Abstract
Aims/Introduction
The role of glucagon abnormality has recently been reported in type 2 diabetes; however, its role in gestational diabetes mellitus (GDM) is still unknown. The glucose intolerance in GDM is heterogeneous, and not all patients require insulin treatment during pregnancy. Here, we investigated whether glucagon abnormality is associated with the requirement for insulin treatment during pregnancy.
Materials and Methods
A total of 49 pregnant women diagnosed with GDM were enrolled. They underwent a 75‐g oral glucose tolerance test during mid‐gestation, and we measured their plasma glucagon levels (by a new sandwich enzyme‐linked immunosorbent assay) at fasting (0 min), and at 30, 60 and 120 min after glucose load in addition to the levels of plasma glucose and serum insulin. All participants underwent another oral glucose tolerance test at postpartum.
Results
Of the 49 patients, 15 required insulin treatment (Insulin group) and 34 were treated with diet therapy alone until delivery (Diet group). The early‐phase glucagon secretion after glucose load, as determined by the changes in glucagon from the baseline to 30 min, was paradoxically augmented during mid‐gestation in the Insulin group, but not in the Diet group. The impaired glucagon suppression during mid‐gestation in the Insulin group was not associated with insulin secretory/sensitivity indexes studied, and was ameliorated postpartum, although the plasma glucose levels remained higher in the Insulin group versus the Diet group.
Conclusions
Impaired early‐phase suppression of glucagon could be associated with the requirement for insulin treatment during pregnancy in patients with GDM.
Keywords: Gestational diabetes, Glucagon, Sandwich enzyme‐linked immunosorbent assay
We evaluated the glucagon responses to oral glucose load in patients with gestational diabetes mellitus using a new sandwich enzyme‐linked immunosorbent assay. We investigated whether the glucagon abnormality is associated with the requirement for insulin treatment during pregnancy.
Introduction
Pregnancy is diabetogenic because of the increased insulin resistance caused by anti‐insulin hormones secreted by the placenta1. Insulin resistance increases as the placenta matures2, and pregnant women are believed to develop gestational diabetes mellitus (GDM) when the insulin secretion fails to compensate for the elevated insulin resistance as the pregnancy advances. It is important to make an accurate diagnosis of GDM and manage it, as GDM is associated with an increased risk of maternal and perinatal complications, such as pre‐eclampsia, macrosomia, shoulder dystocia and neonatal hypoglycemia.
After the adoption of the World Health Organization guideline in 20133 based on the International Association of Diabetes and Pregnancy Study Group criteria in 20104, the prevalence of GDM increased all over the world. It has been reported that up to 12% of all pregnant women in Japan develop GDM5, and one‐third of GDM patients require insulin treatment6. As we reported, the glucose intolerance in some GDM patients improves with appropriate nutrition therapy, but other GDM patients require insulin treatment during pregnancy7. GDM patients complicated with pre‐gravid obesity or excess maternal weight gain8, 9, 10 and women diagnosed with overt diabetes in pregnancy are more likely to require insulin therapy11. It can be difficult to predict the requirement of insulin therapy during pregnancy, even if the patient's insulin secretory capacity is analyzed using an oral glucose tolerance test (OGTT) during mid‐gestation12.
It has been advocated that diabetes is caused not only by insulin action deficiency, but also by insufficient glucagon suppression – the so‐called bi‐hormonal disorder13. Glucagon is a peptide hormone secreted from α‐cells of pancreatic Langerhans islets in response to hypoglycemia, and when a healthy human intakes glucose, the glucagon secretion is suppressed immediately14. In contrast, it is well known that paradoxical hyperglucagonemia after glucose ingestion occurs in patients with type 1 or type 2 diabetes, which plays a significant role in exacerbating hyperglycemia in these patients13. It was also reported that glucagon secretory abnormality can begin to develop even in individuals with mild impaired glucose tolerance15. GDM is a less severe form of glucose intolerance than overt diabetes in pregnancy, and it is suspected that women with GDM might develop glucagon secretory abnormality as a result of a lack of insulin action toward α‐cells, as in impaired glucose tolerance.
Several investigations of the plasma glucagon levels in small numbers of GDM patients and healthy women have been reported. Some of those investigations showed that the fasting levels of plasma glucagon in women with GDM were higher than those of healthy pregnant women16, 17. Other studies have inconsistently shown that plasma glucagon levels in both fasting and a post‐challenge OGTT among women with GDM were almost equal to those in healthy pregnant women18, 19, 20. However, those studies did not fully examine the glucagon secretory responses in GDM, because the levels of plasma glucagon were measured with a radioimmunoassay that uses polyclonal antibodies against the glucagon C‐terminal region. Such assays might not accurately measure plasma glucagon (1–29) because of cross‐reactivity against other proglucagon fragments, including glicentin and miniglucagon (19–29)21.
In 2014, a quantitative sandwich enzyme‐linked immunosorbent assay (ELISA) for glucagon was developed22 in which the plasma glucagon concentration is determined by two monoclonal antibodies against the N‐ and C‐terminal regions of the glucagon peptide with much less cross‐reactivity against other proglucagon fragments compared with the cross‐reactivity afforded by the conventional radioimmunoassay23. Miyachi et al.24 reported that the accuracy of the sandwich ELISA for glucagon measurement is confirmed by comparing with liquid chromatography‐mass spectrometry.
We carried out the present study to evaluate the glucagon secretory response to oral glucose load in patients with GDM, using the new glucagon assay22, and we attempted to determine whether the glucagon abnormality is associated with the requirement of insulin therapy during pregnancy among patients with GDM.
