Abstract
Exposure to 4,4′-methylene diphenyl diisocyanate (MDI) in the occupational setting may lead to development of occupational asthma (OA), and the underlying molecular mechanisms of MDI-induced disease pathogenesis remain an active area of research. Using a nose-only mouse inhalation model, we find that circulating microRNA (miR)-206–3p and miR-381–3p are downregulated after MDI exposure; however, cellular miR-206–3p and miR-381–3p responses after MDI aerosol exposure and their pathophysiological roles in MDI-OA are unknown. We hypothesize that miR-206–3p and miR-381–3p-regulated mechanisms cause increased expression of the inducible nitric oxide synthase (iNOS) after MDI aerosol exposure. We examined cellular miR-206–3p and miR-381–3p, calcineurins, nuclear factors of activated T cells (NFATs), and iNOS levels from both nose-only exposed murine bronchoalveolar lavage cells (BALCs) and differentiated THP-1 macrophages treated with MDI-glutathione (GSH) conjugates. Both in vivo murine MDI aerosol exposure and in vitro MDI-GSH exposures in THP-1 macrophages result in downregulation of endogenous miR-206–3p and miR-381–3p and upregulation of PPP3CA and iNOS expression. Transfection of THP-1 macrophages with miR-inhibitor-206–3p and miR-inhibitor-381–3p resulted in the upregulation of PPP3CA and iNOS. Using RNA-induced silencing complex immunoprecipitation and translational reporter assays, we verified that PPP3CA, but not iNOS, is directly targeted by both miR-206–3p and miR-381–3p. Downregulation of miR-206–3p and miR-381–3p following by MDI exposure induces calcineurin/NFAT signaling-mediated iNOS transcription in macrophages and BALCs.
Keywords: occupational asthma (OA); diisocyanates (dNCOs); 4,4′-methylene diphenyl diisocyanate (MDI); calcineurin/NFAT signaling; PPP3CA; iNOS
Diisocyanates (dNCOs) are low molecular weight crosslinkers, which are essential for polyurethane production. Among diisocyanates, 4,4′-methylene diphenyl diisocyanate (MDI) is the most widely used globally (Allport et al., 2003) and is utilized in spray foam insulation, truck bed liners, paints, adhesives, elastomers, and coatings, as well as coal mining or tunneling rock face stabilizers (Munn et al., 2005). Exposure to MDI has been associated with occupational asthma (OA) (Bernstein et al., 1993; Engfeldt et al., 2013; Jan et al., 2008; Lofgren et al., 2003; NIOSH, 1994a,b, 2004; Redlich and Karol, 2002).
In occupational clinics, physicians diagnose dNCO-OA using patient histories of dNCO exposure and confirm their OA diagnosis by using dNCO specific inhalation challenge (SIC) (Vandenplas et al., 2014a). Although dNCO-OA is considered a heterogeneous disease with both allergic and nonallergic asthmatic phenotypes (Lemiere et al., 2014; Wisnewski and Jones, 2010); many dNCO-OA patients share common functional and morphologic features with allergic asthma (Mapp et al., 2005). One common feature of allergic asthma is elevated fractional exhaled nitric oxide (FeNO) levels in exhaled air (Dweik et al., 2011; Mummadi and Hahn, 2016). In the OA clinical setting, FeNO levels have been found to be elevated in MDI-OA patients after MDI-SIC (Allmers et al., 2000; Barbinova and Baur, 2006; Baur and Barbinova, 2005; Ferrazzoni et al., 2009). In the airways, the major source of nitric oxide (NO) production is from induction of the inducible nitric oxide synthase (iNOS) in airway cells (Brindicci et al., 2007; Lane et al., 2004). In an in vivo inhalation study, Sprague Dawley rats exposed to 2,4-toluene diisocyanate (TDI), a volatile aromatic diisocyanate structurally similar to MDI, demonstrated increased iNOS expression and NO production in the bronchoalveolar lavage cells (BALCs). The alveolar macrophages in BALCs appeared to be one source of NO after TDI inhalation (Huffman et al., 1997). In spite of these findings, the cellular origin(s) and the mechanism(s) by which MDI exposure causes increased FeNO remains unknown.
MicroRNAs (miRs) are single-stranded noncoding RNA molecules ranging from 19 to 24 nucleotides in length that inhibit protein translation by imperfectly binding to their target RNA transcripts in many diverse cell types (Bartel, 2018). MiRs functions have been described in diverse biological processes and dysregulation of miR expression may lead to development of disease (Esteller, 2011; Mendell and Olson, 2012). Dysregulation of miR expression has been found in tissue from asthmatic patients and has been implicated in asthma pathogenesis (Booton and Lindsay, 2014; Lu and Rothenberg, 2013; Pua and Ansel, 2015); however, the effects of MDI-mediated miR responses have not been described. We have previously reported that up/down-regulation patterns of circulating miR-183–5p, miR-206–3p, and miR-381–3p may serve as potential serum biomarkers for MDI exposure using a mouse model (Lin et al., 2019). Circulating miR-183–5p was upregulated, whereas circulating miR-206–3p and miR-381–3p were both downregulated in the serum of mice exposed to MDI aerosol (Lin et al., 2019). In silico, the calcineurin/nuclear factors of activated T cells (NFATs) signaling pathway was one of the most significantly enriched pathways for miR-206–3p and miR-381–3p (Lin et al., 2019). Calcineurins play important roles in regulation of NFAT activities in the cells (Rusnak and Mertz, 2000). NFAT transcription factors have been described as important transcription regulators in many diverse cell types in the immune system, regulating the expression of many immunological mediators including cytokines, chemokines, and inflammatory genes (Bert et al., 2000; Burke et al., 2000; Cockerill et al., 1995; Macian et al., 2000; Peng et al., 2001; Zhang et al., 1999) as well as iNOS (Buxade et al., 2012; Hamalainen et al., 2002; Li et al., 2006; Ranjan et al., 2014). Therefore, we hypothesize that MDI-mediated downregulation of miR-206–3p and miR-381–3p participates in the regulation of iNOS expression after acute MDI aerosol exposure.
The current report is focused on characterizing the MDI-mediated miR responses in BALCs and the downstream molecular mechanistic effects of MDI on iNOS expression after acute MDI exposure using an in vivo murine MDI aerosol inhalation model, as well as an in vitro cell culture model. MDI exposures were performed via either an in vivo nose-only inhalation murine model or an in vitro MDI-glutathione (GSH) conjugates treatment cell culture model using differentiated THP-1 macrophages. Both in vivo (MDI aerosol murine exposure) and in vitro (MDI-GSH conjugates cell culture exposure) models exhibit downregulation of endogenous miR-206–3p and miR-381–3p and subsequent upregulation of NFAT signaling-mediated iNOS transcription via upregulation of endogenous PPP3CA. This report provides a putative miR-regulated mechanism to describe how iNOS transcription is upregulated after acute MDI exposure in macrophages.
MATERIALS AND METHODS
Chemicals and reagents.
High Performance Liquid Chromatography (HPLC) grade acetone, 3-Å molecular sieve (4–8 mesh), phosphate buffered saline (PBS), Tris buffered saline, Tween 20, dimethyl sulfoxide, 98% MDI, phorbol 12-myristate 13-acetate (PMA), and reduced GSH were acquired from MilliporeSigma (St Louis, Missouri). Tacrolimus (FK506) was purchased from Selleckchem (Houston, Texas). RPMI-1640 culture medium, penicillin-streptomycin-glutamine (PSG; 100×), and fetal bovine serum (FBS) were purchased from Thermo Fisher Scientific (Waltham, Massachusetts). Dry acetone was prepared by incubating 10-ml HPLC grade acetone on 3-Å molecular sieve for a minimum of 24 h to adsorb water.
Animals, MDI aerosol exposure, and bronchoalveolar lavage fluid collection.
The BALCs used in the current study were isolated from mice following 1-h nose-only MDI aerosol exposure or control as previously reported (Hettick et al., 2018; Lin et al., 2019). Detail MDI aerosol exposure and collection of BALCs has been previously described (Hettick et al., 2018). Briefly, 6–8-week old female BALB/c mice were obtained from Taconic (Germantown, New York) and were acclimated for at least 5 days before being randomly assigned into 3 different treatment groups. Five mice per treatment group were housed in a ventilated plastic cage with hardwood chip bedding. MDI aerosol exposures were performed on groups of 5 mice by exposing the animals, via an in-house constructed nose-only inhalation exposure system to 4580 ± 1497 μg/m3 MDI aerosol or pure house air, control (Ctl), for 1 h. Of the total MDI aerosol generated during the 1-h exposure, approximately 50% of the total MDI aerosol (2243 ± 903.8 μg/m3) consisted of particles < 3.0 μm in size. Particles smaller than 3.0 μm in diameter have a greater probability to deposit in the lower respiratory tract. Approximately 10% of the total MDI aerosol consisted of particles < 1 μm diameter and were capable of deposition in the alveolar region (Schlesinger, 1985). The current acute exposure represents the total MDI load of approximately 100 h at the NIOSH defined recommended exposure limit (REL) of 0.05 mg/m3, or 10 workdays. The NIOSH REL represents an exposure to which a worker can be subjected day after day without anticipation of suffering detrimental health effects (NIOSH, 1997). These exposures are approximately 15-fold below the immediately deadly to life and health threshold of 75 mg/m3 (NIOSH, 1997). Mice were euthanized at 4h and 24 h after MDI aerosol exposure via intraperitoneal injection of sodium pentobarbital euthanasia solution (200 mg/kg) followed by exsanguination upon a negative response to a toe pinch. Lungs were perfused with 10-ml ice cold PBS, and bronchoalveolar lavage fluid (BALF) was collected via 3× 1ml ice cold PBS lavages. Cells from the BALF were collected by centrifugation at 300 × g for 10min at 4°C, and stored in a −80° C freezer until total RNA isolation. All animal experiments were performed in the AAALAC, International accredited National Institute for Occupational Safety and Health animal facility in accordance with an institutionally approved animal care and use protocol.
THP-1 cell culture and differentiation.
THP-1 cells from American Type Culture Collection (ATCC, Manassas, Virginia) were maintained at 5× 105/ml in RPMI-1640 media supplement with 10% FBS, and 1× PSG. THP-1 cells (2× 106 cells) were differentiated using 50 nM PMA in 10-cm culture dishes for 3 days. After the initial 3-day PMA treatment, differentiation enhancement of PMA treated cells was performed by removal of PMA-containing media, washing twice with PBS and then incubating the cells in fresh media for 3 days. This method has been previously demonstrated to differentiate THP-1 cells into monocyte-derived macrophage-like cells (Daigneault et al., 2010). All in vitro cell experiments described in this study used enhanced differentiated THP-1 macrophages.
MDI-GSH conjugation.
MDI-GSH conjugation products were prepared in a similar manner to the report published by Wisnewski et al. (2013, 2015) with minor modifications. Briefly, 10mM GSH solution was prepared in 200 mM sodium phosphate buffer (pH = 7.4). Ten percent MDI (w/v) stock solutions was freshly prepared in dry acetone. Fifty microliters of 10% MDI solutions (w/v) were added to 25 ml of GSH solution in a 50-ml polypropylene conical tube to achieve approximately 800 μM MDI stock solutions. This concentration is well below the starting concentrations of chemical (50%) typically aerosolized to generate spray foam insulation (Lesage et al., 2007). After adding MDI into GSH solution, the tube was end-over-end mixed at 25°C for 2 h. The solution was then centrifuged at 10 000 × g and filtered with a 0.2-μm syringe filter. Conjugates were immediately added into enhanced differentiated THP-1 macrophages at the indicated concentrations.
Expression analyses.
