Abstract
Aims/Introduction
The purpose of the present study was to observe the relationship between serum α‐klotho (KL) protein level and diabetic retinopathy (DR), and to further examine the effects of KL protein on apoptosis induced by palmitic acid (PA) in human retinal endothelial cells.
Materials and Methods
A total of 17 healthy people and 60 type 2 diabetes patients were included. According to the results from fundus fluorescein angiography, the diabetes patients were divided into three subgroups: without DR, non‐proliferative DR and proliferative DR. Serum KL level was measured by enzyme‐linked immunosorbent assay. In vitro, human retinal endothelial cells were exposed to PA with or without KL protein. Apoptosis rates were analyzed by flow cytometry analysis. Apoptotic‐related protein expressions were detected by western blotting analysis.
Results
Serum KL level was lower in diabetes patients than that in healthy participants (P = 0.007), and was gradually decreased among the without DR, non‐proliferative DR and proliferative DR subgroups (P = 0.045). A logistic regression analysis showed that after adjusting for the other confounding factors, serum KL level was independently and negatively related with DR (P = 0.049). Furthermore, the increased apoptosis rates induced by PA were inhibited with the addition of KL protein. Consistently, KL protein reversed the expression levels of the increased pro‐apoptotic protein Bax and the decreased anti‐apoptotic protein Bcl‐2 induced by PA. However, the anti‐apoptotic effect of KL protein was attenuated by LY294002 through the phosphatidylinositol 3 kinase‐serine∕threonine kinase pathway.
Conclusions
The data suggested that KL protein was probably a potential protective factor against retinopathy in type 2 diabetes patients.
Keywords: α‐Klotho, Apoptosis, Diabetic retinopathy
α‐Klotho plays important vasculoprotective roles. We analyzed the relationship between serum klotho level and diabetic retinopathy by clinical and cell data. The results suggested that klotho protein was probably a potential protective factor against retinopathy in type 2 diabetes patients.
![]()
Introduction
Diabetic retinopathy (DR) is one of the most common microvascular complications of diabetes. The number of persons with visual impairment as a result of DR is increasing worldwide1, 2, 3. Studies showed that the retinal vascular endothelial cells apoptosis have an important role in the pathogenesis of DR4. Thus, it will be important to identify and intervene in the various pathological factors that cause endothelial cells apoptosis.
Although hyperglycemia is the main initiator of DR, dyslipidemia is also an important risk factor. In a meta‐analysis and systematic review, the results showed that lipid‐lowering agents presented a protective effect on retinopathy progression in diabetes patients5. Basic studies showed that dyslipidemia was implicated in the pathological processes of DR and prediabetic retinopathy6, 7, 8. Palmitic acid (PA), a saturated free fatty acid, could induce retinal endothelial cell damage and participates in the progression of DR6, 7.
The α‐klotho (KL) gene was first described in 1997 as an anti‐aging gene and codes for a single‐pass transmembrane protein9. At the cellular level, the extracellular domain of KL protein can be cleaved by proteolytic enzymes and released into the circulation as soluble KL10. Studies have shown that KL protein plays vasculoprotective roles by reducing oxidative stress and apoptosis of vascular endothelial cells, and enhancing endothelial function11. Studies found that hyperlipidemia could downregulate KL gene expression12, and KL gene deficiency promoted the development of high‐fat diet‐induced arterial stiffening and hypertension13. The data suggested that KL protein was likely to play a pivotal role in vascular protection through the antagonism of lipotoxicity.
Previous studies showed that the KL gene had a critical function for retinal health14, 15. Therefore, based on the corresponding theoretical basis, we aimed to observe the relationship between serum KL level and DR in type 2 diabetes patients. Furthermore, in vitro, the effect of recombinant KL protein on apoptosis induced by PA was observed in human retinal endothelial cells (HRECs).
Methods
Participants
The participants included hospitalized patients with type 2 diabetes in November 2015 to November 2016, and the non‐diabetic healthy population who carried out physical examinations in the same period at the First Affiliated Hospital of Chongqing Medical University in Chongqing, China. Diagnosis of diabetes was based on the criteria from the World Health Organization (1999 version). Exclusion criteria: (i) patients with acute diabetic complications; (ii) patients with type 1 diabetes mellitus or other types of diabetes; (iii) patients with acute and chronic infection symptoms; (iv) patients with severe liver and kidney dysfunctions, severe cardiovascular diseases, malignant tumors and so on; (v) patients who smoked more than one cigarette per day, and who drank excessive alcohol (20 g/day or 140 g/week in men and 10 g/day or 70 g/week in women); and (vi) patients who had received eye surgery and had other eye diseases. Eventually, 60 patients with type 2 diabetes and 17 healthy individuals were included. According to the 2002 international clinical standard on the severity of DR16, all diabetes patients underwent fundus fluorescein angiography and were divided into the following groups: without DR (NDR), non‐proliferative DR (NPDR) and proliferative DR (PDR). The healthy population underwent fundus photography and confirmed that there were no abnormities in the fundus.
Informed consent was obtained from all participants before the survey, and this study was approved by the Research Ethics Committee of the First Affiliated Hospital of Chongqing Medical University.
