Abstract
Background
Gastroesophageal reflux disease (GERDs) is a common chronic digestive system disease, in which the symptoms of reflux esophagitis (RE) seriously affect the quality of life.
Aims
We aimed to study the therapeutic effect of Zhujie Hewei granules (ZHG) on reflux esophagitis in model rats.
Materials and Methods
A rat model of RE was established with the steps of half pylorus ligation, cardiotomy, and hydrochloric acid perfusion. The rats in treatment groups were orally administered with 1.30, 2.60, or 5.20 g/kg ZHG once daily for 28 days. Histopathological changes of the esophagus were observed with hematoxylin-eosin staining. The content of total bilirubin and pH in gastric juice was determined. Esophageal mucosal injury was assessed by macroscopic observation scores, mucosal injury index scores, and esophageal inflammation scores. The levels of gastrin (GAS), motilin (MTL), and vasoactive intestinal peptide (VIP) in serum were evaluated by using ELISA kits.
Results
After treatment with ZHG, the body weight of RE rats tended to increase drastically, the macroscopic observation scores of the esophagus mucous membrane decreased (P < 0.05), the mucosal injury index scores decreased (P < 0.05), the gastric pH values increased (P < 0.05), and the levels of serum MTL and VIP decreased (P < 0.05). In addition, the high dose of the ZHG-treated group showed lower serum GAS (P < 0.05), while the high and middle doses of the ZHG-treated groups showed lower esophageal inflammation scores (P < 0.05).
Conclusions
ZHG was effective in treating RE in rats due using mechanisms including improving the pH value of gastric contents, decreasing the gastrointestinal hormones (including GAS, MTL, and VIP), and improving the inflammatory damage.
1. Introduction
Gastroesophageal reflux diseases (GERDs) are the symptoms or complications resulting from the reflux of gastric contents into the esophagus, oral cavity (including larynx), or lung [1]. GERDs are common clinical chronic diseases that up to 40% of the world's population suffer from at least once a month [2]. The prevalence of gastroesophageal reflux in Asia is lower than that in Europe and America, whereas there is an increasing trend in Asia in recent years [3]. The reflux of gastric acid, bile salts, and other noxious agents contained in the refluxed gastric juice is regarded as the major cause of reflux esophagitis (RE) [4].
Proton pump inhibitors, antireflux drugs, gastric motility drugs, and antidepressants are usually used in clinics to treat GERDs. Proton pump inhibitor is the preferred drug of choice [2, 5]. It is the most potent in inhibiting gastric acid secretion and relieving the pain in patients with GERDs, but 20 to 30 percent of patients reported no significant results [6]. Additionally, proton pump inhibitors cannot reduce the key pathogenic factors in GERDs and cannot cure GERDs completely. Moreover, the long-term use of proton pump inhibitors leads to serious complications [7, 8].
Some traditional Chinese medicines (TCMs) had been used to treat GERDs, which were effective in relieving symptoms, increasing cure rate, reducing recurrence, and showing advantages in less adverse reactions [9–11]. Zhujie Hewei prescription, which consists of four herbs including Eriobotryae folium, Atractylodis macrocephalae rhizoma, Gardeniae fructus, and Platycodonis radix, is a clinically used TCM formula designed by the Department of Gastroenterology of Shanghai Traditional Chinese Medicine Hospital. It is effective in improving the symptoms of GERDs and has been engaged in the clinical treatment of GERDs for nearly 20 years and more than 10,000 outpatients. In addition, it is safe and causes no obvious adverse reactions in patients [12–14]. However, the therapeutic mechanism remains to be elucidated.
Here, we aimed to study the therapeutic effect of Zhujie Hewei granules (ZHG) derived from Zhujie Hewei prescription, on RE in rats, focusing on revealing the underlying mechanisms. The study would be helpful in promoting the clinical applications and new drug research and development of ZHG.
2. Materials and Methods
2.1. Materials
Omeprazole enteric-coated capsules (batch number 20160625) were purchased from Changzhou Siyao Pharmaceuticals Co., Ltd (Changzhou, Jiangsu, China). 2-0 and 3-0 sutures were purchased from Jinhuan Medical Supplies Co., Ltd, Shanghai, China. Microplate reader was purchased from BioTek Synergy 2. Gastrin (GAS) linked immunosorbent assay kit was purchased from Wuhan Myhalic Biotechnology Co., Ltd (Wuhan, Hubei, China). Motilin (MTL) linked immunosorbent assay kit was purchased from Wuhan Myhalic Biotechnology Co., Ltd (Wuhan, Hubei, China). Vasoactive intestinal peptide enzyme (VIP) linked immunosorbent assay kit was purchased from Wuhan Myhalic Biotechnology Co., Ltd (Wuhan, Hubei, China). Total bilirubin detection kit (chemical oxidase standard) was purchased from the Nanjing Institute of Bioengineering (Nanjing, Jiangsu, China).
