Abstract
Environmental cues can become predictors of food availability through Pavlovian conditioning. Two forebrain regions important in this associative learning are the basolateral amygdala (BLA) and medial prefrontal cortex (mPFC). Recent work showed the BLA-mPFC pathway is activated when a cue reliably signals food, suggesting the BLA informs the mPFC of the cue’s value. The current study tested this hypothesis by altering the value of two food cues using reversal learning and illness-induced devaluation paradigms. Rats that received unilateral excitotoxic lesions of the BLA and mPFC contralaterally placed, along with ipsilateral and sham controls, underwent discriminative conditioning, followed by reversal learning, and then devaluation. All groups successfully discriminated between two auditory stimuli that were followed by food delivery (CS+) or not rewarded (CS−), demonstrating this learning does not require BLA-mPFC communication. When the outcomes of the stimuli were reversed, the rats with disconnected BLA-mPFC (contralateral condition) showed increased responding to the CSs, especially to the rCS+ (original CS−) during the first session, suggesting impaired cue memory recall and behavioral inhibition compared to the other groups. For devaluation, all groups successfully learned conditioned taste aversion; however, there was no evidence of cue devaluation or differences between groups. Interestingly, at the end of testing, the non-devalued contralateral group was still responding more to the original CS+ (rCS−) compared to the devalued contralateral group. These results suggest a potential role for BLA-mPFC communication in guiding appropriate responding during periods of behavioral flexibility when the outcomes, and thus the values, of learned cues are altered.
Keywords: Basolateral Amygdala, Medial Prefrontal Cortex, Appetitive Learning, Reversal Learning, Devaluation
Introduction
Behavioral flexibility is the ability to appropriately alter one’s behavior in response to an environmental change (Brown & Tait, 2014). To respond appropriately, the subject must recall previously learned information about available cues and integrate that memory with current environmental demands. One example of adaptive learning and behavioral flexibility is attending to and deciphering between cues that signal the availability of food. The current study examined if communication between the basolateral area of the amygdala (BLA) and medial prefrontal cortex (mPFC) underlies behavioral flexibility when there is a change in the value of food and its associated cues.
Using various paradigms, a plethora of studies have shown the BLA is critical for appetitive associative learning (for review, see Wassum & Izquierdo, 2015). The anterior part of the BLA is the first forebrain region activated during cue-food learning (Cole et al., 2013). Interestingly, even though the BLA is activated early during learning, it does not appear to be essential for the acquisition of appetitive conditioning (Hatfield et al., 1996; Holland et al., 2002; Parkinson et al., 2000; Balleine et al., 2003; Corbit & Balleine, 2005); instead, it is needed when the acquired information supports new learning (Hatfield et al., 1996; Schoenbaum et al., 1999; Blundell et al., 2001; Setlow et al., 2002; Nomura et al., 2004; Corbit & Balleine, 2005; Ishikawa et al., 2008; for reviews, see Holland et al., 2001, 2002; Everitt et al., 2003; Holland & Petrovich 2005, Petrovich, 2013; Wassum & Izquierdo, 2015). In particular, the BLA is necessary for driving behavioral responding when the value of the cue changes. The BLA neurons respond to cue value alterations (Tye et al., 2010) and are necessary for successful reversal learning (Schoenbaum et al., 2003; Churchwell et al., 2009) and cue value updating during devaluation (Hatfield et al., 1996; Balleine et al., 2003; Corbit & Balleine, 2005; Ostlund & Balleine, 2008; Coutureau et al., 2009; Johnson et al., 2009; Parkes & Balleine, 2013).
The mPFC has topographically organized, reciprocal connections with the BLA (Gabbott et al., 2005; Hoover & Vertes, 2007; for review see Reppucci & Petrovich, 2016). The mPFC is critical in adaptive behaviors and executive control (Dalley et al., 2004; O’Doherty, 2011), as well as control of feeding behavior (Mena et al., 2011, 2013; Land et al., 2014; Petrovich et al., 2007; Blasio et al., 2014). Two sub-regions of the mPFC, the prelimbic (PL) and infralimbic (ILA) areas, are activated during cue-food learning (Burgos-Robles et al., 2013; Cole et al., 2015a; Moorman & Aston-Jones, 2015; Warren et al., 2016), and BLA neurons that directly communicate with the mPFC are activated when cue-food associations are well-learned, suggesting that the BLA can inform the mPFC of the associative value of a conditioned cue (Keefer & Petrovich, 2017). In turn, the mPFC is important for altering behavioral responding during satiety-induced devaluation (Coutureau et al., 2009), suggesting the mPFC may be needed to integrate previously learned information with current cue value to appropriately alter behavior.
Therefore, the current study investigated if communication between the BLA and mPFC is necessary in appetitive Pavlovian discriminative cue learning and behavioral flexibility when learned information about the cues is altered. We abolished communication between the BLA and mPFC using a contralateral disconnection approach. This method lesions the BLA in one hemisphere and the mPFC in the other hemisphere, functionally disconnecting communication between the structures in both hemispheres but allowing one of each region to interact within other circuitries. We assessed flexibility using two well-known paradigms, reversal learning and illness-induced devaluation, to observe behavioral changes when the outcomes, and thus the values, of previously learned food cues are altered. We hypothesized disconnection of the BLA-mPFC circuitry would not alter the initial discriminative conditioning since these structures are not essential for the initial learning of cue-food associations, but it would interfere with cue value updating during reversal learning and devaluation.
Materials and Methods
Subjects
Experimentally naïve, male Long-Evans rats (250–275 g upon arrival) were obtained from Charles Rivers Laboratories and individually housed and maintained on a 12-hour light/dark cycle (lights on at 07:00). For one week, rats had ad libitum access to food (standard laboratory chow) and water and were acclimated to the colony room and experimenter handling prior to surgical and behavioral procedures. All procedures were in accordance with the National Institutes of Health Guidelines for Care and Use of Laboratory Animals and approved by the Boston College Animal Care and Use Committee.
Surgical Procedures
Under aseptic conditions, subjects were briefly anesthetized with isoflurane gas and given a mixture (1 ml/kg bodyweight; intramuscularly) of ketamine (50 mg/ml) and xylazine (10 mg/ml) prior to stereotaxic frame (Kopf Instruments) placement. Rats received a unilateral 0.3 μl injection into mPFC (coordinates from bregma: anteroposterior, +3.0mm; mediolateral, ±0.7mm; dorsoventral, −4.5mm) and two 0.1 μl injections into either the contralateral or ipsilateral BLA depending on group allocation (coordinates from bregma: anteroposterior, −2.3–2.5mm; mediolateral, ±5.0mm; dorsoventral, −8.4–8.7mm) using a 1 μl 32-gauge Hamilton Neuros syringe driven by a Quintessential Stereotaxic Injector (Stoelting) at a rate of 0.1 μl/min. Excitotoxic lesions were induced by injecting 0.15M N-methyl-D-asparatate (NMDA; Sigma-Aldrich) in a phosphate buffered saline solution (PBS), whereas sham lesions received contralateral injections of PBS alone. The syringe was left in place for 4 min after each infusion to allow for diffusion of solution. After infusions, the scalp was clipped closed and coated with triple antibiotic cream, and rats were given chewable Rimadyl tablets (Bio Serv, Frenchtown, NJ; 2 mg/1 tablet/100 g bodyweight) for two days following surgery. Rats recovered for at least one week prior to behavioral testing and were monitored and weighed daily.
