclinical studies to human clinical studies. Metabolism of NGP 555 was investigated in the presence of human liver microsomes and hepatocytes of rats, dogs, monkeys, and humans and indicated NGP 555 was primarily metabolized via hydroxylation and further oxidation to form a carboxylic acid metabolite (designated M436). These studies revealed moderate metabolism of NGP 555 in all species. M436 metabolite was found as the most significant metabolite in rat, dog, monkey, and human hepatocytes. Upon further investigation, the carboxylic acid (M436) and the hydroxylated analogs of NGP 555 were found as the major metabolites in dog plasma after single and multiple oral dosing; the structure of NGP 555 and its primary metabolite is shown in Fig. 1. This finding is consistent with what was observed during the in vitro metabolism studies conducted with NGP 555 and dog hepatocytes. Overall, the carboxylic acid (M436) analog of NGP 555 was found as the major metabolite in rat, dog, and human plasma after single and multiple oral dosing.
Fig. 1.
NGP 555 is metabolized to form the carboxylic acid (M436) of NGP 555 as the major metabolite. The major metabolite, M436, was present at > 10% of total drug-related material in plasma of rats, dogs, and humans as determined by LC-MS/MS analysis of rat, dog, and human plasma samples.
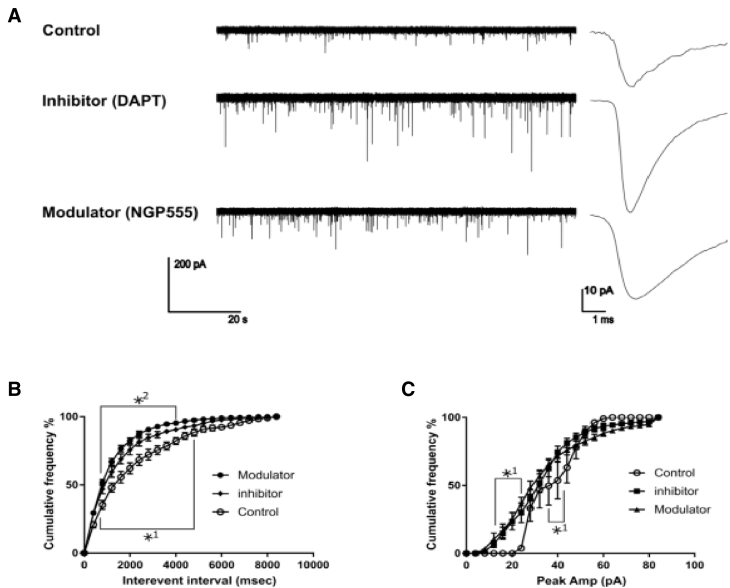
In a study designed to determine if NGP 555 impacted synaptic function, in Fig. 2 we show that NGP 555 is capable of promoting synaptic transmission in wild-type mouse neuronal cultures. The recordings depict higher frequency of events with the treatments compared with the controls. The interevent intervals describe the timeframe between the single minis. The significantly smaller intervals show a higher frequency of events in inhibitor and modulator cells. Furthermore, the modulator cells display higher frequencies, indicated by smaller intervals, than the inhibitor cells. The averaged minis with inhibitor and modulator-treated cells also display a larger amplitude than the controls. In addition, albeit nonsignificant, the modulator cells show a slightly slower decay of the signal compared with control and inhibitor cells. The drug-treated cells show significantly more single quantum (amplitudes below 20 pA) and multiquantal (~35–45 pA) releases compared with the control. Amplitudes of the control cells stop around 70 pA, whereas the drug-treated cells still include events with higher amplitudes. Taken together, these studies demonstrate a clear positive response of NGP 555 on synaptic function.
Fig. 2.
NGP 555 improves synaptic amplitude and frequency. Cells on different coverslips (11 to 13 days in vitro) were treated 24 hours before recording with either dimethyl sulfoxide (control, n = 4), difluorphenacetyl-alanyl phenylglycine t-butyl ester (gamma-secretase inhibitor, n = 5), or NGP 555 (GSM, n = 4) and recorded on the same day. The membrane resistances were 400 ± SEM MΩ (control), 390 ± SEM MΩ (inhibitor), and 450 ± SEM MΩ (modulator) whereas access-resistance varied between 2 and 14 MΩ. The cumulative histogram analysis was used in B and C to bridge the big difference in event numbers between the control cells and the drug-treated cells. (A): Exemplary excerpt from cell culture recordings and their average mini-currents (voltage clamp-spontaneous release). (B): The interevent intervals describe the timeframe between the single minis. (C): The peak amplitude. Statistics: *P < .05, *1 = difference between control and treated cells, *2 = difference between modulator and inhibitor cells, analysis of variance.