2.2. ZHG Formulation
ZHG with batch numbers 20151218, 20151221, and 20151224 were prepared by Shanghai University of Traditional Chinese Medicine. ZHG was stored at room temperature and dissolved in water when used. ZHG was packed with 12.5 g per bag that was equivalent to 4.84 g Atractylodis macrocephalae rhizoma, 3.63 g Eriobotryae folium, 3.63 g Gardeniae fructus, and 0.40 g Platycodonis radix. In brief, the herbal piece of the TCMs was extracted three times with 10 times volumes of boiled water (1 h for each extraction). The extraction was then filtered and concentrated to a relative density of 1.14∼1.16. Finally, with dextrin as the base material, the concentrated solution is sprayed into the fluidized bed for drying and granulation to obtain ZHG. ZHG are stored in the environment of normal temperature and darkness, and the validity period is 24 months. ZHG are dissolved in water when used.
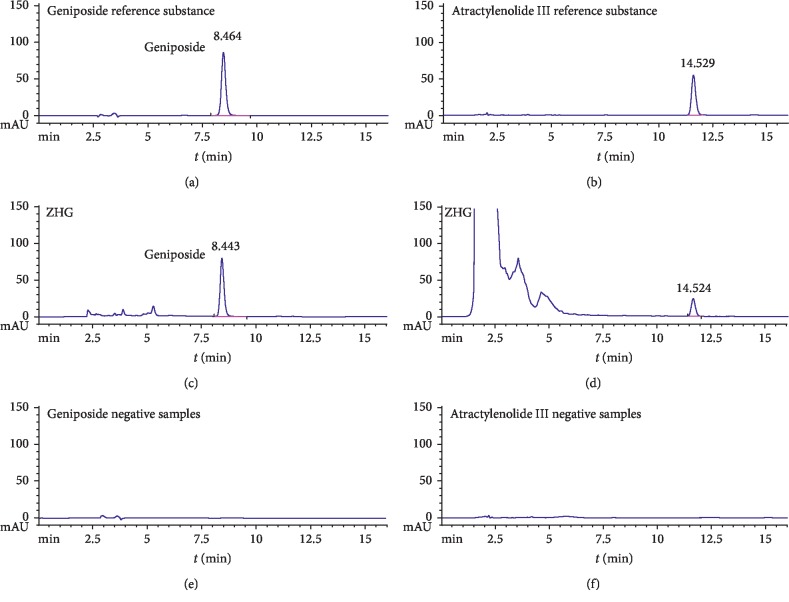
The methods of evaluating the quality of ZHG were established using quantitative high-performance liquid cartography (HPLC). Geniposide in ZHG was determined using a HPLC method with the following conditions: Phenomenex C18 chromatographic column (4.6 × 250 mm, 5 μm), mobile phase acetonitrile-water (85 : 15), flow rate 1.0 mL/min, detection wavelength 238 nm, and column temperature 30°C. In addition, atractylenolide III was measured using a HPLC method with the following conditions: Phenomenex C18 chromatographic column (4.6 × 250 mm, 5 μm), mobile phase acetonitrile-water (50 : 50), flow rate 1.0 mL/min, detection wavelength 220 nm, and column temperature 30°C. The HPLC chromatogram of Zhujie Hewei granules is shown in Figure 1. The stability was investigated by HPLC every 0, 3, 6, 9, 12, 18, and 24 months. The average content of three ZHG batches (20151218, 20151221 and 20151224) of geniposide and atractylenolide III was 14.2 and 1.5 mg/g in ZHG, respectively. After 24 months of study of room temperature stability, the two indexes changed by −3.28%–14.38% and +5.60%–5.09%, respectively.
Figure 1.
HPLC chromatography of geniposide and atractylode III.
2.3. Animals
Sprague–Dawley rats (n = 98, half male and half female) with a body weight of 180–250 g were purchased from the experimental animal center of Lanzhou University. The rats were maintained in an experimental house under controlled temperature (22 ± 2°C) with a 12 h light-dark cycle and free access to water and a standard diet. All animal experimental protocols were approved by the Animal Care and Use Committee of Lanzhou University (Approval number 2013-0002). Prior to the experiments, all rats were given a period of at least 7 days for acclimatization.
2.4. Rat RE Model
The rat model of RE was prepared according to previous studies with modification [15–18]. In brief, the model was established with the steps of hemiplegia ligation, cardiotomy, and hydrochloric acid perfusion. Animals were deprived of food for 24 h prior to surgery. Anesthesia was administered using 0.4% sodium pentobarbital. The animal was tethered in a supine position, then the upper abdomen was entered through a 3 cm midline incision, and the pylorus of the rat was ligated by using 2-0 nonabsorbable suture. After that, the muscular layer was torn vertically upward about 0.70 cm at the esophagogastric junction and sutured again, and the abdomen was closed. Three days after the surgery, the rats were treated with 0.1 mol/L hydrochloride by gavage (1.0 mL/100 g), once a day for seven days. In the sham operation group, the stomach and duodenum were separated for 15 min without ligating the pylorus and duodenum, with water instead of hydrochloric acid by gavage.