Measurements of anthropometric and laboratory parameters
In all participants, the sex, age, height, weight, systolic blood pressure (SBP), diastolic blood pressure (DBP), total cholesterol (TC), triglyceride (TG), low‐density lipoprotein cholesterol (LDL‐c), high‐density lipoprotein cholesterol (HDL‐c), serum creatinine (Scr), blood urea nitrogen (BUN), uric acid (UA) and fasting plasma glucose (FPG) were collected. The information about duration of diabetes, glycated hemoglobin and percentage of diabetic nephropathy (DN) were also collected. DN was diagnosed according to the albuminuria levels: normoalbuminuria (<30 mg/24 h), microalbuminuria (30–300 mg/24 h) and macroalbuminuria (>300 mg/24 h)17. The serum lipid profiles including TC, TG, HDL‐c and LDL‐c were measured by an enzymatic assay (Wako Diagnostics, Tokyo, Japan). The FPG was tested by hexokinase assays (Olympus Diagnostics, Tokyo, Japan). The Scr, BUN and UA were tested by enzymatic methods (Roche Diagnostics, Mannheim, Germany).
Measurement of serum KL level
Fasting venous blood samples were collected in coagulation‐promoting tubes and were centrifuged at 1620 g for 10 min. The serum samples were extracted and stored at −80°C. Serum KL level was measured by enzyme‐linked immunosorbent assay. The assay kit was purchased from Immuno‐Biological Laboratories (Takasaki, Japan), and the detection range was from 93.75 to 6,000 pg/mL.
Cell culture
Human retinal endothelial cells were purchased from TongPai Biotechnology Co., Ltd (Shanghai, China).
Cells were grown in RPMI‐1640 medium (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Gibco), 100 μg/mL streptomycin (HyClone, Logan, UT, USA) and 100 U/mL penicillin (Hyclone) under a humidified atmosphere containing 5% CO2 at 37°C. Cells with passages 3–15 were used. When reaching approximately 80% of confluence in 60‐mm culture dishes, the cells were treated with PA (Sigma‐Aldrich, St. Louis, MO, USA) after pre‐treatment with or without recombinant human KL protein (R&D Systems, Minneapolis, MN, USA) and the phosphatidylinositol 3 kinase‐serine∕threonine kinase (PI3K/Akt) inhibitor, LY294002 (Sigma‐Aldrich). Namely, the cells were randomly divided into a normal control (Ctr) group, PA group, PA + KL group and PA + KL + LY294002 group.
Cell viability assay
To determine the pro‐apoptotic effect of PA on HRECs, cells were first intervened with different concentrations of PA for 24 h, and the viability was measured with a cell counting kit‐8 (CCK‐8; Beyotime Institute of Biotechnology, Shanghai, China) following the manufacturer's instructions. Cells were seeded in a 96‐wells plate and intervened with PA (0, 200, 400, 800 μmol/L) for 24 h. Then cells were incubated with the mixed liquor (10 μL CCK‐8 reagent + 100 μL RPMI‐1640 medium) at 37°C for 1 h. And the optical density value was measured at 450 nm.
Detection of apoptosis by flow cytometry analysis
Apoptosis was carried out using an apoptosis assay kit (Sungene Biotech, Tianjin, China). The apoptotic cells were measured using annexin V‐FITC assay conjugated to propidium iodide by flow cytometry with BD FACSVantage SE cytometer (BD Biosciences, San Jose, CA, USA).
Western blot analysis
Total protein was extracted. The cells were washed with ice‐cold phosphate‐buffered saline and collected in radioimmunoprecipitation assay lysis buffer (Beyotime Institute of Biotechnology). The protein concentrations were measured with BCA method (Boster Biological Technology, Wuhan, China). The Bcl‐2, Bax, Akt and p‐Akt antibodies were ordered (Cell Signaling Technology, Beverly, MA, USA). Phosphospecific protein level was compared with its total protein level, and others were normalized to β‐actin.
Statistical analysis
Statistical analysis was carried out using SPSS 21.0 (SPSS Inc., Chicago, IL, USA).
Clinical data are presented as the mean ± standard deviation for normally distributed variables, and the median (interquartile range [IQR]) for abnormal distributions. Unpaired t‐test and Mann–Whitney U‐test were used for comparisons of normally and abnormally distributed continuous variables between the two groups, respectively. Multiple and pairwise comparisons were determined by analysis of variance and Student–Newman–Keuls tests for normally distributed data, and Kruskal–Wallis and stepwise–step‐down tests for abnormal distributions. Categorical variables are presented as the percentage (%). The χ2‐test was used to compare categorical variables. A logistic regression analysis was used for multivariate analysis to analyze the association between serum KL protein and DR. Data from cells are shown as the mean ± standard error of the mean. P‐values (two‐tailed) <0.05 are regarded as significant differences.
Results
Baseline characteristics
The anthropometric and biochemical characteristics of participants are shown in Table 1. The study included 77 participants, consisting of 47 men and 30 women. Compared with the healthy population (NC group), the SBP, DBP and FPG in diabetes patients (diabetes group) significantly increased (all P < 0.05), whereas the HDL‐c decreased (P < 0.05). There were no significant differences in sex, age, body mass index, TC, LDL‐c, TG, Scr, BUN and UA between the two groups (all P > 0.05).
