Abstract
Aims/Introduction
To investigate the efficacy/safety of dulaglutide once‐weekly monotherapy versus glimepiride in Chinese patients with type 2 diabetes.
Materials and Methods
This was a post‐hoc analysis of a Chinese randomized, double‐blind, non‐inferiority, phase III study. Patients (n = 572) with inadequate glycemic control received dulaglutide 1.5 mg (n = 189) or 0.75 mg (n = 194) once‐weekly or glimepiride (1–3 mg/day; n = 189) for 26 weeks. The primary objective of the study was to investigate the non‐inferiority of dulaglutide 1.5 mg versus glimepiride by the change from baseline to week 26 in glycated hemoglobin (non‐inferiority margin 0.4%).
Results
Dulaglutide 1.5 mg and 0.75 mg were non‐inferior (P < 0.001) and superior (P ≤ 0.002) versus glimepiride for the change in glycated hemoglobin from baseline to week 26. The least‐squares mean differences (95% confidence interval) versus glimepiride were dulaglutide 1.5 mg, −0.53% (−0.74, −0.32) and dulaglutide 0.75 mg, −0.32% (−0.53, −0.12). Significantly more patients attained glycated hemoglobin <7.0% at week 26 in the dulaglutide 1.5 mg (71.7%) versus the glimepiride (57.5%; P = 0.005) group. The decrease from baseline to week 26 in fasting blood glucose was significantly more pronounced in both the dulaglutide groups versus the glimepiride group (P < 0.01). The overall incidence and rate of hypoglycemia were lower in both of the dulaglutide groups versus the glimepiride group. At week 26, bodyweight had increased from baseline in the glimepiride group and decreased from baseline in both dulaglutide groups. The most frequent gastrointestinal drug‐related adverse events with dulaglutide were diarrhea, abdominal distension, nausea and vomiting.
Conclusions
These findings support once‐weekly dulaglutide monotherapy as a treatment for Chinese patients with early stage type 2 diabetes.
Keywords: Chinese, Dulaglutide, Type 2 diabetes mellitus
This study investigated the efficacy and safety of once‐weekly dulaglutide monotherapy versus glimepiride in Chinese patients with type 2 diabetes mellitus. Once‐weekly dulaglutide 1.5 mg or 0.75 mg monotherapy provided superior glycemic control over glimepiride. The safety profile of dulaglutide in Chinese patients with type 2 diabetes mellitus was consistent with that expected for a glucagon‐like peptide‐1 receptor agonist.

Introduction
Type 2 diabetes is becoming increasingly prevalent in China1 and other East Asian countries2. Recent International Diabetes Federation estimates show that nearly 11% of adults in China have diabetes, and that more than half (53.6%) of these adults remain undiagnosed3. With increasing rates of obesity (driven by economic development and consequent lifestyle changes)1, the prevalence and related economic burden of type 2 diabetes in China seems likely to further increase. Therefore, identifying treatment strategies that are effective for Chinese patients with type 2 diabetes is of clear importance.
Dulaglutide is a long‐acting human glucagon‐like peptide‐1 receptor agonist that has been approved as a once‐weekly subcutaneous injection for the treatment of type 2 diabetes based on findings from the phase III AWARD (Assessment of Weekly AdministRation of dulaglutide in Diabetes) studies4, 5, 6, 7, 8, 9, 10. Treatment with dulaglutide is associated with effective glycemic control, a relatively low rate of hypoglycemia, decreased bodyweight and the benefits of once‐weekly dosing11. In addition to being efficacious in combination with other therapies4, 5, 6, 8, 9, 10, dulaglutide monotherapy was found to significantly improve glycemic control (compared with metformin), and was well tolerated in the global, phase III, AWARD‐3 study7. More recently, results from a phase III clinical study in East Asian patients with type 2 diabetes showed that dulaglutide monotherapy (1.5 and 0.75 mg) provided glycemic control superior to that provided by glimepiride (a commonly used oral antidiabetic drug in China12), and had favorable safety and tolerability13.
The aim of the present post‐hoc analysis of the aforementioned phase III East Asian study13 was to assess the efficacy and safety of dulaglutide monotherapy (1.5 and 0.75 mg once‐weekly) versus glimepiride in Chinese patients with inadequately controlled type 2 diabetes who were either naïve to or taking oral anti‐hyperglycemic medication (OAM) as monotherapy.
Methods
Study design
The post‐hoc analyses of Chinese patients with type 2 diabetes were of a previously described13 randomized, multicenter, double‐blind, phase III study carried out in China (29 sites), Taiwan and South Korea (ClinicalTrials.gov: NCT01644500).
Ethical review boards at each study site reviewed and approved the study protocol. The study was carried out as per the Declaration of Helsinki, the Council for International Organizations of Medical Sciences International Ethical Guidelines, the International Conference on Harmonization, Good Clinical Practices Guideline [E6], and applicable local laws and regulations.
All patients gave written informed consent before participating in the study.
Patients
All Chinese patients meeting the previously described inclusion and exclusion criteria13 were included in the post‐hoc analyses. Key inclusion criteria included a body mass index ≥19 and ≤35 kg/m2 and OAM‐naïvety (with glycated hemoglobin [HbA1c] ≥7.0 and ≤10.5% at screening) or discontinuation of OAM monotherapy for ≥3 months before screening (with HbA1c ≥6.5 and ≤10.0% at screening). Key exclusion criteria included any prescription for incretin‐based medications and treatment with insulin within 3 months before screening.
