Abstract
All Microsporidia share a unique, extracellular spore stage, containing the infective sporoplasm and the apparatus for initiating infection. The polar filament/polar tube when exiting the spore, transports the sporoplasm through it into a host cell. While universal, these structures and processes have been enigmatic. This study utilized several types of microscopy, describing and extending our understanding of these structures and their functions. Cryogenically preserved polar tubes vary in diameter from 155 to over 200nm, noticeably larger than fixed-sectioned, or negatively stained samples. The polar tube surface is pleated and covered with fine fibrillar material that projects from the surface and is organized in clusters or tufts. These fibrils may be the sites of glycoproteins providing protection and aiding infectivity. The polar tube surface is ridged with 5–6nm spacing between ridges, enabling the polar tube to rapidly increase its diameter to facilitate the passage of the various cargo including cylinders, sacs or vesicles filled with particulate material and the intact sporoplasm containing a diplokaryon. The lumen of the tube is lined with a membrane that facilitates this passage. Careful examination of the terminus of the tube indicates that it has a closed tip where the membranes for the terminal sac are located.
Keywords: Cargo, cryogenic transmission electron microscopy, plunge freeze, polar filament, polar tube protein, spore germination, sporoplasm, 3D-reconstruction
THE Phylum Microsporidia Balbiani 1882 contains over two hundred genera and approximately thirteen hundred species representing a diverse group of obligate intracellular parasitic protists (Becnel et al., 2014; Cali et al., 2017). Since microsporidia are such a diverse group and infect a wide range of hosts, their life cycles and the nature of the diseases they produce are quite variable (Weiss 2014). Their host range extends from other protists; invertebrates, especially insects and other arthropods; to vertebrates, particularly fish and mammals, including 9 Genera and 17 species reported to infect humans (Weiss and Becnel 2014). Of the human infecting organisms, the genus Anncaliia formerly Nosema; Brachiola, (Franzen et al., 2006) originally isolated from mosquitos (Varvra and Undeen, 1970) has been identified as the cause of muscle, skin, eye, and disseminated infections (Cali et al., 1998, Cali et al., 2010; Coyle et al.,2004; Margileth et al., 1973; Sutrave et al., 2018; Weiss, 2014). In addition to being a human pathogen, Anncaliia has the unique ability to grow and develop in several cell culture systems, fish cells (Monaghan et al., 2010), rabbit cells (Lowman et al., 2000), human HeLa cells (Santianna et al, 2015) and a variety of insect and animal hosts (Undeen and Maddox, 1973).
Despite their diversity, all microsporidia share a specialized, resistant, extracellular stage, the spore, which encloses and protects the infective sporoplasm and the unique mechanism for initiating infection. Spores are generally small, oval or pyriform shaped, and range in size from 1μm – 12 μm (Vavra 1976; Vavra and Larsson 2014). Those infecting mammals are generally 1–4 μm in length (Bryan et al., 1991; Webber et al., 1994). When observed with transmission electron microscopy (TEM) the spores have an electron dense outer spore coat overlying an inner thicker lucent coat followed by a membrane system surrounding the spore contents and polar filament coils. The spore contents include: an anterior anchoring disk complex, tightly packed arrays of membrane clusters, the lamellar polaroplast, and flattened tubules, a centrally located sporoplasm, composed of scant cytoplasm containing a nucleus or pair of abutted nuclei (diplokaryon), tightly packed ribosomes and some endoplasmic reticulum. Surrounding the central region of the spore is a coiled polar filament (termed the polar tube when extruded). There may be a few to many dozens of coils of the polar filament, and it may be arranged in a single to multiple rows, depending on the organism (Cali and Takvorian 2014; Vavra 1976; Varvra and Larsson 2014).
Thelohan’s 1894 drawings of microsporidial spores with extruded polar tubes (PTs) and droplets (sporoplasm) suggested there was a relationship between the spore, polar tube (PT), and sporoplasm (Thelohan 1894). Ohshima’s (1937) report indicating that the sporoplasm exits a hollow PT further defined this relationship. Kramer’s light microscopic study of Geimsa stained spores, enabled him to describe the extruded PT. He illustrated the passage of nuclei through the PT, the presence of a cytoplasmic globule at the tip of the tube, and a sporoplasm containing nuclei, discharged from the polar tubule; clearly demonstrating that the PT served to transport the sporoplasm (Kramer, 1960). High-speed video microscopy, documented the extrusion of the PT and the release of a sporoplasm in less than two seconds using activated Nosema algerae spores (Frixione et al. 1992; 1996). The result of these and other studies, indicate that the method by which microsporidia infect their host, is sporoplasm transfer via the extruded PT, injecting a sporoplasm into a host cell (Keohane and Weiss 1998; Keohane et al.1999; Vavra 1976; Weidner 1972; Weiss et al., 2014). Although being the accepted paradigm for infection for over 100 years, the mechanism of polar filament activation, its translocation inside the spore, its exit from the spore by eversion, and subsequent transport of the infective sporoplasm through the now “hollow” PT continues to be poorly understood, despite having been the subject of several reviews (Keohane and Weiss, 1999; Varvra and Larsson 1999; Weiss et al., 2014; Xu and Weiss 2005).
Due to the small size of the microsporidian spore and its extensive membrane systems, extrusion apparatus, polar filament, and infective sporoplasm, all tightly packed inside this spore, the transmission electron microscope (TEM) has been required to study spore structure and its contents (Weiss and Becnel 2014). While inside the spore, cross sectional images of the polar filament indicate that it is a solid structure composed of alternating concentric rings of electron dense and lucent material surrounding electron dense material in the center, the number of coils and their contents varies in different organisms (Cali et al., 2002; Takvorian and Cali 1986; Varvra 1976; Weiss et al., 2014). During activation and extrusion, the internal organization of the spore undergoes massive membrane reorganization while the polar filament changes; becoming a hollow tube as it everts and exits the spore, thus the nomenclature, polar filament in the spore and polar tube when extruded (Cali et al., 2002; Chioralia, et al., 1998; Varvra 1976; Weiss et al., 2014). Due in part to the role of the long (50–300μm) but narrow (90–120nm) polar filament/tubule transferring the relatively large (1–1.5μm) infective sporoplasm from the spore into a host cell through this narrow tube in less than 2 seconds (Frixione et al., 1992), its’ structure, chemical composition, and function has been extensively studied yet many questions persist (Weiss et al., 2014).
Weidner conducted a number of pioneering studies of the microsporidian invasion apparatus providing insight into PT morphology, chemical composition, and elongation of discharged PTs (Weidner 1972; Weidner 1976; Weidner 1982; Weidner and Byrd 1982). Weidner postulated that the tube is elongated by assembly of dense material, polar tube protein (PTP) that flows through the tube and emerges at the growing tip (Weidner 1982, Weidner et al., 1995). Additionally, TEM images of negatively stained discharged PTs were described as having several morphological features; a tube-within-a-tube, cylinders, cylinders-within-cylinders, and unassembled PTP inside the tube (Weidner 1982). Subsequently, the PT was described as being membrane lined and having membranous cylinders containing monomeric PTP material in its lumen, which then polymerizes as it exits the distal tip of the PT. In addition, a membrane also emerges from the tip of the tube, forming a sporoplasmic sac, housing the emerging sporoplasm contents (Weidner et al., 1995). Cali et al., 2002, conducted an extensive structural study of the Anncaliia algerae (then Brachiola) PT, while inside the spore, during its activation, translocation, extrusion, and during the process of becoming extra-sporal. This study defined a new sporoplasm structure, MIN (Multilayered Interlaced Network), its connection to the PT and proposed function was discussed (Cali et al., 2002). A comprehensive study of A. algerae sporoplasms, utilizing TEM, enzyme histochemistry, and high voltage TEM (HVEM) tomography with computer generated 3D reconstructions, demonstrated that the extruded sporoplasm is connected to the PT by the MIN, which has multiple attachment sites with the PT (Takvorian et al., 2013). Although we (AC/PMT, LMW) and numerous other laboratories, have utilized virtually every type of microscopy and specimen preparation to study the microsporidian spore; the extruding PT and discharge of the sporoplasm remains enigmatic.