Methods
Patients and control participants
The participants were Japanese singleton pregnant women who had been diagnosed with GDM based on the International Association of Diabetes and Pregnancy Study Group/World Health Organization criteria3 using 75‐g OGTTs until 28 gestational weeks. None of the patients had previously been treated with antidiabetic agents including insulin. Patients were excluded if they had been diagnosed with type 1 diabetes, type 2 diabetes, overt diabetes in pregnancy (fasting glucose ≥126 mg/dL and/or 2‐h glucose in a 75‐g OGTT ≥200 mg/dL and/or glycated hemoglobin ≥6.5%) or latent autoimmune diabetes of adults with glutamic acid decarboxylase antibody positivity. Patients whose conditions were complicated with other diseases (including thyroid disease, collagen disease, renal disorder, liver disease, cardiovascular disease and respiratory disease), a history of gastrointestinal surgery or pancreatectomy, alcohol or drug abuse and malignancy were excluded. Patients treated with diabetogenic medication. such as a steroid. were also excluded.
To obtain the reference values of glucagon responses during a 75‐g OGTT in mid‐gestation, we also recruited healthy pregnant women who had been ascertained to be negative in the screening test for GDM; that is, their levels of plasma glucose were <140 mg/dL at 1 h of a 50‐g glucose challenge test.
Study design
This was a single‐center, prospective cohort study carried out at Nagasaki University Hospital from April 2015 to October 2018. The study is registered with UMIN‐CTR, no. 000034337. The study protocol is shown in Figure 1.
Figure 1.
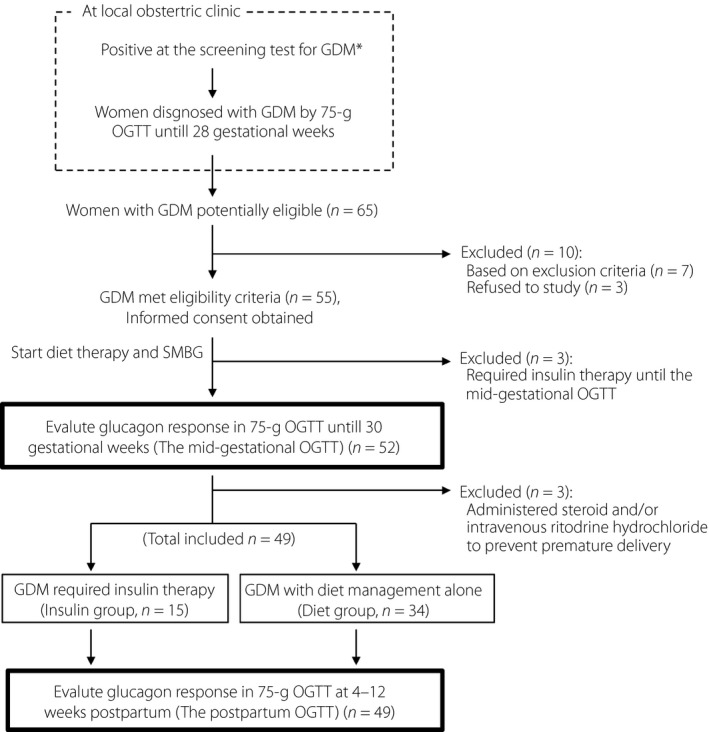
Study design. Flow chart of the patients with gestational diabetes mellitus (GDM; n = 49) and the 16 patients excluded due to predefined criteria or for remedial reasons. *The cut‐off value of the screening test for GDM is a 140 mg/dL plasma glucose level at 1 h during the 50‐g glucose challenge test. OGTT, oral glucose tolerance test; SMBG, self‐monitoring blood glucose.
We recruited 55 singleton pregnant women who had been diagnosed with GDM based on their levels of plasma glucose at fasting, 1 h and 2 h during a 75‐g OGTT until 28 gestational weeks by obstetricians at local clinics. After being enrolled in the present study, all of the patients received diet therapy instructions from a nutritionist and started self‐monitoring blood glucose. The enrolled patients were re‐evaluated with a 75‐g OGTT to evaluate their glucagon secretion responses until 30 gestational weeks (the mid‐gestational OGTT). The patients who required insulin treatment until the re‐evaluation OGTT were withdrawn from the study and referred back to routine antenatal care. The patients who were administered a steroid and/or intravenous ritodrine hydrochloride to prevent premature delivery were also withdrawn from the study.
A diet therapy instruction until delivery for the obese patients (body mass index [BMI] ≥25 kg/m2) was to consume 30 kcal/kg/day of their pre‐gravid ideal bodyweight, comprised of carbohydrate at 50–60% of total calories. The non‐obese patients (BMI <25 kg/m2) were instructed to consume 250 kcal/day during the second trimester and 450 kcal/day during the third trimester in addition to the calories described above for the obese patients.
Insulin treatment was started when a patient's level of fasting glucose >95 mg/dL or postprandial 2‐h glucose >120 mg/dL had continued for >1 week after the initiation of diet therapy. Insulin detemir was used as a supplement to basal insulin, and insulin aspart or insulin lispro was used to supplement bolus insulin. Clinicians were permitted to change the doses of the insulin preparations to keep the levels of fasting and postprandial blood glucose under each limit described above.
We divided the GDM patients into the Insulin group, defined as the patients who required insulin treatment during gestation to achieve the targeted glucose levels described above, and the Diet group, defined as the patients who had maintained their glucose levels within the targeted levels without using insulin until delivery. Both insulin treatment and self‐monitoring blood glucose were discontinued just after delivery for all patients. The patients’ glucagon secretion responses during a 75‐g OGTT were obtained at 4–12 weeks after delivery (the postpartum OGTT). All antenatal, perinatal and postpartum care were carried out as usual throughout the study period.
The healthy pregnant women enrolled into the study underwent a 75‐g OGTT once during gestational weeks 24–28 as control participants. On all occasions, OGTT was carried out in the morning after an overnight (10 h) fast. Written informed consent was obtained from all participants. The study was approved by the ethics committee of Nagasaki University Hospital (approval no. 14032483), and was carried out in accordance with the Declaration of Helsinki.