Apparatus
All habituation and training occurred within the same set of behavioral chambers (30 × 28 × 30 cm; Coulbourn Instruments) located in a separate room from where the colony was housed. The chambers had aluminum top and sides, and one side contained a recessed food cup (3.2 × 4.2 cm). The front and back of the chambers were clear Plexiglas with a hinged front. The floor had stainless-steel rods 5 mm wide and 15 mm apart. Each chamber was contained within an isolation cubicle (79 × 53 × 53 cm; Coulbourn Instruments) composed of monolithic rigid foam walls and a ventilation fan (55 dB). Video cameras were attached to the rear wall of each isolation cubicle and connected to a recording system (Coulbourn Instruments).
The conditioned stimuli (CSs) were a 10s 75dB, 2kHz tone and a 10s 75dB white noise. The unconditioned stimulus (US) was two food pellets (formula 5TUL, 45 mg: Test Diets), delivered into the food cup of each chamber. GraphicState 3.0 software system (Coulbourn Instruments) controlled all stimuli.
Behavioral Training Procedure
Experimental design is outlined in Table 1. Rats were food restricted to maintain 85% of their post-surgery recovery body weight throughout training. A day prior to training, all rats received a 30 min habituation session to the behavioral chambers without stimuli, and then received 1g of the US in their home cage to familiarize them with the pellets.
Table 1.
Experiment Design.
| Discriminative Conditioning | Reversal Learning | Devaluation |
||
|---|---|---|---|---|
| CTA (x2) | Tests | |||
| CS+ ➔ US | ➔ | rCS− ➔ (nothing) | Devalued: US ➔ LiCl | rCS+ |
| CS− ➔ (nothing) | ➔ | rCS+ ➔ US | rCS− | |
| Non-devalued: US; LiCl | ||||
CS: Conditioned Stimulus (tone or white noise); rCS: Reversal Conditioned Stimulus; US: Unconditioned Stimulus (food pellets); LiCl: Lithium Cloride injection (i.p.)
Arrows between Discriminative Conditioning and Reversal Learning columns indicate same CS used but outcome changed
Discriminative Conditioning
Rats received ten 30 min training sessions with two cues, and each session consisted of six intermixed presentations of each cue. One auditory cue (CS+; e.g. white noise) was immediately followed by the delivery of the US, two palatable food pellets, and the other cue (e.g. tone) was presented alone (CS−). Cues that served as the CS+ and CS− were counterbalanced, and the inter-trial intervals (ITIs) were between 60–219s. ITIs and CS order varied across training sessions.
Reversal Learning
Following successful discriminative conditioning, reversal learning commenced for 15 sessions. Reversal sessions were similar in length and number of CS presentations as during discriminative conditioning; however, the outcomes of the CS+ and CS− were reversed. The previous CS+ was not followed by the delivery of food and referred to as the reversal CS− (rCS−), and the previous CS− was followed by the delivery of food and referred to as the reversal CS+ (rCS+).
Devaluation
Conditioned Taste Aversion
Following reversal learning, half of each group received conditioned taste aversion (CTA) in 2 trials over 4 days. All rats were given access to 5g of the US in a ceramic dish in their home cage for 10 min (a measure of baseline consumption), and then half of the rats received a LiCl injection (0.3M LiCl in 0.9% sterile saline; 5 ml/kg; i.p.) to induce malaise immediately following access to the US (Devalued group). The control, Non-devalued group received the same LiCl injections, but 24 hours after US access (in order to not form the US-illness association). Twenty-four hours later the same procedure was repeated to induce stronger CTA. Rats were tested for US consumption the day following each CTA session (consumption test 1 and 2).
CS Devaluation Testing
Subsequently, rats received eight sessions with six presentations of each CS alone, without the US, to test if their responding to the CSs changed due to CTA procedures. We assessed behavioral responding for eight sessions to examine extinction recall and updating of the cues’ values.
Final Consumption Test
Following CS testing, all rats underwent another consumption test with the US in their home cage to confirm that the CTA memory remained intact throughout testing.
Behavioral Measures
The primary measure of learning was conditioned responding to the food cup (“food cup behavior”) during the presentations of the CSs. Food cup behavior refers to the rats standing in front of and directly facing the food cup or demonstrating distinct nose pokes into the food cup. Trained observers unaware of group allocation recorded rats’ behavior every 1.25s during each 10s CS and during the 10s prior to the onset of the CS as a measure of baseline responding (pre-CS). The total number of identified food cup observations during each period was summed and converted to a percentage of total time. Specific learned responding (Elevation) was calculated by subtracting baseline responding during the pre-CS period from responding during the CS for both CS+ and CS−, which was used to calculate the mean value for each group.
For CTA, the total amount of the US consumed during each consumption test was measured in grams and averaged for each group.
Histological Procedures and Immunohistochemistry
After behavioral testing, rats were perfused, and brain tissue was collected to examine the accuracy and extent of the lesions. Rats were given a lethal dose of tribromoethanol (375 mg/kg; 1.5ml/100g bodyweight; i.p.) and transcardially perfused with 0.9% saline followed by ice cold 4% paraformaldehyde in 0.1M borate buffer. Brains were stored overnight in the paraformaldehyde solution with 12% sucrose at 4°C, and then rapidly frozen in hexanes cooled in dry ice and stored at −80°C until sectioning and analysis.
Brains were sliced into 40 μm coronal slices using a sliding microtome (Leica Biosystems) and collected into 3 serially adjacent series. Brain tissue from one series was processed for NeuN detection to verify lesion placements. Tissue was incubated for 1 hour at room temperature in a blocking solution (potassium phosphate-buffered saline solution [KPBS] containing normal horse serum [NHS], Triton X-100, and milk), followed by incubation with anti-NeuN antibody raised in mouse (1:1000, MAB377; Millipore) in the blocking solution for 72hr at 4°C. Tissue was rinsed in a solution containing KPBS, NHS, and milk followed by incubation with biotinylated secondary antibody against mouse (1:500, BA-2001; Vector Laboratories) in the blocking solution for 45 min. After KPBS rinses, tissue was incubated in an avidin biotin complex (ABC, PK-6100; Vector Laboratories) for 45 min, rinsed with KPBS, and treated with nickel-intensified 3, 3’-diaminobenzidine (SK-4100; Vector Laboratories) for color visualization of neurons labeled for NeuN. Sections were rinsed again, mounted onto SuperFrost slides (Fisher Scientific), dried at 45°C, dehydrated through graded alcohols, cleared in xylenes, and coverslipped with DPX Mountant (Electron Microscopy Services).
A second series of tissue sections was mounted from KPBS onto gelatin-coated slides and stained with thionin for the identification of neuroanatomical borders as defined in Swanson’s rat brain atlas (Swanson, 2004).
Image Acquisition and Analysis
Verification and extent of lesions within the mPFC and BLA were analyzed using a 10X objective on an Olympus BX51 light microscope attached to an Olympus DP72 camera using DP2-BSW software (Olympus America Inc, Center Valley, PA). Extent and location of BLA and mPFC lesions were determined based on analysis of NeuN-stained tissue using adjacent thionin-stained tissue to identify nuclear borders and were drawn onto templates from the rat brain atlas (Swanson, 2004). Acceptable lesions ablated more than 60% of the PL and ILA within the mPFC and more than 60% of the anterior and posterior BLA. Subjects with less than 60% damage of either structure were excluded from analysis.
