Abstract
Aims/Introduction
Patients with type 2 diabetes mellitus have an increased hip fracture risk. We investigated the relationship between hip fracture and all‐cause death in patients with type 2 diabetes in comparison with cardiovascular disease (CVD) or end‐stage renal disease (ERSD).
Materials and Methods
In total, 4,923 Japanese participants with type 2 diabetes (mean age 65 years, 2,790 men, 2,133 women) were followed for a median of 5.3 years (follow‐up rate 99.5%). We evaluated the associations between the presence of hip fracture (n = 110), upper limb fracture (n = 801), CVD (n = 1,344), ESRD (n = 104) and all‐cause death by logistic regression analysis.
Results
A total of 309 participants died during follow up. Multivariate‐adjusted odds ratios (ORs) for all‐cause mortality were significantly higher in participants with hip fractures than those without hip fractures (OR 2.67, 95% confidence interval [CI] 1.54–4.41), whereas the ORs for upper limb fracture were not significant. The ORs for all‐cause mortality were significantly higher in participants with CVD than those without CVD (OR 1.78, 95% CI, 1.39–2.70) and ESRD (OR 2.36, 95% CI 1.32–4.05). The ORs for all‐cause mortality of hip fracture were not affected by further adjustment for CVD and ESRD (OR 2.74, 95% CI 1.58–4.54). The cause of death was infection (40.0%), malignant neoplasm (25.0%) and CVD (15.0%) among participants with hip fracture.
Conclusions
Hip fractures were associated with an increased risk of death among Japanese patients with type 2 diabetes, independently of CVD and ESRD.
Keywords: Death, Hip fracture, Type 2 diabetes
Hip fracture is associated with increased mortality in the general population, although few studies have investigated the impact of hip fractures on the risk of death in patients with type 2 diabetes who also have a higher prevalence of fatal diseases, such as cardiovascular disease, renal disease or malignant neoplasia. In this study, the presence of hip fracture was associated with an increased risk of death among Japanese patients with type 2 diabetes, independently of cardiovascular disease and end‐stage renal disease. It should be emphasized that hip fracture is a critical event in the aging population of patients with type 2 diabetes.

Introduction
The recent epidemic of diabetes mellitus, along with advancements in the treatment of diabetes and its complications, has led to a rapid increase in the number of aged patients with diabetes1, 2, 3. A better understanding of geriatrics is required in the clinical management of patients with diabetes4. Osteoporosis occurs with aging and increases the risk of fragility fractures, which cause other comorbidities or increased mortality in persons of advanced age5. Epidemiological studies have shown an increased risk of hip or other fractures in patients with type 2 diabetes than those without type 2 diabetes6. This is partly explained by non‐enzymatic glycation of the collagen within bones, decreased bone turnover, oxidative stress and adverse effects of certain diabetes medications6, 7.
Fragility fractures, especially hip fractures, are associated with increased mortality5, 8, 9, 10, 11, 12. Many factors are associated with the higher mortality rates after hip fracture, including older age, poor physical and cognitive function, comorbid conditions, frailty, and postoperative complications, such as cardiac and pulmonary complications, infections, and an increased risk of thromboembolism, most of which are common in patients with type 2 diabetes. However, few studies have investigated the impact of hip fractures on the risk of death in patients with type 2 diabetes who also have a higher prevalence of fatal diseases, such as cardiovascular disease (CVD)13, renal disease14 or malignant neoplasia15. Because the cause of death in patients with diabetes varies by country and ethnicity, it is important to study the impact of hip fractures on mortality in each country or ethnicity. In this context, we investigated the relationship between hip fracture and all‐cause death in a hospital‐based cohort of Japanese patients with type 2 diabetes in comparison with CVD or end‐stage renal disease (ERSD).
Methods
Study participants
The Fukuoka Diabetes Registry includes 5,131 outpatients who were regularly followed in 16 diabetes specialist clinics in Fukuoka Prefecture, Japan (UMIN Clinical Trial Registry 000002627)16. The participants were registered between April 2008 and October 2010. Exclusion criteria were: (i) patients aged <20 years; (ii) those with drug‐induced diabetes; (iii) those with ESRD under dialysis; and (iv) those with serious diseases other than diabetes mellitus, such as cancer. After excluding 208 patients with type 1 diabetes mellitus, the remaining 4,923 patients were enrolled in the current study. The study was approved by the Kyushu University institutional review board (approval number 290, date of approval 4 January 2008), and followed the ethics of the Helsinki declaration with written informed consent.