Table 1.
Comparison of clinical and biochemical data of subjects
| Variable | NC (n = 17) | DM (n = 60) | P | DM | P | ||
|---|---|---|---|---|---|---|---|
| NDR (n = 27) | NPDR (n = 17) | PDR (n = 16) | |||||
| Sex, males (%) | 52.9% | 63.3% | 0.574 | 55.6% | 64.7% | 68.7% | 0.524 |
| Age (years) | 57.0 ± 5.1 | 57.8 ± 5.6 | 0.595 | 58.0 ± 4.7 | 57.4 ± 5.5 | 57.9 ± 7.1 | 0.925 |
| BMI (kg/m2) | 23.00 ± 3.51 | 24.15 ± 3.20 | 0.228 | 24.00 ± 2.85 | 23.85 ± 3.46 | 24.95 ± 3.68 | 0.623 |
| SBP (mmHg) | 120.0 ± 10.8 | 138.9 ± 20.1 | 0.001 | 135.5 ± 17.0 | 140.6 ± 23.5 | 142.9 ± 21.3 | 0.478 |
| DBP (mmHg) | 75.9 ± 8.6 | 83.0 ± 12.5 | 0.046 | 81.7 ± 12.2 | 82.2 ± 15.0 | 86.2 ± 10.4 | 0.505 |
| HDL‐c (mmol/L) | 1.52 ± 0.34 | 1.12 ± 0.28 | <0.001 | 1.13 ± 0.32 | 1.05 ± 0.18 | 1.18 ± 0.28 | 0.468 |
| LDL‐c (mmol/L) | 2.92 ± 0.44 | 2.81 ± 0.95 | 0.626 | 2.82 ± 0.92 | 2.65 ± 1.08 | 3.01 ± 0.87 | 0.622 |
| TC (mmol/L) | 4.90 ± 0.61 | 4.30 ± 1.21 | 0.053 | 4.36 ± 1.12 | 3.91 ± 1.37 | 4.77 ± 1.06 | 0.176 |
| TG (mmol/L) | 1.37 (1.10–2.25) | 1.38 (0.97–2.35) | 0.837 | 1.42 (0.89–2.13) | 1.33 (0.92–2.51) | 1.41 (1.15–2.65) | 0.759 |
| FPG (mmol/L) | 5.28 ± 0.30 | 8.57 ± 3.04 | <0.001 | 8.21 ± 2.78 | 9.19 ± 3.51 | 8.50 ± 3.02 | 0.588 |
| Scr (μmol/L) | 74.24 ± 12.57 | 74.53 ± 22.69 | 0.959 | 59.41 ± 11.76 | 80.47 ± 21.06* | 93.75 ± 21.59* , ** | <0.001 |
| BUN (mmol/L) | 5.73 ± 1.07 | 6.97 ± 2.91 | 0.090 | 5.56 ± 1.41 | 6.98 ± 2.73 | 9.33 ± 3.52* , ** | <0.001 |
| UA (μmol/L) | 342.29 ± 70.50 | 326.43 ± 84.17 | 0.481 | 278.67 ± 66.13 | 360.47 ± 76.85* | 370.88 ± 80.39* | <0.001 |
| Duration (years) | 6.0 (2.0–10.0) | 9.0 (6.5–12.0) | 10.0 (7.3–18.5) * | 0.032 | |||
| HbA1c (%) | 9.80 ± 2.00 | 10.16 ± 2.30 | 7.94 ± 1.59* , ** | 0.004 | |||
| DN (%) | 40.7% | 52.9% | 56.2% | 0.556 | |||
Data are presented as the mean ± standard deviation for normally distributed variables, and the median (interquartile ranges) for abnormal distributions. Unpaired t‐test and Mann–Whitney U‐test were used for comparisons of normally and abnormally distributed continuous variables between two groups, respectively. Multiple and pairwise comparisons were determined by analysis of variance and Student–Newman–Keuls tests for normally distributed data, and Kruskal–Wallis and stepwise–step‐down tests for abnormal distributions. Categorical variables were presented as the percentage (%). The χ2‐test was used to compare categorical variables. Statistical differences were defined by P‐values (two‐tailed) <0.05.
*P < 0.05 versus without diabetic retinopathy (NDR).
**P < 0.05, proliferative diabetic retinopathy versus non‐proliferative diabetic retinopathy.
BMI, body mass index; BUN, blood urea nitrogen; DBP, diastolic blood pressure; DM, diabetes mellitus; DN, diabetic nephropathy; FPG, fasting plasma glucose; HDL‐c, high‐density lipoprotein cholesterol; LDL‐c, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; Scr, serum creatinine; TC, total cholesterol; TG, triglyceride; UA, uric acid.