One problem with current studies of PTs has been that of sample preparation using conventional resin embedding followed by thin sectioned TEM samples requiring extensive processing which may produce artifacts. An alternative to viewing thin sectioned specimens of PTs is TEM of extruded negative stained tubes. Negatively stained PTs collected immediately after discharge, enable the observation of surface features and some of their internal structures. The PTs are thin enough to image in TEM with negative stains such as phosphotungstic acid, Vanadium, or other heavy metals (Cali et al. 2002; Khohane et al., 1994; Weidner 1972, 1976). Over the last twenty years, the use of cryogenic electron microscopy or Cryo-EM to study cells, isolated organelles, viruses, and macromolecules has become more widespread. Additionally, as cryogenic sample processing equipment and Cryo-TEM’s have become more advanced and available, their use has increased as have the types of specimens imaged (Hutchings and Zanetti, 2018). Cryo-EM overcomes the formation of artifacts inherent in conventional TEM sample processing, and unlike negative stain, the projection images include details from all internal structures. Cryogenic processing of rapidly frozen, fully hydrated biological specimens, embedded in vitreous ice has enabled us to study samples in a near-to-native state when maintained in a liquid nitrogen (LN2) cooled environment and imaged in a Cryo-TEM (Lucic et al., 2013; Milne et al., 2012). These specimens provide high resolution images of unstained, low contrast specimens. Electron tomography enables the reconstruction of three-dimensional (3D) morphological features from two-dimensional (2D) images. This is accomplished by aligning many 2D tilt images of the sample with computer processing software such as IMOD (Kremer, et al., 1996; Mastronarde 1997) and then using the image stacks to produce tomograms and 3D computer generated reconstructions (Lucic et al., 2013; Milne et al., 2012).
Because of Anncaliia’s exceptional ability to infect and successfully develop in such a wide host range, its relatively large (4μm) spore size, and its medical importance, we chose to study this organism’s extrusion apparatus. Here we report the first use of cryo-TEM to study the PT and compare its structure to that observed by conventional TEM of thin sectioned and negative stained isolated PTs. We present cryo-tomography and 3D reconstruction of a portion of the PT. We also present the first cryo-TEM image of an intact sporoplasm traveling through the PT.
MATERIALS AND METHODS
Spore propagation
Anncaliia algerae formerly (Nosema, Brachiola) spores were propagated in rabbit kidney cells (RK-13; ATCC CCL-37), collected from culture media, and stored in sterile distilled water at 4 °C (Cali et al., 2002).
Germination of spores
To identify the time necessary for spore germination/activation, 5 -μL aliquots of A. algerae spores in sterile, distilled water were placed on several microscope-slide cover slips and allowed to air dry. After drying, 5-μL of a modified (Leitch et al. 1993) germinating solution containing, 140mM NaCl, 5mM KCl, 1mM CaCl2, and 10nm Tris-HCl buffer pH 8.6, were added to each of the cover slips. Spore activation was monitored by phase contrast microscopy from time zero to 5-min post addition of germination buffer as previously described (Cali and Takvorian 2001, Cali et al, 2002).
Light microscopy of polar tubes from germinated spores
The extruded PTs from germinated spores were examined with a Zeiss 200 Axiophot inverted microscope (Thornwood, NY) using 63X, and 100X phase-contrast objectives. Images were recorded with an Axiocam 506 CCD camera.
High speed light microscopy
High speed microscopy was performed with an Olympus IX71 microscope (Waltham, Massachusetts) with a 60X N.A. 1.4 planapo lens. Images were collected blind with a video camera modified to collect 200 frames per second using sub-frame collection and interlaced lines. The VHS tape played at standard speed was digitized using a Scion LG3 card in a Windows PC and images were processed using Image J at the Albert Einstein College of Medicine Analytical Imaging Facility, Bronx, NY.
Transmission electron microscopy.
Ten 1-ml aliquots of A. algerae spores (4 × 107 ml) were placed in individual 1.5-ml microfuge tubes, with 0.5-ml of germination solution. Samples of spores suspended in distilled water were prepared for germination using the previously described germination methods. After addition of the germination buffer, the individual samples were then fixed in 0.1 M cacodylate-buffered 2.5% glutaraldehyde at 30-sec intervals up to 5-min. Controls were processed the same way except for the substitution of sterile distilled water in place of germination buffer. The tubes were then centrifuged at 5,000 × g for 30-s to obtain a pellet of intact and discharged spores, discharged PTs, and sporoplasms as previously described (Cali et al., 2002; Takvorian et al., 2013). The samples were post- fixed in OsO4, dehydrated, and processed into resin. Thin sections were stained with uranyl acetate and lead citrate. Samples for negative staining were obtained by removing aliquots (1–2 μL) of spore/germination buffer mixture from selected cover slips (from germination procedure). These aliquots were dispensed onto coated grids, allowed to dry, and stained with Nanovan (Nanoprobes, Inc.,Yaphank, NY). The samples were observed and photographed on Kodak 4489 film (Kodak, Rochester, NY) or with a OneView 16 Megapixel digital camera (Gatan, Pleasanton, CA) with a FEI Tecnai 12 TEM (FEI (Hillsboro OR.) at the Rutgers, Newark Electron Microscopy Facility.
Cryo-electron microscopy of extruded polar tubes
Five microliter aliquots each of A. algerae spores (4 × 107 ml) and germination buffer were placed on a glass slide and observed with a Nikon inverted microscope using a 40X phase contrast objective to monitor spore germination. Five microliters of the buffer containing the germinating spores, were placed on a Quantifoil R2/2 grid (Quantifoil Micro Tools GmbH, Jena, Germany) and plunge frozen in liquid Nitrogen (LN2) cooled liquid ethane in a Vitrobot (FEI, Hillsboro, OR). The PTs frozen in vitreous ice were imaged with a FEI Tecnai 20 LaB6 TEM (FEI, Hillsboro, OR) at 200kV in Low Dose Mode, images were recorded on a TVIPS F224 digital camera (TVIPS GmbH, Gauting, Germany) at the Albert Einstein College of Medicine Analytical Imaging Facility, Bronx, NY.
Additional Cryo-electron microscopy studies of PTs were conducted using a Gatan CP3 Cryoplunge 3 (Gatan, Pleasanton, CA) or an FEI Vitrobot (FEI, Hillsboro, OR) Plunge Freezer. The 3–5 μL aliquots of germinated PT samples were placed on Quantifoil R2/2 grids and were plunged into LN2 cooled liquid ethane. The frozen samples were then imaged with a JEOL 2100 at 200kV in Low Dose Mode, equipped with a TVIPS F224 digital camera (TVIPS GmbH, Gauting, Germany) or a FEI Tecnai 20 LaB6 TEM (FEI, Hillsboro, OR) at 200kV in Low Dose Mode. Samples were imaged at 50,000X at a pixel size of 3.06 Å. Tomograms of selected samples were also imaged, recorded, and aligned using IMOD (Mastronarde, 1997) at the New York Structural Biology Center, NY, NY.
3D reconstructions from tomograms
Tilt series were collected on the Tecnai F20 (200 kV), set magnification 29,000X, using the low-dose mode in Serial EM (Mastronarde, 2005) on a TVIPS F416 CMOS camera at camera binning 2 (pixel size 0.62 nm/pixel). Tilt range was from −50 degrees to +68 degrees with a tilt interval of 2 degrees. Total dose was 100 e−/A2. Tilt series were aligned using IMOD patch tracking (Mastronarde, 1997) and reconstructed with 10–20 iterations of Simultaneous Iterative Reconstruction Technique (SIRT) to enhance contrast. Three-dimensional models of the polar tube surfaces were generated by manual segmentation of features following denoising using median filtering, non-local means filtering, and/or anisotropic diffusion (Frangakis and Hegerl 2001) inside of Amira software (FEI, now Thermo Fisher).
Image Processing
Images obtained during this study were processed with Photoshop® CS-5 Adobe (www.adobe.com/) and measurements were obtained with FIJI Image J (https://imagej.nih.gov/ij/)
RESULTS
Light microscopy of Anncaliia algerae spores.
Using phase-contrast optics, numerous 4 X 2 μm non-germinated and germinated spores were visible. About 20% of the spores germinated during the first minute after addition of the buffer and by five min post exposure (PE) approximately 50–60% had extruded their PTs. Germinated spores were identified by the presence of a thin dark “thread-like” PT attached to their anterior end and protruding several μm away from the spore shell (Fig. 1A). Typically, these extruded PTs ranged in length from 75 – 100 μm and the leading end of fully extruded tubes formed a short hook or “J” that was about 2–4 μm (Fig. 1B). In addition, numerous small dark dot-like structures, pieces of polar tubes, and membrane – like sacs were visible. On rare occasion, one was able to observe the germination process, PT release from the apical end of the spore, the corkscrew-like motion of the elongating tube, and when fully extruded, the curving of the tube’s end into a hook followed by the sporoplasm (Fig. 1C).
Figure 1.
Light and Transmission Electron Microscopy (TEM) of extruded Anncaliia algerae polar tubes.
A. Phase contrast image of several Anncaliia algerae spores (S), some in the process of germinating with their extruding polar tubes (PT) still attached to the spore shell. Germinated spore shells appear dark while ungerminated spores are more refractile and appear white. Several sporoplasms (SP) are visible near their PT terminus. Bar is 10μm.
B. Spore with a fully extruded PT terminating in “J” shaped hook. Bar is 10μm.
C. Spore with a fully extruded PT and sporoplasm at its distal end. Bar is 10μm.
D. Montage of 10 high speed frames of the terminus of an extruding PT and the emerging sporoplasm. Frame (a) is the terminal end of the PT. Frames (b—e) illustrate the PT in the process of curling into its characteristic terminal hook. Frame (f) after the PT hook is complete, the terminal tip of tube widens and material (membrane?) exits (arrowhead). Frames (g—j) the emerging SP (arrowhead) appears as a dense elongated structure as it starts to exit the PT (g). The SP becomes more spherical (h--j) as it completes its emergence from the PT.
