Urology, the surgical specialty addressing the diagnosis and treatment of the urinary tract in women and the reproductive and urinary tracts in men, has a strong history of successfully adopting innovative techniques in advance of other clinical specialties [1]. For example, urology initiated clinical use of the first circulating tumor marker, systemic hormone-based therapy of cancer, and robotic-assisted minimally invasive surgery [2]. Urologists continue to be innovators, having recently adopted innovations in minimally invasive surgery and by conducting clinical and laboratory-based research to develop novel devices, therapies, and diagnostic techniques.
Current urological treatments are varied and include active surveillance, pharmacological therapies, device and prosthetic implants, and ambulatory as well as major surgery, including reconstruction of the genitourinary system after trauma or disease. In addition to cancer, urologists often treat urological complications of aging or systemic disease or injuries, involving repair or restoration of components of genitourinary organs and their innervation and vascularization. These urologic conditions, as well as the innovative nature of this surgical specialty, position urology for clinical application of regenerative medicine and tissue engineering techniques in the very near future; an opinion recently embraced in an important viewpoint article in Nature Reviews Urology [1]. Therefore, urologic applications serve well as examples of the capabilities of regenerative medicine and tissue engineering for a broad variety of clinical applications.
Regenerative medicine addresses the process of replacing, engineering, or regenerating human cells, tissues, or organs to restore or establish normal function, by treatment with either autologous or allogenic stem or stromal cells [3]. Tissue engineering extends regenerative medicine to research growth of organs and tissues ex vivo in the laboratory followed by safe implantation in vivo for applications when the body cannot heal itself, even with regenerative medicine techniques. The utilization of different types of stem or stromal cells with diverse capabilities and capacities could offer effective therapeutic options to patients with a variety of benign urologic disorders, including lower urinary tract dysfunction, urinary incontinence, neurogenic bladder, and erectile dysfunction, as well as urethral and bladder trauma. With aging of the population and the dominance of diseases and disorders of the elderly in urology, novel therapies, once adopted clinically, have great potential to influence care in other clinical specialties as well [4].
No cell-based therapy is currently in clinical practice in urology, although a number of clinical trials have been completed or ongoing in the field [5]. Therefore, the goal of this special issue of Advanced Drug Delivery Reviews is to draw together the expertise of researchers in bioengineering, biomaterials, stem cells, gene therapy, and cell and matrix biology, as well as clinicians, who provide a urological roadmap for future progress. In aggregate, these papers create a body of work that comprehensively and systematically highlights innovations in the field and sets the standard for future research and clinical care. As a result, the collection of outstanding papers in this special double issue of Advanced Drug Delivery Reviews encompasses the current state of the art of research on urological applications of regenerative medicine and tissue engineering and outlines the directions of future work. To date, there have not been any urologic journal issues that have discussed this topic with such a wide overview.
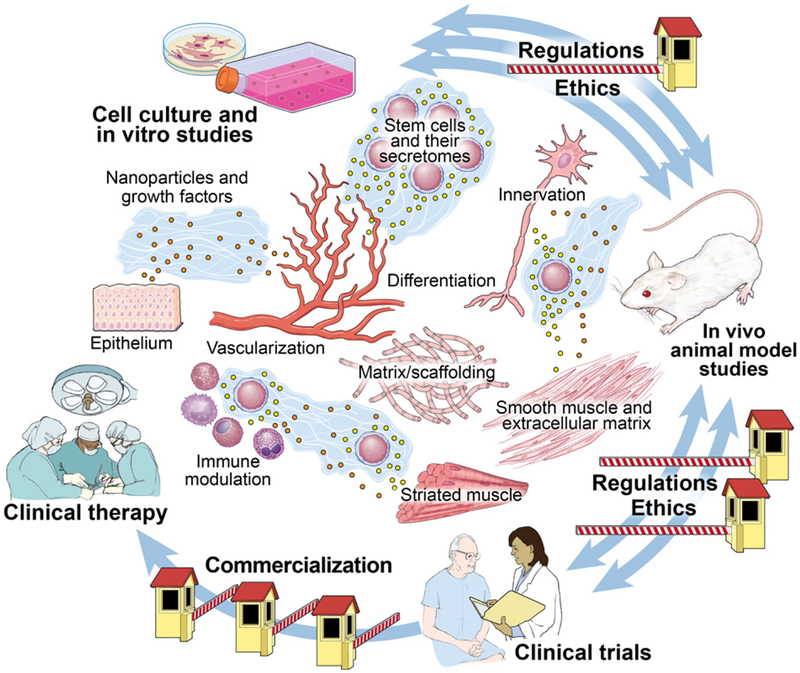
Since a primary mechanism of action of stem cell therapy is via paracrine, autocrine, and endocrine secretions [6], regenerative medicine and tissue engineering could be considered as modern methods of drug and gene therapy, which have great potential as applied to urologic conditions as illustrated in Figure 1. Tran and Damaser reviewed this literature and suggest mechanisms for application of stem cells as modern drug delivery therapies for urological conditions [7]. In addition, gene therapy can be used to manipulate the secretions of stem cells to provide improved therapeutic outcomes, as reviewed by Li et al. [8], suggesting that stem cells can be manipulated prior to transplantation to provide personalized cells for personalized medicine.