Treatment protocol
The study treatment protocol has been described in full previously13. Patients received subcutaneous dulaglutide 1.5 or 0.75 mg once‐weekly, or oral glimepiride 1–3 mg/day for 26 weeks. Glimepiride dosing began at 1 mg/day, and was increased at week 4 to 2 mg/day and at week 8 to 3 mg/day, as tolerated.
Outcome measures
Efficacy
The study outcome measures have been described previously13. The primary and key secondary efficacy outcome measures were the change from baseline to week 26 in HbA1c for dulaglutide 1.5 mg versus glimepiride and dulaglutide 0.75 mg versus glimepiride, respectively. Other secondary efficacy outcomes at week 26 were: the proportion of patients who attained HbA1c <7.0 or ≤6.5%; 7‐point self‐monitored blood glucose (SMBG) concentration profiles; fasting blood glucose (FBG) concentrations; blood glucose concentration excursions; and insulin sensitivity and β‐cell function, as determined using updated homeostasis model assessment (per blood glucose, C‐peptide and insulin levels).
Safety
As previously described13, safety outcomes included treatment‐emergent adverse events (TEAEs), TEAEs leading to discontinuation, serious adverse events, the incidence and the rate of hypoglycemia, bodyweight change, vital signs, laboratory tests, electrocardiograms, dulaglutide anti‐drug antibodies (ADAs), and any allergic or hypersensitivity reactions.
Events that were considered definitive or possible acute pancreatitis were independently adjudicated by an expert committee.
All deaths/non‐fatal cardiovascular adverse events were adjudicated by an external committee.
Statistical analysis
As previously described13, the study sample size was calculated based on a one‐sided significance level of 0.025, a non‐inferiority margin of 0.4% and a standard deviation (SD) of 1.3 for the change from baseline to week 26 in HbA1c. Approximately 789 patients were to be enrolled in the study.
A modified intention‐to‐treat population was used for the efficacy analyses, which comprised all patients with a baseline HbA1c reading and one or more post‐baseline HbA1c reading, and who had received one or more dose of the study drug.
An as‐treated population (the treatment patients actually received) was used for the safety analyses. The population included all patients who received one or more dose of the study drug.
The change from baseline to week 26 in HbA1c was analyzed using a mixed‐model repeated measures analysis. Fixed effects were treatment, visit, treatment‐by‐visit interaction and pre‐study treatment. The covariate was baseline HbA1c, and the random effect was patient. Missing data were not imputed. The treatment difference (dulaglutide 1.5 mg minus glimepiride) at week 26 was estimated using the corresponding 95% confidence interval (CI) of the least‐squares mean (LSM). Dulaglutide 1.5 mg was considered non‐inferior to glimepiride if the upper bound of the 95% CI for the difference between treatments was <0.4%. In the case where non‐inferiority was achieved, the type 1 error rate was controlled at 0.025 using tree gatekeeping14. The superiority of dulaglutide 1.5 mg versus glimepiride, along with the non‐inferiority and superiority of dulaglutide 0.75 mg versus glimepiride, was then assessed. As these were post‐hoc analyses, no adjustments for multiplicity were made.
Secondary efficacy outcomes were analyzed by Fisher's exact test (proportion of patients attaining HbA1c targets), mixed‐model repeated measures analysis for the primary efficacy outcome (FBG, 7‐point SMBG profiles, BG excursions) or analysis of covariance (insulin sensitivity, β‐cell function).
Demographics and baseline characteristics were analyzed by Fisher's exact test or an analysis of variance model for categorical and continuous data, respectively.
Safety outcomes were analyzed using descriptive statistics. Categorical data are summarized using counts and percentages. Continuous data are summarized using the mean, standard deviation and median.
For all comparisons, P < 0.05 was taken to show statistical significance.
Results
Patient disposition/baseline characteristics
Of the 572 patients randomized (dulaglutide 1.5 mg [n = 189]; dulaglutide 0.75 mg [n = 194]; glimepiride [n = 189]), 50 (8.7%) discontinued the study and 522 (91.3%) completed study treatment (Figure 1).
Figure 1.
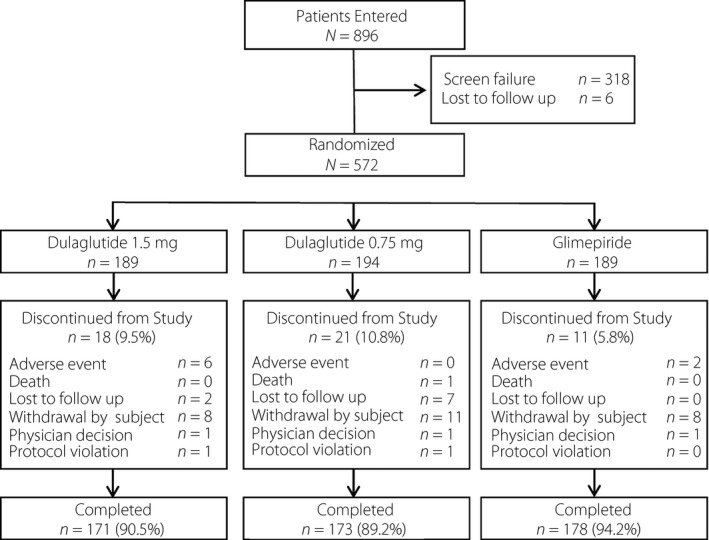
Patient disposition.
Demographic and baseline clinical characteristics were similar across the three treatment groups (Table 1). The overall mean values for key variables at baseline were: HbA1c 8.0% (SD 1.0%); bodyweight, 69.8 kg (SD 11.1 kg); and duration of type 2 diabetes 3.6 years (SD 4.3 years). Slightly >50% of patients had a history of treatment with ≥1 OAM.
Table 1.