Two randomized, placebo-controlled, double-blind, single or multiple ascending dose studies in healthy volunteers were conducted. All pertinent study documents were reviewed by an institutional review board before study initiation. Studies were conducted in compliance with Food and Drug Administration regulations as described in the Code of Federal Regulations 21 parts 50 and 56, Department of Health and Human Services regulations as described in 45 Code of Federal Regulations 46, guidelines resulting from the International Conference on Harmonization, Good Clinical Practice, and potentially the Common Rule as appropriate. All subjects who participated in this study were fully informed about the study in accordance with Good Clinical Practice guidelines, federal regulations, Health Insurance Portability and Accountability Act, and guidelines in accordance with local requirements.
The principal investigator explained the nature of the study to the subjects, in nontechnical terms, and answered all questions regarding the study. Subjects reviewed, signed, and dated the informed consent form.
An equal number of males and females were enrolled, and the gender balance was similar for those on study drug or placebo. A range of ethnicities were enrolled, and overall, the demographic profiles of subjects receiving NGP 555 and placebo were similar. Gender and race enrollment do not suggest any imbalances between treatment groups that may affect the interpretation of study results.
In the first human phase 1 clinical trial, safety, tolerability, PK, and PD (in a subset of subjects) of escalating doses of NGP 555 were evaluated after giving NGP 555 once daily as an oral capsule formulation. Table 1 shows a listing of the adverse events (AEs) that were determined to be possibly related to drug treatment, categorized by system organ class and preferred term. In phase 1a, NGP 555 was well tolerated across all five cohorts, and the maximum tolerated dose was not reached in this study. The most frequently reported AEs by both placebo- and NGP 555-treated subjects were headache, nausea, and dizziness. The most common AEs with a reasonable possibility of relationship to NGP 555 treatment by study cohort were lower abdominal pain, nausea, and sleep disorder (1 subject each). All AEs were mild in severity. Table 1 shows the AEs reported to be possibly or probably related to study drug treatment. These included headache, nausea, vomiting, lack of appetite, and a cough. These AEs occurred more frequently in the placebo subjects in comparison with NGP 555-treated subjects. In phase 1b, the bulk of the adverse events (AEs) in this trial resulted from theCSF collection procedures (49 total including 2 serious adverse events [SAEs]) and were determined to be unlikely related to study drug treatment. The two SAEs (placebo and 200 mg dose) occurred after study completion when subjects were admitted to the hospital for nausea/vomiting (placebo) and headache (200 mg), both were attributed to the CSF collection and determined to be nonstudy drug related. Other AEs (skin irritation, constipation, headache, chapped lips, upper respiratory infection, and difficulty in writing small letters) were determined to be unlikely related to study drug treatment. One subject (at 400 mg), who showed an increase in alanine aminotransferase, grade 2 (moderate), and increase in aspartate aminotransferase, grade 1 (mild) at day 16 only, also received 2 doses of Tylenol and 2 doses of aspirin in the 24 hrs before the collection of plasma for clinical chemistry analysis. This subject did not show increased liver enzymes at day 7 labs, and a second subject at 400 mg did not show any increase in liver enzymes. In addition, no bilirubin changes were noted in any subjects in this trial. There were no other clinically meaningful differences noted between subjects who received NGP 555 and those who received placebo for changes over time in clinical laboratory data (including hematology, clinical chemistry, coagulation, or urinalysis), Columbia Suicide Severity Rating Scale, vital signs, and electrocardiograms.
Table 1.
Treatment-emergent adverse events attributed by the investigator as having a reasonable possibility of relationship to treatment
| Phase 1a (Single-ascending dose) | ||||||||
|---|---|---|---|---|---|---|---|---|
| System organ class preferred term relationship | NGP 555 |
|||||||
| Placebo (N = 10) | Cohort 1 25 mg (N = 6) | Cohort 2 50 mg (N = 6) | Cohort 3 100 mg (N = 6) | Cohort 4 200 mg (N = 6) | Cohort 5 300 mg (N = 6) | All NGP 555 (N = 30) | All subjects (N = 40) | |
| Number (%) of subjects with >=1 TEAE by relationship | ||||||||
| GI disorders | ||||||||
| Abdominal pain lower | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 1 (3.3) | 1 (2.5) |
| Possibly related | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 1 (3.3) | 1 (2.5) |
| Nausea | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 0 (0.0) | 0 (0.0) | 1 (3.3) | 1 (2.5) |
| Probably related | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 0 (0.0) | 0 (0.0) | 1 (3.3) | 1 (2.5) |
| Sleep disorder | ||||||||
| Possibly related | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 0 (0.0) | 1 (3.3) | 1 (2.5) |
| Phase 1b (Multiple-ascending dose) | ||||||
|---|---|---|---|---|---|---|
| System organ class preferred term relationship | Placebo (N = 6) | Cohort 1 100 mg (N = 6) | Cohort 2 200 mg (N = 10) | Cohort 3 400 mg (N = 2) | All NGP 555 (N = 18) | All subjects (N = 24) |
| Number (%) of subjects with >=1 TEAE by relationship | ||||||
| GI disorders | ||||||
| Possibly related | 1 (16.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0) | 1 (4.2) |
| Emesis | 1 (16.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0) | 1 (4.2) |
| Probably related | 1 (16.7) | 0 (0.0) | 1 (10.0) | 0 (0.0) | 1 (5.6) | 2 (8.3) |
| Nausea | 1 (16.7) | 0 (0.0) | 1 (10.0) | 0 (0.0) | 1 (5.6) | 2 (8.3) |
| Metabolism and nutritional disorders | ||||||
| Possibly related | 1 (16.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (4.2) |
| Lack of appetite | 1 (16.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (4.2) |
| Respiratory disorders | ||||||
| Possibly related | 1 (16.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (4.2) |
| Cough | 1 (16.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (4.2) |
| Nervous system disorders | ||||||
| Possibly related | 1 (16.7) | 0 (0.0) | 1 (10.0) | 0 (0.0) | 1 (5.6) | 2 (8.3) |
| Headache | 1 (16.7) | 0 (0.0) | 1 (10.0) | 0 (0.0) | 1 (5.6) | 2 (8.3) |
Abbreviations: GI, gastrointestinal; TEAE, Treatment-emergent adverse event.
