The 2016 World Health Organization (WHO) classification has designated two categories of high-grade B-cell lymphoma (HGBCL): HGBCL with MYC and BLC2 and/or BCL6 rearrangements (often referred to as “double/triple-hit” lymphoma), and HGBCL, not otherwise specified.[1] Optimal management of these aggressive malignancies remains uncertain. Historically, many HGBCL cases were classified as diffuse large B-cell lymphoma (DLBCL) and treated with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), while others were designated as “B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and Burkitt lymphoma (BL)” (BCLU) and treated using high-intensity regimens (e.g. CODOX-M/IVAC or hyper-CVAD +/− rituximab).[2–4] Recently, the less intensive dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab regimen (DA-EPOCH-R) has been successfully applied in BL and in aggressive DLBCL (particularly double/triple-hit lymphomas) [5–9]. One concern with DA-EPOCH-R is that it omits systemic central nervous system (CNS) prophylaxis, while intraparenchymal brain recurrences are increasingly frequent in DLBCL and HGBCL [4, 10–13]. In contrast, high-intensity regimens include systemic high-dose methotrexate (HDMTX) which provides intraparenchymal CNS prophylaxis. Our objective was to describe progression-free survival (PFS) and risk of CNS recurrence among HGBCL and BL patients treated in our academic center, which cares for most patients with lymphoma diagnosed in Rhode Island. We integrated cancer registry and medical records for patients treated for BL or HGBCL between 2005 and 2017. HGBCL was defined as high-grade lymphoma with concurrent MYC and BCL2 and/or BCL6 rearrangements, or BCLU as diagnosed according to the 2008 WHO classification. All cases of BCLU were diagnosed according to the WHO criteria and reviewed by an academic hematopathologist (DOT) for inclusion in this study. Cases of DLBCL were excluded. We compared patient characteristics using Wilcoxon rank-sum (for continuous variables) or Fisher’s exact tests (for categorical variables), PFS using log-rank test, and cumulative incidence of CNS recurrence using Gray’s test. P<.05 was considered statistically significant.
The clinical characteristics of 64 patients (38 BL and 26 HGBCL) are shown in Supplemental Table S1. BL was associated with a higher frequency of CNS involvement at baseline (21% vs. 4%). Among HGBCLs, 58% had MYC rearrangement, whereas 31% had concurrent MYC and BCL2/BCL6 rearrangements. The number of HGBCL cases increased over the study period, mainly due to increase in testing of aggressive lymphomas by fluorescent in situ hybridization (FISH) for MYC/BCL2/BCL6 rearrangements (Fig. 1A). Compared with patients with BL, those with HGBCL more often received DA-EPOCH-R (35% vs. 13%) or R-CHOP (23% vs. 11%) chemotherapy, and less often high-intensity regimens (28% vs. 65%, P=.040). Most HIV-positive patients had BL (11 out of 13), and median CD4 count of diagnosis of 322 cells/mm3. Eight patients who did not receive chemotherapy (median age, 77 years) were excluded from further outcome analysis.
Figure 1.