Patient demographics and baseline clinical characteristics (modified intention‐to‐treat analysis set)
| Variable | Dulaglutide 1.5 mg (n = 184) | Dulaglutide 0.75 mg (n = 186) | Glimepiride (n = 186) | Total (n = 556) |
|---|---|---|---|---|
| Male, n (%) | 109 (59.2) | 107 (57.5) | 109 (58.6) | 325 (58.5) |
| Age, years (mean ± SD) | 52.8 ± 10.40 | 53.8 ± 9.83 | 52.7 ± 9.61 | 53.1 ± 9.95 |
| <65 years, n (%) | 167 (90.8) | 163 (87.6) | 172 (92.5) | 502 (90.3) |
| ≥65 years, n (%) | 17 (9.2) | 23 (12.4) | 14 (7.5) | 54 (9.7) |
| Bodyweight (kg) | 69.7 ± 10.8 | 70.7 ± 12.0 | 69.1 ± 10.6 | 69.8 ± 11.1 |
| BMI (kg/m2) | 25.5 ± 3.21 | 26.0 ± 3.34† | 25.3 ± 2.92 | 25.6 ± 3.17 |
| HbA1c, % (mean ± SD) | 8.0 ± 1.00 | 8.0 ± 1.01 | 7.9 ± 1.01 | 8.0 ± 1.00 |
| <8.5%, n (%) | 129 (70.1) | 127 (68.3) | 134 (72.0) | 390 (70.1) |
| ≥8.5, n (%) | 55 (29.9) | 59 (31.7) | 52 (28.0) | 166 (29.9) |
| Duration of T2D (years) | 4.0 ± 4.67 | 3.2 ± 4.00 | 3.6 ± 4.10‡ | 3.6 ± 4.26 |
| History of ≥1 previous OAM | ||||
| Yes, n (%) | 97 (52.7) | 96 (51.6) | 99 (53.2) | 292 (52.5) |
| Current alcohol use, n (%) | 41 (22.3) | 33 (17.7) | 34 (18.4)§ | 108 (19.5) |
| Current tobacco use,* n (%) | 64 (35.2)¶ | 41 (22.0) | 49 (26.5)†† | 154 (27.8) |
| Systolic BP (mmHg) | 129 ± 13.2 | 129 ± 13.9 | 128 ± 15.0 | 128 ± 14.0 |
| Diastolic BP (mmHg) | 79 ± 9.0 | 79 ± 9.4 | 78 ± 8.8 | 78 ± 9.1 |
| Pulse rate (b.p.m.) | 76 ± 10.1 | 75 ± 9.1 | 77 ± 9.6 | 76 ± 9.6 |
Data are presented as the mean ± standard deviation, except where indicated. *P < 0.05 based on Fisher's exact test. † n = 185; ‡ n = 185; § n = 185; ¶ n = 182; †† n = 185. BMI, body mass index; BP, blood pressure; HbA1c, glycated hemoglobin; OAM, oral antidiabetic medication; SD, standard deviation; T2D, type 2 diabetes.
Efficacy
The LSM decrease in HbA1c from baseline to week 26 was significantly more pronounced in both of the dulaglutide groups (1.5 mg −1.46%, [standard error {SE}] 0.08%; 0.75 mg, −1.25% [SE 0.08%]) versus the glimepiride group (−0.92% [SE 0.08%]); both dulaglutide doses were non‐inferior and superior to glimepiride in reducing HbA1c (all P < 0.01; Figure 2). The LSM difference in HbA1c versus glimepiride was −0.53% for dulaglutide 1.5 mg, and −0.32% for dulaglutide 0.75 mg at week 26. The change from baseline in HbA1c over time for each treatment group is summarized in Figure 3.
Figure 2.
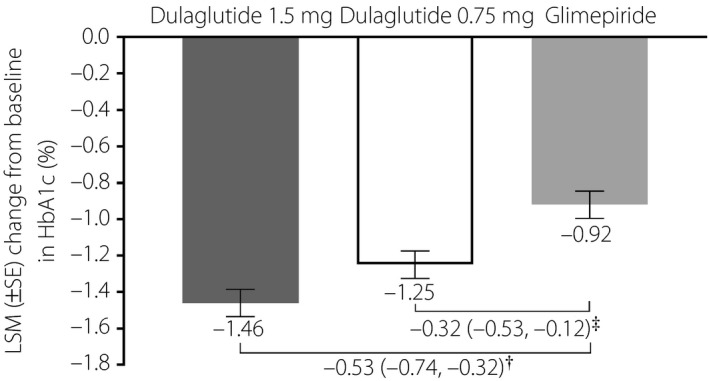
Change in glycated hemoglobin (HbA1c) from baseline to week 26 in Chinese patients with type 2 diabetes who were treated with dulaglutide 1.5 mg, dulaglutide 0.75 mg or glimepiride. †Difference in least‐squares mean (LSM; 95% confidence interval) between dulaglutide 1.5 mg and glimepiride. Dulaglutide 1.5 mg was non‐inferior and superior to glimepiride (both P < 0.001). ‡Difference in LSM (95% confidence interval) between dulaglutide 0.75 mg and glimepiride. Dulaglutide 0.75 mg was non‐inferior (P < 0.001) and superior (P = 0.002) to glimepiride.
Figure 3.