2.5. Treatment
After the RE surgery or sham operation for 10 days, the rats were randomly divided into seven groups (n = 14 each group): normal control, sham, model (RE only), low-dose group of ZHG (RE + 1.3 g/kg ZHG), middle-dose group of ZHG (RE + 2.6 g/kg ZHG), high-dose group of ZHG (RE + 5.2 g/kg ZHG), and omeprazole (RE + 4.20 mg/kg omeprazole). The ZHG, omeprazole, or normal water (for the Normal control, Sham, and Model groups) was orally administered once a day by oral gavage for 28 days.
2.6. Determination of the Levels of the Gastrointestinal Hormones in Serum
The levels of gastrin (GAS), motilin (MTL), and vasoactive intestinal peptide (VIP) in serum were evaluated by using ELISA kits. The serum was separated by centrifugation at 3000×g (4°C) for 10 min. The serum was stored at −20°C before detection. The ELISA plate was read by using a microplate reader (BioTek Synergy 2). The concentrations were determined in accordance with the manufacturer's instructions and the standard curve. All the experiments were performed in triplicate.
2.7. Determination of the pH Value and Gastric Bilirubin Amount of the Gastric Contents
After the rats were euthanized, the cardia and pylorus were ligated and then, the whole stomach was taken out. After cutting along the side of the great curvature of the stomach, the gastric cavity was washed with 5 mL distilled water and the pH values of the supernatant of the gastric content were determined using a pH meter. After filtration with filter paper, the filtrate was centrifuged for 10 min, and the supernatant was taken to determine the amount of total bilirubin by detection kit. The supernatant was stored at −20°C before use in the bilirubin detection kit. Every sample was triplicate.
2.8. Histopathological Examination of Esophagus with H&E Staining
The lower esophagus of rats was dissected and cut open longitudinally. The tissue samples were fixed in 4% paraformaldehyde (Solarbio, Beijing, China) overnight at 4°C. The samples were then rinsed with PBS (Solarbio, Beijing, China) and embedded with paraffin (50–52°C, Solarbio, Beijing, China). Then the samples were serially sectioned at a thickness of 5 μm for histologic analysis. The sections were stained with hematoxylin and eosin (Solarbio, Beijing, China). An optical microscope (Stemi DV4 or Axio Scope A1, Carl Zeiss, Oberkochen, Germany) was used to observe the histopathological changes. The index score of esophageal mucosa injury and the inflammation score of esophageal tissue were determined. The esophagus of the rat was cut longitudinally, and the damaged part was exposed, then the integral pathological grade of the esophagus ware calculated by the diagnostic criteria for RE, which was issued by the Chinese medical association digestive endoscopy society in February 1999 (Tables 1 and 2). Meanwhile, the degree of inflammation was also evaluated using the Harry S. Cooper inflammatory integral calculation method (Table 3).
Table 1.
Esophageal histopathological score.
| Gross manifestations of esophageal mucosa | Score |
|---|---|
| Normal (histologically changeable) | 0 |
| Point or stripe redness, erosion, no fusion phenomenon | 1 |
| Striped redness, erosion, fusion, nonholonomic | 2 |
| Extensive lesion, rubedo, total circumferential erosion, or ulcer | 3 |
Table 2.
Pathological grading of reflux esophagitis.
| Grading | Pathology | Score | ||||||
|---|---|---|---|---|---|---|---|---|
| Hyperplasia of squamous epithelium | Mucosal lamina propria papillae extension | Inflammatory cell infiltration in epithelial cell | Mucosal erosion | Exulceration | Barrett esophageal changes | |||
| Normal | − | − | − | − | − | − | 0 | |
| Mild | + | + | + | − | − | − | 1 | |
| Medium | + | + | + | + | − | − | 2 | |
| Severe | + | + | + | − | + | +/− | 3 | |
Table 3.
Analysis of inflammatory cells in the esophagus.
| Intensity and extent of inflammation | Score |
|---|---|
| There is almost no infiltration of inflammatory cells in the lamina propria | 0 |
| There are a small number of inflammatory cells in the lamina propria and no infiltration of inflammatory cells in the epithelial layer | 1 |
| There are more inflammatory cells in the lamina propria and no infiltration of inflammatory cells in the epithelial layer | 2 |
| There are a lot of inflammatory cells in the lamina propria and a small number of inflammatory cells infiltrate into the epithelial layer | 3 |
| Lymphoid nodules were formed in the lamina propria and a large number of inflammatory cells infiltrated in the epithelial layer | 4 |
2.9. Statistical Analysis
Data were presented as Mean ± SD. Statistically significant differences among groups were calculated by one-way ANOVA. Multiple recombination compartment comparisons were analyzed by using the LSD test. Dunnett's T3 test was used in the uneven variance. P < 0.05 was considered to be statistically significant.
