Abstract
This study aimed to compare serum amyloid processing biomarkers among HIV subtype B (n=25), HIV subtype C (n=26), healthy HIV-negative controls (n=18), and patients with Alzheimer's disease (AD; n=24). Immunoassays were used to measure main soluble Aβ isoforms Aβ38, Aβ40, Aβ42, and Aβ-total in serum and cerebrospinal fluid (CSF). People living with HIV (PLWH) and HIV(−) samples, including AD samples, were compared for gender and age, while HIV subtypes were compared for nadir CD4 and plasma viral load suppression.
CSF/serum ratios of Aβ40, Aβ42, and Aβ-total were lower in HIV-1C group than in HIV-1B group (p=0.020, 0.025, and 0.050 respectively). In serum, these biomarkers were comparable. Serum Aβ isoforms were significantly lower in PLWH than in AD. Serum Aβ42 levels in PLWH were decreased compared to those in control group, thus similar to Aβ42 alterations in CSF; these results were different from those observed in AD. Impaired cellular immunity, low CD4 cell count (nadir or current) influences serum Aβ metabolism in HIV-1B but not HIV-1C. However in PLWH overall, but not in individual HIV subtype groups, greater CD4 recovery, calculated as the difference between current and nadir CD4, correlated with Aβ42/Aβ40 ratio in serum (rs 0.246; p=0.0479). No significant correlation was found with global deficit score (GDS), an index of neurocognitive performance, age or duration of infection. These findings are consistent with those of subtype-dependent amyloid processing in blood in chronic HIV disease.
Keywords: neuronal injury, aging, biomarkers, HIV-associated neurocognitive disorders, subtype
INTRODUCTION
The profile of amyloid processing biomarkers in HIV infection is not yet well defined. Several studies have been carried out on HIV subtype B (HIV-1B) (Brew et al., 2005; Gisslén et al., 2009; Nath et al., 2009; Peterson et al., 2014; Krut et al., 2013; Krut et al., 2014), but data on other subtypes has not been reported. Soluble amyloid-β (Aβ), a small ~4kDa polypeptide, is the product of normal cell metabolism; it is found in various body fluids, including blood and cerebrospinal fluid (CSF) (Mehta et al., 2000). As blood can be obtained through minimal invasive sampling methods, there is an increasing interest on the validation of blood-based neural injury biomarkers. The majority of these validation studies are on Alzheimer’s disease (AD) (Wang et al., 2017).
The association between HIV infection and aging is an increasingly important topic, as the life-expectancy of people with HIV has increased and quality of life has improved (Raboni et al., 2017). HIV infection is associated with the development of age-related physiological disturbances and medical comorbidities earlier in life. HIV-associated neurocognitive disorders (HAND) are more common in older People living with HIV (PLWH). Moreover, PLWH have an epigenetic age 4.9 years older than that of healthy controls (Gross et al., 2016).
Aging is a risk factor for Aβ accumulation and neurocognitive impairment (High et al., 2012). PLWH may show Aβ deposition in the brain at younger ages. This suggests a dysregulation of amyloid processing in the setting of HIV infection. Aβ is the primary component of amyloid plaques (Hardy and Selkoe, 2002); several studies have noted marked increases in the prevalence of diffuse amyloid plaques in PLWH compared to HIV(−) age-matched controls (Green et al., 2005; Rempel and Pulliam, 2005; Esiri et al., 1998).
We previously reported CSF biomarkers suggesting that amyloid processing pathways are altered in a subtype-dependent manner, such that more brain Aβ deposits would occur in chronically infected patients with HIV-1C than in age-matched HIV-1B (de Almeida et al., 2018A; de Almeida et al., 2018B). Additionally, over a prolonged period of time, HIV-1-induced accumulation of Aβ may lower the threshold for dementia (Rempel and Pulliam, 2005). Based on these and previous studies of blood Aβ biomarkers on AD, the authors hypothesized that there is a higher level of serum Aβ in HIV-1C than in HIV-1B group.
This study aimed to compare serum and CSF/serum ratios of amyloid processing biomarkers between HIV-1B and HIV-1C infected participants; additional comparisons between these and healthy HIV(−) and AD patients were done. Here we investigated for the first time the main Aβ isoforms in peripheral blood of PLWH. The use of these biomarkers could influence decisions to treat neurocognitively impaired HIV+ individuals with cholinesterase inhibitors or memantine, a partial antagonist of NMD A receptors. Both drugs registered in many countries for the treatment of cognitive impairment in dementia, particularly for AD (Knight et al., 2018).
METHODS
This was a cross-sectional survey of stored CSF and serum samples; which was approved by institutional review boards (IRB) at Clinicas Hospital, University Federal of Parana (HC-UFPR, Curitiba, Parana, Brazil), the Brazilian National Commission of Ethics in Research (CONEP, Brasilia, Brazil), and the University of California San Diego (UCSD, San Diego, USA).
Subjects
All participants agreed and signed a consent form approved by the IRB. For AD participants, legally responsible caregivers signed the consent. CSF and serum samples were collected under a protocol funded by NIMH (R21 MH076651-01). HIV(+) and AD participants were recruited at HC-UFPR.
A total of 107 paired serum and CSF samples were analyzed. Participants were divided in groups as follows: HIV(+) (n = 65), HIV(−) (n = 18), and AD patients (n = 24). Demographic characteristics, HIV status, and co-infections from each group are summarized in Table 1.
Table 1.
Demographic data, clinical and HIV infection characteristics, and comorbidities of HIV participants, uninfected volunteers and Alzheimer’s disease
| HIV+ (n = 65) | HIV− (n = 18) | AD (n=24) | P | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 43 (35; 48) | 40 (34; 50) | 76.5 (67;79.5) | <0.0001 |
| Education, years | 7 (5;11) | 12 (11;15.5) | 4 (2;6) | 0.0001 |
| Gender, n male (%) | 32 (49) | 14 (77.8) | 8 (33) | 0.0120 |
| Clinical scales | ||||
| GDS | 0.65 (0.3;1.05) | |||
| MMSE | - | - | 14 (9.5;20) | |
| MoCA, (n=8) | 11.5(10.5;12.5) | |||
| FAQ | - | - | 23.5 (15;27.5) | |
| Geriatric Depression scale | - | - | 1 (0.5;3) | |
| Co-morbidities | ||||
| HCV, n (%) | 10(15) | 0 | 0 | - |
| Log Plasma HCV RNA, n | 2.9 (1.7; 5.9) | 0 | 0 | - |
Data are median (IQR) or number of cases (%).
Hepatitis C virus (HCV) status was assessed by antibody testing (Abbott-Architect).
Participants co-infected with HCV were not on treatment with interferon-gamma.
Mini-mental state examination (MMSE); Montreal Cognitive Assessment (MoCA);
Functional Activities Questionnaire (Pfeffer’s FAQ); Global deficit score (GDS)18.
PLWH participants.
As previously mentioned, PLWH, n = 65, were recruited at HC-UFPR, Brazil. Individuals with opportunistic CNS infections were not selected for the study. All volunteers provided both blood and CSF samples, and underwent serological testing to confirm HIV status before enrollment, in accordance with guidelines published by the Brazilian Ministry of Health (Brasil, Ministério da Saúde, 2017). For participants with clinically resistant infections, the infecting HIV strain was genotyped using pol sequences, while env sequences were used for all other participants. Genotyping indicated that 25 individuals were infected with HIV-1 subtype B, and 39 with non-B HIV-1 subtypes (C-26, BF-09, BC-1, CF-1, and F-2 participants). In one participant, the HIV-1 subtype could not be determined.
HIV Uninfected controls.
A control group of 18 age-matched HIV(−) individuals was recruited at the HIV Neurobehavioral Research Center, University of California San Diego, USA. Inclusion criteria and characteristics were described previously (de Almeida et al., 2018B).
Alzheimer disease participants.