NOTE. AEs were monitored throughout the course of the study and TEAEs were reported from the time of study drug administration through Day 23 (follow-up). AEs were mapped to preferred terms and body systems using the Medical Dictionary for Regulatory Activities (MedDRA) coding dictionary version 16.1. The number and percentage of subjects experiencing AEs was summarized by system organ class and preferred term.
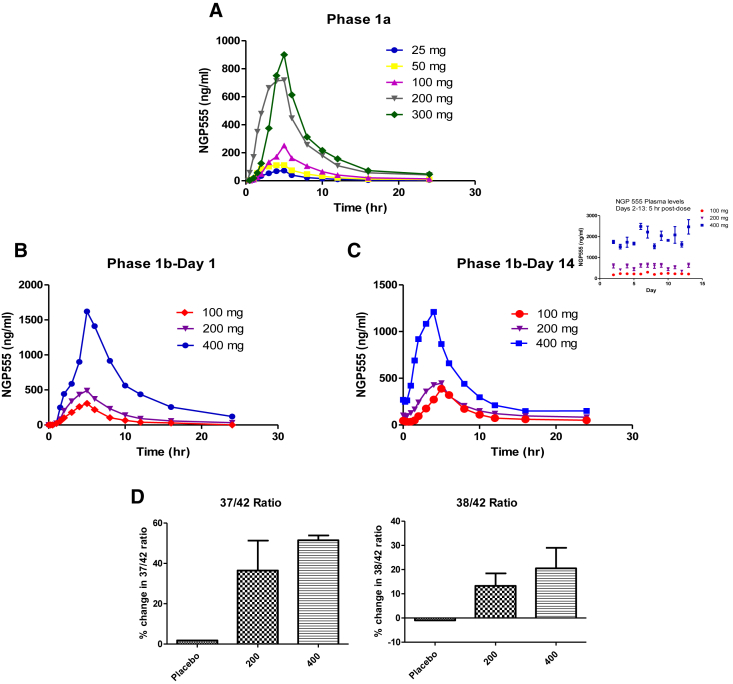
The results of the bioanalytical analysis of NGP 555 in plasma for both studies are depicted in Fig. 3. For phase 1a (single dose), the data (panel A) show that the exposure and Cmax values are generally dose dependent for both NGP 555 and M436, and the profiles are similar when compared with preclinical models. The peak NGP 555 concentration levels appear to be roughly proportional to the dose levels for cohorts 1, 2, 3, and 5. The mean peak concentration level for cohort 4 (200 mg) was larger than that which would have been expected based on dose proportionality. For phase 1b, Fig. 3, panel B (day 1), Fig. 3, panel C (day 14), the median peak plasma concentration levels were ~4 hours after dose. The results of the dose proportionality were as expected for the 100 and 400 mg dose levels with the Cmax showing a 3.6-fold increase and the AUClast, a 4.4-fold increase. The 200 mg dose level showed a less than expected increase in values. The observed peak NGP 555 concentration level for the three dosing levels (100, 200, and 400 mg, respectively) occurred at 3.9, 4.1, 5.0 hours after dose (day 1) and 4.3, 3.7, 3.2 hours after dose (day 14). PK analysis of plasma samples from the phase 1b multiple dose trial for both NGP 555 and the primary metabolite, M436, are shown for day 1 (Table 2) and day 14 (Table 3). For both NGP 555 and the metabolite, M436 plasma concentration levels reached their peak ranging from 3 to 5 hours after dose.
Fig. 3.