For reverse transcription quantitative polymerase chain reaction (RT-qPCR), total RNA was extracted by using the mirVana miR Isolation Kit (Thermo Fisher Scientific) according to manufacturer’s instruction. The mRNA and miR levels were analyzed as previously described (Lin et al., 2011, 2015, 2019; Sharma et al., 2014). Reactions were normalized to beta-2-microglobulin (B2M) for mRNA analysis, or to U6 snRNA for miR analysis. Gene expression assays and miR specific assays used in this study were purchased from Thermo Fisher Scientific and include human PPP3CA (Hs00174223_m1), NOS2/iNOS (Hs01075529_m1), B2M (Hs00187842_m1), mouse Ppp3ca (Mm01317678_m1), Ppp3cb (Mm00920265_m1), Ppp3cc (Mm01318938_m1), Ppp3r1 (Mm01187904_m1), Ppp3r2 (Mm01349242_g1), Nfatc1 (Mm01265944_m1), Nfatc2 (Mm01240677_m1), Nfatc3 (Mm01249200_m1), Nfatc4 (Mm00452375_m1), Nos2/iNos (Mm00440502_m1), B2m (Mm00437762_m1), mmu-miR-183–5p/hsa-miR-183–5p (Assay ID No. 002269; mmu/Mus musculus, hsa/Homo sapiens), mmu-miR-206–3p/hsa-miR-206–3p (No. 000510), mmu-miR-381–3p/hsa-miR-381–3p (No. 000571), and U6 snRNA (No. 001973). PCR reactions were performed on an ABI 7500 Real-Time PCR System (Thermo Fisher Scientific). Expression of mRNAs and miRs was determined by using the △△CT method as previous described (Lin et al., 2011, 2015, 2019; Sharma et al., 2014).
Immunoblot analysis and antibodies.
Cell extracts for immunoblot analysis were prepared as previously described (Lin et al., 2011). Following electrophoresis, proteins were transferred onto nitro-cellulose membranes and probed with either anti-PPP3CA (Cell Signaling Technology, Danvers, Massachusetts) or p-actin (Santa Cruz Biotechnology, Dallas, Texas) antibodies. Bound antibodies were detected using Pierce ECL Western Blotting Substrate (Thermo Fisher Scientific).
Plasmid construction.
pMIR-REPORT firefly luciferase vector and pcDNA3.1+ vector were purchased from Thermo Fisher Scientific. pRL-TK renilla luciferase reporter was obtained from Promega (Madison, Wisconsin). The pcDNA3.1+/c-(k)dyk-PPP3CA cDNA clone, which contains human PPP3CA coding sequences (accession no. NM_000944), was purchased from GenScript (Piscataway, New Jersey). To construct a wildtype (WT) PPP3CA translational reporter, a 2.4-kb cDNA fragment representing the 3′ untranslated region (UTR) of human PPP3CA (NM_000944.4) was generated by PCR using a MluI restriction site containing forward primer (ccgacgcgtCAGTGCCACTTCCTGTTCAC) and a PmeI restriction site containing reverse primer (ccgtttaaacCTTATTTTAATGTCAAATGTTTATTTC) on THP-1 cell cDNA. The PCR amplified PPP3CA 3’UTR cDNA fragment was treated with MluI and PmeI. This fragment was inserted into pMIR-REPORT vector that was prepared by sequential treatment with MluI, PmeI, and CIP.
Transient transfection and translational reporter assays.
For PPP3CA overexpression studies, 1× 106 differentiated THP-1 macrophages were transfected with 2.5 μg of either pcDNA3.1+/ c-(k)dyk-PPP3CA expression plasmid or pcDNA3.1+ empty vector using Mirus TransIT-LT1 transfection reagent in a 6-well plate for 24 h. After 24 h, total RNA was extracted and prepared for RT-qPCR expression analyses. For miR functional analyses, the following mirVana miRNA inhibitors (MH) and miR-mimics (MC) were obtained from Thermo Fisher Scientific and diluted to 20 μM in nuclease-free water: hsa-miR-206–3p (MH10409, MC10409), hsa-miR-381–3p (MH10242, MC10242), MH-negative control no. 1 (4464076), and MC-negative control no. 1 (4464058). Cells were subjected to reverse transfection and 24 h later, forward transfection was performed as previously described (Lin et al., 2011). Twenty-four hours after the start of the forward transfection, cell extracts were prepared for Western blotting and RT-qPCR expression analyses. Translational reporter assays were performed following just one transfection, at 24 h after the start of the reverse transfection. miR-inhibitors or -mimics were cotransfected with PPP3CA 3′UTR luciferase translational reporter plasmid, including the pRL-TK control, and Dual-Luciferase Reporter Assays (Promega) were performed as previously described (Lin et al., 2011).
Validation of miR targets by argonaute (Ago) immunoprecipitation.
Immunoprecipitation (IP) of the miR-containing RNA-induced silencing complex (miR/RISC) and target mRNAs was performed using the miRNA target IP kit (Active Motif, Carlsbad, California) according to the manufacturer’s instructions. Briefly, differentiated THP-1 cells were trypsinized and seeded at 8× 106 cells in 10-cm plates. The cells were transfected with 25 nM miR-mimics of miR-206–3p or miR-381–3p or miR-mimics negative control no. 1 for 24 h. Two 10-cm plates of cells using an equal number of 1.6 × 107 cells were taken for the IP reaction. After cell lysis, the lysates were divided into 2 equal aliquots. Each lysate aliquot underwent IP by using either a pan-Ago antibody that recognizes Ago1, Ago2, and Ago3 to precipitate the RISC containing Agos/miRs/mRNAs or an isotype IgG antibody control. The precipitated complex was collected and the RNA was purified from the complex using mirVana miR Isolation Kit (Thermo Fisher Scientific). The RNA was converted to cDNA using the High Capacity cDNA Reverse Transfection Kit (Thermo Fisher Scientific) and TaqMan qPCR assays of human PPP3CA and iNOS were used for RT-qPCR. The data were analyzed by comparing the cells transfected with miR-mimics or nontarget miR-mimic-control oligonucleotide and the fold enrichment of either PPP3CA or iNOS was calculated from the anti-panAgo and the IgG isotype antibody IP preparations as described by the manufacturer.
Statistical analysis.
Data were analyzed using either the unpaired t test (two-tailed) or one-way analysis of variance followed by Tukey’s multiple comparison ad hoc post-test. Statistical analyses were performed in GraphPad Prism 7.0 (GraphPad Software, La Jolla, California). Differences were considered significant when the analysis yielded p < .05.
RESULTS
Endogenous mmu-miR-206–3p and mmu-miR-381–3p Are Downregulated in BALCs Isolated From Acute MDI Aerosol Exposed Mice
Previously, we reported that up/down-regulation of circulating mmu-miR-183–5p, mmu-miR-206–3p, and mmu-miR-381–3p may serve as putative serum biomarkers for detection of MDI aerosol exposure in mice (Lin et al., 2019); however, in that report, we did not examine the endogenous cellular miR responses to MDI exposure. Given that a proteomics study only identified MDI-conjugated proteins in the BALF and BALCs isolated from the airway of acute MDI aerosol-exposed mice, but not in lung or trachea tissue (Hettick et al., 2018), we proposed that major MDI-mediated responses were likely occurring in the BALCs.
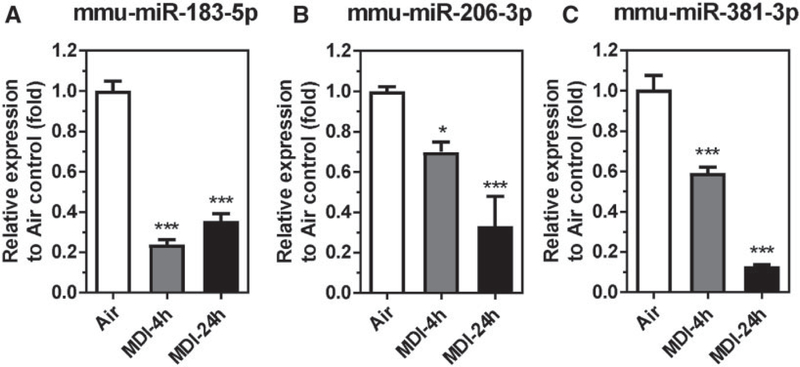
To test this hypothesis, the endogenous cellular mmu-miR-183–5p, mmu-miR-206–3p, and mmu-miR-381–3p responses were determined in the BALCs from MDI aerosol-exposed mice and the results are presented in Figure 1. The endogenous levels of the previously identified candidate mmu-miR-183–5p were downregulated 4.20-fold in BALCs (4-h postexposure) and 2.81-fold (24-h postexposure) after 1h of aerosol MDI exposure compared with house air control (Figure 1A). This finding is in conflict with the previous result that circulating mmu-miR-183–5p was upregulated in serum after MDI aerosol exposure (Lin et al., 2019). The observation of decreased endogenous mmu-miR-183–5p levels in the BALCs after MDI aerosol exposure suggests that the source of the upregulation of the circulating mmu-miR-183–5p in the serum is not the BALCs but rather from other cell types in the airway, eg, lung epithelial cells, fibroblast cells, and airway smooth muscle cells or from a systematic response. However, consistent with the finding that both circulating mmu-miR-206–3p and mmu-miR-381–3p were downregulated in serum of MDI aerosol-exposed mice, the endogenous mmu-miR-206–3p and mmu-miR-381–3p in the BALCs were downregulated 1.43-fold and 1.69-fold (4-h postexposure) and downregulated 3.02-fold and 7.67-fold (24-h postexposure), respectively, following 1-h MDI aerosol exposure compared with house air control (Figs. 1B and 1C). The downregulation of endogenous mmu-miR-206–3p and mmu-miR-381–3p in BALCs correlates well with the downregulation of circulating mmu-miR-206–3p and mmu-miR-381–3p in serum after acute MDI aerosol exposure (Lin et al., 2019); therefore, we decided to pursue the endogenous cellular functions of miR-206–3p and miR-381–3p in response to acute MDI aerosol exposure in the current study.
Figure 1.
Candidate miR levels in bronchoalveolar lavage cells (BALCs) isolated from MDI aerosol-exposed mice. Total RNA isolated from BALCs from MDI aerosol-exposed mice and determined by reverse transcription quantitative polymerase chain reaction (RT-qPCR). Candidate miRs (A) mmu-miR-183–5p, (B) mmu-miR-206–3p, and (C) mmu-miR-381–3p were determined 4 or 24h post MDI aerosol exposure (N = 3; bars, SEM). Air, house air; MDI, 4,4′-methylene diphenyl diisocyanate (*p < .05 and ***p < .001).
MDI Aerosol Exposure In Vivo Upregulate PPP3CA in BALCs
Using an in silico pathway enrichment assay to identify potential targets of the conserved human homologs of the murine miRs (hsa-miR-206–3p and hsa-miR-381–3p), we noted that several immune-related pathways were highly enriched, including Fc gamma R-mediated phagocytosis, the calcineurin/NFAT pathway, chemokine signaling pathway, FMLP pathway, and leukocyte transendothelial migration (Lin et al., 2019). Among these enriched immune-related pathways, the calcineurin/NFAT signaling pathway was selected for further investigation because this pathway regulates transcription of many asthma-related genes (eg, Th1, 2 and proinflammatory cytokines, and chemokines) (Feske et al., 2001; Hermann-Kleiter and Baier, 2010) and dysregulation of this pathway has been associated with asthma (Diehl et al., 2002; Hodge et al., 1996; Keen et al., 2001; Koch et al., 2015; Rengarajan et al., 2002a,b; van Rietschoten et al., 2001). Activation of this pathway requires calcineurin-mediated cytosolic dephosphorylation of NFAT transcription factors which triggers translocation of the NFATs from the cytoplasm into the nucleus where NFAT mediates gene expression in diverse cells (Hogan et al., 2003; Im and Rao, 2004; Macian, 2005). Calcineurin is a heterodimeric protein consisting of a catalytic subunit, calcineurin A (including 3 isozymes: PPP3CA, PPP3CB, and PPP3CC), and a Ca2+-binding regulatory subunit, calcineurin B (including 2 isozymes: PPP3R1 and PPP3R2) (Rusnak and Mertz, 2000). The NFAT protein family consists of 5 members, NFATc1, NFATc2, NFATc3, NFATc4, and NFAT5 (Crabtree and Olson, 2002; Rao et al., 1997; Wu et al., 2007). Of these, NFATc1 through -c4 are Ca2+ dependent and are regulated by calcineurin, whereas NFAT5 is not regulated by calcineurin but rather regulated by osmolality (Macian, 2005; Macian et al., 2001).
