Abstract
Background and Aims
Rice ecosystems in the tropical coastal areas are subject to two types of flooding stress: transient complete submergence and long-term water stagnation (stagnant flooding). Here, we aimed to dissect the mechanisms for stagnant flooding tolerance of rice genotypes carrying SUB1, a quantitative trait locus for submergence tolerance.
Methods
We screened 80 elite genotypes under stagnant flooding stress in the lowland rice fields in the wet and dry seasons, and examined the tolerance mechanisms of promising genotypes for the two following seasons.
Key results
Yield reduction under stagnant flooding averaged 48 % in the dry season and 89 % in the wet season. Elite genotypes carrying SUB1 showed 49 % lower yield than those without SUB1 under stagnant flooding, with no differences under shallow water conditions. However, we identified a few high-yielding Sub1 genotypes that were as tolerant of stagnant flooding as a reference genotype that lacked SUB1. These genotypes had intermediate stature with more shoot elongation in response to rising water than a moderately tolerant Sub1 reference variety, resulting in greater canopy expansion and higher yield. It was important to increase lodging resistance, since plant height >140 cm increased lodging under stagnant flooding. The culm diameter was closely associated with culm strength; reduced aerenchyma formation and increased lignin accumulation in the culm should increase lodging resistance.
Conclusions
The study demonstrated a successful combination of submergence and stagnant flooding tolerance in a rice breeding programme, and identified elite Sub1 genotypes that also tolerate stagnant flooding. Our results will support genetic improvement of Sub1 varieties for stagnant flooding tolerance.
Keywords: Flooding stress, lodging resistance, plant elongation, rainfed lowland rice, stagnant flooding, Sub1 varieties
INTRODUCTION
Coastal rice ecosystems, covering >16 % of rice areas worldwide (20 × 106 ha), are adversely affected by annual flooding (Ismail et al., 2013). Paddy fields in these flood-prone lowlands are subject to either flash floods or long-term flooding (Rumanti et al., 2018). Climate change is increasing the incidence of both types of floods in the tropics (Hirabayashi et al., 2013). Flash floods cause submergence of rice for a few days to 2 weeks, whereas long-term partial submergence to a depth of around 50 cm (medium- or semi-deep), commonly referred to as stagnant flooding, usually persists for a few weeks to several months (Septiningsih and Mackill, 2018). Yield loss due to floods ranges from 10 to 100 %, depending on the rice variety, flood duration, depth and floodwater conditions (Ismail et al., 2013). Traditional rice varieties still predominate in flood-prone lowlands, so rice yield is low, ranging from 0.5 to 2.0 t ha−1, which is equivalent to less than half the yield in more favourable lowlands (Mackill et al., 2012).
To date, a number of high-yielding varieties that can tolerate complete submergence have been released in tropical Asia (Manzanilla et al., 2016). This was made possible by identification of the causal gene in Indian landrace FR13A (Bailey-Serres et al., 2012). A novel quantitative trait locus (QTL) that controls the submergence tolerance of FR13A, named SUB1, was identified on chromosome 9; the underlying gene, named SUB1A, was cloned and found to encode an ethylene-response factor (Xu et al., 2006). Subsequently, submergence-tolerant modern rice varieties were developed by introgression of SUB1 into popular varieties through marker-assisted breeding (Septiningsih et al., 2009; Rumanti et al., 2018). These Sub1 varieties can survive about 2 weeks of submergence (Xu et al., 2006), and they typically provide a yield advantage of 1–3 t ha−1 compared with the original varieties following submergence (Ismail et al., 2013).
Progress in genetic improvement for tolerance of stagnant flooding has been slow. Grain yield of modern rice varieties is considerably reduced (by 34–83 %) by stagnant flooding, even when the plants are not fully submerged (Kato et al., 2014). These conditions also reduce tillering, increase lodging and cause partial mortality (Singh et al., 2011). Here, lodging refers to a situation in which a plant falls over, causing permanent displacement of the above-ground parts towards the ground. Deep-water rice landraces suitable for marshlands have a tall and spindly stature, and perform poorly in flood-prone lowlands due to severe lodging (Vergara et al., 2014). Moreover, the predominant allocation of stem reserves for internode elongation rather than the growth of reproductive organs causes a low harvest index in deep-water rice landraces (Kato et al., 2014). The International Rice Research Institute (IRRI) has initiated a breeding programme to improve rice tolerance of stagnant flooding during the last decade (Collard et al., 2013), and a few modern tolerant varieties that lack the SUB1 QTL were identified after thorough screening of the IRRI germplasm collection and breeding lines (Vergara et al., 2014).
Rice is a semi-aquatic species, and has two contrasting strategies to cope with flooding stress: quiescence (transient dormancy) and escape (Bailey-Serres et al., 2012). The SUB1 QTL induces quiescence by suppressing ethylene-activated shoot elongation during submergence, thereby reducing carbohydrate consumption and increasing survival (Voesenek and Bailey-Serres, 2015). On the other hand, fast flood-induced shoot elongation is beneficial for submerged terrestrial species if it leads to emergence above the water surface during floods with a long duration. This facultative elongation is central to plants that respond to flooding events with an escape strategy, and is generally triggered by the accumulation of the volatile hormone ethylene inside submerged plant tissues (Bailey-Serres et al., 2012). Vigorous leaf emergence above the water surface is crucial for plant survival and high yield under stagnant flooding, which means that shoot elongation must keep up with the increasing water depth (Kato et al., 2014). The progress made by developing plants with a quiescence strategy by introgressing the SUB1 QTL has been remarkable in lowlands that experience flash floods, but ineffective in areas where partial flooding persists for a few months (Singh et al., 2011). To date, none of the released Sub1 varieties are well adapted to this type of stress, with only a few varieties showing moderate tolerance (Vergara et al., 2014). It remains unknown whether the introgression of the SUB1 QTL negatively affects breeding for tolerance of stagnant flooding, whether elite Sub1 genotypes that perform well under stagnant flooding can be selected, and whether selection under control conditions based on agronomic performance characteristics such as inherent stature (i.e. stature under non-stress conditions) and yield potential could affect yield under stagnant flooding.
Attention should be also paid to the lodging that can occur after accelerated shoot elongation under submergence (Vergara et al., 2014). Modern rice varieties that tolerate stagnant flooding show flood-induced elongation of leaf blades and sheaths but only slight elongation of internodes with increasing water depth, indicating that the trait for this escape strategy differs from the trait that deep-water rice landraces commonly possess (Kato et al., 2014). Nevertheless, increased final plant height is inevitable under stagnant flooding, with the increase generally around 20 cm (Kato et al., 2014; Vergara et al., 2014). This can result in a drastic increase in lodging during the grain-filling stage. Breeding of cereal crops has aimed to improve lodging resistance by increasing the bending moment at the breaking point of the culms (i.e. the culm’s breaking resistance), to avoid buckling of the culm at the elongated basal internodes (Kashiwagi and Ishimaru, 2004; Wu and Ma, 2016). The bending moment at this point can be mechanistically dissected into the section modulus and the bending stress (Ookawa et al., 2016). The former is related to morphological traits such as culm diameter and wall thickness, whereas the latter is related to physico-chemical traits such as the composition of structural carbohydrates, such as cellulose and lignin (Wu and Ma, 2016). By applying this model, Ookawa et al. (2010) mapped a novel QTL for culm strength, Strong Culm 2 (SCM2), and discovered a gene for increasing the flexural rigidity of culms and thereby increased lodging resistance in rice. Interestingly, this gene was identical to Aberrant Panicle Organization1 (APO1), which was reported to control panicle structure (Ikeda et al., 2007).
Submergence also triggers the formation of a tissue with gas-filled pores (aerenchyma) in the internodes of rice culms. This typically results from parenchyma cell death mediated by reactive oxygen species (Steffens et al., 2011; Voesenek and Bailey-Serres, 2015). However, this anatomical acclimation to facilitate underwater gas transport may reduce the culm’s breaking resistance. To date, there have been no investigations of the genotypic variation in culm breaking resistance in rice under stagnant flooding. Genotypic variation in traits associated with culm stiffness in relation to stagnant flooding has also not yet been explored.