Statistical Analysis
Data were analyzed using a mixed design analysis of variance (ANOVA) with the following between-subjects factors: Lesion group (Contralateral, Ipsilateral, Sham) and devaluation group (Devalued, Non-devalued), and within-subjects factors: type of CS (CS+, CS− or rCS+, rCS−) and conditioning sessions (discriminative conditioning sessions 1, 5, and 10; reversal learning sessions 1, 5, 10, and 15; consumption tests 1–4; or devaluation tests 1, 4, and 8). The percentage of conditioned responding was the dependent variable for all experiments, except for CTA in which pellet consumption (in grams) was analyzed. A significance value of p < 0.05 was used for all analyses, except for lesion group post-hoc analyses in which Bonferroni adjusted alpha level was used (p = 0.05/3 = 0.017). SPSS software was used for all statistical analyses.
Results
Histology
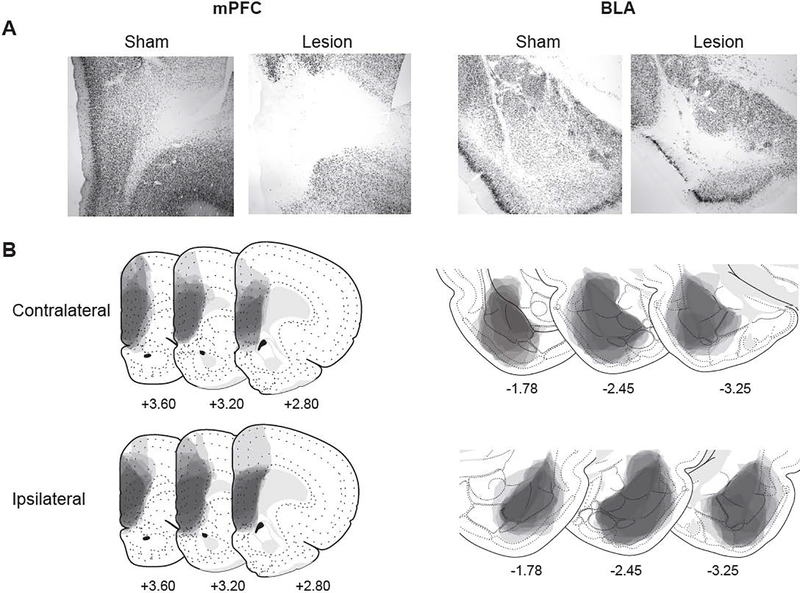
The location and extent of lesions were analyzed throughout the rostro-caudal span of the mPFC and BLA based on the Swanson brain atlas (Swanson, 2004; Fig. 1). Acceptable mPFC lesions ablated at least 60% of the prelimbic cortex (PL; Levels 6–9 [+4.2 to +2.8 mm from Bregma]) and infralimbic cortex (ILA; Levels 8–9 (+3.2 to +2.8 mm from Bregma]) and in the majority of cases, there was, on average, 50% damage to the dorsal anterior cingulate area. Acceptable BLA lesions ablated at least 60% of the anterior BLA (BLAa; Levels 25–29 [−1.53 to −2.85 mm from Bregma]) and the posterior BLA (BLAp; Levels 28–32 [−2.45 to −3.90 mm from Bregma]) with additional damage (~60%) to the lateral amygdala (Levels 28–30 [−2.45 to −3.25 mm from Bregma]). Eleven subjects were excluded due to inadequate damage to the mPFC or BLA, resulting in a total of 25 subjects included in analyses (Sham, n=8; Ipsilateral, n=9; and Contralateral, n=8).
Figure 1.
Extent of lesions identified by NeuN immunohistochemistry. (A) Images show NeuN-stained tissue from mPFC sham (first panel) and lesion (second panel) and BLA sham (third panel) and lesion (fourth panel). (B) Lesion extent of all included subjects with contralateral (top) and ipsilateral (bottom) lesions, drawn with 10% opacity in Adobe Illustrator CS6. Numbers below each schematic refers to distance (in mm) from Bregma and correspond to the following Swanson brain atlas levels: +3.60, Level 7; +3.20, Level 8; +2.80, Level 9; −1.78, Level 26; −2.45, Level 28; and −3.25, Level 30. Scale bar = 100 μm.
Discriminative Conditioning
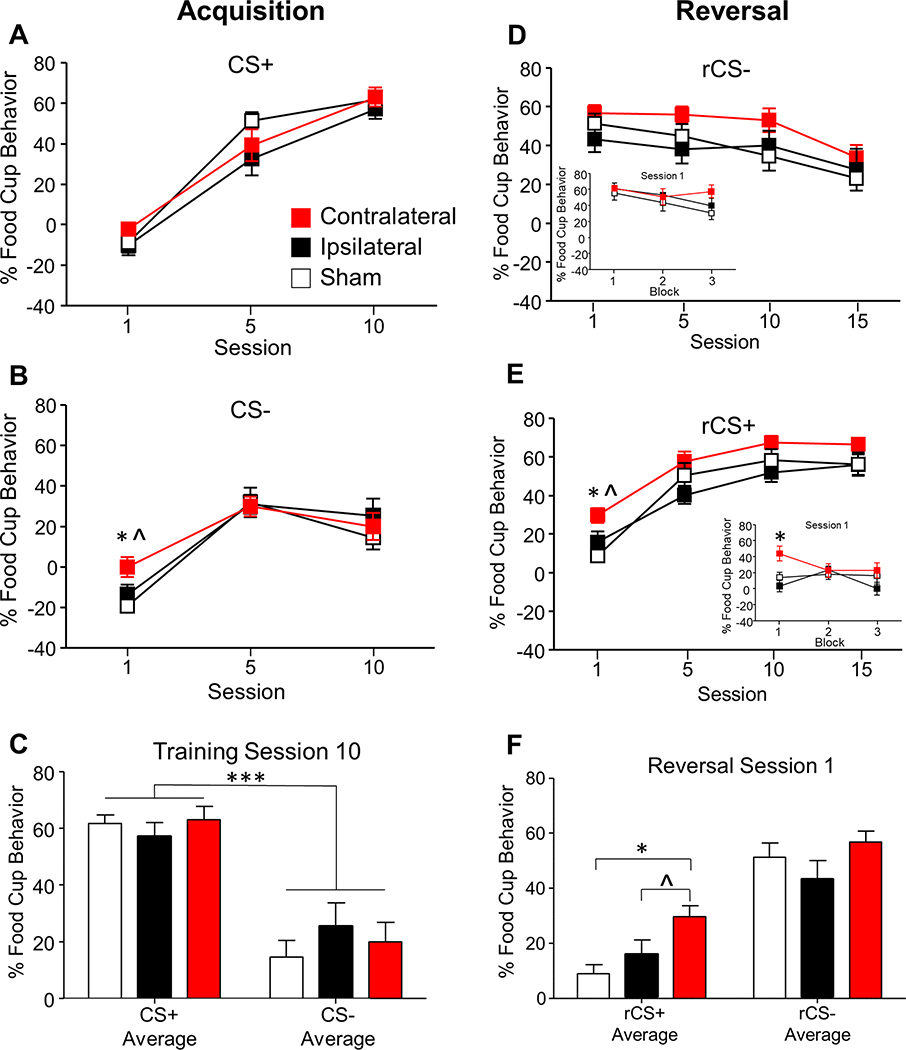
All groups successfully discriminated between the CSs by the end of training, with minimal differences between lesion groups (Fig. 2, left). A Lesion Group (sham, ipsilateral, contralateral) by CS (CS+Elevation, CS−Elevation) by Session (1, 5, 10) repeated measures ANOVA found a main effect of Session (F(2,44) = 148.32, p < 0.001) and CS (F(1,22) = 31.14, p < 0.001) and a Session by CS interaction (F(2,44) = 23.95, p < 0.001), but no effect of Lesion Group (F(2,22) = 0.92, p > 0.05) or any other interactions (F’s < 2.0, p’s > 0.05).