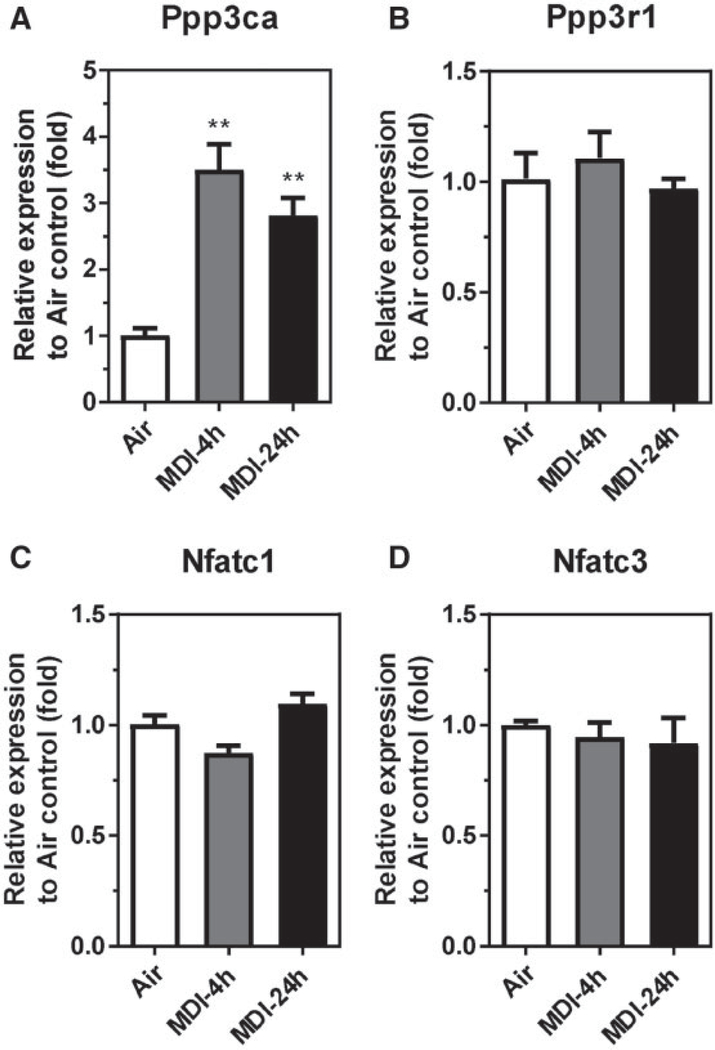
To investigate whether MDI aerosol exposure could regulate calcineurin and/or NFATs expression in the BALCs, we determined the endogenous mRNA expression for Ppp3ca, Ppp3cb, Ppp3cc, Ppp3r1, Ppp3r2, and Nfatc1-4 from BALCs of MDI aerosol-exposed and house air control mice (Figure 2). Of these candidates, only Ppp3ca mRNA was upregulated in response to MDI aerosol exposure in BALCs (Figure 2A). The endogenous Ppp3ca mRNA was upregulated 3.51-fold (4-h postexposure) and 2.82-fold (24-h postexposure) in BALCs collected after MDI aerosol exposure. Additionally, the regulatory calcineurin subunit Ppp3r1 mRNA was not changed in response to MDI aerosol exposure (Figure 2B), whereas the other calcineurin isozymes including Ppp3cb, Ppp3cc, and Ppp3r2 mRNAs were not detected (data not shown) by RT-qPCR. Furthermore, the endogenous Nfatc1 and Nfatc3 mRNAs remained unchanged (Figs. 2C and 2D), whereas Nfatc2 and Nfatc4 were not detected (data not shown) in response to MDI aerosol exposure. These data suggest that MDI aerosol exposure may induce calcineurin/NFAT signaling in BALCs through the upregulation of PPP3CA expression.
Figure 2.
Calcineurin isozymes and Nfats mRNA levels in BALCs isolated from MDI aerosol-exposed mice. Total RNA isolated from BALCs from MDI aerosol-exposed mice and determined by RT-qPCR. Endogenous mRNA expression of (A) Ppp3ca, (B) Ppp3r1, (C) Nfatc1, and (D) Nfatc3 was determined 4 or 24h post MDI aerosol exposure (N = 3; bars, SEM). Air, house air; MDI, 4,4′-methylene diphenyl diisocyanate (**p < .01).
MDI-GSH Conjugates Upregulate Endogenous PPP3CA and Downregulate Endogenous hsa-miR-206–3p and hsa-miR-381–3p in Differentiated THP-1 Macrophages
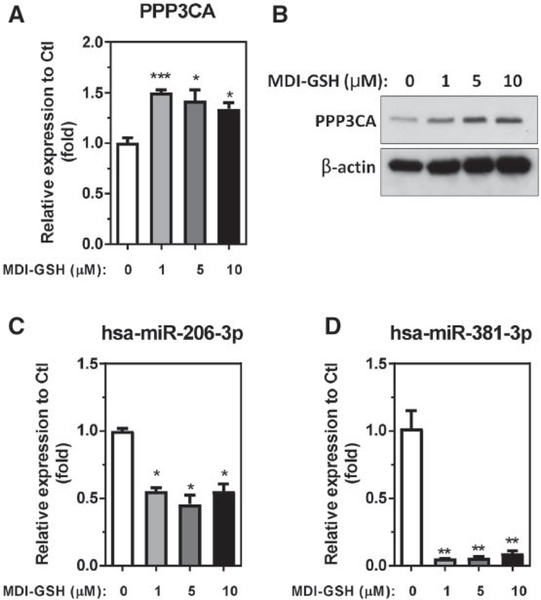
Previously, we have determined that the BALCs in the airway are the major targets for inhaled MDI aerosol (Hettick et al., 2018). Among all cell types in the airway, macrophages are the most abundant, accounting for 80%–90% of the total cell count in the BALF (Heron et al., 2012). In addition, Wisnewski et al. (2015) reported that the macrophage population was among the most significantly induced cell type by intranasal admission of MDI-GSH conjugates in a naïve mouse model; therefore, we used differentiated human THP-1 macrophages as an in vitro cell model to investigate the cellular responses to MDI-GSH conjugates exposure. To investigate whether MDI-GSH conjugates exposure could induce a similar response in a human cell line to the observed Ppp3ca upregulation and miR-206–3p/miR-381–3p downregulation observed in the mouse model (Figs. 1 and 2), differentiated THP-1 macrophages were treated with MDI-GSH conjugates at concentrations of 0, 1, 5, and 10 μM for 24h and the results are presented in Figure 3. In response to MDI-GSH conjugates exposure, endogenous PPP3CA mRNA expression is significantly upregulated between 1.34-fold and 1.51-fold (Figure 3A) and endogenous PPP3CA protein expression is upregulated consistent with this finding (Figure 3B). Furthermore, the endogenous hsa-miR-206–3p and hsa-miR-381–3p are also significantly downregulated from 1.80-fold to 2.19-fold (Figure 3C) and 10.88-fold to 18.74-fold (Figure 3D), respectively. These results confirm that the exposure to MDI in the form of MDI-GSH conjugates can upregulate endogenous PPP3CA expression and decrease endogenous miR-206–3p and miR-381–3p expression in differentiated THP-1 macrophages.
Figure 3.
MDI-GSH conjugate-induced endogenous PPP3CA and hsa-miR-206 and hsa-miR-381–3p expression in differentiated THP-1 macrophages. Differentiated THP-1 macrophages exposed to MDI-GSH conjugates at indicated concentrations for 24h followed by total RNA isolation and determination by TaqMan or miR stem-loop RT-qPCR. A, Endogenous PPP3CA mRNA level (N = 3; bars, SEM). B, Endogenous PPP3CA protein expression visualized by immunoblot (upper panel). β-Actin served as a loading control (lower panel). C, Endogenous miR expression of hsa-miR-206–3p. D, Endogenous miR expression of hsa-miR-381–3p (N = 3; bars, SEM). MDI, 4,4′-methylene diphenyl diisocyanate; GSH, glutathione (*p < .05, **p < .01, and ***p < .001).
PPP3CA as a Potential Target of hsa-miR-206–3p and hsa-miR-381–3p
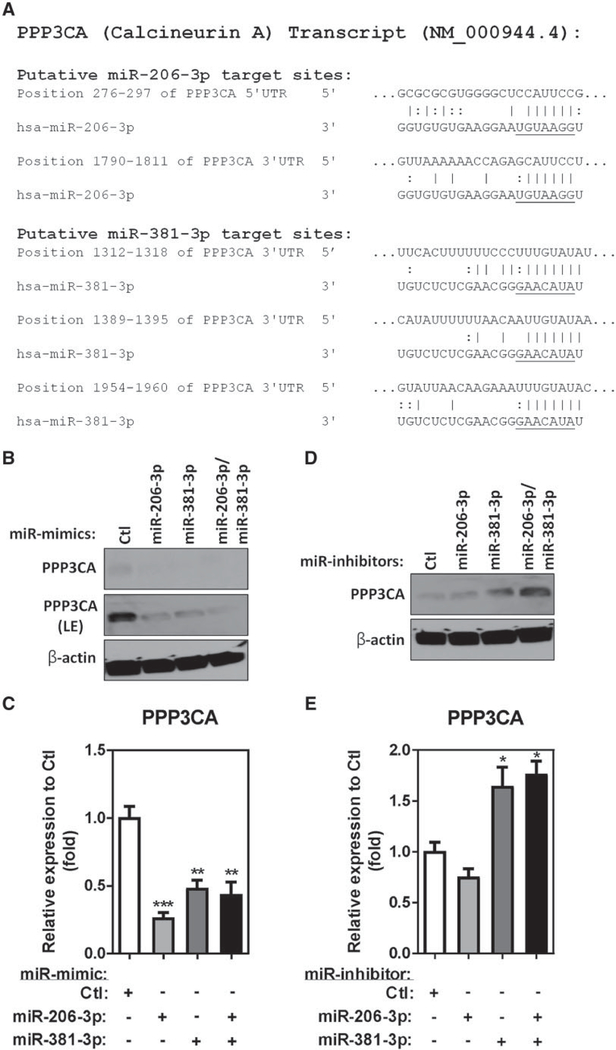
Analysis of the human PPP3CA transcript using the in silico miR-target prediction tool miRDB (Wong and Wang, 2015) led to the identification of 3 hsa-miR-381–3p predicted binding sites in the 3’UTR of the transcript (NM_000944.4) (Figure 4A). Although miRDB did not predict hsa-miR-206–3p would bind the PPP3CA transcript, when we manually searched the seed sequence of miR-206–3p against the human PPP3CA transcript, 2 putative hsa-miR-206–3p binding sites were found on either the 5’UTR or the 3’UTR of the PPP3CA transcript (Figure 4A).
Figure 4.