3. Results
3.1. Effects of ZHG on Body Weight
As shown in Figure 2, the body weight of rats in the model group was significantly lower than those in sham operation groups (F = 2.603, P=0.041). Compared with the model group, ZHG increased body weight, as the treatment with a high dose of ZHG inhibited the body weight loss of the rats (P=0.16). In addition, compared with omeprazole, ZHG at middle and low doses had a better effect in inhibiting weight loss in rats (P=0.870; P=0.951).
Figure 2.
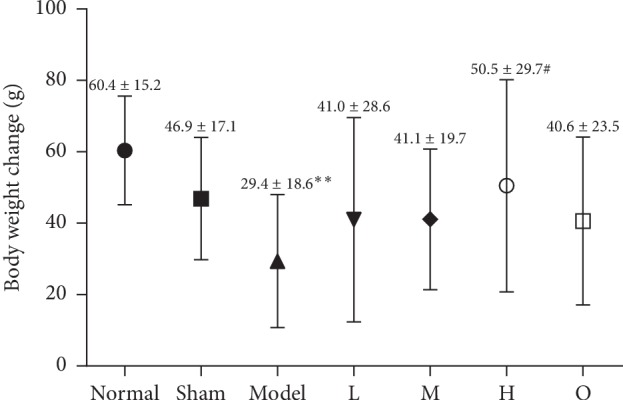
Effects of ZHG on body weight change.
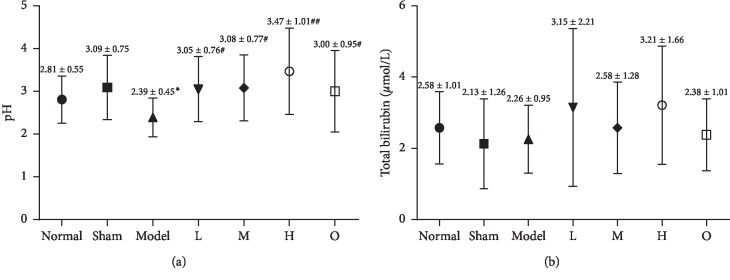
3.2. Effect of ZHG on pH and Total Bilirubin
As shown in Figure 3, compared to the sham group, the model group of reflux-induced esophagitis rats displayed a significant decrease in the gastric pH (F = 2.474, P=0.019). In contrast, the gastric pH in the low, middle, and high ZHG-treated groups was significantly increased (P=0.025; P=0.021; P < 0.00001). But there was no significant difference in the levels of total bilirubin between the normal group, the sham operation control group, the omeprazole group, and the high-, middle-, and low-dose groups of ZHG (all P > 0.05).
Figure 3.
Effects of ZHG on ER-induced changes in pH and total bilirubin in the supernatant of gastric contents in rats.
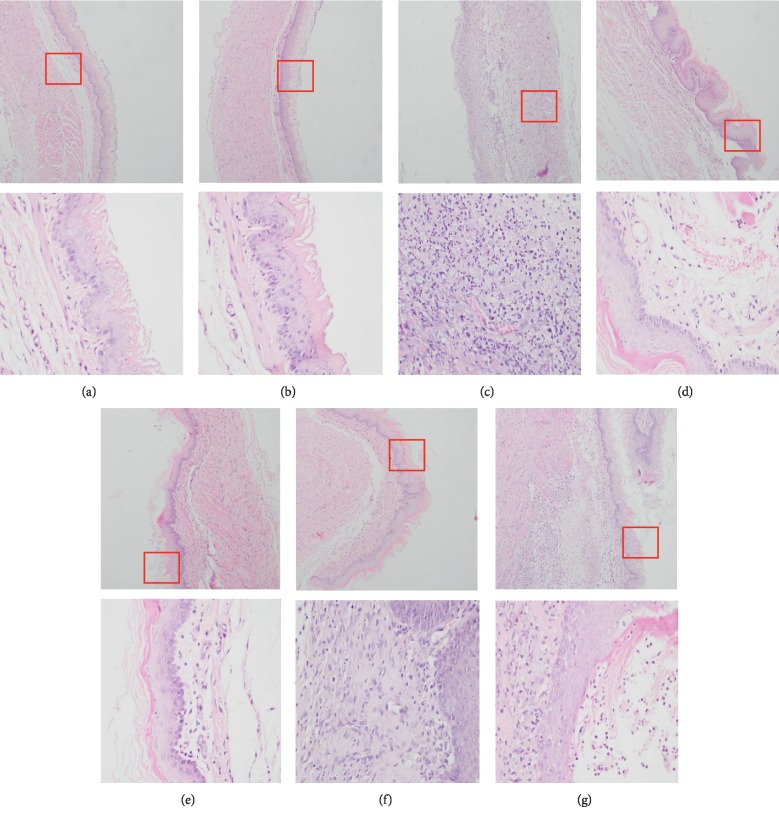
3.3. Effect of ZHG on the Histopathological Changes of Esophageal Mucosa
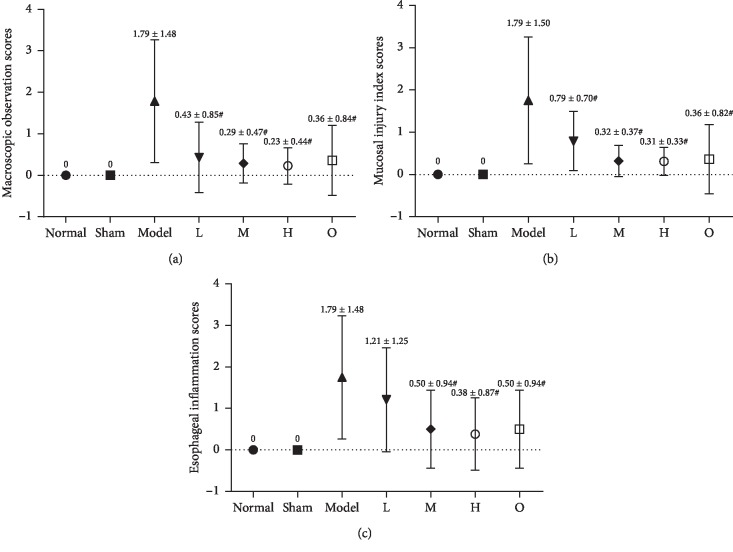
As shown in Figure 4, the esophageal mucosa of rats in the normal and sham operation groups did not show any histological changes. In contrast, the epithelium and lamina propria of esophageal mucosa in the model group were necrotic and exfoliated, and a large amount of exuded cellulose, neutrophils, and necrotic tissue fragments were observed. The esophagus and mucosa of rats in the administration group were improved to varying degrees. As shown in Figure 5, the macroscopic observation scores of the esophagus mucosa of the rats in the ZHG groups and omeprazole groups were significantly lower than those in the model group (F = 9.136, P < 0.00001). There was no significant difference between ZHG groups and the omeprazole group in terms of the change of the macroscopic observation scores of the esophageal mucosa in the rats (P=0.805; P=0.805; P=0.668).