E. TEM image of longitudinal section through an extruded PT. The tube lumen is abutted by a thin electron dense (ED) layer, then a lighter layer, and a dark irregular surface coating containing fine dense fibrils that may be arranged in tufts or clusters (arrows). The diameter of this segment of the tube is uniform. Bar is 100nm.
F. Extruded PT containing membrane defined cylinders (C) that caused the tube to become distended between broad arrows. Bar is 100nm.
G. Cross section of an extruded PT from spores fixed immediately after one minute in germination buffer. The lumen of the tube is filled with about eight alternating concentric thin ED and electron lucent (EL) rings of material. The outer wall of the PT is enclosed by a relatively thick ED wall which is in turn covered by additional rings of material. The outermost PT surface is a ring of medium dense fibrous material with tufts of fibers projecting outward (arrows). Bar is 100nm.
H. Anterior portion of a spore fixed after one minute in germination buffer. The PT is covered with fine fibrillar material as it exits the anterior of the spore. Some of the fibrils extend out from the PT approximately 15nm (arrows). The anterior aperture (A) or collar of the spore is surrounded by the thick electron lucent endospore coat (EN) which is overlain by the electron dense exospore coat (EX). Bar is 100nm.
High speed light microscopy
High speed light microscopy of germinating spores imaged at 200 frames per second, captured the extrusion of the PT and the exit of the sporoplasm, from the A. algerae spore. The images revealed the progressive elongation of the PT, the formation of a “J” hook at the end of the tube, followed by the emergence of a sac from the tube and the slightly delayed sporoplasm. This exit of the sporoplasm from the tube is accompanied by a forward “lurch” of the polar tube and spore shell (supplement S-1, 2). Isolated image frames (A-J) of S-1 illustrate the formation of the terminal end of the PT; the formation of the “J” shaped hook, and the passage of the sporoplasm from the tube (Fig. 1D).
Transmission electron microscopy (TEM) of Anncaliia algerae polar tubes: thin sectioned resin embedded polar tubes.
The extruded PTs of activated A. algerae spores that were fixed and processed into resin, often have a relatively uniform surface and a 90 to 120 nm diameter when observed with a TEM (Fig. 1E), some PTs are not of a uniform diameter and have a bulge in some areas giving the appearance that something inside is distending the tube. Sometimes the structures or material being transported through the tube are enclosed by multiple layers of membrane-like material, significantly expanding the tube diameter as observed in Fig. 1F. The lumen of some PTs contains electron dense (ED) and electron lucent (EL) material, sometimes arranged as alternating concentric rings (Fig. 1G). Spores placed in germination buffer for less than 3 minutes, then rapidly fixed and processed into resin, sometimes have a fine fibrous coating on the surface of the extruded PT. This coating is present on the tube as it is exiting the spore’s anterior opening, which appears as a narrow 190nm hollow collar of endospore coat surrounded by the exospore wall (Fig. 1H). The fibrillar material may extend as far as 25 nm from the PT surface and may be organized as small tufts of material along the tube (Fig. 1G, H).
Transmission electron microscopy (TEM) of Anncaliia algerae polar tubes: negative stained polar tubes.
Negative stained PTs varied in appearance depending upon the contents of the tube and whether one imaged the surface or was able to penetrate the outer surface to visualize the inside of the tube. Polar tubes in the uniform regions still had some variation in the width or diameter, from 115 to 145nm (Fig. 2A). When the interior of the PT is penetrated and imaged, it appears to be composed of three main layers; a medium dense central area bounded by a thin dense layer on either side, which is covered by a variable layer (Fig. 2A). The central region or lumen of the PT is generally 85 −95nm in diameter and the covering is 20 to 25 nm thick, resulting in an overall diameter of 130–145nm (Fig. 2A). The covering of the PT has very fine fibrils projecting 8–10nm from the surface and some fibrils are in small tufts (Fig. 2A). The fibrillar tufts were not present on all regions of the tubes, but when present, the fibers generally projected 8 to 10nm from the surface and a few of the individual fibers were approximately 20nm long (Fig. 2A). The surface of the polar tubes had rows of ridges oriented parallel to the long axis of the PT; these ridges have a uniform spacing and a slightly stippled appearance (Fig. 2B). On occasion, the inside of the PTs had undulating tubular structures containing dense material, giving a tube-within-a-tube appearance (Fig. 2C).
Figure 2.
Transmission Electron Microcrospy- of negatively stained activated Anncaliia algerae extruded polar tubes.
A. Uniform region of an extruded PT with the lumen of the tube enclosed by the thin ED limiting layer. The outer most surface layer is composed of fibrillar material organized into several tufts (arrows). Bar is100nm.
B. Polar tube surface with parallel uniformly spaced rows of stippled material along the length of the tube. Bar is 100 nm.
C. Polar tube containing an undulating tubular structure (T) inside its lumen, enabling one to see a tube-within-a- tube organization. Bar is 100 nm.
Cryo-electron microscopy of extruded polar tubes
Anncaliia algerae spores placed in germination buffer generally started to germinate within 1 to 3 minutes. Aliquots of 3 to 5μL of samples placed on Quantifoil® grids were cryogenically preserved. The unstained PTs were visible in some of the Quantifoil grid holes if the tubes were in a thin vitreous ice layer. Only small segments of the tubes were visible and these varied in appearance; from straight to “S” shaped, occasionally two tubes were present. The tubes are composed of numerous layers of alternating electron light and dark material oriented parallel to the tube shaft (Fig. 3A). The tube’s protein composition or packing presents as a regular array pattern of material at a diagonal to the tube shaft giving it a helical orientation (Fig. 3B).
Figure 3.
Cryo-Transmission Electron Microscopy (CTEM) - of unstained, cryo preserved extruded Anncaliia algerae polar tubes in vitreous ice.
A. A PT whose outer edge is composed by 2–3 layers of alternating electron lucent (EL) arrow and medium dense parallel bands of material. The central portion of the tube contains ED material that is finely stippled. Bar is 50nm.
B. Organized arrays of polar tube protein on the surface of a PT. Bold arrows indicate the angle of protein orientation to the PT shaft, suggesting a helical organization. The fibrillar material visible along the tube is obvious and indicated by arrows. Bar is 50nm.
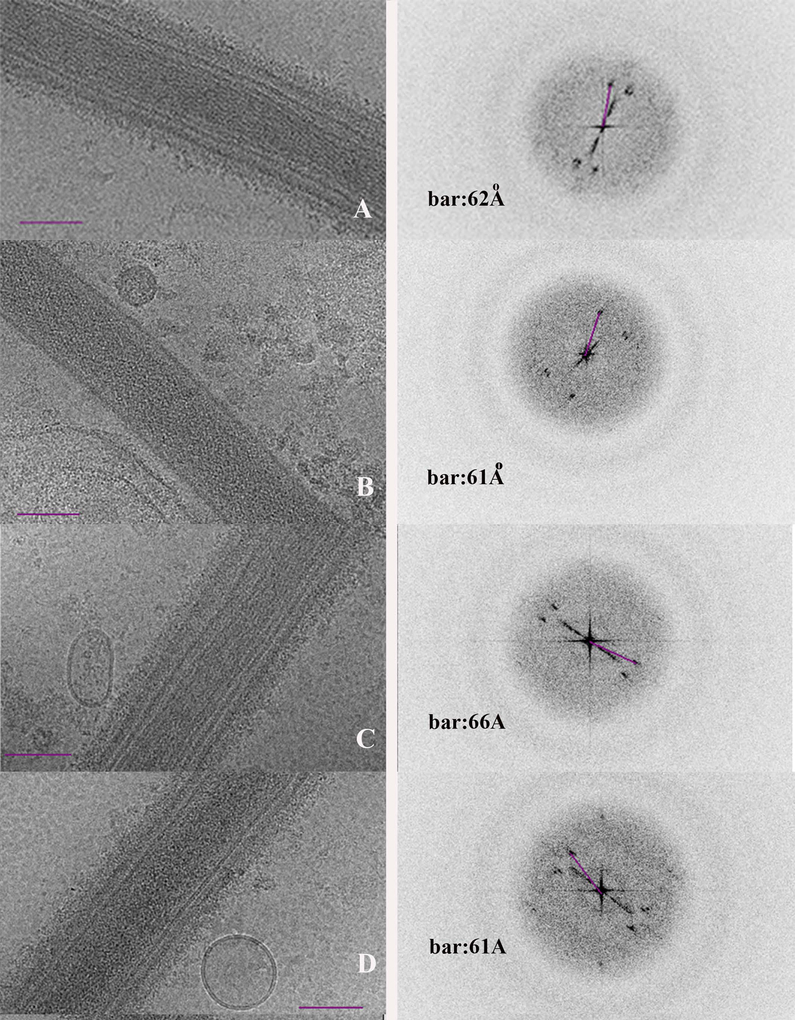
The diffraction patterns of the PTs in Fig. 4 A–D represent a few of the over 100 images collected during this aspect of the project and support the observed structural patterns. We calculated the Fourier transform of the cryo images, and found low order diffraction spots in 65 out of 112 images. The spacing of the low order diffraction varied in the micrographs between 54Å and 74Å, with a mean value of 64Å and standard deviation of 4Å. A histogram of the values is provided in Fig. 5. The PT surface has uniformly spaced ridges that are parallel to their long axis (Fig. 6A), and the ridges are spaced approximately 50 to 60 Å apart.