AD, n=24, were clinically diagnosed by the dementia investigative team from the cognitive dysfunction outpatient clinic, Neurology Unit, HC-UFPR. They all underwent detailed clinical anamnesis and examination routine blood analyses to rule out treatable causes of dementia. All AD patients were HIV, HCV, syphilis, and neurosyphilis negatives. All AD participants met dementia criteria of the Diagnostic and Statistical Manual of Mental Disorders, DSM-V (American Psychiatric Association, 2013), and criteria for probable AD according to the National Institute on Aging and Alzheimer’s Association, NIA-AA (McKhann et al., 2011). Diagnostic methods used on the AD group were previously described (de Almeida et al., 2018B). Clinical diagnoses of AD were made independently of CSF AD biomarkers. AD participants were classified, at the moment of CSF and serum collection, with probable AD, with a clinical dementia rating (CDR) of 2 (2-2.5), indicating moderate dementia, severe decrease of daily instrumental activity, and no associated depression (Table 1). Neuroimaging (n=18), computed tomography or magnetic resonance imaging, showed volumetric reduction of the brain, absence of expansive lesions or extra-axial collections, or pathological calcifications in the brain. The median duration of symptoms was 36 (range 24 to 60) months.
Laboratory Methods
Serum and CSF biomarkers.
Amyloid β isoforms (Aβ38, Aβ40, and Aβ42) were assayed by electrochemiluminescence (MSD, Meso Scale Discovery multi-array; Rockville, MD, USA). The analytic sensitivity of these assays ranged between 0.1 to 10.1 pg/mL. All samples were assayed concurrently in duplicate according to manufacturers’ instructions. The acceptable coefficient of variation (CV) between duplicates was lower than 20%. When results were under the minimum low-detection limit determined by the manufacturer, the corresponding low-detection limit value was used for statistical analysis. Aβ-total was defined as the sum of all three Aβ isoforms quantified, which are the main Aβ isoforms produced. Ratios between Aβ38, Aβ40, Aβ42, and Aβ-total were calculated both for CSF and serum. We calculated Aβ isoforms CSF/serum ratios in order to evaluate the Aβ proportion between the compartments (blood and CSF) on the different groups studied. Not with the intend to identify the amount that crossed the blood brain barrier (BBB); as the transport of Aβ isoforms across the BBB, in both directions, is carrier mediated (Deane et al., 2009; El Ali and Rives, 2013).
Sample collection and storage.
CSF was collected by lumbar punctures with atraumatic spinal needles under aseptic conditions by a trained neurologist. All samples were collected at the same time on sampling days to limit diurnal variations. CSF and serum were collected in parallel. Samples were collected in polypropylene tubes to avoid adherence of the proteins to the tube walls in accordance with instructions from biomarkers reagent manufacturers. CSF total protein, glucose, and WBC counts were measured with standard laboratory methods. CSF and serum aliquots were frozen and stored at −80 °C at HC-UFPR facilities.
Data analyses
The demographic variables (age, gender and education) were compared between the HIV (+), the HIV (−), and the AD groups in pair-wise using Student t-tests for continuous variables and Fisher's exact test for binary and categorical variables. Demographic and HIV disease characteristics were compared between individuals with HIV-1 subtype B and C using similar methods. The CSF and serum biomarkers, the biomarker index, and the biomarker CSF/serum ratios were presented in terms of median (IQR), and log10-transformed prior to statistical analyses if their distributions were not approximately normal.
First, hierarchical comparisons were performed with AD versus HIV (+) groups as primary comparison, and HIV (−) versus AD groups and HIV (−) versus HIV (+) groups as secondary comparisons, without adjustment for multiple comparisons. Age and gender were included as covariates in multivariable linear regression models if they had a p-value of < 0.2 in the adjusted model. If the effect of age was shown statistically significant non-linearity, a smooth age effect was used within a generalized additive model (Wood, 2006). The p-values for the biomarker effects were then corrected for multiple testing with the Benjamini-Hochberg procedure, within each class of biomarkers (serum, CSF, index, and CSF/serum ratio)
Second, the CSF and serum biomarkers, the biomarker index, and the biomarker CSF/serum ratios were compared between HIV-1 subtypes B and C. A multivariable model was applied to control for plasma HIV viral load suppression and nadir CD4 count, which has been shown in previous studies to be associated with increased soluble biomarkers of inflammation and chemotaxis in HIV (Noel et al., 2014; Mooney et al., 2015). Using similar methods as above, the p-values were adjusted for multiple testing. In addition, simple linear regression was used to compare the serum biomarkers and biomarker CSF/serum ratios of controls vs. HIV subtype B, and controls vs. HIV subtype C.
In correlation analysis, the correlation coefficients (rs) were estimated using Spearman’s rank-order method. We tested the correlation of serum biomarkers and biomarker indexes with the variables: global deficit score (GDS); HIV infection characteristics (duration of infection, age at the time of study, age at the time of the beginning of the infection, plasma and CSF HIV RNA, peripheral blood platelets cell count); cell immunity characteristics [nadir CD4, current CD4, CD4 recovery = current CD4 - nadir CD4].