PPP3CA is a target of both hsa-miR-206–3p and hsa-miR-381–3p. A, Alignment of the PPP3CA 5’UTR and 3’UTR regions indicating putative hsa-miR-206–3p and hsa-miR-381–3p binding sites. Seed sequences of hsa-miR-206–3p and hsa-miR-381–3p are underlined. Endogenous PPP3CA protein levels in differentiated THP-1 macrophages transfected with 25 nM of miR-mimic (B) or miR-inhibitor (D) were determined by immunoblot. β-Actin served as a loading control (LE, long exposure). Total RNA isolated from differentiated THP-1 macrophages transfected with 25 nM of miR-mimic (C) or miR-inhibitor (E) determined by PPP3CA TaqMan stem-loop RT-qPCR mRNA assays (N = 3; bars, SEM) (*p < .05, **p < .01, and ***p < .001).
To investigate whether either miR-206–3p and/or miR-381–3p can regulate endogenous PPP3CA expression, we performed gain- and loss-of-function studies by transfecting miR-mimics or miR-inhibitors into differentiated THP-1 macrophages. Transfection of either miR-mimic-206–3p or miR-mimic-381–3p downregulated endogenous PPP3CA protein (Figure 4B) and RNA (Figure 4C) compared with the miR-mimic control. Additionally, cotransfection of both miR-mimic-206–3p and miR-mimic-381–3p further decreased PPP3CA protein level (Figure 4B) but not the mRNA level (Figure 4C) compared with cells transfected with either miR-mimic-206–3p or miR-mimic-381–3p alone, indicating the translation of PPP3CA was further inhibited by the combination of both miR-mimic-206–3p and miR-mimic-381–3p (Figure 4B). Because MDI-GSH conjugates treatment decreased endogenous miR-206–3p and miR-381–3p levels in differentiated THP-1 macrophages (Figs. 3C and 3D), we performed a loss-of-function study by transfecting differentiated THP-1 cells with miR-inhibitor-206–3p, miR-inhibitor-381–3p and a nontargeting miR-inhibitor control to investigate whether the endogenous PPP3CA level would be affected by either miR-206–3p or miR-381–3p. Consistent with PPP3CA as potential target of both miR-206–3p and miR-381–3p, transfection of either miR-inhibitor-206–3p or miR-inhibitor-381–3p increased endogenous PPP3CA protein level (Figure 4D); however, only cells transfected with miR-inhibitor-381–3p or miR-inhibitor-206–3p and miR-inhibitor-381–3p increased endogenous PPP3CA mRNA level (Figure 4E). In addition, transfection of both miR-inhibitor-206–3p and miR-inhibitor-381–3p further increased endogenous PPP3CA protein expression compared with transfection of either miR-inhibitor-206–3p or miR-inhibitor-381–3p alone (Figs. 4D and 4E). These data suggest that PPP3CA may be a potential target of both miR-206–3p and miR-381–3p.
Verification ofPPP3CA as a Direct Target of Either hsa-miR-206–3p or hsa-miR-381–3p
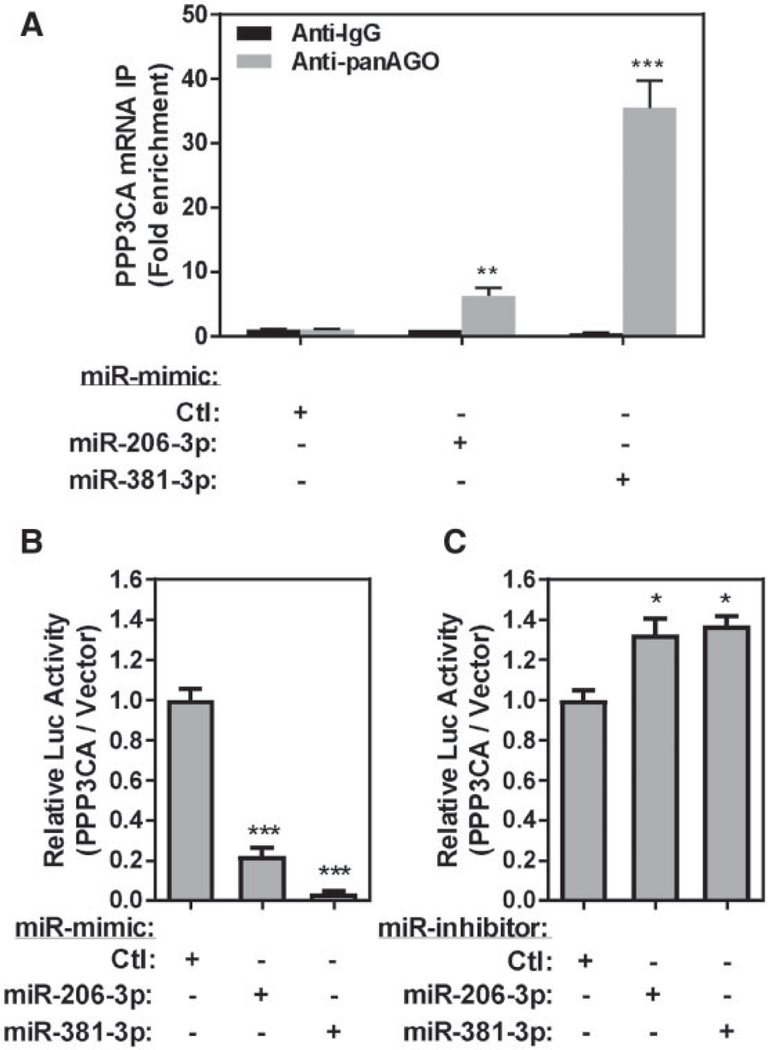
A potential target gene that is regulated by miRs may be either directly regulated by binding of a particular miR or through other miR-regulated pathways (eg, transcriptional or posttran-scriptional regulation) (Inui et al., 2010; Tsang et al., 2007). The translational repression or mRNA transcript degradation of a specific miR-target mRNA is mediated by a miR-containing RISC (Gregory et al., 2005). In the RISC, the miR is bound with Ago proteins (Gregory et al., 2005; Matranga et al., 2005) which guide the complex to the binding sites located in the 3’UTR region of target mRNAs (Gregory et al., 2005). To determine whether or not PPP3CA is directly regulated by either miR-206–3p or miR-381–3p, we first confirmed miR-206–3p and miR-381–3p binding to PPP3CA by performing an Ago antibody pulldown method to precipitate the RISCs which contain Agos/miRs/mRNAs (RISC-IP), and confirmed the roles of miR-206–3p and miR-381–3p on translational repression by using a 3’UTR luciferase translational reporter assay. Because Ago proteins are major components of the RISC and are capable binding to miRs to bring the RISC to potential target mRNA through miR guidance (Matranga et al., 2005), the RISC containing Agos/miRs/mRNAs can be immunoprecipitated by the anti-Ago antibodies to identify target mRNA transcripts. Here, the complex was precipitated using a pan-Ago antibody (specific for Ago-1, Ago-2, and Ago-3). Transfection of miR-mimic-206–3p increased precipitated PPP3CA transcript by 6.22-fold compared with the nontargeting miR-mimic control, whereas transfection of miR-mimic-381–3p increased precipitated PPP3CA transcript by 35.5-fold compared with the nontargeting control (Figure 5A). These results indicate that the PPP3CA mRNA transcript binds to miR-206–3p and miR-381–3p-containing RISCs.
Figure 5.
PPP3CA is a direct target of both hsa-miR-206–3p and hsa-miR-381–3p in differentiated THP-1 macrophages. A, Differentiated THP-1 macrophages transfected with 25 nM of miR-mimic or nontargeting miR-mimic-Control and immunoprecipitated using panAgo or isotype IgG antibody. Fold enrichment of PPP3CA transcript was measured (N = 3; bars, SEM). Differentiated THP-1 macrophages cotransfected with PPP3CA 3’UTR luciferase reporter and 25 nM of miR-mimic or nontargeting miR-mimic-Ctl (B) or miR-inhibitor and nontargeting miR-inhibitor-Ctl (C) (N = 3; bars, SEM) (*p < .05, **p < .01, and ***p < .001).
Furthermore, we addressed the specificity and translational inhibition roles of miR-206–3p and miR-381–3p on the PPP3CA mRNA transcript using the PPP3CA 3’UTR luciferase reporter assay. Transfection of miR-mimic-206–3p decreased PPP3CA luciferase reporter activities by 4.50-fold, whereas transfection of miR-mimic-381–3p downregulated the PPP3CA luciferase reporter activities by 26.61-fold compared with the cells transfected with miR-mimic-Ctl (Figure 5B). In addition, transfection of either miR-inhibitor-206–3p or miR-inhibitor-381–3p increased the PPP3CA luciferase reporter activities by 1.33–1.37-fold compared with transfection of miR-inhibitor-control (Figure 5C). Because both miR-206–3p and miR-381–3p are capable of repressing PPP3CA expression, these data indicate that the PPP3CA transcript is, in fact, a direct target of both miR-206– 3p and miR-381–3p.
MDI Aerosol Exposure In Vivo and MDI-GSH Conjugates Exposure In Vitro Upregulate iNOS mRNA
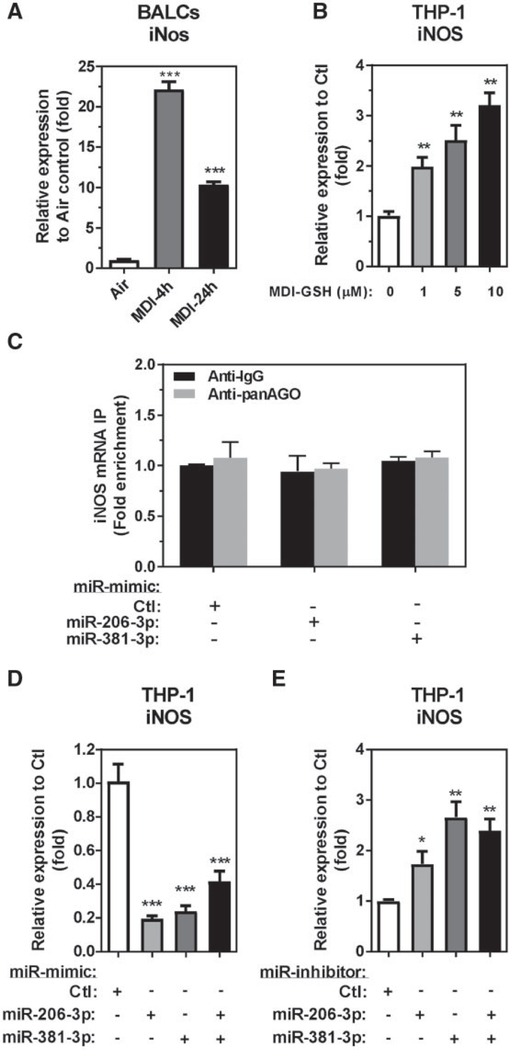
In the asthmatic airway microenvironment, the macrophages secrete multiple mediators such as IL-1, IFN-γ, TNF-α, IL-6, CCL2, CCL3, CXCL8 (IL-8), and others to recruit and activate other inflammatory cells to the airway (Gosset et al., 1999; Rosenwasser, 2000; Vissers et al., 2005). The macrophages also release NO and nitrates, bioactive lipids, platelet activating factor, and reactive oxygen species (ROS) which are reported to affect vascular smooth muscle tone, bronchial epithelial cells, and possibly bronchial smooth muscle tone in the asthmatic airways (Rosenwasser, 2000). Among the mediators secreted by macrophages in the asthmatic airways, only FeNO has been demonstrated to be significantly elevated 24h after MDI-SIC in MDI-OA patients (Barbinova and Baur, 2006; Baur and Barbinova, 2005; Ferrazzoni et al., 2009). The source of NO production in the respiratory tract is largely from conversion of Larginine into L-citrulline and the concomitant release of NO by the iNOS in the airway cells (Brindicci et al., 2007; Lane et al., 2004). Previous reports have shown that activated calcineurin/NFAT signaling can induce iNOS expression in macrophages, epithelial cells, and other different cell types (Buxade et al., 2012; Hamalainen et al., 2002; Li et al., 2006; Ranjan et al., 2014); however, the response of macrophages to MDI exposure and whether or not these cells contribute to the increased NO de nouo synthesis via induction of iNOS after MDI exposure is currently unknown. To determine whether or not iNOS can be induced by either MDI or MDI-GSH conjugates, we measured the expression of endogenous iNos mRNA in the BALCs from MDI aerosol-exposed mice as well as MDI-GSH conjugates-treated THP-1 macrophages. Compared with mice exposed to house air control, iNos mRNA expression in MDI aerosol-exposed mouse BALCs was upregulated 22.15-fold (4-h postexposure) and 10.35-fold (24-h postexposure) (Figure 6A). Similarly, treatments of differentiated THP-1 macrophages with MDI-GSH conjugates also increased endogenous iNOS mRNA (Figure 6B). These results indicate that MDI exposure may increase iNOS expression in the airway.