Figure 2.
Conditioned responding throughout discriminative conditioning (left) and reversal learning (right). Percentage of time (mean ± SEM) rats expressed food cup behavior (elevation above baseline) across discriminative conditioning in response to the CS+ (A) and CS− (B) and average responding during session 10 (C). Percentage of time (mean ± SEM) rats expressed food cup behavior across reversal learning in response to the rCS− (D; previously the CS+) and rCS+ (E; previously the CS−) and average responding during session 1 (F). Inset graphs in D and E are session 1 of the respective rCS, and each block is the average of 2 CS trials. *** p < 0.001; * p < 0.01 Contralateral vs Sham; ^ p = 0.04 Contralateral vs Ipsilateral.
A Lesion Group by Session repeated measures ANOVA on CS+ Elevation responding showed a main effect of Session (F(2,44) = 135.43, p < 0.001), but no effect of Lesion Group or interactions (F’s < 2, p’s > 0.05), confirming all lesion groups similarly increased conditioned responding to the CS+ across training sessions (Fig. 2A).
Additionally, all groups showed a change in conditioned responding during the CS− (Fig. 2B). A Lesion Group by Session repeated measures ANOVA on CS− Elevation responding showed a main effect of Session (F(2,44) = 51.79, p < 0.001), but no effect of Lesion Group or interactions (F’s < 2, p’s > 0.05). Follow-up analyses during the first training session showed a main effect of Lesion Group (F(2,22) = 3.97, p < 0.05), and a one-way ANOVA showed a significant Lesion Group effect on CS− Elevation responding during the first session (F(2,24) = 5.00, p < 0.05). Post-hoc analyses showed the CS− Elevation responding for Contralateral lesion group was higher compared to the Sham group (p < 0.017) and Ipsilateral lesion group (p = 0.036), due to a difference in Pre-CS− responding during the first session (sham: 37.76±16.45; ipsilateral: 28.47±13.38; contralateral: 21.35±8.89; p =0.022 between sham and contralateral). There were no Pre-CS differences in any other sessions (p’s > 0.05), and results are consistently presented as Elevation measures (CS – Pre-CS responding).
To confirm successful discrimination at the end of training, follow-up analyses on training session 10 showed a main effect of CS (F(1,22) = 73.67, p < 0.001) with no differences between lesion groups or interactions (F’s < 1, p’s > 0.05), confirming higher food cup responding to the CS+ compared to the CS− in all groups (Fig. 2C).
Reversal Learning
All rats successfully learned the new outcomes during reversal learning; however, the Contralateral lesion group consistently responded higher to the CSs throughout learning (Fig. 2, right). A Lesion Group by reversal CS (rCS+Elevation, rCS−Elevation) by Session (1, 5, 10, 15) repeated measures ANOVA found an effect of Lesion Group (F(2,22) = 4.11, p < 0.05), CS (F(1,22) = 4.27, p = 0.05), Session (F(3,66) = 12.13, p < 0.001), and a CS by Session interaction (F(3,66) = 49.18, p < 0.001). Post-hoc analysis showed the Contralateral lesion group had higher responding throughout reversal learning compared to Ipsilateral lesion group (p < 0.017) and marginally compared to the Sham group (p = 0.035).
To examine CS simple effects, separate analyses were conducted for rCS+ and rCS−. A Lesion Group by Session repeated measures ANOVA on the rCS+ (Fig. 2E) found a main effect of Lesion Group (F(2,22) = 5.15, p < 0.05) and Session (F(3,66) = 67.13, p < 0.01), but no interaction (F < 2, p > 0.05), indicating that all groups increased responding to the rCS+ throughout reversal learning. Additionally, the Contralateral lesion group consistently responded higher to the rCS+ compared to the Ipsilateral lesion group (p < 0.017) and marginally compared to the Sham group (p = 0.023).
A Lesion Group by Session repeated measures ANOVA on the rCS− (Fig. 2D) found a main effect of Session (F(3,66) = 8.96, p < 0.01), with no effect of Lesion Group (F(2,22) = 2.17, p = 0.14) or interaction (F < 1, p > 0.05), indicating that all groups similarly decreased responding to the rCS− throughout reversal learning.
Follow-up analyses on individual reversal sessions found a significant effect during session 1, which is mostly memory recall test. A rCS by Lesion Group repeated measures ANOVA found a main effect of Lesion group (F(2,22) = 5.48, p < 0.05) and CS (F(1,22) = 53.93, p < 0.001). Post-hoc analyses found an effect of Lesion group during the rCS+ (F(2,24) = 5.51, p < 0.05), but not during the rCS− (F(2,24) = 1.53, p > 0.05). The Contralateral lesion group had higher conditioned responding to the rCS+ compared to the Sham (p < 0.017) and marginally compared to the Ipsilateral lesion group (p = 0.038) during reversal session 1 (Fig 2F). Additionally, we analyzed the first reversal session in more detail in blocks of 2 trials (3 blocks total). A rCS by Lesion Group by Block repeated measures ANOVA found a main effect of Block (F(2,44) = 3.90, p < 0.05). Post-hoc analyses on the individual blocks showed an effect of Lesion group on the first block (F(2,22) = 7.50, p < 0.01). The Contralateral lesion group had higher conditioned responding the rCS+ during the first block compared to the Sham and Ipsilateral lesion groups (p’s < 0.017; Fig 2E inset). There were no other effects on the other rCS+ Blocks nor during any rCS− Blocks (p’s > 0.05).
Devaluation
Devaluation testing commenced following reversal learning. Half of each lesion group received US-LiCl pairings (“Devalued”) and half received LiCl and US unpaired (“Non-devalued”), resulting in Sham: Non-devalued (n=4) and Devalued (n=4); Ipsilateral: Non-devalued (n=6) and Devalued (n=3); and Contralateral: Non-devalued (n=4) and Devalued (n=4).
Conditioned Taste Aversion
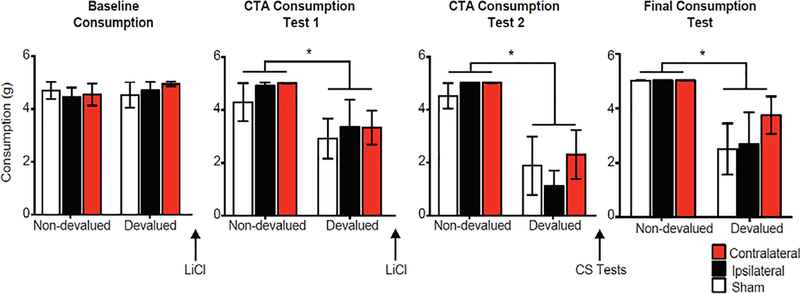
All rats that received the US-LiCl pairings (Devalued groups) showed a decrease in food consumption with no differences between Lesion groups (Fig. 3). A Lesion Group by Devaluation by Consumption Test repeated measures ANOVA found a main effect of Test Session (F(2,38) = 21.16, p < 0.001) and Devaluation (F(1,19) = 16.36, p < 0.001) and a Test Session by Devaluation interaction (F(2,38) = 31.71, p < 0.001). Post-hoc analyses confirmed no differences between Lesion Groups or Devaluation groups (F’s < 1, p’s > 0.05) during the baseline consumption test that occurred prior to LiCl injections. During consumption tests following the LiCl injections, there was an effect of Devaluation during the first (F(1,19) = 14.27, p < 0.01) and second (F(1,19) = 36.39, p < 0.001) tests, but no effect of Lesion Group or interaction (F’s < 1, p’s > 0.05), confirming all Devaluation groups, regardless of lesion condition, decreased consumption of the US.