Results were considered statistically significant at the 5% alpha level. Statistical analyses were implemented using R version 3.2.3, 2015. Cohen’s d effect sizes (and 95% confidence intervals) were reported for differences between groups.
RESULTS
Aβ isoform levels were on average 50-1000 times higher in CSF than in serum in all three groups studied. Aβ isoforms were not significantly correlated between CSF and blood in the HIV (+) group or subtypes (all p>0.05). Aβ isoform CSF/serum ratios in the three groups studied are shown in Table 2. Across all participants, the levels of serum Aβ-40 were higher than the levels of other Aβ isoforms. The concentrations of Aβ38, Aβ40, Aβ42, and Aβ-total in CSF were described previously (de Almeida et al., 2018B).
Table 2.
HIV (+); HIV (−) and Alzheimer’s disease levels of serum amyloid-β isoforms, indexes, and cerebrospinal fluid/serum ratios.
| Biomarker | HIV+ | AD | CTRL | (a) Diff (95% CI) | Pa | (b)Diff (95% CI) | Pb | (c) Diff (95% CI) | pc |
|---|---|---|---|---|---|---|---|---|---|
| Serum | 65 | 24 | 18 | ||||||
| Aβ-38, pg/mL | 2.48 (2.48; 21.39) | 24.60 (9.00; 75.8) | 7.226 (2.48; 22.50) | −0.81 (−1.33, −0.28) | 0.002 | 0.07 (−0.45, 0.59) | 0.79 | 0.71 (0.001, 1.41) | 0.049 |
| Aβ-40, pg/mL | 93.40 (59.55; 132.0) | 167.6 (145.4; 234.5 | 117.9 (108.0; 141.7) | −1.06 (−1.69, −0.43) | <0.001 | 0.18 (−0.38, 0.73) | 0.61 | 0.69 (0.001, 1.38) | 0.049 |
| Aβ-42, pg/mL | 5.14 (2.68; 7.73) | 9.95 (7.51; 14.71) | 7.99 (5.24; 9.55) | −1.68 (−1.46, −0.35) | <0.001 | 0.73 (0.07, 1.38) | 0.023 | 1.71 (0.006, 3.41) | 0.049 |
| Aβ-Total,pg/mL | 109.2 (67.65;143.8) | 213.0 (170.1; 347.9 | 140.6 (121.7; 172.6) | −1.07 (−1.70, −0.44) | <0.001 | 0.2 (−0.36, 0.75) | 0.61 | 0.71 (0.001, 1.42) | 0.049 |
| Indexes in serum | |||||||||
| Aβ-38/Aβ-40 | 0.0796 (0.036; 0.205) | 0.158 (0.077; 0.310) | 0.077 (0.0217; 0.272) | −0.33 (−0.84, 0.19) | 0.19 | 0.021 (−0.54, 0.59) | 0.94 | −0.59 (−0.94, 0.39) | 0.460 |
| Aβ-38/Aβ-Total | 0.069 (0.032; 0.162) | 0.125 (0.061; 0.221) | 0.065 (0.020; 0.174) | −0.32 (−0.83, 0.19) | 0.19 | 0.047 (−0.52, 0.62) | 0.94 | −0.62 (−0.95, 0.38) | 0.460 |
| Aβ-40/Aβ-Total | 0.866 (0.784; 0.914) | 0.790 (0.692; 0.890) | 0.860 (0.734; 0.918) | 0.35 (−0.16, 0.86) | 0.19 | 0.15 (−0.42, 0.72) | 0.94 | −0.23 (−0.89, 0.44) | 0.460 |
| Aβ-42/Aβ-38 | 0.876 (0.270; 1.841) | 0.432 (0.148; 1.290) | 1.291 (0.380; 2.821) | 0.05(−0.42, 0.52) | 0.90 | 0.31(−0.21, 0.83) | 0.24 | 0.31(−0.31, 0.92) | 0.830 |
| Aβ-42/Aβ-40 | 0.06 (0.04; 0.07) | 0.06 (0.05; 0.07) | 0.07 (0.05; 0.10) | −0.86 (−0.98, 0.05) | 0.084 | 0.58 (0.03, 1.13) | 0.045 | −0.03 (−0.69, 0.64) | 0.940 |
| Aβ-42/Aβ-Total | 0.05 (0.03; 0.06) | 0.05 (0.04; 0.06) | 0.06 (0.05; 0.09) | −0.19 (−0.66, 0.28) | 0.43 | 0.54 (0.01, 1.09) | 0.045 | −0.08 (−0.74, 0.59) | 0.940 |
| CSF/serum | |||||||||
| Aβ-38 | 507.9 (91.97; 918.2) | 65.48 (24.11; 265.30) | 322.90 (93.18; 846.60) | 0.86 (0.33, 1.37) | <0.001 | 0.03 (−0.53, 0.59) | 0.92 | −0.84 (−1.61, −0.07) | 0.023 |
| Aβ-40 | 49.11 (27.19; 73.60) | 23.42 (16.52; 32.73) | 38.64 (25.75; 48.01) | 1.02 (0.43, 1.59) | <0.001 | 0.04 (−0.52, 0.60) | 0.92 | −0.77 (−1.47, −0.05) | 0.032 |
| Aβ-42 | 83.17 (56.63; 170.2) | 27.15 (16.41; 44.51) | 76.71(61.58; 132.60) | 1.58 (0.86, 2.29) | <0.001 | 0.21 (−0.35, 0.77) | 0.92 | −1.31 (−2.23, −0.38) | 0.001 |
| Aβ-Total | 66.56 (39.50; 97.51) | 25.26 (18.63; 41.64) | 50.08 (38.31; 68.65) | 1.09 (0.47, 1.71) | <0.001 | 0.06 (−0.50, 0.62) | 0.92 | −0.86 (−1.63, −0.08) | 0.023 |
HIV(+)xAD;
HIV(+)xCTRL;
ADxCTRL. Values in median (IQR); Diff: Group differences presented as Cohen’s d; CI: confidence interval; p: p value; all p values adjusted for multiple testing with the Benjamini-Hochberg (BH) method, (a) and (c) adjusted for gender or age and BH method.
Significant differences are in bold typeface.
Serum Aβ in PLWH, HIV(−), and AD patients
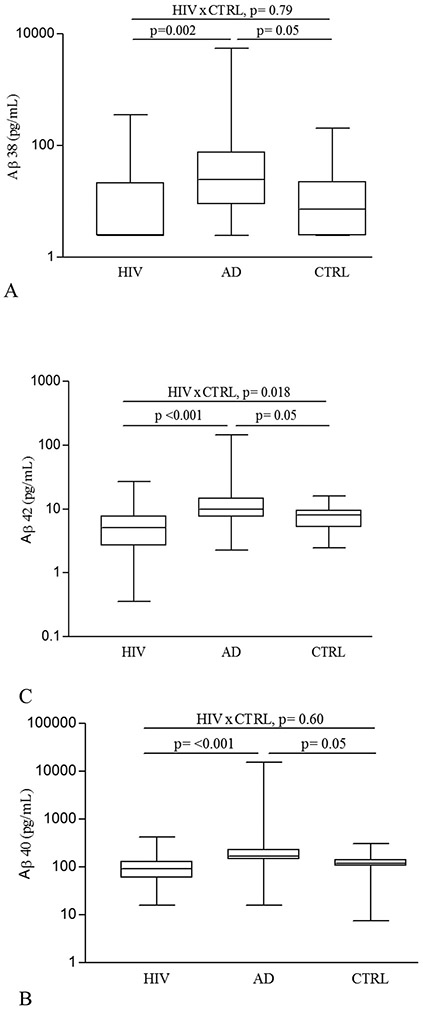
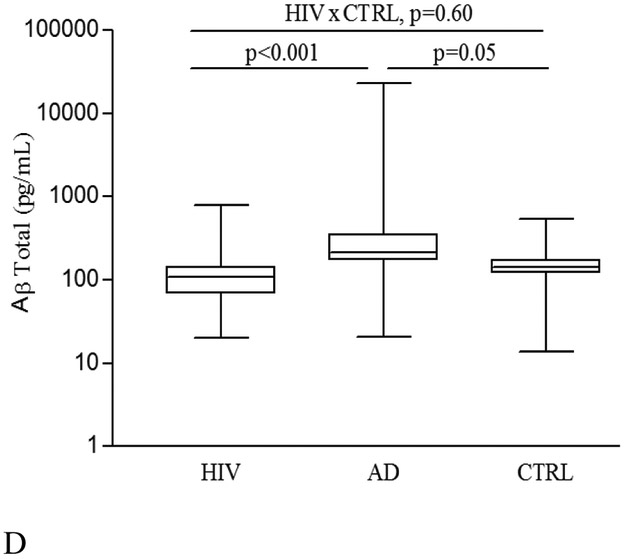
Figure 1 shows that serum levels of Aβ38, Aβ40, Aβ42, and Aβ-total were significantly lower in the HIV(+) group than in the AD group (for Aβ38 p= 0.002; all others p < 0.001). Serum levels of Aβ42 were also lower in PLWH than in the HIV(−) group (p= 0.018). Pair-wise comparisons of Aβ isoforms and ratios are shown in Table 2.
Figure 1. Serum Amyloid-beta (Aβ) alloform concentrations in HIV(+), AD, and HIV(−) control (CTRL) groups.
(A) Aβ38 (pg/mL); (B) Aβ40 (pg/mL); (C) Aβ42 (pg/mL); and (D) Aβ-total (pg/mL). Comparisons between HIV(+) and AD, and AD and CTRL groups were adjusted for gender or age. All p values were adjusted for multiple testing with the Benjamini-Hochberg (BH) method. The line in the center of the box represents median; the superior and inferior borders of the box represents IQRs; the whiskers represent the least and greatest values.
The ratio of serum Aβ42/Aβ40 was lower in PLWH than in the HIV(−) group (p = 0.045; Table 2). The proportion of Aβ42 to Aβ-total in PLWH was lower than that for HIV− (p = 0.045), but did not differ from AD participants (p = 0.43). However, the proportions of the other Aβ isoforms studied did not differ between three groups (Table 2).