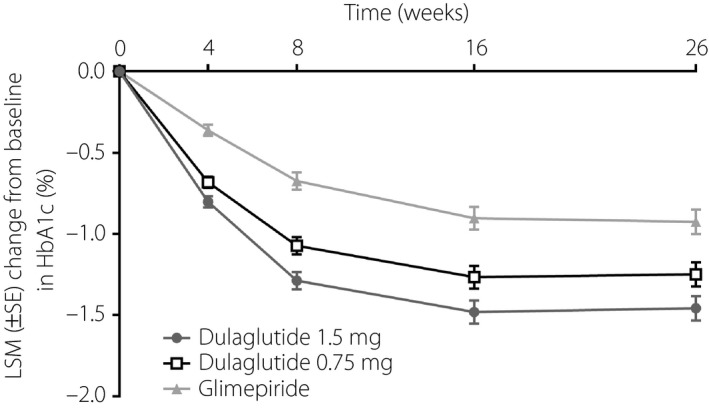
Change in glycated hemoglobin (HbA1c) from baseline (last observation carried forward) over time in Chinese patients with type 2 diabetes who were treated with dulaglutide 1.5 mg, dulaglutide 0.75 mg or glimepiride. LSM, least‐squares mean; SE, standard error.
A significantly greater proportion of patients attained week 26 HbA1c targets of <7.0% and ≤6.5% in the dulaglutide 1.5 mg group (71.7 and 57.1%) compared with the glimepiride group (57.5 and 40.9%, both P < 0.01; Figure 4). There was no significant difference in the proportion of patients attaining week 26 HbA1c targets between the dulaglutide 0.75 mg and glimepiride groups.
Figure 4.
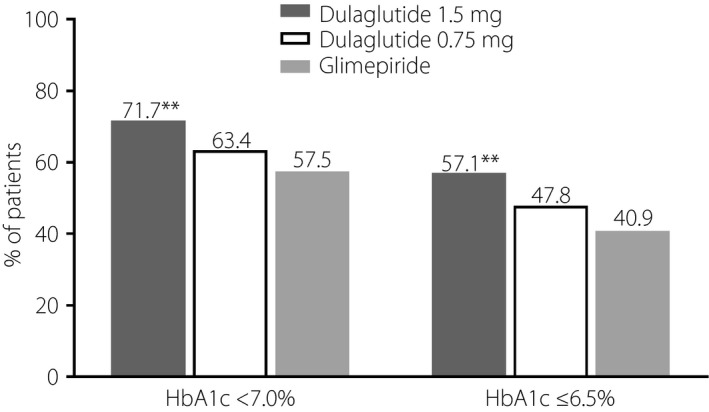
Proportion (last observation carried forward) of Chinese patients with type 2 diabetes who attained glycated hemoglobin (HbA1c) targets at week 26 after treatment with dulaglutide 1.5 mg, dulaglutide 0.75 mg or glimepiride. **P < 0.01 versus glimepiride.
The LSM decrease in FBG from baseline to week 26 was significantly more pronounced in both of the dulaglutide groups (1.5 mg, −2.29 mmol/L [SE 0.14 mmol/L]; 0.75 mg, −1.84 mmol/L, [SE 0.14 mmol/L]) compared with the glimepiride group (−1.41 mmol/L [SE 0.14 mmol/L], both P < 0.05; Figure 5).
Figure 5.
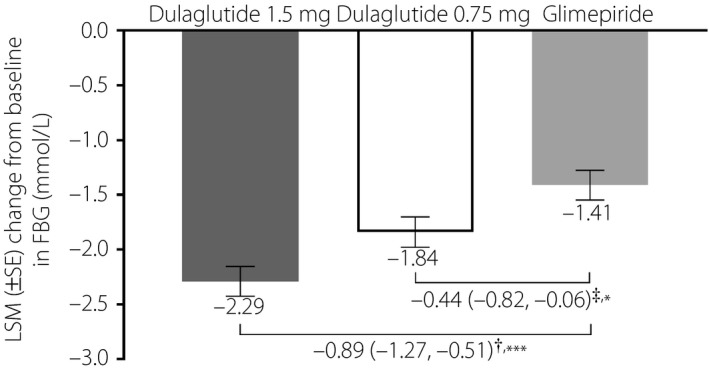
Change in fasting blood glucose (FBG) from baseline to week 26 in Chinese patients with type 2 diabetes who were treated with dulaglutide 1.5 mg, dulaglutide 0.75 mg or glimepiride. †Difference in least‐squares mean (LSM; 95% confidence interval) between dulaglutide 1.5 mg and glimepiride. ‡Difference in LSM (95% confidence interval) between dulaglutide 0.75 mg and glimepiride. *P < 0.05 versus glimepiride; ***P < 0.001 versus glimepiride. SE, standard error.
At week 26, the magnitude of decrease at each assessment time in the 7‐point SMBG profile was significantly more pronounced in the dulaglutide 1.5 mg group versus the glimepiride group (P < 0.01; Figure 6; Table S1). The daily LSM SMBG decrease was also significantly more pronounced in the dulaglutide 1.5 mg group versus the glimepiride group (−2.94 mmol/L [SE 0.12 mmol/L] vs −1.90 mmol/L [SE 0.12 mmol/L]; P < 0.001). Decreases from baseline in all postprandial excursions were significantly more pronounced in the dulaglutide 1.5 mg group versus the glimepiride group (all P < 0.05). At week 26, the magnitude of decrease at the morning 2‐h postprandial, midday 2‐h postprandial and bedtime assessments was significantly more pronounced in the dulaglutide 0.75 mg group versus the glimepiride group (all P < 0.05; Figure 6; Table S1). The daily LSM SMBG decrease was significantly more pronounced in the dulaglutide 0.75 mg group versus the glimepiride group (−2.27 [SE 0.12] mmol/L vs −1.90 [SE 0.12] mmol/L; P = 0.030). The decrease from baseline in the midday 2‐h excursion was significantly more pronounced in the dulaglutide 1.5 mg group versus the glimepiride group (P = 0.022; Table S1).