Figure 3.
Consumption during Conditioned Taste Aversion (CTA) procedures. Graphs show rat’s chow consumption (in grams) that received two sessions of US-LiCl pairings (Devalued) or US and LiCl temporally separated (Non-devalued) during Baseline (prior to LiCl administration), after each training session (CTA Test 1 and 2), and at the end of behavioral testing (Final test). * p < 0.01.
CS Devaluation Testing
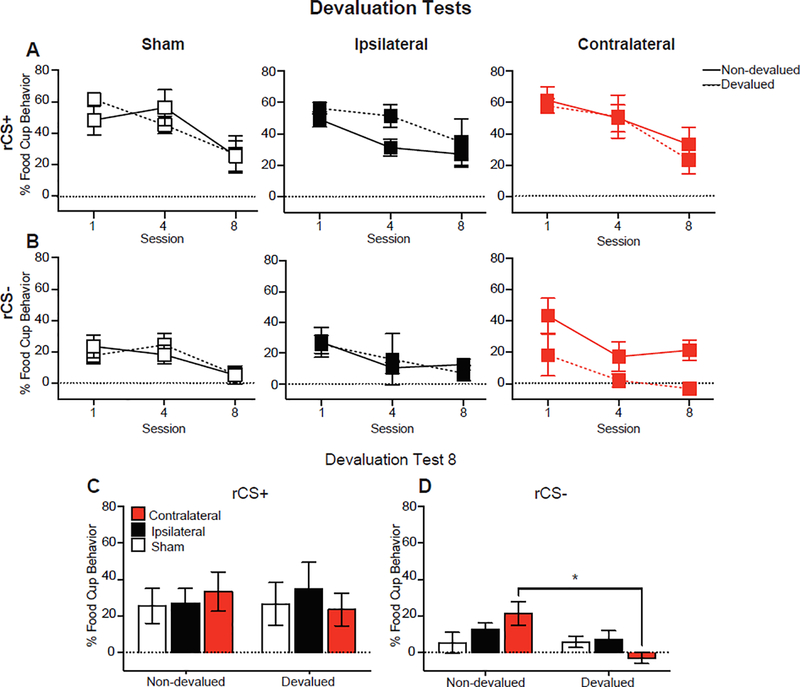
Following CTA, all rats were presented with the CSs (without US). Overall, there was no evidence of devaluation of the CSs (Fig. 4), potentially due to small group sizes within each lesion group (n=3–6). A Lesion Group by Devaluation by CS by Session repeated measures ANOVA showed main effects of CS (F(1,19) = 174.05, p < 0.001) and Session (F(2,38) = 33.71, p < 0.001) and a CS by Devaluation interaction (F(1,19) = 5.71, p < 0.05) and a marginally significant CS by Session interaction (F(2,38) = 2.66, p = 0.08), but no other effects or interactions (F’s < 2, p > 0.05). The devaluation sessions were conducted under extinction resulting in notable differences across testing (decrease in responding across tests). Due to extinction and the main effects of session and CS, follow-up analyses were conducted on conditioned responding to the rCS− and found an effect of session (F(2,38) = 10.50, p < 0.001) and a marginally significant Lesion Group by Devalued interaction (F(2,19) = 2.62, p = 0.099). Individual session analyses on test session 8 showed an effect of Devaluation (F(1,19) = 6.72, p < 0.05) and a Lesion Group by Devaluation interaction (F(2,19) = 3.94, p < 0.05). Independent t-tests showed the Contralateral Non-devalued group had higher food cup responding compared to the Contralateral Devalued group (t (6) =3.44, p < 0.05). There were no differences between devalued and non-devalued groups for Sham or Ipsilateral conditions (p’s > 0.05).
Figure 4.
Conditioned responding throughout CS devaluation testing without the delivery of food. Percentage of time (mean ± SEM) rats expressed food cup behavior (elevation above baseline) during the rCS+ (A) and rCS− (B) across testing and during the last test session (C). * p < 0.01
Final Consumption Test
After CS devaluation testing, rats were given a final consumption to confirm the CTA memory of the US was intact, and, indeed, all rats that received US-LiCl pairings maintained decreased consumption of the US (Fig. 3). A Lesion Group by Devalued ANOVA on US consumption during the final test showed a Devaluation effect (F(1,19) = 19.44, p < 0.001), but no effect of Lesion group or interactions (F’s < 1).
Discussion
The current study examined if communication between the BLA and mPFC is necessary in appetitive Pavlovian cue learning and behavioral flexibility. First, we found discriminative conditioning between a cue paired with food (CS+) and a cue not paired with food (CS−) was similar across all lesion groups. These results indicate that communication between the BLA and mPFC was not necessary for discriminative learning. However, when the outcomes of the cues were reversed, the group that had disconnected BLA-mPFC communication had higher conditioned responding, particularly to the cue that previously did not predict food (rCS+), during the first session of reversal. These results demonstrate that communication between the BLA and mPFC is necessary to incorporate previously learned information during new learning. In contrast, disconnection of the BLA-mPFC circuitry had no effect on CTA or CS devaluation, with a caveat that the control groups (shams and ipsilateral groups) did not show evidence of CS devaluation. Together, these results show communication between the BLA and mPFC is not necessary for discriminative learning or CTA but is necessary for new learning when outcomes of learned cues change.
Contralateral disconnection of the BLA-mPFC circuitry resulted in a small but sustained, increased responding across reversal learning. The effect was most pronounced during the first session of reversal, when the contralateral group had more conditioned responding to the cue that previously did not signal food compared to the ipsilateral and sham groups. The first session of reversal learning is the first test of memory recall, as previously learned contingences are recalled before the new learning of the reversed contingencies occurs. Thus, this session provided an opportunity for CSs memory recall testing at start of reversal learning. Low responding to the rCS+ is expected during the first reversal session since that cue was not followed by food throughout discriminative conditioning (CS−). Higher responding in the contralateral group observed in the current study suggests impairments in the recall and/or their ability to inhibit behavioral responding. Prior learned outcome of the cue needs to be incorporated during learning of the new outcomes, in order to respond appropriately (for review, see Wassum & Izquierdo, 2015). Alternatively, communication between the BLA and mPFC may be needed for behavioral inhibition during learning, as evident in other paradigms. Previous studies have shown that inactivation of the BLA and mPFC impaired behavioral inhibition and, as a result, increases impulsive behavior for rewards. After bilateral BLA or mPFC or contralateral inactivation by muscimol infusions, rats spent less time waiting for a high value reward and decreased preference for that reward, suggesting less behavioral inhibition and more impulsivity (Churchwell et al., 2009). The same study showed that rats with bilateral BLA, but not mPFC, inactivation had more regressive errors in appetitive reversal learning and took longer to successfully learn the new contingencies (Churchwell et al., 2009). Additionally, rats with mPFC lesions showed higher responding during early reversal learning and took longer to display successful reversal learning (Salazar et al., 2004), but minimal impairments during the first few sessions of appetitive learning and discrimination (Salazar et al., 2004; Petrovich et al., 2007). Collectively, prior and current results suggest the BLA-mPFC circuitry is needed to respond appropriately when the cue outcomes have changed. The BLA-mPFC may be needed during a recall and value assessment of learned cues or when the recalled cue value is used to guide and control behavioral responding or inhibition.