Baseline evaluation
Diabetes duration, current smoking habits and current alcohol intake were checked at the baseline. Leisure‐time physical activity (LTPA) was calculated as metabolic equivalent hours per week using Ainsworth's methods17. Blood pressure in the sitting position, bodyweight and height were measured, and body mass index (BMI) was calculated. Information regarding medications including insulin was collected from the medical records. Hemoglobin A1c (HbA1c) was determined by high‐performance liquid chromatography (Tosoh Corp., Tokyo, Japan), serum low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol and creatinine concentrations by enzymatic methods, and serum albumin by the bromocresol purple method. The estimated glomerular filtration rate was calculated based on serum creatinine using the equation proposed by the Japanese Society of Nephrology18. Chronic kidney disease was defined as an estimated glomerular filtration rate of <60 mL/min/1.73 m2. The Geriatric Nutritional Risk Index was calculated by using the following equation: Geriatric Nutritional Risk Index = (1.489 × albumin [g/dL]) + (41.7 × [bodyweight / ideal bodyweight])19. The bodyweight / ideal bodyweight ratio was set to 1 when the patient's bodyweight exceeded the ideal bodyweight calculated from a BMI of 22 kg/m2 as its definition20.
Mortality follow up
The primary outcome of the present study was all‐cause death. All participants underwent an annual follow up by interview, medical record review, telephone, letters and municipal registration of residence. A total of 27 participants were lost to follow up during the follow‐up period (median 5.3 years; follow‐up rate 99.5%). The underlying cause of death was determined based on the medical records and/or death certificate, and coded according to the International Classification of Diseases, 10th revision.
Assessment of fractures, CVD and ESRD
Information regarding the history of fractures and CVD was obtained using a self‐administered questionnaire at enrollment, and the occurrence of fractures, CVD and ESRD was checked annually using a self‐administered questionnaire or by reviewing the medical records during the follow‐up period. CVD and ESRD were confirmed by contacting the participants’ attending specialists. Participants with hip fracture, upper limb fracture or CVD were defined as those with a history of the events at baseline or newly diagnosed events during the follow‐up period. CVD was defined as coronary heart disease and stroke. Participants with ESRD were defined as those who had started renal replacement therapy or died of ESRD during the follow‐up period.
Statistical analysis
Differences in the mean values or proportions at baseline were assessed by Student's t‐test or the χ2‐test, as appropriate. Mortality was calculated in participants with or without hip fracture, upper limb fracture, CVD or ESRD using the person‐years method, and adjusted for age and sex by the direct method using 10‐year age groups. We evaluated the associations between the presence of hip fracture, upper limb fracture, CVD, ESRD and all‐cause death by logistic regression analysis and estimated odds ratios (ORs), and 95% confidence intervals (CIs). The multivariate‐adjusted model included age, sex, diabetes duration, BMI, current smoking habits, current drinking habits, LTPA, HbA1c, systolic blood pressure, low‐density lipoprotein cholesterol and insulin therapy. In our evaluation of the association between the presence of hip fracture and all‐cause death, we further adjusted for the presence of CVD and ESRD. Differences in the proportions of causes of death were evaluated by Fisher's exact test. All statistical analyses were carried out with Statistical Analysis Software (SAS) version 9.4 (SAS institute Inc., Cary, NC, USA). Values of P < 0.05 were considered statistically significant in all analyses.
Results
Baseline characteristics
The baseline characteristics of participants with and without the presence of hip fracture, upper limb fracture, CVD and ESRD are shown in Table 1. In total, 110 participants had hip fractures, 801 had upper limb fractures, 1,344 had CVD and 104 had ESRD. Patients with hip fractures or CVD were older than those without hip fractures or CVD, but patients with upper limb fractures were younger than those without hip fractures or CVD. The proportion of male patients was lower among those with hip fractures than without hip fractures, but higher among those with CVD or ESRD than those without CVD or ESRD. The BMI was lower in patients with hip fractures than those without hip fractures, and higher in patients with upper limb fractures than those without upper limb fractures. The prevalence of current drinkers was lower among patients with hip fractures or ESRD than those without hip fractures or ESRD. LTPA was lower in patients with ESRD than those without ESRD. The HbA1c level was higher in patients with upper limb fractures or CVD than those without upper limb fractures or CVD. The low‐density lipoprotein cholesterol level was lower in patients with hip fracture or CVD than those without hip fracture or CVD. The high‐density lipoprotein cholesterol level was lower in patients with than without CVD or ESRD. The prevalence of chronic kidney disease was higher in patients with hip fracture, CVD or ESRD than those without hip fracture, CVD or ESRD. Systolic blood pressure was higher in patients with CVD or ESRD than those without CVD or ESRD. Diastolic blood pressure was lower in patients with CVD than those without CVD. The prevalence of insulin users was higher among patients with hip fracture, CVD or ESRD than those without hip fracture, CVD or ESRD.
Table 1.