Time-concentration profiles of NGP 555 in humans and CSF biomarker profiles. Panel A shows NGP-555 time-concentration mean profiles for each cohorts 1 through 5, respectively. Panel B: NGP-555 time-concentration mean profiles for each cohorts 1 through 3, respectively (day 1). Panel C presents the day 14 data for the three dosing cohorts. Inset: days 2-13 mean single concentration from 5 hrs. after dose for 3 dosing cohorts. Panel D: mean percentage change in the formation of cerebrospinal fluid Aβ37 or Aβ38 versus Aβ42 from baseline to day 14 in humans. CSF was collected by serial sampling at baseline (day 1) and day 14 (10 hours after dose) after dosing with NGP 555 200 mg (4 subjects), NGP 555 400 mg (2 subjects), or placebo (2 subjects). CSF samples were tested using the Mesoscale discovery Aβ enzyme-linked immunosorbent assay tests for Aβ42 and Aβ38 (triplex kit) or Aβ37 (custom kit). Owing to a large intrasubject variation in values from day 1 to day 14, each subject was compared with their own baseline values at 10 hrs., and data is expressed as mean +/− SD. Panel A shows the ratio of 37/42, and Panel B shows ratio of 38/42.
Table 2.
PK parameters for NGP 555 and B436 metabolite after 1 day of dosing
| Parameter statistics (Geometric mean) | 100 mg NGP 555 (N = 6) | 200 mg NGP 555 (N = 10) | 400 mg NGP 555 (N = 2) |
|---|---|---|---|
| Summary of pharmacokinetic parameters for NGP 555 on day 1 | |||
| Cmax (ng/mL) | 322 | 496 | 1610 |
| tmax (h) | 3.9 | 4.1 | 5.0 |
| AUClast (h∙ng/mL) | 1755.3 | 3252.4 | 11,983.4 |
| λz (h−1) | 0.093 | 0.109 | 0.107 |
| t1/2 (h) | 7.5 | 6.4 | 6.5 |
| Vz (mL) | 567921.9 | 521601.5 | 283269.1 |
| CL (mL/h) | 52,660.4 | 56,696.0 | 30,414.2 |
| Summary of pharmacokinetic parameters for M436 (metabolite) on day 1 | |||
| Cmax (ng/mL) | 16 | 24 | 66 |
| tmax (h) | 4.9 | 5.2 | 5.0 |
| AUClast (h∙ng/mL) | 203.1 | 285.9 | 907.2 |
| λz (h−1) | 0.032 | 0.030 | 0.029 |
| t1/2 (h) | 21.8 | 23.4 | 24.2 |
Abbreviations: Cmax, Maximum plasma concentration; tmax, time to maximum concentration; AUClast, area under curve; λz, slope of the terminal phase; t1/2, half-life associated with the terminal elimination phase; Vz, apparent volume of distribution during terminal phase after administration; CL, apparent total body clearance of drug from plasma.
NOTE. The PK parameters were derived from drug concentrations in plasma using non-compartmental methods with Phoenix WinNonlin Version 6.4. For NGP 555: Cmax, tmax, AUClast, λz, t1/2, VZ and CL and for M436: only Cmax, tmax, AUClast, λz, and t1/2, were derived. Descriptive statistics for PK parameters for NGP 555 and the major metabolite M436 are presented for the Phase 1b study (Day 1).
Table 3.
PK parameters for NGP 555 and B436 metabolite after 14 days of dosing
| Parameter statistics (Geometric mean) | 100 mg NGP 555 (N = 6) | 200 mg NGP 555 (N = 10) | 400 mg NGP 555 (N = 2) |
|---|---|---|---|
| Summary of pharmacokinetic parameters for NGP 555 on day 14 | |||
| Cmax (ng/mL) | 408.7 | 483.7 | 1480.0 |
| tmax (h) | 4.3 | 3.7 | 3.2 |
| AUClast (h∙ng/mL) | 2734.3 | 3875.3 | 11,932.5 |
| λz (h−1) | 0.080 | 0.080 | 0.065 |
| t1/2 (h) | 8.7 | 8.7 | 10.6 |
| Vz (mL) | 391756.7 | 551505.1 | 413223.6 |
| CL (mL/h) | 31,204.8 | 44,142.9 | 26,991.0 |
| Summary of pharmacokinetic parameters for M436 (metabolite) on day 14 | |||
| Cmax (ng/mL) | 75.2 | 76.7 | 167.6 |
| tmax (h) | 4.4 | 3.1 | 3.0 |
| AUClast (h∙ng/mL) | 858.8 | 972 | 2491.8 |
| λz (h−1) | 0.040 | 0.037 | 0.040 |
| t1/2 (h) | 17.4 | 19.0 | 17.2 |
Abbreviations: Cmax, Maximum plasma concentration; tmax, time to maximum concentration; AUClast, area under curve; λz, slope of the terminal phase; t1/2, half-life associated with the terminal elimination phase; Vz, apparent volume of distribution during terminal phase after administration; CL, apparent total body clearance of drug from plasma.