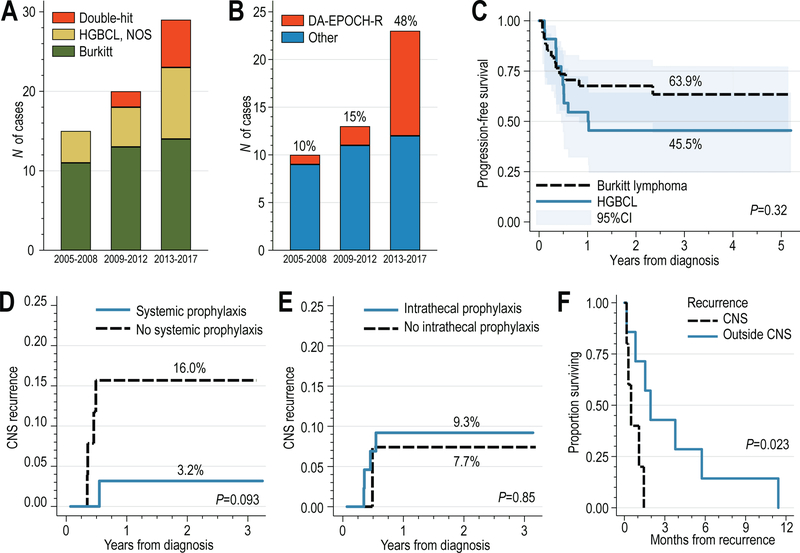
(A) Number of cases of HGBCL (including double-hit lymphomas) and Burkitt lymphoma diagnosed between 2005–2017; (B) Number of HGBCL/Burkitt lymphoma patients treated with DA-EPOCH-R or other high-intensity regimens treated between 2005 and 2017; (C) Progression-free survival, stratified by HGBCL or Burkitt histology; (D) cumulative incidence of CNS recurrence, stratified by receipt of systemic high-dose methotrexate-based chemotherapy, or (D) stratified by receipt of intrathecal central nervous system prophylaxis; (E) overall survival of patients with recurrent HGBCL or Burkitt lymphoma, stratified by site of recurrence (with CNS involvement or not).
The use of DA-EPOCH-R increased from 10% before 2009 to 48% after 2012 (Fig. 1B). The choice of regimen was at the discretion of treating clinician, taking into consideration patient characteristics and preferences. Compared with patients treated using high-intensity regimens, those treated with DA-EPOCH-R were significantly older (median age, 61 vs. 48 years, P=.023) and had more unfavorable International Prognostic Index (P=.027), but there was no significant difference in baseline CNS involvement or receipt of intrathecal prophylaxis between the groups treated with DA-EPOCH-R or high-intensity regimens (Table 1).
Table 1.
Characteristics of patients with HGBCL or Burkitt lymphoma treated with high-intensity regimens or DA-EPOCH-R.
| Variable | High-intensity regimen (n=32) | DA-EPOCH-R (n=14) | P |
|---|---|---|---|
| Age, median (IQR) | 48 (34–61) | 61 (50–69) | .023 |
| Male sex, n (%) | 26 (81%) | 10 (71%) | .46 |
| Race: white, n (%) | 29 (91%) | 11 (79%) | .47 |
| HIV positive, n (%) | 8 (25%) | 3 (21%) | .99 |
| CD4 per mm3, median (IQR) a | 321 (134–745) | 298 (117–479) | .60 |
| Histology, n(%) | |||
| HGBCL | 7 (22%) | 9 (64%) | .008 |
| Burkitt lymphoma | 25 (78%) | 5 (36%) | |
| IPI, n(%) | |||
| 0–2 | 16 (50%) | 2 (14%) | .027 |
| 3–5 | 16 (50%) | 12 (86%) | |
| Stage, n(%) | |||
| I/II | 8 (25%) | 1 (7%) | .24 |
| III/IV/unrecorded | 24 (75%) | 13 (93%) | |
| Involvement, n(%) | |||
| Bone marrow | 11 (34%) | 8 (57%) | .20 |
| CNS | 5 (16%) | 3 (21%) | .68 |
| LDH, IU median (IQR) | 462 (199–1338) | 889 (424–1198) | .14 |
| Intrathecal prophylaxis, n(%) | 30 (94%) | 11 (79%) | .16 |
| High-intensity regimen, n(%) | N/A | ||
| CODOX-M/IVAC±R | 13 (41%) | ||
| Hyper-CVAD±R | 11 (34%) | ||
| Other | 8 (25%) | ||
| Recurrence, n (%) b | |||
| CNS | 0 (0%) | 5 (63%) | .08 |
| Other sites | 4 (100%) | 3 (38%) |
CNS: central nervous system; IPI: international prognostic index; IU: international units; LDH: lactate dehydrogenase; N/A: not applicable
CD4 count at diagnosis, HIV positive patients only
Percent of all recurrences
After median follow-up of 6.2 years, we observed 12 recurrences, of which 5 (42%) occurred in the CNS. Median PFS and overall survival were not reached, whereas 3-year PFS was 57% (95% confidence interval [CI], 43–69%), numerically somewhat better in BL than in HGBCL (64% vs. 45%, P=.32, Fig. 1C). Overall survival at 3 years was 56% (95%CI, 42–68%). Factors associated with shorter PFS included age >60 years (log-rank P=.015), poor performance status (P<.001), high/high-intermediate IPI (P=.0004), and lack of CNS prophylaxis (P=.020), whereas HIV status (P=.53) or baseline CNS involvement (P=.14) were not prognostic. Patients treated with DA-EPOCH-R rather than high-intensity regimens also experienced worse PFS (P=.001), but this was not significant upon stratification by histology and age (P=.14). The small number of recurrences precluded the use of multivariable models.