Fig. 1.
Bench to bed of tissue engineering: Clinical opportunities and hurdles.
Urologic reconstruction and other surgeries often involve implantation of prostheses, such as grafts or mesh, which could potentially be utilized as modern cell or drug delivery systems to improve outcomes as discussed by Hakim et al., using the example of treating stress urinary incontinence [9]. The strength of this special double issue is the comprehensiveness with which this important area of research is addressed. Van Ba and colleagues provide an overview of this field with their paper entitled Bladder tissue engineering: A literature review [10]. Ribeiro-Filho and Sievert systematically summarizes the potential utility of Acellular matrix in urethral reconstruction [11], while Lin and colleagues comprehensively review Biomatrices for bladder reconstruction [12].
Important and relevant areas of research in this field are given particular focus by Vaegler et al. in their paper, Tissue engineering in urothelium regeneration [13] and by Osman et al. with Tissue-engineered buccal mucosa for urethroplasty: Progress and future directions [14]. The interaction between scaffolds, cells, and drug delivery are ably reviewed in the papers by Mauney and colleagues [15] and Sharma and colleagues [16], again suggesting the opportunities for personalized therapies based on an individual patient’s need (outlined in Figure 1).
With ongoing emphasis on improving surgical outcomes, there is great clinical demand to develop advanced reconstructive techniques with improved postsurgical wound healing. In the chapter entitled Wound healing in Urology [17], Ninan and colleagues summarize the current status of wound healing and ongoing experimental research with cell and other therapies to improve outcomes. Hakim and colleagues take an alternate approach and suggest that one successful urologic surgery could be further improved with incorporation of cell-based therapy in their chapter entitled, Sling surgery for urinary incontinence and the application of cell-based therapy [9]. As in all medical fields, accurate and relevant animal models are essential for success of development and testing of novel therapies. The paper by Herrera-Imbrodaa and colleagues [18] provides a background from which judgments can be made regarding appropriate choices of animal models for different investigative paradigms.
Urologists treat localized disease, defects, or disorders that occur as a result of local injury or disease or as complications of systemic diseases such as heart disease, spinal cord injury, or diabetes. Such conditions need local delivery of treatments, but they may also need a more systemic approach and support. Andersson summarizes the current status of investigation of treatments aimed at restoring bladder function in his paper entitled Potential of stem cell treatment in detrusor dysfunction [19], while Klein and colleagues focus on restoration of urethral function in Mesenchymal stromal cells for sphincter regeneration [20]. Alwaal and colleagues review current testing of Stem cell treatment of erectile dysfunction [21] and Sadri-Ardekani et al. review current knowledge regarding Regenerative medicine for the treatment of reproductive system disorders: Current and potential options [22]. The urological complications of spinal cord injury, which are of great importance to patients and increase costs to the healthcare system, are considered by Cruz and colleagues in the paper entitled Biomarkers in spinal cord injury and ensuing bladder dysfunction [23].
Many urologic conditions involve neuromuscular systems, including the somatic system, as well as the sympathetic and parasympathetic autonomic systems, repair of which could be specifically addressed by tissue engineering and regenerative medicine techniques. Current treatment options are limited, urologic diseases are mostly localized, a large population is affected, and the demand for improved treatments is rising. To address this aspect of application of regenerative medicine to urology, Faroni and colleagues review the literature on methods of nerve regeneration, experimental strategies and future perspectives [24] while Handschin and colleagues provide a state of the art assessment of External physical and biochemical stimulation to enhance skeletal muscle bioengineering [25].