Traits responsible for adaptation to transient complete submergence and long-term partial submergence have not yet been successfully combined in modern rice breeding lines. Achievement of this would be agriculturally important because flooding in a given region may sometimes be transient and at other times prolonged, and the relative frequency of the two conditions will change under the predicted climate change. In this paper, we analysed the tolerance of stagnant flooding of advanced breeding lines developed through IRRI’s rice breeding programme. Our key objectives were to elucidate the effect of the SUB1 QTL on the performance of elite genotypes under stagnant flooding and to physiologically characterize promising Sub1 genotypes that can tolerate stagnant flooding. We also dissected the dynamic changes in the porosity (relative volume of internal gas spaces), chemical composition and morphology of the culms under stagnant flooding, as these factors together determine the rice culm’s breaking strength and thus its resistance to lodging.
MATERIALS AND METHODS
Experiment 1: phenotypic evaluation of the yield of advanced-generation elite genotypes
We analysed 80 advanced-generation elite genotypes (fixed breeding lines, >F8 generation; Supplementary Data Table S1). These genotypes were selected on the basis of superior phenotypes under stagnant flooding stress at the F5 and F6 generations. Several reference varieties with or without SUB1 were included, such as IRRI119 (with SUB1) and IRRI154 (without SUB1). These have been used as reference varieties that tolerate stagnant flooding in IRRI’s rice breeding programme (Collard et al., 2013). Submergence tolerance was evaluated using a combination of field-based testing and standard marker genotyping protocols for SUB1 (Septiningsih et al., 2009). Advanced-generation yield trials were conducted at the IRRI farm in Los Baños, the Philippines (14°11′ N, 121°15′ E, elevation 21 m) during the dry season (January to May) and wet season (July to November) of 2012. The soil at the site is an Aquandic Epiaquoll with 25 % sand, 35 % silt and 40 % clay, pH (H2O) 7.4, 23.0 g total C kg−1, 2.0 g total N kg−1, 25.9 mg Bray-II P kg−1, 1.35 cmol exchangeable K kg−1, 0.38 mg exchangeable Zn kg−1, and a cation-exchange capacity of 41.3 cmol kg−1. Mean values of air temperature (24.7 °C in the dry season and 25.0 °C in the wet season), solar radiation (16.6 MJ m−2 d−1 in the dry season and 14.1 MJ m−2 d−1 in the wet season) and total rainfall (487 mm in the dry season and 1464 mm in the wet season) in 2012 were obtained from the meteorological station at the IRRI farm.
Genotypes were arranged in a row–column design with two replicates under shallow water (hereafter, the control) and two under stagnant flooding. Plot size was 2.0 m × 5.4 m. In the control, a water depth of 2–3 cm was maintained from transplanting to a few days before harvest, when the field was drained. Under stagnant flooding, one deep-water pond with irrigation and a drainage facility (0.5 ha) was used for each replicate. Stagnant flooding stress was imposed using a standardized protocol (Kato et al., 2014); in summary, water depth was maintained at 2–3 cm from 0 to 7 d after transplanting (DAT), then increased twice per week at a rate of 1.43 cm d−1 during the early vegetative stage (from 7 to 21 DAT) and three times per week at a rate of 2.14 cm d−1 during the middle of the vegetative stage (from 21 to 35 DAT). A water depth of 50 cm was then maintained from 35 DAT until maturity. There were several typhoons and tropical storms during the wet season, causing a few occasions of temporary (12–24 h) submergence during the vegetative stage when accumulated rainfall exceeded the capacity of the drainage facility.
Two to three 21-d-old seedlings were transplanted per hill at a hill spacing of 20 cm × 20 cm on 3 January and 29 June 2012 for use in the dry and wet seasons, respectively. Fertilizers were incorporated as basal applications of 13 kg P ha−1, 33 kg K ha−1 and 5 kg Zn ha−1 in both seasons. Nitrogen was split-applied at 80 kg ha−1 as a basal application and 40 kg ha−1 at 30 DAT in the dry season, versus 50 kg ha−1 as a basal application and 40 kg ha−1 at 30 DAT in the wet season. Insects, diseases and weeds were carefully controlled using approved pesticides, fungicides and hand weeding.
Plant height was measured weekly using ten plants in each plot during the vegetative stage (from 10 to 50 DAT) to determine the shoot elongation rate. Cumulative plant height was plotted against time (in DAT) and shoot elongation rates were calculated as the slopes of the regression lines for each accession. To evaluate canopy growth, we also determined the fraction of radiation intercepted, tiller number, above-ground biomass and plant survival. The fraction of radiation intercepted was measured at two positions in each plot using a linear photosynthetic active radiation ceptometer (AccuPAR, Decagon Devices, Pullman, WA, USA) at mid-day (1100–1300 h) during the mid-tillering stage (56 DAT). The ceptometer was placed on the water surface, so the values under stagnant flooding reflected the interception by the canopy above the water surface. We then sampled five hills from each plot and counted the tiller number of all plants. We determined the dry weight of shoots after oven-drying at 80 °C for 72 h. Plant survival was determined by counting the number that survived in each plot. At maturity, we measured the height of ten plants in each plot. All plants in each plot were manually harvested to determine grain yield, which was adjusted to a moisture content of 0.14 g H2O g−1.
Experiment 2: characterization of the promising genotypes that tolerated stagnant flooding
We evaluated 11 rice genotypes, including the seven most promising ones that were identified as tolerant of stagnant flooding in Experiment 1 (IR10F109, IR10F365, IR10F339, IR10F571, IR11F186, IR11F195 and IR11F262; Supplementary Data Table S2). Marker-assisted selection for SUB1 and preliminary field testing confirmed that all genotypes except IR11F195, IR10F571 and IR11F262 had SUB1 and were submergence-tolerant (Supplementary Data Table S3). Three varieties that tolerated stagnant flooding (IRRI119, IRRI154 and OR142-99) and one sensitive variety (Swarna-Sub1) that had been identified in previous studies were also used as reference varieties (Kato et al., 2014; Vergara et al., 2014).
Field experiments were conducted at the IRRI farm during the dry and wet seasons of 2013. Mean values were as follows: air temperature, 27.5 °C in the dry season and 27.6 °C in the wet season; solar radiation, 16.4 MJ m−2 d−1 in the dry season and 13.2 MJ m−2 d−1 in the wet season; and total rainfall, 425 mm in the dry season and 1595 mm in the wet season. Genotypes were arranged in a randomized complete block design with four replicates in the control and stagnant flooding treatments. Plot size was 6.0 m × 2.8 m. The water regime, fertilizer application and management of weeds, pests and diseases were the same as in Experiment 1. We transplanted two or three 21-d-old seedlings per hill at a hill spacing of 20 cm × 20 cm on 4 January (dry season) and 9 July 2013 (wet season).
We measured the height of 20 plants in each plot during the vegetative stage (from 10 to 50 DAT, weekly) and a few days after the full emergence of panicles to determine the shoot elongation rate and final shoot height. At 49 DAT, the fraction of radiation intercepted was determined at six positions per plot, and tiller number, the leaf area index above the water surface and the above-ground biomass were measured by sampling 12 hills in each plot. Under stagnant flooding, plants were first cut at the water surface, then the rest of the above-ground biomass (below the water) was pulled out of the soil. Leaf area above the water was measured using a leaf area meter (LI-3000, LI-COR, Lincoln, NE, USA) after separating the samples into green leaves and stems, and was then expressed as the leaf area index. The number of days to heading was recorded. At maturity, the number of panicles was counted in a 5-m2 area where grain yield was subsequently determined. Twelve hills were randomly chosen to measure above-ground biomass, harvest index and yield components. Plants were separated into straw and panicles. Panicles from all 12 hills were hand-threshed, and filled and unfilled spikelets were separated by flotation in tap water and counted to calculate the total spikelets per unit area.