The cumulative incidence of CNS recurrence at 1 year was 9% (95%CI, 3–18%), non-significantly higher in HGBCL than in BL (14% vs. 6%, P=.33), and significantly higher for patients with CNS involvement at baseline (38% vs. 4%, P=.002). All CNS recurrences occurred during the first year of follow-up, and all were observed among patients receiving DA-EPOCH-R (1-year cumulative incidence of 36% vs. 0% for high-intensity regimens, P=.0004). All CNS recurrences involved brain parenchyma, whereas 2 involved leptomeningeal compartment. Administration of HDMTX as part of initial therapy was associated with a numerically lower risk of CNS recurrence (1-year CIF 3% vs. 16%, P=.093, Fig. 1D), whereas there was no difference in CNS recurrence with or without receipt of intrathecal prophylaxis (1-year CIF 9% vs. 8%, P=.85, Fig. 1D). Survival after recurrence was dismal (median, 1 month, 95%CI, 0.2–3.8), despite 58% of patients receiving salvage therapy, and was even worse among patients with CNS recurrence (median 0.5 vs. 1.9 month, P=.023, Fig. 1E).
Our findings, while limited by sample size, single-institutional origin, and retrospective design, reflect trends in the diagnostic and management patterns among patients with HGBCL and BL in the era of routine testing for MYC/BCL2/BCL6 rearrangements, and after the introduction of less intensive chemotherapy for select patients. The HGBCL category has been recently redefined, and more research is needed to establish optimal management using consistent diagnostic approach. Because testing for MYC and BCL2/BCL6 rearrangements became routine gradually over the study period, our analysis likely did not include many cases diagnosed as DLBCL, which would be reclassified now as HGBCL on the basis of FISH results. Clinical behavior and outcomes of HGBCL and BL differ, and likely so should therapeutic strategies. Because of the sample size, we were not able to analyze the associations between CNS recurrence risk and clinical factors or treatments separately for the 2 histologies. Although DA-EPOCH-R showed no survival advantage over R-CHOP in a phase 3 clinical trial in DLBCL [8], the trial may have been compromised by inclusion of many patients with lower risk DLBCL (63% with IPI of 0–2). DA-EPOCH-R remains an important option for highly proliferative tumors including BL, double-hit lymphoma, primary mediastinal B-cell lymphoma, and potentially other HGBCLs. Recent studies show a unique gene expression profile of HGBCL which may help distinguish more patients who could benefit from alternatives to R-CHOP chemotherapy, but the optimal diagnostic and management approach remains to be defined [14]. Our experience shows that a high proportion of HGBCL recurrences occur in the CNS parenchyma and are associated with extremely poor outcomes, indicating persistent major areas of research need. Because CNS recurrences tend to occur within 1 year from diagnosis, early and effective prophylaxis is paramount. Outcomes of DA-EPOCH-R in our institution were likely heavily influenced by selection of patients with unfavorable baseline characteristics for this regimen, so further evaluation is warranted in a multi-institutional setting with a larger sample. However, our data suggest that in HGBCL, systemic HDMTX-based CNS prophylaxis may be essential, and thus support research efforts to augment DA-EPOCH-R with a suitable CNS-directed strategy for high-risk patients.
Supplementary Material
Acknowledgements
Presented in part at the 60th American Society of Hematology Annual Meeting & Exposition, December 1–4, 2018, San Diego, CA. Supported by the grant 128608-RSGI-15–211-01-CPHPS from the American Cancer Society, and U54GM115677 from the National Institute of General Medical Sciences.