HIV-1B and HIV-1C patients
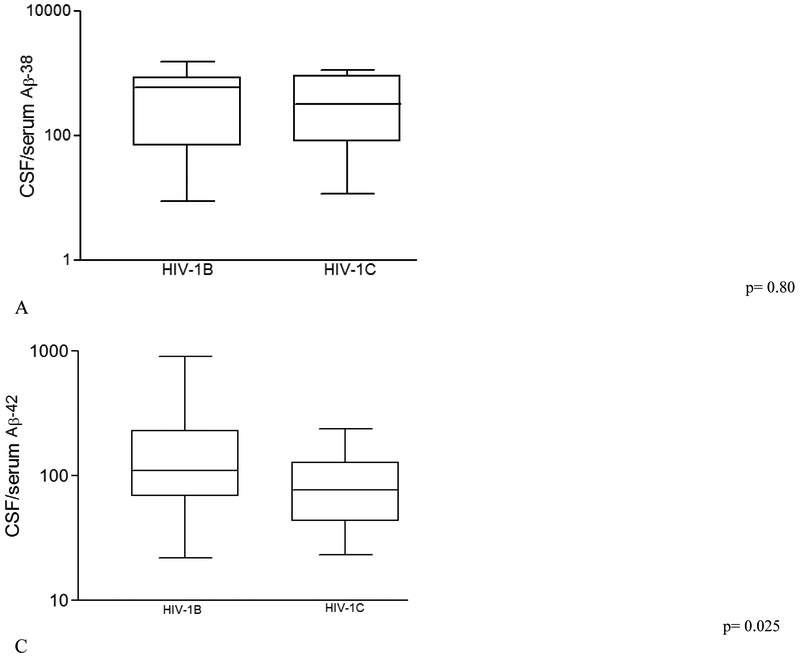
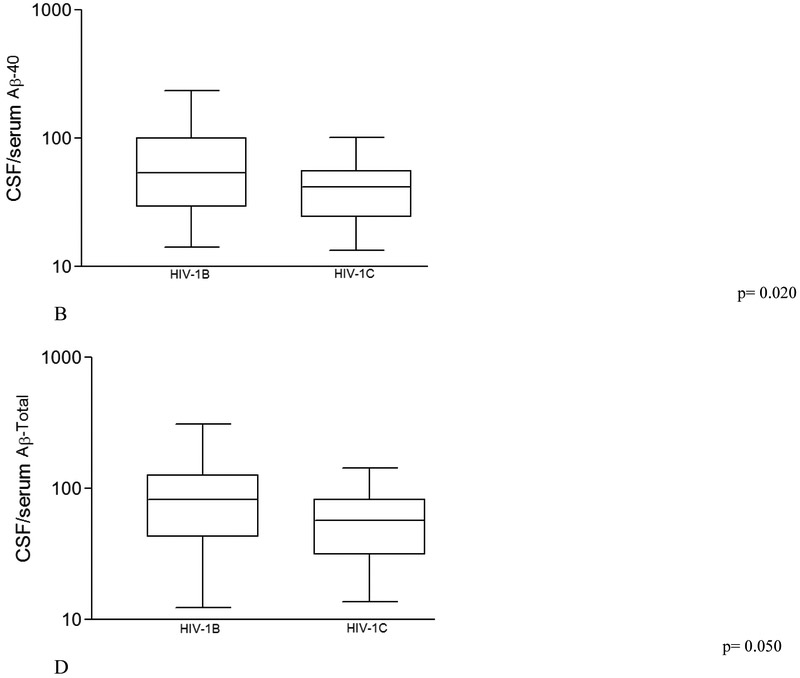
Comparisons of serum Aβ isoforms, between HIV-1 subtypes B and C, and control group, are shown on table 3. CSF/serum ratios of Aβ40, Aβ42, and Aβ-total were lower in HIV-1C than in HIV-1B (p = 0.020, 0.025, and 0.050 respectively; Figure 2); although comparable with the HIV(−) control group.
Table 3.
. HIV-1 subtype B and C levels of serum amyloid-β isoforms, indexes, and cerebrospinal fluid/serum ratios.
| Biomarker | HIV-B | HIV-C | BxC Diff (95% CI) |
BxC pa |
BxC pb |
BxCtrl Diff (95% CI) |
BxCtrl pc |
BxCtrl Pd |
CxCtrl Diff (95% CI) |
CxCtrl pc |
CxCtrl pd |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Serum | 25 | 26 | |||||||||
| Aβ-38, pg/mL | 2.48 (2.48; 24.75) | 4.58 (2.48; 17.69) | −0.01 (−0.59, 0.56) | 0.93 | 0.93 | −0.08 (−0.68, 0.53) | 0.809 | 0.81 | −0.07 (−0.67, 0.53) | 0.83 | 0.92 |
| Aβ-40, pg/mL | 64.30 (43.45; 147.90) | 99.55 (70.95; 133.60) | −0.46 (−1.04, 0.13) | 0.11 | 0.34 | −0.32 (−0.93, 0.29) | 0.307 | 0.41 | 0.03 (−0.57, 0.63) | 0.93 | 0.92 |
| Aβ-42, pg/mL | 3.24 (2.06; 6.83) | 5.67 (3.11; 7.93) | −0.41 (−0.99, 0.17) | 0.19 | 0.34 | −0.93 (−1.54, −0.32) | 0.004 | 0.018 | −0.69 (−1.30, −0.09) | 0.029 | 0.11 |
| Aβ-Total. pg/mL | 78.59 (51.79; 162.30) | 118 (78.66; 147) | −0.37 (−0.95, 0.21) | 0.20 | 0.34 | −0.32 (−0.92, 0.29) | 0.309 | 0.41 | −0.03 (−0.63, 0.57) | 0.92 | 0.92 |
| Indexes in serum | |||||||||||
| Aβ-38/Aβ-40 | 0.114 (0.041; 0.211) | 0.045 (0.031; 0.234) | 0.31 (−0.28, 0.90) | 0.31 | 0.34 | 0.14 (−0.46, 0.75) | 0.646 | 0.77 | −0.10 (−0.70, 0.50) | 0.75 | 0.82 |
| Aβ-38/Aβ-Total | 0.093 (0.037; 0.166) | 0.042 (0.029; 0.184) | 0.31 (−0.28, 0.90) | 0.31 | 0.34 | 0.17 (−0.44, 0.77) | 0.594 | 0.77 | −0.07 (−0.67, 0.53) | 0.82 | 0.82 |
| Aβ40/Aβ-Total | 0.854 (0.784; 0.912) | 0.900 (0,762; 0.923) | −0.29 (−0.89, 0.30) | 0.34 | 0.34 | 0.04 (−0.56, 0.65) | 0.890 | 0.89 | 0.30 (−0.30, 0.90) | 0.34 | 0.65 |
| Aβ-42/Aβ-38 | 0.783 (0.206; 1.277) | 0.931 (0.249; 1.951) | −0.09 (−0.63, 0.45) | 0.55 | 0.55 | −0.42 (−1.03, 0.19) | 0.182 | 0.36 | −0.24 (−0.84, 0.36) | 0.43 | 0.65 |
| Aβ-42/Aβ-40 | 0.06 (0.03; 0.08) | 0.06 (0.05; 0.07) | 0.15 (−0.43, 0.72) | 0.60 | 0.75 | −0.57 (−1.17, 0.04) | 0.075 | 0.22 | −0.69 (−1.29, −0.09) | 0.029 | 0.12 |
| Aβ-42/Aβ-Total | 0.05 (0.03; 0.07) | 0.05 (0.04; 0.06) | 0.10 (−0.48, 0.67) | 0.75 | 0.75 | −0.57 (−1.17, 0.04) | 0.074 | 0.22 | −0.65 (−1.25, −0.05) | 0.039 | 0.12 |
| CSF/serum | |||||||||||
| Aβ-38 | 609.1 (70.16; 882.8) | 328.6 (82.36; 918.2) | 0.08 (−0.51, 0.67) | 0.80 | 0.91 | 0.04 (−0.57, 0.65) | 0.897 | 0.90 | −0.05 (−0.65, 0.55) | 0.87 | 0.87 |
| Aβ-40 | 53.99 (29.33; 99.86) | 41.88 (24.33; 55.54) | 0.73 (0.14, 1.32) | 0.020 | 0.099 | 0.25 (−0.36, 0.85) | 0.431 | 0.60 | −0.28 (−0.88, 0.32) | 0.37 | 0.87 |
| Aβ-42 | 110.3 (68.88; 230.4) | 76.46 (43.43; 128.1) | 0.70 (0.11, 1.29) | 0.025 | 0.099 | 0.56 (−0.04, 1.17) | 0.076 | 0.31 | −0.14 (−0.74, 0.46) | 0.65 | 0.87 |
| Aβ-Total | 82.50 (42.61; 125.5) | 57.13(31.17; 82.76) | 0.61 (0.02, 1.20) | 0.050 | 0.13 | 0.24 (−0.37, 0.84) | 0.450 | 0.60 | −0.24 (−0.84, 0.36) | 0.44 | 0.87 |
Values in median (IQR); significant differences are in bold typeface; Diff: Group differences presented as Cohen’s d; CI: confidence interval; B, HIV-B; C, HIV-C; Ctrl, controls; p, p value;
p values adjusted for plasma VL suppression and CD4 nadir count;
p values adjusted for plasma VL suppression and CD4 nadir count, corrected for multiple testing with the Benjamini-Hochberg (BH) method.
p values not adjusted;
p values corrected for multiple testing with the BH method.
Figure 2. Cerebrospinal fluid /serum ratio of main Amyloid-beta (Aβ) isoforms from HIV-1 subtype B and C samples.