The percentage of lodging was visually estimated from a 5-m2 area at 14 d after heading as (percentage of area where rice was inclined by 30° to 60°)/2 + (percentage of area where rice was completely lodged). Physical and chemical properties of the culms associated with lodging resistance were also measured, as follows. At 20 d after heading, six medium-size plants were pulled from the soil in each plot, then their leaf sheaths were removed and the fourth internodes were dissected from the four biggest culms from each hill (with the internode under the panicle neck regarded as the first internode). The samples were transferred into plastic bags, which were tightly sealed and placed in a cold box at 2–3 °C and carried to the laboratory. Culms with their fourth internodes shorter than 6 cm were not used for further measurements. The bending load at breaking was measured at a distance of 4 cm between supports, as described by Ookawa et al. (2010). In summary, the pulling force was recorded until the culm ruptured, and that maximum force was recorded for 12 culms using a digital pull gauge (Imada, Osaka, Japan). Culm diameter (average of the major and minor axes of an ellipse) and culm wall thickness were measured at eight positions by using a digital calliper. The culm samples were dried at 80 °C for 72 h, ground in an automated mill (TI-200, Fujiwara Seisakusyo, Tokyo, Japan) and used for chemical analysis as described below.
The culm strength parameters were calculated by the following formula:
bending moment at breaking (kgf cm) = section modulus (mm3) × bending stress (kgf mm−2)
Section modulus (mm3) was calculated as π/32 × (a13b1 – a23b2)/a1, where a1 is the diameter of the minor axis in an elliptical cross-section (mm), b1 is the diameter of the major axis in an elliptical cross-section (mm), a2 is the inner diameter (i.e. outer diameter − culm wall thickness) of the minor axis in an elliptical cross-section (mm), and b2 is the inner diameter of the major axis in an elliptical cross-section (mm). Bending stress is a mechanical parameter for lodging resistance that is influenced by the chemical composition of the culm (Ookawa et al., 2010).
We measured the porosity of the culm for six culms in each plot using a buoyancy-based method (Visser and Bögemann, 2003). We divided the fourth internode of the culm into 3-cm-long segments, gently blotted them dry with tissue paper to remove any attached water, then brushed them with 0.1 % Triton X (Sigma-Aldrich, St Louis, MO, USA) to remove gas films on the tissue segments when they were subsequently submerged for the porosity measurement. First, we measured the weight of the culm sample submerged in water in the beaker. We then filled the gaps in the culm wall with water by placing the beaker under a near vacuum for at least 15 min, and then re-weighed the culm sample. The first weight and the difference between the two weights equalled the volume of the segment and the amount of water absorbed by the gaps in the culm wall, respectively, and we converted these mass values into volumes using a basic density of 1 g cm−3 for water at room temperature. The porosity of the culm sample equalled (volume of gas within the tissues [cm3]/volume of tissue [cm3]) × 100 (% v/v).
We expressed the non-structural carbohydrate concentration as the sum of the concentrations of soluble sugars and starch, and determined the lignin content of the oven-dried culm samples. Concentrations of soluble sugars and starch were measured as in Kato et al. (2014). The samples were assayed for soluble sugars using the anthrone reagent (Sigma-Aldrich), and the starch concentration was determined after hydrolysis with amyloglucosidase (Sigma-Aldrich), followed by a glucose assay using glucose oxidase (Sigma-Aldrich). The lignin concentration was measured using the thioglycolate lignin method described by Kashiwagi et al. (2016). In summary, we used ethanol, thioglycolic acid and NaOH (sequentially) to extract thioglycolate lignin from the residue. The absorbance at 280 nm was recorded with a UV spectrophotometer (Shimadzu, Kyoto, Japan).
Statistical analyses
Data were analysed using the generalized linear model procedure (SAS Institute, 2003). In Experiment 1, individual analyses of variance were conducted separately for each treatment and season to assess varietal differences. In Experiment 2, the genotype × season interaction was not significant for any trait, so we have presented the mean values for combined data from both seasons. When the ANOVA result was significant, differences were compared by Fisher’s least-significant difference (LSD) test, with significance defined as P < 0.05. Since we did not replicate the water regimes, as explained above, the effects of water depth and of the genotype × water depth interaction could not be statistically assessed.
RESULTS
Experiment 1: phenotypic evaluation of advanced elite genotypes
On average, stagnant flooding reduced the grain yield by 48 % in the dry season and 89 % in the wet season (Table 1). The survival under stagnant flooding was significantly lower in the wet season (56 %) than the dry season (96 %). Genotypic variation in yield (based on the coefficient of variation) was much larger under stagnant flooding than in the control. The eight pairs of Sub1 and original non-Sub1 varieties widely grown in tropical Asia (BR11, Ciherang, CR1009, IR64, PSBRc18, Samba Mahsuri, Swarna and TDK1) showed little effect of the SUB1 QTL on yield in the control (0–9 % increase), but the presence of this gene reduced yield by 25 % under stagnant flooding in the wet season (Supplementary Data Table S1). When comparing the relative performance of 30 elite genotypes evaluated in both the dry and the wet season, the genotypes carrying the SUB1 QTL had a lower yield than those without the SUB1 QTL under stagnant flooding, by an average of 49 % across seasons, but with no difference under non-stress conditions (Supplementary Data Table S2). Nevertheless, several Sub1 genotypes showed good performance under stagnant flooding.
Table 1.
Yield and key agronomic traits in the advanced-generation yield trial of the flood-tolerant rice breeding programme at the IRRI
| Grain yield (t ha−1) | Plant height (cm) | Mid-tillering stage | ||||||
|---|---|---|---|---|---|---|---|---|
| SER (cm d−1) | FRI | Tillers (m−2) | Biomass (t ha−1) | Survival (%) | ||||
| 2012 DS | ||||||||
| Control | Mean | 4.08ns | 108** | 1.10** | 0.88ns | 450** | 6.73ns | 100ns |
| Range | 2.81–5.34 | 88–140 | 0.80–1.42 | 0.79–0.93 | 295–618 | 3.49–10.34 | – | |
| CV (%) | 14 | 10 | 12 | 3 | 16 | 18 | – | |
| 2012 DS | ||||||||
| SF stress | Mean | 2.13* | 142** | 2.03** | 0.67** | 191** | 5.01** | 96ns |
| Range | 0.20–4.06 | 116–169 | 1.48–2.33 | 0.18–0.90 | 85–290 | 1.27–7.66 | 60–100 | |
| CV (%) | 41 | 8 | 9 | 25 | 23 | 30 | 8 | |
| 2012 WS | ||||||||
| Control | Mean | 3.72** | 121** | 1.35** | 0.75* | 480* | 4.33ns | 100ns |
| Range | 1.09–5.29 | 97–158 | 0.96–1.80 | 0.61–0.85 | 340–628 | 3.10–5.91 | – | |
| CV (%) | 24 | 10 | 13 | 6 | 11 | 14 | – | |
| 2012 WS | ||||||||
| SF stress | Mean | 0.41** | 136** | 1.69* | 0.17** | 86** | 0.83** | 56** |
| Range | 0–2.08 | 103–163 | 1.23–2.08 | 0.05–0.36 | 16–204 | 0.11–2.63 | 13–96 | |
| CV (%) | 114 | 10 | 9 | 44 | 47 | 63 | 36 |
SF, stagnant flooding; CV, coefficient of variation; SER, shoot elongation rate; FRI, fraction of radiation intercepted; DS, dry season; WS, wet season.
Genotypic differences were significant at **P < 0.01, *P < 0.05; ns, not significant.
Plant height and shoot elongation rate were significantly higher under stagnant flooding than in the control in both seasons (Table 1). Although these primary traits had a low coefficient of variation (≤13 %), integrated growth traits such as the fraction of radiation intercepted, tiller number and above-ground biomass had a much higher coefficient of variation (≥23 %) under stagnant flooding than in the control (≤18 %).
The correlation between grain yields under stagnant flooding and in the control was weak and not significant in both seasons (Table 2). Grain yield under stagnant flooding was not significantly correlated with plant height in the control, but was significantly positively correlated with plant height, shoot elongation rate, fraction of radiation intercepted, tiller number, above-ground biomass and percentage survival at the mid-tillering stage in both seasons.