Additionally, the subgroup analyses were carried out among the NDR, NPDR and PDR groups. The results found that the Scr, BUN, UA levels and duration of diabetes among the subgroups were gradually increased (all P < 0.05). Compared with the NDR group, the Scr and UA levels increased in the NPDR and PDR group (all P < 0.05); and the BUN and duration of diabetes obviously increased, whereas the glycated hemoglobin level reduced in the PDR group (all P < 0.05). There were no significant differences in sex, age, BMI, SBP, DBP, HDL‐c, LDL‐c, TC, TG, FPG and percentage of DN among the three groups (all P > 0.05).
Comparison of serum KL level
As shown in Figure 1a, the serum KL level was lower in the diabetes group (566.25 pg/mL, IQR 471.57–705.43 pg/mL) than that in the NC group (768.98 pg/mL, IQR 544.41–1095.52 pg/mL; P = 0.007). Furthermore, subgroup analyses showed that the serum KL levels in the NDR, NPDR and PDR groups were 699.16 pg/mL (IQR 518.91–829.74 pg/mL), 583.40 pg/mL (IQR 471.24–700.70 pg/mL) and 495.88 pg/mL (IQR 407.85–571.11 pg/mL), respectively, and were gradually decreased (P = 0.045). Compared with the NDR group, the serum KL levels were significantly reduced in the PDR group (P = 0.013); however, there was no significant difference between NDR and NPDR groups (P = 0.425), and NPDR and PDR groups (P = 0.123; Figure 1b). Additionally, although there was no obvious statistical difference between the two groups (P = 0.173), the serum KL level was lower in the NDR group than that in the NC group.
Figure 1.
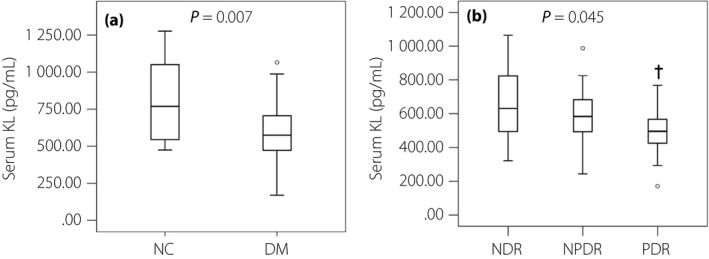
Serum klotho (KL) levels in diabetes and diabetic retinopathy (DR) patients. (a) Comparison of serum KL levels between healthy control participants (NC group; n = 17) and diabetes patients (DM group; n = 60). (b) Comparison of serum KL levels among diabetes patients without diabetic retinopathy (NDR; n = 27), diabetes patients with non‐proliferative diabetic retinopathy (NPDR; n = 17) and diabetes patients with proliferative diabetic retinopathy (PDR; n = 16). Data are represented as box plots. A Mann–Whitney U‐test was used for comparisons of continuous variables between two groups. Kruskal–Wallis and stepwise–step‐down tests were used for multiple and pairwise comparisons. Statistical differences were defined by P‐values (two‐tailed) <0.05. † P < 0.05 versus NDR group.
Association analysis between serum KL level and DR
Diabetic retinopathy was served as the dependent variable, and the sex, age, BMI, SBP, DBP, HDL‐c, LDL‐c, TC and TG, FPG, duration of diabetes, DN state and serum KL level as the independent variables. A logistic regression analysis was carried out to analyze the association between serum KL protein and DR in diabetes patients. The results showed that after adjusting for the above‐mentioned confounding factors, the serum KL level was independently related with DR (B −0.005; SE 0.002; P = 0.049; OR 0.995; 95% CI 0.990–1.000).
Effects of PA on HRECs viability
Human retinal endothelial cells were treated with PA in different concentrations (0, 200, 400, 800 μmol/L) for 24 h, and the cell viability was detected by the CCK‐8 method. The results showed that the cell activity was decreased with the increase of PA concentrations and was shown in a concentration‐dependent manner (Figure 2). Furthermore, the cell viability was significantly decreased in the concentration of 800 μmol/L, which suggested that there might be an obvious cytotoxic effect. Then we selected 400 μmol/L, 24 h as the intervention concentration and time of PA for the subsequent experiments.
Figure 2.
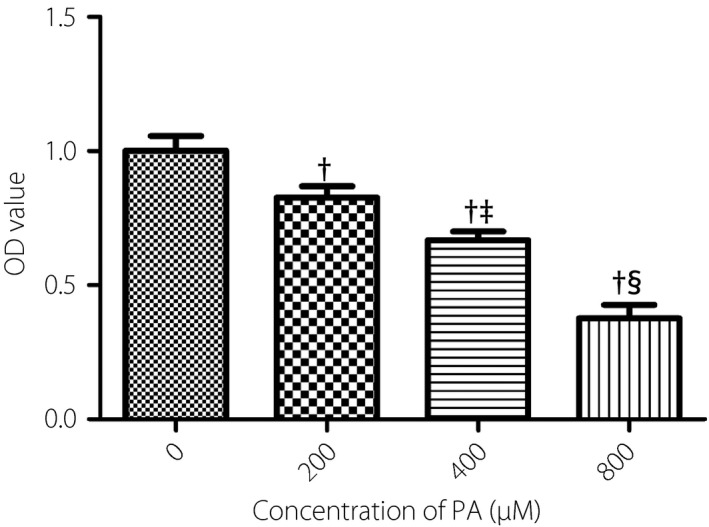
Effects of different concentrations of palmitic acid (PA) on cell viability. human retinal endothelial cells were treated with different concentrations of PA (0, 200, 400, 800 μmol/L). After the treatments for 24 h, cell viability was assessed by cell counting kit‐8 assay. Data are represented as the mean ± standard error of the mean (n = 3). Analysis of variance and Student–Newman–Keuls tests were carried out for multiple and pairwise comparisons. Statistical differences were defined by P‐values (two‐tailed) <0.05. † P < 0.05 versus 0 μmol/L; ‡ P < 0.05 versus 200 μmol/L; § P < 0.05 versus 400 μmol/L. OD, optical density.