Baseline characteristics of participants according to the presence of hip fracture, upper limb fracture, cardiovascular disease and end‐stage renal disease
| Hip fracture | Upper limb fracture | CVD | ESRD | |||||
|---|---|---|---|---|---|---|---|---|
| (−) | (+) | (−) | (+) | (−) | (+) | (−) | (+) | |
| n | 4,813 | 110 | 4,122 | 801 | 3,679 | 1,344 | 4,819 | 104 |
| Age (years) | 65 ± 10 | 71 ± 7*** | 66 ± 10 | 64 ± 12*** | 64 ± 11 | 69 ± 8*** | 65 ± 10 | 65 ± 11 |
| Male (%) | 57 | 45** | 57 | 57 | 53 | 66*** | 56 | 74*** |
| Duration of diabetes (years) | 16 ± 11 | 19 ± 11*** | 16 ± 11 | 15 ± 10 | 15 ± 10 | 18 ± 11*** | 16 ± 11 | 19 ± 10** |
| BMI (kg/m2) | 23.8 ± 3.8 | 22.2 ± 3.3*** | 23.7 ± 3.8 | 24.0 ± 3.8* | 23.7 ± 3.9 | 23.9 ± 3.5 | 23.8 ± 3.8 | 23.9 ± 3.6 |
| Current smoker (%) | 19 | 14 | 18 | 21 | 19 | 18 | 19 | 21 |
| Current drinker (%) | 40 | 20*** | 39 | 40 | 39 | 39 | 39 | 25** |
| LTPA (METs·h/week) | 12 ± 15 | 11 ± 14 | 12 ± 15 | 12 ± 16 | 12 ± 15 | 11 ± 14 | 12 ± 15 | 9 ± 11** |
| HbA1c (%) | 7.43 ± 1.04 | 7.49 ± 1.10 | 7.42 ± 1.04 | 7.53 ± 1.09** | 7.40 ± 1.02 | 7.53 ± 1.09*** | 7.44 ± 1.04 | 7.28 ± 1.23 |
| HbA1c (mmol/mol) | 58 ± 11 | 58 ± 12 | 58 ± 11 | 59 ± 12** | 57 ± 11 | 59 ± 12*** | 58 ± 11 | 56 ± 13 |
| LDL cholesterol (mmol/L) | 2.9 ± 0.7 | 2.7 ± 0.7* | 2.9 ± 0.7 | 2.8 ± 0.7 | 2.9 ± 0.7 | 2.7 ± 0.7*** | 2.9 ± 0.7 | 2.7 ± 0.8 |
| HDL cholesterol (mmol/L) | 1.5 ± 0.4 | 1.5 ± 0.4 | 1.5 ± 0.4 | 1.5 ± 0.4 | 1.5 ± 0.4 | 1.4 ± 0.4*** | 1.5 ± 0.4 | 1.3 ± 0.4*** |
| Serum albumin (g/dL) | 4.4 ± 0.4 | 4.3 ± 0.4*** | 4.4 ± 0.4 | 4.4 ± 0.3 | 4.4 ± 0.3 | 4.3 ± 0.4*** | 4.4 ± 0.3 | 3.7 ± 0.5*** |
| GNRI | 106 ± 6 | 103 ± 7*** | 106 ± 6 | 106 ± 6 | 106 ± 6 | 105 ± 6*** | 106 ± 6 | 96 ± 8*** |
| eGFR <60 mL/min/1.73 m2 | 21 | 31* | 22 | 19 | 17 | 33*** | 20 | 89*** |
| SBP (mmHg) | 131 ± 17 | 128 ± 18 | 131 ± 17 | 131 ± 17 | 130 ± 17 | 133 ± 17*** | 130 ± 17 | 140 ± 25*** |
| DBP (mmHg) | 75 ± 11 | 71 ± 12** | 74 ± 11 | 75 ± 11 | 75 ± 11 | 74 ± 11* | 74 ± 11 | 74 ± 13 |
| Insulin therapy (%) | 29 | 42** | 29 | 30 | 26 | 36*** | 28 | 77*** |
Values are expressed as the mean ± standard deviation or percentage. *P < 0.05, **P < 0.01, ***P < 0.001. BMI, body mass index; CVD, cardiovascular disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; ESRD, end‐stage renal disease; GNRI, Geriatric Nutritional Risk Index; HbA1c, hemoglobin A1c; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; LTPA, leisure‐time physical activity; METs, metabolic equivalents; SBP, systolic blood pressure.