Figure 4.
CTEM polar tube images and their diffraction patterns.
A- D. Four CTEM images representative of the 100 plus samples of PTs and their accompanying diffraction patterns. The calculated Fourier transform of each image’s measured diffraction spot distances are noted on the diffraction image accompanying the CTEM image. Bar is 100nm.
Figure 5.
Histogram of the Fourier transform of the diffraction spots obtained from 65 CTEM images. The spacing of the low order diffraction varied from 54Å to 74Å, mean value of 64Å and SD of 4Å.
Figure 6.
CTEM of unstained, cryo preserved extruded Anncaliia algerae polar tubes containing cargo; consisting of membranes, cylinders, and sacs. Also note the PT surfaces covered with fibrillar and fine particulate structures.
A. Undulating or “S” shaped portion of a PT with layers of parallel alternating electron lucent and dense fine material with approximately 6–7 nm spacing between the dense layers (arrows). Bar is 50nm.
B. Organization of the PT is well preserved and multiple layers of varying densities are visible. The outermost surface edge is covered with fine fibrils (arrows). Bar is 50 nm.
C. Image of two PT segments that contain various forms of cargo. The upper tube has multiple layers of membrane-like material arranged parallel to the orientation of the tube and surrounding a narrow long cylinder (C). The lower PT contains membrane-like and tubular structures, some of which bend around cylinders (C). Bar is 50 nm.
D. Polar tube containing multiple layers of membrane–like material of varying densities some enclosing a cylinder (C). The outer-most surface edge is covered with fine fibrils Bar is 50 nm.
E. Polar tube with dense material or cylinders (C) encompassed by several membrane–like layers. Bar is 50 nm.
F. Polar tube contains a membrane enclosed sac (S) of medium dense particulate material and appears to be pinching off (broad arrows). Bar is 50 nm.
G. Polar tube containing several membrane enclosed sacs (S), of medium dense particulate material. A portion of the tube’s diameter overlaying the large sac is enlarged. The outer surface of the tube is covered with fine fibrillar material (arrows). Bar is 50 nm.
H. Polar tube containing membrane-like structures and dense material. The tube surface is covered with fine fibrils and small “clumps” of dense material at the end of some fibrils (arrows). Bar is 50nm.
I. The PT surface is covered with fine fibrils and dense particulate material (arrows). Inside the PT is a membrane limited undulating tube, giving a-tube-within-a–tube appearance similar to that observed in negative stained tube (Fig. 2.C). Bar is 50 nm.
Projection images through the tube allow one to visualize both part of the tube surface and its internal structure. Most of the PTs have an irregular medium electron dense outer coating of fibrils that abut an electron lucent layer which is parallel to the tube. Abutting this lucent layer is an electron dense layer that outlines and defines the PT itself (Fig. 6B, C).
Inside the PTs are numerous arrays of membrane–like structures in various arrangements. Some are in layers oriented parallel to the shaft (Fig. 6C, D) and others are folded upon themselves (Fig. 6E). Commonly observed structures were a series of membranes enclosing/forming cargo which contain particulate material, often bordered by membranes appearing to outline a lumen (Fig. 6C–E). Other structures inside the lumen of the PT are membrane bound sacs of particulate material (perhaps protein) (Fig. 6 F, G). Some sacs are in the process of pinching off into segments (Fig. 6F), while others contain cylinders that are large enough to distend the diameter of the tube (Fig. 6G).
The PTs varied in overall diameter from 155nm to 212nm, when measured across the outermost layers. The tubes have a fibrillar coating that ranges from 5 to 30nm thick, depending on the image angle (Fig. 3B, 6B, F–H), consequently, the diameter of the PT exclusive of the fibrillar coat varies from a narrow 100nm to 135nm to over 300nm depending on the part of the tube imaged and the cargo inside the tube. Some structures inside the PT (Fig. 6C, G) distended the tube diameter to over 220 nm (Fig. 6G). Some PTs contained structures that appeared to be tubular (Fig. 6H, I) and some were undulating inside the PT, giving a tube-within- a- tube appearance (Fig. 6I). The inner tubes are often filled with particulate material. One 175 nm wide section of PT narrowed to 130 nm, forming a “neck” and then widened to 265nm before it narrowed again (Fig. 7A). The distended tube contained a large amount of electron dense material and curved at almost a 90° angle starting at the neck and continued for over 700nm. Its sharply curved appearance suggests that it might be the beginning of the terminal “J” hooks observed in light micrographs (Fig. 1B).
Figure 7.
CTEM of the distal end of polar tubes.
A. Curved segment of what is the probable terminus or “J” hook near the end of the PT (arrow). The tube diameter is decreased along a long portion of the tube (arrowheads) and then starts to widen at the base of the curve. Electron dense (ED) material is present in the base of the tube and in the apex of the curve. Bar is 50nm.
B. End of the PT containing multiple tightly packed membranes (short arrows) that will give rise to the sporoplasm membrane “terminal sac”. Note the closed tip of the tube. Bar is 50 nm.
A long (700 nm) elliptical or “spear head” shaped section of PT containing clusters of coiled membrane-like structures was observed. The tube’s shape and contents initially suggested it might have been a portion of the sporoplasm, but careful examination indicates this is the closed tip or terminal end of the polar tube, where the membranes for the terminal sac are located. The polar tube neck was 138nm wide, expanding to 255nm then narrowing to an 85nm tip. Inside, the coiled membrane-like structures ranged in size from 105 to 150nm in diameter (Fig. 7B).
A portion of PT that progressively widened from 175nm to over 350nm encased what appeared as a membrane enclosed dense structure (Fig. 8). Additional examination revealed the presence of a 450nm long X 310nm wide ovoid structure inside the tube (Fig. 8). This sperm head–shaped structure appears to be a sporoplasm containing a round 260nm centrally located nuclear region and the outline of a diplokaryon is visible. At the posterior base of the nuclear membrane are 4–5 narrow tubular structures and in the anterior segment of cytoplasm between the nuclear membrane and the outer tip of the sporoplasm, short segments of membrane are visible (Fig. 8).
Figure 8.
Polar tube (PT) contains a membrane enclosed (arrows) sporoplasm (S) inside the tube. The oval or sperm-head shaped sporoplasm has greatly distended a portion of the tube. The sporoplasm contains medium dense material and a membrane (short arrows) encloses two nuclei in a diplokaryon (D) arrangement. The membrane enclosed nuclear region has an indentation and three or four small circular structures are abutted to it (*). Bar is 50nm.
Tomography of CTEM polar tubes.
A segment of PT containing several cylinders and membrane-like structures was observed and imaged at multiple tilt angles. The individual images were then aligned and back-projected using IMOD to produce a tomogram (Supplement 3: Amira movie of model). Manual segmentation of the PT (from outside to inside: green, purple, red, blue, yellow, and pink) and the fibrillary material on the polar tube surface (brown) was performed in Amira. The segmented structures were then overlain on top of the cryo EM image (Fig. 9A). The colors assigned to each of the structures enable one to follow that structure through the PT. Following segmentation, 3D models of the PT were produced (Fig. 9B–D) depicting the observed structures.
Figure 9. Tomogram, segmentation and 3D models of a Polar Tube.
A. Tomogram of a portion of PT containing cargo and membranes, and its surface is covered with tufts of fibrillar material. Segmentation overlay of a portion of PT tomogram (S-3). The different PT structures are color coded to the various densities visible in the stacks of images and identified by color. The tomogram was segmented and 3D models generated from it using Amira® software.
B. Amira generated 3D model of a portion of polar tube obtained from the tomogram images. The colors are assigned to different structures inside and on the surface of the polar tube. The fibril tufts are visible on the surface of the PT. Image was taken at the tilt angle of 0° degrees at Y direction.
C. Amira generated 3D model of a portion of polar tube obtained from the tomogram images. Image was taken at the tilt angle of −60° degrees at Y and Z direction.
D. Amira generated 3D model of a portion of polar tube obtained from the tomogram images. Image was taken at the tilt angle of −60° degrees at X and Y direction.