Figure 6.
miR-206–3p and miR-381–3p indirectly regulate inducible nitric oxide synthase (iNOS) transcription after MDI exposure. Total RNA isolated from MDI aerosol or house air control exposed mice BALCs or from MDI-GSH conjugates exposed differentiated THP-1 macrophages determined by TaqMan stem-loop RT-qPCR. A, Endogenous mouse iNos mRNA level (N = 3; bars, SEM) from BALCs. B, Endogenous human iNOS mRNA (N = 3; bars, SEM) from THP-1 macrophages. C, Differentiated THP-1 macrophages transfected with miR-mimic-206–3p, miR-mimic-381–3p, or nontargeting miR-mimic-Ctl and immunoprecipitated using the panAgo or isotype IgG antibody. Fold enrichment of iNOS transcript was measured (N = 3; bars, SEM). Total RNA isolated from differentiated THP-1 macrophages transfected with 25 nM of miR-mimic (D) or miR-inhibitor (E) determined by iNOS TaqMan stem-loop RT-qPCR mRNA assay (N = 3; bars, SEM). MDI, 4,4′-methylene diphenyl diisocyanate; GSH, glutathione (*p < .05, **p < .01, and ***p < .001).
Hsa-miR-206–3p and hsa-miR-381–3p Indirectly Regulate iNOS Transcription
Given that MDI-GSH conjugates increased PPP3CA expression partially through downregulation of both miR-206–3p- and miR-381–3p-mediated responses in differentiated THP-1 macrophages (Figure 3), and that PPP3CA is involved in NFAT signaling pathways, we hypothesized that MDI-GSH conjugates upregulate iNOS expression through PPP3CA upregulation as a result of miR-206–3p and miR-381–3p suppression in macrophages. In silico targeting analysis did not predict iNOS to be a direct target of either miR-206–3p or miR-381–3p. We performed a RISC-IP experiment to verify that iNOS is not a direct target of either miR-206–3p or miR-381–3p (Figure 6C). Transfection of both miR-mimics-206–3p and -miR-381–3p failed to enrich iNOS mRNA transcripts as determined by RISC-IP using a panAgo antibody (Figure 6C); further verifying that iNOS is not a direct target of either miR-206–3p or miR-381–3p.
To investigate whether either miR-206–3p or miR-381–3p may participate in endogenous iNOS mRNA transcriptional regulation, we used gain- and loss-of-function studies by transfecting miR-mimics or miR-inhibitors into differentiated THP-1 macrophages. Transfection of miR-mimic-206–3p downregulated endogenous iNOS by 5.13-fold and transfection of miR-mimic-381–3p downregulated endogenous iNOS by 4.20- fold (Figure 6D). Furthermore, transfection of miR-inhibitor-206– 3p upregulated endogenous iNOS by 1.73-fold and transfection of miR-inhibitor-381–3p upregulated endogenous iNOS by 2.66-fold (Figure 6E). Transfection of both miR-mimic-206–3p and miR-mimic-381–3p together downregulated endogenous iNOS mRNA levels by 2.40-fold (Figure 6D) and transfection both of miR-inhibitor-206–3p and miR-inhibitor-381–3p together upregulated endogenous iNOS by 2.40-fold (Figure 6E). Given that miR-206–3p and miR-381–3p negatively regulate PPP3CA expression, these results indicate that elevation of iNOS expression in response to MDI is likely to be regulated by miR-206–3p and miR-381–3p-mediated calcineurin/NFAT signaling pathway activation.
Hsa-miR-206–3p and hsa-miR-381–3p Regulate iNOS Transcription Through PPP3CA/Calcineurin/NFAT Signaling
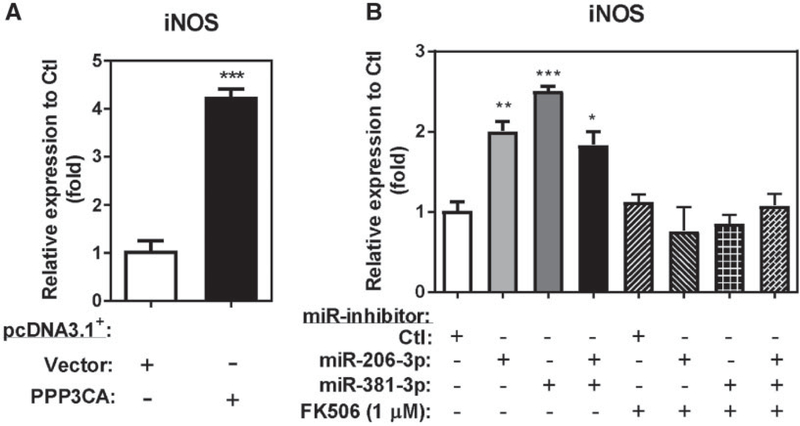
Previous reports have shown that calcineurin inhibition down-regulated iNOS expression in macrophages and other cell types (Hamalainen et al., 2002; Kim et al., 2004; Lima et al., 2001; Trajkovic et al., 1999a,b), these results indicate that calcineurin is an important regulator for iNOS expression. To determine whether miR-206–3p and miR-381–3p-mediated iNOS upregulation is via PPP3CA/calcineurin-regulated NFAT signaling, we performed both a gain-of-function study by overexpression of PPP3CA and a loss-of-function study by using a specific calcineurin A inhibitor, tacrolimus (FK506), to repress PPP3CA/NFAT signaling in miR-inhibitor-206–3p and miR-381–3p transfected THP-1 macrophages. PPP3CA overexpression in differentiated THP-1 macrophages induced endogenous iNOS mRNA by 4.25-fold compared with vector-transfected cells (Figure 7A). Consistent with the finding that miR-inhibitors-206–3p and miR-inhibitor-381–3p upregulated endogenous iNOS mRNA in THP-1 macrophages (Figure 6E), independent transfections of miR-inhibitor-206–3p and miR-inhibitor-381–3p upregulated endogenous iNOS by 2.03-fold and 2.51-fold, respectively (Figure 7B) compared with the miR-inhibitor-Ctl. Furthermore, independent transfection of both miR-inhibitors-206–3p and miR-inhibitor-381–3p together upregulated endogenous iNOS mRNA level by 1.83-fold. Treatment of 1 μM FK506 (PPP3CA inhibitor) blocked iNOS mRNA upregulation by miR-inhibitor-206 and miR-inhibitor-381–3p in THP-1 macrophages (Figure 7B). These results indicate that PPP3CA-mediated signaling is important for the iNOS transcriptional activation by miR-206–3p and miR-381–3p inhibition.
Figure 7.
PPP3CA is required for miR-206–3p and miR-381–3p mediated iNOS transcription. A, Differentiated THP-1 macrophages transfected with 2.5 μg of either a pcDNA3.1+/c-(k)dyk-PPP3CA expression plasmid (PPP3CA) or a pcDNA3.1+ empty vector control (Vector). Total RNA was isolated and the fold enrichment of iNOS transcript was measured by RT-qPCR (N = 3; bars, SEM). B, Differentiated THP-1 macrophages were transfected with 25 nM of miR-inhibitor or nontargeting control with or without 1 μM FK506 treatment. Total RNA was isolated and the fold enrichment of iNOS transcript was measured by RT-qPCR (N = 3; bars, SEM) (*p < .05, **p < .01, and ***p < .001).
DISCUSSION
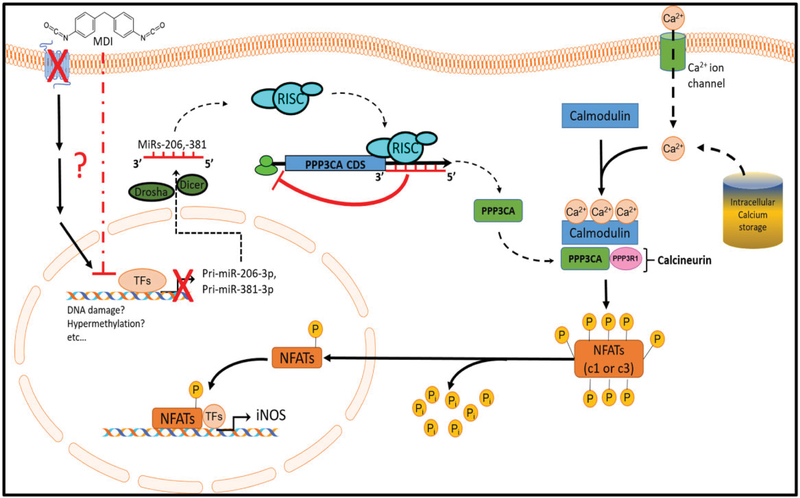
Although elevated FeNO levels have been found in MDI-OA patients following SIC with MDI aerosol (Barbinova and Baur, 2006; Baur and Barbinova, 2005; Ferrazzoni et al., 2009), the molecular mechanism(s) underlying that observation are currently unclear. In the current study, we have identified a potential miR-206–3p- and miR-381–3p-regulated calcineurin/NFAT signaling pathway that may be involved in the upregulation of iNOS transcription after acute MDI exposure. We found that the endogenous miR-206–3p and miR-381–3p were downregulated in both BALCs from an in vivo murine MDI aerosol exposure model and in an in vitro human THP-1 macrophage cell culture MDI-GSH conjugates exposure model. The downregulation of endogenous miR-206–3p and miR-381–3p after MDI exposure results in the upregulation of iNOS transcription via upregulation of PPP3CA and PPP3CA-mediated activation of downstream NFAT signaling (Figure 8). Using an in vitro THP-1 macrophage cell culture model, we have identified that both miR-206–3p and miR-381–3p target the PPP3CA mRNA transcript which suppresses PPP3CA translation. To our knowledge, this is the first report to identify and verify that PPP3CA is a target of either miR-206–3p or miR-381–3p.
Figure 8.
Proposed model of miR-206–3p- and miR-381–3p-regulated PPP3CA/NFAT signaling activation on iNOS transcription after acute MDI exposure. Note: Some illustrated schematics were obtained from motifolio templates (www.motifolio.com, Accessed October 16, 2019).