Mortality with hip fracture, upper limb fracture, CVD and ESRD
Figure 1 shows the age‐ and sex‐adjusted mortality in participants with or without hip fracture, upper limb fracture, CVD or ESRD. The ORs for all‐cause mortality of hip fracture, upper limb fracture, CVD or ESRD are shown in Table 2. The age‐ and sex‐adjusted ORs were significantly higher in those with than without hip fracture, CVD and ESRD, but not in those with upper limb fracture. The multivariate‐adjusted ORs were significantly higher in patients with hip fracture, CVD and ESRD than those without hip fracture, CVD and ESRD (hip fracture OR 2.67, 95% CI 1.54–4.41; CVD OR 1.78, 95% CI 1.39–2.27; ESRD OR 2.36, 95% CI 1.32–4.05). In addition, further adjustment for the presence of CVD and ESRD did not alter the significance in patients with hip fracture (OR 2.74, 95% CI 1.58–4.54).
Figure 1.
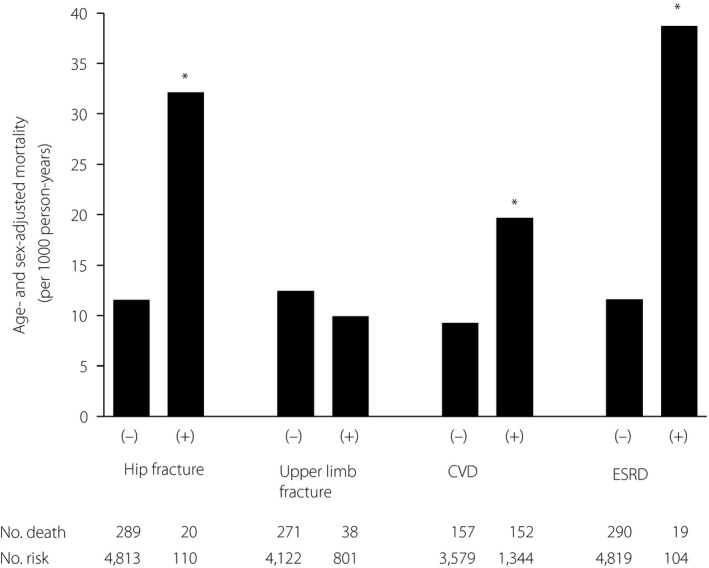
Age‐ and sex‐adjusted mortality in participants with or without hip fracture, upper limb fracture, cardiovascular disease (CVD), or end‐stage renal disease (ESRD). *P < 0.001 compared with those without.
Table 2.
Odds ratios and 95% confidence intervals for all‐cause mortality in participants with hip fracture, upper limb fracture, cardiovascular disease and end‐stage renal disease
| Hip fracture | Upper limb fracture | CVD | ESRD | |||||
|---|---|---|---|---|---|---|---|---|
| (−) | (+) | (−) | (+) | (−) | (+) | (−) | (+) | |
| Unadjusted | 1.00 (Ref.) | 3.48 (2.06–5.61) | 1.00 (Ref.) | 0.71 (0.49–0.99) | 1.00 (Ref.) | 2.78 (2.20–3.51) | 1.00 (Ref.) | 3.49 (2.04–5.70) |
| Age‐ and sex‐adjusted | 1.00 (Ref.) | 2.99 (1.75–4.89) | 1.00 (Ref.) | 0.79 (0.55–1.11) | 1.00 (Ref.) | 2.03 (1.59–2.58) | 1.00 (Ref.) | 3.31 (1.89–5.52) |
| Multivariate‐adjusted | 1.00 (Ref.) | 2.67 (1.54–4.41) | 1.00 (Ref.) | 0.77 (0.53–1.10) | 1.00 (Ref.) | 1.78 (1.39–2.27) | 1.00 (Ref.) | 2.36 (1.32–4.05) |
| Multivariate‐adjusted + CVD + ESRD | 1.00 (Ref.) | 2.74 (1.58–4.54) | ||||||
Multivariate‐adjusted model: age, sex, duration of diabetes, body mass index, current smoking habits, current drinking habits, leisure‐time physical activity, hemoglobin A1c, systolic blood pressure, low‐density lipoprotein cholesterol, and insulin therapy. CVD, cardiovascular disease; ESRD, end‐stage renal disease.
Cause of death
As shown in Figure 2, the main cause of death was infection among participants with hip fracture (40%) and ESRD (37%), cancer among those with upper limb fracture (35%) and in total (37%), and CVD among those with CVD (34%). The difference in the distribution of cause of death was insignificant (P = 0.09). However, the proportion of patients with infection‐related death was significantly higher among those with hip fracture than those without hip fracture (P = 0.03).
Figure 2.