DISCUSSION
Microsporidia possess a unique discharge complex or apparatus which enables the infective sporoplasm to leave the protective spore, traverse the PT and enter a new host cell within seconds without exposure to the extracellular environment. Although it has been almost 60 years since Kramer (1960) demonstrated that the microsporidial sporoplasm was discharged from the spore through the PT and Huger (1960) published electron microscopic images of the polar filament inside a spore, many aspects of PT structure, composition, discharge, and sporoplasm transfer are still enigmatic. Herein we present new information about the extruded A. algerae PT structure and sporoplasm transfer through it. A variety of experiments and imaging methods were used to help us understand this unique process of infection and several new structural aspects of the PT. Additionally, by using a variety of imaging techniques it enables the comparison and confirmation of structures observed with the various methodologies, thus bringing this elusive process to dynamic life.
Phase contrast microscopy of live A. algerae spores in extrusion buffer, enables one to observe the activation and discharge of the PT and release of the sporoplasm. We have observed that the end of fully extruded A. algerae PT often forms a “J” or hook. High speed imaging (S1, S2) captures PT extrusion and formation of this hook and the subsequent release of a terminal sac, and sporoplasm. Figure 1D is a montage of individual high speed frames which clearly illustrates this process. In addition, during spore activation, PT extrusion, and sporoplasm release, we observe that when the sporoplasm exits the PT, its momentum pulls the tube and attached spore shell forward, straightening the tube, indicating that these components remain attached for some period of time. We concur with Frixione et al., (1992), using high speed video enhanced imaging of A. algerae extrusion, indicated that the entire event took less than 2 seconds from spore activation to sporoplasm discharge. They reported that after the complete discharge of the PT there is a 0.2 second delay for sporoplasm emergence.
Most microsporidial spores are ingested, becoming activated, and/or starting their infections in the host digestive tract, which is filled with fluids and food, and they undergo active movement of gut contents down the digestive tract. Anncaliia algerae, originally observed in the mosquito gut (Avery and Anthony, 1983; Vavra and Undeen, 1970), is subject to all of these gut dynamics, and the hooked PT terminus may act like a fish hook, preventing the attached spore and tube from dislodging before the sporoplasm is safely within the host cell. This hooking of the PT terminus was previously observed using high speed light microscopy (Frixione et al., 1992) and by TEM during glycosylation studies of A. algerae extruded PT (Xu et al. 2004). During their high- speed video study of A. algerae extrusion, Frixione et al., (1992) described the initial extrusion of the PT as helicoidal. Similarly, the PT of extruded Encephalitozoon cuniculi often curls appearing like a corkscrew (Bouzahzah et al., 2010) and the PT of E. hellem also takes on this shape when extruded (PMT & AC unpublished observations). Recently, Han et al., (2017) demonstrated a new E. hellem polar tube protein (PTP- 4) that is located at the extreme end of the polar tube just in front of the area of sporoplasm release. A PTP-4 monoclonal antibody was found to be very specific for this terminus of the polar tube and this protein appears to be important for successful infection of the host cell. This location is analogous to the A. algerae portion of the PT which bends to form a hook. Polar tube protein - 4 has also been shown to localize to the terminus of the PT in A. algerae (Weiss and Bechnel 2014). While light microscopic (LM) observations of microsporidia have contributed to our knowledge of these organisms, modern technologies are still providing new insights into their structures and mode of infection.
Transmission electron microscopic (TEM) observations of thin sections of fixed, resin embedded and heavy metal stained microsporidia has been considered the “gold standard” for studying these organisms since Huger’s initial use of TEM (Huger, 1960). Thin sections of extruded PTs usually contain a 100–120nm thick uniform tube with little variation, similar to the structure in Fig. 1E. Cross sections of PTs generally confirm a thin electron dense outer wall surrounding an empty lumen. On occasion, a portion of the PT will be noticeably extended or bulging due to the presence of membrane enclosed cargo inside the lumen, such as we see in Fig. 1F. These dense structures are often thought to be a sporoplasm or some other portion of spore contents moving through the PT. We have previously observed these bulges in the A. algerae PT have been described previously (Cali et al., 2002).
When the activated spores and their extruded PTs are rapidly fixed in < 1 min. after placement in extrusion buffer and then processed for TEM, some of the polar tube cross sections contain a lumen filled with electron dense and electron lucent concentric rings. This material is enclosed by a relatively thick electron dense matrix, all of which is surrounded by a lucent layer delineated by a thin dense ring. This ring may be the sheath described by Weidner (1972). However, the outer surface of the PT in our Fig. 1G has numerous fine radiating projections. We also observe fine fibers or fibrillar material covering the PT outermost surface as it is extruded from the anterior aperture of the spore in Fig. 1H. We have previously observed the presence of these fibers on the PT in this organism (Cali et al., 2002).
Polar tube protein composition studies of different microsporidial genera indicate that a major component of the tube is PTP-1 and it is post-translationally modified by O-linked mannosylation sites which bind concanavalin A (conA) (Bouzahzah and Weiss, 2010; Bouzahzah et al., 2010; Hayman et al., 2005; Peek et al., 2005; Polonais, et al., 2005; Southern et al., 2007). O-glycosylation of PTP-1 plays a role in the function of the PT, as this type of glycosylation can increase the stability of proteins protecting them from degradation. Glycosylation may, therefore, be important in protecting the microsporidian PT from degradation in the gastrointestinal tract of their hosts. Carbohydrate interactions may also be important in creating a “sticky” PT that adheres to as-yet-unidentified cell membrane receptors, such as mannose receptors, facilitating the penetration of the PT into its host cell suggesting that the presence of these glycoproteins on the polar tube surface are important for successful infection of the host cell (Xu and Weiss, 2005). The fibrils observed in the present study may be these glycosylated proteins on the PT surface.
On occasion, the lumen of extruded PTs contains cargo that may be arranged in concentric rings and appears to be composed of or enclosed by membranes as illustrated in Fig. 1G. Chioralia and Trammer (1998) described similar rings of membrane in a cross section of extruded A. algerae PT from the mosquito gut. These materials inside the PT may also be partially organized polar tube proteins as suggested by Weidner (1982). The presence of membranes and their relocation from the spore to the terminal tip of the extruding PT of Spraguea lophii was demonstrated by high speed imaging of Nile-red labeled membranes. Additionally, these emerging membranes, as confirmed by Weidner, formed a sac for the sporoplasm to enter after the PT extrusion was complete (Weidner, 1972; Weidner et al., 1995).
Negatively stained extruded PTs examined with TEM are very useful for observing long segments of unfixed intact tubes, their general appearance, variations in tube width, surface features, projections and/or tufts on the tube (Cali et al., 2002; Keohane et al., 1994). Weidner (1972, 1982) used TEM of negatively stained PTs to study both the composition of chemically treated tubes and the structural organization of PTP from Glugea hertwigi and Nosema michaelis. In the current study, most of the A. algerae PT segments were straight and of relatively uniform width of 115 to 145 nm which is slightly wider than the 100 to 120nm diameter observed in thin sections of resin embedded tubes. The lumen of the tubes in Fig. 2 A, B contain medium electron dense material similar in appearance to what Weidner (1982) described as PTP. The surface and edges of the PTs in our material had some variation with a uniform lumen as seen in Fig. 2 A while others had ridges or stippled material arranged in rows parallel to the long axis of the PT as in Fig. 2B. These ridges clearly demonstrate the surface folds that provide flexibility for the tube to expand during transport of the spore contents as has been previously suggested (Cali et al., 2002; Lom, 1972; Weidner 1972).
The surface of several negatively stained PT (Fig. 2A, B) have medium dense fine material on their surface which appears to be similar to those fibrils observed in Fig. 1G, H obtained from resin embedded thin sections. This fine fibrous material on the PT surface was previously observed on A. algerae PTs (Cali et. al, 2002). The fine fibers that cover the PT are often aggregated in tufts and may be sites of glycoprotein aggregates demonstrated by Weidner (1972) in N. michaelis and in A. algerae by Xu et al., (2004).
One of the internal PT structures was an undulating tube visible in our Fig. 2C. The presence of a similar structure within the extruding polar tube of N. michaelis was described as “cylinders of membrane” Weidner (1972) and as “cylinder-within-a-cylinder” and “tube-within-a-tube” in incompletely discharged G. hertwigi PTs Weidner (1982). These undulating membrane tubes were also previously observed and described as cylinders in A. algerae PT (Cali et. al, 2002).
Cryogenically preserved extruded A. algerae PT, such as observed in Fig. 3 A, appear similar to those observed by negative stain and appear as typical PTs. The cryogenically preserved PTs, however, vary in width from 155 to over 200nm, which is noticeably larger than the 100 to 120nm diameter of fixed, embedded and sectioned PTs and 115 to 145nm of negatively stained samples. The differences in diameter are probably due to the water content of the tubes and organization of PTP. Fixed and resin embedded samples require extensive dehydration in alcohols and transition into non-polar solvents before embedding, greatly reducing the water content, cross linking proteins, and removing soluble fats and lipids. The negative stained PTs are unfixed and air dried, minimally reducing their water content and alteration of proteins. The cryogenically preserved PTs are unfixed, unstained, and instantaneously preserved in a thin layer of vitreous ice, which maintains the sample in a near- natural/native form, greatly reducing any alterations or artifacts. This preservation and imaging demonstrates that the 155 to over 200nm tube diameter is probably closest to actual size. Additionally, since there is no need to fix or dehydrate, all proteins are in their native state, and could in principal be reconstructed via sub-tomogram averaging. The reconstructed tomograms presented in this work, while still at a relatively moderate resolution, present the truest reconstructions of PTs to date. Future collections on direct detectors and higher powered microscopes should reveal higher resolution details and aid in structure identification (Hutchings and Zanetti, 2018).