Laboratory measurements
As described in Study design and in Figure 1, OGTTs were carried out twice just after enrollment in the study during mid‐gestation and at 4–12 weeks postpartum using a 75‐g glucose formulation, the Trelan‐G75 (AY Pharma, Tokyo, Japan). For each OGTT, the levels of plasma glucose (mg/dL), serum insulin (μU/mL) and plasma glucagon (pg/mL) were measured at fasting (0 min), and at 30, 60 and 120 min after the ingestion of the glucose load.
The levels of plasma glucose and serum insulin were measured by the hexokinase ultraviolet method using a JCA‐BioMajesty6070 analyzer (JEOL, Tokyo, Japan), and an ECLusys kit using a Roche Modular Analytics E170 assay (Roche, Basel, Switzerland). Blood sampling for plasma glucagon was carried out using BD P800 tubes (BD, Franklin Lakes, NJ, USA). The blood samples were stored at −80°C, and plasma glucagon was measured after each participant accomplished the study. Plasma glucagon was measured using a sandwich ELISA kit (Mercodia, Uppsala, Sweden)22.
Assessment of glucagon secretion and insulin secretion/sensitivity
To assess the glucagon secretion responses after glucose load, we determined the change in the levels of plasma glucagon from baseline (0 min) to each time point (30, 60 and 120 min) during each 75‐g OGTT. These changes are shown as ΔGlucagon 30 min, ΔGlucagon 60 min and ΔGlucagon 120 min, as described14. The area over the ΔGlucagon curve from 0 to 120 min, shown as AOC ΔGlucagon 0–120 min, was calculated using the trapezoidal rule. Insulin secretion was assessed by the homeostasis model assessment for β‐cell function25, the insulinogenic index26 and the disposition index27. Insulin sensitivity was estimated by the homeostasis model assessment for insulin resistance25 and Matsuda index28.
Statistical analysis
We used the t‐test and the χ2‐test to test differences in maternal characteristics and perinatal outcomes between the Insulin group and Diet groups. The repeated measures analysis of variance (anova) was used to test differences in the values of plasma glucose, serum insulin, plasma glucagon and ΔGlucagon of each time point during the OGTTs between pairs of subject groups. We used the receiver operating characteristic curve to calculate the accuracy of the values that were observed to be significant as a predictive marker. The statistical analyses were carried out using JMP pro version 13.0.0 software (SAS Institute, Cary, NC, USA). The values are presented as the mean ± standard deviation (SD). P‐values <0.05 were considered significant.
Results
One‐third of the GDM patients required insulin therapy
As shown in Figure 1, we excluded six patients from the 55 patients with GDM who met the eligibility criteria. Accordingly, a final total of 49 patients with GDM completed the study. The 49 patients were enrolled at 21.9 ± 5.6 gestational weeks, all the patients received diet therapy instructions just after enrollment. Of the 49 patients with GDM, 15 (30.6%) required insulin treatment to achieve the target glucose levels after they underwent the mid‐gestational OGTT, defined as the Insulin group, and 34 patients (69.4%) were able to keep their glucose levels within the target levels until delivery by diet management alone, defined as the Diet group. Insulin treatment was initiated at 29.6 ± 3.6 gestational weeks in the Insulin group. The mean of the maximum daily dose of insulin in the Insulin group was 39.0 ± 24.8 units (Table 1).
Table 1.
Maternal characteristics and perinatal outcomes
| Total (n = 49) | Insulin group (n = 15) | Diet group (n = 34) | P‐value | |
|---|---|---|---|---|
| Maternal age (years) | 34.1 ± 5.1 | 35.9 ± 4.3 | 33.3 ± 5.2 | 0.10 |
| Primipara (%) | 53.1 | 46.7 | 55.9 | 0.78 |
| Parity (n) | 0.7 ± 0.9 | 0.8 ± 0.8 | 0.7 ± 0.9 | 0.59 |
| Parental history of diabetes (%) | 26.5 | 26.7 | 26.5 | 0.74 |
| History of previous GDM (%) | 18.4 | 33.3 | 11.8 | 0.16 |
| Height (cm) | 157.5 ± 5.8 | 158.8 ± 6.3 | 156.9 ± 5.5 | 0.30 |
| Pre‐gravid bodyweight (kg) | 56.1 ± 9.9 | 60.6 ± 11.2 | 54.1 ± 8.5 | 0.036 |
| Pre‐gravid BMI (kg/m2) | 22.6 ± 3.7 | 23.9 ± 3.7 | 22.0 ± 3.5 | 0.09 |
| Pre‐gravid obesity, BMI ≥25 kg/m2 (%) | 22.4 | 33.3 | 17.6 | 0.40 |
| Gestational weeks at diagnosis of GDM | 20.1 ± 6.0 | 18.0 ± 5.5 | 21.1 ± 5.9 | 0.10 |
| Glucose levels of the diagnostic 75‐g OGTT | ||||
| Fasting (mg/dL) | 80.4 ± 9.0 | 84.2 ± 8.5 | 78.7 ± 8.7 | 0.048 |
| 1‐h (mg/dL) | 179.8 ± 24.9 | 189.1 ± 15.1 | 175.6 ± 27.2 | 0.09 |
| 2‐h (mg/dL) | 158.6 ± 29.5 | 170.4 ± 34.2 | 153.4 ± 25.5 | 0.07 |
| Gestational week study enrollment | 21.9 ± 5.6 | 20.3 ± 5.3 | 22.6 ± 5.6 | 0.19 |
| Gestational week at starting diet therapy | 22.0 ± 5.7 | 21.0 ± 5.3 | 22.8 ± 5.7 | 0.32 |
| Gestational week at starting insulin | NA | 29.6 ± 3.6 | NA | NA |
| Maximum dose of insulin (unit/day) | NA | 39.0 ± 24.8 | NA | NA |
| Basal insulin (unit/day) | NA | 6.8 ± 10.9 | NA | NA |
| Bolus insulin (unit/day) | NA | 32.2 ± 15.3 | NA | NA |
| Total gestational weight gain (kg) | 6.7 ± 4.1 | 6.2 ± 5.0 | 6.9 ± 3.7 | 0.55 |
| Pregnancy‐induced hypertension (%) | 4.1 | 13.3 | 0.0 | 0.16 |
| Pre‐eclampsia (%) | 0.0 | 0.0 | 0.0 | NA |
| Cesarean delivery (%) | 22.4 | 33.3 | 17.6 | 0.40 |
| Gestational week at delivery | 39.4 ± 1.5 | 39.6 ± 1.4 | 39.3 ± 1.5 | 0.46 |
| Preterm delivery (%) | 2.0 | 0.0 | 2.9 | 0.67 |
| Neonatal weight (g) | 3,085 ± 427 | 3,184 ± 449 | 3,041 ± 409 | 0.29 |
| Small for gestational age (%) | 8.2 | 6.7 | 8.8 | 0.76 |
| Heavy for gestational age (%) | 16.3 | 26.7 | 11.8 | 0.38 |
| 1‐min Apgar score <6 (%) | 4.1 | 6.7 | 2.9 | 0.86 |
| 5‐min Apgar score <7 (%) | 2.0 | 0.0 | 2.9 | 0.67 |
| Neonatal hypoglycemia (%) | 0.0 | 0.0 | 0.0 | NA |
| Neonatal hyperbilirubinemia (%) | 4.1 | 6.7 | 2.9 | 0.86 |
| NICU admission (%) | 6.1 | 6.7 | 5.8 | 0.59 |
The values are given as the mean ± standard deviation. P‐values for differences between the insulin treatment group (Insulin group) and the diet therapy alone until delivery group (Diet group) were calculated using the t‐test, χ2‐test or the repeated‐measures anova. BMI, body mass index; GDM, gestational diabetes mellitus; NA, not applicable; NICU, neonatal intensive care unit; OGTT, oral glucose tolerance test.