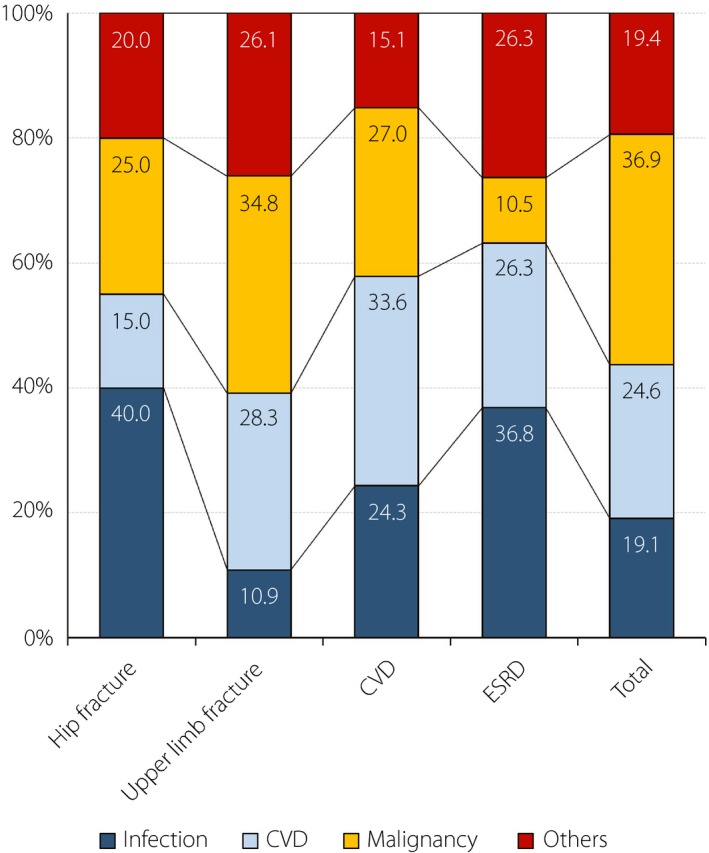
Main causes of death after hip fracture, upper limb fracture, cardiovascular disease (CVD) and end‐stage renal disease (ESRD), and that in all participants. Dark blue, infection; light blue, CVD; yellow, cancer; red, other diseases.
Discussion
In the present study, the presence of hip fracture was associated with an increased risk of death among Japanese patients with type 2 diabetes. This association was significant, even after adjustment for potential confounders including BMI, smoking habit, duration of diabetes, HbA1c, physical activity, CVD and ESRD. In contrast, there was no significant association between upper limb fracture and all‐cause death. Furthermore, the magnitude of the impact of hip fracture on mortality appears to have been greater than that of CVD or ESRD, which are significant risk factors for death in patients with type 2 diabetes.
The risk of mortality persistently increases after hip fracture21. In previous research, the magnitude of the influence of hip fracture on all‐cause mortality was reflected by a hazard ratio (HR) of 2.12 among 122,808 participants from eight cohorts in Europe and the USA, with 4,273 incident hip fractures and 27,999 deaths during a mean of 12.6 years. Higher mortality might be caused by older age, dementia, frailty and postoperative complications, such as CVD and infection, which are common in patients with type 2 diabetes. The increased post‐fracture mortality in patients with type 2 diabetes was first reported in a study of the Spanish population in 2017 (type 2 diabetes, n = 3,861; non‐diabetic, n = 6,616)22. The effect of hip fracture on mortality was significantly higher in patients with type 2 diabetes (HR 2.90) than in those without (HR 2.59) independent of age, sex, BMI, smoking, alcohol intake and history of CVD, thus indicating increased mortality in association with type 2 diabetes (HR 1.28). The magnitude of the relative risk for death was similar to the present results (multivariable‐adjusted OR 2.67).
Four possible mechanisms for the increased mortality in patients with type 2 diabetes with hip fracture in the present study are as follows. First, patients with hip fractures were 6 years older than those without. However, the adjustment for age did not affect the mortality. Second, patients with hip fractures might be physically inactive. However, the registered participants in the present study were attending an outpatient clinic; they were not nursing home residents. Additionally, LTPA was not different between those with and without hip fractures. Third, there were no significant differences in risk factors for fracture, such as glycemic control, blood pressure and smoking. Furthermore, the adjustment for chronic kidney disease and insulin use did not affect the mortality. Finally, malnutrition might contribute to increased mortality in patients with hip fractures. The BMI, serum albumin level and Geriatric Nutritional Risk Index were significantly lower in patients with hip fractures than those without hip fractures. Malnutrition might lead to sarcopenia and induce falls and hip fractures, which could become complicated by fatal infection. In the present study (Figure 2), the leading cause of death was infection among patients with hip fracture (40%), although the leading cause of death was cancer in the total cohort (37%), which was in agreement with the Japanese Report of the Committee on Causes of Death in Diabetes Mellitus (38%)23. A recent study in Taiwan showed that the incidence of infectious disease was significantly higher in post‐fracture patients with type 2 diabetes than those without type 2 diabetes (OR 1.48 for urinary tract infection, OR 1.42 for septicemia and OR 1.13 for pneumonia)24.