Table 2.
Correlation coefficients (Pearson’s r) for the relationship between grain yield under stagnant flooding (SF) stress and the various parameters measured in the flood-tolerant rice breeding programme at the IRRI
| Control | SF stress | |||||||
|---|---|---|---|---|---|---|---|---|
| Grain yield | Plant heighta | Plant heighta | SERb | FRIb | Tiller numberb | Biomassb | Survivalb | |
| 2012 DS | 0.086ns | 0.174ns | 0.685*** | 0.580*** | 0.678*** | 0.484*** | 0.590*** | 0.498*** |
| 2012 WS | 0.213ns | 0.122ns | 0.589*** | 0.470*** | 0.677*** | 0.747*** | 0.607*** | 0.664*** |
aMeasured at maturity.
bMeasured at the mid-tillering stage.
SER, shoot elongation rate; FRI, fraction of radiation intercepted; DS, dry season; WS, wet season.
***P < 0.001; ns, not significant.
Experiment 2: characterization of the promising genotypes that tolerated stagnant flooding
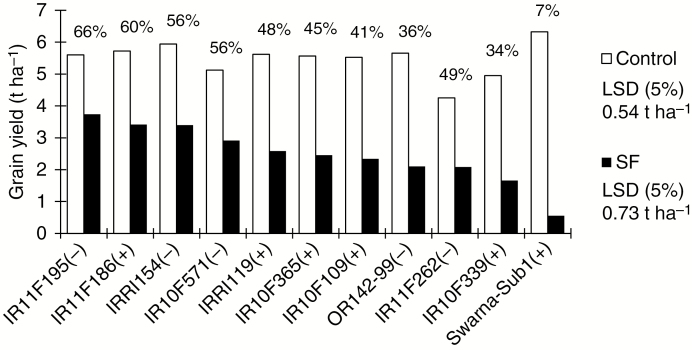
For the 11 selected genotypes that best tolerated stagnant flooding, grain yields were significantly correlated between the dry and wet seasons in 2013 (r = 0.50, P < 0.05 for the control and 0.81, P < 0.01 for stagnant flooding). Stagnant flooding reduced grain yield by an average of 52 % in the dry season and 57 % in the wet season. In the control, Swarna-Sub1 had the highest yield, followed by IRRI154 (Fig. 1; Supplementary Data Table S4). Among the promising genotypes that tolerated stagnant flooding, IR11F186, IR10F365 and IR10F109 (all of which carry the SUB1 QTL) had yields that were not significantly different from that of IRRI154 in the control, but under stagnant flooding the yield of IR11F195 was highest, followed by IR11F186, which had the smallest yield reduction. All the promising genotypes showed significantly higher yield under stagnant flooding than Swarna-Sub1, whose yield reduction was 93 % under this stress.
Fig. 1.
Grain yield in the control and stagnant flooding (SF) trials. The data are mean values averaged over the dry and wet seasons in 2013. The percentage values shown above the bar for each genotype represent the mean relative yields (SF/control). In parentheses, + means that the genotype carries the SUB1 QTL and − means it does not have SUB1.
The promising genotypes had medium to long growth durations, with days to heading generally between that of IRRI154 and that of Swarna-Sub1 (Table 3). Across genotypes, the heading date was delayed under stress by an average of 7 d, with the yield reduction increasing with increasing delay in heading (r = −0.653, P < 0.05). Biomass at maturity, harvest index, panicle number and spikelet number were reduced by stagnant flooding. Only the harvest index was significantly correlated with yield in the control (r = 0.713, P < 0.01), whereas biomass at maturity (r = 0.905, P < 0.01), panicle number (r = 0.935, P < 0.01) and spikelet number (r = 0.907, P < 0.01) were significantly correlated with yield. The biomass at maturity and panicle number were greatest in IR10F571, but its harvest index was lower than in the other genotypes.
Table 3.
Days to heading (DTH), shoot biomass, harvest index and yield components in the control (C) and stagnant flooding (SF) treatments in trials conducted at the IRRI
| Entrya | DTH (d) | Biomass (t ha−1) | Harvest index | Panicles (m−2) | Spikelets (103 m−2) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | SF | C | SF | C | SF | C | SF | C | SF | |
| IR11F195 (−) | 94 | 98 | 11.68 | 8.62 | 0.41 | 0.38 | 226 | 138 | 27.8 | 18.6 |
| IR11F186 (+) | 101 | 109 | 12.74 | 8.55 | 0.38 | 0.35 | 210 | 141 | 28.4 | 18.5 |
| IRRI154 (−) | 88 | 95 | 11.38 | 6.91 | 0.45 | 0.42 | 253 | 133 | 27.8 | 16.0 |
| IR10F571 (−) | 99 | 102 | 11.98 | 8.92 | 0.37 | 0.29 | 230 | 145 | 25.3 | 16.6 |
| IRRI119 (+) | 92 | 92 | 11.24 | 6.88 | 0.43 | 0.33 | 213 | 110 | 19.9 | 10.7 |
| IR10F365 (+) | 88 | 93 | 11.68 | 7.28 | 0.41 | 0.31 | 253 | 119 | 24.3 | 12.7 |
| IR10F109 (+) | 104 | 110 | 13.21 | 6.40 | 0.36 | 0.30 | 230 | 106 | 28.0 | 14.0 |
| OR142-99 (−) | 103 | 111 | 13.28 | 6.27 | 0.37 | 0.28 | 231 | 97 | 29.3 | 13.4 |
| IR11F262 (−) | 105 | 113 | 12.98 | 6.76 | 0.28 | 0.27 | 224 | 108 | 29.2 | 15.2 |
| IR10F339 (+) | 85 | 91 | 10.40 | 4.32 | 0.41 | 0.34 | 244 | 90 | 24.6 | 9.6 |
| Swarna-Sub1 (+) | 102 | 118 | 13.25 | 1.15 | 0.41 | 0.34 | 295 | 18 | 39.4 | 3.0 |
| Mean | 96 | 103 | 12.17 | 6.55 | 0.39 | 0.33 | 237 | 110 | 27.6 | 13.5 |
| LSD (5 %) | 2 | 2 | 1.34 | 1.71 | 0.02 | 0.04 | 29 | 24 | 4.2 | 3.4 |
Values are the means for the dry and wet seasons in 2013.
aIn parentheses, + means that the genotype carries SUB1 and – means that it does not have SUB1.
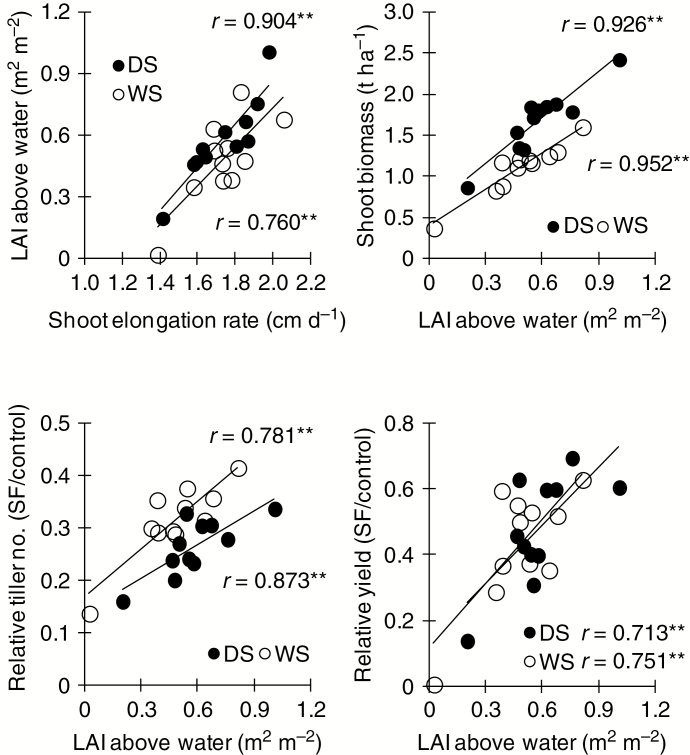
Genotypic variation was high for the traits measured at the late vegetative stage (Table 4). Biomass, leaf area index and shoot elongation rate under stagnant flooding were highest in IR10F571, followed by IR11F195. Facultative shoot elongation under flooding (i.e. high elongation under stagnant flooding relative to that in the control) was greatest in IRRI154 (146 %), followed by IR10F571 (142 %), and lowest in IRRI119 (113 %). The promising Sub1 genotypes that tolerated stagnant flooding, such as IR11F186, showed moderate shoot elongation with increasing water depth. Under stagnant flooding, leaf area index above the water was significantly positively correlated with the shoot elongation rate during the late vegetative stage in both seasons (Fig. 2). Leaf area index was also correlated positively with shoot biomass, relative tiller number (number under stagnant flooding/number in the control) and relative grain yield under stagnant flooding; larger leaf area contributed to more biomass, more tillers and greater yield under stress.