NOTE. The PK parameters were derived from drug concentrations in plasma using non-compartmental methods with Phoenix WinNonlin Version 6.4. For NGP 555: Cmax, tmax, AUClast, λz, t1/2, VZ and CL and for M436: only Cmax, tmax, AUClast, λz, and t1/2, were derived. Descriptive statistics for PK parameters for NGP 555 and the major metabolite M436 are presented for the Phase 1b study (Day 14).
To determine if NGP 555 was engaging its target in humans, we tested the CSF for changes in amyloid biomarkers. The CSF biomarkers are a reflection of the changes happening in the brain. Cerebrospinal fluid samples were collected for determination of Aβ biomarker levels in these samples. CSF samples were collected on day 1 (before study) and day 14 (after study). To accomplish CSF collection, lumbar catheter insertions and serial sampling of ~3 mL per time point occurred. Aβ levels were determined using validated enzyme-linked immunosorbent assays (Mesoscale Discovery services). Calculated PD parameters included individual subject and mean group differences in pre-post change for Aβ42, Aβ38, Aβ37, and their ratios. CSF collection resulted in many AEs, and the Aβ data collected revealed substantial intraday and interday variability within the same subject, making it difficult to interpret changes in a single Aβ alloform. However, because NGP 555 acts by increasing shorter peptides while decreasing Aβ42, this ratio change effectively canceled out variability because of changes in total Aβ production. Fig. 3 (panel D, left side) shows the change in the 37/42 ratio from day 14 to baseline at 10 hr. (after day 14 dosing) while Fig. 3 (panel D, right side) shows the change in the 38/42 ratio. While the study was not powered for statistical significance, the placebo group showed little (or negative change) in the formation of 37/42 or 38/42 whereas both the 200 mg group and 400 mg group showed a positive shift in these ratios after 14 days of dosing. This study which was not powered for statistical significance does show an indication of proof of target engagement in the brain and a trend for relationship to dose given. Although it is difficult to translate the amount of shift in the production from Aβ42 to shorter forms into an actual possibility of preventing amyloid plaque formation and cognitive decline, these data demonstrate favorable shifts in the amyloid biomarker production at safe doses. Preclinical data do suggest that modest shifts in biomarker production (20–40%) turn into significant decreases in amyloid burden while preserving cognitive function [42], [43]. Further studies are necessary to evaluate the impact of NGP 555 on amyloid plaque formation and AD cognitive decline in humans.
4. Discussion
Preclinical results demonstrate NGP 555 has favorable drug properties including good oral absorption, brain penetration, central nervous system activity, and specificity for a lipid-based membrane target (γ-secretase) with limited metabolism [42], [43]. In addition, we report beneficial activity of NGP 555 directed at synaptic function. Mechanistically, these data can be explained by the direct decrease in the production Aβ42 as previously described [45], [46]. Alternately, reports showing beneficial changes on synapse function by disrupting γ-secretase activity may occur via processing of other substrates involved in synaptic function [47], [48], [49], [50]. Further studies to define a possible role of NGP 555 acting on additional known substrates processed by γ-secretase to exert changes in synaptic function are warranted. One approach to determine possible new substrates modulated by NGP 555 treatment involves assessing the products of the γ-cleavage on membrane-anchored proteins expected to impact synaptic function.
Phase 1 studies for NGP 555 were completed for single and multiple dosing in a total of 64 subjects. Safety findings revealed no clinically meaningful differences noted between subjects who received NGP 555 and those who received placebo for changes over time in clinical laboratory data (including hematology, clinical chemistry, coagulation, or urinalysis), Columbia Suicide Severity Rating Scale, vital signs, and electrocardiograms, and the bulk of the adverse events were determined to be nonstudy drug related with the most common AEs with the possibility to be related being nausea, headache, and difficulty sleeping. Importantly, we utilized a novel capsule formulation which demonstrated good PK properties and proof-of-target engagement for its expected brain target. The shapes of the plasma concentration-time profiles are very similar between the three dosing levels in day 1 compared with day 14, and the major metabolite identified preclinically was consistent with the metabolism in humans. While the study was not powered for statistical significance, the subjects on placebo showed little (or negative change) in the formation of Aβ37/Aβ42 or Aβ38/Aβ42 whereas both the 200 mg group and 400 mg group showed a positive shift in these ratios after 14 days of dosing indicating an effect on the CSF Aβ biomarkers. NGP 555 was well tolerated across all cohorts in both the single and multiple dose studies, and the maximum tolerated dose was not identified in either study. In addition, based on the PK profile, it is reasonable to expect that twice-daily dosing would result in an improved outcome on shifting biomarkers. While NGP 555 targets the γ-secretase complex to reduce Aβ42 and Aβ40 and increase Aβ38 and Aβ37, our synaptic study opens up the possibility that NGP 555 has additional activities. Our clinical results demonstrate a clear translation of preclinical to clinical findings; however, further clinical studies are necessary to achieve the ultimate results on prevention of amyloid pathology and reduction of cognitive decline in humans while maintaining adequate safety.
Research in context.
-
1.