The presence of CVD is significantly associated with an increased risk of death. All‐cause mortality of CVD was compared with that of hip fracture in 1,109 hospitalized patients in a community in Italy25. The age‐adjusted mortality rate was 14.5/100 person‐years after hip fracture, 14.3/100 person‐years after stroke, 6.9/100 person‐years after myocardial infarction without coronary revascularization and 2.0/100 person‐years after myocardial infarction with coronary revascularization. Fatality after stroke or myocardial infarction has been declining because of advancements in therapy in recent years26, 27. These findings were compatible with those in the present study; the OR for death was 2.67 in patients with hip fracture and 1.78 in those with CVD.
Patients with ESRD are usually treated with dialysis in Japan, and the prognosis has been poor according to the Japanese Society for Dialysis Therapy (3‐year survival rate of 65% in those who started dialysis in 1992, and 73% in those who started dialysis in 2006)28. Coco and Rush29 reported that the 1‐year mortality rate of hemodialysis patients with hip fractures was 2.7‐fold higher than that of hemodialysis patients without hip fractures, and 2.4‐fold higher than that in the general population with hip fractures. In the present study, however, the impact of hip fracture on mortality was not attenuated after adjusting for the presence of ESRD.
Previous studies have shown that mortality after forearm fractures is the same30, 31 or less32 than that in the general population. In a Swedish prospective cohort study of 2,847 patients with a low‐energy fracture at enrollment, the proportion of surviving patients was lower for hip fractures (41%) than for forearm (74%) or shoulder (64%) fractures at 5 years9. The European Prospective Osteoporosis Study showed that limb fracture was not correlated with aging, unlike hip fracture33. In the present study, there was no significant association between upper limb fracture and all‐cause death.
The main strength of the present study is the high follow‐up rate of death (99.5%), which enabled us to accurately determine the associations of hip fracture or other diabetes‐related complications with death against the background that the follow up of patients with severe comorbidities might be difficult. Furthermore, the cohort in the present study included potential confounders, such as physical activity, smoking habit, laboratory data and medications, and has been used to study fracture risks in patients with type 2 diabetes34, 35, 36.The present study also has several limitations. First, we derived incident fractures from self‐reported data, which might have resulted in misclassification. However, when the accuracy of the self‐administered questionnaire was evaluated in 455 fracture events by comparison with medical records, the agreement rate was 93.0%34. Furthermore, in the Women's Health Initiative Clinical Trial and Observational Study cohort, the validity of self‐reports for hip fracture was higher than that for other sites of fracture37. Second, because all participants in the current study were Japanese, whether the conclusions of the present study can be generalized to other ethnic populations remains unclear. In particular, the incidence of CVD is lower in Japanese than Western populations38. Third, because the current study was observational in nature, other confounding factors, besides those used in the study, might have been present.
The present study showed that the presence of hip fracture was associated with an increased risk of death among Japanese patients with type 2 diabetes, independently of CVD and ESRD. It should be emphasized that hip fracture is a critical event in the aging population of patients with type 2 diabetes during the present era of a better prognosis of CVD. In addition, whether prevention of hip fracture might improve the survival of patients with type 2 diabetes remains to be determined.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
This work was supported in part by The Japan Society for the Promotion of Science KAKENHI from the Ministry of Education, Culture, Sports, Science and Technology of Japan (grant numbers 23249037 and 23659353 to MI and 16K00861 to HF), and the Junior Scientist Development Grant supported by the Japan Diabetes Society (to YK).
The authors thank Drs Yutaka Kiyohara, Yasufumi Doi, Toshiharu Ninomiya, Shigenobu Kanba, Dongchon Kang, Shuzo Kumagai, Shinako Kaizu, Yoichiro Hirakawa, Chisa Matsumoto, Chie Kitaoka (Kyushu University), Nobutaka Tsutsu, Nobuhiro Sasaki (Fukuoka Red Cross Hospital), Kiyohide Nunoi, Yuichi Sato, Yuji Uchizono, Ayumi Yamauchi, Kaori Itoh, Chie Kono (St. Mary's Hospital), Sakae Nohara, Hirofumi Imoto, Kazushi Amano, (Steel Memorial Yawata Hospital), Daisuke Gotoh, Toshitaka Himeno, Masae Toyonaga (Kyushu Central Hospital), Noriyasu Shinohara, Ayako Tsutsumi (Fukuoka Higashi Medical Center), Yasuhiro Idewaki, Masahiro Nakano, Mina Matsuo, Shoko Morimoto, Tomoko Hyodo (Hakujyuji Hospital), Masae Minami (Clinic Minami Masae), Miya Wada (Wada Miya Naika Clinic), Yoshifumi Yokomizo (Yokomizo Naika Clinic), Masanori Kikuchi, Yohei Kikuchi (Kikuchi Naika Clinic), Riku Nomiyama (Suzuki Naika Clinic), Shin Nakamura (Nakamura Naika Clinic), Kenji Tashiro (Oshima Eye Hospital), Mototaka Yoshinari (Yoshinari Naika Clinic), Kojiro Ichikawa (Fukutsu Naika Clinic), Teruo Omae (Hisayama Research Institute For Lifestyle Diseases), Hiroaki Ooboshi and Shigeru Tanaka (Fukuoka Dental College). The authors also thank the clinical research coordinators, Chiho Ohba (Hisayama Research Institute For Lifestyle Diseases), Kayoko Sekioka and Yoko Nishioka (Kyushu University); and those in the administration office, Tomoko Matake (Hisayama Research Institute For Lifestyle Diseases) and Junko Ishimatsu (Kyushu University). Finally, the authors thank Edanz Group (http://www.edanzediting.co.jp) for editing a draft of this manuscript.
