Abstract
Background
Data on the clinical features, optimal treatment, and outcomes of pediatric patients with epithelioid sarcoma (ES) are limited and mostly retrospective.
Methods
A subset analysis of ES patients < 30 years of age enrolled on two international prospective clinical trials conducted between 7/2005 and 11/2015 was performed. Risk-adapted therapy was based on tumor diameter, histologic grade, extent of surgery, and presence/absence of metastases, and included surgery +/− radiotherapy for all patients with the addition of ifosfamide/doxorubicin chemotherapy for intermediate-/high-risk patients. Response to therapy, event-free and overall survival, and pattern and predictors of treatment failure were evaluated.
Results
Sixty-three ES patients (median age 13.1 years, 52% male) were eligible. Clinical features included: 68% extremity, median tumor diameter 3.5 cm, 56% high histologic grade, 14% nodal metastases, 14% distant metastases. Thirty-four low-risk patients underwent surgery (n=30) or surgery/radiotherapy (n=4); 16 intermediate-risk and 13 high-risk patients received chemotherapy +/− surgery +/− radiotherapy. Partial response was observed in 11/22 patients receiving neoadjuvant therapy (50%). Events were local recurrence (n=10) and distant recurrence (n=15); estimated 5-year survival was 86.4%, 63.5%, and 0%, respectively, for low-, intermediate-, and high-risk patients. Loco-regional nodal involvement, invasive tumor, high grade, and lesser extent of resection predicted event-free survival in patients without metastases.
Conclusions
Most low-risk ES patients who have undergone an adequate resection fare well without adjuvant therapy. Large tumor size, high histologic grade, tumor invasiveness, inadequate tumor resection, and metastatic disease predict poorer outcomes in higher risk ES patients, for whom more effective therapies are needed.
Keywords: epithelioid sarcoma, pediatric, soft tissue sarcoma
Introduction
Epithelioid sarcoma (ES) is an aggressive neoplasm of uncertain cellular origin characterized by expression of both mesenchymal and epithelial markers. Over 90% fail to express the SMARCB1/INI1 tumor-suppressor gene product, reflecting deletion or inactivation of this gene located at 22q11.23.1, 2 Although the median age at presentation of ES is 30 years, it is proportionally more common in young patients, accounting for about 2% of pediatric soft tissue sarcomas.3 Pediatric oncologists consider ES among the large and heterogeneous group of non-rhabdomyosarcoma soft tissue sarcomas (NRSTS) that differ from rhabdomyosarcoma (RMS) by their relative insensitivity to chemotherapy and radiotherapy.4, 5
The rarity of ES in both pediatric and adult populations limits the available data on its natural history and treatment, most of which derives from retrospective case series.6–11 More recently, both the North American Children’s Oncology Group (COG) and the European pediatric Soft tissue sarcoma Study Group (EpSSG) included ES in prospective NRSTS clinical trials. In both trials, a risk-adapted treatment program was defined according to features previously determined to predict outcome in pediatric NRSTS: extent of disease, histologic grade and size of the primary tumor, and extent of surgical resection. The current analysis pools data from these two prospective clinical trials to assess clinical features and outcomes of young patients with ES and to identify predictors of treatment failure.
Materials and Methods
Patients under 30 years of age with newly diagnosed ES who enrolled on one of two prospective European and North American clinical trials (EpSSG NRSTS2005, 7/2005–11/2015, or COG ARST0332, 2/2007–2/2012) were eligible for this subset analysis. All participating centers obtained institutional ethics board approval according to the rules of the treating group, and written consent for treatment and data use was obtained from parents/guardians and/or patients according to local research ethics requirements.
Centralized pathology review by expert pediatric soft tissue pathologists confirmed the diagnosis in all cases according to the 2002 World Health Organization (WHO) criteria.12. Although loss of INI1 staining was not mentioned in the 2002 WHO guidelines, the presence or absence of INI1 staining was recorded for cases in which it was performed. Histologic grade was defined by FNCLCC (Fédération Nationale des Centres de Lutte Contre le Cancer) criteria for all patients,13 but the COG study used the POG (Pediatric Oncology Group) grading system for treatment assignment.14 Central review of operative notes, pathology reports, and imaging studies and reports defined the tumor size, sites of disease, and extent of surgery by Intergroup Rhabdomyosarcoma Study (IRS) system criteria.15 Extremity tumors were divided into proximal (shoulder to elbow, buttock to knee) and distal (forearm to finger, lower leg to toe) subsets.
Table 1 shows the treatment assignment specifications for both studies, which were similar. Regional lymph node sampling was mandatory in the COG study but was recommended only for patients with clinically or radiographically suspicious regional lymph nodes in the EpSSG study. The COG study recommended resection of metastases +/− radiotherapy for unresectable disease at the end of therapy; the EpSSG study made no recommendations regarding management of metastases.
Table 1.