Systematic review: The authors reviewed the literature using traditional (e.g., PubMed) sources and presented data. There are numerous descriptions of amyloid approaches and γ-secretase modulators, and they are clearly cited. The innovation with NGP 555 is translation of activity from preclinical to clinical with target specificity, safety margins, biomarker efficacy, metabolism, oral bioavailability, and brain penetration.
-
2.
Interpretation: In characterizing NGP 555, a modulator of Aβ production, we describe our clinical results which demonstrate translation from preclinical studies showing NGP 555 acts by engaging a brain target and affecting amyloid biomarkers in the cerebrospinal fluid when dosed at concentrations below the maximum tolerated dose.
-
3.
Future directions: The manuscript provides key information on the clinical findings of NGP 555 in a phase 1 clinical trial. This study provides the rational to conduct additional clinical studies to further test the amyloid hypothesis in humans with a γ-secretase modulator.
Acknowledgments
The authors would like to thank Niku Hötzel for excellent technical support.
Funding: National Institute of Aging (NIA) supported this work through the R01 AG049702 and National Institute Neurological Disorders and Stroke (NINDS) U44 NS073133. JH is supported by grants from the NIH (R37 HL63762, R01 NS093382, R01 NS108115, and RF1 AG053391 to JH). JH was further supported by the Consortium for Frontotemporal Dementia Research and the Bright Focus Foundation.
We thank Dr. Jeremiah Momper, UCSD, Department of Pharmacology for conducting the statistical analysis of human PK samples.
Footnotes
Competing interests: Drs. Comer and Kounnas are shareholders of NeuroGenetic Pharmaceuticals, Inc.
References
- 1.Alzheimer A., Stelzmann R., Schnitzlein H., Murtagh F. An English translation of Alzheimer's 1907 paper. Uber eine eigenartige Erkankung der Hirnrinde. 1995;90:429–431. doi: 10.1002/ca.980080612. [DOI] [PubMed] [Google Scholar]
- 2.Kidd M. Paired helical filaments in electron microscopy of Alzheimer's disease. Nature. 1963;197:192–193. doi: 10.1038/197192b0. [DOI] [PubMed] [Google Scholar]
- 3.Terry R., Gonatas N., Weiss M. Ultrastructural studies in Alzheimer's presenile studies. Am J Pathol. 1964;44:269–287. [PMC free article] [PubMed] [Google Scholar]
- 4.Younkin S. The role of Abeta 42 in Alzheimer's disease. J Physiol Paris. 1998;92:289–292. doi: 10.1016/s0928-4257(98)80035-1. [DOI] [PubMed] [Google Scholar]
- 5.Shaw L., Vanderstichele H., Knapik-Czajka M., Clark C., Aisen P., Petersen R., Alzheimer's Disease Neuroimaging Initiative Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roe C., Fagan A., Grant E., Hassenstab J., Moulder K., Maue Dreyfus D. Amyloid imaging and CSF biomarkers in predicting cognitive impairment up to 7.5 years later. Neurology. 2013;80:1784–1791. doi: 10.1212/WNL.0b013e3182918ca6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang J., Korecka M., Toledo J., Trojanowski J., Shaw L. Clinical utility and analytical challenges in measurement of cerebrospinal fluid amyloid-β1-42 and τ proteins as Alzheimer disease biomarkers. Clin Chem. 2013;59:903–916. doi: 10.1373/clinchem.2013.202937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y., Fu A., Ip N. Synaptic dysfunction in Alzheimer's disease: mechanisms and therapeutic strategies. Pharmacol Ther. 2019;195:186–198. doi: 10.1016/j.pharmthera.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Lacor P.N., Buniel M.C., Furlow P.W., Clemente A.S., Velasco P.T., Wood M. Aβ oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer's disease. J Neurosci. 2007;27:796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bazzari F., Abdallah D., El-Abhar H. Pharmacological interventions to attenuate Alzheimer's disease progression: the story so far. Curr Alzheimer Res. 2019;16:261–277. doi: 10.2174/1567205016666190301111120. [DOI] [PubMed] [Google Scholar]
- 11.Jadhav S., Avila J., Schöll M., Kovacs G., Kövari E., Skrabana R. A walk through tau therapeutic strategies. Acta Neuropathol Commun. 2019;7:22. doi: 10.1186/s40478-019-0664-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGeer P., Rogers J., McGeer E. Inflammation, antiinflammatory agents, and Alzheimer's disease: the last 22 years. J Alzheimers Dis. 2016;54:853–857. doi: 10.3233/JAD-160488. [DOI] [PubMed] [Google Scholar]
- 13.Reiss A., Arain H., Stecker M., Siegart N., Kasselman L. Amyloid toxicity in Alzheimer's disease. Rev Neurosci. 2018;29:613–627. doi: 10.1515/revneuro-2017-0063. [DOI] [PubMed] [Google Scholar]