J Diabetes Investig 2020; 11: 62–69
Clinical Trial Registry
University Hospital Medical Information Network UMIN000002627
References
- 1. Cheng YJ, Imperatore G, Geiss LS, et al Secular changes in the age‐specific prevalence of diabetes among U.S. adults: 1988‐2010. Diabetes Care 2013; 36: 2690–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gregg EW, Li Y, Wang J, et al Changes in diabetes‐related complications in the United States, 1990‐2010. N Engl J Med 2014; 370: 1514–1523. [DOI] [PubMed] [Google Scholar]
- 3. Rawshani A, Rawshani A, Franzen S, et al Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2018; 379: 633–644. [DOI] [PubMed] [Google Scholar]
- 4. Kirkman MS, Briscoe VJ, Clark N, et al Diabetes in older adults. Diabetes Care 2012; 35: 2650–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Center JR, Nguyen TV, Schneider D, et al Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet 1999; 353: 878–882. [DOI] [PubMed] [Google Scholar]
- 6. Compston J. Type 2 diabetes mellitus and bone. J Intern Med 2018; 283: 140–153. [DOI] [PubMed] [Google Scholar]
- 7. Lecka‐Czernik B. Diabetes, bone and glucose‐lowering agents: basic biology. Diabetologia 2017; 60: 1163–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morin S, Lix LM, Azimaee M, et al Mortality rates after incident non‐traumatic fractures in older men and women. Osteoporos Int 2011; 22: 2439–2448. [DOI] [PubMed] [Google Scholar]
- 9. Johnell O, Kanis JA, Oden A, et al Mortality after osteoporotic fractures. Osteoporos Int 2004; 15: 38–42. [DOI] [PubMed] [Google Scholar]
- 10. Haentjens P, Magaziner J, Colon‐Emeric CS, et al Meta‐analysis: excess mortality after hip fracture among older women and men. Ann Intern Med 2010; 152: 380–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cauley JA, Thompson DE, Ensrud KC, et al Risk of mortality following clinical fractures. Osteoporos Int 2000; 11: 556–561. [DOI] [PubMed] [Google Scholar]
- 12. Bliuc D, Nguyen ND, Milch VE, et al Mortality risk associated with low‐trauma osteoporotic fracture and subsequent fracture in men and women. JAMA 2009; 301: 513–521. [DOI] [PubMed] [Google Scholar]
- 13. Kannel WB, McGee DL. Diabetes and glucose tolerance as risk factors for cardiovascular disease: the Framingham study. Diabetes Care 1979; 2: 120–126. [DOI] [PubMed] [Google Scholar]
- 14. Adler AI, Stevens RJ, Manley SE, et al Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int 2003; 63: 225–232. [DOI] [PubMed] [Google Scholar]
- 15. Ohkuma T, Peters SAE, Woodward M. Sex differences in the association between diabetes and cancer: a systematic review and meta‐analysis of 121 cohorts including 20 million individuals and one million events. Diabetologia 2018; 61: 2040–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ohkuma T, Fujii H, Iwase M, et al Impact of sleep duration on obesity and the glycemic level in patients with type 2 diabetes: the Fukuoka Diabetes Registry. Diabetes Care 2013; 36: 611–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ainsworth BE, Haskell WL, Whitt MC, et al Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 2000; 32: S498–S504. [DOI] [PubMed] [Google Scholar]
- 18. Matsuo S, Imai E, Horio M, et al Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53: 982–992. [DOI] [PubMed] [Google Scholar]
- 19. Bouillanne O, Morineau G, Dupont C, et al Geriatric Nutritional Risk Index: a new index for evaluating at‐risk elderly medical patients. Am J Clin Nutr 2005; 82: 777–783. [DOI] [PubMed] [Google Scholar]
- 20. Shah B, Sucher K, Hollenbeck CB. Comparison of ideal body weight equations and published height‐weight tables with body mass index tables for healthy adults in the United States. Nutr Clin Pract 2006; 21: 312–319. [DOI] [PubMed] [Google Scholar]
- 21. Abrahamsen B, van Staa T, Ariely R, et al Excess mortality following hip fracture: a systematic epidemiological review. Osteoporos Int 2009; 20: 1633–1650. [DOI] [PubMed] [Google Scholar]