Effects of KL protein on PA‐induced apoptosis in HRECs
To determine the effects of KL protein on cell apoptosis induced by PA, HRECs were pretreated with 400 pmol/L recombinant human KL protein for 1 h (the selection of concentration and intervention time based on previous relevant reports18, 19), and then were treated with PA for 24 h. The results showed that after pretreatment with KL protein, the increased apoptosis rate induced by PA was significantly decreased (P < 0.05; Figure 3a,b), and the Bcl‐2 expression was upregulated and Bax expression was downregulated (P < 0.05; Figure 3c,d).
Figure 3.
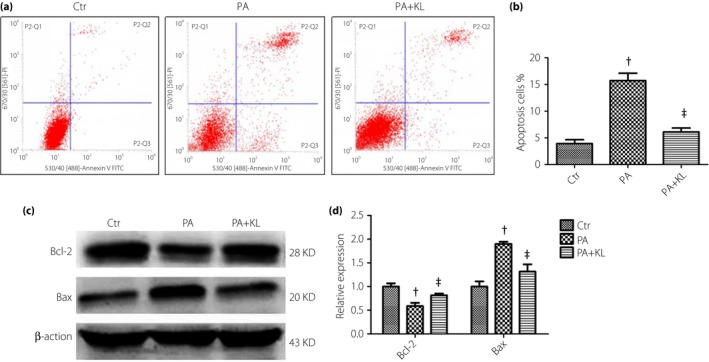
Effects of klotho (KL) protein on palmitic acid (PA)‐induced apoptosis in human retinal endothelial cells. Cells were pretreated with recombinant human KL protein (400 pmol/L) for 1 h and then treated with PA (400 μmol/L) for 24 h. (a,b) The cell apoptosis rate was detected using flow cytometry analysis. (c,d) Levels of Bcl‐2 and Bax were detected by western blot analysis. Data are represented as the mean ± standard error of the mean (n = 3). Analysis of variance and Student–Newman–Keuls tests were carried out for multiple and pairwise comparisons. Statistical differences were defined by P‐values (two‐tailed) <0.05. † P < 0.05 versus control (Ctr) group; ‡ P < 0.05 versus PA group.
The PI3K/Akt pathway plays an important role in regulating cell survival. Akt is a pivotal effector and could be activated through phosphorylation by the products of PI3K. In the present study, the expression of p‐Akt was decreased after exposure of HRECs to PA for 24 h; however, its level was restored to the control level after pretreating with KL protein (Figure 4a,b). Furthermore, to test whether the PI3K/Akt signaling pathway was involved in the inhibitory effects of KL protein on PA‐induced cell damage, the inhibitor of PI3K, LY294002, was applied. HRECs were pretreated with LY294002 (10 μmol/L) for 1 h and 400 pmol/L KL for another 1 h, and then treated with PA for 24 h. The results showed that LY294002 intervention significantly weakened the inhibitory effects of KL protein on cell apoptosis induced by PA (Figure 4c,d).
Figure 4.
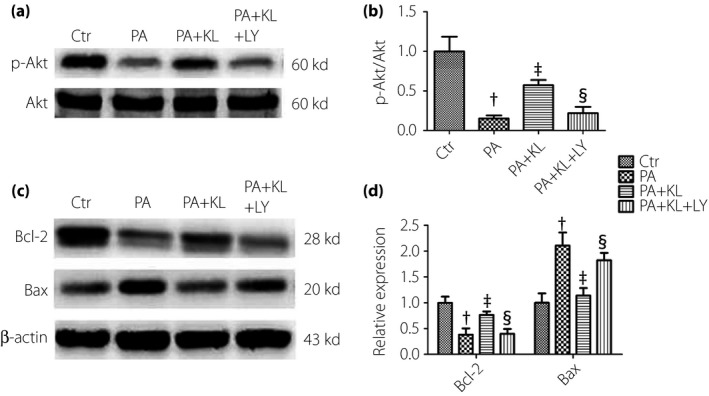
Klotho (KL) protein inhibited palmitic acid (PA)‐induced apoptosis by activating the phosphatidylinositol 3 kinase‐serine∕threonine kinase (PI3K/Akt) pathway in human retinal endothelial cells. Cells were pre‐incubated with the PI3K inhibitor, LY294002 (10 μmol/L), for 1 h, followed by co‐treatment with recombinant human KL protein (400 pmol/L) for 1 h and then with PA (400 μmol/L) for 24 h. (a,b) Level of p‐Akt was detected by western blot analysis. (c,d) Levels of Bcl‐2 and Bax were detected by western blot analysis. Data are represented as the mean ± standard error of the mean (n = 3). Analysis of variance and Student–Newman–Keuls tests were carried out for multiple and pairwise comparisons. Statistical differences were defined by P‐values (two‐tailed) <0.05. † P < 0.05 versus control (Ctr) group; ‡ P < 0.05 versus PA group; § P < 0.05 versus PA + KL group. LY, LY294002.