(A) CSF/serum ratio for Aβ38; (B) CSF/serum ratio for Aβ40; (C) CSF/serum ratio for Aβ42; and (D) CSF/serum ratio for Aβ-total. P values were adjusted for plasma VL suppression and CD4 nadir count, not corrected for multiple testing with the Benjamini-Hochberg (BH) method. We calculated Aβ isoforms CSF/serum ratios in order to evaluate the Aβ proportion between the compartments (blood and CSF) on the different groups studied.
The line in the center of the box represents median; the superior and inferior borders of the box represents IQRs; the whiskers represent the least and greatest values.
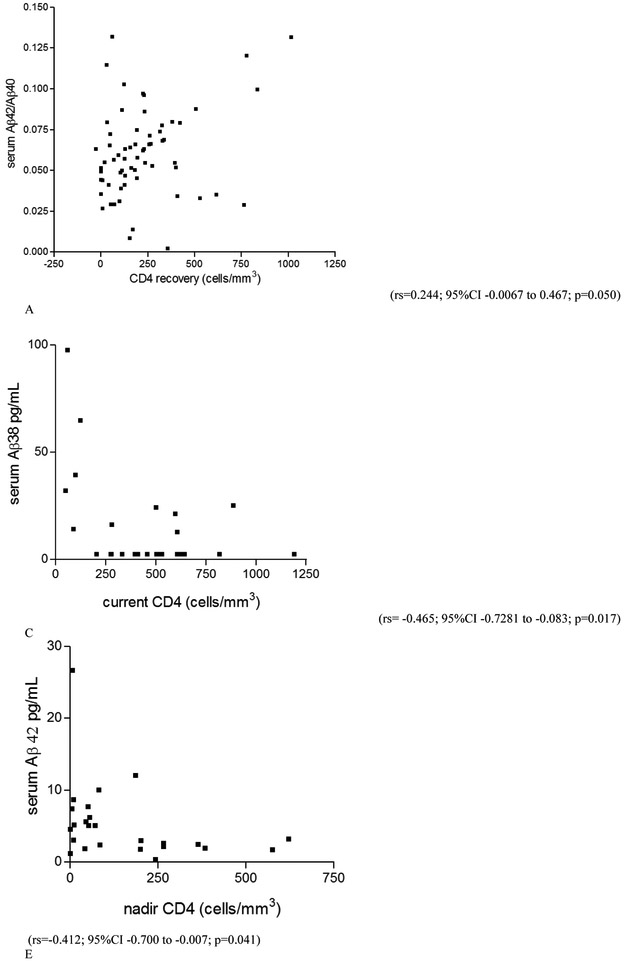
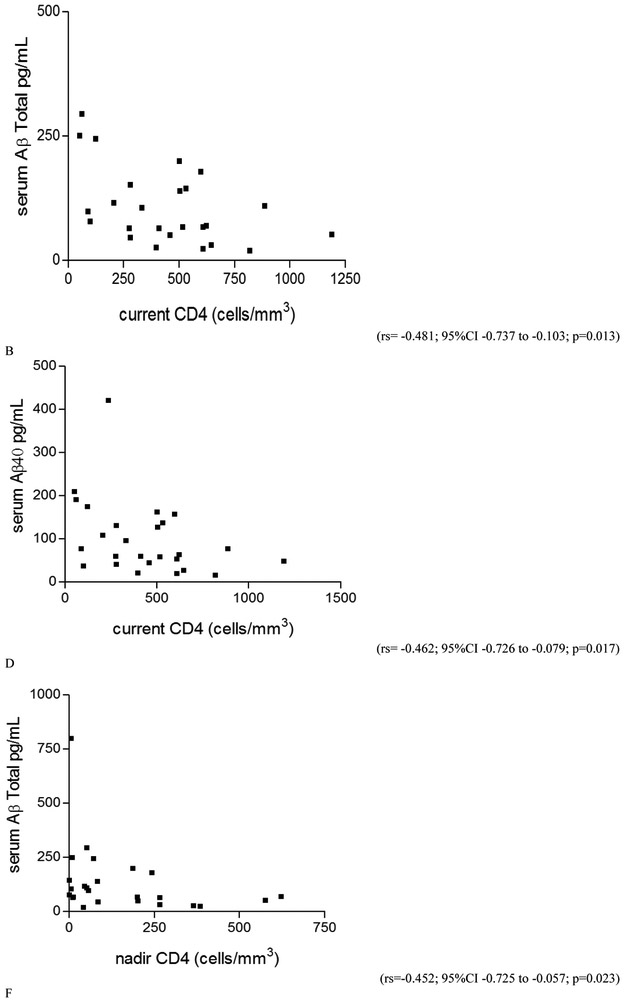
Higher current CD4 was significantly correlated with lower serum Aβ38, Aβ40, and Aβ-total levels in PLWH as a whole and in HIV-1B, but not in HIV-1 subtype C (for PLWH: rs = −0.311, p = 0.012; rs = −0.266, p = 0.032; rs = −0.293, p = 0.018; for HIV-1B: rs = −0.465, p = 0.017; rs = −0.462, p = 0.017; rs = −0.481, p = 0.013, respectively). Nadir CD4 correlated negatively with serum Aβ42 and Aβ-total (rs = −0.412, p = 0.041; rs = −0.452, p = 0.023, respectively) in the HIV-1B group, but not in PLWH as a whole or HIV-1 C group. Greater CD4 recovery correlated with higher Aβ42/Aβ40 ratio in serum (rs = 0.244; p = 0.050) in PLWH as a whole, but not when subtypes were analyzed separately (Figure 3). There was no correlation between serum Aβ isoform levels and other variables studied (p > 0.05).
Figure 3. Correlation between serum Aβ isoforms and cell immunity characteristics in the HIV+ group.
A. CD4 recovery correlated positively with serum Aβ42/Aβ40 ratio in the HIV(+) group as a whole. This ratio represents the proportion between the main Aβ isoform constituent the amyloid plaques (Aβ-42) in brain, and the main AB isoform synthetized (Aβ40). In the HIV-1B group, but not on subtype C: there was moderate negative correlation between: B. current CD4 (cells/mm3) in blood and Aβ-42 (pg/mL) in serum; C. current CD4 (cells/mm3) in blood and Aβ-38 (pg/mL) in serum; D. current CD4 (cells/mm3) in blood and Aβ-40 (pg/mL) in serum; E. nadir CD4 (cells/mm3) in blood and Aβ-42 (pg/mL) in serum; F. nadir CD4 (cells/mm3) in blood and Aβ-Total (pg/mL) in serum.
PLWH participants’ clinical characteristics.
From the group, 52 (80%) patients had AIDS; duration of infection, 87 (29; 135) months; current CD4, 347 (193; 534) cell/mm3; nadir CD4, 88 (28; 267) cell/mm3; Log plasma HIV RNA 1.7 (1.7; 3.6); plasma HIV RNA < 50 copies/mL, 35 (54%); Log CSF HIV RNA, 1.7 (1.7-2.8); CSF HIV RNA < 50 copies/mL, 32 (49%); on combination antiretroviral therapy (cART), 52 (80%); anti-retroviral CNS penetration effectiveness (CPE) (Letendre et al., 2010), 6 (5-9); adherence, antiretroviral treatment adherence was evaluated using AIDS clinical trial group (ACTG) adherence questionnaire (4-day recall), 51 (93%). From those on therapy, the most frequent cART regimen (33 participants; 63%) contained a ritonavir-boosted HIV protease inhibitor (PI) plus two nucleoside/nucleotide reverse transcriptase inhibitors (NRTI and NtRTI); whereas 18 participants (35%) received a non-nucleoside RT inhibitor (NNRTI) with 2 NRTIs, and 4 (8%) participants received other regimen types. Platelets count in the HIV+ group as a whole 223×103(179 ×103; 256 ×103); in the group HIV-1B 227 ×103 (176 ×103; 259 ×103); and HIV-1C 221 ×103 (181 ×103; 253 ×103), p=0.948
DISCUSSION
This exploratory survey provides a broad view of the impact of HIV in the amyloidogenic pathway in blood, over the course of chronic HIV infection. Our findings suggest that impaired cellular immunity (low CD4 cell count, nadir or current) influences serum Aβ in a subtype dependent pattern; in HIV-1B but not HIV-1C low CD4 may increase synthesis or decrease clearance of specific Aβ isoforms. However CD4 recovery was associated with an increased serum Aβ-42/Aβ-40 ratio, independent of subtype. A higher ratio may indicate greater production or decreased clearance of Aβ-42 and higher probability to develop Aβ deposits.