Comparison of maternal characteristics and perinatal outcomes between the Insulin and Diet groups
The mean maternal age, ratio of primipara, parental history of diabetes and history of previous GDM were not significantly different between the Insulin group and Diet group (Table 1). The pre‐gravid bodyweights of the Insulin group (60.6 ± 11.2 kg) were significantly higher than those of the Diet group (54.1 ± 8.5 kg; P = 0.036), whereas there were no significant differences in the pre‐gravid BMI or the ratio of pre‐gravid obese patients (BMI ≥25 kg/m2) between the groups.
The gestational week diagnosed with GDM by the initial OGTT (i.e., the diagnostic OGTT) at the local obstetric clinics was not significantly different between the Insulin and Diet groups (18.0 ± 5.5 vs 21.1 ± 5.9, P = 0.10). The fasting glucose levels (mg/dL) during the diagnostic OGTT in the Insulin group were significantly higher than those in the Diet group (84.2 ± 8.5 vs 78.7 ± 8.7, P = 0.048), although the levels of glucose at 1 and 2 h were not significantly different between the groups. There were no significant differences in total gestational weight gain or perinatal outcomes (including the prevalence of “heavy for gestational age” newborns) between the Insulin and Diet groups.
Differences in the responses of glucagon in addition to glucose and insulin during the mid‐gestational 75‐g OGTT between the Insulin and Diet groups
The gestational week at which the mid‐gestational OGTT was carried out was slightly earlier in the Insulin group compared with the Diet group (26.1 ± 2.4 vs 27.5 ± 1.7, P = 0.026), although the intervals (weeks) between the diagnostic OGTT at a local clinic and the mid‐gestational OGTT of the present study were not significantly different between the Insulin and Diet groups (8.1 ± 5.4 vs 6.4 ± 4.8; P = 0.48; Table S1).
The Insulin group showed higher glucose levels at 60 and 120 min compared with the Diet group during the mid‐gestational OGTT (Figure 2a). There were no significant differences in the concentrations of serum insulin at each time point of the OGTT or the insulin secretory/sensitivity indexes between the Insulin and Diet groups (Figures 2b,3a–e).
Figure 2.
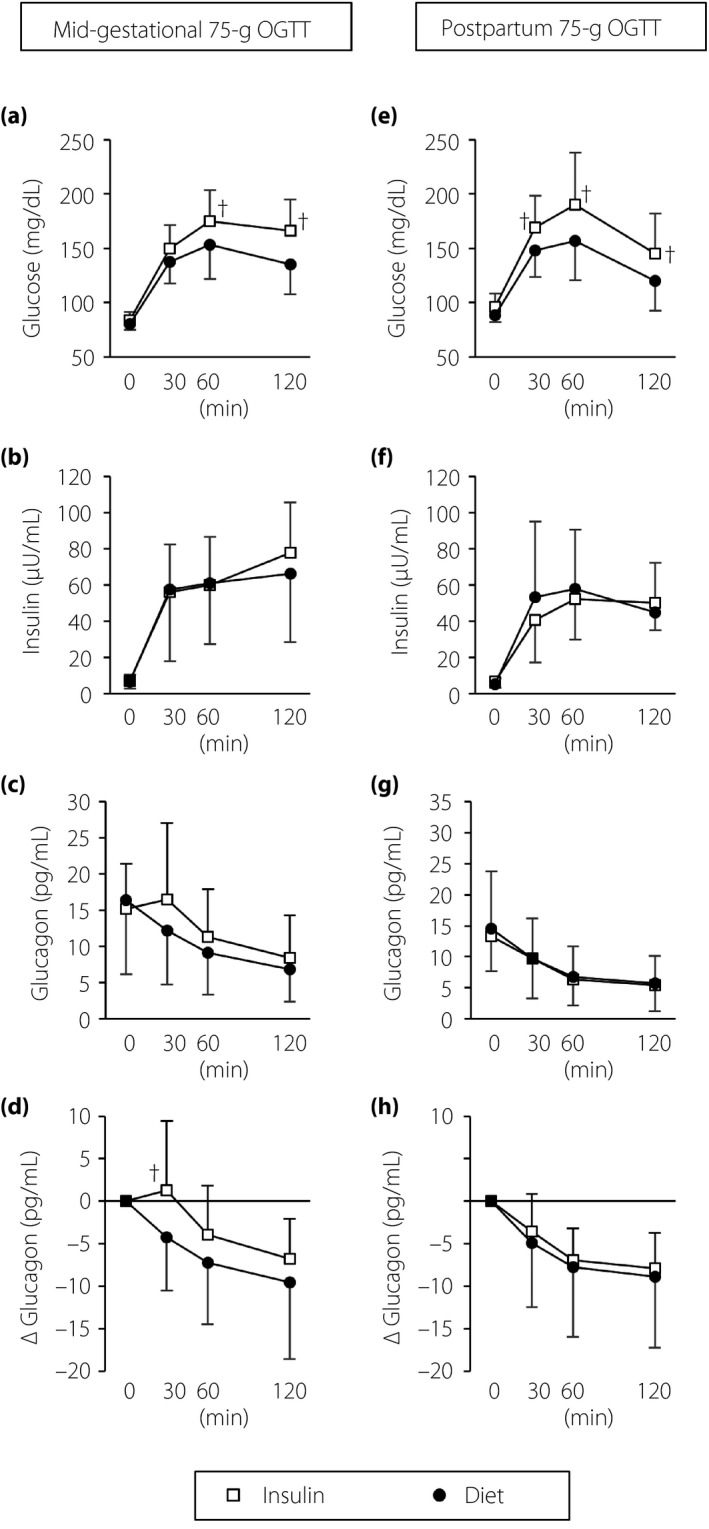
Results of (a–d) the mid‐gestational oral glucose tolerance tests (OGTTs) and (e–h) the postpartum OGTTs. † P < 0.05 for the insulin treatment group (Insulin group) versus the diet therapy alone until delivery group (Diet group) calculated using a repeated‐measures anova. Open squares, the Insulin group; closed circles, the Diet group; bar lines, standard deviation.