COG and EpSSG Treatment Assignment Algorithm
| Number of Patients | |
|---|---|
| COG ARST0332 | 29 |
| No Adjuvant Therapy | 17 |
| IRS group I, any size, POG grade 2 | 13 |
| IRS group I, ≤5 cm, POG grade 3 | 4 |
| Adjuvant Radiotherapy | 1 |
| IRS group II, ≤5 cm, POG grade 3 (55.8 Gy) | 1 |
| Adjuvant Chemotherapy + Radiotherapy | 5 |
| IRS group I/II, >5 cm (55.8 Gy) | 1 |
| IRS group I/II, ≤5 cm, nodal metastases (55.8 Gy)* | 1 |
| IRS group IV, primary tumor grossly resected (55.8 Gy)* | 3 |
| Neoadjuvant Chemotherapy + Radiotherapy, Delayed Resection | 6 |
| IRS group III, any size (45 Gy plus boost based on surgical margin)† | 4 |
| IRS group IV, primary tumor unresected (45 Gy plus boost based on surgical margins)†* | 2 |
| EpSSG NRSTS2005 | 34 |
| No Adjuvant Therapy | 13 |
| IRS group I, ≤5 cm, any FNCLCC grade | 13 |
| Adjuvant Radiotherapy | 3 |
| IRS group I, >5 cm, FNCLCC grade 2 (50.4 Gy) | 1 |
| IRS group II, any size, FNCLCC grade 2 (54 Gy) | 1 |
| IRS group II, ≤5 cm, FNCLCC grade 3 (54 Gy) | 1 |
| Adjuvant Chemotherapy + Radiotherapy | 2 |
| IRS group I, >5 cm, FNCLCC grade 3 (50.4 Gy) | 1 |
| IRS group II, >5 cm, FNCLCC grade 3 (54 Gy) | 1 |
| Neoadjuvant Chemotherapy, Delayed Resection + Pre- or Postoperative Radiotherapy | 16 |
| IRS group III (50.4–59.4 Gy depending on resection timing and surgical margins)‡ | 12 |
| IRS group IV (50.4–59.4 Gy depending on resection timing and surgical margins)‡** | 4 |
FNCLCC: Fédération Nationale des Centres de Luttle Contre Le Cancer; Gy: Gray; IRS: Intergroup Rhabdomyosarcoma Study; POG: Pediatric Oncology Group
Primary tumor total dose 45 Gy following R0 resection, 55.8 Gy following R1 resection, 64.8 Gy for gross tumor
Resectable nodal and distant metastases excised at delayed surgery or at end of therapy; unresectable metastases received 50 Gy RT when feasible.
50.4 Gy pre-operatively, 50.4 Gy postoperatively following R0 resection, 54 Gy postoperatively following R1 resection, 59.4 Gy for gross tumor
Treatment of metastases per investigator choice
For this analysis, patients were divided into low-, intermediate- and high-risk groups according to the treatment administered. Low-risk patients underwent surgery +/− radiotherapy, and comprised predominantly those with non-metastatic, widely or marginally resected ≤ 5 cm tumors. The intermediate-risk group included patients with non-metastatic, high-grade and > 5 cm or unresectable tumors. Those with nodal or distant metastatic disease were high-risk, regardless of tumor grade or size. Patients in both the intermediate- and high-risk groups received chemotherapy, radiotherapy, and delayed surgery for any disease unresected at study entry. Both studies used of 6–7 cycles of ifosfamide (9 g/m2/cycle) and 4–5 cycles of doxorubicin (75 mg/m2/cycle), usually concomitantly depending on radiation timing.
In COG patients, response to therapy was evaluated after 4 cycles of chemotherapy and 45 Gy of radiotherapy by volumetric criteria (0.5 times the product of the 3 largest perpendicular diameters) for the primary tumor and by RECIST version 1.1 criteria for metastases.16 Primary tumor response was defined as complete response (CR): complete disappearance, partial response (PR): ≥ 64% decrease in volume, stable disease (SD): <64% decrease and < 40% increase in volume, or progressive disease (PD): ≥ 40% increase in volume. In EpSSG patients, response was assessed after 3 cycles of chemotherapy and was defined as complete response (CR): complete disappearance of all disease, partial response (PR): ≥ 66% decrease in tumor volume, stable disease (SD): <66% decrease and <40% increase in tumor volume, or progressive disease (PD): ≥ 40% increase in volume or appearance of new disease.
Patients were considered to be event-free until they developed tumor progression or recurrence, toxicity requiring removal from protocol therapy, a second cancer, or died of any cause. Descriptive statistics (frequency and percentage for categorical characteristics; median and range for numerical characteristics) were used to describe the clinical and treatment features of the population. The Kaplan-Meier method was used to construct the event-free survival (EFS) and overall survival (OS) curves, with the standard error computed using the Peto-Pike method. The log-rank test was performed to compare EFS and OS distributions.
Results
Sixty-three patients with ES were eligible for this analysis: 29 enrolled in ARST0332 (2/2007–2/2012), and 34 enrolled in NRSTS2005 (7/2005–11/2015). Table 1 and 2 show the treatment assignment algorithm and clinical features of the study cohort. INI1 staining in the 54 tumors tested showed loss in 47 (87%). An analysis for differences in the distribution of clinical features between patients treated by COG and EpSSG showed a statistically significant difference in the distribution of gender (p=0.0339) but no significant differences in anatomic site, FNCLCC grade, or lymph node status (data not shown).