To further investigate the role of the BLA-mPFC circuitry in cue value updating, rats were tested in a CS devaluation paradigm after reversal learning. All groups that received the US-LiCl pairings successfully acquired CTA, regardless of lesion group, as shown by a decrease in consumption of the US, indicating communication between the BLA and mPFC is not necessary for CTA. This is in agreement with previous studies that have shown that neither the BLA nor mPFC are necessary for the acquisition of CTA (Bahar et al., 2003; Johnson et al., 2009). However, both structures are activated during the maintenance of the CTA memory (Mickley et al., 2005; Xin et al., 2014). Furthermore, protein synthesis inhibition (Akirav et al., 2006) and alterations in BDNF gene expression (Xin et al., 2014) within the mPFC and BLA impair the maintenance of the CTA memory. In the current study, the contralateral disconnection preparation left unilateral BLA and mPFC and their communications with other regions intact that could be sufficient to learn and recall CTA (e.g. the central amygdala and nucleus of the solitary tract [Bahar et al., 2003; Houpt et al., 1994; Schafe and Bernstein, 1996; Sakai and Yamamoto, 1997; Spray & Bernstein, 2004; Yamamoto et al., 1992; for review, see Jahng & Lee, 2015]).
The CS devaluation tests examined how rats responded to the learned cues for food after the food (US) was devalued. Successful CS devaluation is evident by a decrease in conditioned responding to the CS since the associated US was devalued; however, we did not find any evidence of CS devaluation, even in the sham groups. All groups maintained higher responding to the rCS+ compared to the rCS−, likely due to extensive training and experience with the CSs and US (25 learning sessions prior to devaluation). Thus, we cannot conclude from the current data whether BLA-mPFC is needed in CS devaluation. Notably, another study showed that disconnecting the BLA-mPFC circuitry did not interfere with satiety-induced devaluation (Coutureau et al., 2009).
Interestingly, we found a small interaction between BLA-mPFC disconnection and devaluation. The non-devalued contralateral lesion group still had increased conditioned responding during the presentation of the rCS− compared to the devalued contralateral group. This increased responding maybe reflects similar impairments in behavioral inhibition that we found in reversal learning. These results suggest that rats with BLA-mPFC disconnection may not be incorporating previously learned information, including that the rCS− signals the absence of food, to update the value of the cue to appropriately inhibit behavior. However, these results should be interpreted with caution since the group sizes were small, and the sham-lesioned group did not show CS-devaluation.
One limitation of the disconnection preparation is that it does not provide information about the directionality of communication needed. Therefore, we cannot determine if disruption of communication from the BLA to the mPFC or mPFC to BLA or both were responsible for the impairments in the current study. Indeed, both pathways, BLA➔mPFC and mPFC➔BLA may be important but provide different information. It is possible each pathway is involved in distinct aspects of the investigated behavioral paradigms: the BLA could inform the mPFC of the cue’s value (e.g. Keefer & Petrovich, 2017), and the mPFC could have top-down control onto the BLA to guide behavioral inhibition (e.g. Likhtik et al., 2005). This top-down control of behavior has been implicated in Pavlovian fear conditioning and extinction (e.g. for reviews, see Giustino & Maren, 2015; Marek et al., 2018), and similar mechanisms may be controlling behavioral inhibition in appetitive Pavlovian learning. Furthermore, there is anatomical evidence for bidirectional communication (Sesack et al., 1989; Takagishi & Chiba, 1991; Kita & Kitai, 1990; Swanson & Petrovich, 1998; Petrovich et al., 2001; Vertes, 2004; Gabbott et al., 2005; Hoover & Vertes, 2007; Hirai et al., 2012; Little & Carter, 2013; Reppucci & Petrovich, 2016), and functional evidence that the communication between these areas guide reward seeking and learning (Fuchs et al., 2007; Churchwell et al., 2009; Mashhoon et al., 2010; Stefanik et al., 2013; Land et al., 2014; Keefer & Petrovich, 2017; Keistler et al., 2017). Additionally, the BLA has direct influence on activity of the mPFC neurons (Perez-Jaranay & Vives, 1991; McDonald, 1992; Gabbott et al., 2006; Floresco & Tse, 2007; Sotres-Bayon et al., 2012; Sun & Laviolette, 2012; Dilgen et al., 2013), and the mPFC has direct influence onto BLA neurons (Brinley-Reed et al., 1995; Smith et al., 2000; Rosenkranz & Grace, 2001; Likhtik et al., 2005; but see Quirk et al., 2003 for discussion).
In addition to the discussed impairments, the contralateral disconnection of BLA-mPFC could have resulted in the increased contribution of other circuitries that remained intact. In particular, the orbitofrontal cortex (OFC) is critical in reversal learning (e.g. Hervig et al., 2019; Izquierdo et al., 2013), and more generally, it is important in value representation, ultimately resulting in behavioral alteration (for reviews, see Schoenbaum et al., 2011; McDannald et al., 2014). It is plausible the unilateral, intact OFC-BLA circuitry may enhance reversal learning, when BLA communication with the mPFC is absent. Alternatively, impaired communication between the BLA and OFC, due to the BLA lesion that disconnected ipsilateral communication with OFC, could have led to a potentiation of reversal learning, as shown with bilateral BLA lesions by Izquierdo and colleagues (2013).
In summary, the current study examined if communication between the BLA and mPFC is necessary for appetitive cue learning and behavioral flexibility when the outcome of learned cues change. Rats that received disconnection of the BLA-mPFC successfully discriminated between appetitive cues; however, they displayed increased conditioned responding during reversal learning. These results demonstrate that communication between the BLA and mPFC is necessary to respond appropriately when the outcomes of learned food cues have changed.
Acknowledgements
We thank Megan Ebner, Amanda Jenkins, Marissa Kellogg, Sophia Ramos-Bartolomei, Antonio Santos, and Hannah Yoon for technical assistance. We also thank anonymous reviewers for their critical assessments and suggestions that improved the manuscript. This research was supported by the National Institute of Health grant R01DK085721 to G.D.P.