- 22. Martinez‐Laguna D, Nogues X, Abrahamsen B, et al Excess of all‐cause mortality after a fracture in type 2 diabetic patients: a population‐based cohort study. Osteoporos Int 2017; 28: 2573–2581. [DOI] [PubMed] [Google Scholar]
- 23. Nakamura J, Kamiya H, Haneda M, et al Causes of death in Japanese patients with diabetes based on the results of a survey of 45,708 cases during 2001‐2010: report of the Committee on Causes of Death in Diabetes Mellitus. J Diabetes Investig 2017; 8: 397–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liao CC, Lin CS, Shih CC, et al Increased risk of fracture and postfracture adverse events in patients with diabetes: two nationwide population‐based retrospective cohort studies. Diabetes Care 2014; 37: 2246–2252. [DOI] [PubMed] [Google Scholar]
- 25. Carnevale V, Fontana A, Scillitani A, et al Incidence and all‐cause mortality for hip fracture in comparison to stroke, and myocardial infarction: a fifteen years population‐based longitudinal study. Endocrine 2017; 58: 320–331. [DOI] [PubMed] [Google Scholar]
- 26. Carandang R, Seshadri S, Beiser A, et al Trends in incidence, lifetime risk, severity, and 30‐day mortality of stroke over the past 50 years. JAMA 2006; 296: 2939–2946. [DOI] [PubMed] [Google Scholar]
- 27. Fang MC, Coca Perraillon M, Ghosh K, et al Trends in stroke rates, risk, and outcomes in the United States, 1988 to 2008. Am J Med 2014; 127: 608–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Masakane I, Taniguchi M, Nakai S, et al Annual dialysis data report 2015, JSDT Renal Data Registry. Ren Replace Ther 2018; 4: 19. [Google Scholar]
- 29. Coco M, Rush H. Increased incidence of hip fractures in dialysis patients with low serum parathyroid hormone. Am J Kidney Dis 2000; 36: 1115–1121. [DOI] [PubMed] [Google Scholar]
- 30. Cooper C, Atkinson EJ, Jacobsen SJ, et al Population‐based study of survival after osteoporotic fractures. Am J Epidemiol 1993; 137: 1001–1005. [DOI] [PubMed] [Google Scholar]
- 31. Browner WS, Pressman AR, Nevitt MC, et al Mortality following fractures in older women. The study of osteoporotic fractures. Arch Intern Med 1996; 156: 1521–1525. [PubMed] [Google Scholar]
- 32. Olsson H, Hagglund G. Reduced cancer morbidity and mortality in a prospective cohort of women with distal forearm fractures. Am J Epidemiol 1992; 136: 422–427. [DOI] [PubMed] [Google Scholar]
- 33. Ismail AA, Pye SR, Cockerill WC, et al Incidence of limb fracture across Europe: results from the European Prospective Osteoporosis Study (EPOS). Osteoporos Int 2002; 13: 565–571. [DOI] [PubMed] [Google Scholar]
- 34. Komorita Y, Iwase M, Fujii H, et al Impact of body weight loss from maximum weight on fragility bone fractures in Japanese patients with type 2 diabetes: the Fukuoka Diabetes Registry. Diabetes Care 2018; 41: 1061–1067. [DOI] [PubMed] [Google Scholar]
- 35. Komorita Y, Iwase M, Fujii H, et al Serum adiponectin predicts fracture risk in individuals with type 2 diabetes: the Fukuoka Diabetes Registry. Diabetologia 2017; 60: 1922–1930. [DOI] [PubMed] [Google Scholar]
- 36. Komorita Y, Iwase M, Fujii H, et al The serum creatinine to cystatin C ratio predicts bone fracture in patients with type 2 diabetes: the Fukuoka Diabetes Registry. Diabetes Res Clin Pract 2018; 146: 202–210. [DOI] [PubMed] [Google Scholar]
- 37. Chen Z, Kooperberg C, Pettinger MB, et al Validity of self‐report for fractures among a multiethnic cohort of postmenopausal women: results from the Womenʼs Health Initiative observational study and clinical trials. Menopause 2004; 11: 264–274. [DOI] [PubMed] [Google Scholar]
- 38. Yokoyama H, Matsushima M, Kawai K, et al Low incidence of cardiovascular events in Japanese patients with Type 2 diabetes in primary care settings: a prospective cohort study (JDDM 20). Diabet Med 2011; 28: 1221–1228. [DOI] [PubMed] [Google Scholar]