Table 2.
Patient Characteristics (n=63)
| Characteristics | Number (%) |
|---|---|
| Age | |
| Median | 13.1 years |
| Range | 2.7–24.8 years |
| Sex | |
| Male | 33 (52%) |
| Female | 30 (48%) |
| Primary Tumor Site | |
| Head/neck | 10 (16%) |
| Upper extremity | 31 (49%) |
| Body wall | 7 (11%) |
| Visceral | 3 (5%) |
| Lower extremity | 12 (19%) |
| Extremity Tumor Location | |
| Proximal | 9 (21%) |
| Distal | 34 (79%) |
| Loco-regional lymph node involvement | |
| N0 | 54 (86%) |
| N1 | 9 (14%) |
| FNCLCC Grade | |
| 2 | 28 (44%) |
| 3 | 35 (56%) |
| Maximal Tumor Diameter* | |
| Median | 3.5 cm |
| Range | 0.4–19 cm |
| IRS Clinical Group | |
| I | 32 (51%) 6 (10%) |
| II | 16 (25%) |
| III | 9 (14%) |
Tumor diameter not available for 4 patients
Thirty-four of the 63 patients (54%) underwent a procedure to assess lymph node status at diagnosis, including sampling (n=13), sentinel node biopsy (n=19), or node dissection (n=2). Nine of the 34 patients who had lymph node sampling were found to have metastatic tumor in the lymph node(s). Since it was required by protocol, COG patients were more likely than EpSSG patients to undergo a lymph node procedure (97% vs. 18% of patients, respectively).
Low-Risk Patients (n=34)
Thirty patients (48%), including 29 with tumors ≤ 5 cm and 20 with FNCLCC grade 2 tumors, had surgery only. Five events occurred in this group: 2 local recurrence, 1 metastases, 2 combined recurrence. Three patients who received no adjuvant therapy died, including 1 with local recurrence of a 1.6 cm grade 2 perineal tumor that had been widely excised prior to study entry. Four patients received adjuvant radiotherapy following upfront tumor resection. There were no local recurrences in this subgroup, but one of these patients (grade 3, 3.0 cm tumor) experienced a metastatic recurrence and did not survive.
Estimated 5-year EFS and OS for the low-risk cohort were 84.4% (95% CI, 66.2%−93.3%) and 86.4% (95% CI, 67.4%−94.7%), respectively. In a univariate analysis stratified by all risk factors, FNCLCC grade did not predict EFS or OS in low-risk patients overall (p=0.4243 and 0.5085, respectively) or in low-risk patients treated with surgery alone (p=0.8971 and 0.9146, respectively). Due to the small number of patients with large (> 5 cm) tumors, invasive (T2) tumors, and IRS group II resection prior to study entry, it was not possible to reliably determine whether these clinical or treatment factors influenced outcome in low-risk patients.
Intermediate-Risk Patients (n= 16)
The 16 patients with non-metastatic > 5 cm or unresected tumors received neoadjuvant (n= 13) or adjuvant (n=3) therapy. Among the 13 patients who received neoadjuvant therapy (9 chemotherapy/radiotherapy, 4 chemotherapy only), 8 experienced a partial response, 4 had stable disease, and 1 experienced disease progression. Seven of the 13 patients who received neoadjuvant therapy underwent delayed surgery, which achieved negative margins in all patients.
Seven of the 16 intermediate-risk patients experienced an event (5 local, 2 metastatic recurrence) yielding a 5-year EFS estimate of 55.0% (95%CI 27.9–75.6). Among the 5 patients with local progression/recurrence, 4 never underwent tumor resection and 1 had a delayed wide resection but received no radiotherapy. The median radiation dose for patients with and without local recurrence was 45.0 Gy and 50.4 Gy, respectively. Five of the 7 patients whose tumor recurred died, all after a local recurrence. Two patients are alive after pulmonary metastatic recurrence. Estimated 5-year OS for intermediate-risk patients was 63.5% (95% CI 31.5–83.6).
High-Risk Patients (n=13)
Nine patients (14% of the entire patient population) had regional lymph node involvement, 5 of whom also had distant metastases. Among the 9 patients with nodal metastases, the primary tumor was located in the extremity (n=4), viscera (n=2), head/neck (n=2), and body wall (n=1), Six patients with nodal involvement had tumors > 5 cm. Formal lymph node dissection was performed in 3 patients with nodal metastases and one underwent an incomplete nodal dissection. Four patients received radiotherapy to involved lymph nodes. Among the 4 patients without distant metastases, 1 had undergone marginal resection of the primary tumor at study entry. The remaining 3 had gross disease at study entry and received chemotherapy (2 PR, 1 SD); 2 had a delayed resection (1 wide resection, 1 marginal resection). There were no reported local or nodal recurrences in the 9 patients with nodal metastases, but 8 died after distant metastatic recurrence. The sole survivor with nodal metastases had no distant metastases at initial presentation and was alive at 1.2 years from study entry.