The polar tube protein packing and its organization of the tube, is readily visualized in Fig. 3B, 4A–D, and 6A and are similar to the PTP organization described by Weidner, (1972; 1982) and Weiss et al. (2014). The diffraction spots for most images, are spread out into layer lines, consistent with a helical symmetry, indicating the lattice comes from a protein curved around the tube. In a few cases, the tubes seemed to be flattened as the diffraction spots appeared as points rather than lines. We attempted to do helical processing of the layer lines of the better ordered tubes, as in (Diaz et al., 2010), but the phase residuals were very high no matter what helical symmetry was tried, indicating that the lattice is not well ordered. It is not clear what protein is contributing to the scattering, but presumably it is highly packed within the tube. Perhaps the lattice is important for maintaining the rigidity of the tubes.
Although most segments of the PT are straight or slightly curved, the tube in Fig. 6A appears to be undulating and its surface is composed of regularly spaced ridges. Frixione et al., (1992) demonstrated that A. algerae PTs twist in a helical manner as they are in the process of extruding and become straight after they are fully extruded. This suggests that the tube in Fig. 6A was preserved while extruding.
The observed surface ridges and furrows are in agreement with those reported previously (Cali, et al., 2002) and to those present in the negatively stained PT in Fig. 2B and the cryopreserved tube in Fig. 3A, B. The 50 to 60Å spacing between the ridges suggests its organization forms “pleats” that allow the tube diameter to rapidly expand during the transport of spore contents and the sporoplasm into a new host cell. This flexibility of the PT appears to be important during the high increase in osmotic pressure produced by the bulk flow of fluids into the spore by aquaporins (Ghosh, et al., 2012) and the subsequent transport of the sporoplasm during the extrusion process.
The ability to penetrate the PT surface and observe its lumen and the cargo being transported within it is one of the benefits of CryoTransmission Electron Microscopy (CTEM). In Fig. 6B – G we were able to identify numerous membranous structures inside the polar tube. These structures varied from membrane clusters Fig. 6C to cylinders Fig. 6C–E, and sacs Fig. 6F, G. Weidner (1972, 1982) described amorphous material and structures he identified as cylinders inside extruded PTs that were negatively stained. Our CTEM images illustrate the cargo transiting through the PT during extrusion is composed of various structures containing diverse material. The clusters of membranes in Fig. 6C–E are possibly destined to line the inner surface of the PT and for the sporoplasm complex. The various sacs Fig. 6F, G, and cylinders Fig. 6C–E appear to contain particulate material, possibly proteins for the extension of the PT as proposed by Weidner (1982) and Weidner et al., (1995). Unfortunately, microsporidial spore structures processed for CTEM can’t be immunogold labeled, as is usually used for PTP identification (Keohane et al., 1994; 1996; Keohane and Weiss 1999). The presence of a tube or lumen inside the PT (Fig. 6 H, I) has also been observed when negatively stained (Cali et al., 2002; Weidner, 1972; 1992) and in our Fig. 2C. The presence of this membrane lined tube enables unobstructed passage of materials through the PT, from spore to its terminus.
Frixione et al., (1992) and Xu et al., (2004) demonstrated that the A. algerae PT curls into a hook at its terminus and Fig. 1B and montage 1D clearly show this process. During our CTEM studies, the presence of the PT curving to form a hook was observed. In Fig. 7A, the PT narrows to 130 nm, forming a curved “neck” and then widens to 265nm. The apex of the hook contains membranous material and an ED spherical structure. There has been some speculation about whether the terminus of the PT should be open or closed. High speed videos (S1, 2) of extruding PTs suggests that the tube was closed and that the observed slowdown of the sporoplasm just before it emerged from the tube was due to the forced opening of the terminus. The subsequent release of membranes to house the sporoplasm, followed by the emergence of the sporoplasm proper, from the PT into its membrane complex was initially suggested by Frixione et al. (1992). Chioralia et al., (1998) conducted an ultrastructural study of A. algerae extrusion and proposed that the extruding PT is lined with membranes that originate in the polaroplast. Our Fig. 7B is an image of the PT terminus, the neck is 138nm wide, expanding to 255nm then narrowing to an 85nm tip. The wide section of the tube contains masses of tightly folded or stacked membranes that appear to be arranged to unfold outward once the tip is opened. Our image confirms the presence of a sealed tip and masses of folded membranes in the PT terminus, which agrees with the interpretation of Weidner et al., (1984) and Frixione et al., (1992).
A number of studies have suggested that when a bulge in the PT was observed, it was probably the sporoplasm passing through that portion of the tube. As we see in our CTEM Fig. 6 C, G, a number of objects can distend the PT. Chioralia et al., (1998) suggest that the sporoplasm’s diplokaryon transits the PT after the sporoplasm has been modified “liquefied” so that it may rapidly pass through the extruded tube. In our Fig. 8, we present the first CTEM image of an intact membrane enclosed sporoplasm transiting through the PT. The presence of the relatively large sporoplasm caused the tube to expand. The ovoid or “sperm head” shaped sporoplasm was over 300nm in diameter and almost 450nm in length. Inside the membrane enclosed structure (sporoplasm) was a fair amount of amorphous material, some membrane segments, and 4–5 circular or tube-like structures. The sporoplasm contains two membrane encased dense structures, which appear to be the nuclei in a diplokaryotic arrangement, typical of A. algerae.
Based on our CTEM images of the extruded PT and its contents, we demonstrate that the PT is composed of tightly packed PTP arranged in a helical orientation. The entire PT surface is ridged with 5–6nm spacing between each ridge, enabling the PT to rapidly increase its diameter to facilitate the passage of the various cargo including the intact sporoplasm through the tube. In addition, the PT surface is covered with fine fibrillar material that projects as far as 17nm from the surface and these fibers are often organized in clusters or tufts. It is suggested that these fibrils may be the sites of modified glycoproteins (Xu and Weiss, 2005) which provide protection to the PT and aid in infectivity. The interior or lumen of the tube is lined with membrane and may contain cargo that includes; membrane bound cylinders, sacs or vesicles filled with particulate material, and an undulating membrane tube that gives the PT a tube-within-a-tube appearance. This membrane bound lumen facilitates passage of cargo and the sporoplasm through the PT.
The tomogram of a portion of the PT (Fig. 9A), its segmentation, and 3D rendering Fig. 9B–D), enable us to observe each of the various structures that are inside the tube and their relationship to each other. Supplement 3 depicts several aligned CTEM images from the tomogram overlain by the 3D structural rendering of the various structural elements observed inside the PT and on its surface. The PT distal end can be identified by its narrowing and curving into the start of a hook. The terminus of the tube is closed and filled with tightly folded membranes arranged so they can exit to form the membrane sac into which the sporoplasm will be expelled as it exits the tube.
The image of a sperm-head-like shaped membrane bound sporoplasm, containing cytoplasm, and its membrane enclosed nuclei in a typical A. algerae diplokaryotic arrangement while inside the PT, dispels any theories about decompartmentalization or modification by liquefaction of the sporoplasm cytoplasm. In addition, this ultrastuctural image of the artifact free, cryopreserved sporoplasm proves that it leaves the spore and traverses the PT as a fully intact membrane bound cellular entity, during extrusion.
CONCLUSION
This report utilizes and compares many different processing and imaging methodologies to study the extruded polar tube of A. algerae. Additionally, 3D computer generated models of the PT enable one to better understand the structures both inside the PT and on its surface. Although this is the first application of cryo-transmission electron microscopy to study cryo-preserved PT structure, similar observations by traditional methodologies in different genera, confirms the universality of the microsporidial infection apparatus. We believe that the current report and our ongoing research, utilizing a diversity of tools and technologies have enabled us to interpret and confirm the various structures and processes of microsporidial PT extrusion.
This study also represents our commitment to employ both new and traditional methodologies to study the structural and functional components of the microsporidial spore.
Supplementary Material
Video S1. High speed video of a germinating spore, from which selected frames are presented in Fig. 1D. The video captures the extrusion of the approximately 90μm long PT and the exit of the sporoplasm.
Video S2. This high speed video of a germinating spore, illustrates the force generated by the exiting of the sporoplasm and its contents from the spore/PT causing an obvious lurch of the attached spore shell.
Video S3. Movie of a tomogram of the PT containing cargo, including internal membranous structures, the PT surface and its fibrillar tufts, was segmented and a 3D model generated in Amira and an overlay outlines the PT structures as illustrated in Fig. 9A.