Figure 3.
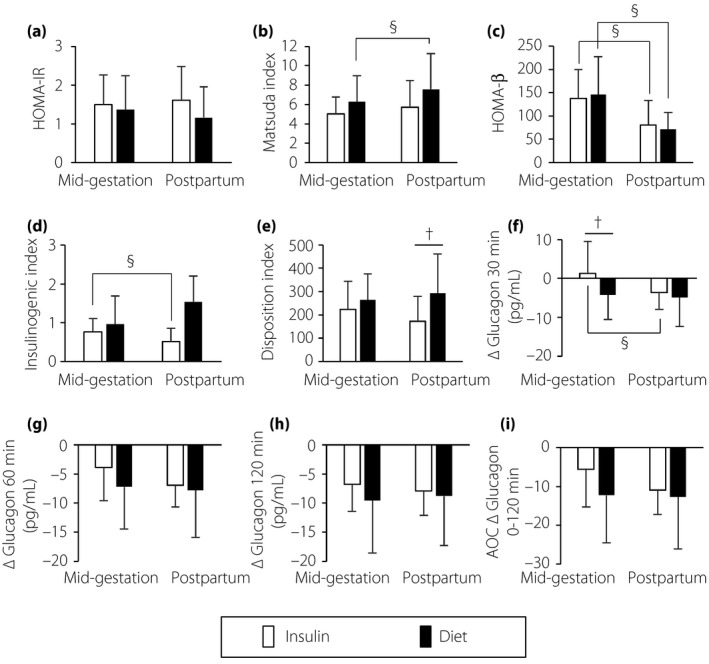
Comparison of indexes of (a,b) insulin sensitivity, (c–e) insulin secretion and (f–i) glucagon responses between the insulin treatment group (Insulin group) and the diet therapy alone until delivery group (Diet group) in mid‐gestation and postpartum. † P < 0.05 for the Insulin group versus the Diet group by the t‐test. § P < 0.05 for mid‐gestation versus postpartum in each group by the paired t‐test. White bars, the Insulin group; black bars, the Diet group; bar lines, standard deviation. ΔGlucagon, change in the levels of plasma glucagon from baseline (0 min) to each time point (30, 60 and 120 min) during each 75‐g oral glucose tolerance test; AOC, area over the ΔGlucagon curve; HOMA‐β, homeostasis model assessment for β‐cell function; HOMA‐IR, homeostasis model assessment for insulin resistance.
The levels of plasma glucagon at 0, 30, 60 and 120 min during OGTTs were not significantly different between the Insulin and Diet groups (Figure 2c). To avoid the influence of the volatility in the levels of fasting plasma glucagon, we evaluated the glucagon responses by using the change in the levels of glucagon from baseline (0 min) to each time point during the OGTT. We observed a paradoxical increase in glucagon secretion at 30 min after the ingestion of glucose; that is, so‐called paradoxical hyperglucagonemia13 in the Insulin group, but not in the Diet group (Figure 2d). The Diet group showed the appropriate suppression of glucagon after the glucose load, but not to the extent of the glucagon suppression observed in the present study's pregnant control participants with normal glucose tolerance (Table S1).
The changes in glucagon concentrations at 30 min from baseline (ΔGlucagon 30 min, pg/dL) were significantly different between the Insulin group and the Diet group (1.3 ± 8.1 vs −4.2 ± 4.0, P = 0.006; Figure 3f). The other indexes of glucagon suppression (ΔGlucagon 60 min, ΔGlucagon 120 min and AOC ΔGlucagon 0–120 min) were not significantly different between the groups (Figure 3g–i).
The receiver operating characteristic analysis showed that the cut‐off value of ΔGlucagon 30 min to predict the future insulin requirement during pregnancy in the patients with GDM was −2.01 pg/mL (area under the curve 0.684, 95% confidence interval 0.511–0.818) with 67% sensitivity and 68% specificity (Figure 4). We carried out multiple linear regression analyses to find correlations between ΔGlucagon 30 min and insulin secretory/sensitivity indexes; no associations were shown (Table S2).
Figure 4.