Figure 6.
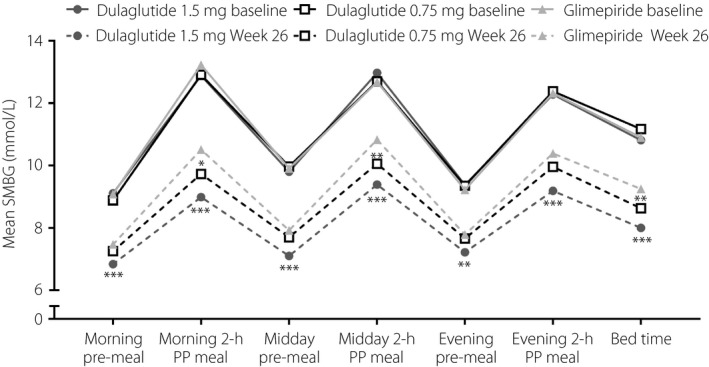
Seven‐point self‐monitored blood glucose (SMBG) profiles by time of assessment at baseline and week 26 in Chinese patients with type 2 diabetes who were treated with dulaglutide 1.5 mg, dulaglutide 0.75 mg or glimepiride. *P < 0.05 versus glimepiride; **P < 0.001 versus glimepiride; ***P < 0.001 versus glimepiride.
At week 26, the increase in C‐peptide and insulin‐based updated homeostasis model assessment for β‐cell function was significantly more pronounced in the dulaglutide groups versus the glimepiride group (all P < 0.001; Table S2).
Safety
The incidence of TEAEs was similar among the dulaglutide and glimepiride treatment groups (Table 2). Overall, 61.6% of all patients reported one or more TEAE. The majority of events were mild‐to‐moderate in severity. Few patients (≤6 in each treatment group) experienced serious adverse events or discontinued treatment due to a TEAE. As already described13, there was one death (due to intentional injury) in the dulaglutide 0.75 mg group that was not considered related to the study drug. TEAEs reported by ≥5% of patients were hyperlipidemia, diarrhea, nasopharyngitis, lipase increased, dyslipidemia, decreased appetite, abdominal distension, nausea and vomiting (Table 2). Drug‐related TEAEs were more common in both of the dulaglutide groups versus the glimepiride group (Table 2), mainly due to a higher incidence of TEAEs in the gastrointestinal system organ class (most frequently reported were: diarrhea, abdominal distension, nausea and vomiting). The incidence of gastrointestinal TEAEs was highest during the initial 2 weeks of treatment with dulaglutide (approximately 20%), but thereafter decreased rapidly (<5% between weeks 2 and 4; Figure 7).
Table 2.
Safety overview
| n (%) | Dulaglutide 1.5 mg (n = 189) | Dulaglutide 0.75 mg (n = 194) | Glimepiride (n = 187) | Total (n = 570) |
|---|---|---|---|---|
| Patients with ≥1 TEAE | 129 (68.3) | 112 (57.7) | 110 (58.8) | 351 (61.6) |
| Patients with ≥1 SAE | 6 (3.2) | 3 (1.5) | 3 (1.6) | 12 (2.1) |
| Patients who discontinued treatment due to a TEAE | 6 (3.2) | 4 (2.1) | 3 (1.6) | 13 (2.3) |
| Patients who died on therapy | 0 (0.0) | 1 (0.5) | 0 (0.0) | 1 (0.2) |
| Patients with ≥1 drug‐related TEAE | 72 (38.1) | 43 (22.2) | 20 (10.7) | 135 (23.7) |
| Most common TEAEs† | ||||
| Hyperlipidemia‡ | 27 (14.3) | 30 (15.5) | 41 (21.9) | 98 (17.2) |
| Diarrhea | 35 (18.5) | 18 (9.3) | 7 (3.7) | 60 (10.5) |
| Nasopharyngitis | 11 (5.8) | 11 (5.7) | 10 (5.3) | 32 (5.6) |
| Lipase increased | 14 (7.4) | 12 (6.2) | 5 (2.7) | 31 (5.4) |
| Dyslipidemia | 8 (4.2) | 9 (4.6) | 11 (5.9) | 28 (4.9) |
| Decreased appetite | 13 (6.9) | 11 (5.7) | 1 (0.5) | 25 (4.4) |
| Abdominal distension | 15 (7.9) | 5 (2.6) | 4 (2.1) | 24 (4.2) |
| Nausea | 18 (9.5) | 5 (2.6) | 1 (0.5) | 24 (4.2) |
| Vomiting | 12 (6.3) | 1 (0.5) | 0 (0.0) | 13 (2.3) |
†Reported by ≥5% of patients in any treatment group. ‡Lipid profile was measured at randomization (visit 3) for the first time with the treatment‐emergent adverse event (TEAE) collected from the lead‐in phase, leading to the high incidence of hyperlipidemia. SAE, serious adverse event.
Figure 7.
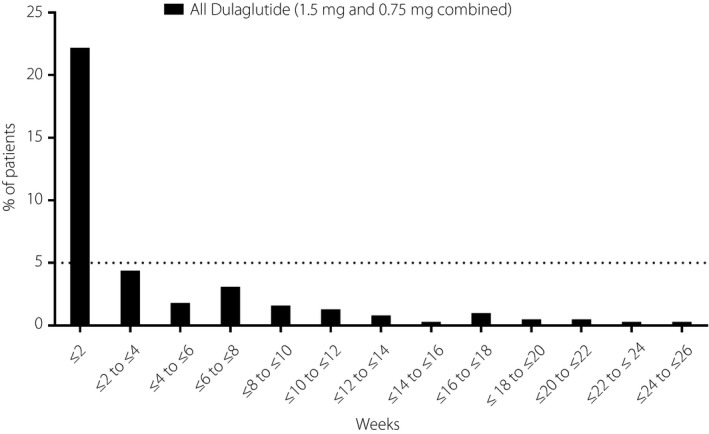
Incidence of gastrointestinal treatment‐emergent adverse events (per system organ class) over time in Chinese patients with type 2 diabetes who were treated with dulaglutide (1.5 and 0.75 mg combined).