Table 4.
Shoot biomass, leaf area index (LAI) above the water surface, tiller number, shoot elongation rate (SER) and plant survival rate 56 d after transplanting in the control (C) and stagnant flooding (SF) treatments at the IRRI
| Entrya | Biomass (t ha−1) | LAI above water (m2 m−2) | Tillers (m−2) | SER (cm d−1) | Survival (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | SF | C | SF | C | SF | C | SF | C | SF | |
| IR11F195 (−) | 3.97 | 1.70 | 2.99 | 0.79 | 416 | 142 | 1.47 | 1.87 | 100 | 89 |
| IR11F186 (+) | 4.02 | 1.52 | 3.19 | 0.50 | 442 | 145 | 1.44 | 1.74 | 100 | 89 |
| IRRI154 (−) | 3.88 | 1.53 | 2.94 | 0.61 | 441 | 149 | 1.23 | 1.80 | 100 | 88 |
| IR10F571 (−) | 3.78 | 1.87 | 2.78 | 0.84 | 427 | 148 | 1.42 | 2.02 | 100 | 90 |
| IRRI119 (+) | 4.17 | 1.48 | 3.26 | 0.50 | 372 | 116 | 1.48 | 1.68 | 100 | 87 |
| IR10F365 (+) | 4.19 | 1.51 | 2.29 | 0.53 | 413 | 107 | 1.47 | 1.86 | 100 | 87 |
| IR10F109 (+) | 3.54 | 1.22 | 2.68 | 0.42 | 543 | 142 | 1.19 | 1.68 | 100 | 85 |
| OR142-99 (−) | 3.33 | 1.08 | 2.90 | 0.42 | 421 | 120 | 1.28 | 1.61 | 100 | 75 |
| IR11F262 (−) | 3.76 | 1.30 | 3.30 | 0.55 | 435 | 110 | 1.37 | 1.64 | 100 | 87 |
| IR10F339 (+) | 3.94 | 1.46 | 2.94 | 0.54 | 397 | 113 | 1.35 | 1.75 | 100 | 79 |
| Swarna-Sub1 (+) | 3.56 | 0.62 | 2.95 | 0.11 | 543 | 81 | 1.00 | 1.40 | 100 | 54 |
| Mean | 3.83 | 1.39 | 2.93 | 0.53 | 441 | 125 | 1.34 | 1.73 | 100 | 83 |
| LSD (5 %) | 0.51 | 0.44 | 0.50 | 0.20 | 48 | 27 | 0.10 | 0.11 | ns | 14 |
Values are the means for the dry and wet seasons in 2013.
aIn parentheses, + means that the genotype carries SUB1 and – means that it does not have SUB1.
ns, not significant.
Fig. 2.
Relationship between vegetative growth parameters and yield for 11 rice genotypes that tolerated stagnant flooding (SF) in the control and under SF stress in the dry season (DS) and wet season (WS) of 2013. **P < 0.01. LAI, leaf area index.
On average, plants were taller under stagnant flooding than in the control, by an average of 20 cm, at the mid-grain-filling stage (Table 5). Severe lodging was observed in IR11F186, OR142-99 and IR11F262 under both conditions. For the six genotypes whose plant height was >140 cm, lodging percentage had a strong, significant, positive correlation with plant height under stagnant flooding (r = 0.965, P < 0.01).
Table 5.
Plant height and lodging 14 d after heading in the control (C) and stagnant flooding (SF) treatments at the IRRI
| Entrya | Plant height (cm) | Lodging (%) | ||
|---|---|---|---|---|
| C | SF | C | SF | |
| IR11F195 (−) | 129 | 144 | 0 | 6 |
| IR11F186 (+) | 147 | 153 | 44 | 50 |
| IRRI154 (−) | 108 | 136 | 0 | 0 |
| IR10F571 (−) | 110 | 142 | 0 | 1 |
| IRRI119 (+) | 128 | 140 | 0 | 0 |
| IR10F365 (+) | 116 | 137 | 3 | 0 |
| IR10F109 (+) | 116 | 134 | 0 | 3 |
| OR142-99 (−) | 127 | 148 | 31 | 19 |
| IR11F262 (−) | 123 | 145 | 16 | 8 |
| IR10F339 (+) | 101 | 122 | 0 | 0 |
| Swarna-Sub1 (+) | 107 | 122 | 4 | 0 |
| Mean | 119 | 139 | 9 | 8 |
| LSD (5%) | 4 | 6 | 12 | 13 |
Values are means for the dry and wet seasons in 2013.
aIn parentheses, + means that the genotype carries SUB1 and – means that it does not have SUB1.
The bending moment at breaking, an indicator of the culm’s physical strength and resistance to lodging (Ookawa et al., 2010), was an average of 25 % higher under stagnant flooding than the control (Table 6), though the lodging percentage did not differ significantly between the two conditions (Table 5). Genotypic variation in the bending moment was significant under both conditions, ranging from 0.717 to 1.268 kgf cm−1 in the control and from 0.903 to 1.532 kgf cm−1 under stagnant flooding. Bending stress decreased by an average of 24 % under stagnant flooding, whereas the section modulus increased by 64 % and culm diameter increased by 23 %. Culm wall thickness was not significantly affected by water depth. Neither the bending moment at breaking nor bending stress under stagnant flooding was significantly correlated with those in the control, but the section modulus under stagnant flooding was significantly positively correlated with that in the control (r = 0.717, P < 0.01). Culm diameter and culm wall thickness under stagnant flooding were also significantly positively correlated with those in the control (r = 0.609 [P < 0.05] and 0.644 [P < 0.05], respectively).
Table 6.
Physical strength of culms (culm bending moment at breaking, bending stress and section modulus), and culm diameter and wall thickness measured at the fourth internode 20 d after heading in the control (C) and stagnant flooding (SF) treatments at the IRRI
| Entrya | Bending moment at breaking (kgf cm) | Bending stress (kgf mm−2) | Section modulus (mm3) | Culm diameter (mm) | Culm wall thickness (mm) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | SF | C | SF | C | SF | C | SF | C | SF | |
| IR11F195 (−) | 0.853 | 1.029 | 0.719 | 0.533 | 12.3 | 20.2 | 5.59 | 6.99 | 0.82 | 0.76 |
| IR11F186 (+) | 1.083 | 1.327 | 0.962 | 1.003 | 11.9 | 13.8 | 5.46 | 5.80 | 0.78 | 0.82 |
| IRRI154 (−) | 0.888 | 0.970 | 0.935 | 0.501 | 9.9 | 20.3 | 4.97 | 6.65 | 0.93 | 0.92 |
| IR10F571 (−) | 0.856 | 0.903 | 1.175 | 0.660 | 9.9 | 14.7 | 4.62 | 6.09 | 0.81 | 0.73 |
| IRRI119 (+) | 1.014 | 1.198 | 0.908 | 0.691 | 11.5 | 18.4 | 5.42 | 6.39 | 0.80 | 0.91 |
| IR10F365 (+) | 1.268 | 1.356 | 0.851 | 0.577 | 15.7 | 24.0 | 5.95 | 7.26 | 0.93 | 0.86 |
| IR10F109 (+) | 0.857 | 1.113 | 1.005 | 0.861 | 8.9 | 13.3 | 4.92 | 5.86 | 0.76 | 0.77 |
| OR142-99 (−) | 0.783 | 1.227 | 1.063 | 1.007 | 7.7 | 12.6 | 4.78 | 5.78 | 0.66 | 0.71 |
| IR11F262 (−) | 1.078 | 1.532 | 1.028 | 0.798 | 10.8 | 20.1 | 5.33 | 6.68 | 0.76 | 0.84 |
| IR10F339 (+) | 1.009 | 1.083 | 1.162 | 0.583 | 9.7 | 19.0 | 5.10 | 6.80 | 0.80 | 0.78 |
| Swarna-Sub1 (+) | 0.717 | 1.310 | 0.769 | 0.795 | 9.8 | 17.1 | 5.13 | 6.24 | 0.80 | 0.89 |
| Mean | 0.946 | 1.186 | 0.962 | 0.728 | 10.7 | 17.6 | 5.21 | 6.41 | 0.80 | 0.82 |
| LSD (5%) | 0.104 | 0.137 | 0.107 | 0.084 | 1.2 | 2.3 | 0.20 | 0.28 | 0.04 | 0.04 |
Values are mean values for the dry and wet seasons in 2013.