In the occupational clinic, an MDI-SIC test is conducted by heating pure MDI to generate MDI aerosol in a chamber to a concentration of ≤ 20ppb, and suspected MDI-OA patients are exposed to this MDI aerosol for a duration of approximately 1 h (Vandenplas et al., 2014b) In the current study, we used MDI-GSH conjugates as a surrogate for pure MDI exposure in cell culture experiments because of the high cytotoxicity of pure MDI on THP-1 macrophages (data not shown). It has been shown that the major antioxidant GSH, which contains free thiol moieties, exists at very high concentration (> 100 μM) in the airway fluid (Cantin et al., 1987), and previous reports have shown that GSH reacts with inhaled MDI aerosol to form MDI-GSH conjugates in vivo, which potentially play important roles in MDI-mediated sensitization (Wisnewski et al., 2013, 2015). Animals exposed to intranasal admission of MDI-GSH conjugates exhibited significantly more airway inflammation and mucus production response than animals which received intranasal admission of either GSH or MDI alone (Wisnewski et al., 2015). In addition, MDI-GSH conjugates have been found to transcarbamoylate albumin in the airway fluid resulting in an MDI-conjugated albumin which may play a role in pathogenic eosinophilic inflammatory responses (Wisnewski et al., 2013).
Mechanistic studies to understand how MDI or MDI-GSH conjugates may downregulate the endogenous miR-206–3p and miR-381–3p in BALCs to initiate this process are not currently available. Given that MDI contains 2 highly reactive electrophilic isocyanate (N=C=O) moieties, our best hypothesis is that MDI reacts rapidly with nucleophilic biomolecules in the lung micro-environment after inhalation. Such reactions will result in covalent modifications which could alter the structure of endogenous proteins, eg, membrane receptors, thereby affecting signal transduction into the cell, and ultimately affect the transcription of the miRs and/or the miR maturation machinery such as the microprocessor complex (ribonuclease III Drosha, and ds-RNA binding protein DGCR*) in the nucleus or endoribo-nuclease Dicer in the cytoplasm (Bartel, 2018). These effects could proceed via either direct binding of MDI to endogenous proteins such as membrane receptors, or via GSH-initiated transcarbamoylation reactions, such as those proposed by Wisnewski et al. (2013).
Pauluhn et al. showed that inhaled MDI deposited in the lung reacts with surfactant molecules to form MDI-conjugated surfactant complexes, which have been demonstrated to be phagocytized by the alveolar macrophages (Pauluhn, 2000, 2002). Although phagocytized MDI-surfactant complexes are thought to be degraded in macrophages, the possibility exists that unreacted MDI from the phagocytized complex may still be capable of reacting with the endogenous cellular proteins involved in the maturation of miRs in the cytoplasm, with the nuclear proteins involved in the export of pri-miRNAs from the nucleus to the cytosol, with genomic DNA regions that contain the miR genes and their regulating molecules in the nucleus. Interestingly, the promoter region of interferon-y has been found to be hypermethylated after specific dNCO inhalation challenge in MDI-OA and other dNCO-OA patients (Ouyang et al., 2013), indicating that MDI can induce epigenetic modification of specific genes in the promoter region, therefore disrupting genes expression. Could such a mechanism be at work to downregulate the transcription and/or the maturation of miR-206–3p and miR-381–3p in the airway macrophages? Further investigation of how MDI exposure affects downregulation of endogenous miR-206–3p and miR-381–3p is needed.
Airway macrophage dysfunctions play important roles in asthma pathogenesis (Fricker and Gibson, 2017). These dysfunctions may include decreasing phagocytosis, efferocytosis, and inflammatory resolution while increasing ROS and immune mediators production and secretion (Fricker and Gibson, 2017). The levels of inflammatory/immune mediators produced by airway macrophages, such as TNF-α, IL-1β, IL-6, IL-8, IL-17, and GM-CSF, have been found elevated in the asthmatic airway from patients with asthma (Ackerman et al., 1994; Hoshi et al., 1995; Song et al., 2008). In vertebrates, the calcineurin/NFAT signaling pathway regulates the expression of many important immune regulatory genes that are involved in both innate and adaptive immunity (Wu et al., 2007). Currently, the levels of airway macrophage-secreted mediators, including TNF-α, IL-1β, IL- 6, IL-8, CCL2, CCL3, and others, in MDI-OA patients’ airway remain unclear. Determination of these mediator concentrations in the BALF from MDI-OA patient airways would be of great value.
By negatively regulating their target genes, miRs affect diverse signaling pathways in the regulation of cell function and disease. For miR-mediated calcineurin/NFAT signaling regulation, only a few miRs have been previously reported, such as miRs-124, −133a, −184, and −528 (Kang et al., 2013; Li et al., 2010, 2014; Weitzel et al., 2009). The current report adds miR-206–3p and miR-381–3p to the list of miRs that directly regulate calcineurin/NFAT signaling. Given that calcineurin/NFAT signaling regulates cytokines, chemokines, and other mediators such as IL-2, −3, −4, −5, −13, INF-γ, TNF-α, and GMCSF (Bert et al., 2000; Burke et al., 2000; Cockerill et al., 1995; Macian et al., 2000; Peng et al., 2001; Zhang et al., 1999) that are implicated in asthma pathogenesis, an important future study will be to determine whether calcineurin/NFAT signaling-dependent cytokines, chemokines and other mediators are regulated by either miR-206–3p or miR-381–3p.
Besides the roles they play in directly regulating genes involved in the calcineurin/NFAT signaling pathway, miR-206–3p and miR-381–3p may be involved in regulation of other important inflammatory genes associated with asthma pathogenesis. For example, elevated C-C motif chemokine ligand 2/Monocyte chemoattractant protein-1 (CCL2/MCP-1) levels were associated with decreased expression of miR-206 in HEV71 encephalitis patients (Zhang et al., 2017). The authors found that CCL2/MCP-1 is a direct target of miR-206 (Zhang et al., 2017). Elevated levels of CCL2/MCP-1 have been found in peripheral blood mononuclear cells (PBMCs) isolated from dNCO-OA patients when compared with normal subjects (Lummus et al., 1998) and CCL2/MCP-1 has been proposed as a potential biomarker for diagnosis of dNCO-OA patients (Bernstein et al., 2002). It is very possible that downregulation of miR-206–3p by MDI exposure may cause upregulation of CCL2/MCP-1 through direct binding of miR-206–3p to the CCL2/MCP-1 3′UTR; further study of this phenomenon is needed. Recently, miR-381–3p has been shown to target the high mobility group box 1 (HMGB1) gene resulting in reduced macrophage mobility and infiltration in polymyositis (Liu et al., 2018). HMGB1 is a highly conserved DNA binding protein that is an important modulator of immune and inflammatory responses in airway inflammation (Yang et al., 2013). Chakraborty et al. (2013) showed that HMGB1 upregulates iNOS expression and NO production in macrophages. Importantly, HMGB1 was found elevated and elevated HMGB1 levels were associated with impaired lung function and more severe disease in asthmatic patients (Hou et al., 2011; Watanabe et al., 2011). Tang et al. (2014) reported that elevated pulmonary HMGB1 level was associated with allergic airway inflammation in a TDI-induced asthmatic mouse model. TDI inhalation upregulates HMGB1 expression in the lung in a TDI-induced asthmatic mouse model via PI3K mediated caspase-1 activation (Liang et al., 2015). Interestingly, NFAT signaling has been implicated in the regulation of HMGB1 release in human monocytes (Liu et al., 2014). Based on the results presented in this report, determination of whether miR-381–3p-mediated NFAT signaling can regulate HMGB1 expression and release following acute MDI exposure is worthy of further investigation.
In conclusion, this report implicates miR-206–3p and miR-381–3p as important regulators of calcineurin/NFAT signaling, ultimately targeting iNOS transcription in airway macrophages after acute MDI exposure. Decreased expression of miR-206–3p and miR-381–3p may be affected by MDI aerosol exposure in an in vivo mouse inhalation model as well as MDI-GSH conjugates exposure in an in vitro human THP-1 macrophage cell culture model. This miR-regulated mechanism may contribute to the upregulation of iNOS transcription in the macrophages following acute MDI exposure.
FUNDING
This work was supported by intramural funds from the National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention.
Footnotes
DECLARATION OF CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention.
REFERENCES
- Ackerman V, Marini M, Vittori E, Bellini A, Vassali G, and Mattoli S (1994). Detection of cytokines and their cell sources in bronchial biopsy specimens from asthmatic patients. Relationship to atopic status, symptoms, and level of airway hyperresponsiveness. Chest 105,687–696. [DOI] [PubMed] [Google Scholar]
- Allmers H, Chen Z, Barbinova L, Marczynski B, Kirschmann V, and Baur X (2000). Challenge from methacholine, natural rubber latex, or 4,4-diphenylmethane diisocyanate in workers with suspected sensitization affects exhaled nitric oxide [change in exhaled NO levels after allergen challenges]. Int. Arch. Occup. Environ. Health 73,181–186. [DOI] [PubMed] [Google Scholar]
- Allport DC, Gilbert DS, and Outterside SM (2003). MDI and TDI: A Safety, Health and the Environment: A Source Book and Practical Guide. John Wiley, New York. [Google Scholar]
- Barbinova L, and Baur X (2006). Increase in exhaled nitric oxide (eNO) after work-related isocyanate exposure. Int. Arch. Occup. Environ. Health 79, 387–395. [DOI] [PubMed] [Google Scholar]
- Bartel DP (2018). Metazoan microRNAs. Cell 173, 20–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur X, and Barbinova L (2005). Increase of exhaled nitric oxide (eNO) after methylene diphenyl diisocyanate (MDI) exposure in isocyanate workers with bronchial hyperresponsiveness. Allergol. Int. 54,151–158. [Google Scholar]
- Bernstein DI, Cartier A, Cote J, Malo JL, Boulet LP, Wanner M, Milot J, L’Archeveque J, Trudeau C, and Lummus Z (2002). Diisocyanate antigen-stimulated monocyte chemoattractant protein-1 synthesis has greater test efficiency than specific antibodies for identification of diisocyanate asthma. Am. J. Respir. Crit. Care Med. 166, 445–450. [DOI] [PubMed] [Google Scholar]
- Bernstein DI, Korbee L, Stauder T, Bernstein JA, Scinto J, Herd ZL, and Bernstein IL (1993). The low prevalence of occupational asthma and antibody-dependent sensitization to diphenylmethane diisocyanate in a plant engineered for minimal exposure to diisocyanates. J. Allergy Clin. Immunol. 92, 387–396. [DOI] [PubMed] [Google Scholar]
- Bert AG, Burrows J, Hawwari A, Vadas MA, and Cockerill PN (2000). Reconstitution of T cell-specific transcription directed by composite NFAT/Oct elements. J. Immunol. 165, 5646–5655. [DOI] [PubMed] [Google Scholar]