The incidence of all forms of hypoglycemia and the overall rate of hypoglycemia were lower in both of the dulaglutide groups versus the glimepiride group (Table 3). There were no events of severe hypoglycemia reported.
Table 3.
Hypoglycemia overview
| Dulaglutide 1.5 mg (n = 189) | Dulaglutide 0.75 mg (n = 194) | Glimepiride (n = 187) | Total (n = 570) | |
|---|---|---|---|---|
| All hypoglycemic episodes | ||||
| No. episodes | 14 | 10 | 76 | 100 |
| Incidence, n (%) | 12 (6.3) | 8 (4.1) | 33 (17.6) | 53 (9.3) |
| Rate, mean ± SD (events/patient/year), | 0.14 ± 0.59 | 0.09 ± 0.51 | 1.39 ± 8.03 | |
| Severe hypoglycemia† | ||||
| No. episodes | 0 | 0 | 0 | 0 |
| Incidence, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Nocturnal hypoglycemia‡ | ||||
| No. episodes | 1 | 1 | 9 | 11 |
| Incidence, n (%) | 1 (0.5) | 1 (0.5) | 5 (2.7) | 7 (1.2) |
| Documented symptomatic hypoglycemia§ | ||||
| No. episodes | 1 | 2 | 32 | 35 |
| Incidence, n (%) | 1 (0.5) | 2 (1.0) | 14 (7.5) | 17 (3.0) |
| Asymptomatic hypoglycemia¶ | ||||
| No. episodes | 6 | 3 | 29 | 38 |
| Incidence, n (%) | 5 (2.6) | 2 (1.0) | 22 (11.8) | 29 (5.1) |
| Probable hypoglycemia†† | ||||
| Number of episodes | 7 | 5 | 15 | 27 |
| Incidence, n (%) | 7 (3.7) | 4 (2.1) | 10 (5.3) | 21 (3.7) |
†Defined as an episode requiring the assistance of another person to actively administer carbohydrate, glucagon or other resuscitative actions. These episodes might be associated with sufficient neuroglycopenia to induce seizure or coma. Plasma glucose measurements might not be available during such an event, but neurological recovery attributable to the restoration of plasma glucose to normal was considered sufficient evidence that the event was induced by a low plasma glucose concentration. ‡Defined as any hypoglycemic event that occurred between bedtime and waking. §Defined as any time a patient felt that he/she was experiencing symptoms and/or signs associated with hypoglycemia and had a plasma glucose concentration ≤3.9 mmol/L. ¶Defined as an event not accompanied by typical symptoms of hypoglycemia, but with a measured plasma glucose concentration of ≤3.9 mmol/L. ††Defined as an event during which symptoms of hypoglycemia were not accompanied by a plasma glucose determination (but that was presumably caused by a plasma glucose concentration ≤3.9 mmol/L). SD, standard deviation.
Throughout the 26‐week treatment period, bodyweight decreased from baseline in both of the dulaglutide groups, but increased from baseline in the glimepiride group (Figure 8). The week 26 LSM changes from baseline in bodyweight were: dulaglutide 1.5 mg, −1.5 kg (SE 0.2 kg); dulaglutide 0.75 mg, −1.0 kg (SE 0.2 kg); glimepiride, 0.9 kg (0.2 kg).
Figure 8.
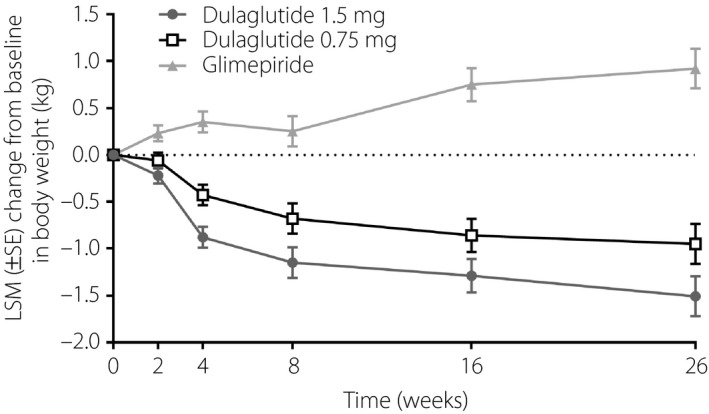
Change in bodyweight from baseline over time in Chinese patients with type 2 diabetes who were treated with dulaglutide 1.5 mg, dulaglutide 0.75 mg or glimepiride. LSM, least‐squares mean; SE, standard error.
Cardiovascular safety findings were generally similar between the treatment groups. The mean decrease from baseline in systolic blood pressure was numerically more pronounced in the dulaglutide groups (dulaglutide 1.5 mg, −1.55 mmHg; dulaglutide 0.75 mg, −1.50 mmHg) versus the glimepiride group (−0.16 mmHg). Likewise, the mean increase from baseline in pulse rate was numerically more pronounced in both of the dulaglutide treatment groups (dulaglutide 1.5 mg, 3.99 b.p.m.; dulaglutide 0.75 mg, 2.66 b.p.m.) versus the glimepiride group (0.42 b.p.m.). The pulse rate interval increased from baseline in both of the dulaglutide groups (dulaglutide 1.5 mg, 3.09 s; dulaglutide 0.75 mg, 3.93 s), and decreased from baseline in the glimepiride group (−0.14 s). One patient treated with dulaglutide 0.75 mg reported a transient ischemic attack, which was confirmed as being a cardiovascular adjudicated event. This event was not fatal.