References
- Akirav I, Khatsrinov V, Vouimba RM, Merhav M, Ferreira G, Rosenblum K, & Maroun M (2006). Extinction of conditioned taste aversion depends on functional protein synthesis but not on NMDA receptor activation in the ventromedial prefrontal cortex. Learning & Memory, 13(3), 254–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahar A, Samuel A, Hazvi S, & Dudai Y (2003). The amygdalar circuit that acquires taste aversion memory differs from the circuit that extinguishes it. European Journal of Neuroscience, 17(7), 1527–1530. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Killcross AS, & Dickinson A (2003). The effect of lesions of the basolateral amygdala on instrumental conditioning. Journal of Neuroscience, 23(2), 666–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasio A, Steardo L, Sabino V, & Cottone P (2014). Opioid system in the medial prefrontal cortex mediates binge-like eating. Addiction biology, 19(4), 652–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell P, Hall G, & Killcross S (2001). Lesions of the basolateral amygdala disrupt selective aspects of reinforce representation in rats. Journal of Neuroscience 21(22): 9018–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulougouris V, Dalley JW, & Robbins TW (2007). Effects of orbitofrontal, infralimbic and prelimbic cortical lesions on serial spatial reversal learning in the rat. Behavioural brain research, 179(2), 219–228. [DOI] [PubMed] [Google Scholar]
- Brown VJ & Tait DS (2014). Behavioral Flexibility: Attentional Shifting, Rule Switching, and Response Reversal In: Stolerman I, Price L. (eds) Encyclopedia of Psychopharmacology. Springer, Berlin, Heidelberg. [Google Scholar]
- Burgos-Robles A, Bravo-Rivera H, & Quirk GJ (2013). Prelimbic and Infralimbic Neurons Signal Distinct Aspects of Appetitive Instrumental Behavior, PLoS One, 8(2): 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchwell JC, Morris AM, Heurtelou NM, & Kesner RP (2009). Interactions between the prefrontal cortex and amygdala during delay discounting and reversal. Behavioral neuroscience, 123(6), 1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S, Hobin MP, & Petrovich GD (2015a). Appetitive associative learning recruits a distinct network with cortical, striatal, and hypothalamic regions. Neuroscience, 286, 187–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S, Mayer HS, & Petrovich GD (2015b). Orexin/Hypocretin-1 Receptor Antagonism Selectively Reduces Cue-Induced Feeding in Sated Rats and Recruits Medial Prefrontal Cortex and Thalamus. Scientific reports, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S, Powell DJ, & Petrovich GD (2013). Differential recruitment of distinct amygdalar nuclei across appetitive associative learning, Learning & Memory, 20:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH & Balleine BW (2003). The role of prelimbic cortex in instrumental conditioning. Behavioural brain research, 146(1–2), 145–157. [DOI] [PubMed] [Google Scholar]
- Corbit LH & Balleine BW (2005). Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of Pavlovian-instrumental transfer, The Journal of Neuroscience, 25: 962–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutureau E, Marchand AR, & Di Scala G (2009). Goal-directed responding is sensitive to lesions to the prelimbic cortex or basolateral nucleus of the amygdala but not to their disconnection. Behavioral neuroscience, 123(2), 443. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, & Robbins TW (2004). Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates, Neuroscience and Biobehavioral Reviews, 28: 771–784. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Cardinal RN, Parkinson JA, & Robbins TW (2003). Appetitive behavior: Impact of amygdala-dependent mechanisms of emotional learning, Annals of the New York Academy of Sciences, 985: 233–250. [PubMed] [Google Scholar]
- Floresco SB, Block AE, & Maric TL (2008). Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behavioural brain research, 190(1), 85–96. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Eaddy JL, Su ZI, & Bell GH (2007). Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. European Journal of Neuroscience, 26(2), 487–498. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Jays PR, Salway P, & Busby SJ (2005). Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. Journal of Comparative Neurology, 492(2), 145–177. [DOI] [PubMed] [Google Scholar]
- Giustino TF & Maren S (2015). The Role of the Medial Prefrontal Cortex in the Conditioning and Extinction of Fear, Frontiers in Behavioral Neuroscience, 9:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield T, Han JS, Conley M, Gallagher M, & Holland P (1996). Neurotoxic lesions of basolateral, but not central, amygdala interfere with Pavlovian second-order conditioning and reinforce devaluation effects, The Journal of Neuroscience, 16: 5256–5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervig ME, Fiddian L, Piilgaard L, Božič T, Blanco-Pozo M, Knudsen C, Olesen SF, Alsiö J, & Robbins TW (2019) Dissociable and Paradoxical Roles of Rat Medial and Lateral Orbitofrontal Cortex in Visual Serial Reversal Learning, Cerebral Cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai Y, Morishima M, Karube F, & Kawaguchi Y (2012). Specialized cortical subnetworks differentially connect frontal cortex to parahippocampal areas. Journal of Neuroscience, 32(5), 1898–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC & Petrovich GD (2005). A neural systems analysis of the potentiation of feedings by conditioned stimuli. Physiology of Behavior 86(5): 747–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC, Petrovich GD, & Gallagher M (2002). The effects of amygdala lesions on conditioned stimulus-potentiated eating in rats, Physiology & Behavior, 76: 117–129. [DOI] [PubMed] [Google Scholar]
- Holland PC, Hatfield T, & Gallagher M (2001). Rats with basolateral amygdala lesions show normal increases in conditioned stimulus processing but reduced conditioned potentiation of eating. Behavioral neuroscience, 115(4), 945. [PubMed] [Google Scholar]
- Hoover WB & Vertes RP (2007). Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat, Brain structure & function, 212: 149–179. [DOI] [PubMed] [Google Scholar]
- Houpt TA, Philopena JM, Wessel TC, Joh TH, & Smith GP (1994). Increased c-fos expression in nucleus of the solitary tract correlated with conditioned taste aversion to sucrose in rats. Neuroscience letters, 172(1–2), 1–5. [DOI] [PubMed] [Google Scholar]
- Ishikawa A, Ambroggi F, Nicola SM, Fields HL (2008). Contributions of the amygdala and medial prefrontal cortex to incentive cue responding. Neuroscience, 155(3), 573–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Darling C, Manos N, Pozos H, Kim C, Ostrander S, Cazares V, Stepp H, & Rudebeck PH (2013). Basolateral amygdala lesions facilitate reward choices after negative feedback in rats, Journal of Neuroscience, 33(9):4105–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahng JW & Lee JH (2015). Activation of the hypothalamic-pituitary-adrenal axis in lithium-induced conditioned taste aversion learning, Eur J Pharmacol, 768–182-8. [DOI] [PubMed] [Google Scholar]
- Johnson AW, Gallagher M, & Holland PC (2009). The basolateral amygdala is critical to the expression of pavlovian and instrumental outcome-specific reinforcer devaluation effects. Journal of Neuroscience, 29(3), 696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefer SE & Petrovich GD (2017). Distinct recruitment of basolateral amygdala-medial prefrontal cortex pathways across Pavlovian appetitive conditioning. Neurobiology of Learning and Memory, 141, 27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killcross S & Coutureau E (2003). Coordination of actions and habits in the medial prefrontal cortex of rats. Cerebral cortex, 13(4), 400–408. [DOI] [PubMed] [Google Scholar]
- Kita H & Kitai ST (1990). Amygdaloid Projections to the Frontal Cortex and the Striatum in the Rat, The Journal of Comparative Neurology, 298:40–49. [DOI] [PubMed] [Google Scholar]
- Land BB, Narayanan NS, Liu RJ, Gianessi CA, Brayton CE, Grimaldi DM et al. (2014). Medial prefrontal D1 dopamine neurons control food intake. Nature neuroscience, 17(2), 248–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhtik E, Pelletier JG, Paz R, & Paré D (2005). Prefrontal control of the amygdala. Journal of Neuroscience, 25(32), 7429–7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little JP & Carter AG (2013). Synaptic Mechanisms Underlying Strong Reciprocal Connectivity between the Medial Prefrontal Cortex and Basolateral Amygdala, The Journal of Neuroscience, 33(39): 15333–15342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek R, Sun Y, & Sah P (2018). Neural circuits for the top-down control of fear and extinction, Psychopharmacology, 236(1): 313–320. [DOI] [PubMed] [Google Scholar]