- Booton R, and Lindsay MA (2014). Emerging role of microRNAs and long noncoding RNAs in respiratory disease. Chest 146, 193–204. [DOI] [PubMed] [Google Scholar]
- Brindicci C, Ito K, Barnes PJ, and Kharitonov SA (2007). Effect of an inducible nitric oxide synthase inhibitor on differential flow-exhaled nitric oxide in asthmatic patients and healthy volunteers. Chest 132, 581–588. [DOI] [PubMed] [Google Scholar]
- Burke TF, Casolaro V, and Georas SN (2000). Characterization of P5, a novel NFAT/AP-1 site in the human IL-4 promoter. Biochem. Biophys. Res. Commun. 270,1016–1023. [DOI] [PubMed] [Google Scholar]
- Buxade M, Lunazzi G, Minguillon J, Iborra S, Berga-Bolanos R, Del Val M, Aramburu J, and Lopez-Rodriguez C (2012). Gene expression induced by Toll-like receptors in macrophages requires the transcription factor NFAT5. J. Exp. Med. 209, 379–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantin AM, North SL, Hubbard RC, and Crystal RG (1987). Normal alveolar epithelial lining fluid contains high levels of glutathione. J. Appl. Physiol. 63,152–157. [DOI] [PubMed] [Google Scholar]
- Chakraborty R, Bhatt KH, and Sodhi A (2013). High mobility group box 1 protein synergizes with lipopolysaccharide and peptidoglycan for nitric oxide production in mouse peritoneal macrophages in vitro. Mol. Immunol. 54,48–57. [DOI] [PubMed] [Google Scholar]
- Cockerill PN, Bert AG, Jenkins F, Ryan GR, Shannon MF, and Vadas MA (1995). Human granulocyte-macrophage colony-stimulating factor enhancer function is associated with cooperative interactions between AP-1 and NFATp/c. Mol. Cell. Biol. 15, 2071–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree GR, and Olson EN (2002). NFAT signaling: Choreographing the social lives of cells. Cell 109(Suppl), S67–S79. [DOI] [PubMed] [Google Scholar]
- Daigneault M, Preston JA, Marriott HM, Whyte MK, and Dockrell DH (2010). The identification of markers of macrophage differentiation in PMA-stimulated THP-1 cells and monocyte-derived macrophages. PLoS One 5, e8668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl S, Chow CW, Weiss L, Palmetshofer A, Twardzik T, Rounds L, Serfling E, Davis RJ, Anguita J, and Rincon M (2002). Induction of NFATc2 expression by interleukin 6 promotes T helper type 2 differentiation. J. Exp. Med. 196, 39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, Olin AC, Plummer AL, and Taylor DR; American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels (FENO) for Clinical Applications. (2011). An official ATS clinical practice guideline: Interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am. J. Respir. Crit. Care Med. 184, 602–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engfeldt M, Isaksson M, Zimerson E, and Bruze M (2013). Several cases of work-related allergic contact dermatitis caused by isocyanates at a company manufacturing heat exchangers. Contact Dermatitis 68,175–180. [DOI] [PubMed] [Google Scholar]
- Esteller M (2011). Non-coding RNAs in human disease. Nat. Reu. Genet. 12,861–874. [DOI] [PubMed] [Google Scholar]
- Ferrazzoni S, Scarpa MC, Guarnieri G, Corradi M, Mutti A, and Maestrelli P (2009). Exhaled nitric oxide and breath condensate pH in asthmatic reactions induced by isocyanates. Chest 136,155–162. [DOI] [PubMed] [Google Scholar]
- Feske S, Giltnane J, Dolmetsch R, Staudt LM, and Rao A (2001). Gene regulation mediated by calcium signals in T lymphocytes. Nat. Immunol. 2, 316–324. [DOI] [PubMed] [Google Scholar]
- Fricker M, and Gibson PG (2017). Macrophage dysfunction in the pathogenesis and treatment of asthma. Eur. Respir. J. 50, 1700196. [DOI] [PubMed] [Google Scholar]
- Gosset P, Tillie-Leblond I, Oudin S, Parmentier O, Wallaert B, Joseph M, and Tonnel AB (1999). Production of chemokines and proinflammatory and antiinflammatory cytokines by human alveolar macrophages activated by IgE receptors. J. Allergy Clin. Immunol. 103, 289–297. [DOI] [PubMed] [Google Scholar]
- Gregory RI, Chendrimada TP, Cooch N, and Shiekhattar R (2005). Human RISC couples microRNA biogenesis and post-transcriptional gene silencing. Cell 123, 631–640. [DOI] [PubMed] [Google Scholar]
- Hamalainen M, Lahti A, and Moilanen E (2002). Calcineurin inhibitors, cyclosporin A and tacrolimus inhibit expression of inducible nitric oxide synthase in colon epithelial and macrophage cell lines. Eur. J. Pharmacol. 448, 239–244. [DOI] [PubMed] [Google Scholar]
- Hermann-Kleiter N, and Baier G (2010). NFAT pulls the strings during CD4+ T helper cell effector functions. Blood 115, 2989–2997. [DOI] [PubMed] [Google Scholar]
- Heron M, Grutters JC, ten Dam-Molenkamp KM, Hijdra D, van Heugten-Roeling A, Claessen AM, Ruven HJ, van den Bosch JM, and van Velzen-Blad H (2012). Bronchoalveolar lavage cell pattern from healthy human lung. Clin. Exp. Immunol. 167, 523–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettick JM, Law BF, Lin CC, Wisnewski AV, and Siegel PD (2018). Mass spectrometry-based analysis of murine bronchoalveolar lavage fluid following respiratory exposure to 4,4′-methylene diphenyl diisocyanate aerosol. Xenobiotica 48, 626–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge MR, Ranger AM, Charles de la Brousse F, Hoey T, Grusby MJ, and Glimcher LH (1996). Hyperproliferation and dysregulation of IL-4 expression in NF-ATp-deficient mice. Immunity 4, 397–405. [DOI] [PubMed] [Google Scholar]
- Hogan PG, Chen L, Nardone J, and Rao A (2003). Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Deu. 17, 2205–2232. [DOI] [PubMed] [Google Scholar]
- Hoshi H, Ohno I, Honma M, Tanno Y, Yamauchi K, Tamura G, and Shirato K (1995). IL-5, IL-8 and GM-CSF immunostaining of sputum cells in bronchial asthma and chronic bronchitis. Clin. Exp. Allergy 25, 720–728. [DOI] [PubMed] [Google Scholar]
- Hou C, Zhao H, Liu L, Li W, Zhou X, Lv Y, Shen X, Liang Z, Cai S, and Zou F (2011). High mobility group protein B1 (HMGB1) in Asthma: Comparison of patients with chronic obstructive pulmonary disease and healthy controls. Mol. Med. 17, 807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman LJ,Judy DJ, Frazer D, Shapiro RE, Castranova V, Billie M, and Dedhia HV (1997). Inhalation of toluene diisocyanate is associated with increased production of nitric oxide by rat bronchoalveolar lavage cells. Toxicol. Appl. Pharmacol. 145, 61–67. [DOI] [PubMed] [Google Scholar]
- Im SH, and Rao A (2004). Activation and deactivation of gene expression by Ca2+/calcineurin-NFAT-mediated signaling. Mol. Cells 18, 1–9. [PubMed] [Google Scholar]
- Inui M, Martello G, and Piccolo S (2010). MicroRNA control of signal transduction. Nat. Reu. Mol. Cell Biol. 11, 252–263. [DOI] [PubMed] [Google Scholar]
- Jan RL, Chen SH, Chang HY, Yeh HJ, Shieh CC, and Wang JY (2008). Asthma-like syndrome in school children after accidental exposure to xylene and methylene diphenyl diisocyanate. J. Microbiol. Immunol. Infect. 41, 337–341. [PubMed] [Google Scholar]
- Kang K, Peng X, Zhang X, Wang Y, Zhang L, Gao L, Weng T, Zhang H, Ramchandran R, Raj JU, et al. (2013). MicroRNA-124 suppresses the transactivation of nuclear factor of activated T cells by targeting multiple genes and inhibits the proliferation of pulmonary artery smooth muscle cells. J. Biol. Chem. 288, 25414–25427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen JC, Sholl L, Wills-Karp M, and Georas SN (2001). Preferential activation of nuclear factor of activated T cells c correlates with mouse strain susceptibility to allergic responses and interleukin-4 gene expression. Am. J. Respir. Cell Mol. Biol. 24, 58–65. [DOI] [PubMed] [Google Scholar]
- Kim Y, Moon JS, Lee KS, Park SY, Cheong J, Kang HS, Lee HY, and Kim HD (2004). Ca2+/calmodulin-dependent protein phosphatase calcineurin mediates the expression of iNOS through IKK and NF-kappaB activity in LPS-stimulated mouse peritoneal macrophages and RAW 264.7 cells. Biochem. Biophys. Res. Commun. 314,695–703. [DOI] [PubMed] [Google Scholar]
- Koch S, Reppert S, and Finotto S (2015). NFATc1 deletion in T lymphocytes inhibits the allergic trait in a murine model of asthma. Clin. Exp. Allergy 45,1356–1366. [DOI] [PubMed] [Google Scholar]
- Lane C, Knight D, Burgess S, Franklin P, Horak F, Legg J, Moeller A, and Stick S (2004). Epithelial inducible nitric oxide synthase activity is the major determinant of nitric oxide concentration in exhaled breath. Thorax 59, 757–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemiere C, Chaboillez S, Bohadana A, Blais L, and Maghni K (2014). Noneosinophilic responders with occupational asthma: A phenotype associated with a poor asthma prognosis. J. Allergy Clin. Immunol. 133,883–885.e3. [DOI] [PubMed] [Google Scholar]
- Lesage J, Stanley J, Karoly WJ, and Lichtenberg FW (2007). Airborne methylene diphenyl diisocyanate (MDI) concentrations associated with the application of polyurethane spray foam in residential construction. J. Occup. Environ. Hyg. 4, 145–155. [DOI] [PubMed] [Google Scholar]
- Li J, Song L, Zhang D, Wei L, and Huang C (2006). Knockdown of NFAT3 blocked TPA-induced COX-2 and iNOS expression, and enhanced cell transformation in Cl41 cells. J. Cell. Biochem. 99, 1010–1020. [DOI] [PubMed] [Google Scholar]
- Li Q, Lin X, Yang X, and Chang J (2010). NFATc4 is negatively regulated in miR-133a-mediated cardiomyocyte hypertrophic repression. Am. J. Physiol. Heart Circ. Physiol. 298, H1340–H1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Kong LB, Li JT, Guo ZY, Xue Q, Yang T, Meng YL, Jin BQ, Wen WH, and Yang AG (2014). MiR-568 inhibits the activation and function of CD4(+) T cells and Treg cells by targeting NFAT5. Int. Immunol. 26, 269–281. [DOI] [PubMed] [Google Scholar]
- Liang J, Zhao H, Yao L, Tang H, Dong H, Wu Y, Liu L, Zou F, and Cai S (2015). Phosphatidylinositol 3-kinases pathway mediates lung caspase-1 activation and high mobility group box 1 production in a toluene-diisocyanate induced murine asthma model. Toxicol. Lett. 236, 25–33. [DOI] [PubMed] [Google Scholar]
- Lima R, Serone AP, Schor N, and Higa EM (2001). Effect of cyclosporin A on nitric oxide production in cultured LLC-PK1 cells. Ren. Fail. 23, 43–52. [DOI] [PubMed] [Google Scholar]
- Lin CC, Law BF, Siegel PD, and Hettick JM (2019). Circulating miRs-183–5p, −206–3p and −381–3p may serve as novel biomarkers for 4,4’-methylene diphenyl diisocyanate exposure. Biomarkers 24, 76–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CC, Liu LZ, Addison JB, Wonderlin WF, Ivanov AV, and Ruppert JM (2011). A KLF4-miRNA-206 autoregulatory feedback loop can promote or inhibit protein translation depending upon cell context. Mol. Cell. Biol. 31, 2513–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CC, Sharma SB, Farrugia MK, McLaughlin SL, Ice RJ, Loskutov YV, Pugacheva EN, Brundage KM, Chen D, and Ruppert JM (2015). Kruppel-like factor 4 signals through microRNA-206 to promote tumor initiation and cell survival. Oncogenesis 4, e155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Zhao Q, Song Q, Zhou F-H, Kang H-J, Pan L, and Yao Y-M (2014). Release of high mobility protein box-1 is greatly regulated by nuclear factor of activated T cell-2 in human monocytes. Eur. J. Inflamm. 12,101–108. [Google Scholar]