Regenerative medicine and tissue engineering have ushered in new methodologies to overcome current clinical shortcomings. These approaches hold much promise, but practical results and treatments available to patients have been slow to materialize, despite the publication of what might appear to be miracle cures, partly due to the difficulty of regulating this new paradigm for therapy which is neither a simple drug nor a device. Much work still needs to be done to move innovation from the laboratory bench to the patient’s bedside, including proof of concept in multiple animal models prior to initiating a clinical study. Existing ethical guidelines and regulations need to be carefully followed to progress from laboratory testing to clinical testing and from clinical trials to clinical practice. These important aspects of innovation development are reviewed by Beriain in his paper entitled The ethics of stem cells revisited [26], Ram-Liebig and colleagues in their paper entitled Regulatory challenges for autologous tissue-engineered products on their way from bench to bedside in Europe [27], and Knoepfler in his paper entitled From bench to FDA to bedside: US regulatory trends for new stem cell therapies [28].
This special double issue of Advanced Drug Delivery Reviews provides a comprehensive review of the current state of regenerative medicine and tissue engineering research in Urology. In so doing it is the first such effort, marking a signal moment in the state of research of the field as we prepare to advance clinical trials to clinical practice. The editors would like to thank all of the authors for their efforts to be both timely and thorough in their reviews. Due to the enormous response of the authors, we were able to accumulate 22 state-of-the-art reviews. We are grateful to those colleagues who reviewed the papers, greatly improving the quality of the papers and producing an exceptional collection of topical reviews. The editors would also like to thank P. A. Toomey, who assisted in the project management and without whose help this issue would never have come to completion. Lastly, the editors would like to thank the Advanced Drug Delivery Reviews journal, its editorial board and staff, as well as the publisher for their support of this special issue to ensure these 22 papers appropriately provided a comprehensive overview of the field.
Footnotes
This preface is part of the Advanced Drug Delivery Reviews theme issue on “Regenerative Medicine Strategies in Urology”.
Contributor Information
Margot S. Damaser, Dept of Biomedical Engineering, Glickman Urological and Kidney Institute, Cleveland Clinic, Cleveland, OH, USA; Advanced Platform Technology Center of Excellence, Louis Stokes Cleveland Department of Veterans Affairs Medical Center, Cleveland, OH, USA.
Karl-Dietrich Sievert, Dept of Urology, UKT, University of Tübingen, Tübingen, Germany; Dept of Urology, UKSH, Campus Lübeck, University of Lübeck, Lübeck, Germany; Dept of Urology and Andrology, Uro-Oncology, Neurourology, Incontinence and Reconstructive Urology, Paracelsus Medical University, Salzburg, Austria.
References
- [1].Albersen M, Cartwright R, Choyke P, Goldenberg SL, Goldman H, Lawrentschuk N, Linehan WM, Murphy D, Nagler H, Scardino P, Shortliffe L, Stenzl A, Theodorescu D, Looking forward, looking back — 10 years in urology, Nat. Rev. Urol (October 28 2014) 649–655. [DOI] [PubMed] [Google Scholar]
- [2].Hussain A, Malik A, Halim MU, Ali AM, The use of robotics in surgery: a review, Int. J. Clin. Pract 68 (11) (November 2014) 1376–1382. [DOI] [PubMed] [Google Scholar]
- [3].Mason C, Dunnill P, A brief definition of regenerative medicine, Regen. Med 3 (1) (January 2008) 1–5. [DOI] [PubMed] [Google Scholar]
- [4].Sievert KD, Amend B, Stenzl A, Tissue engineering for the lower urinary tract: a review of a state of the art approach, Eur. Urol 52 (6) (2007. December) 1580–1589. [DOI] [PubMed] [Google Scholar]
- [5].Garriboli M, Radford A, Southgate J, Regenerative medicine in urology. European journal of pediatric surgery, 24 (3) (June 2014) 227–236. doi: 10.1055/s-0034-1382259. [DOI] [PubMed] [Google Scholar]