Aβ accumulation in brain is one of the pathological hallmarks in AD; and previous reports showed increased levels of blood Aβ in AD participants (Wang et al., 2017). Aβ overproduction is widespread in the peripheral organs and tissues in AD patients. It has been shown that β-secretase activity in the membranes of platelets is increased in patients with mild cognitive decline and AD (Liu et al., 2007; Johnston et al., 2008; Bu et al., 2017), but whether peripherally-generated Aβ contributes to the pathogenesis of AD is uncertain (Citron et al., 1994; Kuo et al., 2000; Deane and Zlokovic, 2007; Bu et al., 2017). It has traditionally been thought that the Aβ deposited in the brain originates in neurons. However, investigators have shown that Aβ crosses the BBB, thus Aβ present in plasma may contribute to the development of Aβ deposits in the brain7 and induce AD-type pathologies such as the induction and promotion of Tau hyperphosphorylation (Bu et al., 2017).
In this study the ratio of serum Aβ42/Aβ40 was lower in PLWH as compared to the HIV(−) group. A previous report found that in AD patients CSF Aβ42/40 ratio was superior to quantification of Aβ42 alone as a marker of amyloid-positivity by PET (Lewczuk et al., 2010).
Previous work has demonstrated a great impact of HIV infection on Aβ metabolisms (Green et al., 2005; Rempel and Pulliam, 2005; Aksenov et al., 2010). Impaired cellular immunity (CD4, CD4/CD8 ratio) is a hallmark of HIV infection. In this study poorer cell-mediated immunity characteristics (current or nadir CD4) were associated with higher levels of Aβ isoforms in PLWH as a whole and among HIV-1B, but not HIV-1 subtype C. These findings are consistent with greater disturbance of amyloid processing as HIV-related immunosuppression worsens. The positive correlation between CD4 recovery, and the serum Aβ-42/Aβ-40 ratio, as well as the negative correlation of Aβ isoforms and nadir CD4, can be result of immunological disbalance, due to an impaired cell immunity recovery with less effective CD4 cells, mainly on those cases on which there was very low CD4 nadir. This was the first study to show the impact of HIV infection on serum Aβ metabolism, future studies examining the mechanism by which improved immunity influences it are necessary.
Our results add to previously published studies, investigating differences between HIV-1 subtypes B and C, in which CSF Aβ42 levels decreased in HIV-1C compared to HIV-1B (de Almeida et al., 2018B). We previously reported that CSF Aβ42 levels in PLWH were lower than HIV− controls (de Almeida et al., 2018B); here we found that serum Aβ42 levels showed a similar pattern. These results were different from reported patterns of Aβ isoforms in AD, where CSF levels are decreased, but serum levels are increased. The majority of AD studies describe increased blood Aβ levels (Mehta et al., 2000; van Oijen et al., 2006; Graff-Radford et al., 2007; Wang et al., 2017); although these results are not uniform (Lopez et al., 2008; Toledo et al., 2013).
In the present study, there was no correlation between Aβ isoforms in serum and CSF. The brain is the main source of Aβ isoforms in blood. In addition to the brain, Aβ is produced in peripheral tissues or cells and secreted into blood circulation, contributing to the pool of circulating Aβ (Bu et al., 2017). Aβ is generated by the proteolytic cleavage of the amyloid precursor protein (APP). APP and the enzymes required for Aβ generation from APP are expressed in many tissues (Deane and Zlokovic, 2007; Bu et al., 2017). In the brain, APP is ubiquitously expressed in neurons, which are believed to be its primary source (Peterson et al., 2014). In peripheral tissues, besides the brain, platelets are the primary source and can generate Aβ by a mechanism similar to that in neurons ((Chen et al., 1995; Evin et al., 2003; Liu et al., 2007; Johnston et al., 2008; Bu et al., 2017). Other possible sources for the production of peripheral Aβ are skin fibroblasts, skeletal muscles, and cerebrovascular smooth muscle cells (Citron et al., 1994; Kuo et al., 2000; Van Nostrand and Melchor, 2001).
One possibility to explain the lower blood Aβ levels in HIV is thrombocytopenia (Marks et al., 2009). This would be expected to lead to less APP and Aβ production, accounting for our observation that Aβ levels in HIV were lower than controls. Thrombocytopenia is a common feature among HIV-positive patients; it is considered a multifactorial disorder, commonly due to immune mechanisms (Marks et al., 2009). However, we found no correlation between platelet counts in peripheral blood and blood Aβ isoforms levels and ratios. Other mechanisms must be considered to explain the blood Aβ isoforms levels in HIV.
Both animal and human studies suggest that HIV can disrupt several steps in the amyloid cascade, from Aβ biogenesis to clearance. The main HIV amyloidogenic protein is Tat (Rempel and Pulliam, 2005; Aksenov et al., 2010 ; Daily et al., 2006; Lan et al., 2011; Giunta et al., 2008; Kim et al., 2013); although several other proteins as NEF (White et al. , 2005); gp41(Mankowski et al., 2002); and Gp120 (Aksenov et al., 2010; Zhang et al., 2011) are amyloidogenic as well. HAART interferes with amyloidogenesis by reducing LRP (Tran et al., 2003), inhibiting Aβ degradation (Hamel, 2007; Lan et al., 2012), increasing production, and decreasing clearance of Aβ peptides (Brown et al., 2014).
In this study, we found that the main Aβ product in blood was Aβ40 as well as in CSF, which are consistent with other research’s results; although we found a higher percentage in serum (~90%) than in CSF (~70%) (de Almeida et al., 2018B; van der Kant and Goldstein, 2015).
The present cross-sectional study is not free of limitations. It does not include a substantial number of older (>60 years) PLWH, who are hypothetically more vulnerable to AD than young PLWH. We did not assess HIV(+) patients with known AD, though this combination of conditions is rare. The HIV(−) group was age and gender matched with the HIV(+) group, in consequence, individuals were younger than those from the AD group. Samples from the control group were collected in a different site than those from the diseased groups (HIV+ and AD). In addition, there are several difficulties in the measurement of Aβ levels in body fluids. Low Aβ concentrations in blood, for example, require sensitive and reliable laboratory assays. Aβ measurements are sometimes variable and imprecise measurements vary between studies and laboratories, indicating that standardization of both preanalytical and analytical procedures is essential (Molinuevo et al., 2013). In regard to blood Aβ measurements, contradicting results have been reached by using different detection methods or research designs (Toledo et al., 2013). The positive points of this study are that, to our knowledge, this is the first study on blood Aβ isoforms in PLWH. Also, most published studies on aging biomarkers from HIV populations only involve HIV-1 subtype B; this is the first study including participants with HIV-1 subtype C. Consequently, this study will contribute to the understanding of the pathophysiology of HIV infection in blood and the CNS, and the impact of HIV-1 genetic diversity in HIV-related metabolic changes.
We conclude that there was impact of HIV infection on blood amyloidal metabolism in a subtype-dependent way, with differences between subtypes B and C. However, it does not appear that measurement of blood Aß isoforms will be useful as a biomarker of HIV-associated dementia, as no correlation between GDS and serum Aß biomarkers was found. The differences between HIV and AD in the patterns of serum Aβ isoforms suggested that HIV infection and AD may not share some of same mechanisms of impairment; corroborating the findings in CSF (da Almeida et al., 2018B). More studies are necessary, to test the usefulness of blood Aβ biomarkers to predict the development of HAND or the differential diagnosis with AD.