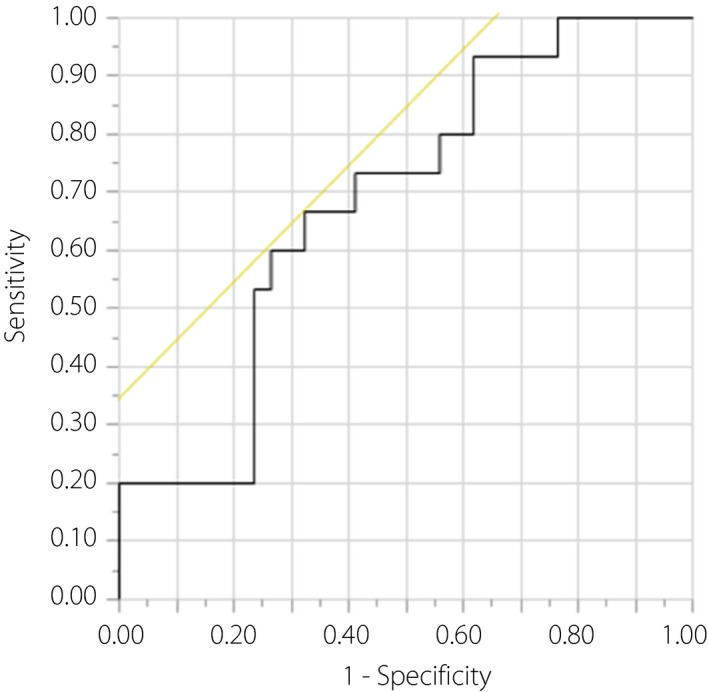
Receiver operating characteristic analysis of change in the levels of plasma glucagon from baseline (0 min) to 30 min (ΔGlucagon 30 min) in the mid‐gestational oral glucose tolerance test for a patient who requires insulin treatment during pregnancy among all of the enrolled gestational diabetes mellitus patients. The cut‐off point of ΔGlucagon 30 min is −2.01 pg/mL. The area under the curve is 0.684 (95% confidence interval 0.511–0.812) with 66.7% sensitivity, 67.6% specificity, 47.6% positive‐predictive value and 82.1% negative‐predictive value.
Results of the postpartum 75‐g OGTT in the Insulin group and the Diet group
Clinical characteristics including bodyweight, BMI, change of weight from delivery, change of weight from pre‐pregnancy and the ratio of lactation intensities were not significantly different between the groups (Table S3). The ratio of patients showing normal glucose tolerance tended to be less in the Insulin group than in the Diet group (46.7 vs 67.6%, P = 0.29).
In the Insulin group, the pattern of paradoxical hyperglucagonemia observed in the mid‐gestational OGTT (Figure 2d) was ameliorated in the postpartum OGTT (Figure 2h), although the levels of glucose at 30, 60 and 120 min during the postpartum OGTT remained higher than those in the Diet group (Figure 2e). The levels of insulin, glucagon and ΔGlucagon at each time point were comparable between the groups (Figure 2f–h). The impaired early‐phase suppression of glucagon secretion (indicated as ΔGlucagon 30 min) was significantly improved at the postpartum period in the Insulin group (Figure 3f).
Discussion
We investigated glucagon secretion using 75‐g OGTTs during mid‐gestation and postpartum in women diagnosed with GDM. The results of our analyses showed that the paradoxical hyperglucagonemia after glucose loading occurred during mid‐gestation in the Insulin group, but not in the Diet group. Oral ingestion of glucose might fail to suppress the glucagon secretion from α‐cells only in patients with GDM who require insulin treatment when insulin resistance increases as the placenta matures through the second and third trimesters.
It was reported that pre‐diabetic patients have already developed paradoxical hyperglucagonemia15, 29. GDM is also a less severe form of glucose intolerance than overt diabetes. A glucagon secretory abnormality can emerge as early as the stage at which insulin secretion cannot compensate for increased insulin resistance during pregnancy. The evaluation of the early‐phase of glucagon suppression after a glucose load could thus be valuable to predict whether a patient with GDM will require insulin treatment during pregnancy.
We reported that the phenotype of early‐onset GDM is heterogeneous; some patients show improved glucose tolerance with appropriate nutrition therapy, but others retain or moreover deteriorate glucose tolerance until mid‐gestation7. We showed the patients who could not improve their glucose intolerance until mid‐gestation included patients with a relatively severe phenotype who required insulin therapy until delivery and more often developed type 2 diabetes after delivery7. The Hyperglycemia and Adverse Pregnancy Outcome follow‐up study showed that children aged 10–14 years of untreated mothers with GDM based on the International Association of Diabetes and Pregnancy Study Group/World Health Organization criteria are more susceptible to glucose intolerance compared with children of mothers without GDM30. These findings suggest that an advanced evaluation of glucose tolerance (including the early‐phase of glucagon suppression during OGTT) might identify the relatively severe form of GDM that requires insulin treatment. Such evaluations might contribute to appropriate maternal glucose management with insulin treatment to reduce the risk of childhood glucose intolerance in offspring.
Several studies have described the relationship between the pathophysiology of GDM and glucagon abnormalities, including higher fasting plasma glucagon levels and the lack of glucagon secretion suppression16, 17. In the present study, we evaluated glucagon responses in GDM patients with the use of a relatively new quantitative sandwich ELISA kit22. We observed not higher levels of fasting glucagon, but a lesser suppression of glucagon after the ingestion of glucose in the GDM patients compared with the healthy pregnant control participants (Table S1). This suggests that glucagon abnormality could be associated with the pathophysiology of GDM, although the present data are insufficient, because only a few healthy pregnant women were enrolled in the present study.
The present analyses showed that the paradoxical hyperglucagonemia during mid‐gestation improved after delivery in patients with GDM who required insulin therapy. The reversibility of glucagon abnormality observed in GDM patients might support the notion that impaired glucagon suppression develops secondarily to a deficiency of insulin action or a hyperglycemic state, rather than representing a primary pathogenic defect in the development of glucose intolerance.