ACKNOWLEDGMENTS
This work was supported by NIH Grants AI091985 (AC) and AI124753 (LMW)
All of the standard transmission electron microscopy was conducted at the Rutgers Newark Electron Microscopy Facility. The high speed light microscopy, initial CTEM feasibility experiments, methodologies, and imaging were conducted at the Albert Einstein Analytical Imaging Facility which is supported in part by: NCI Cancer Center Support Grant P30CA013330 and shared instrumentation grant 1S10OD016214–01A1. Some of this work was performed at the Simons Electron Microscopy Center and National Resource for Automated Molecular Microscopy located at the New York Structural Biology Center, supported by grants from the Simons Foundation (SF349247), NYSTAR, and the NIH National Institute of General Medical Sciences (GM103310). We also thank Michael Cammer for his assistance with the high speed light microscopy and computer processing of the video.
REFERENCES
- Avery SW, and Anthony DW. 1983. “Ultrastructural Study of Early Development of Nosema Algerae in Anopheles Albimanus.” J Invertebr Pathol 42: 87–95. [DOI] [PubMed] [Google Scholar]
- Becnel JJ, Takvorian PM, and Cali A. 2014. “Checklist of Available Generic Names for Microsporidia with Type Species and Type Hosts” In Microsporidia Pathogens of Opportunity, ed. Weiss LM and Becnel JJ : Wiley Blackwell Press; 671–86. [Google Scholar]
- Bouzahzah B, Nagajyothi F, Ghosh K, Takvorian PM, Cali A, Tanowitz HB, and Weiss LM. 2010. “Interactions of Encephalitozoon Cuniculi Polar Tube Proteins.” Infect Immun 78: 2745–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzahzah B, and Weiss LM. 2010. “Glycosylation of the Major Polar Tube Protein of Encephalitozoon Cuniculi.” Parasitol Res 107: 761–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan RT, Cali A, Owen RL, and Spencer HC. 1991. Microsporidia: Opportunistic Pathogens in Patients with Aids Edited by Sun Tsieh. Vol. 2, Progress in Clinical Parasitology. New York: Field & Wood Medical Publishers. [PubMed] [Google Scholar]
- Cali A, Becnel JJ, and Takvorian PM 2017. “Microsporidia” In Handbook of the Protists (Ebook), eds. Archibald John M., Simpson Alastair G.B., Slamovits Claudio H., Margulis Lynn, Melkonian Michael, Chapman David J. and Corliss John O.: Springer International Publishing. [Google Scholar]
- Cali A, Neafie R, Weiss LM, Ghosh K, Vergara RB, Gupta R, and Takvorian PM. 2010. “Human Vocal Cord Infection with the Microsporidium Anncaliia Algerae.” J Euk Microbiol 57: 562–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cali A, and Takvorian PM. 2001. “Brachiola Algerae Sporoplasms.” J Euk Microbiol 48: 81S–82S. [DOI] [PubMed] [Google Scholar]
- Cali A, and Takvorian PM. 2014. “Developmental Morphology and Life Cycles of the Microsporidia” In Microsporidia-Pathogens of Opportunity, ed. Weiss LM and Becnel JJ, 2 ed. 1 vols: Iowa, USA: Wiley Blackwell; 71–133. [Google Scholar]
- Cali A, Takvorian PM, Lewin S, Rendel M, Sian CS, Wittner M, Tanowitz HB, Keohane E, and Weiss LM. 1998. “Brachiola Vesicularum, N. G., N. Sp., a New Microsporidium Associated with Aids and Myositis.” J Euk Microbiol 45: 240–51. [DOI] [PubMed] [Google Scholar]
- Cali A, Weiss LM, and Takvorian PM. 2002. “Brachiola Algerae Spore Membrane Systems, Their Activity During Extrusion, and a New Structural Entity, the Multilayered Interlaced Network, Associated with the Polar Tube and the Sporoplasm.” J Euk Microbiol 49: 164–74. [DOI] [PubMed] [Google Scholar]
- Chioralia G, Trammer T, Maier WA, and Seitz HM. 1998. “Morphologic Changes in Nosema Algerae (Microspora) During Extrusion.” Parasitol Res 84: 123–31. [DOI] [PubMed] [Google Scholar]
- Coyle C, Weiss LM, Rhodes LV, Cali A, Takvorian PM, Brown DF, Visvesvara G, Xiao L, Naktin J, Young E, Gareca M, Colasante G, and Wittner M. 2004. “Fatal Myostitis Due to the Microsporidian Brachiola Algerae, a Mosquito Pathogen.” N Engl J Med 351: 42–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz R, Rice WJ, and Stokes DL. 2010. “Fourier-Bessel Reconstruction of Helical Assemblies.” Methods Enzymol 482: 131–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangakis AS, and Hegerl R. 2001. “Noise Reduction in Electron Tomographic Reconstructions Using Nonlinear Anisotropic Difusion.” J Struct Biol 135: 239–50. [DOI] [PubMed] [Google Scholar]
- Franzen C. 2005. “How Do Microsporidia Invade Cells?”. Folia Parasitologica 52: 36–40. [DOI] [PubMed] [Google Scholar]
- Franzen C, Nassonova ES, Schölmerich J, and Issi IV. 2006. “Transfer of the Members of the Genus Brachiola (Microsporidia) to the Genus Anncaliia Based on Ultrastructural and Molecular Data.” J Euk Microbiol 53: 26–35. [DOI] [PubMed] [Google Scholar]
- Frixione E, Ruiz L, Santillan M, DeVargas LV, Tejero JM, and Undeen AH. 1992. “Dynamics of Polar Filament Discharge and Sporoplasm Expulsion by Microsporidian Spores.” Cell Motil Cytoskeleton 22: 38–50. [Google Scholar]
- Frixione E, Ruiz L, Santillan M, Vega L, Tejero JM, and Undeen AH. 1996. “Dynamics of the Polar Filament Discharge in the Microsporidian Nosema Algerae, Analyzed by Electronically Enhanced Contrast Video-Microscopy.” Cell Motil Cytoskeleton 132: 489–92. [Google Scholar]
- Ghosh K, and Nieves E, Keeling P, Pombert JF, Henrich PP, Cali A, and Weiss LM 2012. “Branching Network of Proteinaceous Filaments within the Parasitophorous Vacuole of Encephalitozoon Cuniculi and Encephalitozoon Hellem.”. Microbes Infect. 14: 324–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B, Polonais V, Sugii T, Yakubu R, Takvorian PM, Cali A, Maier K, Long M, Levy M, Tanowitz H, Pan G, Delbac F, Zhou Z, and Weiss LM. 2017. “The Role of Microsporidian Polar Tube Protein 4 (Ptp4) in Host Cell Infection.” PLoS Pathog 13: 1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman JR, Southern TR, and Nash TE. 2005. “Role of Sulfated Glycans in Adherence of the Microsporidian Encephalitozoon Intestinalis to Host Cells in Vitro.” Infect Immun 73: 841–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchings J and Zanetti G, 2018. Fine details in complex environments: the power of of cryo-electron tomography. Biochem. Soc. Trans June 22, 2018, BST20170351; DOI: 10.1042/BST20170351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keohane E, Takvorian PM, Cali A, Tanowitz HB, Wittner M, and Weiss LM. 1994. “The Identification and Characterization of a Polar Tube Reactive Monoclonal Antibody.” J Euk Microbiol 41: 48S. [PubMed] [Google Scholar]
- Keohane EM, Orr GA, Takvorian PM, Cali A, Tanowitz HB, Wittner M, and Weiss LM. 1999. “Polar Tube Proteins of Microsporidia of the Family Encephalitozoonidae.” J Euk Microbiol 46: 1–5. [DOI] [PubMed] [Google Scholar]
- Keohane EM, and Weiss LM. 1998. “Characterization and Function of the Microsporidian Polar Tube: A Review.” Folia Parasitol (Praha) 45: 117–27. [PubMed] [Google Scholar]
- Keohane EM, and Weiss LM. 1999. “The Structure, Function, and Composition of the Microsporidian Polar Tube” In The Microsporidia and Microsporidiosis, ed. Wittner M. Washington, D. C.: ASM Press; 196–224. [Google Scholar]
- Kramer JP 1960. “Observations on the Emergence of the Microsporidian Sporoplasm.” J Insect Pathol 2: 433–39. [Google Scholar]
- Kremer JR, Mastronarde DN, and McIntosh JR. 1996. “Computer Visualization of Three-Dimensional Image Data Using Imod. “ J Structural Biology 116: 71–76. [DOI] [PubMed] [Google Scholar]
- Leitch GL, He Q, Wallace S, and Visvesvara GS. 1993. “Inhibition of the Spore Polar Filament Extrusion of the Microsporidium, Encephalitozoon Hellem, Isolated from an Aids Patient.” J Euk Microbiol 40: 711–17. [DOI] [PubMed] [Google Scholar]