- Mashhoon Y, Wells AM, & Kantak KM (2010). Interaction of the rostral basolateral amygdala and prelimbic prefrontal cortex in regulating reinstatement of cocaine-seeking behavior. Pharmacology Biochemistry and Behavior, 96(3), 347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDannald MA, Jones JL, Takahashi YK, & Schoenbaum G (2014). Learning theory: a driving force in understanding orbitofrontal function, Neurobiology of Learning and Memory, 108:22–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mena JD, Sadeghian K, & Baldo BA (2011). Induction of Hyperphagia and Carohydrate Intake by μ-Opiod Receptor Stimulation in Circumscribed Regions of Frontal Cortex, The Journal of Neuroscience, 31(9): 3249–3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mena JD, Selleck RA, & Baldo BA (2013). Mu-Opioid Stimulation in Rat Prefrontal Cortex Engages Hypothalamic Orexin/Hypocretin-Containing Neurons, and Reveals Dissociable Roles of Nucleus Accumbens and Hypothalamus in Cortically Driven Feeding, The Journal of Neuroscience, 33(47): 18540–18552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickley GA, Kenmuir CL, Yocom AM, Wellman JA, & Biada JM (2005). A role for prefrontal cortex in the extinction of a conditioned taste aversion. Brain research, 1051(1–2), 176–182. [DOI] [PubMed] [Google Scholar]
- Moorman DE & Aston-Jones G (2015). Prefrontal neurons encode context-based response execution and inhibition in reward seeking and extinction. Proceedings of the National Academy of Sciences, 112(30), 9472–9477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M, Izaki Y, Takita M, Tanaka J, & Hori K (2004). Extracellular level of basolateral amygdalar dopamine responding to reversal of appetitive-conditioned discrimination in young and old rats. Brain research, 1018(2), 241–246. [DOI] [PubMed] [Google Scholar]
- Nowak K, Meyza K, Nikolaev E, Hunt MJ, & Kasicki S (2012). Local blockade of NMDA receptors in the rat prefrontal cortex increases c-Fos expression in multiple subcortical regions. Acta Neurobiol Exp (Wars), 72(3), 207–218. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP (2011). Contributions of the ventromedial prefrontal cortex to goal-directed action selection. Annals of the New York Academy of Science, 1239:118–129. [DOI] [PubMed] [Google Scholar]
- Ostlund SB & Balleine BW (2005). Lesions of medial prefrontal cortex disrupt the acquisition but not the expression of goal-directed learning. Journal of Neuroscience, 25(34), 7763–7770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund SB & Balleine BW (2008). Differential involvement of the basolateral amygdala and mediodorsal thalamus in instrumental action selection. Journal of Neuroscience, 28(17), 4398–4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes SL & Balleine BW (2013). Incentive memory: evidence the basolateral amygdala encodes and the insular cortex retrieves outcome values to guide choice between goal-directed actions. Journal of Neuroscience, 33(20), 8753–8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson JA, Robbins TW, & Everitt BJ (2000). Dissociable roles of the central and basolateral amygdala in appetitive emotional learning. European journal of neuroscience, 12(1), 405–413. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Canteras NS, & Swanson LW (2001). Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems, Brain Research Reviews, 38: 247–89. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Ross CA, Holland PC, & Gallagher M (2007). Medial Prefrontal Cortex is Necessary for an Appetitive Contextual Conditioned Stimulus to Promote Eating in Sated Rats, The Journal of Neuroscience, 27(24): 6436–6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Detrick S, & Kesner RP (1999). Involvement of the prelimbic–infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. Journal of Neuroscience, 19(11), 4585–4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppucci CJ & Petrovich GD (2016). Organization of connections between the amygdala, medial prefrontal cortex, and lateral hypothalamus: a single and double retrograde tracing study in rats. Brain Structure and Function, 221(6): 2937–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz JA & Grace AA (2001). Dopamine attenuates prefrontal cortical suppression of sensory inputs to the basolateral amygdala of rats. Journal of Neuroscience, 21(11), 4090–4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai N & Yamamoto T (1997). Conditioned taste aversion and c-fos expression in the rat brainstem after administration of various USs, Neuroreport, 8(9–10):2215–20. [DOI] [PubMed] [Google Scholar]
- Salazar RF, White W, Lacroix L, Feldon J, & White IM (2004). NMDA lesions in the medial prefrontal cortex impair the ability to inhibit responses during reversal of a simple spatial discrimination. Behavioural Brain Research, 152(2), 413–424. [DOI] [PubMed] [Google Scholar]
- Schafe GE & Bernstein IL (1996). Forebrain contribution to the induction of a brainstem correlate of conditioned taste aversion: I. The amygdala. Brain research, 741(1–2), 109–116. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, & Gallagher M (1999). Neural encoding in orbitofrontal cortex and basolateral amygdala during olfactory discrimination learning. Journal of Neuroscience, 19(5), 1876–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Nugent SL, Saddoris MP, & Gallagher M (2003). Lesions of orbitofrontal cortex and basolateral amygdala complex disrupt acquisition of odor-guided discriminations and reversals. Learning & Memory, 10(2), 129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Takahashi Y, Liu TL, & McDannald MA (2011). Does the orbitofrontal cortex signal value? Annals of the New York Academy of Sciences, 1239:87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, & Bunney BS (1989). Topographical Organization of the Efferent Projections of the Medial Prefrontal Cortex in the Rat: An Anterograde Tract-Tracing Study With Phaseolus vulgaris Leucoagglutinin, Journal of Comparative Neurology, 290: 213–242. [DOI] [PubMed] [Google Scholar]
- Setlow B, Gallagher M, & Holland PC (2002). The basolateral complex of the amygdala is necessary for acquisition but not expression of CS motivational value in appetitive Pavlovian second-order conditioning, European Journal of Neuroscience, 15: 1841–1853. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Sierra-Mercado D, Pardilla-Delgado E, & Quirk GJ (2012). Gating of fear in prelimbic cortex by hippocampal and amygdala inputs. Neuron, 76(4), 804–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spray KJ & Bernstein IL (2004). Afferent and efferent connections of the parvicellular subdivision of iNTS: defining a circuit involved in taste aversion learning. Behavioural brain research, 154(1), 85–97. [DOI] [PubMed] [Google Scholar]
- Stefanik MT & Kalivas PW (2013). Optogenetic dissection of basolateral amygdala projections during cue-induced reinstatement of cocaine seeking. Neural circuits underlying emotion and motivation: Insights from optogenetics and pharmacogenetics, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N & Laviolette SR (2012). Inactivation of the basolateral amygdala during opiate reward learning disinhibits prelimbic cortical neurons and modulates associative memory extinction. Psychopharmacology, 222(4), 645–661. [DOI] [PubMed] [Google Scholar]
- Swanson LW & Petrovich GD (1998). What is the amygdala? Trends in Neuroscience, 21(8): 323–331. [DOI] [PubMed] [Google Scholar]
- Swanson LW (2004). Brain maps: structure of the rat brain A laboratory guise with printed and electronic templates for data, models and schematics. Amsterdam: Elsevier. [Google Scholar]
- Takagishi M & Chiba T (1991). Efferent projections of the infralimbic (area 25) region of the medial prefrontal cortex in the rat: an anterograde tracer PHA-L study. Brain research, 566(1–2), 26–39. [DOI] [PubMed] [Google Scholar]
- Tye KM, Cone JJ, Schairer WW, & Janak PH (2010). Amygdala neural encoding of the absence of reward during extinction. Journal of Neuroscience, 30(1), 116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP (2004). Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse, 51(1), 32–58. [DOI] [PubMed] [Google Scholar]
- Warren BL, Mendoza MP, Cruz FC, Leao RM, Caprioli D, Rubio FJ, … Hope BT (2016). Distinct Fos-expressing neuronal ensembles in the ventromedial prefrontal cortex mediate food reward and extinction memories. Journal of Neuroscience, 36(25), 6691–6703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassum KM & Izquierdo A (2015). The basolateral amygdala in reward learning and addiction. Neuroscience & Biobehavioral Reviews, 57, 271–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin J, Ma L, Zhang TY, Yu H, Wang Y, Kong L, & Chen ZY (2014). Involvement of BDNF signaling transmission from basolateral amygdala to infralimbic prefrontal cortex in conditioned taste aversion extinction. Journal of Neuroscience, 34(21), 7302–7313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Shimura T, Sako N, Azuma S, Bai WZ, & Wakisaka S (1992). C-fos expression in the rat brain after intraperitoneal injection of lithium chloride. Neuroreport: An International Journal for the Rapid Communication of Research in Neuroscience. [DOI] [PubMed] [Google Scholar]