No patients experienced adjudicated acute or chronic pancreatitis. The increases from baseline in total amylase, pancreatic amylase and lipase concentrations were numerically greater in both of the dulaglutide groups versus the glimepiride group (Table S3).
No patients experienced thyroid neoplasm, medullary thyroid carcinoma or C‐cell hyperplasia. Changes from baseline in calcitonin were minimal in all three groups (Table S4).
During the study, a relatively low proportion (19/370; 5.0%) of patients had treatment‐emergent dulaglutide ADAs. Except for two patients, all ADAs developed after baseline (the highest titer reported was 1:32).
Few patients experienced drug‐related hypersensitivity reactions (dulaglutide 1.5 mg, n = 1; dulaglutide 0.75 mg, n = 2 [including 1 event of mild urticaria]; glimepiride, n = 1), and none of the patients who did experience drug‐related hypersensitivity reactions developed treatment‐emergent ADAs.
Discussion
These post‐hoc analyses are the first to examine the efficacy and safety of dulaglutide monotherapy in Chinese patients with type 2 diabetes who had inadequate glycemic control and were either OAM‐naïve or receiving OAM monotherapy. Notably, we found both doses of dulaglutide studied (1.5 and 0.75 mg) were both non‐inferior and superior to glimepiride in providing glycemic control after 26 weeks of treatment. Improvements in secondary efficacy outcomes measures (percentage of patients who attained HbA1c targets, change in FBG, 7‐point SMBG profile, insulin sensitivity and β‐cell function) were also more pronounced (in most cases significantly) with dulaglutide. These improvements in efficacy with dulaglutide coincided with decreased hypoglycemia and weight loss versus glimepiride, and, overall, no new safety concerns. Taken together, these findings show that dulaglutide monotherapy is an efficacious treatment for Chinese patients with type 2 diabetes and has a favorable safety profile.
Our findings from the present post‐hoc analysis that dulaglutide monotherapy (both 1.5 mg and 0.75 mg doses) in Chinese patients with type 2 diabetes resulted in significantly more pronounced decreases in HbA1c from baseline versus glimepiride are consistent with the previously reported overall findings of this study13. Also similar to the overall study findings13, the magnitude of decrease in HbA1c from baseline with dulaglutide in Chinese patients was more pronounced than observed with dulaglutide monotherapy in the AWARD‐3 study7, which included predominantly Caucasian participants. As previously noted and discussed in further detail13, differences in patient factors likely underlie this difference in the magnitude of HbA1c decrease, including body mass index, background therapy and baseline HbA1c. Of interest, a closer examination of the overall study findings suggests that dulaglutide shows low ethnic sensitivity among East Asian patients who have type 2 diabetes. This is evidenced by a similar magnitude of decrease from baseline to week 26 in HbA1c with dulaglutide monotherapy (1.5 and 0.75 mg) in patients from China (−1.46 and −1.25%), the Taiwan region (−1.53 and −1.22%) and Korea (−1.57 and −0.99%; data from Taiwan region and Korea; Eli Lilly and Company, unpubl. data).
Also consistent with the overall study findings13, we found that Chinese patients treated with dulaglutide, in particular dulaglutide 1.5 mg, experienced significantly more pronounced improvements in FBG, 7‐point SMBG profiles (including postprandial glucose excursions), insulin sensitivity and β‐cell function, and more commonly attained HbA1c targets than patients treated with glimepiride.
There were no unexpected safety findings in our post‐hoc analyses of Chinese patients with type 2 diabetes. Of note, dulaglutide treatment was associated with decreased hypoglycemia versus glimepiride, and a decrease in bodyweight loss. These findings are consistent with those of the overall study13 and a previous study that compared another glucagon‐like peptide‐1 receptor agonist, liraglutide, with glimepiride15. Other safety findings, including a transient occurrence at the start of treatment of gastrointestinal drug‐related TEAEs, elevated pancreatic enzymes (with no adjudicated instances of acute or chronic pancreatitis), and few treatment‐emergent dulaglutide ADAs, are also in keeping with the overall study findings13 and other dulaglutide studies4, 5, 6, 7, 8, 9, 10.
The limitations of the study underlying this post‐hoc analysis have been described previously13.
In conclusion, we found that dulaglutide 1.5 or 0.75 mg once‐weekly monotherapy provided superior glycemic control versus glimepiride in Chinese patients with type 2 diabetes. Other efficacy findings also favored dulaglutide, including changes in FBG and SMBG, and the proportion of patients who attained HbA1c targets. Importantly, the safety profile of dulaglutide in this patient population was consistent with that expected for a glucagon‐like peptide‐1 receptor agonist. Taken together, these findings show that once‐weekly dulaglutide monotherapy has a favorable risk‐to‐benefit ratio and, therefore, might be an effective therapeutic option for Chinese patients who have early‐stage type 2 diabetes.
Disclosure
LYD and FW are employees of Lilly Suzhou Pharmaceutical Co. Ltd. LXS, XML, YQS, QML, JHM, YBL and LLC declare no conflict of interest.
Supporting information
Table S1 | Summary of change in 7‐point self‐monitored blood glucose concentrations from baseline to week 26.
Table S2 | Change in β‐cell function and insulin sensitivity from baseline to week 26.