Figure 4.
H&E showing pathological changes in rats. a–g, the surface of the rat's esophagus in the normal, sham operation, model, omeprazole, and high, middle, low dose of ZHG groups, respectively. (original magnification ×100 and 400).
Figure 5.
Effects of ZHG on macroscopic observation scores, mucosal injury index scores, and esophageal inflammation scores in rats.
3.4. Scores of Esophageal Mucosal Injury Index in Different Groups
The esophageal mucosa of rats in the normal group and the sham operation control group was normal under a light microscope. As shown in Figure 5, compared with the model group, the scores of mucosal injury index in the low, middle, and high ZHG-treated groups were decreased, with statistical significance (F = 9.858, P=0.001; P < 0.00001; P < 0.00001). Moreover, there was no significant difference in esophageal mucosal injury index scores between ZHG groups and the omeprazole group (P=0.122; P=0.897; P=0.860).
3.5. Inflammation Scores of Esophagi in Different Groups
The results were shown in Figure 5. Compared with the model group, the inflammatory scores of esophageal tissue in the high and middle dose of ZHG-treated groups and omeprazole group decreased significantly (F = 6.656, P=0.001; P < 0.00001; P < 0.00001). The inflammation scores of the esophagus in the middle and high ZHG-treated groups were not different with the omeprazole treated group (P=1.000; P=0.753).
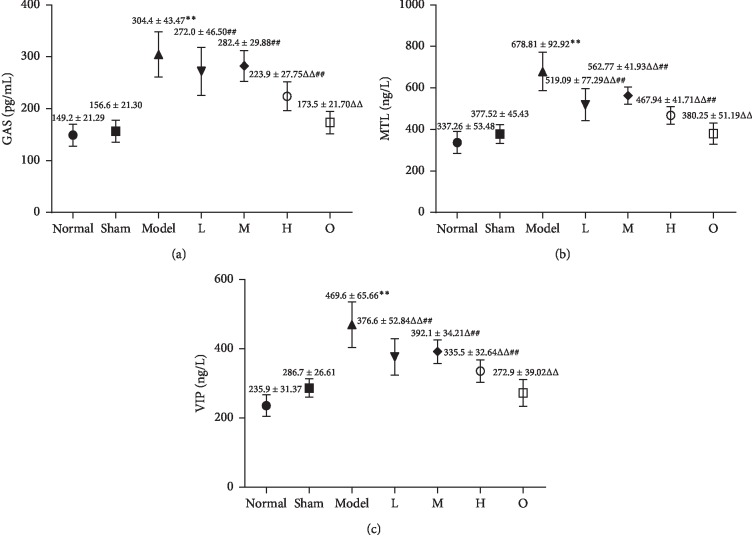
3.6. Effects of ZHG on GAS, MTL, and VIP
As shown in Figure 6, the serum levels of GAS, MTL, and VIP of rats in the model group were significantly higher than those in the sham operation group (F = 71.100, P < 0.00001; F = 56.145, P < 0.00001; F = 50.558, P < 0.00001). However, compared with the model group, the serum levels of these hormones in rats that received ZHG were significantly decreased (All P < 0.05 or P < 0.01).