aIn parentheses, + means that the genotype carries SUB1 and – means that it does not have SUB1.
Culm porosity under stagnant flooding (4.9–15.0 %) was 3.7 times that in the control (1.4–4.2 %; Table 7). The percentage of non-structural carbohydrates and starch weight density increased under stagnant flooding, by averages of 105 % and 267 %, respectively, and were negatively correlated with grain yield under stagnant flooding (r = −0.809 [P < 0.01] and −0.772 [P < 0.01], respectively) and in the control (r = −0.861 [P < 0.01] and −0.848 [P < 0.01], respectively). However, the concentration and weight density of lignin (structural carbohydrate) decreased by averages of 32 % and 27 %, respectively. None of the culm tissue traits was significantly correlated between the water regimes.
Table 7.
Culm porosity, concentrations of non-structural carbohydrates (NSC) and lignin, and starch and lignin weight densities of the culm at the fourth internode 20 d after heading in the control (C) and stagnant flooding (SF) treatments (2013 dry season) at the IRRI
| Entrya | Culm porosity (%) | NSC (%) | Lignin (%) | Starch weight density (μg mm−3) | Lignin weight density (μg mm−3) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | SF | C | SF | C | SF | C | SF | C | SF | |
| IR11F195 (−) | 2.4 | 15.0 | 2.7 | 5.5 | 12.5 | 11.8 | 0.8 | 4.2 | 19.7 | 19.0 |
| IR11F186 (+) | 2.2 | 7.5 | 4.9 | 4.4 | 9.8 | 9.9 | 1.2 | 4.2 | 14.3 | 15.3 |
| IRRI154 (−) | 1.5 | 8.9 | 4.3 | 8.5 | 8.9 | 5.5 | 0.8 | 6.8 | 14.2 | 7.6 |
| IR10F571 (−) | 2.2 | 7.1 | 6.9 | 13.6 | 14.0 | 6.9 | 7.0 | 19.0 | 34.6 | 15.1 |
| IRRI119 (+) | 1.9 | 11.0 | 6.1 | 16.1 | 11.0 | 5.3 | 2.9 | 21.4 | 20.1 | 11.5 |
| IR10F365 (+) | 1.9 | 6.5 | 3.2 | 13.7 | 12.4 | 8.7 | 0.9 | 18.9 | 19.9 | 17.9 |
| IR10F109 (+) | 3.3 | 5.3 | 5.5 | 13.6 | 10.5 | 5.0 | 2.3 | 22.2 | 17.9 | 12.1 |
| OR142-99 (−) | 1.7 | 10.2 | 3.8 | 9.2 | 9.1 | 10.3 | 0.9 | 8.5 | 17.1 | 23.1 |
| IR11F262 (−) | 4.2 | 10.7 | 15.2 | 14.7 | 8.6 | 8.1 | 26.5 | 19.6 | 26.2 | 17.4 |
| IR10F339 (+) | 1.4 | 4.9 | 5.8 | 14.4 | 10.3 | 5.8 | 2.0 | 20.4 | 20.9 | 12.7 |
| Swarna-Sub1 (+) | 2.4 | 5.8 | 3.7 | 14.5 | 14.9 | 5.7 | 0.9 | 24.6 | 22.8 | 14.2 |
| Mean | 2.3 | 8.5 | 5.7 | 11.7 | 11.1 | 7.5 | 4.2 | 15.4 | 20.7 | 15.1 |
| LSD (5%) | 1.0 | 4.7 | 2.2 | 4.1 | 3.5 | 3.1 | 3.5 | 8.8 | 7.9 | 6.4 |
aIn parentheses, + means that the genotype carries SUB1 and – means that it does not have SUB1.
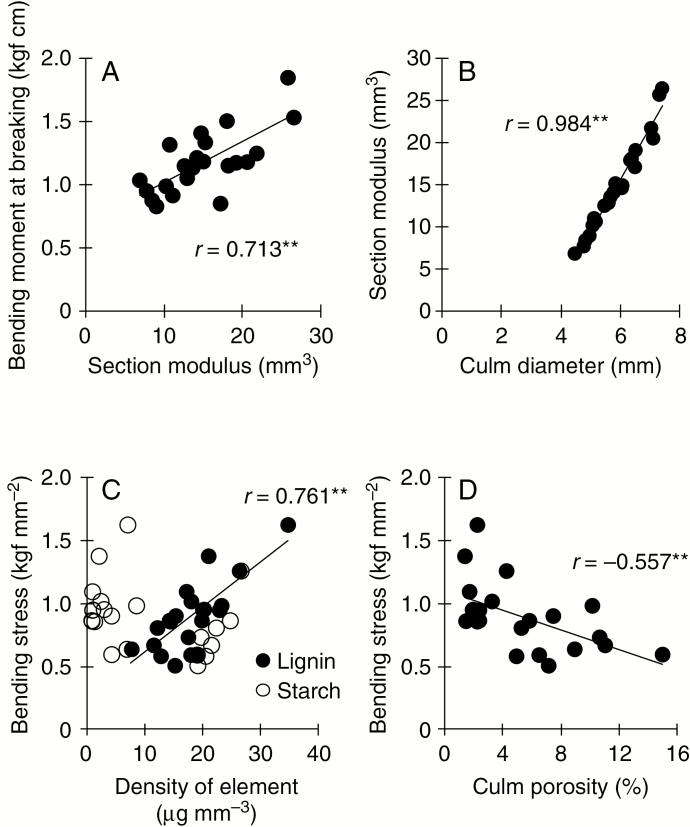
The bending moment at breaking was significantly positively correlated with the section modulus but not significantly correlated with the bending stress (r = −0.238) (Fig. 3). The section modulus was significantly positively correlated with culm diameter. Bending stress was significantly positively correlated with lignin weight density, significantly negatively correlated with culm porosity, but not significantly correlated with starch weight density (r = −0.296).
Fig. 3.
Relationships between culm traits and culm strength for 11 rice genotypes in the control and under stagnant flooding stress in the dry season of 2013. **P < 0.01.
DISCUSSION
The enormous efforts that have been devoted to rice breeding in tropical Asia since the 1980s led to the release of a series of submergence-tolerant high-quality varieties containing the SUB1 QTL in the genetic background of popular varieties (Rumanti et al., 2018; Septiningsih and Mackill, 2018). These varieties can survive about 2 weeks of complete submergence and their yield potential remains unchanged compared with the original varieties. However, the average yield of eight of the Sub1 varieties commercialized in tropical Asia was an average of 25 % lower than that of the original non-Sub1 varieties under stagnant flooding (Supplementary Data Table S1), suggesting that selection for tolerance of stagnant flooding should be combined with selection for the SUB1 QTL. This agrees with recent efforts by our research group to map QTLs for traits related to stagnant flooding tolerance, in which both parents carried the SUB1 QTL (Singh et al., 2017).