Table S3 | Summary of pancreatic enzyme concentrations at baseline and week 26.
Table S4 | Summary of calcitonin concentrations at baseline and week 26.
Acknowledgments
This study was sponsored by Eli Lilly and Company, the manufacturer/licensee of Trulicity®. Medical writing assistance was provided by Luke Carey, PhD, CMPP, and Serina Stretton, PhD, CMPP, of ProScribe – Envision Pharma Group, and was funded by Eli Lilly and Company. ProScribe's services complied with international guidelines for Good Publication Practice (GPP3). Eli Lilly and Company was involved in the study design, data collection, data analysis and preparation of the manuscript.
Eli Lilly and Company provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available on request 6 months after the indication studied has been approved in the USA and European Union, and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once they are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report and blank or annotated case report forms, will be provided in a secure data‐sharing environment for up to 2 years per proposal. For details on submitting a request, see the instructions provided at http://www.clinicalstudydatarequest.com.
The authors express their gratitude to Jia Ning Hou (Lilly Suzhou Pharmaceutical Co. Ltd) for medical review, Bin Zhang (Lilly Suzhou Pharmaceutical Co. Ltd) for statistical review, Yi Ping Zou (Lilly Suzhou Pharmaceutical Co. Ltd) for project management and all study participants.
J Diabetes Investig 2020; 11: 142–150
Contributor Information
Feng Wang, Email: wang_feng3@lilly.com.
Lu Lu Chen, Email: cheria_chen@126.com.
References
- 1. Hu C, Jia W. Diabetes in China: epidemiology and genetic risk factors and their clinical utility in personalized medication. Diabetes 2018; 67: 3–11. [DOI] [PubMed] [Google Scholar]
- 2. Ma RC, Chan JC. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci 2013; 1281: 64–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. International Diabetes Federation . IDF Diabetes Atlas, 8th edn. 2017; Available from: http://www.diabetesatlas.org Accessed June 2, 2019.
- 4. Dungan KM, Weitgasser R, Perez Manghi F, et al A 24‐week study to evaluate the efficacy and safety of once‐weekly dulaglutide added on to glimepiride in type 2 diabetes (AWARD‐8). Diabetes Obes Metab 2016; 18: 475–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wysham C, Blevins T, Arakaki R, et al Efficacy and safety of dulaglutide added onto pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD‐1). Diabetes Care 2014; 37: 2159–2167. [DOI] [PubMed] [Google Scholar]
- 6. Giorgino F, Benroubi M, Sun JH, et al Efficacy and safety of once‐weekly dulaglutide versus insulin glargine in patients with type 2 diabetes on metformin and glimepiride (AWARD‐2). Diabetes Care 2015; 38: 2241–2249. [DOI] [PubMed] [Google Scholar]
- 7. Umpierrez G, Tofe Povedano S, Perez Manghi F, et al Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD‐3). Diabetes Care 2014; 37: 2168–2176. [DOI] [PubMed] [Google Scholar]
- 8. Blonde L, Jendle J, Gross J, et al Once‐weekly dulaglutide versus bedtime insulin glargine, both in combination with prandial insulin lispro, in patients with type 2 diabetes (AWARD‐4): a randomised, open‐label, phase 3, non‐inferiority study. Lancet 2015; 385: 2057–2066. [DOI] [PubMed] [Google Scholar]
- 9. Nauck M, Weinstock RS, Umpierrez GE, et al Efficacy and safety of dulaglutide versus sitagliptin after 52 weeks in type 2 diabetes in a randomized controlled trial (AWARD‐5). Diabetes Care 2014; 37: 2149–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dungan KM, Povedano ST, Forst T, et al Once‐weekly dulaglutide versus once‐daily liraglutide in metformin‐treated patients with type 2 diabetes (AWARD‐6): a randomised, open‐label, phase 3, non‐inferiority trial. Lancet 2014; 384: 1349–1357. [DOI] [PubMed] [Google Scholar]
- 11. Jendle J, Grunberger G, Blevins T, et al Efficacy and safety of dulaglutide in the treatment of type 2 diabetes: a comprehensive review of the dulaglutide clinical data focusing on the AWARD phase 3 clinical trial program. Diabetes Metab Res Rev 2016; 32: 776–790. [DOI] [PubMed] [Google Scholar]
- 12. Weng J, Ji L, Jia W, et al Standards of care for type 2 diabetes in China. Diabetes Metab Res Rev 2016; 32: 442–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen YH, Huang CN, Cho YM, et al Efficacy and safety of dulaglutide monotherapy compared with glimepiride in East‐Asian patients with type 2 diabetes in a multicentre, double‐blind, randomized, parallel‐arm, active comparator, phase III trial. Diabetes Obes Metab 2018; 20: 2121–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dmitrienko A, Tamhane AC. Mixtures of multiple testing procedures for gatekeeping applications in clinical trials. Stat Med 2011; 30: 1473–1488. [DOI] [PubMed] [Google Scholar]
- 15. Yang W, Chen L, Ji Q, et al Liraglutide provides similar glycaemic control as glimepiride (both in combination with metformin) and reduces body weight and systolic blood pressure in Asian population with type 2 diabetes from China, South Korea and India: a 16‐week, randomized, double‐blind, active control trial. Diabetes Obes Metab 2011; 13: 81–88. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 | Summary of change in 7‐point self‐monitored blood glucose concentrations from baseline to week 26.
Table S2 | Change in β‐cell function and insulin sensitivity from baseline to week 26.
Table S3 | Summary of pancreatic enzyme concentrations at baseline and week 26.
Table S4 | Summary of calcitonin concentrations at baseline and week 26.