Nine patients (14% of the entire patient population) had distant metastases involving: lung (n=7), distant lymph nodes (n=3), and other sites (n=6). Compared to those with localized disease, patients with distant metastases were more likely to have non-extremity tumors (67% vs. 26%, p=0.02), tumors > 5 cm in maximal diameter (44% vs. 30%, p=0.40), and lymph node involvement (63% vs. 8%, p<0.0001). Six of the 9 patients with metastases underwent gross resection of the primary tumor (4 prior to study entry, 2 in delayed fashion) but only 1 of these patients underwent complete resection of all metastases. Among the 5 patients with metastases whose disease was evaluable for response, 1 had a partial response, 1 had stable disease, and 3 had progressive disease. All 9 patients with metastases at study entry experienced an event (1 local, 8 metastatic recurrences) at a median of 5.4 months (range 0.8–22.9 months). Only 1 patient remains alive, with persistent metastatic disease.
Response to Neoadjuvant Therapy
In all, 16 non-metastatic and 6 metastatic patients received neoadjuvant therapy including chemotherapy/radiotherapy (n=16) or chemotherapy alone (n=6). Eleven patients had a partial response (50%), 6 had stable disease (27%), and 5 had disease progression (23%). There was no evidence of a difference in response (PR vs. SD vs. PD) based on whether the neoadjuvant therapy was chemotherapy alone or combined with radiotherapy (p=0.69). Of the 22 patients who received neoadjuvant therapy, 11 (50%) underwent gross tumor resection; 10 achieved negative margins.
Tumor Recurrence
Twenty-five of the 63 patients (40%) experienced tumor progression or recurrence, which was local in 10 and distant in 15. Median time to tumor recurrence was 6.6 months (range 0.8–76.1 months). Only 5 patients with tumor recurrence (20%) were alive at the time of this analysis, 1 after local and 4 after metastatic recurrence. The patient with local recurrence has no evidence of disease at 25.8 months from the event. Two of the 4 patients with metastatic recurrence are alive with disease at 11.2 and 19.2 months, respectively, from the event; the remaining 2 are alive with unknown disease status at 12.3 and 33.9 months from the event, respectively.
Predictors of Outcome
Median follow-up of the cohort is 57.8 months (range 8.4–107.9 months). Among the 20 patients who died, cause of death was local tumor progression in 7 and metastases in 13. At 5 years, estimated EFS and OS for the entire cohort were 60.7% (95% CI 47.2–71.8) and 63.6% (95% CI 48.8–75.2), respectively.
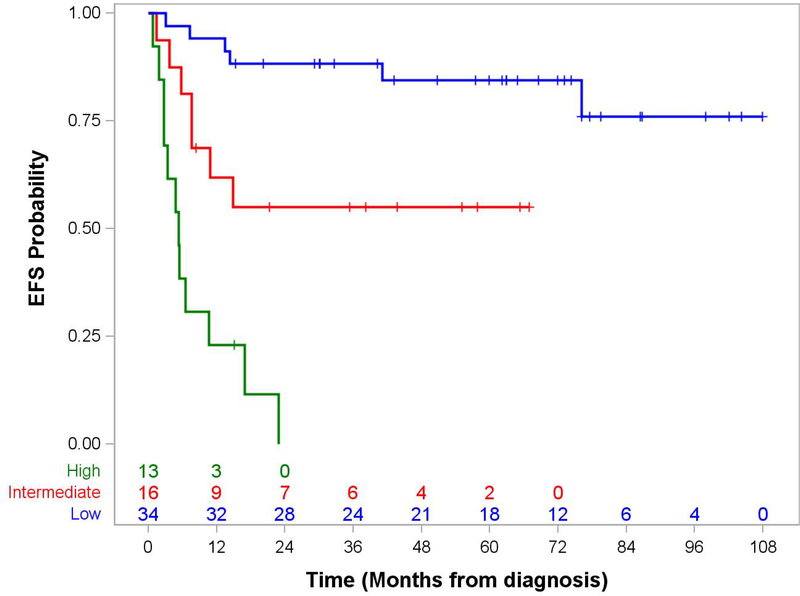
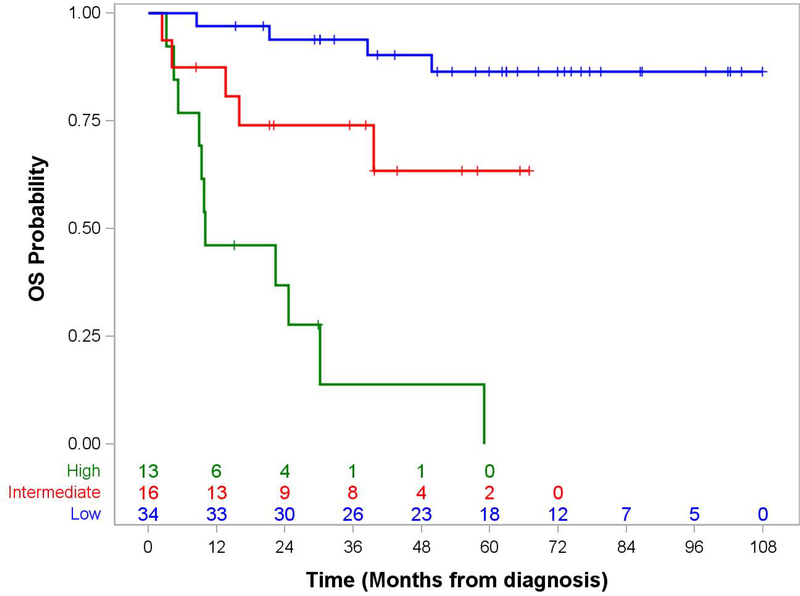
The presence or absence of distant metastatic disease was a strong predictor of outcome: 5-year EFS 71.3% (95% CI, 56.7.0–81.7) for localized disease vs. 0% for metastatic disease, p<0.0001 and OS 75.0% (95% CI, 59.8–85.2) vs. 0%, p=<0.0001 (Figure 1). Table 3 shows the impact of clinical features and treatment response on 5-year EFS and OS for patients with localized disease. Predictors of poorer EFS and OS in non-metastatic patients included invasiveness, more advanced IRS clinical group, higher FNCLCC grade, and loco-regional nodal involvement. Assigned risk group, which incorporated several of these prognostic factors, was also a robust predictor of 5-year overall survival (p<0.0001).