ACKNOWLEDGEMENTS
This work was supported by the following grants: National Institute of Health, NIH R21 MH76651 (Ellis, Ronald J; Almeida, Sergio M.), S10 RR31646 (Letendre, Scott), K24 MH097673(Letendre, Scott); University of California, San Diego, Center for AIDS Research (CFAR), an NIH-funded program (P30 AI036214), which is supported by the following NIH Institutes and Centers: NIAID, NCI, NIMH, NIDA, NICHD, NHLBI, NIA, NIGMS, and NIDDK. The HIV Neurobehavioral Research Center (HNRC) is supported by Center award P30MH062512 from NIMH.The San Diego HIV Neurobehavioral Research Center [HNRC] group is affiliated with the University of California, San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Healthcare System, and includes: Director: Robert K. Heaton, Ph.D., Co-Director: Igor Grant, M.D.; Associate Directors: J. Hampton Atkinson, M.D., Ronald J. Ellis, M.D., Ph.D., and Scott Letendre, M.D.; Center Manager: Thomas D. Marcotte, Ph.D.; Jennifer Marquie-Beck, M.P.H.; Melanie Sherman; Neuromedical Component: Ronald J. Ellis, M.D., Ph.D. (P.I.), Scott Letendre, M.D., J. Allen McCutchan, M.D., Brookie Best, Pharm.D., Rachel Schrier, Ph.D., Debra Rosario, M.P.H.; Neurobehavioral Component: Robert K. Heaton, Ph.D. (P.I.), J. Hampton Atkinson, M.D., Steven Paul Woods, Psy.D., Thomas D. Marcotte, Ph.D., Mariana Cherner, Ph.D., David J. Moore, Ph.D., Matthew Dawson; Neuroimaging Component: Christine Fennema-Notestine, Ph.D. (P.I.), Monte S. Buchsbaum, M.D., John Hesselink, M.D., Sarah L. Archibald, M.A., Gregory Brown, Ph.D., Richard Buxton, Ph.D., Anders Dale, Ph.D., Thomas Liu, Ph.D.; Neurobiology Component: Eliezer Masliah, M.D. (P.I.), Cristian Achim, M.D., Ph.D.; Neurovirology Component: David M. Smith, M.D. (P.I.), Douglas Richman, M.D.; International Component: J. Allen McCutchan, M.D., (P.I.), Mariana Cherner, Ph.D.; Developmental Component: Cristian Achim, M.D., Ph.D.; (P.I.), Stuart Lipton, M.D., Ph.D.; Participant Accrual and Retention Unit: J. Hampton Atkinson, M.D. (P.I.), Jennifer Marquie-Beck, M.P.H.; Data Management and Information Systems Unit: Anthony C. Gamst, Ph.D. (P.I.), Clint Cushman; Statistics Unit: Ian Abramson, Ph.D. (P.I.), Florin Vaida, Ph.D. (Co-PI), Bin Tang, Ph.D., Anya Umlauf, M.S.
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government.
Study funding
This study was funded by CFAR (International Pilot Grant P30 AI036214 and CFAR Visiting Researcher Grant PTHMON7) and NIMH (R21 MH076651-01).
Footnotes
Conflict of Interest:
Sérgio Monteiro de Almeida – the author declare that they have no conflict of interest.
Clea Ribeiro – the author declare that they have no conflict of interest.
Indianara Rotta – the author declare that they have no conflict of interest.
Scott Letendre – the author declare that they have no conflict of interest.
Michael Potter – the author declare that they have no conflict of interest.
Bin Tang – the author declare that they have no conflict of interest.
Meire Silva Batistela – the author declare that they have no conflict of interest.
Florin Vaida – the author declare that they have no conflict of interest.
Ronald Ellis – the author declare that they have no conflict of interest.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- Aksenov MY, Aksenova MV, Mactutus CF, et al. (2010) HIV-1 protein-mediated amyloidogenesis in rat hippocampal cell cultures. Neurosci Lett. 475: 174–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (2013) Diagnostic and Statistical Manual of Mental Disorders DSM-5. Washington, DC: American Psychiatric Association. [Google Scholar]
- Andras IE, Toborek M (2013) Amyloid Beta Accumulation in HIV-1-Infected Brain: the Role of the Blood Brain Barrier. IUBMB Life 65:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasil, Ministério da Saúde (2017) Programa Nacional de DST/AIDS. http://www.aids.gov.br/assistencia/manualdst/item12.htm. [Google Scholar]
- Brew BJ, Pemberton L, Blennow K, et al. (2005) CSF amyloid beta42 and tau levels correlate with AIDS dementia complex. Neurology 65:1490–1492. [DOI] [PubMed] [Google Scholar]
- Brown LAM, Jin J, Ferrell D, et al. (2014) Efavirenz Promotes b-Secretase Expression and Increased Ab1-40,42 via Oxidative Stress and Reduced Microglial Phagocytosis: Implications for HIV Associated Neurocognitive Disorders (HAND). PLoS ONE; 9: e95500. doi: 10.1371/journal.pone.0095500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu XL, Xiang Y, Jin WS, et al. (2017) Blood-derived amyloid-β protein induces Alzheimer’s disease pathologies. Mol.Psychiatry 00:1–9 [DOI] [PubMed] [Google Scholar]
- Chen M, Inestrosa NC, Ross GS, et al. (1995) Platelets are the primary source of amyloidβ-peptide in human blood. Biochem Bioph Res Com. 213:96–103 [DOI] [PubMed] [Google Scholar]
- Citron M, Vigo-Pelfrey C, Teplow DB, et al. (1994) Excessive production of amyloid beta-protein by peripheral cells of symptomatic and presymptomatic patients carrying the Swedish familial Alzheimer disease mutation. Proc Natl Acad Sci USA 91: 11993–11997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daily A, Nath A, Hersh LB (2006) Tat peptides inhibit neprilysin. J NeuroVirology 12:153–160. [DOI] [PubMed] [Google Scholar]
- Deane R, Zlokovic BV (2007) Role of the blood-brain barrier in the pathogenesis of Alzheimer’s disease. Curr Alzheimer Res. 4:191–197. [DOI] [PubMed] [Google Scholar]
- Deane R, Bell RD, Sagare A, Zlokovic BV (2009) Clearance of amyloid-β peptide across the blood-brain barrier: Implication for therapies in Alzheimer’s disease. CNS Neurol Disord Drug Targets 8:16–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida SM, Ribeiro CE, de Pereira AP, et al. (2013) Neurocognitive impairment in HIV-1 clade C- versus B-infected individuals in Southern Brazil. J Neurovirol 19:550–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida SM, Tang B, Ribeiro CE, et al. (2018A) Neprilysin in the Cerebrospinal Fluid and Serum of Patients Infected with HIV1-Subtypes C and B. J Acquir Immune Defic Syndr. 78:248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida SM, Ribeiro CE, Rotta I, et al. (2018B) Biomarkers of neuronal injury and amyloid metabolism in the cerebrospinal fluid of patients infected with HIV-1 subtypes B and C. J Neurovirol. 24:28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Ali A, Rivest S. The role of ABCB1 and ABCA1 in beta-amyloid clearance at the neurovascular unit in Alzheimer’s disease (2013) Front Physiol. 4:45 DOI: 10.3389/fphys.2013.00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esiri MM, Biddolph SC, Morris CS (1998) Prevalence of Alzheimer plaques in AIDS. J Neurol Neurosurg Psychiatry 65:29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evin G, Zhu A, Holsinger RM, et al. (2003) Proteolytic processing of the Alzheimer's disease amyloid precursor protein in brain and platelets. J Neurosci Res. 74:386–392. [DOI] [PubMed] [Google Scholar]
- Gisslén M, Krut J, Andreasson U, et al. (2009) Amyloid and tau cerebrospinal fluid biomarkers in HIV infection. BMC Neurology 9:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giunta B, Zhou Y, Hou H, et al. (2008) HIV-1 TAT Inhibits Microglial Phagocytosis of Abeta Peptide. Int J Clin Exp Pathol. 1: 260–275. [PMC free article] [PubMed] [Google Scholar]
- Graff-Radford NR, Crook JE, Lucas J, et al. (2007) Association of low plasma Abeta42/Abeta40 ratios with increased imminent risk for mild cognitive impairment and Alzheimer disease. Arch Neurol. 64:354–362. [DOI] [PubMed] [Google Scholar]
- Green DA, Masliah E, Vinters HV, et al. (2005) Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS 19:407–11. [DOI] [PubMed] [Google Scholar]