AJO is supported by a Research Scholar Grant from the American Cancer Society Grant (grant number 128608-RSGI-15-211-01-CPHPS) and U54GM115677 from the National Institute of General Medical Sciences.
Footnotes
Disclaimer: Presented in part at the 60th American Society of Hematology Annual Meeting & Exposition, December 1–4, 2018, San Diego, CA.
Conflict of interest: The authors declare no conflict of interest.
References:
- 1.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016; 127: 2375–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lacasce A, Howard O, Lib S, et al. Modified magrath regimens for adults with Burkitt and Burkitt-like lymphomas: preserved efficacy with decreased toxicity. Leuk Lymphoma 2004; 45: 761–767. [DOI] [PubMed] [Google Scholar]
- 3.Thomas DA, Faderl S, O’Brien S, et al. Chemoimmunotherapy with hyper-CVAD plus rituximab for the treatment of adult Burkitt and Burkitt-type lymphoma or acute lymphoblastic leukemia. Cancer 2006; 106: 1569–1580. [DOI] [PubMed] [Google Scholar]
- 4.Perry AM, Crockett D, Dave BJ, et al. B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and burkitt lymphoma: study of 39 cases. Br J Haematol 2013; 162: 40–49. [DOI] [PubMed] [Google Scholar]
- 5.Dunleavy K, Pittaluga S, Shovlin M, et al. Low-intensity therapy in adults with Burkitt’s lymphoma. N Engl J Med 2013; 369: 1915–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roschewski M, Dunleavy K, Abramson JS, et al. Risk-Adapted Therapy in Adults with Burkitt Lymphoma: Results of NCI 9177, a Multicenter Prospective Phase II Study of DA-EPOCH-R. Blood 2017; 130: 188–188. [Google Scholar]
- 7.Dunleavy K, Fanale MA, Abramson JS, et al. Dose-adjusted EPOCH-R (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab) in untreated aggressive diffuse large B-cell lymphoma with MYC rearrangement: a prospective, multicentre, single-arm phase 2 study. Lancet Haematol 2018; 5: e609–e617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartlett NL, Wilson WH, Jung SH, et al. Dose-Adjusted EPOCH-R Compared With R-CHOP as Frontline Therapy for Diffuse Large B-Cell Lymphoma: Clinical Outcomes of the Phase III Intergroup Trial Alliance/CALGB 50303. J Clin Oncol 2019; 37: 1790–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Landsburg DJ, Falkiewicz MK, Maly J, et al. Outcomes of Patients With Double-Hit Lymphoma Who Achieve First Complete Remission. J Clin Oncol 2017; 35: 2260–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ollila TA, Olszewski AJ. Extranodal Diffuse Large B Cell Lymphoma: Molecular Features, Prognosis, and Risk of Central Nervous System Recurrence. Curr Treat Options Oncol 2018; 19: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roschewski M, Dunleavy K. Secondary diffuse large B-cell lymphoma of the central nervous system: the need for better predictors. Leuk Lymphoma 2015; 56: 1583–1584. [DOI] [PubMed] [Google Scholar]
- 12.Schmitz N, Zeynalova S, Nickelsen M, et al. CNS International Prognostic Index: A Risk Model for CNS Relapse in Patients With Diffuse Large B-Cell Lymphoma Treated With R-CHOP. J Clin Oncol 2016; 34: 3150–3156. [DOI] [PubMed] [Google Scholar]
- 13.Nijland M, Jansen A, Doorduijn JK, Enting RH, Bromberg JEC, Kluin-Nelemans HC. Treatment of initial parenchymal central nervous system involvement in systemic aggressive B-cell lymphoma. Leuk Lymphoma 2017; 58: 1–6. [DOI] [PubMed] [Google Scholar]
- 14.Ennishi D, Jiang A, Boyle M, et al. Double-Hit Gene Expression Signature Defines a Distinct Subgroup of Germinal Center B-Cell-Like Diffuse Large B-Cell Lymphoma. J Clin Oncol 2019; 37: 190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.