- Liu Y, Gao Y, Yang J, Shi C, Wang Y, and Xu Y (2018). MicroRNA-381 reduces inflammation and infiltration of macrophages in polymyositis via downregulating HMGB1. Int. J. Oncol. 53, 1332–1342. [DOI] [PubMed] [Google Scholar]
- Lofgren DJ, Walley TL, Peters PM, and Weis ML (2003). MDI exposure for spray-on truck bed lining. Appl. Occup. Environ. Hyg. 18, 772–779. [DOI] [PubMed] [Google Scholar]
- Lu TX, and Rothenberg ME (2013). Diagnostic, functional, and therapeutic roles of microRNA in allergic diseases. J. Allergy Clin. Immunol. 132, 3–13; quiz 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lummus ZL, Alam R, Bernstein JA, and Bernstein DI (1998). Diisocyanate antigen-enhanced production of monocyte chemoattractant protein-1, IL-8, and tumor necrosis factor-alpha by peripheral mononuclear cells of workers with occupational asthma. J. Allergy Clin. Immunol. 102, 265–274. [DOI] [PubMed] [Google Scholar]
- Macian F (2005). NFAT proteins: Key regulators of T-cell development and function. Nat. Reu. Immunol. 5,472–484. [DOI] [PubMed] [Google Scholar]
- Macian F, Garcia-Rodriguez C, and Rao A (2000). Gene expression elicited by NFAT in the presence or absence of cooperative recruitment of Fos and Jun. EMBO J. 19,4783–4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macian F, Lopez-Rodriguez C, and Rao A (2001). Partners in transcription: NFAT and AP-1. Oncogene 20, 2476–2489. [DOI] [PubMed] [Google Scholar]
- Mapp CE, Boschetto P, Maestrelli P, and Fabbri LM (2005). Occupational asthma. Am. J. Respir. Crit. Care Med. 172, 280–305. [DOI] [PubMed] [Google Scholar]
- Matranga C, Tomari Y, Shin C, Bartel DP, and Zamore PD (2005). Passenger-strand cleavage facilitates assembly of siRNA into Ago2-containing RNAi enzyme complexes. Cell 123, 607–620. [DOI] [PubMed] [Google Scholar]
- Mendell JT, and Olson EN (2012). MicroRNAs in stress signaling and human disease. Cell 148,1172–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mummadi SR, and Hahn PY (2016). Update on exhaled nitric oxide in clinical practice. Chest 149,1340–1344. [DOI] [PubMed] [Google Scholar]
- Munn SAR, Aschberger K, Cosgrove O, Pakalin S, PayaPerez A, Pellegrini G, Schwarz-Schulz B, and Vegro S (2005). European Union Risk Assessment Report: Methylenediphenyl Diisocyanate (MDI), Vol. 59 European Commission, Office for Official Publications of the European Communities, Luxembourg. [Google Scholar]
- NIOSH. (1994a). Letter From NIOSH to Distinctive Designs International Inc With a Study Report. US Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health, Cincinnati, OH. [Google Scholar]
- NIOSH. (1994b). Letter From NIOSH to Jim Walter Resources, Inc With a Study Report. US Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health, Cincinnati, OH. [Google Scholar]
- NIOSH. (1997). NIOSH Pocket Guide to Chemical Hazards P. H. S. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, Cincinnati, OH. [Google Scholar]
- NIOSH. (2004). A Summary of Health Hazard Evaluations: Issues Related to Occupational Exposure to Isocyanates, 1989 to 2002. US Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health, Cincinnati, OH. [Google Scholar]
- Ouyang B, Bernstein DI, Lummus ZL, Ying J, Boulet LP, Cartier A, Gautrin D, and Ho SM (2013). Interferongamma promoter is hypermethylated in blood DNA from workers with confirmed diisocyanate asthma. Toxicol. Sci. 133, 218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauluhn J (2000). Acute inhalation toxicity of polymeric diphenyl-methane 4,4′-diisocyanate in rats: Time course of changes in bronchoalveolar lavage. Arch. Toxicol. 74, 257–269. [DOI] [PubMed] [Google Scholar]
- Pauluhn J (2002). Short-term inhalation toxicity of polyisocyanate aerosols in rats: Comparative assessment of irritant-threshold concentrations by bronchoalveolar lavage. Inhal. Toxicol. 14, 287–301. [DOI] [PubMed] [Google Scholar]
- Peng SL, Gerth AJ, Ranger AM, and Glimcher LH (2001). NFATcl and NFATc2 together control both T and B cell activation and differentiation. Immunity 14,13–20. [DOI] [PubMed] [Google Scholar]
- Pua HH, and Ansel KM (2015). MicroRNA regulation of allergic inflammation and asthma. Curr. Opin. Immunol. 36, 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjan R, Deng J, Chung S, Lee YG, Park GY, Xiao L, Joo M, Christman JW, and Karpurapu M (2014). The transcription factor nuclear factor of activated T cells c3 modulates the function of macrophages in sepsis. J. Innate Immun. 6, 754–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A, Luo C, and Hogan PG (1997). Transcription factors of the NFAT family: Regulation and function. Annu. Reu. Immunol. 15, 707–747. [DOI] [PubMed] [Google Scholar]
- Redlich CA, and Karol MH (2002). Diisocyanate asthma: Clinical aspects and immunopathogenesis. Int. Immunopharmacol. 2, 213–224. [DOI] [PubMed] [Google Scholar]
- Rengarajan J, Mowen KA, McBride KD, Smith ED, Singh H, and Glimcher LH (2002a). Interferon regulatory factor 4 (IRF4) interacts with NFATc2 to modulate interleukin 4 gene expression. J. Exp. Med. 195,1003–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengarajan J, Tang B, and Glimcher LH (2002b). NFATc2 and NFATc3 regulate T(H)2 differentiation and modulate TCR-responsiveness of naive T(H)cells. Nat. Immunol. 3,48–54. [DOI] [PubMed] [Google Scholar]
- Rosenwasser LJ (2000). New immunopharmacologic approaches to asthma: Role of cytokine antagonism. J. Allergy Clin. Immunol. 105, S586–S591; discussion S591–2. [DOI] [PubMed] [Google Scholar]
- Rusnak F, and Mertz P (2000). Calcineurin: Form and function. Physiol. Reu. 80,1483–1521. [DOI] [PubMed] [Google Scholar]
- Schlesinger RB (1985). Comparative deposition ofinhaled aerosols in experimental animals and humans: A review. J. Toxicol. Environ. Health 15,197–214. [DOI] [PubMed] [Google Scholar]
- Sharma SB, Lin CC, Farrugia MK, McLaughlin SL, Ellis EJ, Brundage KM, Salkeni MA, and Ruppert JM (2014). MicroRNAs 206 and 21 cooperate to promote RAS-extracellular signal-regulated kinase signaling by suppressing the translation of RASA1 and SPRED1. Mol. Cell. Biol. 34, 4143–4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Luo L, Lei Z, Li B, Liang Z, Liu G, Li D, Zhang G, Huang B, and Feng ZH (2008). IL-17-producing alveolar macrophages mediate allergic lung inflammation related to asthma. J. Immunol. 181,6117–6124. [DOI] [PubMed] [Google Scholar]
- Suuronen K, Suojalehto H, and Cullinan P (2014). Handbook of procedures for specific inhalation challenge testing in the diagnosis of occupational asthma. Eur. Respir. J. 43. [DOI] [PubMed] [Google Scholar]
- Tang H, Zhao H, Song J, Dong H, Yao L, Liang Z, Lv Y, Zou F, and Cai S (2014). Ethyl pyruvate decreases airway neutrophil infiltration partly through a high mobility group box 1-dependent mechanism in a chemical-induced murine asthma model. Int. Immunopharmacol. 21, 163–170. [DOI] [PubMed] [Google Scholar]
- Trajkovic V, Badovinac V, Jankovic V, and Mostarica Stojkovic M (1999a). Cyclosporin A inhibits activation of inducible nitric oxide synthase in C6 glioma cell line. Brain Res. 816,92–98. [DOI] [PubMed] [Google Scholar]
- Trajkovic V, Badovinac V, Jankovic V, Samardzic T, Maksimovic D, and Popadic D (1999b). Cyclosporin A suppresses the induction of nitric oxide synthesis in interferon-gamma-treated L929 fibroblasts. Scand. J. Immunol. 49, 126–130. [DOI] [PubMed] [Google Scholar]
- Tsang J, Zhu J, and van Oudenaarden A (2007). MicroRNA-me-diated feedback and feedforward loops are recurrent network motifs in mammals. Mol. Cell 26, 753–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rietschoten JG, Smits HH, van de Wetering D, Westland R, Verweij CL, den Hartog MT, and Wierenga EA (2001). Silencer activity of NFATc2 in the interleukin-12 receptor beta 2 proximal promoter in human T helper cells. J. Biol. Chem. 276, 34509–34516. [DOI] [PubMed] [Google Scholar]
- Vandenplas O, Suojalehto H, Aasen TB, Baur X, Burge PS, de Blay F, Fishwick D, Hoyle J, Maestrelli P, Munoz X, et al. (2014a). Specific inhalation challenge in the diagnosis of occupational asthma: Consensus statement. Eur. Respir. J. 43, 1573–1587. [DOI] [PubMed] [Google Scholar]
- Vandenplas O, Suojalehto H, Aasen TB, Baur X, Burge PS, de Blay F, Fishwick D, Hoyle J, Maestrelli P, Munoz X, et al. (2014b). Specific inhalation challenge in the diagnosis of occupational asthma: Consensus statement. Eur Respir J. 43(6):1573–1587. Suppl. Final Hand book. p23–24. [DOI] [PubMed] [Google Scholar]
- Vissers JL, van Esch BC, Hofman GA, and van Oosterhout AJ (2005). Macrophages induce an allergen-specific and long-term suppression in a mouse asthma model. Eur. Respir. J. 26, 1040–1046. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Asai K, Fujimoto H, Tanaka H, Kanazawa H, and Hirata K (2011). Increased levels of HMGB-1 and endogenous secretory RAGE in induced sputum from asthmatic patients. Respir. Med. 105, 519–525. [DOI] [PubMed] [Google Scholar]
- Weitzel RP, Lesniewski ML, Haviernik P, Kadereit S, Leahy P, Greco NJ, and Laughlin MJ (2009). microRNA 184 regulates expression of NFAT1 in umbilical cord blood CD4+ T cells. Blood 113, 6648–6657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisnewski AV, and Jones M (2010). Pro/Con debate: Is occupational asthma induced by isocyanates an immunoglobulin E-mediated disease? Clin. Exp. Allergy 40,1155–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisnewski AV, Liu J, and Colangelo CM (2015). Glutathione reaction products with a chemical allergen, methylene-diphenyl diisocyanate, stimulate alternative macrophage activation and eosinophilic airway inflammation. Chem. Res. Toxicol. 28, 729–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisnewski AV, Liu J, and Redlich CA (2013). Connecting glutathione with immune responses to occupational methylene diphenyl diisocyanate exposure. Chem. Biol. Interact. 205, 38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong N, and Wang X (2015). miRDB: An online resource for microRNA target prediction and functional annotations. Nucleic Acids Res. 43, D146–D152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Peisley A, Graef IA, and Crabtree GR (2007). NFAT signaling and the invention of vertebrates. Trends Cell Biol. 17, 251–260. [DOI] [PubMed] [Google Scholar]
- Yang H, Antoine DJ, Andersson U, and Tracey KJ (2013). The many faces of HMGB1: Molecular structure-functional activity in inflammation, apoptosis, and chemotaxis. J. Leukoc. Biol. 93,865–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DH, Yang L, Cohn L, Parkyn L, Homer R, Ray P, and Ray A (1999). Inhibition of allergic inflammation in a murine model of asthma by expression of a dominant-negative mutant of GATA-3. Immunity 11,473–482. [DOI] [PubMed] [Google Scholar]
- Zhang G, Wang J, Yao G, and Shi B (2017). Downregulation of CCL2 induced by the upregulation of microRNA-206 is associated with the severity of HEV71 encephalitis. Mol. Med. Rep. 16, 4620–4626. [DOI] [PMC free article] [PubMed] [Google Scholar]