Figure 6.
Effect of ZHG on GAS, MTL, and VIP in rats.
4. Discussion
After oral ZHG, the body weight of rats increased, especially in the high dose group; the result shows that the ZHG is beneficial to the treatment of RE rats; the pH value of supernatant of gastric contents in rats increased, which indicated that ZHG had inhibitory effect on gastric acid and acid reflux. The secretion of total bilirubin has no obvious effect, which showed that ZHG will not affect the secretion of bile. The macroscopic observation scores, mucosal injury index scores, and esophageal inflammation scores were decreased, which presented that ZHG protected the mucosal injury and inflammatory damage; the levels of GAS, MTL, and VIP in serum of rats with reflux esophagitis were decreased; the result shows that the ZHG regulates the serum gastrointestinal hormone levels.
The determination of the pH value of the supernatant of gastric contents in rats showed that the pH value of the gastric contents of the model group was reduced, which was related to the model replicating method. The “semipyloric ligation” of the model could block the gastric emptying, increase the content of gastric contents, increase the fermentation, and produce acid [19–21]. Our results of pH test of rat gastric contents supernatant suggested that ZHG had an inhibitory effect on the release and/or reflux of gastric acid. However, the underlying mechanism is not clear yet. It may be related to the inhibition of gastric acid secretion and pepsin activity and promoting esophageal acid clearance.
In addition, the results showed that the levels of GAS, MTL, and VIP in the serum of rats with RE were increased but were decreased after treatment with ZHG. Gastrin (GAS) is the first gastrointestinal hormone that has been found to increase the lower esophageal sphincter pressure [22]. Motilin (MTL) is a hormone produced by the endocrine cells of the duodenal mucosa, which can promote the strong contraction of the stomach and accelerate the peristalsis of the intestine [23]. Vasoactive intestinal peptide (VIP), a kind of neurotransmitter, has dual functions of organisms, both gastrointestinal hormones and neuropeptides [24]. The change of GAS, MTL, and VIP levels are closely related to gastrointestinal diseases. By detecting the levels of GAS, MTL, and VIP in serum, we can judge the therapeutic effect of ZHG on RE rats. According to the results, ZHG can reduce the reflux degree of gastric contents in RE rats, reduce the inflammatory reaction and reduce the relaxation of the lower esophageal sphincter.
In the theoretical system of traditional Chinese medicine, GERDs are mainly manifested as acid reflux, stomach reflux, plum nucleus gas, chest arthralgia, and others [25]. The lesions of this disease are located in the stomach and the esophagus, which are closely related to the liver, the spleen, the lung, the kidney, and the gallbladder. Many factors such as diet and emotion lead to the dysfunction of the spleen and the stomach and then produce the symptoms of GERDs [26, 27]. ZHG applies traditional Chinese medicine theory of Yin Yang balance to syndrome differentiation and treatment. It is made up of four TCMs according to the law of compatibility of traditional Chinese medicine. The four TCMs complement each other and act on the human body together, which has achieved a good curative effect. Among them, Eriobotryae folium contains ursolic acid and oleanolic acid, which have obvious anti-inflammatory and gastroprotective effects and can alleviate the symptoms of RE [28, 29]; Atractylodis macrocephalae rhizoma is a popularly used TCM to treat gastrointestinal diseases. It contains atractylenolide III, which is the main gastric protective constituent [30]; Gardeniae fructus contains geniposide, which has the effects of anti-inflammation, regulating bile secretion and improving intestinal mucosal damage [31–33]; Platycodonis radix contains platycodon saponin D, which has a certain therapeutic effect on RE [34]. These components in the TCMs may work alone or synergistically to enhance the therapeutic effect. In brief, the therapeutic effects of ZHG may be attributed to the bioactive compounds discussed above but it still needs further research.
5. Conclusions
ZHG was effective in treating RE rats including improving the body weight, regulating the pH value in the stomach, protecting the mucosal injury and inflammatory damage, and regulating the GAS, MTL, and VIP levels of serum gastrointestinal hormone. Besides, as the TCM formula, Zhujie Hewei prescription has also been safely used clinically and achieved a curative effect without obvious adverse reactions in patients.
Acknowledgments
This work was sponsored by the Foundation of Shanghai Municipal Science and Technology Commission (No. 16401900700) and the Xinglin Young Scholar Program of Shanghai University of Traditional Chinese Medicine.
Contributor Information
Jia-liang Hu, Email: hujialiang19830302@126.com.
Ji-quan Zhang, Email: jiquan007@139.com.
Fei Wu, Email: wufei_shutcm@126.com.
Data Availability
The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
All authors contributed to the design, drafting, and final approval/preparation of the article.