Figure 1. Event-Free and Overall Survival by Risk Group in Patients with Epithelioid Sarcoma.
Panel A. Event-Free Survival
Panel B. Overall Survival
Table 3.
Predictors of EFS and OS in Patients with Localized Disease (n=54)
| Characteristic | # | 5-year EFS (95% CI) | Log-rank Test p value | 5-year OS (95% CI) | Log-rank Test p value |
|---|---|---|---|---|---|
| Clinical Trial Group | |||||
| COG | 24 | 61.5% (38.9–77.9) | 0.21 | 69.2% (45.7–84.1) | 0.38 |
| EpSSG | 30 | 79.6% (60.1–90.3) | 80.6% (58.6–91.7) | ||
| Gender | |||||
| Female | 28 | 61.2% (38.9–77.5) | 0.24 | 68.5% (46.0–83.2) | 0.18 |
| Male | 26 | 80.8% (59.8–91.5) | 82.2% (58.7–93.1) | ||
| Age | |||||
| 1–9 years | 18 | 77.8% (51.1–91.0) | 0.62 | 75.4% (46.4–90.2) | 0.97 |
| 10–17 years | 31 | 68.9% (48.1–82.7) | 0.62 | 73.7% (51.7–86.8) | 0.97 |
| ≥ 18 years | 5 | -- | -- | ||
| FNCLCC Grade | |||||
| 2 | 24 | 91.7% (70.6–97.8) | 0.01 | 89.8% (64.3–97.4) | 0.02 |
| 3 | 30 | 54.4% (34.2–70.8) | 62.8% (41.2–78.4) | ||
| Tumor Invasiveness^ | |||||
| T1 | 36 | 86.1% (69.8–94.0) | 0.0002 | 84.1% (65.7–93.2) | 0.01 |
| T2 | 17 | 34.3% (11.0–59.5) | 52.3% (23.4–74.8) | ||
| Maximal Tumor Diameter^ | |||||
| ≤ 5 cm | 37 | 77.2% (59.2–88.0) | 0.09 | 78.0% (58.8–89.0) | 0.21 |
| > 5 cm | 16 | 55.6% (28.6–75.9) | 66.6% (37.2–84.7) | ||
| Lymph Node Involvement° | |||||
| No | 48 | 78.2% (63.0–87.7) | 0.0002 | 83.7% (68.5–91.9) | <0.0001 |
| Yes | 4 | -- | -- | ||
| IRS Group | |||||
| I | 32 | 86.7% (68.1–94.8) | 0.01 | 89.1% (69.7–96.4) | 0.004 |
| II | 6 | 50.0% (11.1-80.4) | 66.7% (19.5–90.4) | ||
| III | 16 | 48.6% (22.9–70.3) | 48.1% (18.8–72.6) | ||
| Treatment Response§ | |||||
| PR | 10 | 58.3% (23.0–82.1) | 0.68 | 51.4% (14.3–79.6) | 0.93 |
| SD | 5 | -- | -- | ||
| Site within Extremity* | |||||
| Distal | 31 | 75.8% (55.3–87.8) | 0.77 | 82.3% (62.3–92.3) | 0.69 |
| Proximal | 9 | 88.9% (43.3–98.4) | 87.5% (38.7–98.1) | ||
CI: confidence intervals; COG: Children’s Oncology Group; EFS: event-free survival; EpSSG: European paediatric Soft Tissue Sarcoma Group; FNCLCC: Fédération Nationale des Centres de Lutte Contre le Cancer; IRS: Intergroup Rhabdomyosarcoma Study; OS: overall survival; PR: partial response; SD: stable disease
1 patient with tumor invasiveness and maximal tumor diameter unknown
2 patients with loco-regional lymph node status un known
1 patient with progressive disease excluded
40 patients with extremity tumors
Discussion
This analysis of the clinical features and outcomes of young patients with ES treated with a standardized approach on prospective clinical trials demonstrates the value of international collaboration for investigating rare entities and validates prior observations from retrospective case series. Like previously published smaller retrospective case series,8, 10, 17 we found that pediatric ES has a slight male predominance and occurs most often in adolescents. About two thirds of these tumors arose in the extremities, usually distally.