In the current study, the elite Sub1 genotypes showed significantly lower yield (by an average of 49 %) than those that lacked the SUB1 QTL under stagnant flooding, with no significant difference in the control (Supplementary Data Table S2). The yield differences between the Sub1 and non-Sub1 groups under stagnant flooding were greater than those in near-isogenic pairs of varieties with or without the SUB1 QTL, indicating that the intensive selection for tolerance of stagnant flooding in the breeding programme would favour genotypes without the SUB1 QTL. This has extremely important implications for developing the optimal breeding strategy to combine multiple flood tolerances. Notwithstanding the repeated claims of negative impacts of Sub1 on tolerance of stagnant flooding (Singh et al., 2011; Collard et al., 2013), we identified a few Sub1 genotypes, such as IR11F186, that were as tolerant of stagnant flooding as the current non-Sub1 genotypes, such as the reference accession IRRI154 (Fig. 1). It should be noted that these stagnant-flooding-tolerant Sub1 genotypes were as submergence-tolerant as other Sub1 varieties, such as Swarna-Sub1, and were more submergence-tolerant than popular varieties, such as Swarna and IRRI154 (Supplementary Data Table S3). We also demonstrated the importance of selection under the given stress conditions; agronomic performance under non-stress conditions was weakly and non-significantly correlated with yield under stagnant flooding (Table 2), but it is still necessary to consider performance under non-stress conditions to ensure high yield potential.
The main objective of crop improvement is to select elite genotypes that can provide high yield under the target growth conditions. Thus it would be useful if strongly correlated traits could be used to permit indirect selection of difficult-to-achieve traits such as tolerance of stagnant flooding (Collard et al., 2013). In the present study, the yield under stagnant flooding was strongly correlated with plant height, shoot elongation rate, fraction of radiation intercepted, tiller number, biomass and survival percentage under stress (Table 2). These parameters could be non-destructively measured well before the heading stage. In particular, the fraction of radiation intercepted should be targeted for high-throughput phenotyping using technology such as unmanned aerial vehicles (Yang et al., 2017), as this parameter could strongly predict yield (R2 = 0.46 in both the dry and the wet season). We also confirmed that small differences in the primary phenotypic traits among accessions contributed to high variation in the integrated traits for stress tolerance (Table 1), as has often been argued in the stress physiology literature (Bernier et al., 2009; Kato et al., 2011).
Our results could help rice breeders to select breeding materials that can tolerate stagnant flooding for use in flood-prone environments. An efficient breeding strategy may be to only screen for tolerance of stagnant flooding after the SUB1 QTL became fixed in the breeding material in order to ensure that new breeding lines would possess tolerance of both submergence and stagnant flooding, a combination that is needed for resilience in rainfed and flood-prone areas. This would also be most efficient in terms of the screening facilities for stagnant flooding, which generally have limited capacity. Recently, a rapid generation advance breeding method (Collard et al., 2017) has become popular at IRRI because it shortens breeding cycles and the time required for line development, thereby lowering the cost and increasing the breeding efficiency. This method could be highly suitable for combining flooding tolerance traits, especially if marker-assisted selection is used to select for genotypes that have become fixed for the SUB1 QTL.
Although introgression of the SUB1 QTL slightly reduced shoot elongation under stagnant flooding (by 8–12 %) (data not shown), a few promising Sub1 genotypes showed moderate shoot elongation under stress that was better than in the tolerant reference varieties (Table 4), thereby leading to greater leaf growth above water and increased shoot biomass and tiller number (Fig. 2). The increased vegetative growth contributed to the production of more panicles and grains per unit area (Table 3), which were closely associated with the ultimate grain yield under stagnant flooding. We previously identified two strategies in the modern high-yielding varieties that are associated with tolerance of stagnant flooding (Kato et al., 2014): slow facultative elongation combined with intermediate plant height, as in IRRI119 (a Sub1 variety), and moderate elongation induced by stagnant flooding, with a semi-dwarf stature, as in IRRI154 (a non-Sub1 variety). Interestingly, the shoot elongation response to increasing water depth for all of the promising Sub1 genotypes that tolerated stagnant flooding was between those of IRRI154 and IRRI119, whereas their heights under non-stress conditions were greater than that of IRRI154 (Table 4). It is likely that the extreme elongation strategies used for survival in deep-water areas are not effective for tolerance of stagnant flooding. We also showed that breeding elite Sub1 genotypes with a moderate facultative shoot elongation under stagnant flooding is possible and can lead to varieties that are better adapted to flood-prone areas where both submergence and stagnant flooding are experienced in different seasons, or sometimes even within the same season (Mackill et al., 2012). The role of ethylene- and potential non-ethylene-dependent pathways in the coexistence of two contrasting tolerance mechanisms in one rice genotype awaits future investigation.
However, the risk of lodging increases with increasing plant height unless the culm strength increases enough to compensate (Kashiwagi and Ishimaru, 2004). An increase of 2 % in lodging would reduce grain yield by 1 % (Setter et al., 1997). The critical threshold of plant height for lodging will depend on the panicle weight and on biophysical conditions such as soils and climate. We showed that plant height >140 cm significantly increased the risk of lodging under stagnant flooding (Table 5), and that plant height was not associated with any traits related to culm morphology (Table 6).
Our results also provided evidence that rice culm physico-chemical properties significantly affect its responses to stagnant flooding. The culm strength, as estimated by the bending moment at breaking, increased by an average of 25 % under stagnant flooding due to the increased culm diameter and thus to the increased section modulus (Table 6; Fig. 3A, B). Previous studies also suggested that rice produces a smaller number of thicker stems under stagnant flooding (Kato et al., 2014; Vergara et al., 2014). Trade-offs between the size and number of tillers per unit area were reported under variable planting densities (Zhang and Yamagishi, 2010).
The bending stress decreased by an average of 24 % under stagnant flooding, which was consistent with the increase in culm porosity (by 270 %) and the decrease in the lignin weight density (by 27 %) (Table 7; Fig. 3C, D). Among the changes in the cell wall components in rice, lignin accumulation plays the most important role in determining the culm strength (Ookawa and Ishihara, 1993). This indicates that the values of the physico-chemical properties associated with rice culm strength decrease under stagnant flooding, resulting in lower lodging resistance. The content of non-structural carbohydrates and the starch weight density of the culm both increased under stagnant flooding, possibly because fewer grains are filled under stress (Kato et al., 2014). However, the starch accumulation was not significantly related to the culm strength under stagnant flooding (Fig. 3C), which agrees with a study under non-stress conditions (Ookawa et al., 2016). Although culm physical strength was most strongly associated with culm diameter in IRRI’s elite rice genotypes in this study, bending stress could be improved by reducing aerenchyma formation and promoting lignin accumulation in the culm. The aerenchyma in rice culms is often suggested to be important for underwater gas transport (Steffens et al., 2011), but there seems to be a trade-off, with higher culm porosity resulting in lower lodging resistance. More research is needed to identify the minimum degree of aerenchyma formation in culms required for effective oxygen transport under stagnant flooding, as well as the contribution of aerenchyma in leaf sheaths and leaf gas films to oxygen transport (Winkel et al., 2014). Interestingly, plant height under stagnant flooding was positively correlated with the concentration of lignin in the stems (r = 0.668*), but negatively correlated with the weight density of starch (r = −0.650*). Whether the increase in plant height through breeding affects lignin accumulation and changes in bending stress under stagnant flooding awaits further investigation.