References
- 1.Katz P. O., Gerson L. B., Vela M. F. Guidelines for the diagnosis and management of gastroesophageal reflux disease. American Journal of Gastroenterology. 2013;108(3):308–328. doi: 10.1038/ajg.2012.444. [DOI] [PubMed] [Google Scholar]
- 2.Alzubaidi M., Gabbard S. GERD: diagnosing and treating the burn. Cleveland Clinic Journal of Medicine. 2015;82(10):685–692. doi: 10.3949/ccjm.82a.14138. [DOI] [PubMed] [Google Scholar]
- 3.Bor S., Yuksel E. S. How is the gastroesophageal reflux disease prevalence, incidence, and frequency of complications (stricture/esophagitis/Barrett’s esophagus/carcinoma) in Turkey compared to other geographical regions globally? The Turkish Journal of Gastroenterology. 2017;28(1):S4–S9. doi: 10.5152/tjg.2017.03. [DOI] [PubMed] [Google Scholar]
- 4.Spechler S. J. Carcinogenesis at the gastroesophageal junction: free radicals at the frontier. Gastroenterology. 2002;122(5):1518–1520. doi: 10.1053/gast.2002.33368. [DOI] [PubMed] [Google Scholar]
- 5.Salehi M., Karegar-Borzi H., Karimi M., Rahimi R. Medicinal plants for management of gastroesophageal reflux disease: a review of animal and human studies. The Journal of Alternative and Complementary Medicine. 2017;23(2):82–95. doi: 10.1089/acm.2016.0233. [DOI] [PubMed] [Google Scholar]
- 6.Savarino E., Zentilin P., Marabotto E., et al. A review of pharmacotherapy for treating gastroesophageal reflux disease (GERD) Expert Opinion on Pharmacotherapy. 2017;18(13):1333–1343. doi: 10.1080/14656566.2017.1361407. [DOI] [PubMed] [Google Scholar]
- 7.Gyawali C. P. Proton pump inhibitors in gastroesophageal reflux disease: friend or foe. Current Gastroenterology Reports. 2017;19(9):p. 46. doi: 10.1007/s11894-017-0586-5. [DOI] [PubMed] [Google Scholar]
- 8.Yasawy M. I., Randhawa M. A. GERD is becoming a challenge for the medical profession: is there any remedy? Hepato-Gastroenterology. 2014;61(134):1623–1626. [PubMed] [Google Scholar]
- 9.Ho C. E., Goh Y. L., Zhao X. X., Yu C. Y., Zhang C. GERD: an alternative perspective. Psychosomatics. 2016;57(2):142–151. doi: 10.1016/j.psym.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Teschke R., Wolff A., Frenzel C., Eickhoff A., Schulze J. Herbal traditional Chinese medicine and its evidence base in gastrointestinal disorders. World Journal of Gastroenterology. 2015;21(15):4466–4490. doi: 10.3748/wjg.v21.i15.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ling W., Li Y., Jiang W., Sui Y., Zhao H. L. Common mechanism of pathogenesis in gastrointestinal diseases implied by consistent efficacy of single Chinese medicine formula A PRISMA-compliant systematic review and meta-analysis. Medicine. 2015;94(27):p. e1111. doi: 10.1097/md.0000000000001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang H. Treatment of 45 cases of liver-stomach reflux esophagitis with He Zhong Ping Ni prescription. Chinese Medicine Modern Distance Education of China. 2014;12(8):44–45. [Google Scholar]
- 13.Xu S., Zhu L. Y. Effect of He Zhong Ping Ni prescription on proton pump inhibitor in the treatment of gastroesophageal reflux disease. Henan Traditional Chinese Medicine. 2014;34(12):2427–2429. [Google Scholar]
- 14.Qin Y., Zhu L. Y., Liu Y., Fan H. L., Somg H. W. The clinic study on curative effect of the He zhong ping Ni prescription in treating liver stomach heat accumulation syndrome of reflux esophagitis(RE) Progress in Modern Biomedicine. 2012;12(13):2511–2514. [Google Scholar]
- 15.Li Y., Wo J. M., Ellis S., Ray M. B., Jones W., Martin R. C. A novel external esophageal perfusion model for reflux esophageal injury. Digestive Diseases and Sciences. 2006;51(3):527–532. doi: 10.1007/s10620-006-3165-4. [DOI] [PubMed] [Google Scholar]
- 16.Omura N., Kashiwagi H., Yano F., Suzuki Y., Aoki T. The establishment of chronic acid reflux esophagitis in rats. Gastroenterology. 2000;118(4):p. A487. doi: 10.1016/s0016-5085(00)84068-8. [DOI] [PubMed] [Google Scholar]
- 17.Omura N., Kashiwagi H., Chen G., Yano F., Aoki T. The establishment of surgically introduced chronic reflux esophagitis in rats. Gastroenterology. 2000;116(4):p. A272. doi: 10.1080/003655299750025020. [DOI] [PubMed] [Google Scholar]