As in prior studies,1, 2 loss of INI1 staining was very common but not universal, occurring in 87% of cases evaluated. In current practice, INI1 immunostains are used as part of the ancillary diagnosis of epithelioid sarcoma. However, this was not a part of the WHO guidelines in 2002 and indeed, there is an INI1 retention rate of 5 to 25% in several series which appears to be related to alterations in other components of the SWI-SNF complex.18 Due to these factors, data regarding INI1 expression were not uniformly available in our cases, nor is loss of it required for diagnosis.
We found that the vast majority of patients with adequately excised small tumors can be safely treated with surgery alone. Our findings are similar to those of a previously published joint COG-EpSSG analysis that confirmed the safety of a surgery-only approach for low-risk synovial sarcoma,19 suggesting that even histologically aggressive soft tissue sarcomas that are < 5 cm and adequately excised may not need adjuvant therapy. Despite their overall good outcome, a small handful of low-risk ES patients died of disease progression. We were unable to identify tumor features that differentiated these patients from the rest of the low-risk group, suggesting that more work is needed to identify biologic predictors of outcome that more effectively identify those who would benefit from therapy intensification.
Patients with intermediate-risk (non-metastatic, > 5 cm or unresected) tumors, had an EFS around 50%. Half of our patients with unresected tumors experienced a partial tumor response after neoadjuvant chemotherapy +/− radiotherapy. This finding is consistent with the 43% response rate reported in a smaller retrospective pediatric case series in which only chemotherapy was given preoperatively.8 In adults, response rates following chemotherapy alone are in the 0–15% range.11, 20 Whether the higher response rate we observed was due to more frequent use of radiotherapy in combination with chemotherapy or to differences in tumor biology in children compared to adults is unclear. Since nearly three-quarters of our neoadjuvant therapy patients received both chemotherapy and radiotherapy, we evaluated whether combination therapy produced a higher response rate than chemotherapy alone but could find no evidence of a differential response. However, we cannot definitively exclude radiotherapy as a contributor to tumor response given the small number of patients in our series. Administering radiotherapy prior to tumor resection carries several potential benefits, including lower prescribed doses, smaller field sizes, and resection of irradiated tissue that may lower the risk of secondary neoplasia in young patients with many years to develop this treatment complication. About half of our patients with gross disease at study entry were able to undergo complete resection after neoadjuvant therapy. Therefore, combined modality neoadjuvant therapy should be considered for patients who are anticipated to require both treatment modalities, including those with initially unresectable disease.
Because all patients with > 5 cm tumors received chemotherapy, we cannot definitively confirm the benefit of chemotherapy for preventing metastatic recurrence. However, the fact that distant metastases were the site of first failure in only 2 of 16 patients (13%) with initially non-metastatic large tumors suggests a potential benefit for chemotherapy, considering that distant metastases are the most common site of recurrence and cause of death in adults who succumb to ES.6, 9, 11
Although lymph node metastases are rare in most soft tissue sarcomas, nodal involvement is present in 13–21% of adults with ES.21, 22 Fourteen percent of our patients had nodal involvement, a proportion similar to the largest previously published pediatric ES series.8 The COG and EpSSG studies utilized a different strategy for lymph node staging. Sampling of lymph nodes was mandatory in the COG study and required only for clinical or radiographic nodal enlargement in the EpSSG study. Although the EpSSG approach could potentially underestimate (and therefore undertreat) patients with occult nodal involvement, an as yet unpublished COG analysis of lymph node metastases in non-rhabdomyosarcoma soft tissue sarcomas including epithelioid sarcoma found that 19 of 20 patients with nodal involvement had radiographic evidence of lymph node metastases (the remaining patient did not have the nodal bed imaged). Based on these findings and the complete absence of nodal recurrences in our international analysis of epithelioid sarcoma, we believe that lymph node sampling is only warranted for patients with clinically or radiographically involved nodes. The optimal approach to management of nodal metastases is uncertain, although most of our patients had local control of their lymph nodes and no lymph node recurrences were observed. Unfortunately, aggressive local control of nodal metastases did not translate into better outcomes because most patients with nodal involvement died of distant metastatic disease. Regional lymph node metastases therefore portend a very poor prognosis, and efforts to improve systemic therapy may benefit these patients.
As described in the published literature,6, 11, 23 outcome was dismal for patients with distant metastases at study entry. These patients were more likely to have a non-extremity primary tumor, a tumor > 5 cm in size, and lymph node metastases, and only one achieved gross resection of all sites of disease. Despite their poor survival, 5 of our 9 metastatic patients with measurable disease achieved stable disease or a partial response after neoadjuvant therapy, suggesting some potential for intensive chemotherapy +/− radiotherapy to prevent disease progression at least in the short term. In addition to ifosfamide/doxorubicin as used in our study, others have documented the clinical benefit of gemcitabine, with or without docetaxel.24, 25
Predictors of EFS and OS in this analysis were similar to other studies of pediatric and adult patients with soft tissue sarcomas: FNCLCC grade, invasiveness, extent of resection, and nodal or distant metastases.4, 5, 26–28 Small retrospective case series and population dataset analyses restricted to ES have mostly documented these same prognostic factors.6, 8, 11, 23, 29 Although utilizing the FNCLCC system for grading epithelioid sarcoma in adults is not recommended, our finding that FNCLCC grade predicted EFS and OS in our cohort agrees with the findings of the largest published pediatric case series8 and suggests that this system may effectively predict outcome in pediatric patients. The largest published pediatric case series also found that tumor size > 5 cm did not predict outcome, a finding that was confirmed in our larger patient cohort.