- Gross AM, Jaeger PA, Kreisberg JF, et al. (2016) Methylome-wide Analysis of Chronic HIV Infection Reveals Five-Year Increase in Biological Age and Epigenetic Targeting of HLA. Mol Cell 62:157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel F (2007) HIV Protease Inhibitors Inhibit Insulin-degrading Enzyme (IDE) Function. 67th Scientific Sessions. American Diabetes Association. [Google Scholar]
- Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297: 353–356. [DOI] [PubMed] [Google Scholar]
- High KP, Brennan-Ing M, Clifford DB, et al. (2012) HIV and aging: state of knowledge and areas of critical need for research. A report to the NIH Office of AIDS Research by the HIV and Aging Working Group. J Acquir Immune Defic Syndr. 60: S1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JA, Liu WW, Coulson DT, et al. (2008) Platelet beta-secretase activity is increased in Alzheimer's disease. Neurobiol Aging 29: 661–668. [DOI] [PubMed] [Google Scholar]
- Kim J, Yoon JH, Kim YS (2013) HIV-1 Tat interacts with and regulates the localization and processing of amyloid precursor protein. PLoS One. 29;8:e77972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight R, Khondoker M, Magill N, Stewart R, Landau S (2018) A Systematic Review and Meta-Analysis of the Effectiveness of Acetylcholinesterase Inhibitors and Memantine in Treating the Cognitive Symptoms of Dementia. Dement Geriatr Cogn Disord 45:131–151. [DOI] [PubMed] [Google Scholar]
- Krut JJ, Zetterberg H, Blennow K, et al. (2013) Cerebrospinal fluid Alzheimer’s biomarker profiles in CNS infections. J Neurol. 260:620–26. [DOI] [PubMed] [Google Scholar]
- Krut JJ, Gisslen M, Hagberg L, et al. (2014) Hyperphosphorylated Tau in Cerebrospinal fluid: a biomarker for neurological aging in HIV? CROI #453. [Google Scholar]
- Kuo YM, Kokjohn TA, Watson MD, et al. (2000) Elevated abeta42 in skeletal muscle of Alzheimer disease patients suggests peripheral alterations of AbetaPP metabolism. Am J Pathol. 156: 797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan X, Xu J, Kiyota T et al. (2011) HIV-1 reduces Abeta-degrading enzymatic activities in primary human mononuclear phagocytes. J Immunol. 186: 6925–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan X, Kiyota T, Hanamsagar R, et al. (2012) The Effect of HIV Protease Inhibitors on Amyloid-β Peptide Degradation and Synthesis in Human Cells and Alzheimer’s Disease Animal Model. J Neuroim Pharmacol. 7: 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letendre S, Ellis R, Deutsch R, et al. (2010) Correlates of time-to-loss-of-viral response in CSF and plasma in the CHARTER cohort. Program and abstracts of the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA 16-19 February (poster 430). [Google Scholar]
- Lewczuk P, Matzenc A, Blennowd K, et al. (2017) Cerebrospinal fluid AB42/40 Corresponds better than AB42 to amyloid PET in Alzheimer’s Disease. Journal of Alzheimer’s Disease 55: 813–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WW, Todd S, Craig D, et al. (2007) Elevated platelet beta-secretase activity in mild cognitive impairment. Dement Geriatr Cogn Disord. 24: 464–468. [DOI] [PubMed] [Google Scholar]
- Lopez OL, Kuller LH, Mehta PD, et al. (2008) Plasma amyloid levels and the risk of AD in normal subjects in the Cardiovascular Health Study. Neurology 70:1664–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankowski JL, Queen SE, Tarwater PM, et al. (2002) Accumulation of b-amyloid precursor protein in axons correlates with CNS expression of SIV gp41. J Neuropathol Exp Neurol. 61: 85–90 [DOI] [PubMed] [Google Scholar]
- Marks KM, Clarke RM, Bussel JB, et al. (2009) Risk factors for thrombocytopenia in HIV-infected persons in the era of potent antiretroviral therapy. J Acquir Immune Defic Syndr. 52:595–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, et al. (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7:263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta PD, Pirttila T, Mehta SP, et al. (2000) Plasma and Cerebrospinal fluid levels of amyloid BProteins 1-40 and 1-42 in Alzheimer disease. Arch Neurol. 57:100–105. [DOI] [PubMed] [Google Scholar]
- Molinuevo JL, Gispert JD, Dubois B, et al. (2013) The AD-CSF-Index discriminates Alzheimer’s disease patients from healthy controls: a validation study. J Alzheimers Dis.36:67–77. [DOI] [PubMed] [Google Scholar]
- Mooney S, Tracy R, Osler T, et al. (2015) Elevated biomarkers of inflammation and coagulation in patients with HIV are associated with higher Framingham and VACS risk index scores. PLoS One 10: e0144312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A, Hersh LB (2005) Tat and amyloid: multiple interactions. AIDS 19: 203–4. [DOI] [PubMed] [Google Scholar]
- Noel N, Boufassa F, Lecuroux C, et al. (2014) Elevated IP10 levels are associated with immune activation and low CD4(+) T-cellcounts in HIV controller patients. AIDS 28: 467–476. [DOI] [PubMed] [Google Scholar]
- Peterson J, Gisslen M, Zetterberg H, et al. (2014) Cerebrospinal Fluid (CSF) Neuronal Biomarkers across the Spectrum of HIV Infection: Hierarchy of Injury and Detection. PLoS ONE 9: e116081. doi: 10.1371/journal.pone.0116081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulliam L (2009) HIV regulation of amyloid beta production. J Neuroim Pharmacol. 4:213–217. [DOI] [PubMed] [Google Scholar]
- Raboni SM, Ribeiro CE, Almeida SM, et al. (2017) Impact of public health strategies on reducing AIDS mortality in southern Brazil. Int J STD AIDS 28:54–62. [DOI] [PubMed] [Google Scholar]
- Rempel HC, Pulliam L (2005) HIV-1Tat inhibits neprilysin and elevates amyloid beta. AIDS 19:127–35. [DOI] [PubMed] [Google Scholar]
- Toledo JB, Shaw LM, Trojanowski JQ (2013) Plasma amyloid beta measurements - a desired but elusive Alzheimer's disease biomarker. Alzheimers Res Ther. 5: 8. doi: 10.1186/alzrt162. PubMed: 23470128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran H, Robinson S, Mikhailenko I, et al. (2003) Modulation of the LDL receptor and LRP levels by HIV protease inhibitors. J Lipid Res. 44:1859–1869. [DOI] [PubMed] [Google Scholar]
- van der Kant R, Goldstein LSB (2015) Cellular Functions of the Amyloid Precursor Protein from Development to Dementia. Dev. Cell 32: 23. [DOI] [PubMed] [Google Scholar]
- Van Nostrand WE, Melchor JP (2001) Disruption of pathologic amyloid beta-protein fibril assembly on the surface of cultured human cerebrovascular smooth muscle cells. Amyloid 8: 20–27. [PubMed] [Google Scholar]
- van Oijen M, Hofman A, Soares HD, et al. (2006) Plasma Abeta(1-40) and Abeta(1-42) and the risk of dementia: a prospective case-cohort study. Lancet Neurol. 5:655–660. [DOI] [PubMed] [Google Scholar]
- Wang MJ, Yi SH, Han J, et al. (2017) Oligomeric forms of amyloid-β protein in plasma as a potential blood-based biomarker for Alzheimer’s disease. Alzheimer's Res Ther. 9:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JA, Manelli AM, Holmberg KH, et al. (2005) Differential effects of oligomeric and fibrillar amyloid-beta 1–42 on astrocyte-mediated inflammation. Neurobiol Dis. 18:459–65. [DOI] [PubMed] [Google Scholar]
- Wood SN (2006) Generalized Additive Models: An Introduction with R. Boca Raton, FL: CRC Press, LLC. [Google Scholar]
- Xu J, Ikezu T (2009) The comorbidity of HIV-associated neurocognitive disorders and Alzheimer’s disease: a foreseeable medical challenge in post-HAART era. J Neuroim Pharmacol. 4: 200–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Liu J, Katafiasz B, et al. (2011) HIV-1 gp120- induced axonal injury detected by accumulation of beta-amyloid precursor protein in adult rat corpus callosum. J Neuroim Pharmacol. 6: 650–7. [DOI] [PMC free article] [PubMed] [Google Scholar]