- 14.Hardy J., Selkoe D. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 15.Golde T., Petucell C., Lewis J. Targeting Aβ and tau in Alzheimer's disease, an early interim report. Exp Neurol. 2009;223:252–266. doi: 10.1016/j.expneurol.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki K., Iwata A., Iwatsubo T. The past, present, and future of disease-modifying therapies for Alzheimer's disease. Proc Jpn Acad Ser B Phys Biol Sci. 2017;93:757–771. doi: 10.2183/pjab.93.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lao K., Ji N., Zhang X., Qiao W., Tang Z., Gou X. Drug development for Alzheimer's disease: review. J Drug Target. 2018;20:1–10. doi: 10.1080/1061186X.2018.1474361. [DOI] [PubMed] [Google Scholar]
- 18.Das B., Yan R. A Close Look at BACE1 Inhibitors for Alzheimer's Disease Treatment. CNS Drugs. 2019;33:251–263. doi: 10.1007/s40263-019-00613-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imbimbo B., Giardina G.A. γ-secretase inhibitors and modulators for the treatment of Alzheimer's disease: disappointments and hopes. Curr Top Med Chem. 2011;11:1555–1570. doi: 10.2174/156802611795860942. [DOI] [PubMed] [Google Scholar]
- 20.Tomita T. Secretase inhibitors and modulators for Alzheimer's disease treatment. Expert Rev Neurother. 2009;9:661–679. doi: 10.1586/ern.09.24. [DOI] [PubMed] [Google Scholar]
- 21.Wolfe M.S. γ-Secretase as a target for Alzheimer's disease. Adv Pharmacol. 2012;64:127–153. doi: 10.1016/B978-0-12-394816-8.00004-0. [DOI] [PubMed] [Google Scholar]
- 22.Xia W. G-secretase and its modulators: twenty years and beyond. Neurosci Lett. 2019;701:162–169. doi: 10.1016/j.neulet.2019.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evin G. γ-secretase modulators: hopes and setbacks for the future of Alzheimer's treatment. Expert Rev Neurother. 2008;8:1611–1613. doi: 10.1586/14737175.8.11.1611. [DOI] [PubMed] [Google Scholar]
- 24.Crump C., Johnson D., Li Y. Development and mechanism of γ-secretase modulators for Alzheimer's disease. Biochemistry. 2013;52:3197–3216. doi: 10.1021/bi400377p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gijsen J., Bischoff F. Secretase inhibitors and modulators as a disease modifying approach against Alzheimer's disease. Annu Rep Med Chem. 2012;47:55–69. [Google Scholar]
- 26.Borgegard T., Juréus A., Olsson F., Rosqvist S., Sabirsh A., Rotticci D. First and second generation γ-secretase modulators (GSMs) modulate amyloid-β (Aβ) peptide production through different mechanisms. J Biol Chem. 2012;287:11810–11819. doi: 10.1074/jbc.M111.305227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kukar T., Ladd T., Bann M., Fraering P., Narlawar R., Maharvi G. Substrate-targeting γ-secretase modulators. Nature. 2008;453:925–930. doi: 10.1038/nature07055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tamayev R., D'Adamio L. Inhibition of gamma-secretase worsens memory deficits in a genetically congruous mouse model of Danish dementia. Mol Neurodegener. 2012;7:1–7. doi: 10.1186/1750-1326-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milano J., McKay J., Dagenais C., Foster-Brown L., Pognan F., Gadient R. Modulation of Notch processing by γ-secretase inhibition causes intestinal goblet cell metaplasia and induction of genes known to specify gut secretory lineage differentiation. Toxicol Sci. 2004;82:341–358. doi: 10.1093/toxsci/kfh254. [DOI] [PubMed] [Google Scholar]
- 30.van Es J., van Gijn M., Riccio O., van den Born M., Vooijs M., Begthel H. Notch/γ-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- 31.Green R., Schneider L., Amato D., Beelen A., Wilcock G., Swabb E., Tarenflurbil Phase 3 Study Group Effect of tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer disease: a randomized controlled trial. JAMA. 2009;16:2557–2564. doi: 10.1001/jama.2009.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu Y., Logovinsky V., Schuck E., Kaplow J., Chang M., Miyagawa T. Safety, tolerability, pharmacokinetics, and pharmacodynamics of the novel γ-secretase modulator, E2212, in healthy human subjects. J Clin Pharmacol. 2014;54:528–536. doi: 10.1002/jcph.249. [DOI] [PubMed] [Google Scholar]
- 33.Nakano-Ito K., Fujikawa Y., Hihara T., Shinjo H., Kotani S., Suganuma A. E2012-Induced Cataract and its predictive biomarker. Tox Sci. 2014;137:249–258. doi: 10.1093/toxsci/kft224. [DOI] [PubMed] [Google Scholar]