Outcomes for patients with recurrent or progressive disease in our study were poor. A significant proportion of the local failures were due to unresectable tumor, emphasizing the importance of surgery in the treatment of ES. Given that some patients with metastatic recurrence may survive for several years beyond the recurrence, salvage therapy aimed at eliminating metastatic disease may be a consideration in appropriate candidates.
To summarize, this joint analysis of clinical features and outcomes of prospectively treated pediatric ES patients largely confirms the findings of previously published retrospective studies. Most low-risk patients with adequately excised ≤ 5 cm tumors are cured with surgery alone. We documented a relatively high rate of response to chemotherapy +/− radiotherapy in pediatric ES, suggesting a potentially important role for multimodality therapy in the management of patients with high-risk features including unresectable disease. Unfortunately, outcomes for these patients and those with recurrent disease continue to be poor, so more effective therapeutic approaches are needed. As our experience demonstrates, prospective clinical trials and international collaboration are feasible and hold promise for future efforts to improve outcomes for patients with rare tumors like ES.
Highlights.
Large series of prospectively treated young patients with epithelioid sarcoma
Most low-risk patients treated with surgery alone fared well
Half of patients receiving neoadjuvant therapy had a partial tumor response
Poorer outcome predicted by large tumor size, high histologic grade, tumor invasiveness, metastatic disease, and inadequate resection
Acknowledgments
We gratefully acknowledge the patients and their families, care providers, and research personnel who participated in this study.
Funding Sources
Financial support for ARST0332 was provided by Children’s Oncology Group Grants U10CA180886, U10CA180899, U10CA098543, and U10CA098413, and by the St. Baldrick’s Foundation. The European Paediatric Soft Tissue Sarcoma Study Group is supported by Fondazione Città della Speranza, Italy.
Footnotes
Conflict of Interest Statement
The authors have declared no conflicts of interest
Clinical Trial Registration
COG ARST0332: ClinicalTrials.gov Identifier
EpSSG NRSTS 2005: European Union Drug Regulating Authorities Clinical Trials No. 2005-001139-31
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hornick JL, Dal Cin P, Fletcher CD. Loss of INI1 expression is characteristic of both conventional and proximal-type epithelioid sarcoma. Am J Surg Pathol. 2009;33: 542–550. [DOI] [PubMed] [Google Scholar]
- 2.Modena P, Lualdi E, Facchinetti F, et al. SMARCB1/INI1 tumor suppressor gene is frequently inactivated in epithelioid sarcomas. Cancer Res. 2005;65: 4012–4019. [DOI] [PubMed] [Google Scholar]
- 3.Spunt SL, Pappo AS. Childhood nonrhabdomyosarcoma soft tissue sarcomas are not adult-type tumors. J Clin Oncol. 2006;24: 1958–1959; author reply 1959–1960. [DOI] [PubMed] [Google Scholar]
- 4.Ferrari A, Casanova M, Collini P, et al. Adult-type soft tissue sarcomas in pediatric-age patients: experience at the Istituto Nazionale Tumori in Milan. J Clin Oncol. 2005;23: 4021–4030. [DOI] [PubMed] [Google Scholar]
- 5.Spunt SL, Hill DA, Motosue AM, et al. Clinical features and outcome of initially unresected nonmetastatic pediatric nonrhabdomyosarcoma soft tissue sarcoma. J Clin Oncol. 2002;20: 3225–3235. [DOI] [PubMed] [Google Scholar]
- 6.Baratti D, Pennacchioli E, Casali PG, et al. Epithelioid sarcoma: prognostic factors and survival in a series of patients treated at a single institution. Ann Surg Oncol. 2007;14: 3542–3551. [DOI] [PubMed] [Google Scholar]
- 7.Callister MD, Ballo MT, Pisters PW, et al. Epithelioid sarcoma: results of conservative surgery and radiotherapy. Int J Radiat Oncol Biol Phys. 2001;51: 384–391. [DOI] [PubMed] [Google Scholar]
- 8.Casanova M, Ferrari A, Collini P, et al. Epithelioid sarcoma in children and adolescents: a report from the Italian Soft Tissue Sarcoma Committee. Cancer. 2006;106: 708–717. [DOI] [PubMed] [Google Scholar]
- 9.Chbani L, Guillou L, Terrier P, et al. Epithelioid sarcoma: a clinicopathologic and immunohistochemical analysis of 106 cases from the French sarcoma group. Am J Clin Pathol. 2009;131: 222–227. [DOI] [PubMed] [Google Scholar]