- [6].Madrigal M, Rao KS, Riordan NH, A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods, J. Transl. Med 12 (1) (October 11 2014) 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tran C, Damaser MS, Stem cells as drug delivery methods: application of stem cell secretome for regeneration, Adv. Drug Deliv. Rev 82–83 (2015) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Li L, Zhang D, Li P, Damaser M, Zhang Y, Virus integration and genome influence in approaches to stem cell based therapy for andro-urology, Adv. Drug Deliv. Rev 82–83 (2015) 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hakim L, de Ridder D, Van Der Aa F, Slings for urinary incontinence and the application of cell-based theraphy. Adv Drug Deliv Rev 82–83 (2014. November 22) 22–30. [DOI] [PubMed] [Google Scholar]
- [10].Lam Van Ba O, Aharon S, Loutochin O, Corcos J, Bladder tissue engineering: a literature review, Adv. Drug Deliv. Rev 82–83 (2015) 31–37. [DOI] [PubMed] [Google Scholar]
- [11].Alves Ribeiro-Filho L, Sievert KD, Acellular matrix in urethral reconstruction, Adv. Drug Deliv. Rev 82–83 (2015) 38–46. [DOI] [PubMed] [Google Scholar]
- [12].Lin H-K, Madihally SV, Palmer B, Frimberger D, Fung K-M, Kropp BP, Biomatrices for bladder reconstruction, Adv. Drug Deliv. Rev 82–83 (2015) 47–63. [DOI] [PubMed] [Google Scholar]
- [13].Vaegler M, Maurer S, Toomey PA, Amend B, Sievert KD, Tissue engineering aspects in urothelium regeneration, Adv. Drug Deliv. Rev 82–83 (2015) 64–68. [DOI] [PubMed] [Google Scholar]
- [14].Osman NI, Hillary C, Bullock AJ, MacNeil S, Chapple CR, Tissue engineered buccal mucosa for urethroplasty: progress and future directions, Adv. Drug Deliv. Rev 82–83 (2015) 69–76. [DOI] [PubMed] [Google Scholar]
- [15].Mauney JR, Adam RM, Dynamic reciprocity in cell–scaffold interactions, Adv. Drug Deliv. Rev 82–83 (2015) 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sharma AK, Cheng EY, Growth factor and small molecule influence on urological tissue regeneration utilizing cell seeded scaffolds, Adv. Drug Deliv. Rev 82–83 (2015) 86–92. [DOI] [PubMed] [Google Scholar]
- [17].Ninan N, Thomas S, Grohens Y, Wound healing in urology, Adv. Drug Deliv. Rev 82–83 (2015) 93–105. [DOI] [PubMed] [Google Scholar]
- [18].Herrera-Imbrodaa B, Lara MF, Izeta A, Sievert KD, Hart ML, Urinary incontinence animal models as a tool to study cell-based regenerative therapies targeting the urethral sphincter, Adv. Drug Deliv. Rev 82–83 (2015) 106–116. [DOI] [PubMed] [Google Scholar]
- [19].Andersson K-E, Potential of stem cell treatment in detrusor dysfunction, Adv. Drug Deliv. Rev 82–83 (2015) 117–122. [DOI] [PubMed] [Google Scholar]
- [20].Hart ML, Klein G, Brinchmann JE, Rolauffs B, Stenzl A, Sievert KD, Aicher WK, Mesenchymal stromal cells for sphincter regeneration, Adv. Drug Deliv. Rev 82–83 (2015) 123–136. [DOI] [PubMed] [Google Scholar]
- [21].Alwaal A, Zaid UB, Lin C-S, Lue TF, Stem cell treatment of erectile dysfunction, Adv. Drug Deliv. Rev 82–83 (2015) 137–144. [DOI] [PubMed] [Google Scholar]
- [22].Sadri-Ardekani H, Atala A., Regenerative medicine for the reproductive system disorders: current and potential options, Adv. Drug Deliv. Rev 82–83 (2015) 145–152. [DOI] [PubMed] [Google Scholar]
- [23].Duarte Cruz C, Coelho A, Antunes-Lopes T, Cruz F, Biomarkers of spinal cord injury and ensuing bladder dysfunction, Adv. Drug Deliv. Rev 82–83 (2015) 153–159. [DOI] [PubMed] [Google Scholar]
- [24].Faroni A, Mobasseri SA, Kingham PJ, Reid AJ, Peripheral nerve regeneration: experimental strategies and future perspectives, Adv. Drug Deliv. Rev 82–83 (2015) 160–167. [DOI] [PubMed] [Google Scholar]
- [25].Handschin C, Mortezavi A, Plock J, Eberli D, External physical and biochemical stimulation to enhance skeletal muscle bioengineering, Adv. Drug Deliv. Rev 82– 83 (2015) 168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].de Miguel-Beriain I, The ethics of stem cells revisited, Adv. Drug Deliv. Rev 82–83 (2015) 176–180. [DOI] [PubMed] [Google Scholar]
- [27].Ram-Lieblig G, Bednarz J, Stuerzebecher B, Fahlenkamp D, Balsmeyer U, Spiegler ME, Liebig S, Knispe H, Regulatory challenges for autologous tissue engineered products on their way from bench to bedside in Europe, Adv. Drug Deliv. Rev 82–83 (2015) 181–191 (Growth factor). [DOI] [PubMed] [Google Scholar]
- [28].Knoepfler PS, From bench to FDA to bedside: US regulatory trends for new stem cell therapies, Adv. Drug Deliv. Rev 82–83 (2015) 192–196. [DOI] [PMC free article] [PubMed] [Google Scholar]