Discussion
The present data showed that the serum KL level was decreased in DR patients, and it was independently and negatively correlated with DR. In addition, KL protein could ameliorate the apoptosis induced by PA in HRECs, and the mechanisms might be involved in the activation of PI3K/Akt signaling pathway.
A variety of pathophysiological factors in vivo could affect KL gene expression. Studies have shown that hyperglycemia, oxidative stress, inflammation and angiotensin II significantly inhibited KL gene expression20, 21, 22, 23. The present results showed that the serum KL protein level was evidently reduced in diabetes patients, which was consistent with previous studies24, 25, 26, 27, 28.
The reduced serum KL level was probably an independent risk factor for DR. In the study of Słomiński et al.29, the findings suggested that the KL gene polymorphism protected against the development of retinopathy in patients with type 1 diabetes, which might be involved in the increased KL protein levels and/or activity and/or trafficking of the protein that improved the inflammatory status and delayed endothelial dysfunction. The present results showed that serum KL protein levels showed a downward trend with the increase of severity of retinopathy. However, it should be pointed out that serum KL protein was mainly from the kidney, and the renal and serum KL protein levels were significantly reduced in the DN state28, 30, 31, 32. Consistent with previous results, the subgroup analyses were carried out according to the patients without DN (NDN group) or with DN (DN group) among diabetes patients, and the results showed that compared with the NDN group (682.60 pg/mL, IQR 526.52–829.74 pg/mL), the serum KL level in the DN group (474.18 pg/mL, IQR 381.78–604.76 pg/mL) was significantly decreased (P < 0.001). The DN state served as one of the important adjustment variables, and a logistic regression analysis was carried out to analyze the association between serum KL and DR. The results showed that the serum KL level was independently and negatively related with DR after adjusting for the DN status and other variables. In a brief summary, according to the above‐mentioned results, we speculated that if the serum KL level was raised, there might be great improvement effects on DR. Consequently, we further observed the effects of KL protein in in vitro cell culture experiments.
The synthesis and decomposition function of adipose tissue is impaired in diabetes. In addition to hyperglycemia, dyslipidemia has also been found to be closely related with the DR progression. The present clinical data showed that the serum HDL‐c level was significantly decreased in the diabetes patients compared with the healthy participants; however, there were no differences in TC, LDL‐c and TG between the two groups, and furthermore, the blood lipid levels had no obvious differences in each subgroup according to the severity of retinopathy. We inferred that the use of lipid‐lowering drugs in diabetes patients might be the main reasons leading to no obvious differences. PA, one of the main sources of serum free fatty acid, was significantly increased in the diabetes patients and closely associated with DR33. In accordance with previous studies6, 7, 34, the present results showed that PA could significantly induce retinal endothelial cell apoptosis. Fenofibrate, a peroxisome proliferator‐activated receptor alpha agonist, could improve dyslipidemia. However, studies have confirmed that the improvement effects of fenofibrate on DR might not be due to its lipid‐lowering effects, but mainly depended on its anti‐inflammatory, anti‐oxidant and anti‐apoptosis functions35. This suggested that if there was a molecule against lipotoxicity‐induced injury, it would have important values to delay the progression of retinopathy or other diabetic complications.
Previous studies showed that KL protein probably had an important role in vascular protection through the antagonism of lipotoxicity12, 13. In clinical data, we found that the serum KL protein level was lower in the DR population and was an independent risk factor for DR. Therefore, we further observed the effects of KL protein through an in vitro cell experiment. The results showed that the administration of recombinant KL protein could evidently inhibit apoptosis induced by PA in HRECs, suggesting that KL protein might have a direct protective effect on DR.
The PI3K/Akt pathway plays an important role in regulating cell survival, and Akt is a pivotal effector. Studies reported that KL protein could activate the PI3K/Akt pathway to play protective roles. In the study of Zeldich et al.19, administration of recombinant KL protein could inhibit the glutamate and amyloid‐induced neuronal cell oxidative stress injury, whereas PI3K inhibitors LY294002 significantly weakened the protective effect of KL protein. In the present study, our results showed that KL protein significantly reversed the decreased expression level of p‐Akt induced by PA. Additionally, although KL protein attenuated apoptosis caused by PA in HRECs, the inhibitory effects of KL were obviously abolished by LY294002. This suggested that KL protein could inhibit HRECs damage induced by PA through activating the PI3K/Akt signaling pathway. Nevertheless, contrary to the study of Yamamoto et al.36, the results showed that KL protein could activate the fork transcription factor and then enhance the resistance of cells to oxidative stress, but the mechanism was involved in inhibiting the insulin/insulin‐like growth factor receptor 1/PI3K/Akt signaling pathway. Therefore, further experiments are necessary to elaborate the effects of KL protein on the Akt signaling pathway.