It was shown that pregnancy is associated with both reduced postprandial glucagon‐like peptide‐1 responses and increased postprandial glucose‐dependent insulinotropic polypeptide responses, both of which normalize at the postpartum period when normal glucose homeostasis is re‐established20. These phenomena are pronounced in women with GDM compared with healthy pregnant women. The glucagon secretion from α‐cells is suppressed by glucagon‐like peptide‐1 and increased by glucose‐dependent insulinotropic polypeptide31. Thus, changes in incretin hormones during pregnancy might contribute to the development of impaired glucagon suppression in GDM patients.
It was reported that β‐cells secrete serotonin by a signaling of prolactin and placental lactogen when stimulated by increases in the glucose concentration during normal pregnancy. Serotonin secreted from β‐cells stimulates insulin secretion and β‐cell proliferation32, but also decreases cyclic adenosine monophosphate levels in neighboring α‐cells through 5‐hydroxytryptamine 1F receptors, and it inhibits glucagon secretion33. The proportion of 5‐hydroxytryptamine 1F receptor‐positive α‐cells was reported to be reduced in patients with type 2 diabetes34, which suggests that serotonin's glucagonostatic effect might be impaired in patients with diabetes. According to these findings, increased serotonin input to α‐cells is an additional mechanism that helps maintain glucose homeostasis during pregnancy. We did not evaluate the patients’ glucagon secretion through serotonin signaling in the present study. As the potential role of serotonin in the pathophysiology of GDM remains speculative, further investigations are required to evaluate serotonin signaling as part of the strategy of diabetes care for pregnant women.
The present study had several limitations. First, the sample size was small. Second, we could not fully compare the glucagon responses in GDM and those in healthy pregnant women, because we enrolled only a few (n = 5) pregnant control participants (Table S1). Third, we did not determine the patients’ glucagon responses during the initial OGTT carried out to identify GDM at the local obstetric clinics. It is thus unknown whether an evaluation of glucagon responses in the initial OGTT (i.e., the diagnostic OGTT) can be used to anticipate the requirement of insulin treatment during pregnancy among all patients with GDM. Fourth, no precise rule was followed to initiate insulin treatment by clinicians, except that insulin should be started when the targeted glucose levels at fasting and the 2‐h postprandial time point could not be maintained for >1 week, as described earlier. Fifth, we could not evaluate the patients’ compliance with the diet therapy. These could have affected the decision‐making regarding the GDM patients’ requirement for insulin.
In summary, the results of our present study showed that the insulin requirement during pregnancy in a patient with GDM was associated with impaired early‐phase glucagon suppression (as shown by ΔGlucagon 30 min) during a 75‐g OGTT in mid‐gestation. However, the role of glucagon remains less clear compared with the roles of insulin action in glucose metabolism. The present findings might contribute to the understanding of glucagon pathophysiology and could be used to help improve diabetes care, especially the care for patients with GDM.
Disclosure
The authors declare no conflict of interest.
Supporting information
Table S1| Results of the mid‐gestational 75‐g oral glucose tolerance tests.
Table S2| Multiple linear regression analyses of the change in the levels of plasma glucagon from baseline (0 min) to 30 min (ΔGlucagon 30 min) values of the mid‐gestational oral glucose tolerance tests.
Table S3| Results of the postpartum 75‐g oral glucose tolerance tests.
Acknowledgments
We thank Mr Masaki Miwa and Dr Takeshi Nakamura for their precise assays of glucagon and configuration of the diagrams.
J Diabetes Investig 2020; 11: 232–240
Clinical Trial Registry
University Hospital Medical Information Network UMIN‐CTR000034337
References
- 1. Cousins L. Insulin sensitivity in pregnancy. Diabetes 1991; 40(Suppl 2): 39–43. [DOI] [PubMed] [Google Scholar]
- 2. Catalano PM, Tyzbir ED, Roman NM, et al Longitudinal changes in insulin release and insulin resistance in nonobese pregnant women. Am J Obstet Gynecol 1991; 165: 1667–1672. [DOI] [PubMed] [Google Scholar]
- 3. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: a World Health Organization Guideline. Diabetes Res Clin Pract 2014; 103: 341–363. [DOI] [PubMed] [Google Scholar]
- 4. Metzger BE, Gabbe SG, Persson B, et al International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010; 33: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morikawa M, Yamada T, Akaishi R, et al Change in the number of patients after the adoption of IADPSG criteria for hyperglycemia during pregnancy in Japanese women. Diabetes Res Clin Pract 2010; 90: 339–342. [DOI] [PubMed] [Google Scholar]
- 6. Sugiyama T, Saito M, Nishigori H, et al Comparison of pregnancy outcomes between women with gestational diabetes and overt diabetes first diagnosed in pregnancy: a retrospective multi‐institutional study in Japan. Diabetes Res Clin Pract 2014; 103: 20–25. [DOI] [PubMed] [Google Scholar]
- 7. Horie I, Kawasaki E, Sakanaka A, et al Efficacy of nutrition therapy for glucose intolerance in Japanese women diagnosed with gestational diabetes based on IADPSG criteria during early gestation. Diabetes Res Clin Pract 2015; 107: 400–406. [DOI] [PubMed] [Google Scholar]
- 8. Sugiyama T, Nagao K, Metoki H, et al Pregnancy outcomes of gestational diabetes mellitus according to pre‐gestational BMI in a retrospective multi‐institutional study in Japan. Endocr J 2014; 61: 373–380. [DOI] [PubMed] [Google Scholar]