- 34.Soares H., Gasior M., Toyn J., Wang J., Hong Q., Berisha F. Gamma secretase modulator, BMS-932481, modulates Aβ peptides in the plasma and CSF of healthy volunteers. JPET. 2016;358:138–150. doi: 10.1124/jpet.116.232256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bursavich M., Harrison B., Blain J. Gamma secretase modulators, new Alzheimer's drugs on the horizon? J Med Chem. 2016;59:7389–7409. doi: 10.1021/acs.jmedchem.5b01960. [DOI] [PubMed] [Google Scholar]
- 36.Xie L., Am Ende C., Pettersson M., Rankic D., Sach N., Sakya S. Synthesis of pyridopyrazine-1,6-dione γ-secretase modulators via selective 4-methylimidazole N1- Buchwald arylation. J Org Chem. 2019;84:4921–4925. doi: 10.1021/acs.joc.8b02953. [DOI] [PubMed] [Google Scholar]
- 37.Gerlach K., Hobson S., Eickmeier C., Groß U., Braun C., Sieger P. Discovery of tetrahydroindazoles as a novel class of potent and in vivo efficacious gamma secretase modulators. Bioorg Med Chem. 2018;26:3227–3241. doi: 10.1016/j.bmc.2018.04.053. [DOI] [PubMed] [Google Scholar]
- 38.Sekioka R., Honjo E., Honda S., Fuji H., Akashiba H., Mitani Y. Discovery of novel scaffolds for γ-secretase modulators without an arylimidazole moiety. Bioorg Med Chem. 2018;26:435–442. doi: 10.1016/j.bmc.2017.11.049. [DOI] [PubMed] [Google Scholar]
- 39.Zhao Z., Pissarnitski D., Huang X., Palani A., Zhu Z., Greenlee W. Discovery of a Tetrahydrobenzisoxazole Series of γ-Secretase Modulators. ACS Med Chem Lett. 2017;8:1002–1006. doi: 10.1021/acsmedchemlett.7b00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blain J., Bursavich M., Freeman E., Hrdlicka L., Hodgdon H., Chen T. Characterization of FRM-36143 as a new γ-secretase modulator for the potential treatment of familial Alzheimer's disease. Alzheimers Res Ther. 2016;8:34. doi: 10.1186/s13195-016-0199-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wagner S., Rynearson K., Duddy S., Zhang C., Nguyen P., Becker A. Pharmacological and toxicological properties of the potent oral γ-secretase modulator BPN-15606. J Pharmacol Exp Ther. 2017;362:31–44. doi: 10.1124/jpet.117.240861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kounnas M., Danks A., Cheng S., Tyree C., Ackerman E., Zhang X. Modulation of γ-secretase reduces β-amyloid deposition in a transgenic mouse model of Alzheimer's disease. Neuron. 2010;67:769–780. doi: 10.1016/j.neuron.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kounnas M., Lane-Donovan C., Nowakowski D., Herz J J., Comer W. NGP 555, a γ-secretase modulator, lowers the amyloid biomarker, Aβ42, in cerebrospinal fluid while preventing Alzheimer's disease amyloid pathology and cognitive decline in Rodents. Alzheimers Dement. 2017;3:65–73. doi: 10.1016/j.trci.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Q., Waltz S., Woodruff G., Ouyang J., Israel M., Herrera C. Effect of potent γ-secretase modulator in human neurons derived from multiple Presenilin 1-Induced pluripotent stem cell mutant carriers. JAMA Neuro. 2014;71:1481–1489. doi: 10.1001/jamaneurol.2014.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klyubin I., Betts V., Welzel A., Blennow K., Zetterberg H., Wallin A. Amyloid beta protein dimer-containing human CSF disrupts synaptic plasticity: prevention by systemic passive immunization. J Neurosci. 2008;28:4231–4237. doi: 10.1523/JNEUROSCI.5161-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Z., Jackson R., Hong W., Taylor W., Corbett G., Moreno A. Human brain-derived Aβ oligomers bind to synapses and disrupt synaptic activity in a manner that requires APP. J Neurosci. 2017;37:11947–11966. doi: 10.1523/JNEUROSCI.2009-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parent A., Barnes N., Taniguchi Y., Thinakaran G. Sisodia SS “Presenilin attenuates receptor-mediated signaling and synaptic function”. J Neurosci. 2005;25:1540–1549. doi: 10.1523/JNEUROSCI.3850-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taniguchi Y., Kim S., Sisodia S. Presenilin-dependent gamma-secretase processing of Deleted in Colorectal Cancer (DCC) J Biol Chem. 2003;278:30425–30428. doi: 10.1074/jbc.C300239200. [DOI] [PubMed] [Google Scholar]
- 49.Duncan R., Song B., Koulen P. Presenilins as drug targets for Alzheimer's disease—recent insights from cell biology and electrophysiology as novel opportunities in drug development. Int J Mol Sci. 2018;19:1621–1629. doi: 10.3390/ijms19061621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haapasalo A., Kovacs D. The many substrates of presenilin/γ-secretase. J Alzheimers Dis. 2011;25:3–28. doi: 10.3233/JAD-2011-101065. [DOI] [PMC free article] [PubMed] [Google Scholar]