It should be pointed out that there were several limitations. Regarding clinical data, first, the sample size was relatively small in the present study. Second, the detailed history of medication, especially the use of angiotensin II receptor antagonist and insulin, should be obtained, and it would be better to carry out subgroup analyses. Regarding cell data, first, when exploring the mechanisms, we only discussed the PI3K/Akt signaling pathway, and other pathways were unclear. In addition to the PI3K inhibitor, the Akt inhibitor needed to be used in order to elaborate this signal pathway. Second, the concentration of KL protein was selected mainly according to the previous reports, and we examined the effects of KL protein only in in vitro experiments. Third, PA stimulation alone did not represent the state of diabetes. By all accounts, further large sample studies are required to exhaustively analyze the relationship between the serum KL level and DR. Further basic studies in vivo should be carried out to explore the protective roles and dose–effect of KL protein on DR.
In conclusion, the serum KL level was independently and negatively correlated with retinopathy in type 2 diabetes patients. Furthermore, KL treatment can inhibit apoptosis induced by PA in HRECs, and the potential mechanism might be involved in the activation of the PI3k/Akt signaling pathway. The data suggested that KL protein was likely to be a new and important target for DR.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
We specially thank the Laboratory of Lipid and Glucose Metabolism, the First Affiliated Hospital of Chongqing Medical University, Chongqing, P.R. China, for technical support. This study was supported by research grants from the National Nature Science Foundation of China (81070639, 81270911, 30771038 and 30570744) and National Key Clinical Specialties Construction Program of China.
J Diabetes Investig 2020; 11: 162–169
References
- 1. Leasher JL, Bourne RR, Flaxman SR, et al Global estimates on the number of people blind or visually impaired by diabetic retinopathy: a meta‐analysis from 1990 to 2010. Diabetes Care 2016; 39: 1643–1649. [DOI] [PubMed] [Google Scholar]
- 2. Lin S, Gupta B. Visual impairment certification due to diabetic retinopathy in North and Eastern Devon. Acta Ophthalmol 2017; 95: e756–e762. [DOI] [PubMed] [Google Scholar]
- 3. Song P, Yu J, Chan KY, et al Prevalence, risk factors and burden of diabetic retinopathy in China: a systematic review and meta‐analysis. J Glob Health 2018; 8: 010803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eshaq RS, Aldalati AMZ, Alexander JS, et al Diabetic retinopathy: breaking the barrier. Pathophysiology 2017; 24: 229–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shi R, Zhao L, Wang F, et al Effects of lipid‐lowering agents on diabetic retinopathy: a meta‐analysis and systematic review. Int J Ophthalmol 2018; 11: 287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kumar B, Kowluru A, Kowluru RA. Lipotoxicity augments glucotoxicity‐induced mitochondrial damage in the development of diabetic retinopathy. Invest Ophthalmol Vis Sci 2015; 56: 2985–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mohamed IN, Hafez SS, Fairaq A, et al Thioredoxin‐interacting protein is required for endothelial NLRP3 inflammasome activation and cell death in a rat model of high‐fat diet. Diabetologia 2014; 57: 413–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kowluru RA, Mishra M, Kowluru A, et al Hyperlipidemia and the development of diabetic retinopathy: comparison between type 1 and type 2 animal models. Metabolism 2016; 65: 1570–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Matsumura Y, Aizawa H, Shiraki‐Iida T, et al Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun 1998; 242: 626–630. [DOI] [PubMed] [Google Scholar]
- 10. Chen CD, Tung TY, Liang J, et al Identification of cleavage sites leading to the shed form of the anti‐aging protein klotho. Biochemistry 2014; 53: 5579–5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mencke R, Hillebrands JL. The role of the anti‐ageing protein Klotho in vascular physiology and pathophysiology. Ageing Res Rev 2017; 35: 124–146. [DOI] [PubMed] [Google Scholar]
- 12. Sastre C, Rubio‐Navarro A, Buendia I, et al Hyperlipidemia‐associated renal damage decreases Klotho expression in kidneys from ApoE knockout mice. PLoS One 2013; 8: e83713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lin Y, Chen J, Sun Z. Antiaging gene Klotho deficiency promoted high‐fat diet‐induced arterial stiffening via inactivation of AMP‐activated protein kinase. Hypertension 1979; 2016: 564–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reish NJ, Maltare A, McKeown AS, et al The age‐regulating protein klotho is vital to sustain retinal function. Invest Ophthalmol Vis Sci 2013; 54: 6675–6685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang Y, Wang L, Wu Z, et al The expressions of Klotho family genes in human ocular tissues and in anterior lens capsules of age‐related cataract. Curr Eye Res 2017; 42: 871–875. [DOI] [PubMed] [Google Scholar]