- 18.Omura N., Kashiwagi H., Chen G., Suzuki Y., Yano F., Aoki T. Establishment of surgically induced chronic acid reflux esophagitis in rats. Scandinavian Journal of Gastroenterology. 1999;34(10):948–953. doi: 10.1080/003655299750025020. [DOI] [PubMed] [Google Scholar]
- 19.Asaoka D., Nagahara A., Izumi Y., et al. Effect of lansoprazole on extraesophageal manifestations of GERD in a rat chronic acid reflux esophagitis model. Gastroenterology. 2008;134(4):A170–A171. doi: 10.1016/s0016-5085(08)60794-5. [DOI] [Google Scholar]
- 20.Terashima S., Matsusaka Y., Nishio H., Kato S., Takeuchi K. Orally administered glycine is highly effective against acid reflux esophagitis in rats. Gastroenterology. 2007;132(4):p. A490. [Google Scholar]
- 21.Li Y., Ellis S. F., Martin R. C., Ray M. B., Jones W., Wo J. M. A novel external esophageal perfusion rat model for reflux esophagitis. Gastroenterology. 2004;126(4):p. A238. [Google Scholar]
- 22.Ferrand A., Wang T. C. Gastrin and cancer: a review. Cancer Letters. 2006;238(1):15–29. doi: 10.1016/j.canlet.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 23.Luttikhold J., de Ruijter F. M., van Norren K., et al. Review article: the role of gastrointestinal hormones in the treatment of delayed gastric emptying in critically ill patients. Alimentary Pharmacology & Therapeutics. 2013;38(6):573–583. doi: 10.1111/apt.12421. [DOI] [PubMed] [Google Scholar]
- 24.Bryant M. G., Bloom S. R., Polar J. M., Albuquerque R. H., Modlin I., Pearse A. G. E. Possible dual role for vasoactive intestinal peptide as gastrointestinal hormone and neurotransmitter substance. The Lancet. 1976;307(7967):991–993. doi: 10.1016/s0140-6736(76)91863-8. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y. F., Zhang J. Q., Wu F., Runa K. F. Update on the pathogenesis and clinical treatment of gastroesophageal reflux disease. World Chinese Journal of Digestology. 2013;21(34):3821–3827. doi: 10.11569/wcjd.v21.i34.3821. [DOI] [Google Scholar]
- 26.Qin Y., Sun Z. G. Progress in research on pathogenesis and treatment of gastroesophageal reflux disease with traditional Chinese and western medicine. Chinese Journal of Integrated Traditional and Western Medicine on Digestion. 2015;23(2):147–149. [Google Scholar]
- 27.Wang X. Y., Zhu L. Y. Progress in treatment of gastroesophageal reflux disease by traditional Chinese medicine and western medicine. World Chinese Journal of Digestology. 2014;22(4):488–493. doi: 10.11569/wcjd.v22.i4.488. [DOI] [Google Scholar]
- 28.López-Hortas L., Pérez-Larrán P., González-Muñoz M. J., Falqué E., Domínguez H. Recent developments on the extraction and application of ursolic acid. A review. Food Research International. 2018;103:130–149. doi: 10.1016/j.foodres.2017.10.028. [DOI] [PubMed] [Google Scholar]
- 29.Liu J. Pharmacology of oleanolic acid and ursolic acid. Journal of Ethnopharmacology. 1995;49(2):57–68. doi: 10.1016/0378-8741(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 30.Wang K.-T., Chen L.-G., Wu C.-H., Chang C.-C., Wang C.-C. Gastroprotective activity of atractylenolide III from Atractylodes ovataon ethanol-induced gastric ulcerin vitroandin vivo. Journal of Pharmacy and Pharmacology. 2010;62(3):381–388. doi: 10.1211/jpp.62.03.0014. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Z., Li Y., Shen P., et al. Administration of geniposide ameliorates dextran sulfate sodium-induced colitis in mice via inhibition of inflammation and mucosal damage. International Immunopharmacology. 2017;49:168–177. doi: 10.1016/j.intimp.2017.05.033. [DOI] [PubMed] [Google Scholar]
- 32.Xiao W., Li S., Wang S., Ho C.-T. Chemistry and bioactivity of Gardenia jasminoides. Journal of Food and Drug Analysis. 2017;25(1):43–61. doi: 10.1016/j.jfda.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang L., Wu G., Wu F., Jiang N., Lin Y. Geniposide attenuates ANIT-induced cholestasis through regulation of transporters and enzymes involved in bile acids homeostasis in rats. Journal of Ethnopharmacology. 2017;196:178–185. doi: 10.1016/j.jep.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 34.Cho S. Y., Song C. H., Lee J. E., Choi S. H., Park S. J., Ku S. K. Effects of platycodin D on reflux esophagitis due to modulation of antioxidant defense systems. Toxicology Letters. 2014;229:p. S214. doi: 10.1016/j.toxlet.2014.06.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.