- 9. Sugiyama T, Metoki H, Hamada H, et al A retrospective multi‐institutional study of treatment for mild gestational diabetes in Japan. Diabetes Res Clin Pract 2014; 103: 412–418. [DOI] [PubMed] [Google Scholar]
- 10. Santos MJ, Fernandes V, Marques O, et al Effect of maternal body mass index and weight gain in women with gestational diabetes on the incidence of large‐for‐gestational‐age infants. Diabetes Metab. 2016; 42: 471–474. [DOI] [PubMed] [Google Scholar]
- 11. Saisho Y, Miyakoshi K, Tanaka M, et al Beta cell dysfunction and its clinical significance in gestational diabetes. Endocr J 2010; 57: 973–980. [DOI] [PubMed] [Google Scholar]
- 12. Ikenoue S, Miyakoshi K, Saisho Y, et al Clinical impact of women with gestational diabetes mellitus by the new consensus criteria: two‐year experience in a single institution in Japan. Endocr J 2014; 61: 353–358. [DOI] [PubMed] [Google Scholar]
- 13. Unger RH, Cherrington AD. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J Clin Invest 2012; 122: 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Horie I, Abiru N, Eto M, et al Sex differences in insulin and glucagon responses for glucose homeostasis in young healthy Japanese adults. J Diabetes Investig 2018; 9: 1283–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yabe D, Kuroe A, Watanabe K, et al Early phase glucagon and insulin secretory abnormalities, but not incretin secretion, are similarly responsible for hyperglycemia after ingestion of nutrients. J Diabetes Complications 2015; 29: 413–421. [DOI] [PubMed] [Google Scholar]
- 16. Beis C, Grigorakis SI, Philippou G, et al Lack of suppression of plasma glucagon levels in late pregnancy persists postpartum only in women with previous gestational diabetes mellitus. Acta Diabetol 2005; 42: 31–35. [DOI] [PubMed] [Google Scholar]
- 17. Grigorakis SI, Alevizaki M, Beis C, et al Hormonal parameters in gestational diabetes mellitus during the third trimester: high glucagon levels. Gynecol Obstet Invest 2000; 49: 106–109. [DOI] [PubMed] [Google Scholar]
- 18. Kuhl C. Etiology and pathogenesis of gestational diabetes. Diabetes Care 1998; 21(Suppl 2): B19–B26. [PubMed] [Google Scholar]
- 19. Kuhl C. Glucose metabolism during and after pregnancy in normal and gestational diabetic women. 1. Influence of normal pregnancy on serum glucose and insulin concentration during basal fasting conditions and after a challenge with glucose. Acta Endocrinol 1975; 79: 709–719. [PubMed] [Google Scholar]
- 20. Bonde L, Vilsboll T, Nielsen T, et al Reduced postprandial GLP‐1 responses in women with gestational diabetes mellitus. Diabetes Obes Metab 2013; 15: 713–720. [DOI] [PubMed] [Google Scholar]
- 21. Bak MJ, Albrechtsen NW, Pedersen J, et al Specificity and sensitivity of commercially available assays for glucagon and oxyntomodulin measurement in humans. Eur J Endocrinol 2014; 170: 529–538. [DOI] [PubMed] [Google Scholar]
- 22. Wewer Albrechtsen NJ, Hartmann B, Veedfald S, et al Hyperglucagonaemia analysed by glucagon sandwich ELISA: nonspecific interference or truly elevated levels? Diabetologia 2014; 57: 1919–1926. [DOI] [PubMed] [Google Scholar]
- 23. Matsuo T, Miyagawa J, Kusunoki Y, et al Postabsorptive hyperglucagonemia in patients with type 2 diabetes mellitus analyzed with a novel enzyme‐linked immunosorbent assay. J Diabetes Investig 2016; 7: 324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miyachi A, Kobayashi M, Mieno E, et al Accurate analytical method for human plasma glucagon levels using liquid chromatography‐high resolution mass spectrometry: comparison with commercially available immunoassays. Anal Bioanal Chem 2017; 409: 5911–5918. [DOI] [PubMed] [Google Scholar]
- 25. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004; 27: 1487–1495. [DOI] [PubMed] [Google Scholar]
- 26. Tura A, Kautzky‐Willer A, Pacini G. Insulinogenic indices from insulin and C‐peptide: comparison of beta‐cell function from OGTT and IVGTT. Diabetes Res Clin Pract 2006; 72: 298–301. [DOI] [PubMed] [Google Scholar]
- 27. Kahn SE, Prigeon RL, McCulloch DK, et al Quantification of the relationship between insulin sensitivity and beta‐cell function in human subjects. Evidence for a hyperbolic function. Diabetes 1993; 42: 1663–1672. [DOI] [PubMed] [Google Scholar]
- 28. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999; 22: 1462–1470. [DOI] [PubMed] [Google Scholar]
- 29. Yosten GLC. Alpha cell dysfunction in type 1 diabetes. Peptides 2018; 100: 54–60. [DOI] [PubMed] [Google Scholar]
- 30. Lowe WL Jr, Scholtens DM, Kuang A, et al Hyperglycemia and Adverse Pregnancy Outcome Follow‐up Study (HAPO FUS): maternal gestational diabetes mellitus and childhood glucose metabolism. Diabetes Care 2019; 42: 372–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Holst JJ, Christensen M, Lund A, et al Regulation of glucagon secretion by incretins. Diabetes Obes Metab 2011; 13(Suppl 1): 89–94. [DOI] [PubMed] [Google Scholar]
- 32. Kim H, Toyofuku Y, Lynn FC, et al Serotonin regulates pancreatic beta cell mass during pregnancy. Nat Med 2010; 16: 804–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Almaca J, Molina J, Menegaz D, et al Human beta cells produce and release serotonin to inhibit glucagon secretion from alpha cells. Cell Rep 2016; 17: 3281–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bennet H, Balhuizen A, Medina A, et al Altered serotonin (5‐HT) 1D and 2A receptor expression may contribute to defective insulin and glucagon secretion in human type 2 diabetes. Peptides 2015; 71: 113–120. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1| Results of the mid‐gestational 75‐g oral glucose tolerance tests.
Table S2| Multiple linear regression analyses of the change in the levels of plasma glucagon from baseline (0 min) to 30 min (ΔGlucagon 30 min) values of the mid‐gestational oral glucose tolerance tests.
Table S3| Results of the postpartum 75‐g oral glucose tolerance tests.