- 16. Wilkinson CP, Ferris FL 3rd, Klein RE, et al Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003; 110: 1677–1682. [DOI] [PubMed] [Google Scholar]
- 17. He BB, Xu M, Wei L, et al Relationship between anemia and chronic complications in Chinese patients with type 2 diabetes mellitus. Arch Iran Med 2015; 18: 277–283. [PubMed] [Google Scholar]
- 18. Yang K, Nie L, Huang Y, et al Amelioration of uremic toxin indoxyl sulfate‐induced endothelial cell dysfunction by Klotho protein. Toxicol Lett 2012; 215: 77–83. [DOI] [PubMed] [Google Scholar]
- 19. Zeldich E, Chen CD, Colvin TA, et al The neuroprotective effect of Klotho is mediated via regulation of members of the redox system. J Biol Chem 2014; 289: 24700–24715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang JS, Chuang CT, Liu MH, et al Klotho attenuates high glucose‐induced fibronectin and cell hypertrophy via the ERK1/2‐p38 kinase signaling pathway in renal interstitial fibroblasts. Mol Cell Endocrinol 2014; 390: 45–53. [DOI] [PubMed] [Google Scholar]
- 21. Mitobe M, Yoshida T, Sugiura H, et al Oxidative stress decreases klotho expression in a mouse kidney cell line. Nephron Exp Nephrol 2005; 101: e67–e74. [DOI] [PubMed] [Google Scholar]
- 22. Moreno JA, Izquierdo MC, Sanchez‐Nino MD, et al The inflammatory cytokines TWEAK and TNFalpha reduce renal klotho expression through NFkappaB. J Am Soc Nephrol 2011; 22: 1315–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhou Q, Lin S, Tang R, et al Role of fosinopril and valsartan on Klotho gene expression induced by angiotensin II in rat renal tubular epithelial cells. Kidney Blood Press Res 2010; 33: 186–192. [DOI] [PubMed] [Google Scholar]
- 24. Keles N, Dogan B, Kalcik M, et al Is serum Klotho protective against atherosclerosis in patients with type 1 diabetes mellitus? J Diabetes Complications 2016; 30: 126–132. [DOI] [PubMed] [Google Scholar]
- 25. Liu JJ, Liu S, Morgenthaler NG, et al Association of plasma soluble alpha‐klotho with pro‐endothelin‐1 in patients with type 2 diabetes. Atherosclerosis 2014; 233: 415–418. [DOI] [PubMed] [Google Scholar]
- 26. Nie F, Wu D, Du H, et al Serum klotho protein levels and their correlations with the progression of type 2 diabetes mellitus. J Diabetes Complications 2017; 31: 594–598. [DOI] [PubMed] [Google Scholar]
- 27. Wu C, Wang Q, Lv C, et al The changes of serum sKlotho and NGAL levels and their correlation in type 2 diabetes mellitus patients with different stages of urinary albumin. Diabetes Res Clin Pract 2014; 106: 343–350. [DOI] [PubMed] [Google Scholar]
- 28. Zhang L, Liu T. Clinical implication of alterations in serum Klotho levels in patients with type 2 diabetes mellitus and its associated complications. J Diabetes Complications 2018; 32: 922–930. [DOI] [PubMed] [Google Scholar]
- 29. Slominski B, Ryba‐Stanislawowska M, Skrzypkowska M, et al The KL‐VS polymorphism of KLOTHO gene is protective against retinopathy incidence in patients with type 1 diabetes. Biochim Biophys Acta 2018; 1864: 758–763. [DOI] [PubMed] [Google Scholar]
- 30. Lee EY, Kim SS, Lee JS, et al Soluble alpha‐klotho as a novel biomarker in the early stage of nephropathy in patients with type 2 diabetes. PLoS One 2014; 9: e102984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Inci A, Sari F, Coban M, et al Soluble Klotho and fibroblast growth factor 23 levels in diabetic nephropathy with different stages of albuminuria. J Investig Med 2016; 64: 1128–1133. [DOI] [PubMed] [Google Scholar]
- 32. Maltese G, Fountoulakis N, Siow RC, et al Perturbations of the anti‐ageing hormone Klotho in patients with type 1 diabetes and microalbuminuria. Diabetologia 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang X, Gao Y, Zhou Z, et al Familial clustering of diabetic retinopathy in Chongqing, China, type 2 diabetic patients. Eur J Ophthalmol 2010; 20: 911–918. [DOI] [PubMed] [Google Scholar]
- 34. Yamagishi S, Okamoto T, Amano S, et al Palmitate‐induced apoptosis of microvascular endothelial cells and pericytes. Mol Med 2002; 8: 179–184. [PMC free article] [PubMed] [Google Scholar]
- 35. Noonan JE, Jenkins AJ, Ma JX, et al An update on the molecular actions of fenofibrate and its clinical effects on diabetic retinopathy and other microvascular end points in patients with diabetes. Diabetes 2013; 62: 3968–3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yamamoto M, Clark JD, Pastor JV, et al Regulation of oxidative stress by the anti‐aging hormone klotho. J Biol Chem 2005; 280: 38029–38034. [DOI] [PMC free article] [PubMed] [Google Scholar]