- 10.Gross E, Rao BN, Pappo A, et al. Epithelioid sarcoma in children. J Pediatr Surg. 1996;31: 1663–1665. [DOI] [PubMed] [Google Scholar]
- 11.Levy A, Le Pechoux C, Terrier P, et al. Epithelioid sarcoma: need for a multimodal approach to maximize the chances of curative conservative treatment. Ann Surg Oncol. 2014;21: 269–276. [DOI] [PubMed] [Google Scholar]
- 12.Guillou L, Kaneko Y. Epithelioid sarcoma In: Fletcher CDMUK, Mertens F, editor. Pathology and Genetics of Tumours of Soft Tissue and Bone. Lyon, France: IARC Press, 2002:205–207. [Google Scholar]
- 13.Guillou L, Coindre JM, Bonichon F, et al. Comparative study of the National Cancer Institute and French Federation of Cancer Centers Sarcoma Group grading systems in a population of 410 adult patients with soft tissue sarcoma. J Clin Oncol. 1997;15: 350–362. [DOI] [PubMed] [Google Scholar]
- 14.Parham DM, Webber BL, Jenkins JJ 3rd, Cantor AB, Maurer HM. Nonrhabdomyosarcomatous soft tissue sarcomas of childhood: formulation of a simplified system for grading. Mod Pathol. 1995;8: 705–710. [PubMed] [Google Scholar]
- 15.Lawrence W, Jr., Gehan EA, Hays DM, Beltangady M, Maurer HM. Prognostic significance of staging factors of the UICC staging system in childhood rhabdomyosarcoma: a report from the Intergroup Rhabdomyosarcoma Study (IRS-II). J Clin Oncol. 1987;5: 46–54. [DOI] [PubMed] [Google Scholar]
- 16.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45: 228–247. [DOI] [PubMed] [Google Scholar]
- 17.Kodet R, Smelhaus V, Newton WA Jr., et al. Epithelioid sarcoma in childhood: An immunohistochemical, electron microscopic, and clinicopathologic study of 11 cases under 15 years of age and review of the literature. Pediatr Pathol. 1994;14: 433–451. [DOI] [PubMed] [Google Scholar]
- 18.Kohashi K, Yamamoto H, Yamada Y, et al. SWI/SNF Chromatin-remodeling Complex Status in SMARCB1/INI1-preserved Epithelioid Sarcoma. Am J Surg Pathol. 2018;42: 312–318. [DOI] [PubMed] [Google Scholar]
- 19.Ferrari A, Chi YY, De Salvo GL, et al. Surgery alone is sufficient therapy for children and adolescents with low-risk synovial sarcoma: A joint analysis from the European paediatric soft tissue sarcoma Study Group and the Children’s Oncology Group. Eur J Cancer. 2017;78: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones RL, Constantinidou A, Olmos D, et al. Role of palliative chemotherapy in advanced epithelioid sarcoma. Am J Clin Oncol. 2012;35: 351–357. [DOI] [PubMed] [Google Scholar]
- 21.Daigeler A, Kuhnen C, Moritz R, et al. Lymph node metastases in soft tissue sarcomas: a single center analysis of 1,597 patients. Langenbecks Arch Surg. 2009;394: 321–329. [DOI] [PubMed] [Google Scholar]
- 22.Keung EZ, Chiang YJ, Voss RK, et al. Defining the incidence and clinical significance of lymph node metastasis in soft tissue sarcoma. Eur J Surg Oncol. 2018;44: 170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jawad MU, Extein J, Min ES, Scully SP. Prognostic factors for survival in patients with epithelioid sarcoma: 441 cases from the SEER database. Clin Orthop Relat Res. 2009;467: 2939–2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frezza AM, Jones RL, Lo Vullo S, et al. Anthracycline, Gemcitabine, and Pazopanib in Epithelioid Sarcoma: A Multi-institutional Case Series. JAMA Oncol. 2018;4: e180219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pink D, Richter S, Gerdes S, et al. Gemcitabine and docetaxel for epithelioid sarcoma: results from a retrospective, multi-institutional analysis. Oncology. 2014;87: 95–103. [DOI] [PubMed] [Google Scholar]
- 26.Pisters PW, Leung DH, Woodruff J, Shi W, Brennan MF. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol. 1996;14: 1679–1689. [DOI] [PubMed] [Google Scholar]
- 27.Spunt SL, Poquette CA, Hurt YS, et al. Prognostic factors for children and adolescents with surgically resected nonrhabdomyosarcoma soft tissue sarcoma: an analysis of 121 patients treated at St Jude Children’s Research Hospital. J Clin Oncol. 1999;17: 3697–3705. [DOI] [PubMed] [Google Scholar]
- 28.Pappo AS, Rao BN, Jenkins JJ, et al. Metastatic nonrhabdomyosarcomatous soft-tissue sarcomas in children and adolescents: the St. Jude Children’s Research Hospital experience. Med Pediatr Oncol. 1999;33: 76–82. [DOI] [PubMed] [Google Scholar]
- 29.Gasparini P, Facchinetti F, Boeri M, et al. Prognostic determinants in epithelioid sarcoma. Eur J Cancer. 2011;47: 287–295. [DOI] [PubMed] [Google Scholar]