Conclusions
Genetic improvement of Sub1 rice varieties to withstand stagnant flooding has been targeted in IRRI’s rainfed rice breeding programmes. The required tolerance mechanisms for these flood stresses differ, with submergence-tolerant Sub1 genotypes showing an average of 49 % lower yield than non-Sub1 genotypes under stagnant flooding. Nevertheless, we identified a few promising high-yielding Sub1 genotypes that were as tolerant of stagnant flooding as the non-Sub1 variety IRRI154, which was previously identified as the accession most tolerant of stagnant flooding. These genotypes had intermediate stature, like IRRI119, but were capable of moderate shoot elongation in response to rising water under stagnant flooding. The extent of canopy cover above water during the late vegetative stage strongly predicted the yield under stagnant flooding (R2 = 0.46), which suggests it may be a suitable indirect selection criterion for high-throughput phenotyping. Further genetic improvement for tolerance of stagnant flooding should also focus on culm strength to enhance lodging resistance. We found that increasing stem porosity and reducing lignin weight density in the culm decreased its strength under stagnant flooding. Collectively, these traits can be targeted in breeding to develop genotypes that tolerate both submergence and stagnant flooding, thereby providing higher and more stable yields in flood-affected rainfed rice ecosystems.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Table S1: grain yield of 80 genotypes in the advanced-generation yield trial of the flood-tolerant rice breeding programme at the International Rice Research Institute in 2012. Table S2: relative performance, expressed as a proportion of the site mean yield, for 30 advanced elite lines in the flood-tolerant rice breeding programme at the International Rice Research Institute in 2012. Table S3: submergence tolerance score of the selected rice genotypes evaluated in the field in the wet season of 2012. Table S4: grain yield and shoot biomass in the control and stagnant flooding trials in dry and wet seasons in 2013.
FUNDING
We acknowledge the contribution of the Stress-Tolerant Rice for Africa and South Asia (STRASA) project, funded by the Bill and Melinda Gates foundation and the Consortium for Unfavorable Rice Environments (CURE) project, funded by the International Fund for Agricultural Development (IFAD), in supporting this research.
LITERATURE CITED
- Bailey-Serres J, Lee SC, Brinton E. 2012. Waterproofing crops: effective flooding survival strategies. Plant Physiology 160: 1698–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier J, Serraj R, Kumar A, et al. 2009. The large-effect drought-resistance QTL qtl12.1 increases water uptake in upland rice. Field Crops Research 110: 139–146. [Google Scholar]
- Collard BCY, Septiningsih EM, Das SR, et al. 2013. Developing new flood-tolerant varieties at the International Rice Research Institute (IRRI) towards 2025. SABRAO Journal of Breeding and Genetics 45: 42–56. [Google Scholar]
- Collard BCY, Beredo JC, Lenaerts B, et al. 2017. Revisiting rice breeding methods – evaluating the use of rapid generation advance (RGA) for routine rice breeding. Plant Production Science 20: 337–352. [Google Scholar]
- Hirabayashi Y, Mahendran R, Koirala S, et al. 2013. Global flood risk under climate change. Nature Climate Change 3: 816–821. [Google Scholar]
- Ikeda K, Ito M, Nagasawa N, Kyozuka J, Nagato Y. 2007. Rice ABERRANT PANICLE ORGANIZATION 1, encoding an F‐box protein, regulates meristem fate. Plant Journal 51: 1030–1040. [DOI] [PubMed] [Google Scholar]
- Ismail AM, Singh US, Singh S, Dar MH, Mackill DJ. 2013. The contribution of submergence-tolerant (Sub1) rice varieties to food security in flood-prone rainfed lowland areas in Asia. Field Crops Research 152: 83–93. [Google Scholar]
- Kashiwagi T, Ishimaru K. 2004. Identification and functional analysis of a locus for improvement of lodging resistance in rice. Plant Physiology 134: 676–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwagi T, Munakata J, Ishimaru K. 2016. Functional analysis of the lodging resistance QTL BSUC11 on morphological and chemical characteristics in upper culms of rice. Euphytica 210: 233–243. [Google Scholar]
- Kato Y, Henry A, Fujita D, Katsura K, Kobayashi N, Serraj R. 2011. Physiological characterization of introgression lines derived from an indica rice cultivar, IR64, adapted to drought and water-saving irrigation. Field Crops Research 123: 130–138. [Google Scholar]
- Kato Y, Collard BCY, Septiningsih EM, Ismail AM. 2014. Shoot growth and other traits associated with tolerance of stagnant flooding in rice. AoB PLANTS 6: plu058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackill DJ, Ismail AM, Singh US, Labios RV, Paris TR. 2012. Development and rapid adoption of submergence-tolerant (Sub1) rice varieties. Advances in Agronomy 115: 303–356. [Google Scholar]
- Manzanilla D, Singh RK, Kato Y, Johnson D. 2016. Climate-ready technologies: combating poverty by raising productivity in rainfed rice environments in Asia. Los Baños, Philippines: International Rice Research Institute. [Google Scholar]
- Ookawa T, Ishihara K. 1993. Varietal difference of the cell wall components affecting the bending stress of the culm in relation to the lodging resistance in paddy rice. Japanese Journal of Crop Science 62: 378–384. [Google Scholar]
- Ookawa T, Hobo T, Yano M, et al. 2010. New approach for rice improvement using a pleiotropic QTL gene for lodging resistance and yield. Nature Communications 1: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ookawa T, Aoba R, Yamamoto T, et al. 2016. Precise estimation of genomic regions controlling lodging resistance using a set of reciprocal chromosome segment substitution lines in rice. Scientific Reports 6: 30572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumanti IA, Hairmansis A, Nugraha Y, et al. 2018. Development of tolerant rice varieties for stress-prone ecosystems in the coastal deltas of Indonesia. Field Crops Research 223: 75–82. [Google Scholar]
- SAS Institute 2003. SAS user’s guide: statistics. Version 9.1 2002–2003. Cary, NC: SAS Institute. [Google Scholar]
- Septiningsih EM, Mackill DJ. 2018. Genetics and breeding of flooding tolerance in rice. In: Sasaki T, Ashikari M, eds. New waves in rice genomics, genetics, and breeding. Singapore: Springer, 275–295. [Google Scholar]
- Septiningsih EM, Pamplona AM, Sanchez DL, et al. 2009. Development of submergence-tolerant rice cultivars: the Sub1 locus and beyond. Annals of Botany 103: 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setter TL, Laureles EV, Mazaredo AM. 1997. Lodging reduces yield of rice by self-shading and reductions in canopy photosynthesis. Field Crops Research 49: 95–106. [Google Scholar]
- Singh A, Carandang J, Gonzaga ZJC, Collard BC, Ismail AM, Septiningsih EM. 2017. Identification of QTLs for yield and agronomic traits in rice under stagnant flooding conditions. Rice 10: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Mackill DJ, Ismail AM. 2011. Tolerance of longer-term partial stagnant flooding is independent of the SUB1 locus in rice. Field Crops Research 121: 311–323. [Google Scholar]
- Steffens B, Geske T, Sauter M. 2011. Aerenchyma formation in the rice stem and its promotion by H2O2. New Phytologist 190: 369–378. [DOI] [PubMed] [Google Scholar]
- Vergara GV, Mackill DJ, Ismail AM. 2014. Variation in tolerance of partial stagnant flooding in rice. AoB PLANTS 6: plu055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser EJW, Bögemann GM. 2003. Measurement of porosity in very small samples of plant tissue. Plant and Soil 253: 81–90. [Google Scholar]
- Voesenek LACJ, Bailey-Serres J. 2015. Flood adaptive traits and processes: an overview. New Phytologist 206: 57–73. [DOI] [PubMed] [Google Scholar]
- Winkel A, Pedersen O, Ella E, Ismail AM, Colmer TD. 2014. Gas film retention and underwater photosynthesis during field submergence of four contrasting rice genotypes. Journal of Experimental Botany 65: 3225–3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Ma BL. 2016. A new method for assessing plant lodging and the impact of management options on lodging in canola crop production. Scientific Reports 6: 31890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Xu X, Fukao T, et al. 2006. Sub1A is an ethylene responsive-factor-like gene that confers submergence tolerance in rice. Nature 442: 705–708. [DOI] [PubMed] [Google Scholar]
- Yang G, Liu J, Zhao C, et al. 2017. Unmanned aerial vehicle remote sensing for field-based crop phenotyping: current status and perspectives. Frontiers in Plant Sciences 8: 1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Yamagishi J. 2010. Response of spikelet number per panicle in rice cultivars to three transplanting densities. Plant Production Science 13: 279–288. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.