- Lowman PM, Takvorian PM, and Cali A. 2000. “The Effects of Elevated Temperatures and Various Time-Temperature Combinations on the Development of Brachiola (Nosema) Algerae N. Comb. In Mammalian Cell Culture.” J Euk Microbiol 47: 221–34. [DOI] [PubMed] [Google Scholar]
- Lucic V, Rigort A, and Baumeister W. 2013. “Cryo-Electron Tomography: The Challenge of Doing Structural Biology in Situ.” J Cell Biol 202: 407–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margileth M, Strano AJ, Chandra R, and Neifie R. 1973. “Disseminated Nosematosis in an Immunolgically Compromised Infant.” Arch Pathol 95: 145–50. [PubMed] [Google Scholar]
- Mastronarde DN 2005. “Automated Electron Microscope Tomography Using Robust Prediction of Specimen Movements.” J Struct Biol 152: 36–51. [DOI] [PubMed] [Google Scholar]
- Mastronarde DN 1997. “Dual- Axis Tomography: An Approach with Alignment Methods That Preserve Resolution “. J Struct Biol 120: 343–52. [DOI] [PubMed] [Google Scholar]
- Milne J, Borgnia MJ, Bartesaghi A, Tran EEH, Earl LA, Schauder DM, Lengyel J, Pierson J, Patwardhan A, and Subramaniam S. 2013. “Cryo‐Electron Microscopy – a Primer for the Non‐Microscopist.” FEBS J 280: 28–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan SR, Rumney RL, Vo NTK, Bols NC, and Lee LEJ. 2011. “In Vitro Growth of Microsporidia Anncaliia Algerae in Cell Lines from Warm Water Fish.” In Vitro Cell Dev Biol Anim 47: 104–13. [DOI] [PubMed] [Google Scholar]
- Ohshima K 1937. “On the Function of the Polar Filament of Nosema Bombycis.” Parasitology 29: 220–24. [Google Scholar]
- Peek R, Delbac F, Speijer D, Polonais V, Greve S, Bonnema EW, Ringrose J, and van Gool T. 2005. “Carbohydrate Moieties of Microsporidian Polar Tube Proteins Are Targeted by Immunoglobulin G in Immunocompetent Individuals.” Infect Immun 73: 7906–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polonais V, Prensier G, Metenier G, Vivares C, and Delbac F. 2005. “Microsporidian Polar Tube Proteins: Highly Divergent but Closely Linked Genes Encode Ptp1 and Ptp2 in Members of the Evolutionarily Distant Antonospora and Encephalitozoon Groups.” Fungal Genet Biol 42: 791–803. [DOI] [PubMed] [Google Scholar]
- Santiana M, Takvorian PM, Altan-Bonnet N, and Cali A. 2015. “A Novel Fluorescent Labeling Method Enables Monitoring of Spatio-Temporal Dynamics of Developing Microsporidia “. J Euk Microbiol 63: 318–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern TR, Jolly CE, Lester Melissa E., and Hayman JR. 2007. “Enp1, a Microsporidian Spore Sall Protein That Enables Spores to Adhere to and Infect Host Cell in Vitro.” Eukaryot Cell 6: 1354–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutrave G, Maundrell A, Keighley C, Jennings Z, Brammah S, Wang M, Pamphlett R, Webb CE, Stark D, Englert H, Gottlieb D, Bilmon I, and Watts MR. 2018. “Anncaliia Algerae Microsporidial Myositis, New South Wales, Australia.” Emerg Infect Dis 24: 1528–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takvorian PM, Buttle KF, Mankus D, Mannella CA, Weiss LM, and Cali A. 2013. “The Multilayered Interlaced Network (Min) in the Sporoplasm of the Microsporidium Anncaliia Algerae Is Derived from Golgi.” J Euk Microbiol 60: 166–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takvorian PM, and Cali A. 1986. “The Ultrastructure of Spores (Protozoa: Microspora) from Lophius Americanus, the Angler Fish.” J Protozool 33: 570–75. [DOI] [PubMed] [Google Scholar]
- Thelohan P 1894. “Sur La Presence D’une Capsule a Filament Dans Les Spores Des Microsporidies.” C R Acad Sci 118 1425–27. [Google Scholar]
- Undeen AH, and Maddox JV. 1973. “The Infection of Nonmosquito Hosts by Injection with Spores of the Microsporidian Nosema Algerae.” J Invertebr Pathol 22: 258–65. [DOI] [PubMed] [Google Scholar]
- Vavra J 1976. “Structure of the Microsporidia” In Biology of the Microsporidia eds. Vavra J and Sprague V. Vol. 1, Comparative Pathobiology New York: Plenum Press; 1–85. [Google Scholar]
- Vavra J, and Larsson JIR. 1999. “Structure of the Microsporidia” In The Microsporidia and Microsporidiosis, eds. Wittner M and Weiss LM. Washington, D. C.: ASM Press; 7–84. [Google Scholar]
- Vavra J, and Larsson JIR. 2014. “Structure of the Microsporidia” In Microsporidia Pathogens of Opportunity, ed. Weiss LM and Becnel JJ 1 vols. Vol. 1 Iowa, USA: Wiley Blackwell; 1–70. [Google Scholar]
- Vavra J, and Undeen AH. 1970. “Nosema Algerae N. Sp. (Cnidospora, Microsporida) a Pathogen in a Laboratory Colony of Anopheles Stephensi Liston (Diptera, Culicidae).” J Protozool 17: 240–49. [DOI] [PubMed] [Google Scholar]
- Weber R, Bryan RT, Schwartz DA, and Owen RL. 1994. “Human Microsporidial Infections.” Clin Microbiol Rev 7: 426–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner E 1982. “The Microsporidian Spore Invasion Tube. Iii. Tube Extrusion and Assembly.” J Cell Biol 93: 976–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner E 1982. 1976. “The Microsporidian Spore Invasion Tube. The Ultrastructure, Isolation, and Characterization of the Protein Comprising the Tube.” J Cell Biol 71: 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner E 1972. “Ultrastructural Study of Microsporidian Invasion into Cells.” Z Parasitenk 40: 227–42. [DOI] [PubMed] [Google Scholar]
- Weidner E, and Byrd W. 1982. “The Microsporidian Sporeinvasion Tube. Ii. Role of Calcium in the Activation of Invasion Tube Discharge.” J Cell Biol 93: 970–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner E, Byrd W, Scarborough A, Pleshinger J, and Sibley D. 1984. “Microsporidian Spore Discharge and the Transfer of Polaroplast Organelle Membrane into Plasma Membrane.” J Protozool 31: 195–98. [Google Scholar]
- Weidner E, Manale SB, Halonen SK, and Lynn JW. 1995. “Protein-Membrane Interaction Is Essential to Normal Assembly of the Microsporidian Spore Invasion Tube.” Biol Bull 188: 128–35. [DOI] [PubMed] [Google Scholar]
- Weiss LM 2014. “Clinical Syndromes Associated with Microsporidiosis” In Microsporidia Pathogens of Opportunity, ed. Weiss LM and Becnel JJ 1 vols. Vol. 1 Iowa, USA: Wiley Blackwell; 371–401. [Google Scholar]
- Weiss LM, and Becnel JJ, eds. 2014. Microsporidia-Pathogens of Opportunity. 2 ed: Iowa, USA: Wiley Blackwell; 1–709. [Google Scholar]
- Weiss LM, Delbac F, Hayman JR, Pan G, Dang X, and Zhu Z. 2014. “The Microsporidian Polar Tube and Spore Wall” In Microsporidia Pathogens of Opportunity: Vol. 1 Iowa, USA: Wiley Blackwell; 261–306. [Google Scholar]
- Xu Y, Takvorian PM, Cali A, Orr G, and Weiss LM. 2004. “Glycosylation of the Major Polar Tube Protein of Encephalitozoon Hellem, a Microsporidian Parasite That Infects Humans.” Infect Immun 72: 6341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Takvorian PM, Cali A, and Weiss LM. 2003. “Lectin Binding of the Major Polar Tube Protein (Ptp1) and Its Role in Invasion.” J Euk Microbiol Supplement to vol 50: 600–01. [DOI] [PubMed] [Google Scholar]
- Xu Y, and Weiss LM. 2005. “The Microsporidian Polar Tube: A Highly Specialised Invasion Organelle.” Int J Parasitol 35: 941–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. High speed video of a germinating spore, from which selected frames are presented in Fig. 1D. The video captures the extrusion of the approximately 90μm long PT and the exit of the sporoplasm.
Video S2. This high speed video of a germinating spore, illustrates the force generated by the exiting of the sporoplasm and its contents from the spore/PT causing an obvious lurch of the attached spore shell.
Video S3. Movie of a tomogram of the PT containing cargo, including internal membranous structures, the PT surface and its fibrillar tufts, was segmented and a 3D model generated in Amira and an overlay outlines the PT structures as illustrated in Fig. 9A.